Abstract
Sodium-glucose linked transporter 2 (SGLT2) inhibitors are a new and promising class of antidiabetic agents which target renal tubular glucose reabsorption. Their action is based on the blockage of SGLT2 sodium-glucose cotransporters that are located at the luminal membrane of tubular cells of the proximal convoluted tubule, inducing glucosuria. It has been proven that they significantly reduce glycated hemoglobin (HbA1c), along with fasting and postprandial plasma glucose in patients with type 2 diabetes mellitus (T2DM). The glucosuria-induced caloric loss as well as the osmotic diuresis significantly decrease body weight and blood pressure, respectively. Given that SGLT2 inhibitors do not interfere with insulin action and secretion, their efficacy is sustained despite the progressive β-cell failure in T2DM. They are well tolerated, with a low risk of hypoglycemia. Their most frequent adverse events are minor: genital and urinal tract infections. Recently, it was demonstrated that empagliflozin presents a significant cardioprotective effect. Although the SGLT2 inhibitors’ efficacy is affected by renal function, new data have been presented that some SGLT2 inhibitors, even in mild and moderate renal impairment, induce significant HbA1c reduction. Moreover, recent data indicate that SGLT2 inhibition has a beneficial renoprotective effect. The role of this review paper is to explore the current evidence on the renal effects of SGLT2 inhibitors.
Keywords: albuminuria, glucosuria, hyperfiltration, renal impairment, renoprotection, SGLT2 inhibitors, SGLT transporters, tubulointerstitial fibrosis, type 2 diabetes
Introduction
According to the World Health Organization fact sheet for diabetes, 422 million people worldwide have diabetes; 90% of whom have type 2 diabetes mellitus (T2DM). In 2012 approximately 3.7 million people died from diabetes and high blood glucose. It is estimated that diabetes will be the seventh leading cause of death in 2030 [World Health Organization, 2016:fact sheet 138 and 312]. Thus, T2DM has taken on the characteristics of an epidemic along with, in westernized civilizations, physical inactivity and obesity [James, 2008]. Although the main pathophysiologic origin of T2DM is associated with β-cell failure and insulin resistance in liver and muscles (the triumvirate), multiple derangements are taking place in other organs like fat cells (increased lipolysis), the gastrointestinal tract (incretin deficiency/ resistance), the alpha-cell (hyperglucagonemia), the kidney (enhanced glucose reabsorption) and the brain (insulin resistance) comprising the Ominous Octet [DeFronzo, 2009].
Sodium-glucose linked transporter (SGLT) 2 inhibitors represent a new class of recently developed antidiabetic agents that improve glycemic control, decrease glycated hemoglobin (HbA1c), and reduce body weight, presenting also a low risk for hypoglycemia. This review paper aims to describe the mechanism of action, the efficacy and the safety of SGLT2 inhibitors, both in patients with normal renal function and with chronic kidney disease (CKD); mild, moderate or severe renal impairment. In addition, this review manuscript will explore the beneficial effects of SGLT2 inhibitors on renal function and especially on albuminuria, diabetic tubulointerstitial fibrosis and hyperfiltration in in vitro, in animal and in human studies.
SGLT-mediated glucose transportation
Although sustained hyperglycemia may be harmful for the whole body, leading to diabetic complications, glucose is recognized by the body as a valuable source of energy. Thus, a large number of complicated transport systems and biochemical processes have been integrated to serve its retention. The kidneys preserve glucose via tubular glucose reabsorption, by SGLTs, secondary active cotransporters and facilitative glucose transporters (GLUTs) [Wright et al. 2007]. SGLT1 and SGLT2, which are members of the SLC5 gene family [Wright et al. 2004], are secondary active sodium-glucose cotransporters located in the brush border (luminal membrane) of the tubular cells at the proximal tubule [Guyton and Hall, 2006]. SGLT2 transporters are situated at the convoluted (S1/2) segment of the proximal tubule [Vallon et al. 2011; Wright et al. 2011] and reabsorb approximately 80–90% of the filtered glucose [Wright et al. 2007; Abdul-Ghani and DeFronzo, 2008; Vallon et al. 2011; Wright et al. 2011]. Although they present a rather low affinity for glucose, they have very high capacity for glucose transportation and 1:1 sodium-glucose coupling [Barfuss and Schafer, 1981; Turner and Moran, 1982a, 1982b]. SGLT1 transporters are located in the straight (S3) segment of the proximal tubule and reabsorb the remaining 10–20% of glucose that ‘escapes’ SGLT2 reabsorption [Wright et al. 2011]. SGLT1 transporters present high affinity/ low capacity for glucose and 2:1 sodium-glucose coupling [Turner and Moran, 1982a, 1982b]. Apart from the kidney, SGLT1 transporters are mainly expressed in the enterocytes of the small intestine facilitating both glucose and galactose absorption [Wright et al. 1994] (Table 1).
Table 1.
Transporters that mediate glucose reabsorption in the proximal convoluted tubule of the kidney.
| SGLTs | GLUTs | ||
|---|---|---|---|
| SLC5 gene family | Major Facilitator super family of membrane transporters |
||
| Secondary active sodium/glucose cotransporters | Facilitated glucose diffusion | ||
| Brush border (luminal membrane) of tubular cells | Basolateral membrane of tubular cells | ||
| SGLT1 | SGLT2 | GLUT1 | GLUT2 |
| High affinity/low capacity | High capacity/low affinity | S3 segment of proximal straight tubule | S1/2 segment of proximal convoluted tubule |
| S3 segment of proximal straight tubule | S1/2 segment of proximal convoluted tubule | ||
| Reabsorbs 10–20% of filtered glucose | Reabsorbs 80–90% of filtered glucose | ||
| Sodium+ /glucose or galactose co- transporter | Sodium+/glucose co- transporter | ||
| Stoichiometry 2:1 for sodium:glucose | Stoichiometry 1:1 for sodium:glucose |
||
GLUT, glucose transporters; SGLT, sodium-glucose linked transporter.
Facilitative glucose transporters (GLUTs)
The GLUT1 and GLUT2 facilitative glucose transporters, which belong to the GLUT (SLC2A) protein family of human transporters, are located in the basolateral membrane of renal proximal tubular cells [Augustin, 2010]. GLUT2 facilitates glucose diffusion from the tubular cells intracellular space to the renal interstitial through the basolateral membrane at proximal convoluted (S1/2 segment) tubule while GLUT1 serves the same function at the proximal straight tubule (S3 segment) [Hediger and Rhoads, 1994].
Mechanism of tubular glucose reabsorption
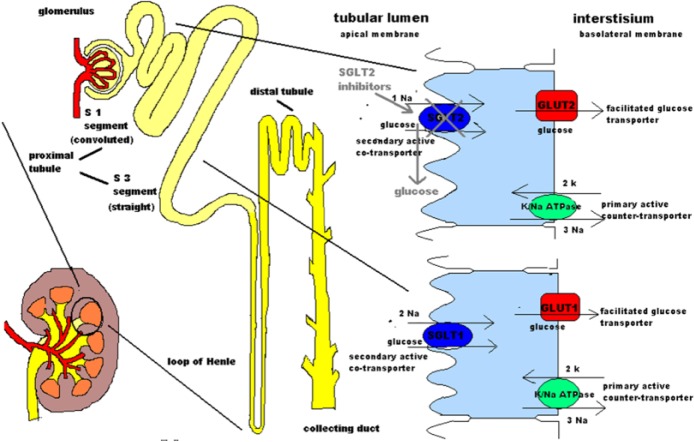
Sodium-potassium adenosine triphosphate (ATP)ase active transporter, which is located in the basolateral membrane of proximal tubular cells, constitutes the source of energy for the glucose reabsorption transport system. Energy released by the ATP hydrolysis is the main fuel for the transportation of sodium ions to the kidney interstitium through the basolateral membrane of tubular cells. This results in a reduction of the intracellular sodium concentration creating a negative electrical potential that induces sodium diffusion from the tubular lumen to the tubular intracellular space through SGLT sodium-glucose cotransporters, driving glucose transportation at the same time, against its gradient, from the lumen into the tubular cells. After entering the tubular cells, glucose is diffused to the kidney interstitium through GLUTs at the basolateral membrane [Hediger and Rhoads, 1994; Guyton and Hall, 2006] (Figure 1).
Figure 1.
A model for renal glucose reabsorption.
The sodium-potassium ATPase pump that is located is at the tubular cells of the S1 and S3 segment of the proximal convoluted tubule is a primary active counter-transporter. Its function is to export sodium to the interstitium and import potassium. ATP-ADP transformation provides the required energy for this function. Therefore, this pump creates the sodium gradient needed for the operation of the secondary active SGLT cotransporter which imports both sodium and glucose from the tubular lumen into tubular cells. Glucose moves against its electrochemical gradient following sodium transportation along its gradient. Intracellular glucose passive diffusion to the interstitium is facilitated by GLUTs which are located at the basolateral membrane of the tubular cells. Concerning SGLTs, SGLT1 is found at the S3 segment and has 2:1 sodium to glucose stoichiometry, whereas SGLT2 is located at the S1 segment and has 1:1 stoichiometry. SGLTs are on the brush border of tubular cells.
The pharmacological effect of SGLT2 inhibitors, which block SGLT2 transporters, is also shown. Thus they induce glucosuria.
ADP, adenosine diphosphate; ATP, adenosine triphosphate; GLUT, glucose transporter; SGLT, sodium-glucose linked transporter.
