Abstract
Mobile phones and Wi-Fi radiofrequency radiation are among the main sources of the exposure of the general population to radiofrequency electromagnetic fields (RF-EMF). Previous studies have shown that exposure of microorganisms to RF-EMFs can be associated with a wide spectrum of changes ranged from the modified bacterial growth to the alterations of the pattern of antibiotic resistance. Our laboratory at the nonionizing department of the Ionizing and Non-ionizing Radiation Protection Research Center has performed experiments on the health effects of exposure to animal models and humans to different sources of electromagnetic fields such as cellular phones, mobile base stations, mobile phone jammers, laptop computers, radars, dentistry cavitrons, magnetic resonance imaging, and Helmholtz coils. On the other hand, we have previously studied different aspects of the challenging issue of the ionizing or nonionizing radiation-induced alterations in the susceptibility of microorganisms to antibiotics. In this study, we assessed if the exposure to 900 MHz GSM mobile phone radiation and 2.4 GHz radiofrequency radiation emitted from common Wi-Fi routers alters the susceptibility of microorganisms to different antibiotics. The pure cultures of Listeria monocytogenes and Escherichia coli were exposed to RF-EMFs generated either by a GSM 900 MHz mobile phone simulator and a common 2.4 GHz Wi-Fi router. It is also shown that exposure to RF-EMFs within a narrow level of irradiation (an exposure window) makes microorganisms resistant to antibiotics. This adaptive phenomenon and its potential threats to human health should be further investigated in future experiments. Altogether, the findings of this study showed that exposure to Wi-Fi and RF simulator radiation can significantly alter the inhibition zone diameters and growth rate for L monocytogenes and E coli. These findings may have implications for the management of serious infectious diseases.
Keywords: radiofrequency radiation, bacteria, Wi-Fi, antibiogram
Introduction
Antibiotic resistance is one of the most important threats to global health.1 According to World Health Organization, this problem is rising dangerously to high levels worldwide, which leads to longer hospitalization, higher medical costs, and raised mortality.2
Bacteria are becoming resistant to almost all commonly available antibiotics and this is a worldwide problem.1 Today, greater use of telecommunication technologies like Global System for Mobile communication (GSM), cordless phones, mobile base stations, wireless personal, and local area networks, such as bluetooth, has led to ever increasing exposure to radiofrequency electromagnetic fields (RF-EMF).3 Therefore, living organisms are now being exposed to microwaves and radiofrequency radiation signals from various sources.4 The effects of these radiations on the biological functions of living cells shows an emerging area of interest in human health with respect to environmental effects.5 Several studies were conducted to confirm the effects of electromagnetic radiation on cell functions6–8; however, the findings obtained in these studies were controversial. In particular, it was proven that EMF can affect functional parameters (cell growth and antimicrobial susceptibility).9–12
Listeria monocytogenes is a gram-positive, facultative anaerobe, nonspore-forming, motile, and rod-shaped bacterium.13 In 1952, it was recognized as the main cause of neonatal infection, meningitis, and sepsis.14 Listeria infection in adult patients is related to immunocompromised systems like HIV infection,15 organ transplants, individuals who have received corticosteroids, and immunosuppressant drugs for their malignancies. Escherichia coli known as E coli, a gram-negative, rod-shaped, facultatively anaerobic bacterium,16 is a common cause of life-threatening infections such as bloodstream and urinary tract infections, otitis media, and other complications.17
Our laboratory at the nonionizing department of the Ionizing and Non-ionizing Radiation Protection Research Center has performed experiments on the health effects of exposure to animal models and humans to different sources of electromagnetic fields such as cellular phones,18–20 mobile base stations,21 mobile phone jammers,22,23 laptop computers,24 radars,25 dentistry cavitrons,26 magnetic resonance imaging,27,28 and Helmholtz coils.29,30 In this study, we assessed whether the exposure to 900 MHz and 2.4 GHz RF-EMF emitted from GSM and a common Wi-Fi router could change the susceptibility of microorganisms to different antibiotics.
Materials and Methods
Antibiotic Susceptibility Test
In the current study, L monocytogenes ATCC 19115 was used and E coli strain was isolated from patients in Faghihi hospital, Shiraz, Iran. Escherichia coli strain was characterized by conventional methods including morphological and biochemical tests and confirmed using API 20 E method. The pure cultures of L monocytogenes and E coli were diluted in Mueller-Hinton Broth to reach 0.5 McFarland turbidity standards to get 1.5 × 108 CFU/mL as the total count.31 Bacterial suspensions were spread on plates and cultured with a set of 6 antimicrobial substances; they were tested by disk diffusion method (Kirby-Bauer method) on Mueller-Hinton agar (MHA-Biolife, Italy) plates and E coli ATCC 25922 was used as the quality control for antibiotic susceptibility tests, according to the Clinical and Laboratory Standards Institute guidelines (CLSI, 2013). The incubation period was 18 to 24 hours at 35°C, and then inhibition zones for each disk were measured.
Antimicrobial Agents
Antibiotics used for E coli tests were imipenem (10 μg), levofloxacin (LEVO 5 μg), aztreonam (30 μg), ciprofloxacin (CIPR 5 μg), cefotaxime (CTX 30 μg), and piperacillin (100 μg). Listeria monocytogenes tests were conducted using doxycycline (DOX 30 μg), sulfamethoxazole–trimethoprim (SXT 25 μg), LEVO 5μg, CTX 30 μg, CIPR 5 μg, and ceftriaxone (CTR 30 μg) antibiotics.
