Abstract
Nanocages (NCs) have emerged as a new class of drug-carriers, with a wide range of possibilities in multi-modality medical treatments and theranostics. Nanocages can overcome such limitations as high toxicity caused by anti-cancer chemotherapy or by the nanocarrier itself, due to their unique characteristics. These properties consist of: (1) a high loading-capacity (spacious interior); (2) porous structure (analogous to openings between the bars of the cage); (3) enabling smart release (a key to unlock the cage); and (4) a low likelihood of unfavorable immune responses (the outside of the cage is safe). In this review, we cover different classes of NC structures such as virus-like particles (VLPs), protein NCs, DNA NCs, supramolecular nanosystems, hybrid metal-organic NCs, gold NCs, carbon-based NCs and silica NCs. Moreover, NC-assisted drug delivery including modification methods, drug immobilization, active targeting, and stimulus-responsive release mechanisms are discussed, highlighting advantages, disadvantages and challenges. Finally, translation of NCs into clinical applications, and an up-to-date assessment of the nanotoxicology considerations of NCs are presented.
Graphical abstract
1- Introduction
Nanotechnology not only has led to tremendous advancements in various fields of technology and science 1-3, but also its pivotal impact on biomedicine, particularly therapy and diagnosis of miscellaneous diseases is inevitable 3-5. The advent of drug delivery systems (DDSs) has opened new horizons with a wide range of capabilities and applications 6-9. DDSs are used to alter the solubility, pharmacokinetics, and biodistribution of their drug cargos, or more specifically to control the duration and rate of drug delivery and release10. “Smart drug carriers” are specifically designed to target specific cells, tissues or organs of the human body, and to release the cargo, which can be either drug, genes or diagnostic reporter molecule only when they arrive at their destination 6, 11, 12. These smart carriers frequently operate in a stimulus-responsive manner when an internal or external stimulus alters the carrier to release the cargo. DDSs have also been designed to enhance the efficiency and safety of administration of potentially highly toxic therapeutic agents, especially cytotoxic drugs used to combat cancer. Although highly active cytotoxic cancer drugs such as doxorubicin (DOX) and paclitaxel (PTX) are now available, the non-specific systemic toxicity these compounds display towards rapidly dividing and metabolically active normal cells in the gut, skin, bone marrow and heart are often dose-limiting, and it has sometimes been said the “treatment is worse than the disease”. DDS allow the side effects of these cancer drugs to be controlled, due to the ability to manipulate and control the time and rate of drug release13. Many of these DDSs take the form of nanoparticles (NPs) whose structure and function is based on their particular formulation 14, 15. The most common NPs that have been employed in DDS applications are chitosan-based NPs 16, bacteriophage-inspired NPs17, bacterial-based NPs 18, albumin-based NPs19, polymeric micelles 20, water-soluble hydrogels21, 22, lipid-based NPs 23, nanorods24, 25, nano-hydrogels11, 21, metal and metal oxide NPs26, 27, silica-based NPs28, quantum dots29, 30, carbon nanotubes (CNTs)31, graphene and graphene oxide32-34.
There are several shortcomings that may occur when NPs are used as DDSs. (1) It is possible that the drug becomes deactivated once it is attached to the NP. Attaching the drug to the NPs requires careful consideration, since it demands that the bonding between the drug and NPs should be strong enough to prevent premature release, but when the cargo has reached the specific target such as a cancer cell, the bonds should predictably degrade according to the desired release rate. On the other hand, binding between the drug and NPs, e.g. NCs, should not alter the activity of the drug molecules in the environmental conditions where they need to be active. (2) The amount of the drug that can be linked to the NPs can be relatively small; It is noteworthy that high concentration of NPs delivered in vivo may result in symptoms like high blood pressure and kidney malfunction, therefore, importantly NPs should be highly efficient as carriers of drugs. (3) NPs can undergo agglomeration, which can result in rapid elimination from the bloodstream because macrophages and other phagocytes engulf the aggregates, preventing them from reaching the target cells. (4) Uncontrolled release can occur, which is known as the “burst effect.” If the burst effect cannot be regulated, administration of NPs loses one important advantage of nanocarrier structures, namely the prevention of common side effects produced by cancer drugs. To overcome these drawbacks and limitations, it is often necessary to use relatively high concentrations of NPs, which in turn can have toxic effects unrelated to the cargo thus negating the original goal35, 36.
An important class of NPs that is under investigation for DDS is known by the term “nanocages” (NCs). NCs are a class of NP-based DDSs that have a hollow body structure that can encapsulate large amounts of drugs inside. NCs have a higher loading capacity than other NPs and can be loaded with hundreds or even thousands of cargo molecules. Therefore a lower dose of NPs is required to deliver a therapeutically effective dose of the drug. As a result, the overall cytotoxicity of the NCs is decreased. Another important feature is the ability of NCs to encapsulate and protect the drugs from the environment fully so that they can be used as carriers of highly lipophilic drugs and remain stable in hydrophilic environments such as the bloodstream. When the NCs reach the target tissue, the more lipophilic the environment is, the easier drug release is triggered 36, 37. The term “cage” implies that it can be unlocked, and therefore NCs are often designed to be stimulus-responsive, taking advantage of specific physical or chemical differences in the environment at their target to alter their molecular structure.
NCs can be divided into two basic groups. Firstly, organic NCs which consist of supramolecular nanosystems such as cyclodextrins (CDs) and protein-based NCs such as vaults and virus-like NPs. These kinds of nanostructures, due to their organic composition, can readily interact with living cells, especially protein-based NPs constructed from endogenous proteins. Protein-based nanoplatforms have another advantage of often not being readily recognized as foreign, and therefore rapidly eliminated by macrophages. Another important feature of protein cage structures is their uniformity of size, i.e. they can show a highly homogeneous size distribution. Protein-based cages can be biologically or chemically engineered or modified using different approaches (genetic or chemical), which can make them uniquely suitable for drug delivery applications 7, 37-39. One example is the “vault” or vault cytoplasmic ribonucleoprotein, which is a eukaryotic organelle found in cells of many different life forms 40. These molecules form a hollow icosahedral vault 41.
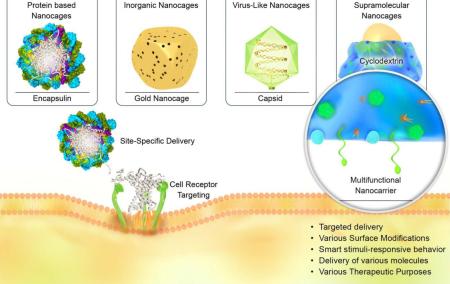
The second group is inorganic NCs, which can be made from metals such as gold, or from non-metallic oxides such as silica. This group of NCs also includes carbon-based NCs, which consist of different allotropes of carbon, such as graphene, fullerenes or CNT-derived NPs. Inorganic NCs are usually mesoporous which gives them special properties such as (1) the volume of pores combined with the large surface area (the latter due to the porous structure) provides a high capacity for drugs to be adsorbed and loaded within the pore channels42; (2) the mesoporous structure with adjustable pore size43 can offer better control over the kinetics of drug loading and release; (3) the chemically modifiable surface can be used to attach targeting ligands, gatekeepers or valves on the pores, which enable control of drug release helping to reduce non-specific cytotoxicity44, 45; (4) inorganic NCs have shown promising in vivo safety results with regard to systemic toxicity46-48, biodistribution49-51, biodegradation, and excretion52, 53; (5) the possibility of using a reporter molecule for tracing, thereby combining simultaneous therapy and bioimaging of these NPs (theranostics) using luminescent, radioactive or magnetic reporters 54-56. Figure 1 schematically illustrates various types of NCs as multifunctional nanocarriers, and their ability for targeted delivery toward cells in biological environments.
Figure 1.
Schematic illustration of various nanocaged platforms, their ability to target cells and intracellular delivery via receptor-mediated uptake.
Another point is that, as stated before, the distinction between NCs and NPs is not crystal clear in several cases and it depends on the definition of the structures. For instance, while most researchers use the term “cage” for protein-based nanostructures like ferritin 57 or inorganic structures like Au NCs 58, while others have not used the term “cage” to apply to virus-like particles in their report 57. Table.1 summarizes the advantages and disadvantages of NCs with some examples and in the following sections, the present paper reviews key studies that have been conducted on the use of NCs as DDSs in the past few years.
Table 1.
Summary of advantages and disadvantages of NCs.
| Advantages | Example |
|---|---|
| Small dimensions | Au NCs are up to 40 nm in diameter compared to Au shells and Au core-shells (>100 nm) used as contrast agents 53. |
| Prevention of premature drug degradation | Au NCs carrying Dox, conjugated with hyalorunic acid-dopamine (HA-DA) and showed no sign of degradation (no trace of DA oxidation) 54. |
| Specific targeting by means of functionalization with chemical compounds | Specific binding peptide, neuropilin 1 was attached to protein NCs to target pancreatic cancer cells 55. |
| Enable enhancing permeability and retention (EPR) effect especially for cancer therapies. | Heat-shock protein 32 applied to increase vascular permeability and enhance accumulation of macromolecular drugs in solid tumors 56. |
| Higher efficiency, lower costs and proper outcome in intracellular delivery | Viral delivery of siRNA is considered to be a matter of safety concern, and nonviral delivery systems are now as being created with equal efficiency, protein-based nanocages are being investigated as a carrier for siRNA 57. |
| Disadvantages | Example |
|---|---|
| Biosafety issues | Apoferritin NCs are considered as a good carrier for delivery of several drugs but their biosafety in various conditions, and combination with different drugs demand further investigation to determine the mechanism 58. |
| Functionalization | Functionalization of the inner walls of hollow NC structures is difficult to control 59. |
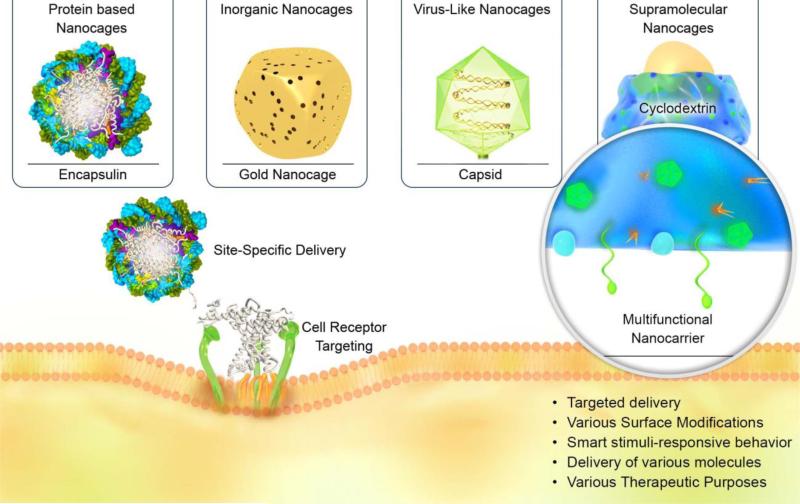
As previously noted, caged nanoplatforms have demonstrated a wide range of capabilities and applications in nanomedicine and as DDSs. Their role in the therapy of various diseases, indicating their multifunctional role, are briefly presented in Figure 2. In the following sections, various NCs that have been tested as DDSs, their therapeutic and diagnostic applications, as well as synthesis methods are discussed. Afterwards, in two sections, translation of NCs into clinical applications and their nanotoxicology are considered.
Figure 2.
Schematic illustration presenting the wide range of capabilities and applications of caged nanoplatforms in nanomedicine and DDSs for delivery of various therapeutic drugs and imaging agents.
2- Modification and targeting approaches for NC DDSs
One of the most important and crucial factors to be considered when NPs are employed in DDSs, is precise control over modification and functionalization of these nanocarriers. In this regard, two main concepts must be considered, surface modification and targeting; however, in some cases these two concepts may be used for the same purpose. Generally, modification procedures are used for different purposes aside from targeting, including protection of the nanocarrier and the loaded diagnostic/therapeutic agent from the immune system 66, enhancing blood circulation time of NPs, and for site specific targeting 67. Surface modification is mainly dependent on the component of NPs, their size and surface charge.
One of the main advantages of NCs is that they can be constructed from various materials, natural or synthetic; therefore, a range of procedures have been utilized for modification. For example, protein-based NCs have the possibility be to be functionalized at three distinct molecular locations or interfaces, which are classified as external, internal, and inter-subunit. Herein, genetic modification of protein NCs have been employed in order to change the amino-acid sequence of the surface exposed residues 68, 69. Modification can also be carried out by changing some specific amino acids into others, or by altering existing amino acids using chemical reactions 70. This molecular functionalization can be used to exert control over the surface charge, improve particle stability and drug encapsulation. On the other hand, several methods have been proposed for surface modification and functionalization of inorganic NCs, such as gold and silica NCs 71. Coating of hydrophilic polymers onto the surface of NCs, is the major approach to modify these structures, especially those with an inherently hydrophobic nature; for example, porous coordination NCs or metal-organic polyhedrons (MOPs), also called “metal organic frameworks” (MOF). Some of the common materials and techniques exploited for surface modification are smart polymers 72, polyethylene glycol (PEG) 72, 73, poly (N-isopropylacrylamide) (NIPAAm), and poly-(diallyl dimethyl ammonium) chloride (PDDA) (a water-soluble cationic polyelectrolyte) 74. These methods can be used concurrently during synthesis, or as a post-synthesis modification 75. For example, Wang et al. 72 modified the surface of hollow mesoporous silica (HMS) NCs by covalent bonding between the succinimidyl groups of the PEG and the surface amine groups of rhodamine B isothiocyanate (RITC)-labeled HMS NCs, and reported high biocompatibility using in-vitro toxicity evaluation. Another study reported the surface modification of porous coordination NCs as MOPs by the “click chemistry” approach, where alkyne groups were attached to the MOPs, followed by grafting with PEG containing azide groups by copper catalysis made the MOPs water-soluble76.