Glucose reabsorption physiology
Normally, blood glucose is freely filtered by the glomerular capillary membrane and passes into the tubular lumen. Given that in normoglycemic conditions the glomerular filtration rate (GFR) is about 180 l per day and the blood glucose concentration is 90–100 mg/dl, approximately 162–180 g of glucose passes from the Bowman’s capsule into the tubular lumen. Since glucose is not normally excreted in the urine, about 162–180 g of glucose per day is reabsorbed by the tubular GLUTs [Guyton and Hall, 2006; Wright et al. 2007; Abdul-Ghani and DeFronzo, 2008]. Tubular glucose reabsorption increases linearly with increasing blood glucose levels, until the glucose transport system becomes saturated and SGLTs reach their maximum transport capacity. Transport maximum (TmG) for the glucose tubular transport system in adults is about 375 mg/min [Guyton and Hall, 2006]. TmG corresponds with a blood glucose level of approximately 300 mg/dl, which is referred to as the threshold for glucose (300 mg/dl is the quotient of TmG 375 mg/min and GFR 125 ml/min). Above the level of TmG, the excess glucose filtered is not reabsorbed and passes into urine. Remarkably, even from 200 mg/dl, a small amount of glucose begins to appear in the urine. This discrepancy is imputed to both anatomical and physiologic heterogeneity of separate nephrons. Thus, for blood glucose level from 200 mg/dl to 300 mg/dl some nephrons reabsorb all the amount of filtered glucose while others exceed their threshold, they cannot reabsorb all the amount of filtered glucose and glucose starts to appear into urine. Consequently, while 300 mg/dl is the theoretical threshold for glucose, 200 mg/dl is the actual threshold and in the glucose titration curve the connection between blood glucose level and glucose reabsorption from 200 mg/dl to 300 mg/dl is not linear (as is the case <200 mg/dl) but curvilinear. This difference between the actual and the theoretical threshold for glucose is called ‘splay’. Similar to threshold, the actual TmG is <375 mg/min [Abdul-Ghani and DeFronzo, 2008; DeFronzo et al. 2012; Guyton and Hall, 2006].
Alterations of renal glucose reabsorption in diabetes
Concerning renal glucose reabsorption in diabetic patients, TmG presents as 20% elevated compared with healthy patients [Farber et al. 1951]. Thus, the mechanism of energy retention through glucose reabsorption becomes maladaptive and even in hyperglycemic states glucose reabsorption is enhanced and hyperglycemia is exacerbated [Abdul-Ghani and DeFronzo, 2014]. In a human study by Rahmoune and colleagues, a threefold increase of renal glucose uptake was shown in T2DM patients compared with the control group [Rahmoune et al. 2005]. In the same study, it was reported that there was an increase of SGLT2 mRNA and SGLT2 protein along with GLUT2 mRNA and GLUT2 protein in patients with T2DM [Rahmoune et al. 2005].
Glucosuria due to gene mutation
Familial renal glucosuria (FRG) is a genetic disease in which a mutation in the SGLT2 cotransporter gene suspends its function, causing glucosuria. Generalized kidney function and blood glucose levels remain normal. Heterozygosity for SGLT2 mutations induce mild glucosuria (<10 g/day) whereas homozygosity induces severe glycosuria (>10 g/day). FRG is usually associated with polyuria, enuresis, growth and maturation delay [Scholl-Burgi et al. 2004] and only in severe cases with dehydration and ketosis during starvation and pregnancy [Oemar et al. 1987] or urinary tract infections (UTIs) [de Marchi et al. 1983]. FRG is typically considered rather a benign differentiation of glucose retention rather than a disease [Santer and Calado, 2010]. These benign features of FRG and the encouraging results of the first studies on SGLT2 inhibitors triggered the research into SGLT2 inhibition as a therapeutic intervention for T2DM.
First SGLT2 inhibitors
The first SGLT2 inhibitor, known as phlorizin, was isolated from the bark of the apple tree in 1835 and was used firstly as an antipyretic. The glucosuric effect of phlorizin was discovered only 50 years later [White, 2010] and its mechanism of action was found to be localized at the proximal convoluted tubule in the 1970s [Vick et al. 1973]. Its molecule consists of a glucose ring connected via an oxygen atom (O-glucoside) to two phenol rings and it presents a tenfold higher affinity for SGLT2 versus the SGLT1 transporter [Vick et al. 1973]. Animal studies on phlorizin demonstrated normalization of both fasting and postprandial plasma glucose concentrations in diabetic rats [Rossetti et al. 1987b], improvement in peripheral insulin sensitivity, [Rossetti et al. 1987b], improvement in insulin secretion, [Rossetti et al. 1987a] and decline of elevated plasma glucagon levels in diabetic dogs [Starke et al. 1985]. Due to poor oral bioavailability (15%), low SGLT2 selectivity and gastrointestinal side effects from SGLT1 inhibition, phlorizin failed to progress to use in humans [Ehrenkranz et al. 2005]. Nevertheless, its beneficial effects on diabetic patients triggered research on other agents that inhibit SGLT2 transporters. T-1095 presented mild selectivity for SGLT2 transporter, thus it was not developed further [Oku et al. 1999; Misra, 2012]. O-glycoside linkages of sergliflozin, another SGLT2 inhibitor, sustain hydrolysis by intestinal β-glucosidase and as a result it has a restricted plasma elimination half-life (0.5–1 h) [Misra, 2012; Hussey et al. 2010]. Many other SGLT2 inhibitors followed these early agents. Some of them have already been approved for use in the United States, Japan and Europe while others are in different stages of clinical development (Table 2).
Table 2.
SGLT2 inhibitors currently in clinical development.
| Drug | Manufacturing company | Development phase | Reference |
|---|---|---|---|
| Dapagliflozin | Bristol-Meyers Squibb Company and AstraZeneca plc | FDA and EMA approved | [FDA, 2014; Astra Zeneca, 2012] |
| Canagliflozin | Mitsubishi Tanabe Pharma Corp., Janssen Pharmaceuticals, Inc. | FDA and EMA approved | [FDA, 2013; Johnson and Johnson, 2013] |
| Ipragliflozin | Astellas Pharma Inc., Kotobuki Pharmaceutical Co., Ltd | Approved in Japan | [Nainggolan, 2014] |
| Tofogliflozin | Chugai Pharmaceuticals Co., Kowa Co., and Sanofi S.A. | Approved in Japan | [Poole and Prossler, 2014] |
| Empagliflozin | Boehringer Ingelheim GmbH and Eli Lilly and Company | FDA and EMA approved | [Mechcatie, 2014] |
| Luseogliflozin (TSO71) | Taisho Pharmaceutical Holdings Co., Ltd | Approved in Japan | [Markaham and Elkinson, 2014] |
| Ertugliflozin (PF 04971729) | Merck & Co., Inc | Phase III | [Merck, 2015] |
| Remogliflozin etabonate | BHV Pharma and Islet Sciences, Inc. | Phase II | [Yahoo Finance, 2014] |
EMA, European Medicines Agency; FDA, Food and Drug Administration; SGLT2, sodium-glucose linked transporter 2.
Efficacy of SGLT2 inhibitors on glycemic control
In animal studies, treatment of Zucker diabetic fatty (ZDF) rats with canagliflozin reduces the renal threshold for glucose (RTg) from 415 mg/dl to 94 mg/dl. Furthermore, it was shown that when the blood glucose level was <90 mg/dl, glucose excretion was minimal [Liang et al. 2012]. In human studies, it has also been reported that canagliflozin lowers the RTg in diabetic patients from 240 mg/dl (in normal individuals this value is 180 mg/dl) to approximately 80–90 mg/dl [Rosenstock et al. 2012; Devineni et al. 2012]. Additionally, a significant HbA1c reduction has been exhibited in diabetic patients treated with dapagliflozin [Wilding et al. 2009; Bailey et al. 2010; Ferrannini et al. 2010; Strojek et al. 2011; List et al. 2009], canagliflozin [Stenlof et al. 2013], empagliflozin [Ferrannini et al. 2014] and tofogliflozin [Kaku et al. 2014]. Furthermore, treatment with ipragliflozin for 12 weeks induced a dose-dependent decrease in HbA1c from −0.49% to −0.81% [Fonseca et al. 2013]. Administration of dapagliflozin produces glucosuria-related urinary calorie loss (200–300 calories/day) [Ferrannini et al. 2010]. In addition, inhibition of renal glucose reabsorption from empagliflozin ranged from 13.1–49.6% with single doses from 1–100 mg and the amount of glucose excreted in the urine within the first 24 h after empagliflozin administration ranged from 19.6 g for a 1 mg dose to 74.3 g for a 100 mg dose [Sarashina et al. 2013]. Urinary glucose excretion (UGE) in ipragliflozin-treated patients was dose-dependent and reached approximately a maximum of 59 g glucose [Veltkamp et al. 2011]. Dapagliflozin significantly decreases both fasting plasma glucose (FPG) [Wilding et al. 2009; Ferrannini et al. 2010; Strojek et al. 2011; List et al. 2009] and postprandial glucose levels (PPG-level), [Wilding et al. 2009; List et al. 2009]. Similar effects were presented for canagliflozin, with significant reduction of both FPG [Devineni et al. 2012; Stenlof et al. 2013; Yale et al. 2013] and PPG-level [Devineni et al. 2012; Stenlof et al. 2013]. In a 28-day study in patients with T2DM, multiple doses of empagliflozin induced significant reductions in both mean FPG and mean plasma glucose (MPG) compared with the baseline values [Heise et al. 2013; Scheen 2014]. Finally, 24 weeks of tofogliflozin administration with doses from 10 mg to 40 mg significantly decreased fasting plasma glucose [Kaku et al. 2014].