All antibiotic disks were purchased from ROSCO Diagnostica (DK-2630 Taastrup, Denmark). Results of antibiotic susceptibility tests before and after exposure to either Wi-Fi or GSM mobile phone radiation were measured and analyzed. The inhibition zone of each plate was recorded as the average of at least 2 different measurements (in millimeters). Three replicate agar plates were used for each regime, according to CLSI guidelines (2013).
Wi-Fi Router
A D-Link Wi-Fi router (D-Link, D-Link Corporation, Taiwan) was used in this study as the exposure source. During the exposure period, data were exchanged between the modem and a laptop computer that was placed in another room (5 m away from the Wi-Fi router).
The Wi-Fi router operated with a power level of 1 W and the specific absorption rate at the distance 14 cm between the bacterial suspension (broth medium) and Wi-Fi router was 0.13 W/kg. During the exposure, bacterial samples were collected in different times 3, 6, 9, and 12 hours after being exposed using sterile swabs.
Radiofrequency Simulator
In this study, all exposures were performed using a GSM 900 MHz mobile simulator operating in the “Talk mode.” This mobile phone simulator was developed at the Department of Medical Physics and Biomedical Engineering, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran, by the collaboration of the private sector.
Outgrowth Curve
For the evaluation of radiofrequency exposure effect on the growth rate of bacteria, optical density (OD) was measured. For each bacterium, a precisely specified concentration of bacterial suspension inoculated in the broth medium and then divided into 2 series as a control and RF simulator exposure groups. For estimating the number of bacterial cells in a broth medium, the turbidity of each group was recorded in 625 nm absorption at different times using a spectrophotometer (UNICO UV-2100 Spectrophotometer, UNICO, USA).
Statistical Analysis
All experiments were replicated 3 times for exposed and nonexposed groups. The means were compared using the nonparametric Mann-Whitney U test, and statistical significance of any difference observed among the mean values was determined using SPSS version 15. P < .05 was considered significant.
Results and Discussions
In our study, we have evaluated E coli and L monocytogenes for their in vitro susceptibility to various antibiotics in the presence of radiofrequency radiation. For each antibiotic, inhibition zone was measured and the test was repeated 3 times. Data obtained for exposed and nonexposed (control) bacteria are summarized in Table 1.
Table 1.
Inhibition Zone Diameters Before and After Exposure to RF and Wi-Fi Radiofrequency Radiation for Escherichia coli.
| Wi-Fi Exposure | RF Simulator | Wi-Fi Exposure | |||||
|---|---|---|---|---|---|---|---|
| Exposure Time | Drug | Control (Mean ± SD) | Exposure (Mean ± SD) | P Value | Control (Mean ± SD) | Exposure (Mean ± SD) | P Value |
| 3 hours | PIPRA | 26.30 ± 0.58 | 24.67 ± 0.58 | .0262a | 25.67 ± 0.58 | 25.30 ± 0.58 | .5608 |
| IMI | 31.67 ± 0.58 | 25.30 ± 0.58 | .0002a | 29.67 ± 0.58 | 25.30 ± 0.58 | .0008a | |
| LEVO | 34.67 ± 0.58 | 30.30 ± 0.58 | .0008a | 34.67 ± 0.58 | 31.67 ± 0.58 | .0032a | |
| AZT | 35.30 ± 0.58 | 29.30 ± 0.58 | .0002a | 34.67 ± 0.58 | 32.30 ± 0.58 | .0083a | |
| CIPR | 33.67 ± 0.58 | 28.67 ± 0.58 | .0005a | 33.30 ± 1.20 | 30.67 ± 0.58 | .0247a | |
| CTX | 36.67 ± 0.58 | 31.30 ± 0.58 | .0001a | 34.67 ± 0.58 | 30.30 ± 0.58 | .0008a | |
| 6 hours | PIPRA | 26.30 ± 0.58 | 22.30 ± 0.58 | .0011a | 25.67 ± 0.58 | 24.67 ± 0.58 | .1023 |
| IMI | 31.67 ± 0.58 | 23.67 ± 0.58 | .0001a | 29.67 ± 0.58 | 26.67 ± 0.58 | .0032a | |
| LEVO | 34.67 ± 0.58 | 26.30 ± 0.58 | .0001a | 34.67 ± 0.58 | 30.67 ± 0.58 | .0011a | |
| AZT | 35.30 ± 0.58 | 25.67 ± 0.58 | <.0001a | 34.67 ± 0.58 | 30.67 ± 0.58 | .0011a | |
| CIPR | 33.67 ± 0.58 | 26.30 ± 0.58 | .0001a | 33.30 ± 1.20 | 33.67 ± 0.58 | .7165 | |
| CTX | 36.67 ± 0.58 | 28.30 ± 0.58 | .0001a | 34.67 ± 0.58 | 29.30 ± 0.58 | .0004a | |
| 9 hours | PIPRA | 26.30 ± 0.58 | 22.67 ± 0.58 | .0016a | 25.67 ± 0.58 | 24.67 ± 0.58 | .1023 |
| IMI | 31.67 ± 0.58 | 25.67 ± 0.58 | .0002a | 29.67 ± 0.58 | 25.67 ± 0.58 | .0011a | |
| LEVO | 34.67 ± 0.58 | 28.30 ± 0.58 | .0002a | 34.67 ± 0.58 | 29.67 ± 0.58 | .0005a | |
| AZT | 35.30 ± 0.58 | 26.67 ± 0.58 | .0001a | 34.67 ± 0.58 | 28.67 ± 0.58 | .0002a | |
| CIPR | 33.67 ± 0.58 | 30.67 ± 0.58 | .0032a | 33.30 ± 1.20 | 30.30 ± 0.58 | .0176a | |
| CTX | 36.67 ± 0.58 | 28.67 ± 0.58 | .0001a | 34.67 ± 0.58 | 28.67 ± 0.58 | .0002a | |
| 12 hours | PIPRA | 26.30 ± 0.58 | 23.67 ± 0.58 | .0051a | 25.67 ± 0.58 | 24.30 ± 0.58 | .0516 |
| IMI | 31.67 ± 0.58 | 28.67 ± 0.58 | .0032a | 29.67 ± 0.58 | 25.67 ± 0.58 | .0011a | |
| LEVO | 34.67 ± 0.58 | 30.30 ± 0.58 | .0008a | 34.67 ± 0.58 | 32.30 ± 0.58 | .0083a | |
| AZT | 35.30 ± 0.58 | 27.67 ± 0.58 | .0001a | 34.67 ± 0.58 | 33.67 ± 0.58 | .1023 | |
| CIPR | 33.67 ± 0.58 | 35.30 ± 0.58 | .0262a | 33.30 ± 1.20 | 34.30 ± 0.58 | .2636 | |
| CTX | 36.67 ± 0.58 | 31.67 ± 0.58 | .0005a | 34.67 ± 0.58 | 35.30 ± 0.58 | .2134 | |
Abbreviations: AZT, aztreonam; CIPR, ciprofloxacin; CTX, cefotaxime; IMI, imipenem; LEVO, levofloxacin; PIPRA, piperacillin; RF, radiofrequency.