In regard to virus like particle (VLP) based NCs, various genetic/chemical modification methods have been suggested to provide advantages such as loading hydrophobic cargos, attachment of imaging/therapeutic agents, efficient gene/drug loading and delivery, stimuli-triggered drug release, targeting various cancers, etc. 68,70, 77-79. For example, bacteriophage MS2 VLPs have been reported to encapsidate a variety of cargos including nucleic acids, imaging agents, anticancer drugs, etc. via chemical conjugation or genetic insertion 80.
Ferritin based NCs can be modified or functionalized with various agents in order to: enhance cancer targeting by using targeting motifs 81; improve cargo loading efficiency 82; enable simultaneous diagnostics and therapy 83; provide a “stealth effect” to enhance the half-life of the therapeutic cargos 84; etc.
In previous reports, modification of small heat shock proteins (sHSPs) has been carried out employing genetic or chemical methods 85, and results such as enhanced cargo delivery and cell targeting have been obtained86.
Modification of inorganic NCs can be implemented to enhance their stability and increase the blood circulation time e.g. by using polyethylene glycol (PEG) groups 87; increase biocompatibility along with lowered nanotoxicity72; and provide good flexibility in modification e.g. the facile formation of thiolate-Au bonds in AuNCs 88.
In summary many functionalization methods, materials and related factors can be implemented to enhance site-specific targeting, reduce side effects, and increase the blood circulation half-life of NCs.
The main aim of targeting to manipulate the structure of NPs (including NCs), to reach the intended target site. Two major approaches that have been introduced for this purpose in recent years are passive and active targeting, which will be discussed in the next sections.
2-1 Passive targeting by caged nanoplatforms
The traditional technique for delivering NPs to tissues and cells is passive targeting. This strategy allows NPs to be exposed to target tissue/cell for a longer period of time compared to other non-nanoscale carriers 89. This targeting strategy is affected by NP characteristics such as their size, and also by physiological features of the tissue such as pathological conditions. Particle size, shape and surface characteristics are three key elements that are modified during passification of nanostructures 90, and can result in surface protection, coating, increasing loading capacity and providing caps to the pores. One of the main factors that affects both cell uptake and biodistribution of the NCs is the size (diameter) of the nanostructure. Among NP characteristics, the size of NP plays the major role in passive targeting. It has been proved that decreasing the overall particle size causes longer circulation half-life, consequently a higher accumulations in the intended tissue. However, other issues such as tissue variation and NC-protein interactions in the blood circulation (formation of a protein corona) may also influence the final results91. In this regard Wang et al.73 designed a study to investigate in vivo pharmacokinetics of different-sized AuNCs. A mouse model of mammary carcinoma (EMT-6) was employed, and PEG-AuNC particles with two different mean diameters of 30nm and 55nm were compared. The 30 nm PEG-AuNCs showed better in vivo pharmacokinetics and decreased uptake by the reticuloendothelial system (RES) with an enhanced blood circulation time. The authors proposed that this system could act as a powerful theranostic tool using positron emission tomography (PET) when the NCs were labeled with Cu.
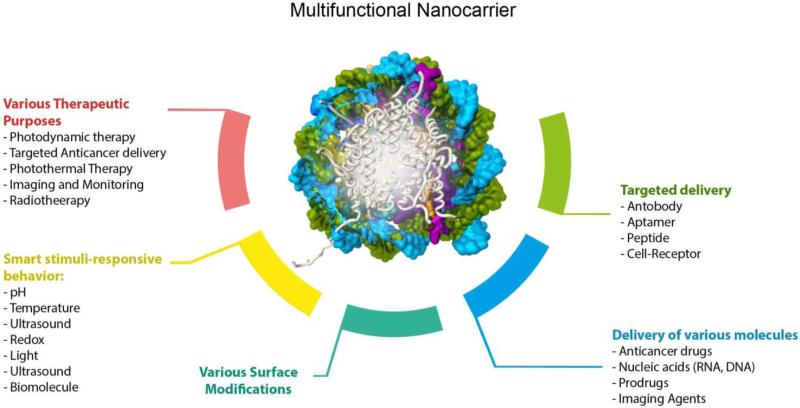
Furthermore, particle shape is another essential factor in improving the cell uptake. Rod-shaped and spherical-shaped particles larger than 100 nm show the highest uptake respectively, followed by NPs with cylindrical and cubic shapes. For particles smaller than 100 nm, spherical shaped NPs show the highest cell uptake 91. In a study, the effect of NC structures on localization of nanocarriers in and around the cells and their cellular uptake was investigated by Rampersaud et al. 92. Here, longer dwell-time of NPs at the outer membrane of cancer cells was a priority to effectively release the ion channel blocking agent. It was found that non-spherical iron oxide NCs compared to their spherical counterparts induced apoptosis of cancer cells through inhibition of glutamate receptors and blockade of sodium ion channels on the cell membrane. However the spherical NPs underwent accelerated endocytosis, resulting in less effective ion channel blocking and inefficient apoptosis. Furthermore, the efficiency of delivery and the cytotoxicity of the anticancer drug, riluzole loaded in NCs was higher than drug loaded in spherical NPs. This difference was proposed to be related to the charge-screening effect of NC surface. Riluzole inhibited the secretion of glutamate by cancer cells via blocking the sodium ion channels, leading to prevention of glutamate receptor activation, thus lowering cancer cell growth (Figure 3-a) 92. Particle surface charge also plays a significant role in NP-cell interactions and cell uptake. For instance, positively charged NPs demonstrate higher cell uptake than their negatively charged or neutral counterparts. Slight negative charge of the cell membrane governs cell uptake via electrostatic attractions. In addition, surface charged NPs accumulate more protein corona, so negatively or positively charged surfaces, can result in different composition of the formed protein corona, which affects the NPs fate 91.
Figure 3.
a) Schematic illustration of (i) riluzole loaded in iron oxide NC and (ii) blocking sodium ion channels by this NC, which prevents glutamate receptor activation and glutamate secretion, therefore reducing cancer cell growth. Reprinted with permission from ref. 92 Copyright 2016 American Chemical Society, b) Schematic illustration of cancer treatment in mice with RBC-coated Au NCs (RBC-AuNCs). Reprinted with permission from ref. 100 Copyright 2014 the American Chemical Society, c) Schematic of receptor-mediated cellular uptake of a biotin-functionalized truncated octahedral DNA NC by LOX-1 overexpressing cells (e.g. fibroblast cells) Reprinted with permission from ref. 101 Copyright 2016 the American Chemical Society.
Moreover, physiological properties of a targeted tissue is a very significant factor for passively targeted delivery of NCs. In a similar manner to most nanostructure-based DDSs, the enhanced permeability and retention (EPR) effect is the major route for accumulation of NCs in targeted sites in vivo. This phenomenon describes the leaky tortuous blood vessels, combined with poor or absent lymphatic drainage that is typical of tumors and some other pathological lesions 93. In addition, a higher degree of vascularization and a greater “pore size” between cells can be important in governing accumulation of NPs in tissues, as particularly seen in tumors. Taking the advantage of the EPR effect, more accumulation of NPs (and macromolecules) can be achieved, so-called as “passive” targeting, which has been investigated in different experimental studies of NCs. The interplay of cage-shaped NPs with EPR effect and cellular internalization in tumor tissues have been also investigated 94,92.
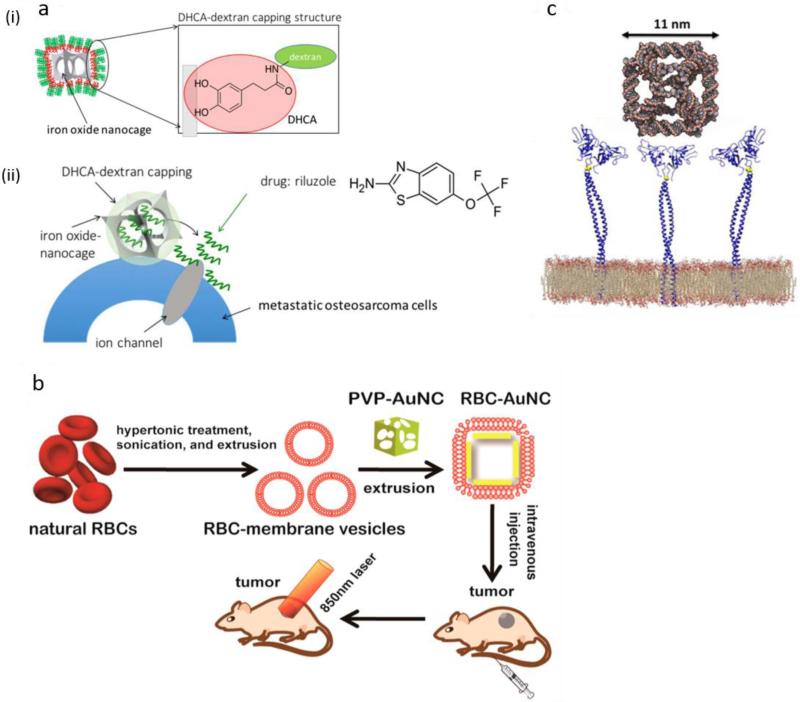
The conventional method for passive targeting and reducing the immunological responses against cage nanostructures (e.g. MOF systems) has been the attachment of hydrophilic polymers, in the case of PEG with different molecular weights, known as PEGylation 95. Despite the advantages of conventional modification, the translation of this approach into in vivo experiments has still been limited. Poor tumor uptake and rapid clearance from the bloodstream are limitations that have hindered more clinical application of these nanostructures. In order to overcome these barriers, new coatings and modification techniques have been developed for NCs, such as glycan shielding 96 , polyketals 97 or AuNC/SiO2 hybrid structures known as “nanorattles” 98. A very recent study reported that AuNCs could be coated with red blood cell (RBC) membranes that gave them a long circulation life time and enhanced in-vivo blood retention compared to a polyvinylpyrrolidone (PVP) coating 99. These NCs could mediate effective photothermal therapy (PTT) in in-vivo cancer treatment, without any serious damage to the animals 100 (Figure 3-b).
2-2 Active targeting by caged nanoplatforms
Surface modification of nanostructures with active-targeting ligands such as antibodies, peptides or receptor-ligands has advantages over the passive targeting approach especially in reducing side-effects to normal tissues and increasing cytotoxicity to targeted pathologic cells 90. Active targeting of NC structures can be divided into two major mechanisms; the first uses naturally-occurring NP structures, for example there is an arginine-glycine-aspartate (RGD) sequence naturally occurring in adenoviruses that has been used to design protein NCs for active targeting of integrin receptors on tumor cells 68, 102. The second major approach uses the attachment of targeting ligands onto the surface of NCs to achieve active targeting of desired tissues or cells. For example, different antibodies and peptides can be exploited to provide targeting for sHSP NCs e.g. for cancer delivery approaches103. In a recent study, a biotin-functionalized truncated octahedral DNA NC was designed for targeting fibroblast cells that overexpress the receptor “oxidized low-density lipoprotein receptor-1” (LOX-1), which is considered a biomarker for cardiovascular diseases and tumors. Figure 3-c schematically illustrates the cellular uptake of this NC. So the DNA NCs efficiently internalized LOX-1 expressing cells via a receptor-mediated uptake. Here, streptavidin (HRP)-biotin reaction was used to detect the DNA NCs that are internalized into the cells 101. Several different classes of ligands have been used for active targeting of NC structures that are summarized below.
