Summary
Background
Small intestinal bacterial overgrowth (SIBO) is a heterogeneous syndrome, characterised by an increased number and/or abnormal type of bacteria in the small bowel. Over the past decades, rifaximin has gained popularity for this indication despite its use is not evidence based.
Aim
To perform a systematic review and meta‐analysis to summarise evidence about the efficacy and safety of rifaximin to eradicate SIBO in adult patients.
Methods
MEDLINE, EMBASE, CCRCT, Scopus and Web of Science were searched from inception to March 16, 2015 for RCTs and observational studies. Furthermore, abstract books of major European, American and Asian gastroenterological meetings were also examined.
Results
Thirty‐two studies involving 1331 patients were included. The overall eradication rate according to intention‐to‐treat analysis was 70.8% (95% CI: 61.4–78.2; I 2 = 89.4%) and to per protocol analysis 72.9% (95% CI: 65.5–79.8; I 2 = 87.5%). Meta‐regression identified three covariates (drug dose, study design and co‐therapy) independently associated with an increased eradication rate. The overall rate of adverse events was 4.6% (95% CI: 2.3–7.5; I 2 = 63.6%). In the subset of studies (n= 10) allowing the analysis, improvement or resolution of symptoms in patients with eradicated SIBO was found to be 67.7% (95% CI: 44.7–86.9; I 2 = 91.3%).
Conclusions
Rifaximin treatment seems to be effective and safe for the treatment of SIBO. However, the quality of the available studies is generally poor. Well‐designed RCTs are needed to substantiate these findings and to establish the optimal regimen.
Introduction
Small intestinal bacterial overgrowth (SIBO) is a heterogeneous syndrome characterised by an increased number and/or abnormal type of bacteria in the small bowel, and it is a well‐recognised cause of maldigestion and malabsorption.1, 2
The recent discovery of an association between SIBO and functional gut symptoms, albeit controversial, has renewed interest in this mimicry. SIBO represents indeed an umbrella term, under which some different functional (e.g. irritable bowel syndrome, chronic constipation, diarrhoea) or organic (e.g. inflammatory bowel disease, coeliac disease, diverticular disease, etc.) conditions can be included, as – in each of them – bacterial proliferation (and consequent inflammation) may, at least in part, trigger similar abdominal symptoms.1
The overall, true prevalence of SIBO – which is usually under‐diagnosed – is unknown.2, 3 Indeed, patients may not seek healthcare and SIBO may not be properly diagnosed by medical investigations. In addition, the diagnostic yield depends on the methodology adopted, so that results from different studies are difficult to compare.4, 5
The mainstay of the SIBO treatment is based on the use of antimicrobial agents, whose aims should not be to eradicate the entire bacterial flora but rather to modify the intestinal microecology in order to get symptoms relief.1 Ideally, the choice of antimicrobials should reflect in vitro susceptibility testing, but this is usually impractical because intestinal bacterial cultures need invasive methodology to collect samples under sterile conditions.6 Therefore, hydrogen breath test (HBT) is widely used as non‐invasive means to diagnose SIBO. As consequence, in clinical practice antibiotic treatment, which should cover both aerobic and anaerobic bacteria, remains primarily empiric.4, 5, 6
Several antibiotic regimens proved to be effective over the past 50 years, with treatment success ranging from 27% to 100%.7 Till the end of 90s, only systemic antimicrobials were used, whose adverse events (AEs) and detrimental effects on gut microbiota are today well known.8 Poorly absorbed antibiotics, unlike systemic ones, allow localised targeting of enteric pathogens and are associated with minimal risk of systemic toxicity or AEs. The restricted use of drugs only for enteric‐infections should also reduce the development of widespread resistance, especially of enterobacteria, a major limitation of current antibiotics.8
Rifaximin is a product of synthesis experiments designed to modify the parent compound, rifamycin, in order to achieve low gastrointestinal absorption while retaining good antibacterial activity.9, 10, 11 Both experimental and clinical pharmacology have clearly shown that this compound is a poorly absorbed antibiotic with a broad spectrum of antibacterial activity, covering Gram‐positive and Gram‐negative microorganism, both aerobes and anaerobes.10, 11, 12, 13
Rifaximin fulfils all the characteristics set by DuPont and Ericsson14 for the ideal antimicrobial that should be used for the treatment of gastrointestinal infections (including dysbiosis and SIBO). As a consequence, over the past decades, rifaximin has been largely used to treat SIBO1, 7 even if there is currently a lack of a critical summary of evidence. To bridge this gap, a systematic review and meta‐analysis of randomised and nonrandomised studies was performed to evaluate the clinical effectiveness of and safety rifaximin to eradicate SIBO in adult patients.
Methods
Search strategy and study selection
This meta‐analysis was developed according to the PRISMA15 and to the MOOSE16 statement guidelines. A search of the medical literature was conducted using MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, Scopus and Web of Science from inception to 16 March 2015. Detailed eligibility criteria for study inclusion are provided in Table 1. The search strategy had two sets of terms joined together with the ‘AND’ operator. The first included the condition of interest: ‘small intestine, intestinal diseases, bacteria, bacterial infections, blind loop syndrome, breath tests, glucose, lactulose, xylose, sucrose, irritable bowel syndrome’ (both as Medical Subject Heading terms and free text term), and ‘small bowel bacterial overgrowth, small intestine bacterial overgrowth, SIBO, small bowel, small intestine, malabsorption, syndromes, irritable bowel syndrome (IBS), functional diseases, HBT, glucose breath test, lactulose breath test, xylose test, sucrose breath test, jejunal aspirate’ (as free text term). The second included the treatment evaluated: ‘rifaximin’ (as subject heading and free text term in Embase and as free text term in the other databases). A search of the abstract books from the Digestive Disease Week (2000–2014), American College of Gastroenterology (2004–2014), United European Gastroenterology Week (2000–2014), British Society of Gastroenterology (2001–2014), and Asian Pacific Digestive Week (2003–2014), was also performed. Bibliographies of all identified relevant studies were used to perform a recursive search. There were no language restrictions. Abstracts of the papers identified by the initial search were evaluated independently and in a blinded manner by the two authors for appropriateness. The primary outcome was to assess the efficacy of rifaximin to eradicate SIBO, and the secondary outcome was to evaluate its safety.
Table 1.
Inclusion criteria
| Randomised controlled trials (RCTs) and observational studies using rifaximin to eradicate SIBO |
| Patients aged ≥18 years |
| Test to diagnose SIBO reported |
| Criteria to consider a test positive for SIBO reported |
| Follow‐up performed to assess eradication |
| Rifaximin regimens reporteda |
| Studies not including patients with neoplastic diseases |
Studies using cyclic treatment of rifaximin or reporting more than one dosage of rifaximin tested but not indicating the number of patients treated with each dosage were not included.