Additional beneficial effects of SGLT2 inhibitors
Apart from their beneficial effect in glucose metabolism, these new antidiabetic agents have proven to be advantageous in other systems too. Body weight exhibited significant reduction in dapagliflozin [Wilding et al. 2009; Ferrannini et al. 2010; Strojek et al. 2011; List et al. 2009; Nauck et al. 2011], canagliflozin [Yale et al. 2013; FDA Advisory Committee Meeting, 2013] and tofogliflozin [Kaku et al. 2014]. With ipragliflozin body weight decreased up to 1.7 kg in 12 weeks [Veltkamp et al. 2011]. Also, in patients treated with dapagliflozin and tofogliflozin a significant waist circumference reduction was observed [Kaku et al. 2014; Bolinder et al. 2012].It has been suggested that body weight decrease may be attributed to visceral fat tissue lipolysis and enhanced lipid metabolism [Kaku et al. 2014; Bolinder et al. 2012].
Mean systolic and diastolic blood pressure (BP) were found to be lower in patients treated with dapagliflozin, possibly because of osmotic diuresis and volume reduction [Wilding et al. 2009; Ferrannini et al. 2010; List et al. 2009; Nauck et al. 2011], or canagliflozin [Devineni et al. 2012; Stenlof et al. 2013; Yale et al. 2013; FDA Advisory Committee Meeting, 2013]. Similar effects on systolic and diastolic BP have been reported for tofogliflozin [Kaku et al. 2014]. BP reduction was also observed in ipragliflozin-treated T2DM patients [Wilding et al. 2013]. SGLT2 inhibitors also demonstrated beneficial effects on the lipidemic profile of T2DM patients. High-density lipoprotein (HDL) cholesterol significantly increased with canagliflozin [Stenlof et al. 2013] and triglycerides presented a small reduction [Stenlof et al. 2013; FDA Advisory Committee Meeting, 2013], although only one trial reached significance [FDA Advisory Committee Meeting, 2013]. Dapagliflozin produced a small increase in HDL cholesterol without altering patients’ lipidemic profile [Bailey et al. 2010; Strojek et al. 2011; Nauck et al. 2011], whereas in tofogliflozin-treated patients there were significant improvements in both HDL cholesterol and triglyceride levels [Kaku et al. 2014]. β-cell function and HOMA2-%B were improved in patients treated with canagliflozin [Rosenstock et al. 2012; Stenlof et al. 2013]. Additionally, treatment with empagliflozin reduced insulin secretion and tissue glucose disposal, increased endogenous glucose production and glucagon-like peptide-1 response and improved β-cell function, and insulin sensitivity of tissue glucose uptake [Ferrannini et al. 2014]. In a recent study, male ZDF rats were treated with empagliflozin. It was shown from this study that SGLT2 inhibitors evoked beneficial effect on β-cell mass and retarded the loss of β-cells in T2DM, possibly by lowering glucose toxicity [Hansen et al. 2014; Novikov and Vallon, 2015]. Administration of tofogliflozin also had beneficial effect on pancreatic β-cell function [homeostatic model assessment (HOMA-B)] and insulin resistance (HOMA-IR) [Kaku et al. 2014].
According to a recent study, SGLT2 cotransporters are also expressed and are functional in glucagon-secreting α-cells of the pancreatic islets [Bonner et al. 2015]. In the same study, it was demonstrated that in diabetic patients the SGLT2 expression is reduced on α-cells and SGLT2 inhibitors induce glucagon release [Bonner et al. 2015]. Therefore, SGLT2 inhibition stimulates hepatic gluconeogenesis by glucagon production and reduced blood glucose levels [Novikov and Vallon, 2015]. On the other hand, it is exhibited in other recent studies that SGLT2 inhibition reduced kidney gluconeogenesis, which is up-regulated in diabetes [Gerich 2010; Vallon et al. 2014; Novikov and Vallon, 2015].
Main adverse effects
According to the existing clinical studies, the most common side effects of SGLT2 inhibitors are the increased incidence of genital infections (GIs) and UTIs that could be attributed to the glucosuric effect of these agents [Ferrannini et al. 2010; Nyirjesy et al. 2012; Rosenstock et al. 2012; Devineni et al. 2012; Stenlof et al. 2013; Yale et al. 2013; Rosenstock et al. 2013; Ferrannini et al. 2013a]. Especially, according to a study with dapagliflozin, females were more susceptible to both GIs and UTIs than males and the incidence of these infections was connected with the dapagliflozin dose [Nauck et al. 2011]. Due to their mechanism of action, SGLT2 inhibitors have a very low risk of hypoglycemia [Wilding et al. 2009; Ferrannini et al. 2014; Sarashina et al. 2013; Kaku et al. 2014; Wilding et al. 2013; Rosenstock et al. 2013; Ferrannini et al. 2013a; Kovacs et al. 2013]. The reported hypoglycemic episodes were mild-to-moderate in severity [Wilding et al. 2009]. Other side effects that can be observed with SGLT2 inhibitors are pollakiuria and thirst which can be assigned to osmotic diuresis due to the glucosuric effect of SGLT2 inhibitors [Stenlof et al. 2013; Yale et al. 2013; Janssen Research & Development LLC, 2012; FDA Advisory Committee Meeting, 2013; Kaku et al. 2014; Rosenstock et al. 2013]. Hyperketonemia and ketonuria were also observed [Kaku et al. 2014], as a result of increased lipolysis and mobilization of lipids and free fatty acids [Kaku et al. 2014]. The increased risk of ketoacidosis in patients treated with SGLT2 inhibitors was presented in May 2015 by the US Food and Drug Administration (FDA) [http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm]. It was recently described that the potential mechanisms which may be responsible for ketoacidosis in patients treated with SGLT2 inhibitors include lower insulin and higher glucagon levels that lead to increased lipolysis and ketogenesis and potentially increased renal tubular reabsorption of ketones [Taylor et al. 2015]. On the other hand, in the EMPA-REG OUTCOME study (Empagliflozin Cardiovascular Outcome Event Trial in T2DM patients) it was demonstrated that there was no difference in rates of ketoacidosis in patients with T2DM treated with empagliflozin versus placebo [Zinman et al. 2015]. Pertinent to osmotic diuresis, volume depletion events like hypotension, hypovolemia, dizziness and dehydration, have been reported as slightly elevated [Janssen Research & Development LLC, 2012; FDA Advisory Committee Meeting, 2013; Rosenstock et al. 2012]. Finally, it is noteworthy that the number of discontinuations of SGLT2 inhibitors due to adverse events was similar to the placebo group [Berhan and Barker, 2013].
According to the new drug application for dapagliflozin which was submitted to the US FDA in 2011, a numerical imbalance was observed in bladder and breast cancer cases between treatment groups: 9 out of 5478 patients on dapagliflozin and 1 out of 3156 patients in the control group reported bladder cancer in the pooled dapagliflozin studies and 9 patients on dapagliflozin and 1 in the control group reported breast cancer [United States Food and Drug Administration, 2011]. A post hoc pooled analysis of dapagliflozin clinical trials was published recently that confirmed the numerical imbalance of bladder cancer cases [Ptaszynska et al. 2015]. It was reported in the same study that none of the preclinical studies indicated that dapagliflozin is carcinogenic [Reilly et al. 2014; Tirmenstein et al. 2013]. Furthermore, neither the drug itself nor the drug-induced glucosuria have been found to induce the growth of human bladder cancer cells [Ptaszynska et al. 2015]. Moreover, it has been reported that the patients with urothelial cell carcinomas were over 60 years old, male, and most of them were current or former smokers and typical of the general population [Ptaszynska et al. 2015]. It was also demonstrated that the dapagliflozin trials were not large enough and were not designed to assess the risk of bladder cancer. Finally, it is concluded that there was not a causal relationship between dapagliflozin and bladder cancer and the numerical imbalance of bladder cancer cases may be attributed to chance [Ptaszynska et al. 2015]. Similar results have been reported by another study, in which it was suggested that the imbalance of bladder cancer and breast cancer with dapagliflozin could be a result of early diagnosis of preexisting cancer [Lin and Tseng, 2014]. However, it is suggested that additional clinical data are necessary for firm conclusions and ongoing trials of dapagliflozin will carefully investigate the risk of bladder and breast cancer [Lin and Tseng, 2014; Ptaszynska et al. 2015; ClinicalTrials.gov Identifier: NCT02695121].
Data regarding the efficacy and safety of SGLT2 inhibitors in patients with T2DM and CKD present a special interest, given that renal failure is a common complication of T2DM [Kramer and Molitch, 2005] and UGE is related to GFR and blood glucose levels [Yale et al. 2013]. According to a dapagliflozin study on diabetic patients with CKD, decreasing GFR restrains the ability of dapagliflozin to inhibit tubular glucose reabsorption [Kohan et al. 2014]. In another study on T2DM patients with stage 3 CKD (30 ⩽ GFR ⩽ 60 ml/min), the administration of dapagliflozin resulted in a 50% lower UGE than in T2DM patients with normal or mildly impaired renal function [Kohan et al. 2014]. Although the use of dapagliflozin in patients with GFR between 45–59 ml/min resulted in a modest decrease in FPG and in HbA1c, there was no significant reduction of these parameters in patients with more advanced CKD [Kohan et al. 2014]. Although dapagliflozin-treated patients with CKD did not achieved a glycemic benefit, they presented a significant weight improvement. Finally, it was demonstrated that dapagliflozin administration lowered albumin excretion in CKD patients compared with placebo [Kohan et al. 2014].