2-2-1 Peptides
Surface modification of NPs with peptide ligands has been extensively investigated as a mechanism for active targeting leading to increased cell uptake compared to passive targeting. The advantages of peptide ligands include the capability for large scale production, and the ability to provide localized delivery systems with high drug loading efficiency and lower side effects .The benefits of peptide ligands can be especially exploited in oral protein delivery, because of their potential for localized targeting of the gastrointestinal (GI) tract as shown by in-vitro, ex-vivo and in-vivo experiments 104. These nanosystems mostly enter into cells via endocytosis. In addition, peptides can be used for cell specific delivery through employing nonspecific electrostatic interactions of positive charged peptides (e.g. TAT peptide), cell penetrating peptides (e.g. penetratin) and αvβ3 integrin receptors–ligand interactions (with peptides like RGD) 89. As an example, bacteriophage and phage-inspired nanocarriers are promising vehicles for synthesis of specific peptide ligands 17, 104. Exogenous peptides/proteins can be coated onto the phage surface by insertion of DNA coding sequences into the phage genome through the phage display technique. A phage display library is provided via this technique in which insertion of random oligonucleotide sequences by recombinant DNA technology results in different surface peptides being expressed on the phage NP. Thus the final surface peptide that binds with high-affinity to the desired cells can be isolated by screening techniques. Decoration of NCs with peptides through different mechanisms can also be utilized to target specific cells. One approach is the genetic incorporation of peptides for cell targeting into proteins that form on the surface of the NC. A recent study investigated NPs formed from a ferritin cage bearing an IL-4 receptor-targeting peptide, AP1, fused onto the ferritin surface, (i.e. AP1-peptide bunches on the NCs), in an in-vitro study. This nanocarrier provided super affinity and bi-specificity, and was used for an in-vivo treatment of allergic asthma symptoms 105. Another study used cowpea mosaic virus (CPMV)-NCs with bombesin peptides attached in order to target gastrin-releasing peptide receptors over-expressed in prostate cancer cells 106. Bacteriophage MS2-derived NCs with HIV-1 Tat peptide attached was used to target Huh7 human hepatoma cells 107. The second approach is attachment of targeting peptides onto the particle surface for targeted delivery of therapeutic cargo. Viruses, bacteriophages, and some specific proteins can be used for attachment of specific targeting peptides 17, 108. For instance, a study employed heat shock protein (HSP)-based NCs with attached iRGD peptides to target pancreatic cancer cells through the caspase cascade activation 61. In another study, HSP NCs were modified with SP94 peptides to target hepatocellular carcinoma cells (HCC) cancer cells86.
Other studies have been carried out in this field, e.g. using CPMV-based NCs decorated with peptide F56 where specific binding to the vascular endothelial growth factor receptor 1 was shown 109.
Other critical points that should be considered in active targeting using NC-peptide bioconjugation, include chemical and biological/cellular considerations. The most important factor in the chemistry is homogeneous attachment of peptides onto the NPs (choosing the appropriate approach from current options, e.g. electrostatic assembly, non-covalent interactions or covalent binding) especially for optimum display orientation and affinity 89. Further investigations are needed on these aspects of peptide-NCs based targeted delivery systems particularly protein corona, organelle targeting and toxicity.
2-2-2 Aptamers
DNA or RNA based aptamers are single-stranded oligonucleotides that bind and target various cognate molecules in a specific (but largely unknown) manner. Traditional limitations of typical targeting ligands including the immunogenicity of antibodies, enzymatic degradation of peptides in the blood circulation, and relatively low targeting efficiency of molecular recognition systems such as folate 110 have encouraged researchers to take advantage of aptamer recognition systems. Aptamer ligands show exceptional properties such as an easily modified structure, good target specific interactions and high binding affinity, and appropriate tissue penetration 110. Aptamers can be used as a targeting ligand in combination with NPs, and for efficient targeting of cells when attached to NC-based DDSs. For example, AuNCs coated with an aptamer through electrostatic interactions were developed as a molecular gate to control drug release 74. This bio-responsive system was able to release the loaded molecules in response to binding of targeted biomolecules to the aptamer in a controlled manner, without any external stimuli for theranostic applications. Aptamer modification was also used for targeting Jurkat leukemia T cells using bacteriophage MS2-derived NCs 111.
2-2-3 Receptor-specific ligands
The attachment of receptor-specific ligands can be a possibility to target the up-regulated receptors expressed on specific tissues especially in cancer cells and tumors 68. Several different ligands can be used in active targeting strategies of nanostructures including antibodies, folate (folic acid) 112 and transferrin 113, which undergo ligand-receptor recognition with specific cellular receptors. Numerous investigations using this strategy have been conducted for active targeting of NC-based DDSs, for example a PTX-conjugated adenovirus carrier was developed by Cui et al. for in vivo targeting of folate receptor-expressing cancer cells 114. Folic acid-PEG conjugated CPMV NCs were used for specific targeting of folate receptors in tumor cells 115. Folate receptor expression is up-regulated in many tumors such as ovarian carcinomas, non-Hodgkin's lymphomas and osteosarcomas. Folate receptor binds with folate containing NPs with high affinity and then internalizes the particle 90. VLP NCs have been reported to be functionalized to target growth factor receptors of various cancers79. MS2 bacteriophage VLPs have been reported to target cancer cells via the overexpressed biomarkers such as p120 messenger RNAs 116. Additionally, protein NCs bearing epidermal growth factor (EGR) have been used to target EGF-receptor (EGFR) expressing breast cancer cells MCF-10A and MDA-MB-231 117. The AuNC- hyaluronic acid (HA) platform (see below) was attached to the SV119 ligand for specific targeting of sigma-2 receptors 118 and can also take advantage of HA-CD44 interactions 60. Overexpression of the transferrin receptor on cancer cells can be used for selective binding of iron-containing proteins attached to NPs e.g. NCs and their internalization through endocytosis 90. High doses of DOX were loaded into H-ferritin (HFn)-based NCs for targeting and destroying tumors over-expressing the transferrin receptor (Tfr). Here, the release of DOX into the lysosomes was obtained after receptor-mediated endocytosis. In vivo studies in a murine model showed higher intra-tumoral drug accumulation, inhibition of tumor growth and excellent safety profile 119. Additional studies have employed similar mechanisms for targeting non-cancerous sites. For example, one study designed a poly(acrylic acid)-b-poly(methyl acrylate) NC stabilized itraconazole nanodrug with wheat germ agglutinin (WGA) ligand fortargeting Listeria monocytogenes, a foodborne pathogen, in order to mimic transportation across the intestinal epithelial barrier 120. Binding of WGA to its receptor on the cell surface (N-acetyl-D-glucosamine and sialic acid) encouraged clathrin- and caveolae-mediated endocytosis. Moreover, experiments showed enhanced drug uptake (in vitro), accumulation on the apical side of the epithelium cells, reachingdown to the lamina propria, and improved oral bioavailability (in vivo).
2-3 Smart targeting and release
When drugs are covalently attached to a NC, this binding may provide better protection of the pharmaceutical cargo from degradation in physiological conditions, but special methods have to be implemented in order to ensure that the covalently bound drugs are fully released once they reach the target. Stimuli-responsive moieties and smart materials have had beneficial impacts in biomedical sciences 3, 121, 122 especially in DDSs 8, 123-126, and can be used for achieving enhanced targeting and controlled drug release. Drug release can be achieved by taking advantage of specific physiological differences in the target environment, for example, reducing environments enable the cleavage of labile disulfide bonds, while in acidic environments, hydrazone bonds can be cleaved by protons 6, 68, 70, 75. Therefore choosing the appropriate linkage that can respond to specific changes in the microenvironment is crucial in targeted delivery by smart NCs.
Disassembly of the NP structure is the main mechanism leading to drug release in a smart NP-based delivery. In such mechanisms, a condition or factor (stimulus) that interferes with the normal structure of the NP is required. Various physical (thermal, magnetic, electrical, light, mechniacl and ultrasound) and chemical (pH and redox) stimuli can be used for this purpose. For example, many types of different stimuli have been employed to control opening of the pores on AuNCs such as temperature, near-infrared (NIR) laser irradiation, low pH, as well as high-intensity focused ultrasound (HIFU) 74, 88, 127, 128. Other NCs such as VLPs 129, ferritins 130-133), Encapsulins 134, and DNA NCs 135, 136 have shown responsiveness to various external or internal stimuli. For example a study demonstrated that AuNCs covered with poly(N-isopropylacrylamide-co-acrylamide) (NIPAAm-co-AAm) copolymers could function as a thermally-responsive nanosystem, which could be activated with HIFU to control and localize the release of the dye R6G (used as a model drug) 76. HIFU caused an increase in the local temperature, which destroyed the structure of the polymer coating and increased the rate of drug release. Another study also reported an effective and controlled antitumor activity with NIR responsive yolk-shell NCs loaded with amino-coumarin photo-trigger and chlorambucil anticancer drug in an animal tumor model 137.
In another study, an engineered peptide cage was integrated with a switchable GALA peptide that provided the ability to self-assemble via a coil-to-helix transition occurring at acidic pH (i.e. reversible disassembly and assembly at pH 7 and 4, respectively) 138. Moreover, DNA NCs can be modified to show stimuli-responsive release behavior in response to changes in exogenous or endogenous parameters 135, 136. VLP NCs have been also designed to exhibit stimuli-responsive behavior, for example redox-sensitive drug release 139.
Multi-responsive release systems are an interesting research field with the same mechanisms, but using combinations of more than one triggering stimuli. For instance, a phenylboronic acid-functionalized AuNC including redox- and thermal-sensitive arylboronic esters was designed for H2O2–responsive controlled release of a metal chelator. This structure (in addition to NIR absorbance of the AuNC) was also used for decreasing cellular reactive oxygen species (ROS), inhibition of amyloid-β aggregate formation, and dissolution of amyloid deposits of amyloid-β in an Alzheimer's disease model 140.
3- Drug loading and immobilization techniques
In order to prepare an appropriate DDS, cargo molecules should be integrated into NC structure to be efficaciously stabilized and carried toward the specific target site. In this regard, different methods have been developed for loading and immobilization of cargos based on different combinations of NCs and drugs. A common mechanism of drug loading is diffusion, based upon the porous nature of most NCs. In this regard, different incubation periods and various conditions (pH, temperature) may be used for diffusion of cargo into cage structure and achieving optimized drug loading. Two fundamental techniques exist for drug immobilization: chemical immobilization, which is mainly dependent on covalent NC–drug conjugation, and physical (non-covalent) interactions between metals/drugs and the internal cavity/surface of the NCs. The main approach in physical immobilization is the alteration of the NC (especially proteins) core structure, based on changing the physicochemical properties of the NPs in response to environmental stimuli. This mechanism generally happens via electrostatic interactions between the positive charges of proteins and the negative charges of cargo through assembly process 108. For example, in protein-based NCs, the reversible environmental-triggered opening and closing of the interior pores can be used to load drugs (both hydrophobic and charged polar molecules) into the NCs. To release the drug, the reverse mechanism can be employed with the help of a triggering factor present in the target tissue. Zhou et al. 141 prepared an amorphous silica NC that encapsulated the photosensitizer hypocrellin A, using hydrogen bonding. The NCs were water-soluble and highly monodisperse, and could preserve the optical and photochemical properties of hypocrellin A. These NCs were stable in the aqueous suspension of target cancer cells, localized in mitochondria and were claimed to be highly promising for dual photodynamic therapy (PDT) and bio-imaging purposes 141. In a similar study, a Dox-loaded coated AuNC incorporated with a hyaluronic (HA) coating as a capping agent with multi-stimuli responsiveness was designed for intracellular drug release, using simple drug incubation for loading, and physical interaction for delivery of the drug-carrier 60. Efficient endocytosis via HA-CD44 ligand receptor interaction was achieved, in addition to controlled drug release in different physiological and pathological conditions (negligible and accelerated drug release at pH 7.4 and pH 5.5, respectively) and NIR irradiation for photothermal activation. The second mechanism is based on covalent attachment of the cargo in the interior cavity or the surface of the NC. For example, a study investigated plant virus Cowpea mosaic virus (CPMV)-DOX covalent conjugation by EDC/NHS method 142. In this case, binding occurred between an average of eighty DOX molecules and the external surface of carboxylates CPMV. The product showed greater cytotoxicity compared to free DOX toward HeLa cells. In this approach, protein cages can also be re-engineered, for example by incorporating peptides that can respond by opening the cage, which can be therapeutic in their own right 68.
To avoid any stability or drug release/efficacy problem, utilization of the proper chemical or physical loading strategy is of paramount importance. Selecting the proper drug immobilization method depends on the type of NC, the drug formulation, modification moieties (if applied), the therapeutic purpose and the drug loading mechanism. For example in a previously reported study142, CPMV-DOX and CPMV-SS-DOX (DOX conjugated to CPMV via a disulfide bridge) were used as the control and test groups, respectively. Chemical conjugation induced significant effects on the cellular toxicity in a concentration-independent and time-delayed manner. Thus, this kind of chemical modification could be used for preserving effective drug in stabilized nanostructures. On the other hand, several investigators showed good end-capping efficiency and stability of the structure using physical interactions between therapeutic agents and NCs. Altogether, chemical stability could be proposed to be a more specific and controllable approach. In this approach each molecule contains its specific functional groups and reaction mechanism, consequently all these components are involved togther in the stability. In other words, chemical stability is drug specific 143; for each drug it may be necessary to employ different chemical reactions for attachment and degradation.
It should be noted that structural features such as a highly porous shell and a tunable cavity can be considered for optimization of drug immobilization in NCs 76. As an example, such properties typically can be seen in AuNCs that have hollow interiors and porous walls 76. In Another example, drug molecules could be anchored inside amorphous NCs, which had correct intermolecular spacing to avoid self-aggregation of the drug molecules and concentration quenching 141. Similar considerations have been addressed in other studies conducted to investigate various NCs such as Dox-loaded H-ferritin (HFn) NCs 119, platinum anticancer drugs encapsulated in apoferritin NCs 144, RhB-loaded aptamer-gated AuNCs 74, as well as NCs such as a curcumin/Gd-loaded apoferritin for theranostic applications 145. Recently, a computational simulation study was conducted where the physical interactions of a drug, cyclophosphamide, on the surface of a C60-fullerene NC were optimized 146.