Data extraction
The two reviewers independently extracted data concerning the efficacy and the safety of rifaximin using predesigned data extraction forms, as dichotomous data. In addition, the following clinical data were extracted for each trial: rifaximin regimen (dose and duration), type of study (randomised controlled trial (RCT), cohort studies, etc.), type of test used to diagnose and follow‐up SIBO, sample size, time between end of treatment and eradication assessment (follow‐up), country where the study was carried out, concomitantly use of fibre, mesalazine, pre‐ or probiotics, AEs, whether the study was performed in a gastrointestinal (GI) setting, and if presence of IBS was specifically assessed. Finally, the studies reporting lower GI symptom assessment before and after treatment with rifaximin were identified and evaluated. Any disagreement was resolved by discussion between the two Authors. Distinction between cohort and case series was made according to the definition provided by Dekkers and co‐workers'.17 Risk of bias for RCTs was assessed as described in the Cochrane handbook.18 The Newcastle–Ottawa scale (NOS, possible highest score: 9) was used to assess the quality of case–control studies if included.19 Cohort studies and case series were evaluated using the 20‐items quality appraisal checklist developed by the Institute of Health Economics (IHE, Canada).20
Data synthesis and statistical analysis
Data for primary and secondary outcomes were pooled from all kinds of studies using a random effects model as there is generally no reason to assume that trials included in the analysis are identical in the sense that the true effect size is exactly the same in all the studies.21, 22 In case of cross‐over studies, data from first and second period were combined, if possible. Intention‐to‐treat analysis (ITT) was adopted where possible. To obtain an estimate of the maximum potential benefits, a per protocol analysis was also performed.23 Where possible, data from RCTs were pooled using a random effects model,21 results expressed as relative risk (RR) for success of SIBO eradication, and number need to treat (NNT) calculated as described in the Cochrane handbook.24 Heterogeneity between trials was assessed by χ2 test for heterogeneity, and I 2 statistic with 95% CIs was also calculated.25 Its value ranges from 0% to 100%, with 0% representing no observed heterogeneity, and larger values indicating increasing heterogeneity. A value below 25% was chosen to represent low levels of heterogeneity.25 When the degree of statistical heterogeneity was greater than this cut‐off, for both primary and secondary outcomes, possible explanations were investigated with sub‐group analysis and meta‐regression, using the residual maximum likelihood with random effects weighting and the Knapp and Hartung t‐distribution.26 Prior to analysis, adjusted proportions were calculated using a logit transformation.27 For the primary outcome, only studies where intention‐to‐treat analysis was possible were considered, and the covariates used in meta‐regression and sub‐group analysis were: (i) duration of treatment; (ii) dosage of rifaximin; (iii) type of study (dichotomised as RCT or no‐RCT); (iv) type of test used to diagnose and follow‐up SIBO; (v) sample size of the study (dichotomised as ≥50 patients vs. <50 patients); (vi) time between end of treatment and eradication assessment categorised as: within 7 days after the end of treatment; within 2–4 weeks after the end of treatment; and >4 weeks after the end of treatment; (vii) country where the study was performed (dichotomised as Italy vs. not Italy since most studies were performed in this Country); (viii) concomitantly use of fibre, mesalazine, pre‐ or probiotics (dichotomised as not concomitant use vs. concomitant use). For the secondary outcome, covariates used in meta‐regression and sub‐group analysis were: (i) duration of treatment; (ii) dosage of rifaximin; (iii) type of the study; (iv) sample size of the study; (v) country where the study was performed; (vi) concomitantly use of fibre, mesalazine, pre‐ or probiotics.
We also performed a sub‐group analysis to evaluate the eradication rate in patients with IBS and in patients enrolled in extra‐gastrointestinal settings (e.g. patients with diabetes, rosacea, etc).
Studies reporting lower GI symptom assessment before and after treatment with rifaximin were evaluated in order to identify those showing symptoms relief after therapy from those which did not.
StatsDirect v. 3.0.165 (StatsDirect, Ltd., Cheshire, UK) and stata (StataCorp, 2013, Stata Statistical Software: Release 13.1; StataCorp LP, College Station, TX, USA) were used to generate Forest plots for primary and secondary outcomes with 95% CIs, as well as Funnel plots. The latter were assessed for evidence of asymmetry and possible publication bias or other small study effects using the Egger's linear regression.28 Stata and Comprehensive Meta‐Analysis v. 3.3.070 (Biostat, Inc., Englewood, NJ, USA) were used to perform meta‐regression analyses.
Results
The search strategy employed identified 292 citations, 227 of which were excluded after examining title and abstract. There was a total of 65 studies that were retrieved and evaluated in more detail. Of these, 33 were excluded for various reasons, leaving 32 studies29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60 (2 of which were abstracts36, 54) that were eligible for inclusion involving 1331 patients as shown in Figure 1. 24 studies were cohort studies,29, 32, 33, 35, 36, 37, 39, 40, 41, 42, 43, 45, 46, 49, 50, 52, 53, 54, 55, 56, 57, 58, 59, 60 seven randomised controlled trials (RCTs).30, 31, 34, 44, 47, 48, 51 Finally, one study was a randomised cross‐over study38: since all patients received rifaximin (before or after placebo), they were all included in the proportion meta‐analysis for pooled eradication rates and pooled AEs rate. In two studies, rifaximin was used in patients under mesalazine therapy,30, 35 in other two studies, rifaximin was given to patients taking also fibres,38, 51 and in one study, it was employed in association with probiotics.37
Figure 1.
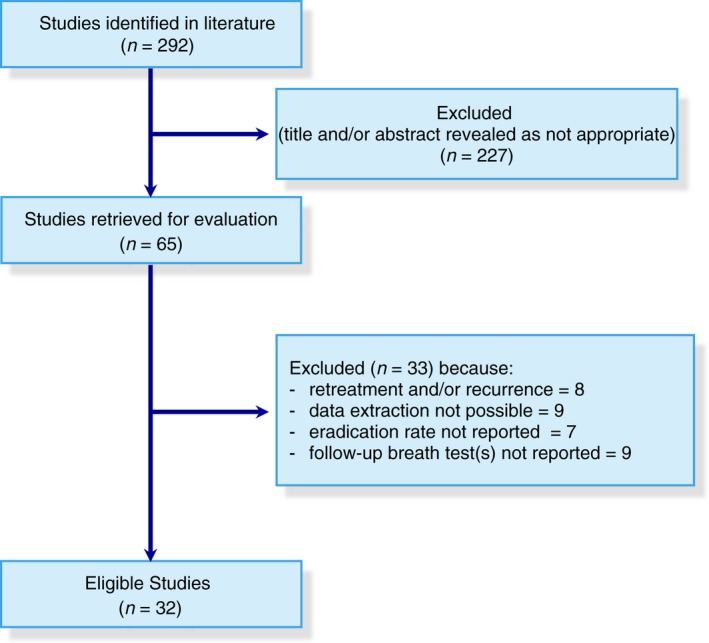
PRISMA flow diagram of the systematic review.
The glucose hydrogen breath test (GHBT) and the lactulose hydrogen breath test (LHBT) were used to diagnose and follow‐up SIBO in 17 (53.1%),30, 31, 34, 36, 37, 40, 41, 42, 44, 48, 49, 51, 52, 53, 54, 59, 60 and 13 studies (40.6%)29, 32, 33, 35, 38, 39, 43, 45, 46, 50, 55, 57, 58 respectively. Two studies47, 56 used both breath tests to identify SIBO. However, only one56 of those assessed also eradication by combined GHBT and LHBT.
Doses of rifaximin used ranged from 600 mg/die to 1600 mg/die, and duration of treatment ranged from 5 to 28 days. Seventy‐five percentage of the studies were performed in Italy. Detailed characteristics of studies included in the meta‐analysis are provided in Table S1A. No RCT was at low risk of bias (Table S1B). Quality cohort studies ranged between 10/20 and 18/20, according to quality appraisal checklist developed by the IHE20 (Table S1C). ITT evaluation was possible in all but six studies.37, 38, 41, 45, 52, 55
Overall eradication rates
Intention‐to‐treat analysis
Intention‐to‐treat analysis was possible in 26 studies29, 30, 31, 32, 33, 34, 35, 36, 39, 40, 42, 43, 44, 46, 47, 48, 49, 50, 51, 53, 54, 56, 57, 58, 59, 60 including 1141 patients. The pooled eradication rate of SIBO was 70.8% (95% CI: 61.4–78.2; Figure 2) with evidence of significant heterogeneity (Cochrane Q: P < 0.0001; I 2 = 89.4%; 95% CI: 86.1–91.6), and Funnel plot asymmetry (Egger test: −4.16; 95% CI: −6.40 to −1.93; P<0.0001, Figure S1A). Being only two the studies where both breath tests were used,47, 56 these were not included in the regression and sub‐group analysis.