aStatistically significant diffidence.
According to Table 1, for E coli, exposure to Wi-Fi and RF simulator decreased the inhibition zone diameters that show an antibacterial resistance pattern. At first, there was no change in sensitivity, but after increasing the exposure time, a specific range of antibacterial resistance was observed.
After 24 hours of exposure, as it can be seen in Table 1 and Figures 1 and 2, the bacteria that were exposed to radiation showed less resistance compared to early-time exposure. However, they didn’t return to time 0 exposure condition.
Figure 1.
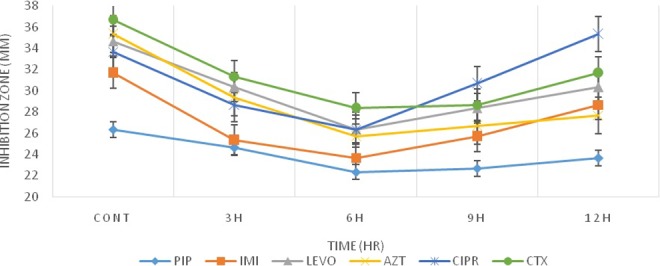
Inhibition zone diameters preexposure and postexposure to radiofrequency (RF) simulator radiation for Escherichia coli.
Figure 2.
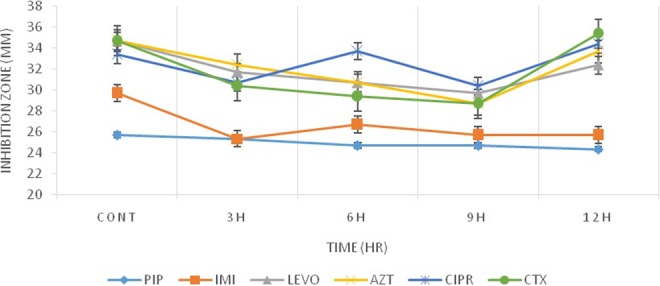
Inhibition zone diameters preexposure and postexposure to Wi-Fi radiation for Escherichia coli.
According to Figures 3 and 4, for L monocytogenes, comparison of data obtained from exposed and nonexposed groups did not show any significant changes in their antibacterial activity except for DOX. However, for E coli, there was a significant change in antimicrobial activities that suggest exposure condition to radiation could influence the degree of antibiotic susceptibility of E coli more than Listeria. In a similar pattern, for L monocytogenes, a specific window of response was observed (Figures 3 and 4). Listeria monocytogenes response to each antibiotic was different, for DOX, and the window response occurred after 6 hours of exposure to Wi-Fi and RF simulator radiation. However, for other antibiotics, these changes were only observed at the ninth hour of exposure to Wi-Fi while this response could not be observed for RF simulator radiation. After 9 hours of exposure to Wi-Fi for CIPR and SXT antibiotics, bacteria had a tendency to become more resistant. This was in contrast to the pattern observed for LEVO, CTX, and CTR antibiotics, which an increased sensitivity was observed. As mentioned above, for Listeria, limited antibacterial changes were observed for DOX after exposure to Wi-Fi and RF simulator radiation. On the other hand, we have previously addressed the bioeffects of the exposure of bacteria to electromagnetic radiations and investigated different aspects of the challenging issue of the ionizing or nonionizing radiation-induced alterations in the susceptibility of microorganisms to antibiotics.19,32–34
Figure 3.
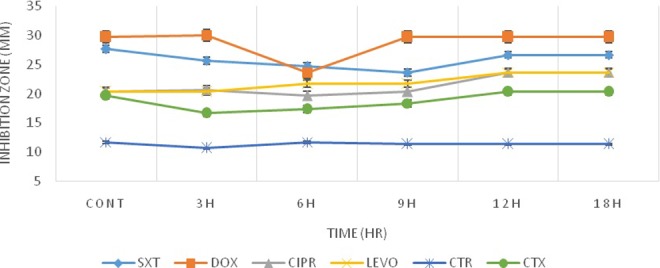
Inhibition zone diameters preexposure and postexposure to radiofrequency (RF) simulator radiation for Listeria monocytogenes.