There are many methods for drug loading and drug release when VLP are used as a protein cage. Loading methods are: assembly/disassembly; covalent modification (genetic or chemical); pore entry. Release strategies are: pH triggered release; reducing environment triggered release; biodegradation; and simple drug diffusion 68. Modification of VLPs can be also done to make loading of hydrophobic cargos possible 77.
It is notable that stimuli-responsive disassembly and reassembly of NCs can be used to load the cargos inside the NC cavity, as indicated in a study for pH-sensitive ferritin NCs 147. NCs that have indicated stimuli-responsive assembly/disassembly include ferritin, apoferritin and ferrihydrite (pH-responsiveness130, 131, 133), Encapsulin NCs (e.g. pH responsiveness) 134, DNA NCs (e.g. responsive to temperature 135, pH 30 and biochemical changes (ATP) 136), AuNCs conjugated with stimuli-responsive moiety showing photoacoustic responsiveness 127 or temperature128, and Cowpea chlorotic mottle virus (CCMV)-based VLPs (response to pH, temperature, ionic and strength129).
In the following sections, we review and summarize different commonly-used NC nanoplatforms that have been reported for DDSs.
4- NCs as platforms for DDSs
4-1 Viral NCs: “VLPs”
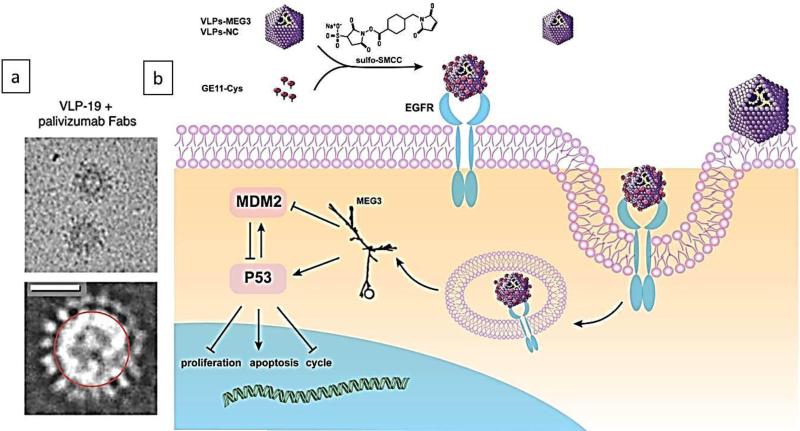
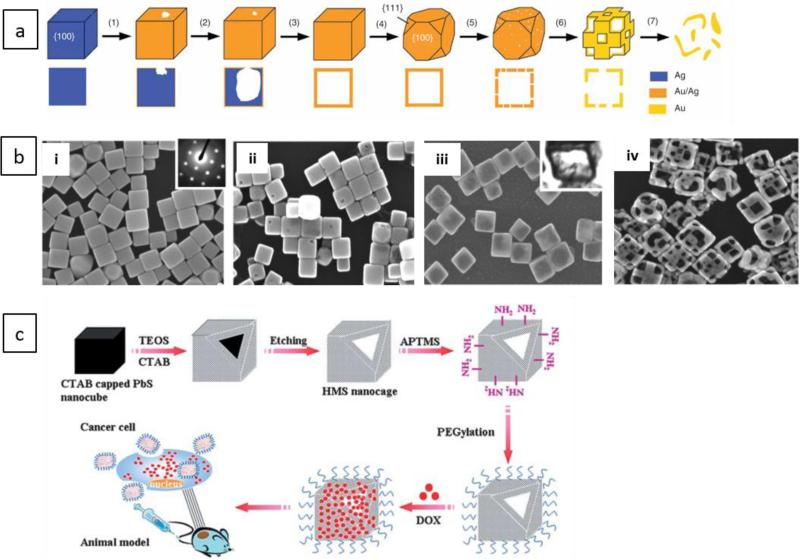
Viruses are infectious nucleic acid-based agents that are covered with external proteins, and are dependent on living cells for their replication. Viral capsid proteins can form hollow spherical structures, as caged platforms, with monolayer walls called “virus-like particles (VLPs)” that have icosahedral symmetry, and as a relatively new class of biomaterials have many applications in medicine such as platforms for therapeutic cargo delivery 76, 148-150. Virus-inspired delivery systems have been reviewed in recent literature 151, 152. In fact, VLPs are devoid of nucleic acids therefore not considered infectious153. Intact viral NPs can be transformed into VLP by pH-induced swelling and alkaline hydrolysis after the nucleic acids have been released. The protein subunits of the coat of viral NPs can be disassembled and reassembled into VLPs154, 155. VLPs such as CCMV based NCs have shown to have disassembly/reassembly behavior and morphological changes in response to pH, temperature and ionic strength alterations induced by protein-protein and protein-RNA interactions 129. Such nanosystems can be appropriate for transport of various cargos. Figure 4-a shows a cryo-electron microscopy (cryo-EM) image of VLP-19 with an approximately 30 nm diameter, to which palivizumab Fabs were bound via the spikes on the surface 156.
Figure 4.
Cryo-EM image of a VLP-19 with approximately 30 nm diameter, to which palivizumab Fabs are bound through the spikes on its surface. Scale bar: 20 nm, Reprinted from ref. 156 copyright 2015, “American Society for Clinical Investigation” (ASCI) (open access), b) Schematic illustrating a GE11 polypeptide-crosslinked MS2 VLP vector, and its uptake into cells by clathrin-mediated endocytosis for delivery of MEG3 RNA and targeting of the EGFR-positive HCC cancer cells, Reprinted from ref.79 Copyright 2016 “Impact Journals” (open access).
VLPs have several important features that make them appropriate vehicles for drug delivery, including: (1) they are very stable and formed by self-assembly having a size range of 10nm to 200nm; (2) they are amenable to simple large-scale purification; (3) all the particles are identical for each type of virus and VLP, hence they can be considered as monodisperse NPs157; and (4) they exhibit good geometry and uniformity149. VLP-based NCs can be used to facilitate delivery and transportation of nucleic acids (DNA, RNA) in diverse environments such as low or high pH, high temperatures and the presence of the host immune systems158.
Naturally occurring viral capsid is a VLP, consisting of three chemically distinct interfaces including the interior surface, the exterior surface, and the interface between subunits 158 (Figure 4-b and c). The exterior surface of viral capsids can be functionalized with peptides, fluorescent dyes, polymers, carbohydrates and oligonucleotides with the help of well-designed bio-conjugation reactions. Moreover, the amino-acid sequence of the capsid proteins can be modified by genetic engineering techniques, providing a large range of possibilities. The interior surface of some viral capsids can also act as a template for the growth of inorganic nanocrystals159. There are two methods for using VLPs as cargo-bearing carriers: (1) encapsulation of the cargo inside the capsid; and (2) binding of the cargo to the surface of the viral capsid160. Hitherto, plant, animal and bacterial viruses (bacteriophage) have all been employed to produce VLPs. Plant viruses and bacteriophages are proper choices because of their chemical and structural stability, their inherent lack of toxicity for human and animals, and their facile production 161-163. Viruses that have been employed to produce genome-free, empty capsid shells include: MS2 bacteriophage; CCMV; tobacco mosaic virus (TMV); red clover necrotic mosaic virus (RCNMV); brome mosaic virus (BMV); and cowpea mosaic virus (CPMV)164. The features such as the shape and size of the capsid, determine the balance maintained between the size of the polymeric cargo and the curvature of the coat protein165.
Furthermore, VLPs can be used to deliver hydrophobic drugs by modification of the protein constituents to produce discrete hydrophobic pockets77. Some therapeutics such as proteins and oligonucleotides may be degraded by the acidic environment of endo/lysosomes after cell uptake; thus VLPs could protect such therapeutics from harsh conditions166.
VLPs can be engineered by chemical and/or genetic modifications to carry genes, drugs, or be used as vaccines167, 168. Recently, Chang et al. 79 synthesized a GE11 polypeptide-crosslinked MS2 VLP vector for delivery of long non-coding RNAs (lncRNAs), MEG3 RNA. This nanocarrier targeted epidermal growth factor receptor (EGFR)-positive HCC cancer cells, and suppressed tumor growth in vivo (Figure 4-b). Genetic engineering techniques allow modification of the capsid proteins in order to provide a platform for viral cage synthesis with diverse shapes and sizes169, 170. Point mutations such as a cysteine point mutation can be utilized to provide new attachment sites for drug conjugation and better control of drug loading amounts171. Another study demonstrated the miRNA delivery capability of a bacteriophage MS2 VLP in which VLP encapsulated pre-miR 146a RNA and was conjugated with HIV-1 Tat47–57 cell-penetrating peptide. Results showed higher RNA expression in-vivo and in-vitro leading to suppression of the target gene172. Changes in environmental conditions, such as reduction in the level of magnesium and calcium ions, can promote opening of pores in some VLPs; for example, red clover necrotic mosaic virus can undergo non-selective drug release within the target cells173. A study in 2016 reported simultaneous imaging and therapy using anti-EGFR antibody-MS2 viral capsid nano-conjugates, which targeted EGF receptor-overexpressing breast cancer cells. The nano-conjugates were labeled with a radio-isotope (i.e. 64Cu isotopes) and their localization were monitored with tomography imaging technique, (PET/CT), and scintillation counting of the organs ex-vivo. The results also indicated highly prolonged circulation time, moderately enhanced tumor uptake, but extravasation effect by tumors limited the targeting effect of antibodies, leading to negligible enhancement of NP uptake by tumors 174.
The possible immunogenicity of VLPs is the main concern when using these NCs as DDSs in vivo, considering the mammalian immune system has evolved to protect against many viruses as a potent threat. Rather high titers of IgG and activation of B-lymphocytes was observed following the use of CCMV and CPMV as DDS. Systemic administration can create potentially severe inflammatory responses because of immune recognition of these carriers 175, 176. Conventionally PEGylation has been used to provide immune evasion and to improve the pharmacokinetics of these types of DDSs. PEGylation can decrease immune cell uptake of adenovirus and other protein-based NCs, since PEG reduces immune recognition by creating a “stealth” effect. For example, PEGylated CPMV did not show any change in its physical structure or the stability of the NPs68. Zhao et al.149 studied self-assembly of VP6, a rotavirus capsid protein that could be used to produce VLPs, which could then be conjugated to the anticancer drug DOX. They showed DOX-VP6 conjugates could be self-assembled into VLPs under the proper conditions; then lactobionic acid (LA) was employed as a chemical ligand on the VLPs surface to target the hepatoma cell line HepG2149.
On the other hand, immunogenicity generated by VLPs has been also studied. An enveloped VLP design using hemagglutinin derived from the influenza virus (A/California/04/09 strain) as a vaccine candidate. Here, the VLP-based vaccine elicited robust HA inhibition antibody responses in vivo (mice) without any adjuvant, and indicated capabilities for clinical evaluations177.
Protein cages can be modified genetically by introducing lysine and cysteine residues to allow attaching of drugs and imaging agents to reactive amino and sulfhydryl groups78. For example, Klem et al. 178 genetically modified CCMV protein cage by replacing alanine with a cysteine residue at position 163 of the coat protein to use the viral protein cage (virion) as a drug carrier. This genetic modification provided 180 exposed sulfhydryls on the exterior surface of the individual virion without disturbing the symmetry of the virion. Next, a synthetic approach including a solid-phase step was utilized to break the symmetry in order to better prevent uncontrolled aggregation.
In another approach, DNA-containing amphiphiles, with oligonucleotides covalently bonded to synthetic hydrophobic units 179, were employed as templates to modulate the self-assembly of the coat proteins of CCMV. The particles had a negative charge that stimulated capsid formation and permitted many small oligonucleotides to be trapped. Preloading of micelles with hydrophobic compounds in the core, or even hydrophilic compounds through sequence-specific hybridization resulted in encapsulation of different small molecules in the viral capsids used as nano-vehicles180.