Figure 2.
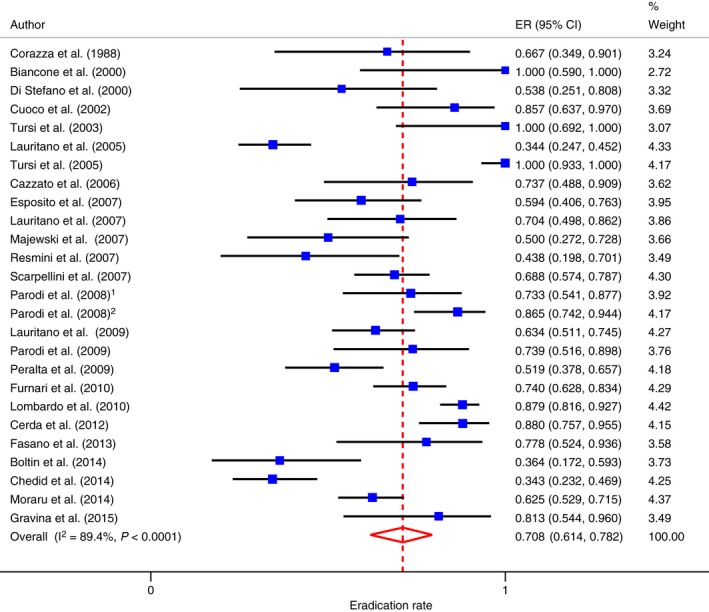
Forest plot of SIBO eradication rate according to ITT analysis.
Meta‐regression showed that eradication significantly increased for unit increase in dosage of rifaximin (Figure 3), in non‐RCTs, and in studies where fibres, mesalazine, pre‐ or probiotics were concomitantly used with rifaximin (Table S1D). A sub‐group analysis was also performed according to the same variables used for the meta‐regression analysis (Table S1E).
Figure 3.
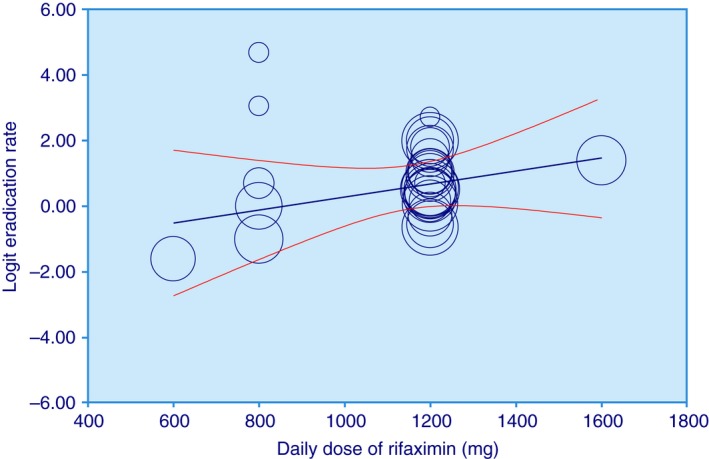
Meta‐regression plot: logit of eradication rate vs. daily dose of rifaximin (adjusted for all the other covariates evaluated).
Per protocol analysis
The PP analysis included overall 1274 patients from all the 32 studies (the 26 studies where ITT analysis was possible,29, 30, 31, 32, 33, 34, 35, 36, 39, 40, 42, 43, 44, 46, 47, 48, 49, 50, 51, 53, 54, 56, 57, 58, 59, 60 and from additional 6 trials where only PP analysis could be accomplished37, 38, 41, 45, 52, 55). The pooled eradication rate of SIBO was 72.9% (95% CI: 65.5–79.8) with evidence of significant heterogeneity (Cochrane Q: P < 0.0001; I 2 = 87.5%; 95% CI: 83.8–90.0), and Funnel plot asymmetry (Egger test: −3.47; 95% CI: −5.28 to −1.67; P = 0.0005, Figures S1B and S1C).
Eradication rates in IBS patients
Fourteen studies37, 39, 41, 42, 44, 45, 48, 49, 50, 53, 54, 55, 58, 59 were performed in patients with IBS. In 4 of them42, 44, 48, 58 it was not possible to extract data concerning the SIBO eradication rate, leaving 10 studies available for the analysis.
Intention‐to‐treat analysis was possible in six studies39, 49, 50, 53, 54, 59 involving 311 patients. The pooled eradication rate of SIBO was 71.6% (95% CI: 56.7–84.4; Figure 4) with evidence of significant heterogeneity (Cochrane Q: P < 0.0001; I 2 = 86.4%; 95% CI: 70.3–92.0), but without evidence of Funnel plot asymmetry (Egger test: −4.80; 95% CI: −15.4–5.86; P = 0.279, Figure S1D).
Figure 4.
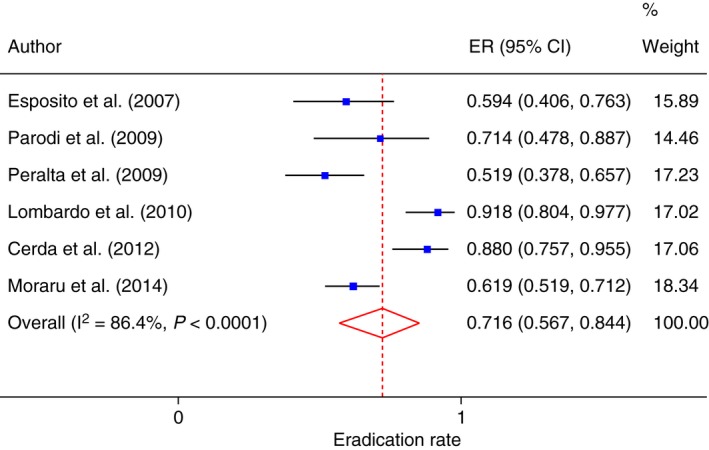
Forest plot of SIBO eradication rate in IBS patients according to ITT analysis.
The PP analysis included overall 427 patients from all the 10 studies (the eight studies where ITT analysis was possible plus additional four trials where only PP analysis could be accomplished37, 41, 45, 55). The pooled eradication rate of SIBO was 75.4% (95% CI: 65.0–84.5; Figure S1E) with evidence of significant heterogeneity (Cochrane Q: P < 0.0001; I 2 = 81.7%; 95% CI: 65.2–88.5), barely without evidence of Funnel plot asymmetry (Egger test: −3.73; 95% CI: −7.69–0.23; P = 0.067, Figure S1F).
Eradication rates in non‐GI settings
Seven studies32, 40, 43, 46, 47, 56, 60 involving 182 patients were performed in non‐GI settings.
According to ITT analysis, the reported overall eradication rate was 74.0% (95% CI: 62.9–83.7; Figure S1G) with evidence of significant heterogeneity (Cochrane Q: P = 0.0149; I 2 = 62%; 95% CI: 0–81.4), and without evidence of Funnel plot asymmetry (Egger test: −3.61; 95% CI: −7.94–0.71; P = 0.08; Figure S1H).
According to PP analysis, the overall eradication rate reported was 76.8% (95% CI: 69.2–83.6; Figure S1I) without evidence of significant heterogeneity (Cochrane Q: P = 0.2424; I 2 = 24.5%; 95% CI: 0–67.9), but with evidence of Funnel plots asymmetry (Egger test: −2.62; 95% CI: −5.01 to −0.239; P = 0.036; Figure S1J).