Figure 4.
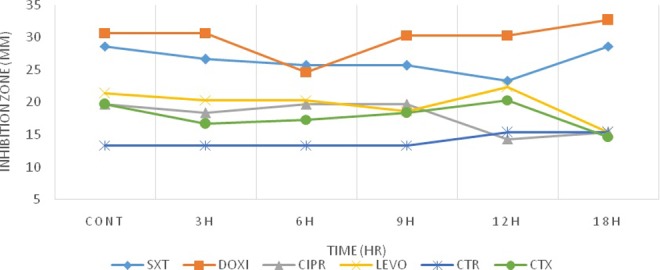
Inhibition zone diameters preexposure and postexposure to Wi-Fi radiation for Listeria monocytogenes.
In the current study, the pattern of the response of E coli to Wi-Fi and RF simulator radiation was identical. The maximum differences in the diameters of inhibition zones were observed between 6 and 9 hours of the bacterial exposure to radiation (Figures 1 and 2). After 12 hours of exposure, the bacterial responses to radiation as a stressor led to returning to the preexposure status. This observation is in line with the previous reports of Mortazavi et al,18,19,34–36 who showed that the radiation-induced stimulatory/beneficial effects in bacteria can be observed only within a narrow window of radiation dose. Based on this theory, when the radiation level is within the window (between the lower and upper levels of the window), stimulatory effects of ionizing or nonionizing radiation can be detected. Therefore, the response of the bacteria and other microorganisms to any environmental stressors can be determined by some key factors such as the magnitude of the dose and dose rate. This type of response was previously confirmed in Klebsiella pneumoniae.34
We have also evaluated the effect of radiofrequency radiation on the growth rate of bacteria. As shown in Figures 5 and 6, during each investigated time period, remarkable differences were observed in the rate of bacterial growth in exposed and nonexposed groups (Table 2). In particular, gram-negative (E coli) and gram-positive bacteria (L monocytogenes) showed a significant growth after exposure. Moreover, the time to reach the logarithmic phase in the growth curve of the bacteria was faster in exposed groups. However, after 8 hours, based on OD625 absorbance, the total count of E coli bacteria in the exposed group was less than that of the control group. These observations are in line with the finding of Akbal et al.37 However, the total counts of L monocytogenes after 24 hours of exposure was higher than that of the control group. At a broader view, our data confirm previous studies that showed that radiofrequency radiation could induce changes in cell growth and antibiotic sensitivity in E coli.
Figure 5.
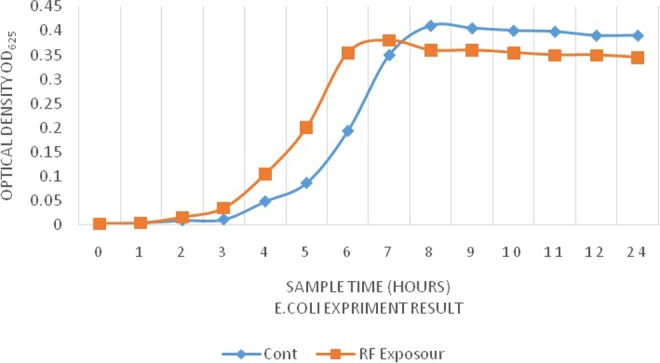
Growth curves in Escherichia coli broth medium preexposure and postexposure.
Figure 6.
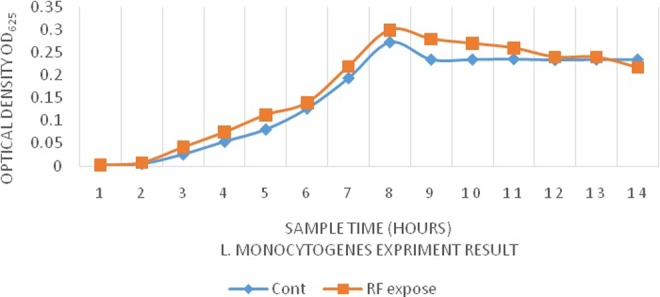
Growth curves Listeria monocytogenes in broth medium preexposure and postexposure.
Table 2.
Average Optical Density (OD625) Results for Escherichia coli and Listeria Monocytogenes Preexposure and Postexposure.
