Abstract
The low stringency-polymerase chain reaction (LS-PCR) and loop-mediated isothermal amplification (LAMP) assays were used to detect the presence of S. mansoni DNA in (1) Brazilian intermediate hosts (Biomphalaria glabrata, B. straminea, and B. tenagophila) with patent S. mansoni infections, (2) B. glabrata snails with prepatent S. mansoni infections, (3) various mixtures of infected and noninfected snails; and (4) snails infected with other trematode species. The assays showed high sensitivity and specificity and could detect S. mansoni DNA when one positive snail was included in a pool of 1,000 negative specimens of Biomphalaria. These molecular approaches can provide a low-cost, effective, and rapid method for detecting the presence of S. mansoni in pooled samples of field-collected Biomphalaria. These assays should aid mapping of transmission sites in endemic areas, especially in low prevalence regions and improve schistosomiasis surveillance. It will be a useful tool to monitor low infection rates of snails in areas where control interventions are leading towards the elimination of schistosomiasis.
1. Introduction
In Africa and Neotropical region there are several Biomphalaria (Gastropoda: Planorbidae) species that act as the intermediate snail hosts of Schistosoma mansoni Sambon, 1907. In Brazil, there are 11 species but only Biomphalaria glabrata (Say, 1818), Biomphalaria tenagophila (Orbigny, 1835), and Biomphalaria straminea (Dunker, 1848) have been found naturally infected by this trematode. The diagnosis is routinely performed through detection of Schistosoma mansoni cercariae by means of artificial light exposure or by squeezing snails between glass slides and observing larval stages of the parasite under the microscope.
However, these diagnostic methods are limited and may not detect early prepatent infections in snails. In addition many field-collected snails may be infected with different trematode species and sometimes snails may die before reaching the laboratory. Therefore, molecular techniques have been developed to detect this parasite within snails and in the environment. The polymerase chain reaction (PCR) assays for amplification of the Sm1-7 repeated sequence of S. mansoni [1] and the low stringency-PCR (LS-PCR) for amplification of tandem DNA region of S. mansoni [2] have been used to detect parasites during the prepatent period. Moreover, Hamburger et al. [3] used the PCR technique to detect S. mansoni DNA in a pool of molluscs as well as in water after filtering using “Browne Capsule” [4].
The loop-mediated isothermal amplification (LAMP) assay was used to detect S. mansoni and Schistosoma haematobium in molluscs, one day after exposure to miracidia [5]. Later, this technique was applicable in labs with limited facilities, since the procedure does not require a thermocycler and, sometimes, it does not demand electrophoresis apparatus [6]. LAMP is a technique developed by Notomi et al. [7] to detect simple DNA, and it is sensitive and fast, using Bst DNA polymerase enzyme to DNA amplification in a constant temperature, which varies from 60 to 65°C. This technique was initially described using two pairs of primers, one outer pair (F3 and B3) and another inner pair (FIP and BIP), which amplify six DNA different regions, making this technique very specific. In 2002, Nagamine et al. [8] added two new primers named loop primers, which target new regions to star DNA amplification, providing a more specific and rapid method.
In this study, the LAMP assay was used with loop primers for mass detection of S. mansoni in Biomphalaria spp. Simultaneously, the S. mansoni DNA was amplified by LS-PCR, a technique used with success in our lab to detect the parasite in molluscs. The work aimed at establishing if these techniques could be used to monitor infected molluscs in endemic areas in order to predict the risk and dissemination of the disease, as well as assess the value of the approach for schistosomiasis control and elimination program when interruption of transmission needs to be verified.
2. Material and Methods
Biomphalaria glabrata, B. tenagophila, and B. straminea molluscs and AL, SJ, and LE strains of S. mansoni used in this study were maintained and raised in the “Lobato Paraense” Mollusc Rearing of René Rachou Research Center, CPqRR/Fiocruz, in Belo Horizonte, MG, Brazil. The LE strain was isolated, in 1968, from a patient residing in Belo Horizonte, MG (Brazil). The SJ strain was isolated, in 1975, from naturally infected snails from São José dos Campos, São Paulo (Brazil). The AL strain was isolated in 1980 from B. glabrata originated from Alagoas state (Brazil).