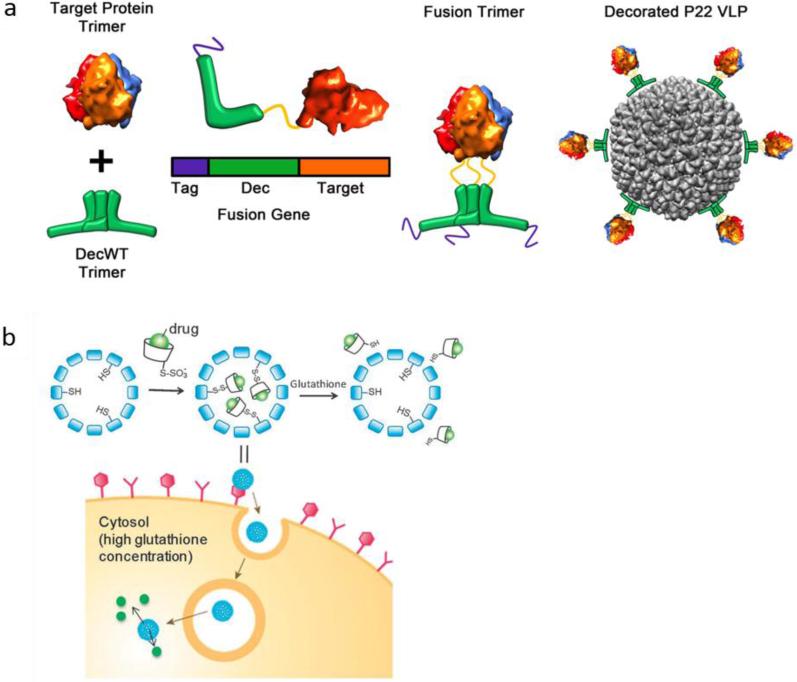
A range of specific types of proteins can be used as decoration (or coat proteins) on VLPs. For example, double-stranded DNA bacteriophages have been reported as a robust and modular tool to spatially present various guest molecules (e.g. complex protein moieties) on the exterior/interior surface of VLPs that lack a native decoration protein. Schwarz et al. modified the exterior surface of a P22 VLP capsid with a bacteriophage trimeric decoration protein, in order to bind a target bioactive protein onto the exterior surface of the VLP with a high affinity. Herein, the target protein was presented on the exterior surface of the VLP via a genetic fusion to the C-terminus of the decoration protein, while its function was maintained. Also, the decoration protein was attached via the N-terminus domain to the VLP capsid. This surface functionalization strategy produced high-affinity binding, lowered cell uptake, and enhanced circulation times for P22 VLP 181 (Figure 5-a).
Figure 5.
a) Presentation of a target protein onto the exterior of a P22 VLP via a decoration protein (Dec). A genetic fusion facilitated binding of the target protein to the C-terminus of the Dec (green). A poly histidine tag (purple) was retained intact on the N-terminus of Dec to facilitate purification. The formed structure was used for exterior decoration of a P22 VLP (grey). Reprinted with permission from ref. 181, Copyright 2015 American Chemical Society, b) Encapsulation of a CD–drug complex in VLP NCs via disulfide bonds and glutathione (GSH)-triggered release. Reprinted with permission from ref. 139 Copyright 2013 the Royal Society of Chemistry.
Linking drugs to VLPs can help increase the stability and obtain higher cytotoxicity. Aljabali et al. investigated the ability of CPMV to deliver DOX to HeLa cancer cells. In this case, DOX was covalently bound to external surface carboxyl groups of the viral NPs. the results showed that DOX conjugated to CMPV (even at very low dosage) had greater cytotoxicity than free DOX142. Recently, Niikura et al. synthesized VLPs coupled through disulfide bonds with cyclodextrins (CDs) that acted as hydrophobic pockets into which hydrophobic drugs could be incorporated. They reported intracellular delivery of hydrophobic dyes or drugs encapsulated in the VLP-CD conjugates with high efficiency, and their subsequent controlled release in recipient cells in response to glutathione (GSH). Additionally, PTX–CD was encapsulated inside the VLPs, which exhibited a dose-dependent cytotoxic effect with a 20-fold smaller IC50 than that for free PTX dissolved in DMSO 139 (Figure 5-b).
Eun-JuKo et al. 182 employed a VLP that was a combination of recombinant human respiratory syncytial virus (RSV) VLP and plasmid DNA, as a vaccine to confer protection against RSV infection. The DNA and VLP-combined vaccine could induce both innate and adaptive immune responses. The VLPs produced by the HE antigen of hepatitis E virus (HEV) were shown to be highly immunogenic and protective against infection. The efficacy of this vaccine after administering three doses was reported to be 100.0% 183, 184.
Ashley et al. used MS2 bacteriophage VLPs to deliver various therapeutic cargos such as RNA- and DNA-based drugs (e.g. siRNA), anticancer drugs, and protein toxins to human hepatocellular carcinoma cells (HCC), and also showed their ability to deliver non-nucleic acid cargos such as quantum dots, chemotherapy drugs and protein toxins (if the cargoes were linked to MS2 pac sites). The anticancer drug (doxorubicin, cisplatin, and 5-fluorouracil)-loaded VLPs were modified with SP94 (an HCC-specific peptide) indicated a 104-fold more avidity for HCC cancer cells compared to other cell types (monocytes, lymphocytes, normal hepatocytes, or endothelial cells), and could deliver high concentrations of the encapsulated cargos to the cytosol of HCC cells. Moreover, siRNA-encapsulated SP94-targeted VLPs silenced expression of cyclin family proteins in Hep3B cells 80. Moreover, antisense oligodeoxynucleotides (ODNs) (for instance targeting p120 messenger RNAs) have been shown to be delivered by MS2 bacteriophage VLPs to increase killing of cancer cells 116.
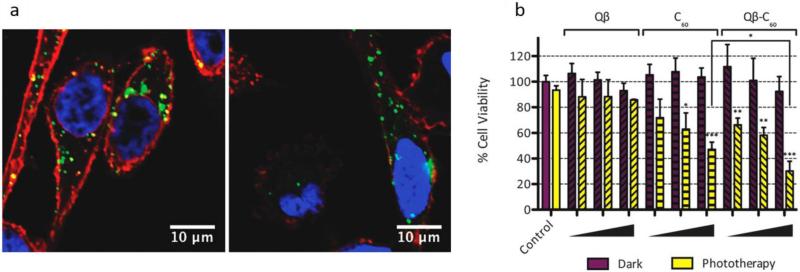
Photodynamic based therapies have utilized the potential of VLP NCs. Stephanopoulos et al. targeted and killed up to 76% of Jurkat leukemia T cells using bacteriophage MS2 as a multivalent vehicle to deliver photosensitizers (PSs) for photodynamic therapy after only 20 minutes incubation. They modified the interior surface of the capsid in order to enhance singlet oxygen generation from the PS, and the outside of the capsid had cell receptor-specific DNA aptamers attached in order to provide targeting. They found that this dual-functionalized capsid could lead to targeting and photo-killing of Jurkat cells while erythrocytes in the mixture were left unharmed. This multivalent system had the advantage of modularity, allowing the attachment of any maleimide-functionalized drug to the inside, and any chosen aptamer to the outside111. In another study, an assembly nanoplatform was synthesized composed of a viral NP (i.e. bacteriophage Qβ) conjugated with a buckyball (C60) and modified with Oregon Green 488 dye (O488), which was synthesized via click chemistry. This NP showed no toxicity in the absence of light therapy, but a PTT effect occurred after a white light irradiation, which led to enhanced cellular internalization and cancer cell killing. Figure 6 (a and b) shows the cellular internalization of the NPs including Qβ–O488 and C60-conjugated Qβ–O488 and the cell viability assay 185.
Figure 6.
a) Confocal microscopy images of incubation of PC-3 cells with viral NCs including Qβ–O488 and C60-conjugated Qβ–O488 and the subsequent cellular uptake (green). Cell nucleus and cell membrane were stained as shown in blue and red, b) cell viability using Qβ–O488 and C60-conjugated Qβ–O488 with and without phototherapy, indicating efficacy of cancer cell killing, Reprinted with permission from ref. 185, Copyright 2012 Royal Society of Chemistry.
VLPs have potential as vehicles for intracellular delivery of enzymes, because they provide stability for the biocatalytic enzyme activity, and have high encapsulation efficiency. In a study cytochrome P450 (CYP) was encapsulated inside the capsid derived from the bacteriophage P22. The product CYPBM3 acted as an enhanced peroxygenase, because the capsid could protect the cargo enzyme against proteases. The CYP cargo retained 70 % of the catalytic activity and showed a slightly higher affinity for hydrogen peroxide as compared to free enzymes. Thus, this potential VLP carrier was suggested to be utilized as an enzyme prodrug therapy for cancer 186.
4-2 Protein-based NCs
4-2-1 Ferritin
Laufberger was the first to isolate the iron-containing protein, ferritin from horse spleen in 1937. Ferritin is also found in many 2human organs and in other life-forms, such as plants, fungi and bacteria187. Ferritin proteins from different species have numerous variations in their amino acid sequence. They are intercellular proteins that have an important role in iron storage and detoxification 188. When ferritin is found as an extracellular protein, it is characteristic of some diseases such as inflammation, angiogenesis, and tumors189.
Ferritin iron storage proteins have a spherical shape, and are composed of 24 separate subunits. These subunits include H-chains and L-chains and form a cavity with an interior and exterior diameter of 8 nm and 12nm (Figure 6). Apoferritin is the iron-free form of ferritin, while the iron-containing form of ferritin is called holoferritin. Although both of these molecules have potential to act as nanocarriers, in most cases apoferritin has been used as the drug carrier. However, one shortcoming of apoferritin is that only those drugs containing anthracyclines or metals (chemotherapeutics) can be loaded into it. On the other hand, apoferritin is a self-assembled molecule with high biocompatibility and long lifetime 190.
The subunits of ferritin-based NCs are arranged to form from two to four folds. The three-fold form contains eight hydrophilic channels191 that allow various cargos such as metal ions, nuclear isotopes or semiconductors 78 to be loaded into the ferritin.
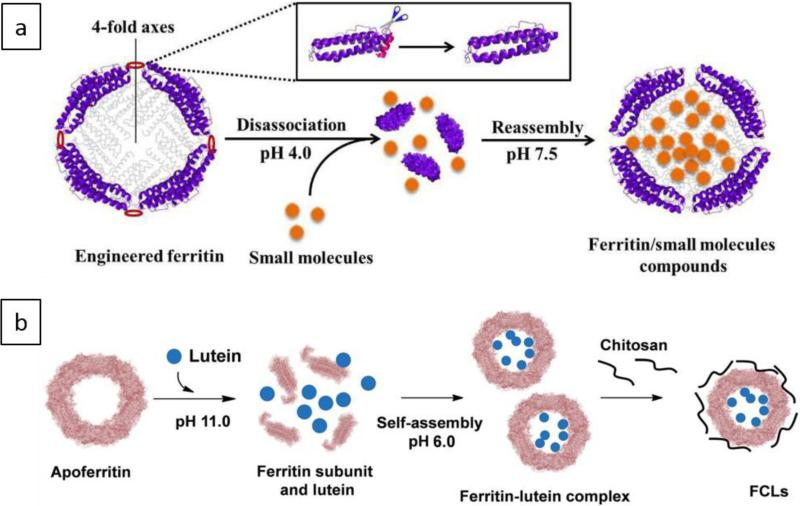
Due to its particular structure, ferritin is widely used in for delivery of various materials. Furthermore, reversible assembly/disassembly behavior and changes in its functionality at different pH values make it an interesting protein to study and designing efficient nanocarriers 130, 131, 133. Drug delivery applications of ferritin and appoferritin have been reviewed in literature 64, 84, 190, 192. Figure 7 (a and b) schematically illustrates the structure of ferritin and apoferritin NCs and their capability to encapsulate drugs via pH-responsive disassembly/reassembly process.
Figure 7.
Schematics illustrating (a) fabrication of a ferritin NC, followed by encapsulation of cargo molecules in its cavity through a reversible disassembly/reassembly process. This NC could be disassembled into its subunits at pH equal to 4.0, and then reassembled into the protein NC form at pH=7.5, b) apoferritin NC disassembled to its subunits at pH=11.0, followed by encapsulation of cargo molecules (lutein) and NC assembly at pH=6.0. Chitosan was used to form a polyelectrolyte complex of FCLs via electrostatic interactions with exterior surface of the apoferritin. a and b Reprinted with permission from ref. 193 and 194 Copyright 2016, Royal Society of Chemistry.
Redox cycling producing free radical generation can be caused by soluble iron supplements and this phenomenon can have adverse effects on the gastrointestinal tract, which is a critical barrier in the treatment of anaemia, an iron deficiency disease. Ferritins can be considered as an effective side effect-free agent for iron supplementation in treatment of anemia. In an attempt to design a safe and efficacious oral iron delivery. Powell et al. synthesized nano-sized (< 5 nm) nano-disperse ferrihydrite, by using tartrate modification to produce low-cost ferritin mimics. Here, tartrate was used to fabricate an enlarged lattice structure, and encapsulate more iron cargos 195.
The high potential of ferritin NCs in Cancer therapy and anticancer delivery has been variously studied. One of the parameters that define the efficiency of nanocarriers is the half-life of drug release. When ferritin is employed as a carrier, the half-life of drugs (e.g. DOX) can be significantly increased. For anticancer delivery, this results in increasing drug accumulation in tumors 192. in a study, Han et al. 196 developed nanoplatforms to produce dendritic cell-based vaccines using ferritin NCs for cancer treatment. The ferritin protein NCs contained antigenic peptides, and composed of SIINFEKL (OT-1) or ISQAVHAAHAEINEAGR (OT-2), either in the interior cavity, or else attached to the surface of the ferritin NCs, and were effectively taken up into the dendrite cells.
In addition, ferritin can be used for targeted delivery of cancers or tumors. In a study, Liang et.al showed that high doses of DOX could be loaded into ferritin NCs and used without functionalization with targeting ligands to kill tumors. DOX was loaded into the H-ferritin and was internalized into tumor cells by interaction with the transferrin receptor TfR1, and subsequently released DOX into the lysosomes 119. Elsewhere, apoferritin was shown to function as a nanocarrier by using ferritin modified with an amino acid sequence (RGD4C) to target integrin αvβ3. Tumor tissue was successfully targeted by using the RGD motif without any breakdown of the NCs. When DOX was loaded into the functionalized nanocarrier, drug delivery efficacy was improved by almost 13% through implementing a genetic modification method81.