In another study with canagliflozin, in patients with stage 3 diabetic CKD (Estimated Glomerular Filtration Rate (eGFR) ⩾ 30 and <50 ml/min/1.73 m2) significant reduction of HbA1c from the baseline was demonstrated. In the same study, the number of patients in the canagliflozin group who achieved HbA1c < 7.0% was significantly higher than those in the placebo group, while the comparison with FPG did not present statistically different reductions in the two groups [Yale et al. 2013]. In both groups it was observed that there was a beneficial body weight reduction, a decrease in systolic and diastolic BP, an increase in HDL cholesterol and a decrease in low-density lipoprotein cholesterol [Yale et al. 2013]. It was also demonstrated that SGLT2 inhibitors are not anticipated to produce significant effects in patients with eGFR < 30 ml/min/1.73 m2 or in patients on dialysis [Yale et al. 2013]. Although the adverse events of canagliflozin in CKD patients and patients with normal renal function did not differ (GIs, UTIs, osmotic diuresis-related events) their frequency was lower in the former group of patients as a result of a moderate glucosuric effect of canagliflozin in these patients [Yale et al. 2013]. Regarding the effect of canagliflozin on renal function, although the onset of the treatment with canagliflozin induced an eGFR reduction in the first 3 weeks of administration, eGFR levels returned to normal over the 26-week treatment period [Yale et al. 2013]. Finally, in CKD patients, aggravation of albuminuria was more frequent in the placebo group compared with canagliflozin treated patients [Yale et al. 2013].
The effect of the SGLT2 inhibitor ipragliflozin on T2DM patients with CKD has also been evaluated. In this study, it was demonstrated that the level of glucosuria after administration of a single dose of ipragliflozin was related to the patients’ GFR and the plasma glucose concentration [Ferrannini et al. 2013b]. In addition, it was calculated that for each 20 ml/min/1.73 m2 reduction of the GFR, glucosuria was decreased by 15 g/day and for each 10 mg/dl increase of fasting glucose glucosuria was increased by 7 g/day [glycosuria (g/day) = −87 ± 14 + 0.77 ± 0.12 × eGFR (ml/min/ 1.73 m2) + 0.70 ± 0.10 × fasting glucose (mg/dl)]; r = 0.81; p < 0.0001) [Ferrannini et al. 2013b]. This equation could be helpful for the prediction of glucosuria, providing a quantification of the pharmacodynamics of ipragliflozin in T2DM patients with CKD [Ferrannini et al. 2013b].
The efficacy of empagliflozin was also evaluated in patients with CKD. In a study in patients with CKD from mild to end-stage, it was demonstrated that UGE was decreased with the increase of the renal impairment stage [Macha et al. 2014]. It was also exhibited that a single dose of 50 mg empagliflozin was well tolerated regardless of the stage of renal impairment and there was no need for dose adjustment in CKD patients [Macha et al. 2014]. In another study in T2DM patients with stage 2 and 3 CKD (eGFR ⩾ 60 to <90 and eGFR ⩾ 30 to <60, respectively) empagliflozin reduced HbA1c during 24 weeks of administration (stage 2: −0.52% for empagliflozin 10 mg, −0.68% for empagliflozin 25 mg, stage 3: −0.42% for empagliflozin 25 mg) [Barnett et al. 2014].
Renoprotection of SGLT2 inhibitors
As reported in recent studies, SGLT2 inhibitors, as well as improving glucose control, may also present a beneficial effect in renal function in patients withT2DM.
It has been proven that the glucose entry into kidney tubular cells is essential for the development of diabetic nephropathy [Johnson et al. 1998; Panchapakesan et al. 2004; Qi et al. 2006] leading to a histological change which is described as tubulointerstitial fibrosis, involving both proximal tubular cell (PTCs) basement membrane alterations and interstitial fibrosis [Gilbert and Cooper, 1999; Tervaert et al. 2010]. The inflammatory and profibrotic effects in PCTs are directly derived from high glucose or mediated by the hyperplasic and profibrotic cytokine transforming growth factor β (TGFβ) [Johnson et al. 1998; Panchapakesan et al. 2004, 2005; Holian et al. 2008; Qi et al. 2005].
In an in vitro study, human PTCs were exposed to high glucose or to TGFβ1 factor for 72 h in the presence or absence of empagliflozin [Panchapakesan et al. 2013]. The results of the study suggested that SGLT2 expression was not directly regulated by high glucose, while high glucose levels increased TGFβ1 secretion from PTCs, which in turn, raised SGLT2 expression [Panchapakesan et al. 2013]. It was also reported that high glucose and SGLT2 inhibition did not affect SGLT1 and GLUT2 expression [Panchapakesan et al. 2013]. Finally it was exhibited that SGLT2 inhibition might reverse the tubulointerstitial inflammatory processes, caused by high glucose, like Toll-like receptor 4 expression, interleukin-6 secretion, nuclear factor-κB binding activity, activator protein 1 binding activity and collagen IV expression [Panchapakesan et al. 2013].
In an animal study by Vallon and colleagues investigating the effects of empagliflozin in type 1 diabetic Akita mice, it was reported that SGLT2 inhibition decreased GFR and defended diabetic glomerular hyperfiltration [Vallon et al. 2014]. SGLT2 inhibition was also responsible for blood glucose reduction, preventing also albuminuria and inflammation processes in the early stages of diabetic nephropathy.
In another animal study by Nagata and colleagues in type 2 diabetic mice (db/db), the effect of an 8-week administration of the SGLT2 inhibitor tofogliflozin was investigated on renal and β-cell function compared with the angiotensin II receptor antagonist losartan [Nagata et al. 2013]. The results of this study suggested that tofogliflozin reduced plasma glucose and HbA1c, while preserving the pancreatic β-cell mass and plasma insulin levels. It was also demonstrated that tofogliflozin might be protective for the glomerular hypertrophy decreasing urinary albumin/creatinine ratio [Nagata et al. 2013]. Additionally, losartan had no effect on glycemic control while presenting a significant improvement in albumin/creatinine ratio. Finally it was estimated that tofogliflozin may have the same beneficial effect on progression of diabetic nephropathy in T1DM patients as well [Nagata et al. 2013].
According to the study by Younis and colleagues on Cohen–Rosenthal diabetic hypertensive (CRDH) rats treated with empagliflozin for 18 weeks, there was a significant reduction in hyperglycemia and improvement of insulin resistance as measured by HOMA-IR [Younis et al. 2014]. Furthermore, the administration of empagliflozin improved diabetic nephropathy and pancreatic damage by reduction of proteinuria and decrease of pancreatic fatty infiltration, respectively [Younis et al. 2014].
Mark and colleagues, in their study on type 1 diabetic Akita mice, hypertensive type 2 diabetic Dahl-STZ (streptozotocin) rats, and normotensive and hypertensive BTBR ob/ob (BTBR means black and tan, brachyuric mouse strain and the ob/ob mutation causes lack of the hormone leptin.) mice treated with empagliflozin for 9–15 weeks, it was demonstrated that the administration of this SGLT2 inhibitor induced significant blood glucose reductions in all groups and BP reduction in Akita mice and Dahl-STZ rats [Mark et al. 2014],. Furthermore, in the same study it was shown that empagliflozin has an important renal benefit which derives from the significant decrease of proteinuria. Additionally, the proteinuria restriction was observed in several preclinical models of nephropathy and was independent of diabetes type and BP status [Mark et al. 2014].