2.1. Samples
2.1.1. Trematode Groups
The pools of cercariae used were (1) LE, SJ, and AL strains of S. mansoni, (2) Cercaria macrogranulosa (simple tail), (3) Cercaria caratinguensis (longifurcate tail), and (4) Cercaria ocellifera (brevifurcate tail). Trematodes from groups 2–4 were obtained from field snails Biomphalaria from Minas Gerais, Brazil.
2.1.2. Molluscs
(A) Biomphalaria glabrata, B. tenagophila, and B. straminea were individually used in different conditions: (1) patent infections shedding S. mansoni cercariae (experimental infection with LE, SJ, and AL, resp.); (2) during the prepatent period (11 days after experimental infection); and (3) noninfected (not exposed to infection).
(B) S. mansoni-infected B. glabrata were dead for eight weeks. In summary, two specimens (10–12 mm), shedding S. mansoni cercariae (experimental infection with LE strain: 10 miracidia/mollusc), were recovered from the aquarium and kept in Petri dishes at room temperature for eight weeks; after this period the soft tissues were decomposed. Then, the empty shells and dried tissues were used for DNA extraction [9].
(C) Field-collected B. glabrata were from Ribeirão das Neves/Minas Gerais (Brazil), shedding S. mansoni cercariae.
(D) Mollusc Pools. This group was performed in triplicate in order to check the reproducibility of the techniques: B. glabrata specimens were exposed to 8 miracidia/mollusc from LE strain. Two snail samples during the prepatent period (11 days after exposure) were squashed and sporocysts were found. The squashed molluscs were added to negative B. glabrata (different ages) pools, separately, as follows: 1 positive snail in a pool of 20 negative (1 : 20) snails (weighing nearly 8 g) and 1 : 300 (104 g).
Four B. glabrata shedding cercariae (patent infections) were added separately to pools of negative B. glabrata (different ages), as follows: 1 : 20 (8 g); 1 : 300 (104 g); 1 : 500 (204 g); and 1 : 1000 (390 g).
B. straminea specimens were exposed to 20 miracidia/mollusc from the AL strain. One of these, in prepatent period (11 days after exposure), was squashed and sporocysts were observed. This squashed mollusc was added to a pool of negative B. straminea (different ages), as follows: 1 : 100 (12 g).
One B. straminea snail shedding cercariae was added to a pool of negative B. straminea (different ages) as follows: 1 : 100 (12 g).
2.1.3. Molluscs Pool Preparation for DNA Extraction
The pools of snails were mashed in one container and submitted to the pepsin digestion protocol by Wallace & Rosen [10] and followed by sedimentation method of Baermann adapted [11]. After sedimentation, the material was centrifuged at 12,000g for 10 min and the precipitate was cryopreserved (−70°C) prior to DNA extraction.
2.2. DNA Extraction
In the individual mollusc group the extraction process was performed using all the body. Cercariae were pooled prior to DNA extraction. In the groups involving pooled snails, a small part of precipitated cryopreserved tissue (approximately 20 mg) was used as template for the DNA extraction.
All DNA extractions were performed using the DNA Wizard Genomic Purification kit (Promega, Madison, USA) according to the manufacturer's instructions.
2.3. LAMP Primer Design
The species-specific oligonucleotide primers initially used for the LAMP assay amplification were selected based on
mitochondrion S. mansoni minisatellite DNA region gene (genbank: L27240.1);
internal transcript spacer of rDNA gene (genbank: AY446082.1);
Schistosoma mansoni SM750 gene (genbank: M63265.1);
partial region of S. mansoni DNA mitochondrion (genbank: AF130787.1).
Thus, each sequence was submitted to LAMP Designer 1.13 software (PREMIER Biosoft International) to design the six-primer set.
2.4. LAMP Assays
Each LAMP reaction contained 1x ThermoPol Reaction Buffer (20 mM Tris-HCl, pH 8.8, 10 mM KCl, 10 mM (NH4)2SO4, 2 mM MgSO4, and 0.1% Triton X-100), 200 μM of each dNTP, 40 pmol/μL of each internal primer (FIP/BIP), 20 pmol/μL of LoopF and LoopB, 5 pmol/μL of each external primer (F3/B3), 0.8 M of Betaine, 6.4 U of Bst DNA polymerase, large fragment (New England Biolabs), and 2 μL DNA (concentration depends on the experiment), in a final volume of 25 μL. A negative control (no DNA) was included in all experiments. The mixture was incubated at 65°C for 40 min and then heated at 80°C for 10 min to terminate the reaction.