Ferritin NPs have been recently used to target pancreatic tumors. Cargo-loaded ferritin NPs could either passively target the tumor via the EPR effect, or by actively binding to transferring receptor (TfR1) expressed on the tumor cells. Here a dual approach was exploited to attack the cancer cells and control their progression. Ferritin NPs loaded with carbachol were used to activate the tumor microenvironment; and ferritin NPs loaded with atropine acted to block the “neural niche” of pancreatic cancer cells, which was significantly effective in impairing neurogenesis in tumors 197.
Stimuli-responsive behavior has been integrated into ferritin NCs in recent publications. In this regard, some modifications have been implemented to enhance drug encapsulation and delivery efficiency; for example a study reported pH-responsive disassembly and reassembly of ferritin NCs 148. Herein, when the NCs were exposed to low pH, they disassembled, and when the pH was raised, the NCs would subsequently reassemble with the drugs trapped inside them.
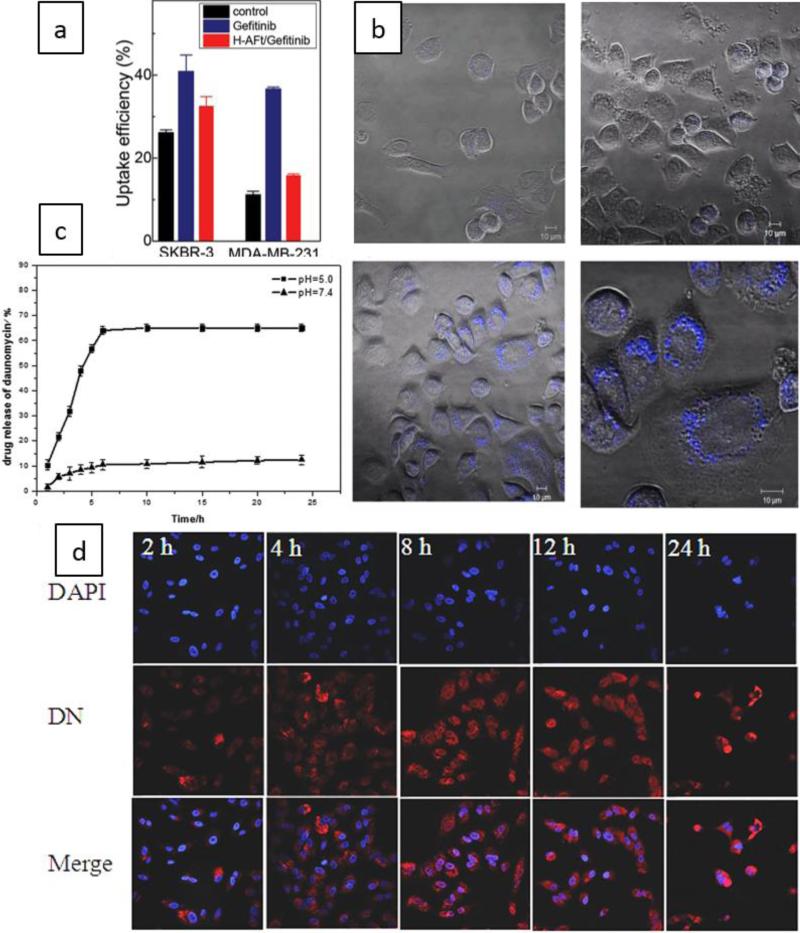
Furthermore, smart ferritin based nanocarriers have been used in DDSs. For instance, in a study on cancer therapy, ten molecules of Gefitinib (an EGFR tyrosine kinase inhibitor) were passively loaded in each apoferritin molecule. This nanocarrier was used to target HER2-overexpressing SKBR3 cancer cell lines. The release of Gefitinib molecules in the acidic environment (i.e. pH 2.0) was enhanced compared to higher pH values (pH=7.4), leading to higher cytotoxicity, which affected the HER-2 and TtR1 receptors in breast cancer cells. Figure 8 (a and b) shows the cellular uptake results obtained from flow cytometry and confocal imaging microscopy 198.
Figure 8.
a) Mean uptake efficiency by SKBR3 and MDA-MB-231 cells obtained from flow cytometry, b) Images of confocal microscopy indicating cellular uptake of control sample (upper images) and Gefitinib-loaded apoferritin NCs by SKBR3 cells (lower images) 198, Open Access, John Wiley & Sons, Inc., c) The curve indicating enhanced drug release for a DN-loaded pH-sensitive HA surface-modified apoferritin in more acidic conditions, d) Confocal images showing Internalization of the nanocarrier into A549 cells at different times (2, 4, 8, 12, and 24 hour), Reprinted with permission from ref. 199, Copyright 2015 Royal Society of Chemistry.
Lung cancer can be targeted by smart ferritin based DDSs. Luo et al. developed a pH-sensitive hyaluronic acid (HA) surface-conjugated apoferritin loaded with daunorubicin (DN) able to bind to the HA-receptor, CD44 on cells. The hydrophobic drug, DN was encapsulated in the hydrophobic channels of the apoferritin via electrostatic absorption at a slightly acidic pH, and a polymer, negatively charged poly- L -aspartic acid (PLAA), further enhanced absorption of the positively charged DN. This nanocarrier showed no activation of the immune system indicating its safety, and also had high cellular uptake. pH-controlled intracellular release of DN inside high CD44-receptor expressing (CD44-positive) lung cancer A549 cells was obtained via targeted receptor-mediated endocytosis, compared to low CD44-expressing (CD44-negative) human embryonic lung MRC-5 cells (Figure 8-c). The more acidic environment (pH=5) led to a higher release rate than a neutral environment (pH=7.4) (Figure 8-d) 199.
Giulio et al. developed a nanosystem responsive to matrix-metalloproteinases (MMPs). This nanocarrier was composed of a human ferritin heavy chain (HFt) containing a MMP-sensitive short peptide linker between ferritin subunits, and an exterior shielding polypeptide sequence. The linker could undergo intratumoral cleavage of the nanocarrier and then removal of the protective shield leading to cargo release. Thus, the nanocarrier delivered an anticancer drug, DOX with subcellular localization, and a high therapeutic efficacy in pancreatic and lung cancer cells was achieved with successful in vivo results in xenogenic mouse models132.
Heavy-chain subunit containing ferritin, so-called H-chain ferritin, can act as an efficient bioactive delivery nanovehicle known as a “Trojan Horse”, which provided an intrinsic targeting of cell nuclei due to its self-triggered nuclear translocation of the intact cage-like architecture. This approach led to substantial advantages including elimination of any further modifications, facilitated translocation and delivery of the encapsulated therapeutic molecules into cell nucleus, simultaneously decreasing required drug doses and effectively bypassing multidrug resistance of cells, and reducing drug-activated iron dysregulation in cells 200, 201. In this regard, H-chain ferritin based DDSs have attracted various studies in cancer therapy 119, 200, 201. Recently, Liang et al. prepared a natural H-ferritin NC loaded with DOX, which was administered for cancer cell killing via a single-dose injection. The cellular internalization of the ferritin NCs occurred through cellular overexpression of TfR1. A high concentration of NC DOX was delivered to cancer cells compared to free DOX, with release in the lysosomes, and noticeably delayed tumor growth. Also, this nanocarrier showed reduced toxicity, a four-fold enhanced maximum tolerated dose compared to DOX, and longer median survival times compared to Doxil, the clinically approved drug 119.
In another study, a rapid nuclear delivery into carcinoma cells by a non-modified DOX-encapsulated H-ferritin NCs was reported where along with the shielding effect of the apoferritin shell reduced the cytoplasm exposure of DOX inside cells. this prevented premature release of DOX 201.
Gene therapy, and gene delivery by ferritins has been another important approach for effective cancer therapies. This is due to the fact that conventional small interfering RNA (siRNA) delivery systems using viral and non-viral nanovehicles suffers from limitations, therefore, their efficiency and safety should be improved. In a study, a siRNA-human apoferritin protein nanoparticle complex was designed with advantages such as protecting and stabilizing negatively-charged siRNA, various surface-functionalized peptides including cancer cell targeting and penetrating peptides, siRNA-capturing cationic peptides, and enzymatic-cleavable peptides inducing intracellular release of siRNA. Here, the nanovehicle efficiently delivered siRNA, which then suppressed expression of the red fluorescent protein (RFP) in RFP-expressing cancer cells. in addition, this study indicated facile genetic surface modification of protein nanoparticles with different functional peptides, safe siRNA delivery for gene treatment 63.
In addition to targeted drug delivery, ferritin has been helpful for other purposes, like enhancing the image resolution and contrast. Ferritins have been employed to detect tumors by employing encapsulated metallic cations as imaging agents 202, 203 that could replace the Fe3+ ions. To this end, in a study the ferritin was cationized by coupling N,N-dimethyl-1,3 propanediamine using 1-ethyl-3-carbodiimide activation of carboxyl groups. When the functionalized ferritin was injected into animal models, better MRI image resolution was produced 78. In another study, Cao et al. generated a single-crystal magnetite core at the nano-scale level by attaching (NH4)2Fe(SO4)2 to H-ferritin at pH 8.5; this NC could also increase the MRI image contrast204. Finally, Wang et al. reported ultra-small copper sulfide NPs that could be accumulated inside the cavity of ferritin NCs (CuS–Fn NCs) to improve the PET imaging of tumors (Cu64). CuS–Fn NCs were also used to nediate photothermal therapy to cure the tumor 205.
NCs can simultaneously increase the sensitivity of both magnetic resonance imaging (MRI) and photo-acoustic imaging (PAI) by attaching effective targeting modules that effectively accumulate in cancers. In a study by Min et al TfR1-receptor targeted apoferritin NCs were fabricated as a theranostic platform. Here, loading metal ions (Fe3+) and melanin nanoparticles as the cargos of the apoferritins, enhanced MRI sensitivity, as well improved PAI sensitivity due to the synergistic effect of integration of Fe3+ and apoferritin. This nanoplatform also demonstrated noticeable bio-stability, high metal ion loading capacity, multimodal imaging (PET/MRI/PAI) capability, higher tumor specificity and uptake by HT29 tumor due to EPR effect. Through these alterations the stability of target module can be improved, and better targeted imaging can be achieved by increasing the metal loading in the apoferritin206.
Ferritin NCs can also be utilized for delivery of luminescent imaging agents. In a recent study, hydrophobic ruthenium(II) polyridyl complexes, Ru(bpy)2dppz2+ and Ru(phen)2dppz2+ were encapsulated in apoferritin where high loading efficiency, inhibition of Ru complex-triggered protein aggregation, and maintaining of protein native architecture were reported. This nanocarrier when compared to free Ru complexes, had enhanced water solubility, facile manipulation, higher cell uptake, and lowered cytotoxicity133.
Furthermore, other applications such as radiotherapy and PTT can be accomplished using ferritins. Ferritins can deliver radioactive isotopes207 such as yttrium(90) and lutetium(177), which can also be used for radioisotope therapy applications 78. In regard to PTT, Zhen et al 82 loaded the photosensitizer ZnF16Pc into Cys-Asp-Cys-Arg-Gly-Asp-Cys-Phe-Cys (RGD4C)-modified apoferritin with an acceptable efficiency (63 wt%). This nanocarrier showed an enhanced photodynamic effect.
It is also worth mentioning that ferritin NCs can be fabricated in combination with other materials. One example is magnetic-carbon-quantum dot probe-labeled apoferritin NCs 208. The idea of engineering such particles comes from the fact that carbon dots (CDs) are biocompatible carbon-based nanostructures used for biological applications, namely optical imaging. Here, natural apoferritin NC was used as a carrier that could encapsulate high concentration of DOX , and could be targeted as their surface was functionalized with a tumor-targeting molecule (folic acid).
4-2-2 Heat shock proteins (HSPs)
Heat shock proteins (HSPs) can be found in all living organisms. They exert a chaperone activity to protect potentially damaged proteins, and they can also function as suitable carriers for drug delivery and medical treatments via several genetic and chemical engineering approaches. In cellular stress conditions, such as high temperatures, inflammation, infection and hypoxia, HSPs are highly over-expressed 184, 209, 210 in order to prevent protein aggregation by their chaperone role. HSP100, HSP90, HSP70, HSP60, HSP27 and the small heat-shock proteins (sHSPs), are six families of HSPs that all exhibit chaperone activity and are components of normal cells211.
The chaperone role of HSPs comes from their ability to form stable complexes with their protein client molecules, where the protein clients bind to nanoscale internal cavities of the chaperone-like structure leading to protein preservation and refolding to restore the preferred secondary structure212, 213. The chaperonin-enclosed NPs can keep their chemical and thermal stability in aqueous solutions for more than one year and they are able to release their contents via ATP-binding and hydrolysis214.