According to a human study by Cherney and colleagues, SGLT2 inhibition and renal hyperfiltration plays a very important role in diabetic nephropathy progression and SGLT2 inhibitors are considered as a very promising method for its prevention [Cherney et al. 2014]. Hyperfiltration is an early renal hemodynamic abnormality which is associated with increased intraglomerular pressure [Hostetter et al. 1981, 1982; Brenner et al. 1981], it is estimated to be present in 60% of Type 1 Diabetes Mellitus (T1DM) patients and it has been related to the development of diabetic nephropathy [Magee et al. 2009; Ruggenenti et al. 2012; Jerums et al. 2010]. The pathogenesis of hyperfiltration can be attributed both to neurohormonal/vascular factors and to tubuloglomerular feedback mechanisms [Sasson and Cherney, 2012]. According to the vascular hypothesis, the treatment of hyperfiltration concerns, so far, renal arteriolar tone regulators such as cyclooxygenase-2 inhibitors, renin–angiotensin–aldosterone system (RAAS) blockers, nitric oxide (NO) synthase inhibitors and protein kinase C inhibitors [Cherney et al. 2008, 2009, 2012; Sochett et al. 2006]. According to the tubular hypothesis, the increased glucose reabsorption due to chronic hyperglycemia in diabetes induces sodium reabsorption along with glucose. As a result, the low concentration of sodium at the macula densa is detected as a low effective circulating volume and induces afferent arteriole vasodilation which increases GFR and causes hyperfiltration [Vallon et al. 1999]. The participants in this study, who were patients with T1D, were separated into two groups: patients with normal filtration rate [GFR: 90–134 ml/min/1.73 m2 (T1D-N)] and patients with renal hyperfiltration [GFR ⩾ 134 ml/min/1.73 m2 (T1D-H)] and treated with empagliflozin 25 mg for 8 weeks [Cherney et al. 2014]. According to the results, empagliflozin administration induced a significant GFR reduction both under euglycemic and hyperglycemic conditions (reduction of GFR by 33 ml/min/1.73 m2 and 44 ml/min/1.73 m2, respectively) in the T1D-H group [Cherney et al. 2014]. However, GFR was not significantly decreased in T1D-N patients [Cherney et al. 2014]. In addition in the T1D-H group, apart from the GFR reduction, there were other beneficial effects like a significant decline in effective renal plasma flow and renal blood flow, a significant rise in renal vascular resistance and a significant reduction of systolic BP [Cherney et al. 2014]. In the same study it was presented in the T1D-H group that there was a significant increase in circulating RAAS mediators and reduction of plasma NO levels, whereas in the T1D-N group only aldosterone levels were increased significantly while there was no effect on NO [Cherney et al. 2014]. In both groups HbA1c declined significantly, accompanied by a decrease in total daily insulin dose. It was also presented that the body weight and the body mass index were decreased, while 24-h UGE was significantly increased in both groups. In the T1D-H group the changes in UGE were even greater [Cherney et al. 2014]. Additionally, the 24 h urine volume was significantly increased but only in the T1D-H group [Cherney et al. 2014]. Finally it was concluded that the efficacy of SGLT2 inhibitors on GFR reduction and hyperfiltration is equivalent to pharmacological RAAS blockage [Cherney et al. 2014].
Further observation of a cohort of normotensive, normoalbuminuric patients, revealed that vasodilation of the afferent arteriole characterized by lower afferent renal arteriolar resistances (RA) was exhibited in T1D–H participants compared with T1D–N participants [Škrtić et al. 2014]. It was also demonstrated that treatment with empagliflozin for 8 weeks decreased RA and also reduced glomerular hydrostatic pressure (PGLO) in T1D–H patients, with no effect on efferent renal arteriolar resistances (RE). These hemodynamic changes that were induced by empagliflozin significantly improved intraglomerular hypertension. Consequently SGLT2 inhibition is shown to be beneficial on early renal abnormalities that characterize T1DM and further investigation could enlighten us as to whether this renal beneficial effect is sustainable [Škrtić et al. 2014]
Furthermore, more investigation is needed to prove whether SGLT2 inhibitors, which have beneficial effects on the afferent renal arteriole (constriction of the afferent arteriole), can be combined with RAAS inhibition, which induces changes in RE (efferent arteriole vasodilatation) and has cumulative renoprotective effect [Škrtić et al. 2014]
According to the EMPA-REG OUTCOME study, empagliflozin has a protective effect against the progression of kidney disease in T2DM. Patients with T2DM and estimated GFR of at least 30 ml/min received either empagliflozin or placebo once daily. It was demonstrated that the number of empagliflozin-treated patients whose nephropathy was worse was lower compared with placebo. Also, the number of empagliflozin-treated patients who doubled their serum creatinine level was lower compared with placebo and the initiation of renal replacement therapy was significantly lower in the empagliflozin group. From this study it was concluded that empagliflozin decreases the progression of kidney disease and lowers the rate of renal relevant events [Wanner et al. 2016]
Impact of SGLT2 inhibitors on cardiovascular disease
The beneficial effect of SGLT2 inhibitors on BP has already been demonstrated from the early clinical trials of these agents. A recent review on the effect of dapagliflozin and canagliflozin on both hypertensive and normotensive patients with T2DM confirmed a 4–10 mmHg reduction of systolic BP [Oliva and Bakris, 2014]. Furthermore, in a recent study on empagliflozin, 825 patients with T2DM and hypertension were randomized to 10 mg or 25 mg empagliflozin or placebo once daily for 12 weeks. At week 12, mean 24 h systolic BP [ambulatory BP monitoring (ABPM)] was −3.44 mmHg on 10 mg empagliflozin and −4.16 mmHg on 25 mmHg empagliflozin versus placebo and mean 24 h diastolic BP was −1.36 mmHg on 10 mg empagliflozin and −1.72 mmHg on 25 mg empagliflozin versus placebo; therefore empagliflozin significantly reduces not only HbA1c but also BP [Tikkanen et al. 2015]. SGLT2 inhibitors have been proven to improve more than one cardiovascular risk factor, reducing HbA1c, body weight and BP and present a beneficial effect on the lipidemic profile at the same time [Majewski and Bakris, 2015]
The effect of SGLT2 inhibition on cardiovascular disease was confirmed by the results of the EMPA-REG OUTCOME study. A total of 7020 patients with T2DM who were drug-naïve or on background glucose-lowering therapy and were at high risk of cardiovascular events, were randomized and treated with empagliflozin 10 mg, empagliflozin 20 mg or placebo. It was demonstrated that in the pooled empagliflozin group there were significantly lower rates of the primary outcome of the study (cardiovascular death, nonfatal myocardial infarction, nonfatal stroke) compared with placebo [hazard ratio (HR) = 0.86; 95% confidence interval (CI) = 0.74–0.99; p = 0.04 for superiority]. Also there were significantly lower rates of death from cardiovascular causes (3.7% versus 5.9% in the placebo group; 38% relative risk reduction), hospitalization for heart failure (2.7% and 4.1%, respectively; 35% relative risk reduction), and death from any cause (5.7% and 8.3%, respectively; 32% relative risk reduction). There were no significant results for nonfatal myocardial infarction, nonfatal stroke, nor hospitalization for unstable angina [Zinman et al. 2015].
Further investigation was conducted in patient subgroups, including those with or without baseline heart failure. It was shown that empagliflozin improved heart failure outcomes, in terms of hospitalization for heart failure or death from heart failure [2.8 versus 4.5%; HR, 0.61 (0.47–0.79); p < 0.001] and was related to a reduction of all-cause hospitalization [36.8 versus 39.6%; HR, 0.89 (0.82–0.96); p = 0.003] [Fitchett et al. 2016].
According to the results of a recent meta-analysis regarding the effects of SGLT2 inhibitors on cardiovascular events, in adults with T2DM, it was shown that SGLT2 inhibitors protect against the risk of major adverse cardiovascular events, cardiovascular death, heart failure and death from any cause. Also, there was no significant benefit for nonfatal myocardial infarction or angina and an adverse effect was shown only for nonfatal stroke. Finally, in the same study it was suggested that there was no clear evidence about the differing effect of the various drugs in cardiovascular outcomes or death [Wu et al. 2016]
As well as the EMPA-REG study there are many cardiovascular trials on SGLT2 inhibitors, the results of which are expected in the next few years (Table 3).
Table 3.
Cardiovascular ongoing endpoint studies with SGLT2 inhibitors.
| Drug | Study | Purpose | Final results | ClinicalTrials.gov identifier |
|---|---|---|---|---|
| Dapagliflozin | Dapagliflozin Effect on Symptoms and Biomarkers in Diabetes Patients with Heart Failure (DEFINE-HF) | The primary purpose of this study is to evaluate the impact of dapagliflozin, as compared with placebo, on heart failure disease-specific biomarkers, symptoms, health status, and quality of life in patients with type 2 diabetes and chronic heart failure with reduced systolic function. | Expected May 2017 | NCT02653482 |
| Dapagliflozin | Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI58) | This study is being carried out to see if dapagliflozin when added to a patients current anti-diabetes therapy is effective in reducing cardiovascular events such as myocardial infarction, ischemic stroke, and cardiovascular-related death, compared with placebo (inactive study medication) | Expected April 2019 | NCT01730534 |
| Canagliflozin | CANVAS - CANagliflozin cardioVascular Assessment Study (CANVAS) | The study will assess canagliflozin (JNJ-28431754) in the treatment of patients with T2DM with regard to cardiovascular risk for major adverse cardiac events. Other objectives include evaluating the overall safety, tolerability, and effectiveness of canagliflozin | Expected June 2017 | NCT01032629 |
| Canagliflozin | Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants with Diabetic Nephropathy (CREDENCE) | The goal of this study is to assess whether canagliflozin has a renal and vascular protective effect in reducing the progression of renal impairment relative to placebo in participants with T2DM, Stage 2 or 3 CKD and macroalbuminuria, who are receiving standard of care including a maximum tolerated labeled daily dose of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker | Expected June 2019 | NCT02065791 |
| Empagliflozin | (Empagliflozin) Cardiovascular Outcome Event Trial in T2DM Patients (EMPA-REG OUTCOME) | The aim of the present study is to investigate the safety of BI 10773 treatment in patients with T2DM and high cardiovascular risk | Published September 2015 |
NCT01131676 |
| Ertugliflozin | Cardiovascular Outcomes Following Treatment with Ertugliflozin in Participants With T2DM and Established Vascular Disease (MK-8835-004) | A study of the cardiovascular outcomes following treatment with ertugliflozin in participants with T2DM and established vascular disease. The main objective of this study is to assess the cardiovascular safety of ertugliflozin. This trial includes a predefined glycemic substudy in participants receiving background insulin with or without metformin and another predefined glycemic substudy in participants receiving background sulfonylurea monotherapy | Expected June 2020 | NCT01986881 |
CKD, chronic kidney disease; SGLT2, sodium-glucose linked transporter 2; T2DM, type 2 diabetes mellitus.