To determine the sensitivity of infection detection by LAMP, DNA was extracted from 200 cercariae from LE strain of S. mansoni (yielding 8.0 ng) and from one negative B. glabrata (yielding 13.4 ng). DNA concentrations were determined by Qubit 2.0 high sensitivity (Invitrogen) and different amounts of parasite DNA were mixed with a constant amount of negative snails in artificial infection. Thus, the DNA negative snails were diluted to 1.3 ng and mixed with 8.0 pg, 0.8 pg, 80 fg, and 8 fg of cercariae DNA and the LAMP was carried out as described above.
2.5. LS-PCR Assay
DNA was amplified using 0.8 units of taq polymerase Platinum (Invitrogen), 200 μM dNTPs, 1.5 mM MgCl2, 50 mMKCl, 10 mM Tris-HCl, pH 8.5, 5 pmol of each primer (ER 5′ ACCTACCGTACTATGACG and EF 5′ GGTTTCTTAGTGTTATAGCC) [12], and 1 μL DNA (concentration depends on the experiment) in a final volume of 10 μL. The pair of primers used in these reactions was designed to amplify across adjacent in tandem minisatellite units from S. mansoni mtDNA [12]. A negative control (no DNA) was included in all experiments. DNA was amplified in thermocycler, using 35 cycles of amplification under the following conditions: an initial denaturing step at 95°C for 3 min, annealing 40°C for 1 min, and extension at 72°C for 1 min. For the following 34 cycles, the denaturing step was reduced to 45 sec. A final extension step (72°C) was added at the last 5 min of reaction.
The sensitivity LS-PCR was 0.1 pg S. mansoni DNA in 0.1 ng of DNA snail, as showed by Jannotti-Passos et al. [2].
2.6. Amplicon Detection
2.6.1. Gel Electrophoresis to LAMP and LS-PCR
Three microliters of LAMP products was examined on a 6% polyacrylamide gel and stained with silver. The remaining was used in the colorimetric detection to LAMP.
2.6.2. Colorimetric Detection to LAMP
DNA intercalating dye SYBR Green I [5] and SYBR Safe DNA gel stain (both by Invitrogen) was added to the solution after the reaction was completed. When the LAMP reaction is positive the color of the reaction solution changed from orange to yellow/green in the presence of LAMP amplicon.
3. Results
3.1. LAMP Primer Design
Each set of six primers was submitted to LAMP reaction and only one set was specific to amplify S. mansoni DNA. This set of primers was from ITS rDNA of S. mansoni and were named:
LAMPsmITS_F3 (5′ACGCACATTAAGTCGTGG 3′); LAMPsmITS_B3 (5′AACCAGAGACAAGATCAAGTG 3′); LAMPsmITS_FIP (5′ACCGCAGCATCTCAATCAAGTCAGAGGCTCCGTCCTAAT 3′); LAMPsmITS_BIP (5′TTGTGCTCGAGTCGTGGCGCATACGATAGGTGCGAAT 3′); LAMPsmITS_LoopF (5′ATCTAGACCAGACTAGGCTGT3′); LAMPsmITS_LoopB (5′GACATTATACACGCTCGGGAT 3′). Thus, this set was used in all LAMP assay.
3.2. LS-PCR and LAMP Assays
The LAMP sensitivity was evaluated with a limit of detection observed at 70 fg of DNA (Figure 1). The specificity LAMP assay was tested by amplification of DNA from S. mansoni cercariae of the strains LE, SJ, and AL, while the amplification of DNA from cercariae C. macrogranulosa, C. caratinguensis, and C. ocellifera were not detectable (Figure 2). The specificity and sensitivity of infection detection by LS-PCR have been made previously by Jannotti-Passos et al. [2].
Figure 1.