In case of Small HSPs, they prevent protein aggregation in stressful conditions by partially bonding as molecular chaperones to denatured proteins 215, thus recovering cell viability, when environmental stresses have threatened cells 216. Different homologues of sHSPs are found in most existing life-forms 217-220. sHSPs form a particular group of HSPs that have an α-crystallin domain (an 80-100 amino acid sequence)221 bordered by variable amino- and carboxy-terminal extensions 215.
In 1998, Kim et al. 222 elucidated the crystal structure of the sHSP from the hyper-thermophilicarchaeon, Methanococcus jannaschii. It was shown that this sHSP had a spherical structure with 24 subunits, an exterior diameter of 12 nm and an internal diameter of 6.5 nm. It also had approximately 3nm pores, which were larger than the usual pores found in other NCs. Solute exchange was easily accomplished between the interior cavity and the exterior bulk solvent. The structural and functional properties of sHSP16.5 have been studied by Bova et al.85 who used chemical and genetic approaches to alter the structure of sHSPs. It was found that the protein structure could remain unaltered over a wide range of temperatures and pH values. In protein NCs including HSPs, presence of lysine residues both on the inner and outer surfaces of the cage allows various molecules to be attached by the formation of amide bonds. On the other hand, mutagenesis can also provide different conjugation sites by introducing cysteine amino acid residues with reactive thiol functionality. In previous studies, sHSPs have been modified with PEG linkers and SP94 peptide attachments to increase the affinity of the cages toward HCC cells. Here, selective delivery of DOX was achieved due to the combined cell-targeting and drug-delivery properties of the SP94-modified sHSP cages 86.
sHSP can be utilized as a container for iron oxide NPs, prepared in a monodisperse formulation using a size-constrained synthetic process223. In normal cells, HSP70 is expressed only at a very low level or not expressed at all, while it is highly expressed in several cancer cell lines. Thus, it can be a potential biomarker of cancer prognosis or a marker of cancer progression224, as has been reported in the case of completely resected non-small cell lung cancer (NSCLC) patients that received platinum-based adjuvant chemotherapy225. HSP27 was also reported to inhibit a component in the apoptotic signaling pathway in a model of drug-resistant oral cancer cells 226. Since conventional chemotherapeutic agents attack malignant and normal proliferating cells non-specifically, and moreover tend to exhibit sub-optimal pharmacokinetic and biopharmaceutical characteristics227, the development of DDSs that deliver drug molecules to the specific site without harming normal organs at the same time is required. Table 2 summarizes several sHSPs used in drug delivery approaches.
Table 2.
Various sHSPs used in drug delivery, their structures, engineering methods, targets, encapsulated drug and targeting peptides are listed.
| Name | Structure | encapsulates | Specified method | Target | Targeting peptide | Ref. |
|---|---|---|---|---|---|---|
| HSP16.5 | • 400 kDa • Homogenous complex • 24 subunits • Forms nanoscale, hollow, spherical capsule with small pores |
• Drugs • MRI agents |
Genetic engineering | Liver cells | preS1 peptide from HBV | 103 |
| Encapsulin | • Assembled from 60 copies of identical 31 kDa monomers • thin and icosahedral T = 1 symmetric cage structure with interior and exterior diameters of 20 and 24 nm, respectively |
has a large enough central cavity (20 nm inner diameter) to encapsulate a large amount of therapeutic and/or diagnostic reagents | Genetic and chemical engineering | HepG2 cells | Hepatocellular carcinoma (HCC)-targeting peptide (SP94-peptide, SFSIIHTPILPL) | 236 |
| sHspG41C | 24 interior thiols fully occupied | Doxorubicin | Genetic engineering | melanoma and lymphocytes | amino acid cysteine has been genetically engineered within the interior of sHsp | 237 |
HSP NCs have been genetically modified to attach peptides and antibodies for targeting of specific cancer cell types, such as melanoma and lymphocytic leukemia103. The genetic changes can introduce cysteine residues with reactive thiol groups to allow covalent binding with ligands bearing maleimide groups. As an example, the interior surface of a mutant sHSP was linked to the (6-maleimidocaproyl)-hydrazone derivative of DOX, which accomplished the selective-release of the entrapped drug in acidic (lysosomal) compartments where the hydrazone linkage was hydrolyzed103.
Murata et al. 228 prepared a NC derived from HSP16.5, a sHSP isolated from called M. jannaschii, which was mentioned above229-231. As sHSPs cannot display any tissue or organ specificity when administered systemically175, a targeting peptide sequence was added to the recombinant HSP16.5 by a genetic engineering approach. By incorporating the preS1 peptide (which was originally derived from the envelope proteins of hepatitis B virus HBV) onto the exterior surface of the NCs, targeted nanocapsules were constructed with good specificity for liver cells232, 233. Amino acids 21-47 of preS1 were responsible for its cell attachment (which is the most important single step in the entry of a virus into the cell). A study by Dash et al. 234 showed that this 27-mer preS1 peptide had high affinity to bind to HepG2 cells depending on its concentration. These NCs were localized in the cytoplasm mediated by clathrin-dependent endocytosis and showed no inherent cytotoxicity. Modification of the N-terminal region of the preS1 domain of HBV was carried out by attaching myristic acid in order to increase the cell uptake efficiency232, 235. Conjugation of HSPG41C-preS1 NCs with a well-chosen lipid increased the specificity for targeting hepatocytes in vivo.
4-2-3 Encapsulins
Encapsulins are newly discovered NC-like structures that are proteins of non-viral origin (due to not having any viral genes) derived from Thermotoga maritime (and other thermophilic bacteria) and Brevibacterium linens238. Encapsulins are bacterial nano-components and are the smallest example of bacterial protein cages, which show spontaneous preassembly behavior in-vivo as intact NCs 239. Encapsulins have been recently introduced as a promising drug delivery platform. Biomedical applications of encapsulins such as cell-specific optical nanoprobing, targeted therapeutic delivery, multi-functionality, acting as a specific nanocontainer, and as an enzymatic nanoreactor have been reviewed recently 240. Bacterial encapsulin NCs are considered to possess a range of advantages including the ability to encapsulate smaller proteins (e.g. ferritin-like proteins and dye-decolorizing proteins) compared to viral protein cages. These include better biocompatibility, robust stability toward temperature and pH changes, more facile chemical modification with no loss of stability (e.g. integration of stimuli-responsive moieties into encapsulins) and promising carriers of functional molecules with cell-penetrating behavior 239, 241. Determination of its crystal structure showed that encapsulin formed a thin icosahedral T = 1 symmetric cage structure that was composed of 60 identical copies of 31 kDa monomers. This NC has an interior diameter of 20 and an exterior diameter of 40 nm. Although the function of encapsulin in its bacterial host is not yet completely clear, studies have shown that this protein is involved in responses against oxidative stress by encapsulating proteins such as dye-decolorizing peroxidase (DyP) and ferritin like protein (FLP). The encapsulated protein binds to the interior surface of encapsulin with a specific peptide tag at the C terminus. The central cavity is therefore large enough to encapsulate several different therapeutic and diagnostic payloads239. In a study by Moon et al.236, as shown in Figure 9-a, chemical and genetic approaches allowed the HCC-cell binding peptide (SP94, SFSIIHTPILPL) as targeting ligand to be attached onto the exterior surface of the encapsulin (Encap_loophis42C123). Using a thiol-maleimide Michael-type addition, fluorescent probes such as fluorescein-5-maleimide (F5M) and drug molecules such as prodrug Aldoxorubicin (AlDox) could be attached to the encapsulin NC. Thus, the SP94 peptide-targeted encapsulin NCs could specifically enter the acidic compartments of target HepG2 cells, followed by intracellular release of the drug, AlDox, leading to efficacious cancer cell killing than free drugs. These results illuminated simultaneous targeting, diagnostics and therapeutic effect of the developed nanocarrier as a multifunctional nanosystem.
Figure 9.
a) Encapsulin NCs for targeting the surface markers of HCC cells using SP94-peptide (blue) with linker (yellow) genetically or chemically attached (green) to the exterior surface of the NC. AlDOX is chemically attached to Encap_loophis42C123 and delivered to the target cell. Reprinted with permission from ref. 236 Copyright 2014 the American Chemical Society. b) Schematic showing integration of a light-responsive moiety (spiropyran) with an encapsulin NC at its surface through amine-succinimide reaction (i), and UV light-activated isomerization of spiropyran moiety (blue) with no fluorescence to merocyanine moiety (red) with high fluorescent activity. This reaction is reversible via a visible light irradiation (ii) Reprinted with permission from ref. 241 Copyright 2016, John Wiley & Sons, Inc.
In a study, Tamura et al. used the encapsulin derived from Rhodococcus erythropolis N771 (Reencapsulin) as a nanocarrier, to encapsulate different proteins such as green fluorescent protein (EGFP) and firefly luciferase in its internal cavity through fusion of the C-terminal 37-amino-acid sequence of the R. erythropolis N771 DypB peroxidase to the C-terminus. Here, a 28 nm-spherical particle could be formed via the assembly of 60 Reencapsulin monomers. Externally packaging potential of target proteins on the external surface of Reencapsulin was also demonstrated 242.
Encapsulins nanocontainers can be modified by certain procedures (e.g. the action of enzymes) to package foreign cargos. Such encapsulated cargos and procedures can affect the mechanical properties of the encapsulin compartments e.g. their shell. For example, in a study by Snijder et al., rigid-enzyme bacterial containers including B. linens and T. maritima encapsulins were formed via the assembly of encapsulin dimers as highly stable substructures, in order to overcome mechanically compromised characteristics resulting in destabilization due to cargo loading. However these encapsulin nanocompartments showed intrinsically solid mechanical properties e.g. high stiffness that suggested they would be suitable nanoplatforms for nanomedical applications and nanocarriers of DDSs238.
Cancer immunotherapy can be implemented through activation of cytotoxic CD8+ T cell immune responses as a promising approach, especially via using targeted nanocarriers. In this regard, a type of antigen presenting cells (APCs), i.e. dendritic cells can induce initiation and regulation of antigen-specific cytotoxic CD8+ T cells to eradicate their target cancer cells, and also tumor rejection by secretion of IFN-γ cytokine. In a research, Choi et al. incorporated the OT-1 peptide (SIINFEKL) of ovalbumin (OVA) protein into encapsulin subunits, to fabricate antigenic peptide nanocarriers. Here, significant uptake of the encapsulin nanocarriers by DCs was followed by phagosome processing. Thus, OT-1 peptides were efficaciously delivered and presented to naïve CD8+ T cells, leading to antigen-specific cytotoxic CD8+ T cell proliferation. The results suggested that cytotoxicity caused through activation of DC-mediated antigen-specific T cells by presention of the antigen contained in encapsulin nanocarriers as well as IFN-γ secretion by tumor-infiltrating lymphocytes led to enhanced tumor growth inhibition, and melanoma tumor destruction in-vivo 243.
Reversible assembly/disassembly behavior of encapsulin nanocompartments in response to pH alteration was reported by Rahampour et al. Here, integration of the encapsulin with Rhodococcus jostii RHA1 peroxidase DypB, (a bacterial lignin peroxidase) gave rise to pH-responsiveness, where treatment at pH 3.0 led to disassembly and at pH 7.0 to reassembly 134. Encapsulin based NCs can be functionalized with stimuli-responsive moieties, particularly to provide non-invasive imaging and cell-tracking. Recently Putri et al. in 2016 reported a bacterial derived encapsulin NC covalently grafted with a photo-switchable non-fluorescent spiropyran label. Here, exposure to UV-light irradiation photo-isomerized the spiropyran to its fluorescent photo-isomer, merocyanine, and a visible light reversed this photo-isomerization. These results demonstrated a reversible ON/OFF photo-switch that facilitated imaging of this NC via super-resolution microscopy (Figure 9-b)241.
4-2-4 Vault protein NCs
The vault cytoplasmic ribonucleoprotein (also known as “vault”) is a eukaryotic organelle (a special subunit inside cells) whose precise function is not yet clear 244. Vaults were first discovered and isolated by Nancy Kedersha (cell biologist) and Leonard Rome (biochemist) at UCLA School of medicine245. The size of the vaults has been measured by different techniques such as STEM, Cryo-TEM, and negative staining to be between 25-60 nm 41. The protein structure of vaults consists of several major vault proteins (MVPs) bound to other minor vault proteins. The complex structure of vaults consists of two large complexes of major and minor vault proteins located close together which form a barrel-like vault. Their barrel-like structure with a large hollow body allows encapsulation of drugs40. Vaults also have a high capacity for drug loading, and their protein-based structure has been found to be relatively non-immunogenic. Their relative ease of bioengineering is another advantage that makes them suitable for drug delivery 246, 247.
Vault NPs have been used as a protein shell for NPs with a lipophilic core248. These vaults consisted of an amphipathic R-helix produced from protein 5A of hepatitis C virus, and a novel aspect of these NPs was the lumen of the vault was able to encapsulate lipophilic compounds in a lipophilic microenvironment reversibly. Electron microscopy showed that the vault waist was greater than 35 nm, and the vaults could encapsulate hundreds to thousands of drug molecules such as bryostatin1. Bryostatin1 is a lipophilic macrolide lactone that functions as a modulator of protein kinase C and can inhibit angiogenesis and cause apoptosis in cancer cells. In another study, Buehler et al.249 manipulated the structure of the vault to be a carrier for all-trans retinoic acid (ATRA), which is a potentially toxic hydrophobic compound. Lipid bilayer nanodisks were formed from the vault-binding lipoprotein complex and these bound the ATRA inside the vault, protecting it from the environment. Cryoelectron tomography demonstrated that vault could completely encapsulate the ATRA, which showed lower toxicity towards the HCC cell line HepG2249.