SGLT2 inhibitors and T1DM
There are limited data about the therapeutic effects of SGLT2 inhibitors on T1DM individuals and only two studies (on dapagliflozin and empagliflozin) have been published until now [Henry et al. 2013; Cherney et al. 2014]. The results of these studies demonstrated a significant reduction in HbA1c, in FPG, in body weight and in the total daily number of insulin units required to achieve therapeutic glycemic control. It was also shown a dose-dependent increase in mean UGE from baseline. Furthermore, the co-administration of SGLT2 inhibitors and insulin did not increase the risk for hypoglycemia. Finally SGLT2 inhibitors reduce glomerular hyperfiltration and result in a renoprotective effect [Henry et al. 2013; Cherney et al. 2014; Lamos et al. 2014].
Conclusion
It is the first time in diabetes history that the kidneys have been used as a therapeutic target. SGLT2 inhibitors, this new class of antidiabetic agents, via glucosuria induction, significantly decrease HbA1c and FPG levels. Given that these agents do not interfere with insulin secretion and action, β-cell progressive failure does not attenuate their efficacy. Nevertheless, as the glucosuric effect depends on renal function, the efficacy of these agents decreases along with the stages of renal impairment. SGLT2 inhibition also provides a significant beneficial effect on body weight and BP reduction due to calorie loss and osmotic diuresis from glucosuria. SGLT2 inhibitors are generally well tolerated with the most common adverse events being UTIs and GIs and with low risk for hypoglycemia. Apart from the exciting cardioprotective results of empagliflozin (the EMPA-REG study) one more advantageous feature that was recently revealed is the renoprotective effect of empagliflozin and tofogliflozin against diabetic nephropathy, as discussed above, in in vitro, in animal and in human studies.
Since even more agents of this new class are completing their clinical programs and reaching market authorization (Table 2) our antidiabetic armamentarium is now equipped with more promising and effective weapons in the fight against diabetes.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: John Doupis has received honoraria for lecturing on SGLT2 inhibitors, from AstraZeneca, Janssen, and Boehringer Ingelheim (Ingelheim, Germany). The other authors have no conflict of interest to disclose.
Contributor Information
Vasileios Andrianesis, Athens Naval Hospital, Athens, Greece.
Spyridoula Glykofridi, Metaxa General Hospital, Athens, Greece.
John Doupis, Iatriko Paleou Falirou Medical Center, 36 Areos Street, 17562, Paleo Faliro, Athens, Greece.
References
- Abdul-Ghani M., DeFronzo R. (2008) Inhibition of renal glucose reabsorption: a novel strategy for achieving glucose control in type 2 diabetes mellitus. Endocr Pract 14: 782–790. [DOI] [PubMed] [Google Scholar]
- Abdul-Ghani M., DeFronzo R. (2014) Lowering plasma glucose concentration by inhibiting renal sodium-glucose co-transport. J Intern Med 276: 352–363. DOI: 10.1111/joim.12244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astra Zeneca. (2012) Forxiga™ (dapagliflozin) now approved in European Union for treatment of type 2 diabetes. Available at: https://www.astrazeneca.com/media-centre/press-releases/2012/FORXIGA-dapagliflozin-now-approved-in-European-Union-for-treatment-of-type-2-diabetes-14112012.html#! (accessed 28 October 2016).
- Augustin R. (2010) The protein family of glucose transport facilitators: it’s not only about glucose after all: critical review. IUBMB Life 62: 315–333. [DOI] [PubMed] [Google Scholar]
- Bailey C., Gross J., Pieters A., Bastien A., List J. (2010) Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 375: 2223–2233. [DOI] [PubMed] [Google Scholar]
- Barfuss D., Schafer J. (1981) Differences in active and passive glucose transport along the proximal nephron. Am J Physiol 240: F322–F332. [DOI] [PubMed] [Google Scholar]
- Barnett A., Mithal A., Manassie J., Jones R., Rattunde H., Woerle H., et al. (2014) Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2: 369–384. [DOI] [PubMed] [Google Scholar]
- Berhan A., Barker A. (2013) Sodium glucose co-transport 2 inhibitors in the treatment of type 2 diabetes mellitus: a meta-analysis of randomized double-blind controlled trials. BMC Endocrine Disorders 13: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolinder J., Ljunggren Ö., Kullberg J., Johansson L., Wilding J., Langkilde A., et al. (2012) Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 97: 1020–1031. [DOI] [PubMed] [Google Scholar]
- Bonner C., Kerr-Conte J., Gmyr V., Queniat G., Moerman E., Thévenet J., et al. (2015) Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 21: 512–517. [DOI] [PubMed] [Google Scholar]
- Brenner B., Hostetter T., Olson J., Rennke H., Venkatachalam M. (1981) The role of glomerular hyperfiltration in the initiation and progression of diabetic nephropathy. Acta Endocrinol Suppl (Copenh) 242: 7–10. [PubMed] [Google Scholar]
- Cherney D., Konvalinka A., Zinman B., Diamandis E., Soosaipillai A., Reich H., et al. (2009) Effect of protein kinase Cbeta inhibition on renal hemodynamic function and urinary biomarkers in humans with type 1 diabetes: a pilot study. Diabetes Care 32: 91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney D., Miller J., Scholey J., Bradley T., Slorach C., Curtis J., et al. (2008) The effect of cyclooxygenase-2 inhibition on renal hemodynamic function in humans with type 1 diabetes. Diabetes 57: 688–695. [DOI] [PubMed] [Google Scholar]
- Cherney D., Perkins B., Soleymanlou N., Maione M., Lai V., Lee A., et al. (2014) The renal hemodynamic effect of SGLT2 inhibition in patients with type 1 diabetes. Circulation 129: 587–597. DOI: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- Cherney D., Reich H., Jiang S., Har R., Nasrallah R., Hebert R., et al. (2012) Hyperfiltration and the effect of nitric oxide inhibition on renal and endothelial function in humans with uncomplicated type 1 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol 303: R710–R718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marchi S., Cecchin E., Basile A., Proto G., Donadon W., Schinella D., et al. (1983) Is renal glycosuria a benign condition? Proc Eur Dial Transplant Assoc 20: 681–685. [PubMed] [Google Scholar]
- DeFronzo R. (2009) From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58: 273–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R., Davidson J., Del Prato S. (2012) The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 14: 5–14. [DOI] [PubMed] [Google Scholar]
- Devineni D., Morrow L., Hompesch M., Skee D., Vandebosch A. (2012) Canagliflozin improves glycemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab 14: 539–545. [DOI] [PubMed] [Google Scholar]
- Ehrenkranz J., Lewis N., Kahn C., Roth J. (2005) Phlorizin: a review. Diabetes Metab Res Rev 21: 31–38. [DOI] [PubMed] [Google Scholar]
- Farber S., Berger E., Earle D. (1951) Effect of diabetes and insulin of the maximum capacity of the renal tubules to reabsorb glucose. J Clin Invest 30: 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E., Berk A., Hantel S., Pinnetti S., Hach T., Woerle H., et al. (2013a) Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care 36: 4015–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E., Muscelli E., Frascerra S., Baldi S., Mari A., Heise T., et al. (2014) Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 124: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E., Ramos S., Salsali A., Tang W., List J. (2010) Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double- blind, placebo-controlled, phase III trial. Diabetes Care 33: 2217–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E., Veltkamp S., Smulders R., Kadokura T. (2013b) Impact of chronic kidney disease and sodium-glucose cotransporter 2 inhibition in patients with type 2 diabetes. Diabetes Care 36: 1260–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitchett D., Zinman B., Wanner C., Lachin J., Hantel S., Salsali A., et al. (2016) Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. Eur Heart J 26. pii: ehv728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca V., Ferrannini E., Wilding J., Wilpshaar W., Dhanjal P., Ball G., et al. (2013) Active- and placebo-controlled dose-finding study to assess the efficacy, safety, and tolerability of multiple doses of ipragliflozin in patients with type 2 diabetes mellitus. J Diabetes Complications 27: 268–267. [DOI] [PubMed] [Google Scholar]
- Gerich J. (2010) Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med 27: 136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R., Cooper M. (1999) The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int 56: 1627–1637. [DOI] [PubMed] [Google Scholar]
- Guyton A., Hall J. (2006) Chapter 27: Urine formation by the kidneys: II tubular processing of the glomerular filtrate. In Guyton A, Hall J. (Ed.), Textbook of Medical Physiology (11th ed.; pp. 327–347). Philadelphia, Pennsylvania: Elsevier Saunders. [Google Scholar]
- Hansen H., Jelsing J., Hansen C., Hansen G., Vrang N., Mark M., et al. (2014) The sodium glucose cotransporter type 2 inhibitor empagliflozin preserves beta-cell mass and restores glucose homeostasis in the male Zucker diabetic fatty rat. J Pharmacol Exp Ther 350: 657–664. [DOI] [PubMed] [Google Scholar]
- Hediger M., Rhoads D. (1994) Molecular physiology of sodium-glucose cotransporters. Physiol Rev 74: 993–1026. [DOI] [PubMed] [Google Scholar]
- Heise T., Seewaldt-Becker E., Macha S., Hantel S., Pinnetti S., Seman L., et al. (2013) Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab 15: 613–621. [DOI] [PubMed] [Google Scholar]
- Henry R., Rosenstock J., Edelman S., Mudaliar S., Chalamandaris A., Kasichayanula S., et al. (2015) Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double- blind, placebo-controlled pilot study. Diabetes Care 38(3): 412–419. [DOI] [PubMed] [Google Scholar]
- Holian J., Qi W., Kelly D., Zhang Y., Mreich E., Pollock C., et al. (2008) Role of Kruppel-like factor 6 in transforming growth factor-beta1-induced epithelial-mesenchymal transition of proximal tubule cells. Am J Physiol Renal Physiol 295: F1388–F1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetter T., Rennke H., Brenner B. (1982) The case for intrarenal hypertension in the initiation and progression of diabetic and other glomerulopathies. Am J Med 72: 375–380. [DOI] [PubMed] [Google Scholar]
- Hostetter T., Troy J., Brenner B. (1981) Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int 19: 410–415. [DOI] [PubMed] [Google Scholar]
- Hussey E., Clark R., Amin D., Kipnes M., O’Connor-Semmes R., O’Driscoll E., et al. (2010) Single-dose pharmacokinetics and pharmacodynamics of sergliflozin etabonate, a novel inhibitor of glucose reabsorption, in healthy volunteers and patients with type 2 diabetes mellitus. J Clin Pharmacol 50: 623–635. [DOI] [PubMed] [Google Scholar]
- James W. (2008) The fundamental drivers of the obesity epidemic. Obes Rev 9(Suppl. 1): 6–13. [DOI] [PubMed] [Google Scholar]
- Jerums G., Premaratne E., Panagiotopoulos S., MacIsaac R. (2010) The clinical significance of hyperfiltration in diabetes. Diabetologia 53: 2093–2104. [DOI] [PubMed] [Google Scholar]
- Johnson and Johnson (2013) Invokana™ (Canagliflozin) approved in the European Union for treatment of adults with type 2 diabetes. Available at: http://www.investor.jnj.com/releasedetail.cfm?ReleaseID=809160. (accessed 7 July 2014).