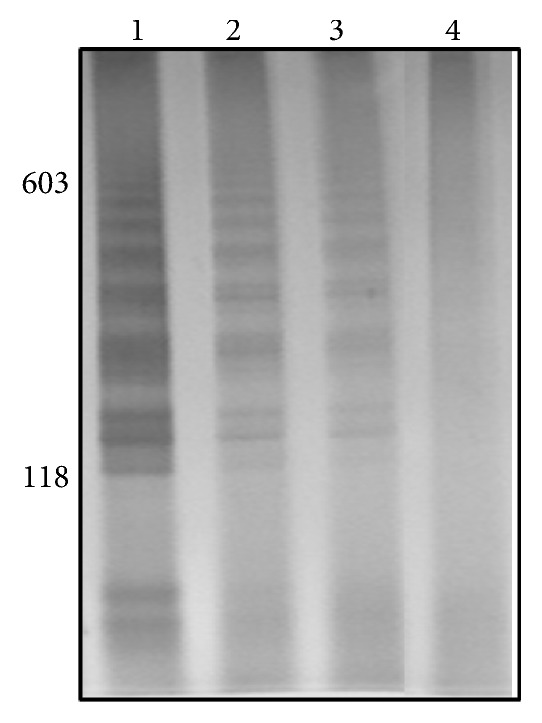
Silver-stained 6% polyacrylamide gel showing LAMP products from sensitivity test. Lane 1: DNA negative snails (1.3 ng) mixed with 8.0 pg from cercariae DNA, Lane 2: DNA negative snails (1.3 ng) mixed with 0.8 pg of cercariae DNA, Lane 3: DNA negative snails (1.3 ng) mixed with 80 fg of cercariae DNA, and Lane 4: DNA negative snails (1.3 ng) mixed with 8 fg of cercariae DNA. Molecular-size markers are shown on the left of the gel.
Figure 2.
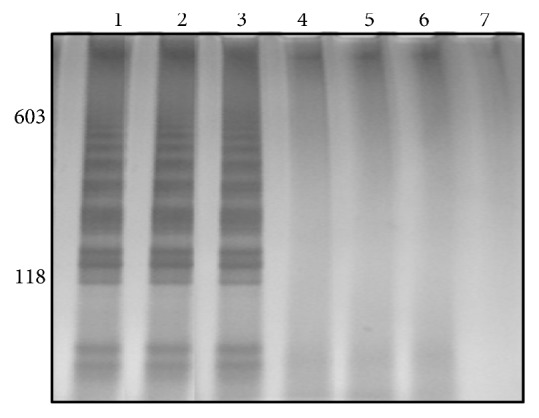
Silver-stained 6% polyacrylamide gel showing LAMP products from specificity test. Lane 1: DNA from Schistosoma mansoni cercariae LE strain; Lane 2: DNA from S. mansoni cercariae SJ strain; Lane 3: DNA from S. mansoni cercariae AL strain; Lane 4: macrogranulosa cercariae; Lane 5: caratinguensis cercariae; Lane 6: ocelifera cercariae; Lane 7: negative control. Molecular-size markers are shown on the left of the gel.
LAMP and LS-PCR amplifications presented characteristic profile to S. mansoni in B. glabrata, B. tenagophila, and B. straminea shedding S. mansoni cercariae; infected B. glabrata, B. tenagophila, and B. straminea in prepatent period; S. mansoni-infected B. glabrata dead for eight weeks and B. glabrata shedding S. mansoni cercariae from Ribeirão das Neves/MG (Brazil) (Figures 3(a) and 3(b)).
Figure 3.