Comparative studies
Rifaximin vs. placebo
Only one RCT47 compared rifaximin alone to placebo and it was performed in patients with rosacea. 87.5% (95% CI: 71.0–96.4) of the 32 patients randomised to rifaximin were eradicated, whilst all patient (n = 20) randomised to placebo remained positive. Those were successively treated with rifaximin and the eradication found was 85.0% (95% CI: 64.0–94.8), giving an overall eradication rate of 86.5% (95% CI: 74.2–94.4). No data on AEs were reported in this study.
Rifaximin vs. other antimicrobials
In two studies rifaximin (1200 mg for 7 days) was compared to chlortetracycline (333 mg t.d.s for 7 days)31 or metronidazole (750 mg/die for 7 days)48 respectively, including overall 168 patients. According to ITT analysis, the overall eradication rate was 61.6% (95% CI: 51.1–71.6) and 37.6% (95% CI: 21.1–55.6) in patients randomised to rifaximin and other antimicrobials respectively, with a difference in eradication rate of 24% (95% CI: 6.2–35.5) in favour of rifaximin. The pooled RR of eradicating SIBO was 1.50 (95% CI: 1.11–2.04; Figure S1K) without evidence of significant heterogeneity (Cochrane Q: P = 0.418; I 2 = 0%). Egger's test was not performed due to the low number of the studies. NNT was 5 (95% CI: 2–43). According to PP analysis, the overall eradication rate was 64.6% (95% CI: 53.9–74.6) and 42.5% (95% CI: 27.7–58.6) in patients randomised to rifaximin and other antimicrobials respectively, with a difference that was not significant (P = 0.079). The pooled RR of eradicating SIBO was 1.53 (95% CI: 0.95–2.45; Figure S1L), without evidence of significant heterogeneity (Cochrane Q: P = 0.256; I 2 = 22.4%).
In the first study, there were no AEs.31 In the second study,48 AEs were significantly more frequent in the metronidazole (22.5%; 95% CI: 14.4–33.5) than in rifaximin group (8.5%; 95% CI: 3.9–17.2; difference in AEs: 14.1%; 95% CI: 2.1–26). Furthermore, six patients (8.5%; 95% CI: 3.9–17.2) in the metronidazole group were obliged to discontinue the study due to the severity of AEs.
Combination studies
Rifaximin plus fibres
In two studies38, 51 rifaximin was given in patients taking fibres. The first one was a randomised crossover trial where patients with SIBO and symptomatic uncomplicated diverticular disease taking insoluble fibre (i.e. bran) were randomised to receive rifaximin or placebo.38 The eradication rates found according to PP analysis were 83.3% (95% CI: 55.1–95.3) for rifaximin and 10% (95% CI: 1.8–40.4) for placebo with a difference in eradication significantly in favour of rifaximin (difference in eradication: 73.3%; 95% CI: 32.8–90.9). During the second phase of the study, patients not eradicated with placebo were treated with rifaximin reporting an eradication rate of 77.7% (95% CI: 39.9–97.1). The overall eradication rate (including the first and the second period) was 80.9% (95% CI: 59.9–92.3). AEs were not reported in details. However, no patient had to discontinue the study due to AEs of rifaximin.
The second study51 was a RCT where patients with SIBO were randomised to receive either rifaximin alone or in combination with partially hydrolysed guar gum. The eradication rate found in the latter group was 85% (95% CI: 70.1–94.2) according to ITT analysis and 87.1% (95% CI: 72.5–95.7) according to PP analysis, and it was significantly higher than that obtained in patients treated with rifaximin alone (62.1%; 95% CI: 44.7–77.5 according to both ITT and PP analysis; difference for eradication rate according to ITT analysis: 22.8%; 95% CI: 3.18–41.5; difference for eradication rate according to PP analysis: 25%; 95% CI: 5.6–43.4).51 AEs were not reported in details. However, no patient had to discontinue the study due to AEs of rifaximin.
Rifaximin plus mesalazine
In two studies rifaximin was given in patients taking mesalazine. The first study was a quite small RCT 30 where patients with Crohn's disease and SIBO were randomised to receive either rifaximin or placebo. After the end of treatment, SIBO was eradicated in all patients receiving rifaximin (100%; 95% CI: 59.0–100), and in only 28.5% (95% CI: 3.6–70.9) of those randomised to placebo (difference in eradication: 71.4%; 95% CI: 23.2–92.1). No data on AEs were reported.
The second study was a performed in patients with acute uncomplicated diverticular disease of the colon35 where rifaximin was able to eradicate SIBO in all patients treated (100%; 95% CI: 93.3–100).
Rifaximin plus probiotics
In one study37 SIBO positive patients were treated with rifaximin followed by a cycle of probiotics (Lactobacilli and Bifidobacteria based preparation) for twenty‐day. Follow‐up was performed 4–5 months after the end of treatment and revealed an eradication rate of 82.6% (95% CI: 61.2–95). Treatment did not cause any significant AEs.
Symptom relief
The evaluation of studies assessing symptoms before and after treatment with rifaximin (Table S1F) showed that different symptoms were measured in different ways. A thorough analysis of these studies pointed out that symptoms improved after therapy in a large proportion (≥75%) of trials, an effect seen more frequently in studies including IBS patients (Table S1F and Figure S1M). Furthermore, it was possible to extract and pool data concerning the improvement or resolution of symptoms (according to the definitions provided by the investigators) before and after eradication in only 10 trials.29, 30, 32, 33, 38, 41, 45, 46, 51, 57 The overall improvement or resolution of symptoms in eradicated patients was 67.7% (95% CI = 44.7–86.9; Figure S1N), with evidence of heterogeneity (Cochrane Q: P < 0.0001; I 2 = 91.3%; 95% CI: 86.9–93.7), but without Funnel plot asymmetry (Egger test: 7.97959; 95% CI: −1.290–17.249, P = 0.0833; Figure S1O).
Adverse events
Adverse events were reported in 17 studies involving 815 patients where only rifaximin was used.31, 32, 34, 39, 40, 41, 42, 43, 44, 46, 48, 50, 53, 55, 56, 57, 58 As shown in Figure 5, the overall rate of AEs was 4.6% (95% CI = 2.3–7.5), with evidence of heterogeneity (Cochrane Q: P = 0.0002; I 2 = 63.6%; 95% CI: 31.2–77.1), but without Funnel plot asymmetry (Egger test: 0.8794; 95% CI: −0.543–2.301, P = 0.2074; Figure S1P). Meta‐regression and sub‐group analysis revealed that non‐RCTs presented a significant lower incidence of AEs, when compared to RCTs (Table S1G and Table S1H).
Figure 5.
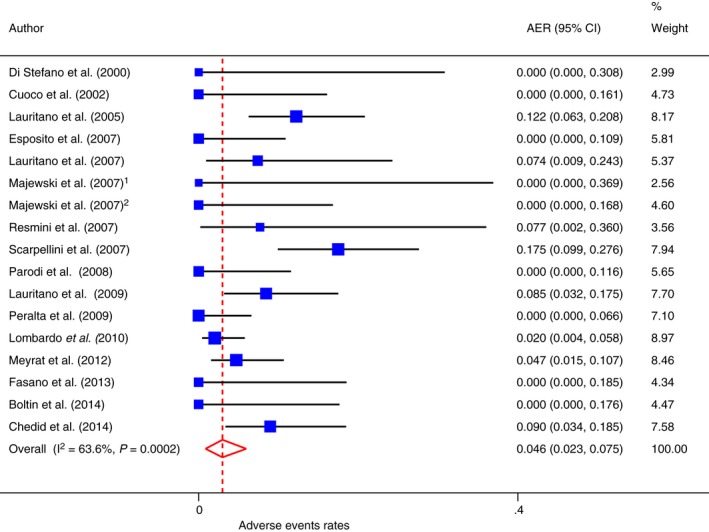
Forest plot of adverse events in patients taking rifaximin alone.