| Experimental Results | ||||
|---|---|---|---|---|
| Time | E Coli | L Monocytogenes | ||
| OD625 | OD625 | |||
| Control | Exposure | Control | Exposure | |
| 0 hour | 0.003 ± 0.001 | 0.003 ± 0.006 | 0.002 ± 0.0011 | 0.002 ± 0.0006 |
| 1 hour | 0.009 ± 0.006 | 0.004 ± 0.001 | 0.005 ± 0.006 | 0.008 ± 0.0006 |
| 2 hours | 0.01 ± 0.001 | 0.016 ± 0.006 | 0.026 ± 0.001 | 0.042 ± 0.0003 |
| 3 hours | 0.012 ± 0.006 | 0.035 ± 0.001 | 0.054 ± 0.001 | 0.075 ± 0.001 |
| 4 hours | 0.049 ± 0.001 | 0.105 ± 0.006 | 0.081 ± 0.006 | 0.113 ± 0.001 |
| 5 hours | 0.087 ± 0.001 | 0.201 ± 0.001 | 0.127 ± 0.001 | 0.14 ± 0.001 |
| 6 hours | 0.194 ± 0.001 | 0.355 ± 0.002 | 0.194 ± 0.001 | 0.22 ± 0.002 |
| 7 hours | 0.35 ± 0.01 | 0.38 ± 0.002 | 0.273 ± 0.001 | 0.3 ± 0.001 |
| 8 hours | 0.41 ± 0.006 | 0.36 ± 0.002 | 0.235 ± 0.006 | 0.28 ± 0.001 |
| 9 hours | 0.405 ± 0.006 | 0.36 ± 0.002 | 0.235 ± 0.001 | 0.27 ± 0.001 |
| 10 hours | 0.4 ± 0.01 | 0.355 ± 0.002 | 0.236 ± 0.001 | 0.26 ± 0.002 |
| 11 hours | 0.398 ± 0.003 | 0.35 ± 0.002 | 0.234 ± 0.006 | 0.24 ± 0.006 |
| 12 hours | 0.39 ± 0.01 | 0.35 ± 0.002 | 0.235 ± 0.001 | 0.24 ± 0.001 |
| 24 hours | 0.39 ± 0.01 | 0.345 ± 0.002 | 0.235 ± 0.001 | 0.217 ± 0.006 |
Some researchers have indicated that organisms acquire resistance through several known factors such as patient noncompliance or in vitro exposure to radiofrequency radiation.38–40 Nowadays, our world is surrounded by enormous radiofrequency sources such as Wi-Fi routers and laptop computers that can lead to serious health problems. When someone is infected with a microorganism that obtained its resistance from the host environment, it causes a serious problem for health-care systems and treatment failure or receiving a higher dosage of antibiotics will be possible. Therefore, this may lead to more side effects and finally prolonged hospitalization.
In several studies,10,41 it was shown that antimicrobial sensitivity alterations were affected by the intensity of electromagnetic fields. Antibacterial sensitivity also depends on the physical properties of the electromagnetic fields such as frequency and magnetic flux density, exposure duration, and type of bacteria. Based on this point, evaluation of the effect of radiofrequency radiation on bacteria is not only essential to investigate their environmental effects, but it is also vital for detecting the antibiotic resistance pattern in the clinical laboratories and environment.42–45
Since the frequency of Wi-Fi router is 2.4 GHz while it is 900 MHz for the mobile simulator, we can conclude that the difference in response to Wi-Fi and the mobile simulator is possibly due to the frequency of radiation.46 In several studies on bacteria,34,47,48 one of the factors that influenced antibacterial sensitivity was the cell wall structure of bacteria and peptidoglycan (PG) nature in gram-positive and gram-negative bacteria. In gram-positive ones like Listeria, cell wall thickness is greater than that of gram negatives. The thicker the PG,49 the permeability of the cell wall to permit the entrance of molecules to the cells will be decreased. According to these findings, the frequency of radiation can make some changes in PG of cell wall and enhance the permeability of the membrane to antibiotics.8,50 Torgomyan showed that alteration in the oxidoreduction state of proteins in the bacterial cell membrane can be the major membranous mechanism after exposure to low-intensity electromagnetic field.51
Also, the effect of electromagnetic radiation on E coli cultures was studied by Justo et al,52 which found that cell growth could be changed (stimulation or inhibition) under magnetic field. Furthermore, the exposure of E coli ATCC 25992 to the magnetic field of 2 mT at the frequency of 50 Hz caused significant alterations in the morphology, growth curves, structural parameters, and the sensitivity to certain antibiotics such as nalidixic acid, amoxicillin, and erythromycin.9,53 These results were confirmed by the study of Stansell et al,54 who found that static magnetic fields at moderate intensities are able to decrease the antibiotic sensitivity and make E coli WHMC 4202 more resistant.
In our study, we used several antibiotics that act through various mechanisms including protein and DNA synthesis inhibition, cell wall inhibition, and dihydrofolate reductase inhibition (it is summarized in Table 3). Each antibiotic enters the cell via a specific pathway. Some of them enter via efflux pumps in the cell membrane,34,55,56 and others enter via ion channels through the cell wall.57 All of these antibiotics may enter the cell via a nonspecific mechanism such as endocytosis. In this mechanism, molecules pass the membrane based on the permeability of the cell wall.58–60 Considering our results, we believe that Wi-Fi and mobile exposure can serve as physical methods to alter the antibacterial susceptibility of microorganisms. In this light, the permeability of the membrane can be changed by radiofrequency radiation. It seems that the radiation can alter the sensitivity of the efflux pumps or ion channels by permitting the entrance of the molecules through the cell wall. In order to verify these theories, it would be better if this study is replicated with other pathogenic bacteria both gram-positive and gram-negative ones with various forms of antibiotics.
Table 3.
Antibiotics Classification.
| Antibiotic Classification | Mechanism | Antimicrobial Agents | Abbreviation |
|---|---|---|---|
| Sulfonamide | DHFRI | Trimethoprim/sulfamethoxazole | SXT |
| Penicillin | Inhibits cell wall synthesis | Ceftriaxone | CTR |
| Cefotaxime | CTX | ||
| Piperacillin | PIPRA | ||
| Imipenem | IMI | ||
| Aztreonam | AZT | ||
| Tetracycline | Protein synthesis inhibition (30 s) | Doxycycline | DOX |
| Fluoroquinolones | Nucleic acid synthesis inhibition | Ciprofloxacin | CIPR |
| Levofloxacin | LEVO | ||
| Aminoglycoside | Protein synthesis inhibition (30 s) | Amikacin | AMI |
Abbreviation: DHFRI, dihydrofolate reductase inhibitors.