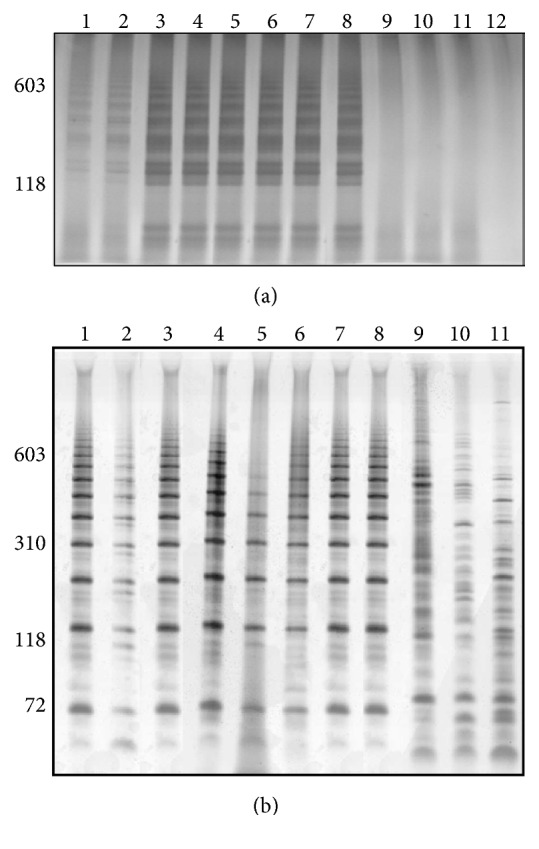
Silver-stained 6% polyacrylamide gel showing LAMP (a) and LS-PCR (b) products. Lane 1: Biomphalaria glabrata shedding Schistosoma mansoni cercariae; Lane 2: Biomphalaria tenagophila shedding S. mansoni cercariae; Lane 3: Biomphalaria straminea shedding S. mansoni cercariae; Lane 4: infected B. glabrata in prepatent period; Lane 5: infected B. tenagophila in prepatent period; Lane 6: infected B. straminea in prepatent period; Lane 7: S. mansoni-infected B. glabrata dead for eight weeks; Lane 8: field-collected B. glabrata shedding S. mansoni cercariae from Ribeirão das Neves/MG (Brazil); Lane 9: negative B. glabrata; Lane 10: negative B. tenagophila Lane 11: negative B. straminea; and Lane 12: negative control (only in LAMP). Molecular-size markers are shown on the left of the gel.
For molluscs pool amplifications LAMP and LS-PCR assays presented characteristic profile to S. mansoni in all amplifications, except in pool of 300 negative snails and one B. glabrata in prepatent period and in pool of 100 negative snails and one B. straminea in prepatent period (Figures 4(a) and 4(b), Lanes 2 and 7, resp.). All triplicates from molluscs pool group were equal (data not shown).
Figure 4.
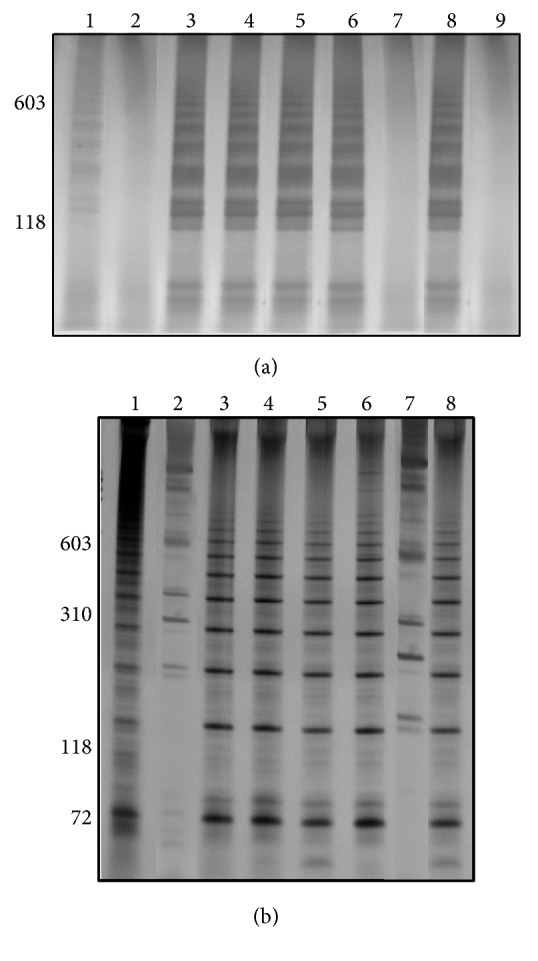
Silver-stained 6% polyacrylamide gel showing LAMP (a) and LS-PCR (b) from molluscs pool. Lanes 1 and 2: infected Biomphalaria glabrata in prepatent period: 1 positive snail in a pool of 20 negative snails and 1 : 300; Lanes from 3 to 6: infected B. glabrata shedding cercariae: 1 : 20; 1 : 300; 1 : 500, and 1 : 1000; Lane 7: infected Biomphalaria straminea in prepatent period: 1 : 100; Lane 8: infected B. straminea shedding cercariae; Lane 9: negative control (only in LAMP). Molecular-size markers are shown on the left of the gel.