Only in one study55 the 0.47% (95% CI = 0.01–10.6) of patients who experience AEs had to discontinue the therapy prematurely for this reason.
A case of C. difficile infection (CDI) – post treatment – was reported to occur in one patient of a study were rifaximin was used at the dosage of 1200 mg daily for 4 weeks. However, no information about either the time elapsed between the end of antibiotic therapy and the occurrence of the CDI or the presence of concurrent risk factors for the infection was provided.58 The same paper reported also a case of anaphylaxis to rifaximin, again without providing any information on the severity of this AE.
Discussion
Small intestinal bacterial overgrowth is a very heterogeneous syndrome characterised by an increased number and/or abnormal type of bacteria in the small bowel,3 and is becoming a common finding in clinical practice. The management of SIBO should be centred on identifying and correcting underlying causes, treating the overgrowth, and addressing the nutrition deficiencies, where detected.3, 61
Several broad‐spectrum systemic antibiotics such as fluoroquinolones, metronidazole, tetracycline, amoxicillin‐clavulanic acid, chloramphenicol, etc., have been used to manage SIBO.7 However, they are usually associated with several and sometimes severe AEs.61, 62
Rifaximin is a poorly absorbed antibiotic that has been largely used to treat SIBO over the past decades.10, 11 Both experimental and clinical pharmacology clearly show that this compound displays a broad spectrum of antibacterial activity, covering Gram‐positive and Gram‐negative organisms, both aerobic and anaerobic.10, 11 Being virtually non‐absorbed, its bioavailability within the gastrointestinal tract is rather higher with intraluminal and faecal drug concentrations largely exceeding the minimal inhibitory concentration values observed in vitro against a wide range of pathogenic organism. Furthermore, it has been found that rifaximin is able to preserve colonic flora and increase the relative abundance of Lactobacilli and Bifidobacteria, showing ‘eubiotic’ effects.63, 64
The results of our meta‐analysis provide evidence that rifaximin is clinically effective in eradicating SIBO. A significant heterogeneity was found and multivariate meta‐regression identified three covariates (namely the drug dose, the study design and co‐therapy) independently associated with an increased eradication rate. Two studies reported a dose‐dependent eradication rate: the higher the daily dose of rifaximin, the higher the eradication rate.34, 44 In addition, the treatment success was significantly higher in non‐randomised trials.65 Despite RCTs are usually preferred to evaluate the efficacy of therapeutic interventions, a large amount of evidence is often accumulated through non‐randomised studies. For this reason, we decided to include them in our analysis. It is worthwhile mentioning that RCTs and non‐randomised studies show a high correlation in their estimates of efficacy. However, it is more frequent to find larger treatment effects in non‐randomised studies compared to than the opposite.66, 67, 68 This was indeed the case in our study. Finally, concomitant administration of rifaximin with fibres (both soluble and insoluble), probiotics (Lactobacilli and Bifidobacteria), or mesalazine, three gut microbiota‐directed therapies,69, 70, 71, 72, 73, 74, 75 consistently gave higher eradication rate. The global effectiveness of rifaximin in eradicating SIBO was maintained in the sub‐group of patients with IBS, where a significant heterogeneity was still present. It is worth mentioning that the IBS studies were all non‐RCTs.
The analysis of the studies including symptom evaluation points to an association between symptom improvement and rifaximin treatment. It was possible to evaluate the effect of eradication on symptoms only in 10 studies. Symptoms improved or disappeared in more than two‐thirds of patients (67.7%). However, the sample size was relatively small (205 patients overall) and there was also an incomplete ‘outcome bias’ since, in most studies, data regarding symptoms in non‐eradicated patients were not available. Therefore, the above findings should be interpreted with caution. Nevertheless, two recent studies76, 77 have shown that a positive H2BT does predict symptomatic response to antibiotic therapy in patients with IBS. A thoughtful Editorial78 actually suggested that breath testing for SIBO could represent a mean to enrich rifaximin responders amongst IBS patients. By using SIBO as a biomarker of IBS, the therapeutic gain of rifaximin over placebo, reported by the TARGET trials,79 may well be extended to reach a clinically significant figure.
All the studies included in our meta‐analysis employed to diagnose SIBO (as well as to evaluate eradication) GHBT or LHBT, which – although widely used – are less sensitive and specific than bacterial culture, till now considered as the gold standard.6 Each substrate has its own advantages and disadvantages, with GHBT favouring specificity over sensitivity, while the reverse is true for LHBT.80 However, whatever breath test is used, the effectiveness of rifaximin in eradicating SIBO remains the same, as evidenced by meta‐regression analysis.
Several antimicrobials have been found effective in reducing gas production, albeit with various success rates for (review see 7). However, only few head to head comparisons were performed. Conversely from our study, a recent meta‐analysis on antibiotic efficacy in treating SIBO narrowed the inclusion criteria to RCTs, showing that antibiotics were more effective than placebo (OR: 2.55; 95% CI: 1.29–5.04).81 In their subsequent analysis on efficacy of rifaximin vs. placebo, the Authors selected three RCTs, two of which were not included in our own meta‐analysis. The first trial82 was performed in children whilst our study was devoted to adults only. The second study83 had some methodological drawbacks. Since LHBT was performed after randomisation, patients did receive treatment independently from the presence of SIBO. Additionally, two criteria for establishing SIBO diagnosis were used, which produced significantly different results (55% positivity with the first criteria vs. 8% positivity with the second criterion). Finally, several different outcomes were adopted to evaluate rifaximin efficacy, which makes difficult to compare the results obtained with other studies.
Besides efficacy, our systematic review carefully looked at rifaximin safety and tolerability. Evidence for harms of medical interventions is important when weighting the benefits and risks of treatments in clinical decision‐making. However, such evidence is often suboptimal.84, 85 We found that 4.6% of patients treated with rifaximin reported AEs, but only the 0.47% of them had to discontinue the therapy. Meta‐regression revealed that, among the covariates analysed, only non‐RCTs were significantly associated with a lower rate of AEs when compared to RCTs. Although non‐RCTs are considered conservative in estimating risks of harms (as it happened in our study), evaluation of a broad range (i.e. randomised as well non‐randomised) of studies can help to build a complete picture of any potential harm and improve the generalisability of the analysis, without loss of validity.86
When considering the results of this meta‐analysis, several important limitations should be acknowledged. As with any systematic review and meta‐analysis, the results rely on the quality and reporting of the trials. There were no studies using culture to diagnose and follow‐up the eradication. We found a significant heterogeneity among trials and for this reason meta‐regression analysis was performed. However, the results of this analysis are to be interpreted with caution as meta‐regression has its own limitations. Covariates used were merely related to the study design and not to the clinical condition. Furthermore, since meta‐regression describes observational associations across trials, it can suffer from confounding. In addition, as the number of studies and sample size do influence the results of meta‐regression, the lack of an association does not necessarily mean its ‘true’ absence88 The associations found in a meta‐regression should therefore be considered more hypothesis‐generating and not regarded as proof of causality.87, 88 Only 25% of studies included in the meta‐analysis were RCTs.30, 34, 38, 44, 47, 48, 51, 89 No RCT resulted to be at low risk of bias, and all had problems with concealment of allocation and blinding.90 Furthermore, for the sake of homogeneity, it was possible to pool the results of only two RCTs.31, 48 Most of the studies included were therefore non‐RCTs, which are susceptible to selection bias and, as mentioned before, tend to find larger effects.68, 91, 92, 93 Moreover, data concerning the improvement or resolution of symptoms in eradicated patients were limited. Finally, funnel plots asymmetry suggested not only publication bias but the presence of other types of biases, depending on other sources (e.g. heterogeneity, poor methodological quality, etc.).94 All the above limitations clearly affect the quality and the strength of the provided evidence and, therefore, the results of this meta‐analysis should be considered with caution.95
In conclusion, rifaximin therapy is effective and safe for the treatment of SIBO. Since the quality of the available studies is generally poor, well‐designed, large RCTs (with well‐established criteria to assess SIBO and to evaluate symptoms before and after therapy according to the eradication status) are needed to substantiate these findings and to establish the optimal regimen (i.e. daily dose and duration) of rifaximin to treat this increasingly common condition.