- Johnson D., Saunders H., Brew B., Poronnik P., Cook D., Field M., et al. (1998) TGFbeta 1 dissociates human proximal tubule cell growth and Na(+)-H+ exchange activity. Kidney Int 53: 1601–1607. [DOI] [PubMed] [Google Scholar]
- Kaku K., Watada H., Iwamoto Y., Utsunomiya K., Terauchi Y., Tobe K., et al. (2014) Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol 13: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohan D., Fioretto P., Tang W., List J.(2014) Long-term study of patients with type 2 diabetesT2DM and moderate renal impairment shows that dapagliflozin reduces weigh and blood pressure but does not improve glycemic control. Kidney Int 85: 962–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs C., Seshiah V., Swallow R., Jones R., Rattunde H., Woerle H., et al. (2013) Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab 16(2): 147–158. [DOI] [PubMed] [Google Scholar]
- Kramer H., Molitch M. (2005) Screening for kidney disease in adults with diabetes. Diabetes Care 28: 1813–1816. [DOI] [PubMed] [Google Scholar]
- Lamos E., Younk L., Davis S. (2014) Empagliflozin, a sodium glucose co-transporter 2 inhibitor, in the treatment of type 1 diabetes. Expert Opin Investig Drugs 23: 875–882. [DOI] [PubMed] [Google Scholar]
- Liang Y., Arakawa K., Ueta K., Matsushita Y., Kuriyama C., Martin T., et al. (2012) Effect of canagliflozin on renal threshold for glucose, glycemia, and body weight in normal and diabetic animal models. PLoS One 7: e30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Tseng C. (2014) A review on the relationship between SGLT2 inhibitors and cancer. Int J Endocrin 2014: 719578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nainggolan L. (2014) Ipragliflozin (Suglat) first of new diabetes drug class in Japan. Medscape Medical News. Available at: http://www.medscape.com/viewarticle/819447 (accessed 7 July 2014).
- List J., Woo V., Morales E., Tang W., Fiedorek F. (2009) Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 32: 650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macha S., Mattheus M., Halabi A., Pinnetti S., Woerle H., Broedl U. (2014) Pharmacokinetics, pharmacodynamics and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in subjects with renal impairment. Diabetes Obes Metab 16: 215–222. [DOI] [PubMed] [Google Scholar]
- Magee G., Bilous R., Cardwell C., Hunter S., Kee F., Fogarty D. (2009) Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia 52: 691–697. [DOI] [PubMed] [Google Scholar]
- Majewski C., Bakris G. (2015) Blood pressure reduction: an added benefit of sodium glucose cotransporter 2 inhibitors in patients with type 2 diabetes. Diabetes Care 38: 429–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark M., Vallon V., Hugo C., Roman R., Mayoux E. (2014) Albuminuria, the sentinel marker of renal failure, is consistently decreased with empagliflozin in several preclinical models of diabetic nephropathy. EASD Virtual Meeting. ePoster #824. [Google Scholar]
- Markaham A., Elkinson S. (2014) Luseogliflozin: first global approval. Drugs 74: 945–950. doi: 10.1007/s40265-014-0230-8. [DOI] [PubMed] [Google Scholar]
- Mechcatie E. (2014) FDA approves empagliflozin for adults with type 2 diabetes. Clinical Endocrinology News. Available at: http://www.clinicalendocrinologynews.com/home/article/fda-approves-empagliflozin-for-adults-with-type-2-diabetes/e8e500442424cf39148f889d6fe6b16c.html (accessed 2 March 2016).
- Merck. (2015) Merck pipeline. Available at: http://www.merck.com/research/pipeline/home.html (accessed 2 March 2016, updated 31 October 2015).
- Misra M. (2012) SGLT2 inhibitors: a promising new therapeutic option for treatment of type 2 diabetes mellitus: review. J Pharm Pharmacol 65: 317–327. [DOI] [PubMed] [Google Scholar]
- Nagata T., Fukuzawa T., Takeda M., Fukazawa M., Mori T., Nihei T., et al. (2013) Tofogliflozin, a novel sodium-glucose co-transporter 2 inhibitor, improves renal and pancreatic function in db/db mice. Br J Pharmacol 170: 519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck M., Del Prato S., Meier J., Duran- Garcia S., Rohwedder K., Elze M., et al. (2011) Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care 34: 2015–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikov A., Vallon V. (2015) Sodium glucose cotransporter 2 inhibition in the diabetic kidney: an update. Curr Opin Nephrol Hypertens 24: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyirjesy P., Zhao Y., Ways K., Usiskin K. (2012) Evaluation of vulvovaginal symptoms and Candida colonization in women with type 2 diabetes mellitus treated with canagliflozin, a sodium glucose co-transporter 2 inhibitor. Curr Med Res Opin 28: 1173–1178. [DOI] [PubMed] [Google Scholar]
- Oemar B., Byrd D., Brodehl J. (1987) Complete absence of tubular glucose reabsorption: a new type of renal glucosuria (type 0). Clin Nephrol 27: 156–160. [PubMed] [Google Scholar]
- Oku A., Ueta K., Arakawa K., Ishihara T., Nawano M., Kuronuma Y., et al. (1999) T-1095, an inhibitor of renal Na+-glucose cotransporters, may provide a novel approach to treating diabetes. Diabetes 48: 1794–1800. [DOI] [PubMed] [Google Scholar]
- Oliva R., Bakris G. (2014) Blood pressure effects of sodium-glucose co-transport 2 (SGLT2) inhibitors. J Am Soc Hypertens 8: 330–339. [DOI] [PubMed] [Google Scholar]
- Panchapakesan U., Pegg K., Gross S., Komala M., Mudaliar H., Forbes J., et al. (2013) Effects of SGLT2 inhibition in human kidney proximal tubular cells renoprotection in diabetic nephropathy? Plos One 8: e54442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchapakesan U., Pollock C., Chen X. (2004) The effect of high glucose and PPAR-gamma agonists on PPAR-gamma expression and function in HK-2 cells. Am J Physiol Renal Physiol 287: F528–F534. [DOI] [PubMed] [Google Scholar]
- Panchapakesan U., Sumual S., Pollock C., Chen X. (2005) PPAR-gamma agonists exert antifibrotic effects in renal tubular cells exposed to high glucose. Am J Physiol Renal Physiol 289: F1153–F1158. [DOI] [PubMed] [Google Scholar]
- Poole R., Prossler J. (2014) Tofogliflozin: first global approval. Drugs 74: 939–944. [DOI] [PubMed] [Google Scholar]
- Ptaszynska A., Cohen S., Messing E., Reilly T., Johnsson E., Johnsson K. (2015) Assessing bladder cancer risk in type 2 diabetes clinical trials: the dapagliflozin drug development program as a ‘case study’. Diabetes Ther 6: 357–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W., Chen X., Holian J., Mreich E., Twigg S., Gilbert R., et al. (2006) Transforming growth factor-beta1 differentially mediates fibronectin and inflammatory cytokine expression in kidney tubular cells. Am J Physiol Renal Physiol 291: F1070–F1077. [DOI] [PubMed] [Google Scholar]
- Qi W., Twigg S., Chen X., Polhill T., Poronnik P., Gilbert R., et al. (2005) Integrated actions of transforming growth factor-beta1 and connective tissue growth factor in renal fibrosis. Am J Physiol Renal Physiol 288: F800–F809. [DOI] [PubMed] [Google Scholar]
- Rahmoune H., Thompson P., Ward J., Smith C., Hong G., Brown J. (2005) Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non–insulin-dependent diabetes. Diabetes 54: 3427–3434. [DOI] [PubMed] [Google Scholar]
- Reilly T., Graziano M., Janovitz E., Dorr T., Fairchild C., Lee F., et al. (2014) Carcinogenicity risk assessment supports the chronic safety of dapagliflozin, an inhibitor of sodium-glucose co-transporter 2, in the treatment of type 2 diabetes mellitus. Diabetes Ther 5: 73–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstock J., Aggarwal N., Polidori D., Zhao Y., Arbit D., Usiskin K., et al. (2012) Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care 35: 1232–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstock J., Seman L., Jelaska A., Hantel S., Pinnetti S., Hach T., et al. (2013) Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab 15: 1154–1160. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Shulman G., Zawalich W., DeFronzo R. (1987a) Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest 80: 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L., Smith D., Shulman G., Papachristou D., DeFronzo R. (1987b) Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 79: 1510–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggenenti P., Porrini E., Gaspari F., Motterlini N., Cannata A., Carrara F., et al. (2012) Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care 35: 2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santer R., Calado J. (2010) Familial renal glucosuria and SGLT2: from a Mendelian trait to a therapeutic target: In-depth review. Clin J Am Soc Nephrol 5: 133–141. [DOI] [PubMed] [Google Scholar]