The amplicons of LAMP were observed by gel (Figures 1–4) and colorimetrics methods. However, the colorimetric detection (SYBR Green I and SYBR Safe DNA) was not reproducible and caused concern about false-positives and false-negatives. Figure 5 illustrates the inconsistency in the interpretation of results after staining with SYBR Green I and exposure to ultraviolet light. The samples are the same as those applied to the gel of Figure 4.
Figure 5.
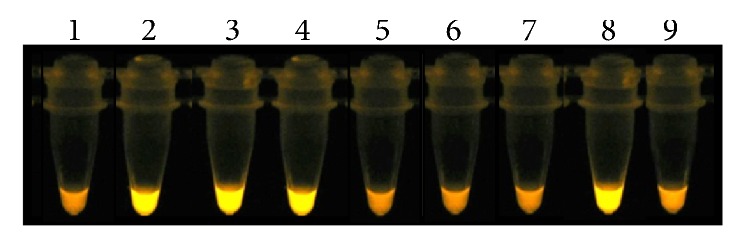
Fluorescence of LAMP products from molluscs pool using SYBR Green I staining followed by ultraviolet illumination at 320 nm. Tubes 1 and 2: infected Biomphalaria glabrata in prepatent period: 1 positive snail in a pool of 20 negative snails and 1 : 300; Tubes from 3 to 6: infected B. glabrata shedding cercariae: 1 : 20; 1 : 300; 1 : 500; and 1 : 1000; Tube 7: infected Biomphalaria straminea in prepatent period: 1 : 100; Tube 8: infected B. straminea shedding cercariae; Tube 9: negative control.
4. Discussion
The possibility of diagnosing S. mansoni in pooled snails will accelerate the detection of new snail habitats and could assist schistosomiasis control programs by highlighting freshwater bodies associated with schistosomiasis transmission. Thus, the main contribution of this work was the use of molecular assays for DNA detection of parasite that showed that it was possible to detect one infected snail in a pool of 1000 negative B. glabrata. LS-PCR and LAMP assays were sensitive and specific to detection. The LAMP assay saves time and does not require an expensive thermocycler since all reactions can be performed at a constant temperature [13]. In same experiments colorimetric assays are used as dyes that are added to the solutions before or after LAMP reaction. Later, these authors [13] compared different dyes to analyze the sensitivity of LAMP assay, including hydroxy naphthol blue (HNB), calcein, and SYBR Green: the first two being added before the reaction and the last one after the reaction. These authors observed that the HNB sensitivity was equivalent to SYBR Green. But the tubes with HNB dye were not required to be opened to determine the results, reducing the risk of cross-contamination. LAMP technique was used to detect Leishmania DNA directly from infected sand fly extract, using colorimetric malachite green (MG) by naked eye visualization [14]. However, the MG and HNB dyes are difficult to discern by eye due to the subtleness in the difference between positive and negative results [14–17]. The main disadvantage of using SYBR Green I is the possibility of cross-contamination. Indeed, Abbasi et al. [5] observed that the detection by SYBR Green I was not possible in the case of examining snails because uninfected snails also showed positive fluorescent signals given the amount of snail DNA present in the reaction mixture. Similarly, when we used SYBR Green I and SYBR Safe DNA in the LAMP reactions from S. mansoni-infected Biomphalaria, interpretation problems were encountered with the possibility of false-positives (see Figure 5). Therefore our preference was to run the LAMP products on 6% polyacrylamide gels and to use silver staining.
LAMP assays have been used for the detection of different species of Schistosoma, either in intermediate or in definitive hosts [5, 18–20]. Hamburger et al. [6] adapted the LAMP tool for application in field laboratories in endemic areas for S. haematobium and S. mansoni. These field laboratories lacked the facility and expert teams in molecular biology. They demonstrated that a DNA amplification tool suitable for field laboratories ensures a broader examination of LAMP efficacy in various epidemiologic settings. LAMP assay was used to detect Schistosoma japonicum infection in pooled samples of field-collected Oncomelania in China and they constructed a transmission of schistosomiasis risk map using ArcMap, based on the positive proportion, which will be a surveillance and response strategies guide in high risk areas [21]. Kumagai et al. [22] reported that LAMP and conventional PCR could amplify S. japonicum DNA from a group of 100 normal snails artificially mixed with one infected snail; consequently, the detection from pooled samples has opened the door for large-scale low-cost screening.