Authorship
Guarantors of the article: Dr Luigi Gatta and Professor Carmelo Scarpignato.
Author contributions: Luigi Gatta and Carmelo Scarpignato designed the study, did the literature search, analysed and interpreted the data, wrote and critically reviewed the paper.
All authors approved the final version of the manuscript.
Supporting information
Figure S1. Figures A to P.
Table S1. Tables A to H.
Acknowledgements
We are indebted to Jonathan Belsey, MB BS (Sudbury, UK) for the useful suggestions dealing with the statistical approach to data analysis. We are also grateful to the following investigators for providing us with additional data concerning their studies: Prof. R.W. McCallum (El Paso, USA), Dr L. Lombardo (Torino, Italy), Prof. M. Pimentel (Los Angeles, USA), Dr R. D'Incà (Padova, Italy), Prof. A. Gasbarrini (Rome, Italy), Dr A. De Stefano (Pavia, Italy), Dr E. Cerda (Mexico).
Declaration of personal interests: Dr Luigi Gatta has no conflicts of interest to disclose while Professor Carmelo Scarpignato is member of the Speakers' Bureau and of the Scientific Advisory Board of Alfa Wassermann, the manufacturer and marketer of rifaximin.
Declaration of funding interests: None.
As part of AP&T's peer‐review process, a technical check of this meta‐analysis was performed by Dr Y Yuan. The Handling Editor for this article was Professor Roy Pounder, and it was accepted for publication after full peer‐review.
References
- 1. Scarpignato C, Gatta L. Commentary: towards an effective and safe treatment of small intestine bacterial overgrowth. Aliment Pharmacol Ther 2013; 38: 1409–10. [DOI] [PubMed] [Google Scholar]
- 2. Quigley EM. Small intestinal bacterial overgrowth: what it is and what it is not. Curr Opin Gastroenterol 2014; 30: 141–6. [DOI] [PubMed] [Google Scholar]
- 3. Quigley EM, Abu‐Shanab A. Small intestinal bacterial overgrowth. Infect Dis Clin North Am 2010; 24: 943–59. [DOI] [PubMed] [Google Scholar]
- 4. Khoshini R, Dai SC, Lezcano S, Pimentel M. A systematic review of diagnostic tests for small intestinal bacterial overgrowth. Dig Dis Sci 2008; 53: 1443–54. [DOI] [PubMed] [Google Scholar]
- 5. Rana SV, Sharma S, Kaur J, Sinha SK, Singh K. Comparison of lactulose and glucose breath test for diagnosis of small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Digestion 2012; 85: 243–7. [DOI] [PubMed] [Google Scholar]
- 6. Abu‐Shanab A, Quigley EMM. Diagnosis of small intestinal bacterial overgrowth: the challenges persist!. Expert Rev Gastroenterol Hepatol 2009; 3: 77–87. [DOI] [PubMed] [Google Scholar]
- 7. Corazza GR, Di Stefano M, Scarpignato C. Treatment of functional bowel disorders: is there room for antibiotics? Digestion 2006; 73(Suppl. 1): 38–46. [DOI] [PubMed] [Google Scholar]
- 8. Sullivan A, Edlund C, Nord CE. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis 2001; 1: 101–14. [DOI] [PubMed] [Google Scholar]
- 9. Marchi E, Montecchi L, Venturini AP, Mascellani G, Brufani M, Cellai L. 4‐Deoxypyrido[1',2':1,2]imidazo[5,4‐c]rifamycin SV derivatives. A new series of semisynthetic rifamycins with high antibacterial activity and low gastroenteric absorption. J Med Chem 1985; 28: 960–3. [DOI] [PubMed] [Google Scholar]
- 10. Scarpignato C, Pelosini I. Rifaximin, a poorly absorbed antibiotic: pharmacology and clinical potential. Chemotherapy 2005; 51(Suppl. 1): 36–66. [DOI] [PubMed] [Google Scholar]
- 11. Calanni F, Renzulli C, Barbanti M, Viscomi GC. Rifaximin: beyond the traditional antibiotic activity. J Antibiot 2014; 67: 667–70. [DOI] [PubMed] [Google Scholar]
- 12. Jiang ZD, Dupont HL. Rifaximin: in vitro and in vivo antibacterial activity‐a review. Chemotherapy 2005; 51(Suppl. 1): 67–72. [DOI] [PubMed] [Google Scholar]
- 13. Adachi JA, Dupont HL. Rifaximin: a novel nonabsorbed rifamycin for gastrointestinal disorders. Clin Infect Dis 2006; 42: 541–7. [DOI] [PubMed] [Google Scholar]
- 14. DuPont HL, Ericsson CD. Prevention and treatment of traveler's diarrhea. N Engl J Med 1993; 328: 1821–7. [DOI] [PubMed] [Google Scholar]
- 15. Liberati A, Altman DG, Tetzlaff J, et al The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151: W65–94. [DOI] [PubMed] [Google Scholar]
- 16. Stroup DF, Berlin JA, Morton SC, et al Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–12. [DOI] [PubMed] [Google Scholar]
- 17. Dekkers OM, Egger M, Altman DG, Vandenbroucke JP. Distinguishing case series from cohort studies. Ann Intern Med 2012; 156: 37–40. [DOI] [PubMed] [Google Scholar]
- 18. Hoffman JT, Altman DG, Sterne JAC. Assessing risk of bias in included studies In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011): The Cochrane Collaboration, 2011. Available at: http://handbook.cochrane.org (accessed 10 December 2016) [Google Scholar]
- 19. Wells GA, Shea B, O'Connell D, et al The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available at: http://www.ohri.ca/programs/ clinical_epidemiology/oxford.htm (accessed May 2015).