- Sarashina A., Koiwai K., Seman L., Yamamura N., Tanigughi A., Negishi T., et al. (2013) Safety, tolerability, pharmacokinetics and pharmacodynamics of single doses of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, in healthy Japanese subjects. Drug Metab Pharmacokinet 28: 213–219. [DOI] [PubMed] [Google Scholar]
- Sasson A., Cherney D. (2012) Renal hyperfiltration related to diabetes mellitus and obesity in human disease. World J Diabetes 3: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheen A. (2014) Pharmacokinetic and pharmacodynamic profile of empagliflozin, a sodium glucose co-transporter 2 inhibitor. Clin Pharmacokinet 53: 213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl-Burgi S., Santer R., Ehrich J. (2004) Long-term outcome of renal glucosuria type 0: The original patient and his natural history. Nephrol Dial Transplant 19: 2394–2396. [DOI] [PubMed] [Google Scholar]
- Škrtić M., Yang G., Perkins B., Soleymanlou N., Lytvyn Y., Eynatten M., et al. (2014) Characterisation of glomerular haemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia 57: 2599–2602. [DOI] [PubMed] [Google Scholar]
- Sochett E., Cherney D., Curtis J., Dekker M., Scholey J., Miller J. (2006) Impact of rennin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. J Am Soc Nephrol 17: 1703–1709. [DOI] [PubMed] [Google Scholar]
- Starke A., Grundy S., McGarry J., Unger R. (1985) Correction of hyperglycemia with phloridzin restores the glucagon response to glucose in insulin deficient dogs: implications for human diabetes. Proc Natl Acad Sci USA 82: 1544–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenlof K., Cefalu W., Kim K., Alba M., Usiskin K., Tong C., et al. (2013) Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 15: 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strojek K., Yoon K., Hruba V., Elze M., Langkilde A., Parikh S. (2011) Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab 13: 928–938. [DOI] [PubMed] [Google Scholar]
- Taylor S., Blau J., Rother K. (2015) SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 100: 2849–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervaert T., Mooyaart A., Amann K., Cohen A., Cook H., Drachenberg C., et al. (2010) Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 21: 556–563. [DOI] [PubMed] [Google Scholar]
- Tikkanen I., Narko K., Zeller C., Green A., Salsali A., Broedl U., et al. (2015) Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 38: 420–428. [DOI] [PubMed] [Google Scholar]
- Tirmenstein M., Dorr T., Janovitz E., Hagan D., Abell L., Onorato J., et al. (2013) Nonclinical toxicology assessments support the chronic safety of dapagliflozin, a first-in-class sodium-glucose cotransporter 2 inhibitor. Int J Toxicol 32: 336–350. [DOI] [PubMed] [Google Scholar]
- Turner R., Moran A. (1982a) Heterogeneity of sodium-dependent D-glucose transport sites along the proximal tubule: evidence from vesicle studies. Am J Physiol Renal Fluid Electrolyte Physiol 242: F406–F414. [DOI] [PubMed] [Google Scholar]
- Turner R., Moran A. (1982b) Further studies of proximal tubular brush border membrane D-glucose transport heterogeneity. J Membr Biol 70: 37–45. [DOI] [PubMed] [Google Scholar]
- United Stated Food and Drug Administration (2011) Dapagliflozin. Background document [database on the Internet]. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM449865.pdf.
- United States Food and Drug Administration (2013) FDA approves Invokana to treat type 2 diabetes. Available at: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm345848.htm (accessed 7 July 2014).
- United States Food and Drug Administration (2014) FDA approves Farxiga to treat type 2 diabetes. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm380829.htm (accessed 7 July 2014).
- United States Food and Drug Administration Advisory Committee (2013) FDA briefing document. NDA 204042. Invokana (canagliflozin) Tablets. Applicant: Janssen Pharmaceuticals, Inc. Available at:www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM334550.pdf (accessed 5 May 2013). [Google Scholar]
- Vallon V., Gerasimova M., Rose M., Masuda T., Satriano J., Mayoux E., et al. (2014) SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic akita mice. Am J Physiol Renal Physiol 306: F194–F204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V., Platt K., Gunard R., Schroth J., Whaley J., Thomson S., et al. (2011) SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 22: 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallon V., Richter K., Blantz R., Thomson S., Osswald H. (1999) Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol 10: 2569–2576. [DOI] [PubMed] [Google Scholar]
- Veltkamp S., Kadokura T., Krauwinkel W., Smulders R. (2011) Effect of ipragliflozin (ASP1941), a novel selective sodium-dependent glucose co-transporter 2 inhibitor, on urinary glucose excretion in healthy subjects. Clin Drug Investig 31: 839–851. [DOI] [PubMed] [Google Scholar]
- Vick H., Diedrich D., Baumann K. (1973) Reevaluation of renal tubular glucose transport inhibition by phlorizin analogs. Am J Physiol 224: 552–557. [DOI] [PubMed] [Google Scholar]
- Wanner C., Inzucchi S., Lachin J., Fitchett D., Eynatten M., Mattheus M., et al. (2016) Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375: 323–334. [DOI] [PubMed] [Google Scholar]
- White J. (2010) Apple trees to sodium glucose co-transporter inhibitors: a review of SGLT2 inhibition. Clin Diabetes 28: 5–10. [Google Scholar]
- Wilding J., Ferrannini E., Fonseca V., Wilpshaar W., Dhanjal P., Houzer A. (2013) Efficacy and safety of ipragliflozin in patients with type 2 diabetes inadequately controlled on metformin: a dose-finding study. Diabetes Obes Metab 15: 403–409. [DOI] [PubMed] [Google Scholar]
- Wilding J., Norwood P., T’joen C., Bastien A., List J., Fiedorek F. (2009) A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care 32: 1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2016) Diabetes fact sheet No 312. Available at: http://www.who.int/mediacentre/factsheets/fs312/en/ (accessed 18 October 2016).
- World Health Organization (2016) Diabetes fact sheet No 138. Available at: http://www.who.int/mediacentre/factsheets/fs138/en/ (accessed 18 October 2016).
- Wright E., Hirayama B., Loo D. (2007) Active sugar transport in health and disease. J Int Med 261: 32–43. [DOI] [PubMed] [Google Scholar]
- Wright E., Hirayama B., Loo D., Turk E., Hager K. (1994) Intestinal sugar transport. In: L. Johnson (Ed.), Physiology of Gastrointestinal Tract (3rd ed.; pp. 1751–1772). New York, NY: Raven Press. [Google Scholar]
- Wright E., Turc E. (2004) The sodium/glucose cotransport family SLC5. Pflugers Arch 447: 510–518. [DOI] [PubMed] [Google Scholar]
- Wright E., Loo D., Hirayama B. (2011) Biology of human sodium glucose transporters. Physiol Rev 91: 733–794. [DOI] [PubMed] [Google Scholar]
- Wu J., Foote C., Blomster J., Toyama T., Perkovic V., Sundström J., et al. (2016) Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 4: 411–419. DOI: 10.1016/S2213-8587(16)00052-8. [DOI] [PubMed] [Google Scholar]
- Yahoo Finance (2014) Islet sciences to acquire BHV pharma and phase 2 SGLT2 inhibitor remogliflozin etabonate indicated for T2DM and NASH. Available at: http://finance.yahoo.com/news/islet-sciences-acquire-bhv-pharma-131500341.html (accessed 2 March 2016).
- Yale J., Bakris G., Cariou B., Yue D., David- Neto E., Xi L., et al. (2013) Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 15: 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis F., Abassi Z., Mayoux E., Hollander K., Rath-Wolfson L., Rosenthal T. (2014) Empagliflozin, a selective SGLT2 inhibitor, ameliorated hyperglycaemia and insulin resistance, while preserving the integrity of pancreas and kidney in CRDH rats. EASD Virtual Meeting. Abstract #801. [Google Scholar]
- Zinman B., Wanner C., Lachin J., Fitchett D., Bluhmki E., Hantel S., et al. (2015) Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128. [DOI] [PubMed] [Google Scholar]