Regarding costs and time per reaction, the LAMP is approximately half the price and the time compared with LS-PCR. If the cost of thermocyclers for DNA amplification is also included, LAMP becomes comparatively even cheaper given that it can be performed using only a heat block. On the other hand, samples without S. mansoni DNA presence show no specific amplicons when LS-PCR technique is used, providing a more accurate result, since it works as an internal control.
The principal contribution of this study was to detect one B. glabrata infected with S. mansoni in a pool of 1000 negative snails, regardless of the technique used. This strategy is interesting to provide a low-cost, rapid, and effective method to detect S. mansoni DNA in field-collected Biomphalaria. The next step is to validate this strategy using snails collected from the field and to carry out mapping of transmission sites. This assay might improve schistosomiasis surveillance by providing a much needed tool to monitor infections in snails especially in low transmission areas.
Acknowledgments
This work was partially supported by grants from FAPEMIG, CNPq (304121/2014-2), and Fiocruz. The authors would like to thank the “Lobato Paraense Mollusc Rearing” and Dr. Luciano Moreira and Daniela da Silva Gonçalves of René Rachou Research Center for the support and discussion for this research and Dr. David Rollinson for manuscript suggestions.
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contributions
Roberta Lima Caldeira, Liana Konovaloffi Jannotti-Passos, and Omar Dos Santos Carvalho conceived and designed the study and wrote the paper. Roberta Lima Caldeira and Omar Dos Santos Carvalho were responsible for the acquisition of the financial support for the study. Roberta Lima Caldeira and Liana Konovaloffi Jannotti-Passos performed the experiments, analyzed the data, and proved biological samples.
References
- 1.Hamburger J., Turetski T., Kapeller I., Deresiewicz R. Highly repeated short DNA sequences in the genome of Schistosoma mansoni recognized by a species-specific probe. Molecular and Biochemical Parasitology. 1991;44(1):73–80. doi: 10.1016/0166-6851(91)90222-r. [DOI] [PubMed] [Google Scholar]
- 2.Jannotti-Passos L. K., Vidigal T. H. D. A., Dias-Neto E., et al. PCR amplification of the mitochondrial DNA minisatellite region to detect Schistosoma mansoni infection in Biomphalaria glabrata snails. Journal of Parasitology. 1997;83(3):395–399. doi: 10.2307/3284400. [DOI] [PubMed] [Google Scholar]
- 3.Hamburger J., He-Na, Xu Y. X., Ramzy R. M., Jourdane J., Ruppel A. A polymerase chain reaction assay for detecting snails infected with bilharzia parasites (Schistosoma mansoni) from very early prepatency. American Journal of Tropical Medicine and Hygiene. 1998;59(6):872–876. doi: 10.4269/ajtmh.1998.59.872. [DOI] [PubMed] [Google Scholar]
- 4.Hamburger J., Yu-Xin X., Ramzy R. M., Jourdane J., Ruppel A. Development and laboratory evaluation of a polymerase chain reaction for monitoring Schistosoma mansoni infestation of water. American Journal of Tropical Medicine and Hygiene. 1998;59(3):468–473. doi: 10.4269/ajtmh.1998.59.468. [DOI] [PubMed] [Google Scholar]
- 5.Abbasi I., King C. H., Muchiri E. M., Hamburger J. Detection of Schistosoma mansoni and Schistosoma haematobium DNA by loop-mediated isothermal amplification: identification of infected snails from early prepatency. American Journal of Tropical Medicine and Hygiene. 2010;83(2):427–432. doi: 10.4269/ajtmh.2010.09-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamburger J., Abbasi I., Kariuki C., et al. Evaluation of loop-mediated isothermal amplification suitable for molecular monitoring of schistosome-infected snails in field laboratories. American Journal of Tropical Medicine and Hygiene. 2013;88(2):344–351. doi: 10.4269/ajtmh.2012.12-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Notomi T., Okayama H., Masubuchi H., et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Research. 2000;28(12, article E63) doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagamine K., Hase T., Notomi T. Accelerated reaction by loop-mediated isothermal amplification using loop primers. Molecular and Cellular Probes. 2002;16(3):223–229. doi: 10.1006/mcpr.2002.0415. [DOI] [PubMed] [Google Scholar]