- 20. Guo B, Moga C, Harstall C, Schopflocher D. A principal component analysis is conducted for a case series quality appraisal checklist. J Clin Epidemiol 2016; 69: 199–207. e2. [DOI] [PubMed] [Google Scholar]
- 21. DerSimonian R. Meta‐analysis in the design and monitoring of clinical trials. Stat Med 1996; 15: 1237–48. [DOI] [PubMed] [Google Scholar]
- 22. Borenstein M. Introduction to Meta‐Analysis. Chichester, UK: John Wiley & Sons, 2009. [Google Scholar]
- 23. Sedgwick P. What is per protocol analysis? BMJ 2013; 346: f3748–f48. [Google Scholar]
- 24. Schünemann J, Oxman AD, Vist GE, et al Interpreting results and drawing conclusions In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011): The Cochrane Collaboration. Available at: http://handbook.cochrane.org (accessed 10 December 2016). [Google Scholar]
- 25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; 327: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harbord RM, Higgins JP. Meta‐regression in Stata. Stata J 2008; 8: 493–519. [Google Scholar]
- 27. Kleinbaum DG, Klein M, Pryor ER. Logistic Regression: A Self‐Learning Text. 3rd ed New York: Springer, 2010. [Google Scholar]
- 28. Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Corazza GR, Ventrucci M, Strocchi A, et al Treatment of small intestine bacterial overgrowth with rifaximin, a non‐absorbable rifamycin. J Int Med Res 1988; 16: 312–6. [DOI] [PubMed] [Google Scholar]
- 30. Biancone L, Vernia P, Agostini D, Ferrieri A, Pallone F. Effect of rifaximin on intestinal bacterial overgrowth in Crohn's disease as assessed by the H2‐Glucose Breath Test. Curr Med Res Opin 2000; 16: 14–20. [DOI] [PubMed] [Google Scholar]
- 31. Di Stefano M, Malservisi S, Veneto G, Ferrieri A, Corazza GR. Rifaximin versus chlortetracycline in the short‐term treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2000; 14: 551–6. [DOI] [PubMed] [Google Scholar]
- 32. Cuoco L, Montalto M, Jorizzo RA, et al Eradication of small intestinal bacterial overgrowth and oro‐cecal transit in diabetics. Hepatogastroenterology 2002; 49: 1582–6. [PubMed] [Google Scholar]
- 33. Tursi A, Brandimarte G, Giorgetti G. High prevalence of small intestinal bacterial overgrowth in celiac patients with persistence of gastrointestinal symptoms after gluten withdrawal. Am J Gastroenterol 2003; 98: 839–43. [DOI] [PubMed] [Google Scholar]
- 34. Lauritano EC, Gabrielli M, Lupascu A, et al Rifaximin dose‐finding study for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2005; 22: 31–5. [DOI] [PubMed] [Google Scholar]
- 35. Tursi A, Brandimarte G, Giorgetti GM, Elisei W. Assessment of small intestinal bacterial overgrowth in uncomplicated acute diverticulitis of the colon. World J Gastroenterol 2005; 11: 2773–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cazzato A, Scarpellini E, Gabrielli M, et al Small intestinal bacterial overgrowth (SIBO) in patients with non‐erosive reflux esophagitis (NERD). Gastroenterology 2006; 130(Suppl. 2): W1083. [Google Scholar]
- 37. Cuoco L, Salvagnini M. Small intestine bacterial overgrowth in irritable bowel syndrome: a retrospective study with rifaximin. Minerva Gastroenterol Dietol 2006; 52: 89–95. [PubMed] [Google Scholar]
- 38. D'Inca R, Pomerri F, Vettorato MG, et al Interaction between rifaximin and dietary fibre in patients with diverticular disease. Aliment Pharmacol Ther 2007; 25: 771–9. [DOI] [PubMed] [Google Scholar]
- 39. Esposito I, de Leone A, Di Gregorio G, et al Breath test for differential diagnosis between small intestinal bacterial overgrowth and irritable bowel disease: an observation on non‐absorbable antibiotics. World J Gastroenterol 2007; 13: 6016–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lauritano EC, Bilotta AL, Gabrielli M, et al Association between hypothyroidism and small intestinal bacterial overgrowth. J Clin Endocrinol Metab 2007; 92: 4180–4. [DOI] [PubMed] [Google Scholar]
- 41. Majewski M, McCallum RW. Results of small intestinal bacterial overgrowth testing in irritable bowel syndrome patients: clinical profiles and effects of antibiotic trial. Adv Med Sci 2007; 52: 139–42. [PubMed] [Google Scholar]
- 42. Majewski M, Reddymasu SC, Sostarich S, Foran P, McCallum RW. Efficacy of rifaximin, a nonabsorbed oral antibiotic, in the treatment of small intestinal bacterial overgrowth. Am J Med Sci 2007; 333: 266–70. [DOI] [PubMed] [Google Scholar]
- 43. Resmini E, Parodi A, Savarino V, et al Evidence of prolonged orocecal transit time and small intestinal bacterial overgrowth in acromegalic patients. J Clin Endocrinol Metab 2007; 92: 2119–24. [DOI] [PubMed] [Google Scholar]
- 44. Scarpellini E, Gabrielli M, Lauritano CE, et al High dosage rifaximin for the treatment of small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2007; 25: 781–6. [DOI] [PubMed] [Google Scholar]
- 45. Yang J, Lee HR, Low K, Chatterjee S, Pimentel M. Rifaximin versus other antibiotics in the primary treatment and retreatment of bacterial overgrowth in IBS. Dig Dis Sci 2008; 53: 169–74. [DOI] [PubMed] [Google Scholar]
- 46. Parodi A, Sessarego M, Greco A, et al Small intestinal bacterial overgrowth in patients suffering from scleroderma: clinical effectiveness of its eradication. Am J Gastroenterol 2008; 103: 1257–62. [DOI] [PubMed] [Google Scholar]
- 47. Parodi A, Paolino S, Greco A, et al Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol 2008; 6: 759–64. [DOI] [PubMed] [Google Scholar]
- 48. Lauritano EC, Gabrielli M, Scarpellini E, et al Antibiotic therapy in small intestinal bacterial overgrowth: rifaximin versus metronidazole. Eur Rev Med Pharmacol Sci 2009; 13: 111–6. [PubMed] [Google Scholar]
- 49. Parodi A, Dulbecco P, Savarino E, et al Positive glucose breath testing is more prevalent in patients with IBS‐like symptoms compared with controls of similar age and gender distribution. J Clin Gastroenterol 2009; 43: 962–6. [DOI] [PubMed] [Google Scholar]
- 50. Peralta S, Cottone C, Doveri T, Almasio PL, Craxi A. Small intestine bacterial overgrowth and irritable bowel syndrome‐related symptoms: experience with Rifaximin. World J Gastroenterol 2009; 15: 2628–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Furnari M, Parodi A, Gemignani L, et al Clinical trial: the combination of rifaximin with partially hydrolysed guar gum is more effective than rifaximin alone in eradicating small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2010; 32: 1000–6. [DOI] [PubMed] [Google Scholar]
- 52. Lauritano EC, Valenza V, Sparano L, et al Small intestinal bacterial overgrowth and intestinal permeability. Scand J Gastroenterol 2010; 45: 1131–2. [DOI] [PubMed] [Google Scholar]
- 53. Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol 2010; 8: 504–8. [DOI] [PubMed] [Google Scholar]
- 54. Cerda E, Minero Alfano JJ, Gerrera Gonzalez J, et al Effect of rifaximin in small intestine bacterial overgrowth in patients with irritable bowel syndrome. Gut 2012; 61: A422. [Google Scholar]
- 55. Meyrat P, Safroneeva E, Schoepfer AM. Rifaximin treatment for the irritable bowel syndrome with a positive lactulose hydrogen breath test improves symptoms for at least 3 months. Aliment Pharmacol Ther 2012; 36: 1084–93. [DOI] [PubMed] [Google Scholar]
- 56. Fasano A, Bove F, Gabrielli M, et al The role of small intestinal bacterial overgrowth in Parkinson's disease. Mov Disord 2013; 28: 1241–9. [DOI] [PubMed] [Google Scholar]