Conclusion
Based on our results, it can be concluded that the bacterial strains used in this study respond differently to EMFs. These bacteria were capable of responding to environmental stresses that act by activating some specific systems such as ion channels, change via the membrane, DNA repair system, and probably ion efflux pumps in the membrane as well as interactions of molecules and antibacterial agents.61 There are some ambiguities that need further investigations regarding answering questions such as which cellular mechanism is responsible for adaptation? Which factors are involved in alterations of antibacterial sensitivity? And subsequently, what are the differences in the response to radiation in gram-negative and gram-positive bacteria? Moreover, experiments on different bacterial strains with various electromagnetic fields should be performed in the future to better clarify these uncertainties.
Acknowledgments
The authors would like to thank the Research Consultation (RCC) of Shiraz University of Medical Sciences for their invaluable assistance in editing this article.
Authors’ Note: This study was technically supported by the Ionizing and Non-ionizing Radiation Protection Research Center (INIRPRC), Shiraz University of Medical Sciences (SUMS), Shiraz, Iran.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was financially supported by the Research Deputy of Kerman University of Medical Sciences, Kerman, Iran.
References
- 1. Bush K, Courvalin P, Dantas G, et al. Tackling antibiotic resistance. Nat Rev Microbiol. 2011;9(12):894–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, et al. Cost and mortality associated with postoperative acute kidney injury. Ann Surg. 2015;261(6):1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hardell L, Sage C. Biological effects from electromagnetic field exposure and public exposure standards. Biomed Pharmacother. 2008;62(2):104–109. [DOI] [PubMed] [Google Scholar]
- 4. Balmori A. Radiotelemetry and wildlife: highlighting a gap in the knowledge on radiofrequency radiation effects. Sci Total Environ. 2016;543(pt A):662–669. [DOI] [PubMed] [Google Scholar]
- 5. Foletti A, Lisi A, Ledda M, de Carlo F, Grimaldi S. Cellular ELF signals as a possible tool in informative medicine. Electromagn Biol Med. 2009;28(1):71–79. [DOI] [PubMed] [Google Scholar]
- 6. Gye MC, Park CJ. Effect of electromagnetic field exposure on the reproductive system. Clin Exp Reprod Med. 2012;39(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sarookhani M, Asiabanha Rezaei M, Safari A, Zaroushani V, Ziaeiha M. The influence of 950 MHz magnetic field (mobile phone radiation) on sex organ and adrenal functions of male rabbits. Afr J Biochem Res. 2011;5(2):65–68. [Google Scholar]
- 8. Oncul S, Cuce EM, Aksu B, Inhan Garip A. Effect of extremely low frequency electromagnetic fields on bacterial membrane. Int J Radiat Biol. 2016;92(1):42–49. [DOI] [PubMed] [Google Scholar]
- 9. Gaafar E, Hanafy MS, Tohamy E, Ibrahim MH. Stimulation and control of E. coli by using an extremely low frequency magnetic field. Rom J Biophys. 2006;16(4):283–296. [Google Scholar]
- 10. Belyaev I. Toxicity and SOS-response to ELF magnetic fields and nalidixic acid in E. coli cells. Mutat Res. 2011;722(1):56–61. [DOI] [PubMed] [Google Scholar]
- 11. Ahmed I, Istivan T, Cosic I, Pirogova E. Evaluation of the effects of extremely low frequency (ELF) pulsed electromagnetic fields (PEMF) on survival of the bacterium Staphylococcus aureus. EPJ Nonlinear Biomed Phys. 2013;1(1):1–17. [Google Scholar]
- 12. Ibraheim MH, El-Din Darwish D. 50 Hz frequency magnetic field effects on Pseudomonas aeruginosa and Bacillus subtilis bacteria. IOSR J Appl Phys. 2013;5(3):2278–4861. [Google Scholar]
- 13. Walker DK. Listeria monocytogenes and Clostridium perfringens Bacteremia complicated by brain abscess and cerebral venous sinus thrombosis: a case report. Infect Dis Clin Pract. 2015;23(6):330–332. [Google Scholar]
- 14. Girard KF, Sbarra AJ, Bardawil WA. Serology of Listeria monocytogenes I. Characteristics of the soluble hemolysin. J Bacteriol. 1963;85(2):349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schlech WF, Acheson D. Foodborne listeriosis. Clin Infect Dis. 2000;31(3):770–775. [DOI] [PubMed] [Google Scholar]
- 16. Lantos F, Hernyik O, Madacsy H, Tóth E. Effect of hexagon field energy on the microbiological infected irrigation and drinking water. Lucrări Ştiinţifice Management Agricol. 2015;17(1):120. [Google Scholar]
- 17. Falagas ME, Giannopoulou KP, Kokolakis GN, Rafailidis PI. Fosfomycin: use beyond urinary tract and gastrointestinal infections. Clin Infect Dis. 2008;46(7):1069–1077. [DOI] [PubMed] [Google Scholar]
- 18. Mortazavi S, Mosleh-Shirazi M, Tavassoli A, et al. A comparative study on the increased radioresistance to lethal doses of gamma rays after exposure to microwave radiation and oral intake of flaxseed oil. Iran J Radiat Res. 2011;9(1):9–14. [Google Scholar]