- 9.Caldeira R. L., Jannotti-Passos L. K., Lira P. M., Carvalho O. S. Diagnostic of Biomphalaria snails and Schistosoma mansoni: DNA obtained from traces of shell organic materials. Memorias do Instituto Oswaldo Cruz. 2004;99(5):499–502. doi: 10.1590/s0074-02762004000500007. [DOI] [PubMed] [Google Scholar]
- 10.Wallace G. D., Rosen L. Techniques for recovering and identifying larvae of Angiostrongylus cantonensis. Malacology. 1969;7:427–439. [Google Scholar]
- 11.Moraes R. G. Contribuição para o estudo do Strongyloides stercoralis e da estrongiloidiase no Brasil. Revista de Saude Publica. 1948;1:507–624. [Google Scholar]
- 12.Pena H. B., Souza C. P., Simpson A. J. G., Pena S. D. J. Intracellular promiscuity in Schistosoma mansoni: nuclear transcribed DNA sequences are part of a mitochondrial minisatellite region. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(3):915–919. doi: 10.1073/pnas.92.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goto M., Honda E., Ogura A., Nomoto A., Hanaki K.-I. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. BioTechniques. 2009;46(3):167–172. doi: 10.2144/000113072. [DOI] [PubMed] [Google Scholar]
- 14.Nzelu C. O., Gomez E. A., Cáceres A. G., et al. Development of a loop-mediated isothermal amplification method for rapid mass-screening of sand flies for Leishmania infection. Acta Tropica. 2014;132(1):1–6. doi: 10.1016/j.actatropica.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Tomita N., Mori Y., Kanda H., Notomi T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nature Protocols. 2008;3(5):877–882. doi: 10.1038/nprot.2008.57. [DOI] [PubMed] [Google Scholar]
- 16.Jothikumar P., Narayanan J., Hill V. R. Visual endpoint detection of Escherichia coli o157: H7 using isothermal genome exponential amplification reaction (GEAR) assay and malachite green. Journal of Microbiological Methods. 2014;98(1):122–127. doi: 10.1016/j.mimet.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Arimatsu Y., Kaewkes S., Laha T., Hong S.-J., Sripa B. Rapid detection of Opisthorchis viverrini copro-DNA using loop-mediated isothermal amplification (LAMP) Parasitology International. 2012;61(1):178–182. doi: 10.1016/j.parint.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J., Rong R., Zhang H. Q., Shi C. J., Zhu X. Q., Xia C. M. Sensitive and rapid detection of Schistosoma japonicum DNA by loop-mediated isothermal amplification (LAMP) International Journal for Parasitology. 2010;40(3):327–331. doi: 10.1016/j.ijpara.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Xu J., Guan Z.-X., Zhao B., et al. DNA detection of Schistosoma japonicum: diagnostic validity of a LAMP assay for low-intensity infection and effects of chemotherapy in humans. PLOS Neglected Tropical Diseases. 2015;9(4) doi: 10.1371/journal.pntd.0003668.e0003668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernández-Soto P., Arahuetes J. G., Hernández A. S., López-Abán J., Santiago B. V., Muro A. A Loop-Mediated Isothermal Amplification (LAMP) assay for early detection of Schistosoma mansoni in stool samples: a diagnostic approach in a murine model. PLoS Neglected Tropical Diseases. 2014;8(9, article e3126) doi: 10.1371/journal.pntd.0003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tong Q.-B., Chen R., Zhang Y., et al. A new surveillance and response tool: risk map of infected Oncomelania hupensis detected by Loop-mediated isothermal amplification (LAMP) from pooled samples. Acta Tropica. 2015;141:170–177. doi: 10.1016/j.actatropica.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Kumagai T., Furushima-Shimogawara R., Ohmae H., et al. Detection of early and single infections of Schistosoma japonicum in the intermediate host snail, Oncomelania hupensis, by PCR and loop-mediated isothermal amplification (LAMP) assay. American Journal of Tropical Medicine and Hygiene. 2010;83(3):542–548. doi: 10.4269/ajtmh.2010.10-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]