- 57. Boltin D, Perets TT, Shporn E, et al Rifaximin for small intestinal bacterial overgrowth in patients without irritable bowel syndrome. Ann Clin Microbiol Antimicrob 2014; 13: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chedid V, Dhalla S, Clarke JO, et al Herbal therapy is equivalent to rifaximin for the treatment of small intestinal bacterial overgrowth. Glob Adv Health Med 2014; 3: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Moraru IG, Moraru AG, Andrei M, et al Small intestinal bacterial overgrowth is associated to symptoms in irritable bowel syndrome. Evidence from a multicentre study in Romania. Rom J Intern Med 2014; 52: 143–50. [PubMed] [Google Scholar]
- 60. Gravina A, Federico A, Ruocco E, et al Helicobacter pylori infection but not small intestinal bacterial overgrowth may play a pathogenic role in rosacea. UEG J 2015; 3: 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bohm M, Siwiec RM, Wo JM. Diagnosis and management of small intestinal bacterial overgrowth. Nutr Clin Pract 2013; 28: 289–99. [DOI] [PubMed] [Google Scholar]
- 62. Baker DE. Rifaximin: a nonabsorbed oral antibiotic. Rev Gastroenterol Disord 2005; 5: 19–30. [PubMed] [Google Scholar]
- 63. Maccaferri S, Vitali B, Klinder A, et al Rifaximin modulates the colonic microbiota of patients with Crohn's disease: an in vitro approach using a continuous culture colonic model system. J Antimicrob Chemother 2010; 65: 2556–65. [DOI] [PubMed] [Google Scholar]
- 64. Ponziani FR, Scaldaferri F, Petito V, et al Rifaximin treatment increases lactobacillus abundance in patients with different gastrointestinal and liver diseases. UEG Journal 2015; 3(5S): A138. [Google Scholar]
- 65. Pocock SJ. Clinical Trials: A Practical Approach. Chichester, West Sussex; New York: Wiley, 1983. [Google Scholar]
- 66. Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 2000; 342: 1887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med 2000; 342: 1878–86. [DOI] [PubMed] [Google Scholar]
- 68. Ioannidis JP, Haidich AB, Pappa M, et al Comparison of evidence of treatment effects in randomized and nonrandomized studies. JAMA 2001; 286: 821–30. [DOI] [PubMed] [Google Scholar]
- 69. Andrews CN, Griffiths TA, Kaufman J, Vergnolle N, Surette MG, Rioux KP. Mesalazine (5‐aminosalicylic acid) alters faecal bacterial profiles, but not mucosal proteolytic activity in diarrhoea‐predominant irritable bowel syndrome. Aliment Pharmacol Ther 2011; 34: 374–83. [DOI] [PubMed] [Google Scholar]
- 70. Kaufman J, Griffiths TA, Surette MG, Ness S, Rioux KP. Effects of mesalamine (5‐aminosalicylic acid) on bacterial gene expression. Inflamm Bowel Dis 2009; 15: 985–96. [DOI] [PubMed] [Google Scholar]
- 71. Xue L, Huang Z, Zhou X, Chen W. The possible effects of mesalazine on the intestinal microbiota. Aliment Pharmacol Ther 2012; 36: 813–4. [DOI] [PubMed] [Google Scholar]
- 72. Marchesi JR, Adams DH, Fava F, et al The gut microbiota and host health: a new clinical frontier. Gut 2016; 65: 330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Angelakis E, Merhej V, Raoult D. Related actions of probiotics and antibiotics on gut microbiota and weight modification. Lancet Infect Dis 2013; 13: 889–99. [DOI] [PubMed] [Google Scholar]
- 74. Hamaker BR, Tuncil YE. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J Mol Biol 2014; 426: 3838–50. [DOI] [PubMed] [Google Scholar]
- 75. Wallace TC, Guarner F, Madsen K, et al Human gut microbiota and its relationship to health and disease. Nutr Rev 2011; 69: 392–403. [DOI] [PubMed] [Google Scholar]
- 76. Ghoshal UC, Srivastava D, Ghoshal U, Misra A. Breath tests in the diagnosis of small intestinal bacterial overgrowth in patients with irritable bowel syndrome in comparison with quantitative upper gut aspirate culture. Eur J Gastroenterol Hepatol 2014; 26: 753–60. [DOI] [PubMed] [Google Scholar]
- 77. Kasir R, Zakko S, Zakko P, et al Predicting a response to antibiotics in patients with the irritable bowel syndrome. Dig Dis Sci 2016; 61: 846–51. [DOI] [PubMed] [Google Scholar]
- 78. Gupta A, Chey WD. Breath testing for small intestinal bacterial overgrowth: a means to enrich rifaximin responders in IBS patients? Am J Gastroenterol 2016; 111: 305–6. [DOI] [PubMed] [Google Scholar]
- 79. Pimentel M, Lembo A, Chey WD, et al Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 2011; 364: 22–32. [DOI] [PubMed] [Google Scholar]
- 80. Saad RJ, Chey WD. Breath testing for small intestinal bacterial overgrowth: maximizing test accuracy. Clin Gastroenterol Hepatol 2013; 12: 1964–72. [DOI] [PubMed] [Google Scholar]
- 81. Shah SC, Day LW, Somsouk M, Sewell JL. Meta‐analysis: antibiotic therapy for small intestinal bacterial overgrowth. Aliment Pharmacol Ther 2013; 38: 925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Collins BS, Lin HC. Double‐blind, placebo‐controlled antibiotic treatment study of small intestinal bacterial overgrowth in children with chronic abdominal pain. J Pediatr Gastroenterol Nutr 2011; 52: 382–6. [DOI] [PubMed] [Google Scholar]
- 83. Chang MS, Minaya MT, Cheng J, Connor BA, Lewis SK, Green PH. Double‐blind randomized controlled trial of rifaximin for persistent symptoms in patients with celiac disease. Dig Dis Sci 2011; 56: 2939–46. [DOI] [PubMed] [Google Scholar]
- 84. Ioannidis JP, Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA 2001; 285: 437–43. [DOI] [PubMed] [Google Scholar]
- 85. Ioannidis JP, Evans SJ, Gotzsche PC, et al Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med 2004; 141: 781–8. [DOI] [PubMed] [Google Scholar]
- 86. Golder S, Loke YK, Bland M. Meta‐analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview. PLoS Med 2011; 8: e1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Baker WL, White CM, Cappelleri JC, et al Understanding heterogeneity in meta‐analysis: the role of meta‐regression. Int J Clin Pract 2009; 63: 1426–34. [DOI] [PubMed] [Google Scholar]
- 88. Yusuf S, Wittes J, Probstfield J, Tyroler HA. Analysis and interpretation of treatment effects in subgroups of patients in randomized clinical trials. JAMA 1991; 266: 93–8. [PubMed] [Google Scholar]
- 89. Di Stefano M, Miceli E, Missanelli A, Mazzocchi S, Corazza GR. Absorbable vs. non‐absorbable antibiotics in the treatment of small intestine bacterial overgrowth in patients with blind‐loop syndrome. Aliment Pharmacol Ther 2005; 21: 985–92. [DOI] [PubMed] [Google Scholar]
- 90. Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001; 323: 42–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Reeves BC, Deeks JJ, Higgins JP, Welles GA. Including non‐randomized studies In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011): The Cochrane Collaboration. Available at: http://handbook.cochrane.org (accessed 10 December 2016). [Google Scholar]
- 92. Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L. Do the findings of case series studies vary significantly according to methodological characteristics? Health Technol Assess 2005; 9: iii–iv, 1–146. [DOI] [PubMed] [Google Scholar]
- 93. Stein K, Dalziel K, Garside R, Castelnuovo E, Round A. Association between methodological characteristics and outcome in health technology assessments which included case series. Int J Technol Assess Health Care 2005; 21: 277–87. [DOI] [PubMed] [Google Scholar]
- 94. Sterne JA, Sutton AJ, Ioannidis JP, et al Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]
- 95. Guyatt GH, Oxman AD, Vist GE, et al GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Figures A to P.
Table S1. Tables A to H.