- 19. Mortazavi SM, Motamedifar M, Namdari G, Taheri M, Mortazavi AR, Shokrpour N. Non-linear adaptive phenomena which decrease the risk of infection after pre-exposure to radiofrequency radiation. Dose Response. 2014;12(2):233–245. doi:10.2203/dose-response. 12-055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mortazavi S, Mosleh-Shirazi M, Tavassoli A, et al. Increased radioresistance to lethal doses of gamma rays in mice and rats after exposure to microwave radiation emitted by a GSM mobile phone simulator. Dose Response. 2013;11(2):281–292. doi:10.2203/dose-response.12-010.Mortazavi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mortazavi SMJ. Safety issue of mobile phone base stations. J Biomed Phys Eng. 2013;3(1):1–2. [Google Scholar]
- 22. Parsanezhad ME, Mortazavi SMJ, Doohandeh T, Namavar-Jahromi B. Exposure to radiofrequency radiation emitted from mobile phone jammers adversely affects the quality of human sperm. Int J Radiat Res (IJRR). In press. [Google Scholar]
- 23. Mortazavi SMJ, Parsanezhad ME, Kazempour M, Ghahramani P, Mortazavi A, Davari M. Male reproductive health under threat: short term exposure to radiofrequency radiations emitted by common mobile jammers. J Hum Reprod Sci. 2013;6(2):124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mortazavi SMJ, Tavasoli AR, Ranjbari F, Moamaei P. Effects of laptop computers’ electromagnetic field on sperm quality. J Reprod Infertil. 2011;11(4):251–258. [Google Scholar]
- 25. Mortazavi SMJ, Taeb S, Dehghan N. Alterations of visual reaction time and short term memory in military radar personnel. Iran J Public Health. 2013;42(4):428–435. [PMC free article] [PubMed] [Google Scholar]
- 26. Mortazavi SM, Vazife-Doost S, Yaghooti M, Mehdizadeh S, Rajaie-Far A. Occupational exposure of dentists to electromagnetic fields produced by magnetostrictive cavitrons alters the serum cortisol level. J Nat Sci Biol Med. 2012;3(1):60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mortazavi SMJ, Neghab M, Anooshe SMH, et al. High-field MRI and mercury release from dental amalgam fillings. Int J Occup Environ Med. 2014;5(2):101–105. [PMC free article] [PubMed] [Google Scholar]
- 28. Mortazavi SMJ, Daiee E, Yazdi A, et al. Mercury release from dental amalgam restorations after magnetic resonance imaging and following mobile phone use. Pak J Biol Sci. 2008;11(8):1142–1146. [DOI] [PubMed] [Google Scholar]
- 29. Haghnegahdar A, Khosrovpanah H, Andisheh-Tadbir A, et al. Design and fabrication of helmholtz coils to study the effects of pulsed electromagnetic fields on the healing process in periodontitis: preliminary animal results. J Biomed Phys Eng. 2014;4(3):83–90. [PMC free article] [PubMed] [Google Scholar]
- 30. Paknahad M, Shahidi S, Mortazavi SMJ, Mortazavi G, Moghadam MS, Nazhvani AD. The effect of pulsed electromagnetic fields on microleakage of amalgam restorations: an in vitro study. Shiraz E-Med J. 2016;17(2):e32329. [Google Scholar]
- 31. Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3(2):163–175. [DOI] [PubMed] [Google Scholar]
- 32. Mortazavi SM. Isolation a new strain of Kocuria rosea capable of tolerating extreme conditions. J Environ Radioact. 2015;147:153–154. [DOI] [PubMed] [Google Scholar]
- 33. Mortazavi SM, Darvish L, Abounajmi M, et al. Alteration of bacterial antibiotic sensitivity after short-term exposure to diagnostic ultrasound. Iran Red Crescent Med J. 2015;17(11):e26622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taheri M, Mortazavi SM, Moradi M, et al. Klebsiella pneumonia, a microorganism that approves the non-linear responses to antibiotics and window theory after exposure to Wi-Fi 2.4 GHz electromagnetic radiofrequency radiation. J Biomed Phys Eng. 2015;5(3):115–120. [PMC free article] [PubMed] [Google Scholar]
- 35. Mortazavi S, Motamedifar M, Mehdizadeh A, et al. The effect of pre-exposure to radiofrequency radiations emitted from a GSM mobile phone on the susceptibility of BALB/c mice to Escherichia coli. J Biomed Phys Eng. 2012;2(4):139–146. [Google Scholar]
- 36. Mortazavi S. Letter to the Editor: Window theory in non-ionizing radiation-induced adaptive responses. Dose Response. 2013;11(2):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Akbal A, Balik HH. Investigation of antibacterial effects of electromagnetic waves emitted by mobile phones. Pol J Environ Stud. 2013;22(6):1589–1594. [Google Scholar]
- 38. Latif IA, AL-Azawy AF, AL-Assie AH. Assessment of genetic effects of bacterial cells after exposure to mobile phone radiation using RAPD. Iraqi J Biotechnol. 2013;12(2):63–74. [Google Scholar]
- 39. Mineta M, Katada R, Yamada T, et al. Bacterial mutation in high magnetic fields and radiofrequency radiation [in Japanese]. Nihon Igaku Hoshasen Gakkai zasshi. 1999;59(9):467–469. [PubMed] [Google Scholar]
- 40. Zohre R, Ali Y, Mostafa J, Samaneh R. Nondrug antimicrobial techniques: electromagnetic fields and photodynamic therapy. Biomed Pharmacol J. 2015;8(March Spl Edition):147–155. [Google Scholar]
- 41. Tadevosian A, Trchunian A. Effect of coherent extremely high-frequency and low-intensity electromagnetic radiation on the activity of membrane systems in Escherichia coli [in Russian]. Biofizika. 2008;54(6):1055–1059. [PubMed] [Google Scholar]
- 42. Torgomyan H, Trchounian A. Escherichia coli membrane-associated energy-dependent processes and sensitivity toward antibiotics changes as responses to low-intensity electromagnetic irradiation of 70.6 and 73 GHz frequencies. Cell Biochem Biophys. 2012;62(3):451–461. [DOI] [PubMed] [Google Scholar]
- 43. Belyaev IY, Shcheglov V, Alipov YD, Polunin V. Resonance effect of millimeter waves in the power range from 10-19 to 3 × 10-3 W/cm2 on Escherichia coli cells at different concentrations. Bioelectromagnetics. 1996;17(4):312–321. [DOI] [PubMed] [Google Scholar]
- 44. Torgomyan H, Ohanyan V, Blbulyan S, Kalantaryan V, Trchounian A. Electromagnetic irradiation of Enterococcus hirae at low-intensity 51.8-and 53.0-GHz frequencies: changes in bacterial cell membrane properties and enhanced antibiotics effects. FEMS Microbiol Lett. 2012;329(2):131–137. [DOI] [PubMed] [Google Scholar]
- 45. Torgomyan H, Tadevosyan H, Trchounian A. Extremely high frequency electromagnetic irradiation in combination with antibiotics enhances antibacterial effects on Escherichia coli. Curr Microbiol. 2011;62(3):962–967. [DOI] [PubMed] [Google Scholar]
- 46. Bayır E, Bilgi E, Şendemir-Ürkmez A, Hameş-Kocabaş EE. The effects of different intensities, frequencies and exposure times of extremely low-frequency electromagnetic fields on the growth of Staphylococcus aureus and Escherichia coli O157: H7. Electromagn Biol Med. 2015;34(1):14–18. [DOI] [PubMed] [Google Scholar]
- 47. Inhan-Garip A, Aksu B, Akan Z, Akakin D, Ozaydin AN, San T. Effect of extremely low frequency electromagnetic fields on growth rate and morphology of bacteria. Int J Radiat Biol. 2011;87(12):1155–1161. [DOI] [PubMed] [Google Scholar]
- 48. Ayari S, Dussault D, Millette M, Hamdi M, Lacroix M. Changes in membrane fatty acids and murein composition of Bacillus cereus and Salmonella typhi induced by gamma irradiation treatment. Int J Food Microbiol. 2009;135(1):1–6. [DOI] [PubMed] [Google Scholar]
- 49. Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2(5):a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nguyen THP, Shamis Y, Croft RJ, et al. 18 GHz electromagnetic field induces permeability of gram-positive cocci. Sci Rep. 2015;16(5):10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Torgomyan H, Trchounian A. Low-intensity electromagnetic irradiation of 70.6 and 73 GHz frequencies enhances the effects of disulfide bonds reducer on Escherichia coli growth and affects the bacterial surface oxidation–reduction state. Biochem Biophys Res Commun. 2011;414(1):265–269. [DOI] [PubMed] [Google Scholar]
- 52. Justo OR, Pérez VH, Alvarez DC, Alegre RM. Growth of Escherichia coli under extremely low-frequency electromagnetic fields. Appl Biochem Biotechnol. 2006;134(2):155–163. [DOI] [PubMed] [Google Scholar]
- 53. Gaafar E, Hanafy MS, Tohamy E, Ibrahim MH. The effect of electromagnetic field on protein molecular structure of E. coli and its pathogenesis. Rom J Biophys. 2008;18(2):145–169. [Google Scholar]
- 54. Stansell MJ, Winters WD, Doe RH, Dart BK. Increased antibiotic resistance of E. coli exposed to static magnetic fields. Bioelectromagnetics. 2001;22(2):129–137. [PubMed] [Google Scholar]
- 55. Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178(20):5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Segatore B, Setacci D, Bennato F, Cardigno R, Amicosante G, Iorio R. Evaluations of the effects of extremely low-frequency electromagnetic fields on growth and antibiotic susceptibility of Escherichia coli and Pseudomonas aeruginosa. Int J Microbiol. 2012;2012:587293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Corringer PJ, Poitevin F, Prevost MS, Sauguet L, Delarue M, Changeux JP. Structure and pharmacology of pentameric receptor channels: from bacteria to brain. Structure. 2012;20(6):941–956. [DOI] [PubMed] [Google Scholar]
- 58. Walleczek J. Electromagnetic field effects on cells of the immune system: the role of calcium signaling. FASEB J. 1992;6(13):3177–3185. [DOI] [PubMed] [Google Scholar]
- 59. Bersani F, Marinelli F, Ognibene A, et al. Intramembrane protein distribution in cell cultures is affected by 50 Hz pulsed magnetic fields. Bioelectromagnetics. 1997;18(7):463–469. [DOI] [PubMed] [Google Scholar]
- 60. Marchionni I, Paffi A, Pellegrino M, et al. Comparison between low-level 50 Hz and 900 MHz electromagnetic stimulation on single channel ionic currents and on firing frequency in dorsal root ganglion isolated neurons. Biochim Biophys Acta. 2006;1758(5):597–605. [DOI] [PubMed] [Google Scholar]
- 61. Tadevosyan H, Kalantaryan V, Trchounian A. Extremely high frequency electromagnetic radiation enforces bacterial effects of inhibitors and antibiotics. Cell Biochem Biophys. 2008;51(2-3):97–103. [DOI] [PubMed] [Google Scholar]