Another beneficial feature of vaults is their target selectivity 250, 251, especially for delivery of anti-cancer agents 252, 253. Kickhoefer et al. 254 prepared vaults with C-terminal peptide extensions at the top and the bottom of the vault that could be used to attach ligands with specificity for targeting epithelial cancer cells (A431). Three different tags were employed bound to the C-terminus of MVP, the 55 amino acid epidermal growth factor (EGF), a 33 amino acid IgG-binding peptide, and an 11 amino acid epitope tag, altogether 96 copies of MVPs. The baculovirus expression technique was used to modify the vaults.
Recently, vault protein nanocarriers have been reported that possess stimuli-responsive features. In a study by Matsumato etl al. 255, a dual-sensitive vault protein NP was synthesized, which was responsive to both temperature (with varying low critical solution temperature (LCST) at different pH values) and pH (e.g. aggregation at pH=6.0). Another experiment showed that decreasing the pH weakened the bonds between proteins. It was proposed that the observed responses of the vaults at low pH values were due to the strong polar characteristic of the lateral interaction between adjacent proteins 256. It also has been shown that the vault maintained its structure at pH values ranging from 6.0 to 7.5, but the particle was reported to be 15% shorter in length at pH 6.0 compared to its length at pH 7.5. The measurements confirmed that reducing the pH will not open the vaults in two halves, as had been suggested previously 257-259. Reducing pH will destabilize the particle which can be seen through the weakening of interactions of the shoulder regions with the barrel region.
4-3 Supramolecular nanosystems
Supramolecular-based nanosystems have the potential to form host-guest complexes with hydrophobic drugs, mitigate toxicity of anticancer drugs, and enhance bioavailability and stability 260. In order to optimize these nanosystems, it is necessary to rationally design them for their intended target, type of drug interaction (i.e. surface adsorption, encapsulation, etc.), and to introduce stimuli-responsive release under intrinsic physiological conditions or external stimuli e.g. magnetic, electrochemical, mechanical, etc. 261. These factors play a key role in designing advanced DDSs with tailored storage, transport and confined/controlled release properties 262-264. Their potential as theranostics also has been further investigated in recent studies. The following section summarizes the important endeavors in DDSs of various supramolecular NCs.
4-3-1 Cyclodextrins (CDs)
CDs are a family of α-1,4-glycosidic bonded macrocyclic oligosaccharides with an abundance of hydroxyl groups on the molecule. They have a hydrophobic cavity that can encapsulate different cargos e.g. proteins, ions, small molecules and oligonucleotides, and their application is not only limited to DDSs 265. For example, they can be used as stabilizers or flavoring modifiers in the food industry to reduce any unpleasant odor and taste 266. Several properties make CDs very attractive especially their good biocompatibility, versatile functionalization capacity and excellent pharmaceutical features 265. Different CD complexes and CD-conjugated polymers for various applications have been reviewed 265,267. In a recent study, a highly stable NC based on multivalent host–guest interactions of a polymer–CD conjugate with a polymer–PTX conjugate for passive and active targeting of PTX to cancer cells. This study employed ester linkages between the drug and an anhydride moiety on the polymer backbone that underwent degradation inside the cells leading to efficient release of PTX and significant activity in vivo in a murine tumor model 268 (Figure 10-a and b).
Figure 10.
a) Conjugation of CD and PTX molecules via their hydroxyl groups to copolymer backbone led to enzyme degradation of the ester linkages hydrolysis inducing apoptosis in cancer cells . b) Growth profile of MDA-MB-231 tumors, respectively treated with different groups. (pPTX / pCD: polymeric PTX/ polymeric CD. AP-1: peptide ligand). Reproduced with permission from ref. 268 Copyright 2014 Nature Publishing Group.
Porphyrin-cyclodextrin can also be conjugated to PTX or DOX, and such dual-function NC could be used in a synergistic combination of chemotherapy and photodynamic therapy for in vitro and in vivo studies 269. Another study looked at intermolecular interactions of two new β-CD derivatives with DOX 270. CD-based nanogels can form inclusion complexes that allow sustained release of 3-methylbenzoic acid (3-MBA) (used as a model drug) through a controlled mechanism271. Other applications have been also reviewed38 highlighting the excellent properties of CDs as DDSs.
4-3-2 Hybrid metal-organic NCs
Metal–organic frameworks (MOFs) are compounds consisting of metal ions or clusters coordinated to organic molecules to form one-, two-, or three-dimensional structures that can be porous. MOFs have good structural stability and a stable pore structure both of which are important factors for DDSs. Several investigations have been conducted on MOF-based DDSs for example their use for 5-fluorouracil (5-FU) adsorption and delivery 272; adsorption, delivery and release of bioactive gases (for example NO) 273, 274; and other biomedical applications 272. Yi et al. 275 prepared water-soluble metal-NCs based on arene ruthenium complexes for cancer targeting, which could encapsulate pyrenyl-nucleosides in a host–guest system, These NCs exhibited effective anti-proliferative characteristic on ovarian cancer cell lines, (including cisplatin-resistant strains) functioning as a DDS for floxuridine with enhanced cellular uptake and better bioavailability 275. A similar strategy was applied by Mattson et al. 276 to deliver lipophilic pyrenyl derivatives. Zhang et al. 277 prepared a series of well-defined hollow MOF-based NCs using a facile solvothermal method, which were claimed to be a novel class of functional nanomaterials with promising applications in various fields.
4-3-3 Cucurbiturils (CBs)
CBs are macrocyclic oligomeric molecules constructed from a small number of glycoluril monomer units (for instance 5-7) linked by methylene bridges. CBs were first synthesized in 1905 by Behrend who condensed glycoluril with formaldehyde 278, but their structure was not elucidated until 1981 279. These compounds have become popular in the field of catalysis based on their molecular recognition properties 280. Their interesting structure and their ability to solubilize water-insoluble drugs make them a good candidate for DDSs. In one study, cucurbituril-7 (CB7) was used to investigate the delivery of triamterene (a potassium-sparing diuretic used for the treatment of hypertension) by enhancing its solubility and bioavailability. The results demonstrated the formation of a stable host-guest complex in aqueous solution, better solubility (1.6 times), and increased oral bioavailability in rats 281. Other applications of CBs include their use in cisplatin anticancer complexes 282, 283, and crossing the cell membrane using CB complexed with basic dyes 284,285.
4-3-4 Calixarenes
Calixarenes are macrocyclic oligomers (often tetramers) based on a hydroxyl-alkylation product formed from reactions between phenols and an aldehyde, named after “calyx” (the Greek word for chalice) 286. Their properties such as easy functionalization, availability, and ability to form host-guest complexes with various molecules e.g. organic molecules and the moieties of large molecules and supramolecules (e.g. amino acids, proteins, and DNA), and metal ions, make them appropriate candidates for biomedical applications263, 287. Construction of a NC from a cobalt-containing Co4-calixarene was recently reported by Su et al.288 as the first open pentameric (Co20) calixarene coordination compound with possibilities as a DDS. This group has also reported other calixarene NCs e.g. thiacalix[4]arene-based polyhedral coordination cages289, high-nuclearity M4n (M = Co or Ni, n equals to 2–6) coordination cages 290, and “heterometallic calixarene-based cages”291. In another study, construction of a water-soluble platinum–calixarene complex, called “Calixplatin”, exhibited enhanced anticancer activity compared to carboplatin (and other platinum drugs) against a human NSCLC cell line 292. The biopharmaceutical and biological applications of calixarenes have been reviewed 263, 287. Examples include 4-tert-butylcalix[4]arene containing penicillin V and nalidixic acid 293; calixarene–cyclodextrin heterodimers for sustained release of docetaxel 294; and a hybrid liposomal PEGylated calix[4]arene for delivery of curcumin 295. Other supramolecular structures (or hybrids formed with other nanostructures) that have been used for biomedical applications based on host-guest interactions include cryptands, crown ethers, and pillararenes 260, 263, 296-298.
4-4 DNA NCs
DNA possesses important features of molecular recognition, predictable base hybridization, well-defined secondary structure, satisfactory chemical stability, and good commercial availability; therefore it has been chosen as a powerful building block for the fabrication of many nanostructures 299. In recent years, various approaches have been proposed for assembly of 3D DNA nanostructures, such as employing a biomimetic strategy which was inspired from natural biological supramolecules (i.e. viruses), and is based on self-assembly of identical components (symmetric star motifs, tiles etc.) and has allowed construction of DNA polyhedral structures 300. The strategy of employing “n-point star tiles” has been adapted as a general approach for precise hierarchical assembly of various DNA NCs and large complex supramolecular structures 301. In recent literature, DNA nanostructures have been used to impart a variety of capabilities in DDSs 302, and also miscellaneous biomedical applications of DNA NCs have been reviewed 303. Furthermore, recent studies have shown that DNA 3D nanostructures do not possess serious cytotoxicity or immunogenicity issues 304. They can exhibit good stability against nuclease degradation in biological media, and can non-invasively enter living cells without use of transfection reagents, remaining intact within cytoplasm 305, 306. Along with these features, investigation of site-specific sub-cellular targeting capability of DNA NCs, suggested that these structures could serve as highly efficient smart drug delivery vehicles 307.
DNA NCs can encapsulate guest molecules and release them under specific conditions, which makes them amenable for controlled delivery of therapeutics 308. Juul et al. 135 developed a non-covalent temperature-controlled approach to encapsulate horseradish peroxidase (HRP) into a DNA NC structure (3T). Modification of the basic cage structure with hairpin-forming DNA strands offered flexibility and could facilitate opening of the cage and release of the cargo at 37°C while retaining it at 4°C. Moreover, Liu et al 309 reported the reversible assembly/disassembly of tetrahedral DNA NCs, which were responsive to external pH with potential applications for on-demand drug release. They used star-shaped nanomotifs to assemble the DNA cage structure and introduced a cytosine-containing DNA triplex to the sticky-end regions of each branch of the motif with two complementary single-stranded overhangs. It was found that cytosine could be protonated in acidic media (e.g., pH 5.0) and interact with G-C base pairs to form C+G-C, while it lost a proton in neutral media (e.g., pH 8.0) and dissociated. Elsewhere, a smart multilayered DNA NC was synthesized by a layer-by-layer assembly method. The layers of this NC could be separated from each other by exposure to a chemical trigger, ATP 136. This ATP-activated layer separation of DNA NCs can be applied in design of smart DNA DDSs.
A recent study used both DNA NCs and gold NPs, to prepare DNA-caged gold NPs and investigated their controlled release behavior when exposed to both pH variation and a DNA degrading enzyme. The polyhedral NCs were assembled using four-point-star motifs with three complementary strands and a fourth strand that was attached to the surface of the AuNPs, which was modified with single stranded-DNA. Subsequently, DOX was incorporated into the AuNPs-NCs, and drug release was observed upon adding an exonuclease enzyme, and also when the pH was reduced from 7.2 to 6.86 310. Another interesting release strategy was proposed based on reversible on/off switching of the surface pores of the NC structure via a DNA-based capping technique. The faces of a DNA tetrahedron capped the surface pores binding to a capping motif (CM) using DNA hybridization, and uncapping occurred upon introducing another cap-removal strand (CR) which resulted in CM strand displacement increasing the pore size 308.
Setyawati et al. 311 produced a novel DNA 3D structure with theranostic applications by preparing a DNA nanopyramid platform that incorporated Au nanoclusters emitting red-light and protected with glutathione. These could be used for bacterial detection and could also contain actinomycin D as a therapeutic agent. The results indicated successful cell recognition, rapid uptake of the nanostructure inside Escherichia coli and Staphylococcus aureus bacterial cells and high killing efficacy by actinomycin D. Another nanotheranostic platform was proposed by Jiang et al. 312 based on a DNA origami (DO)-gold nanorod (GNR) complex, which combined the photothermal conversion properties of GNR and the structural properties of DNA origami. The NC was delivered to human breast tumor cells (MCF7) and showed efficient cell uptake, two-photon cell imaging capability, and photothermal cytotoxicity.
4-5 Gold NCs
Gold (Au) nanostructures with unique physical and chemical properties have attracted tremendous attention in recent years in the field of nanomedicine 313-315. In 2002, a novel class of Au NCs was invented known as AuNCs. AuNCs are characterized by a hollow interior surrounded by ultrathin and porous walls 316. Their other properties include compact size (approximately 20-500 nm), straightforward and precise tuning of localized surface plasmon resonance (LSPR), and large absorption cross-section per unit volume. The main advantages of AuNCs are: (1) can be produced in large quantities with high quality; (2) capability for loading and releasing of drugs due to their porous walls and hollow interiors; (3) possibility of controllable size to enhance their permeation through tissues; (4) being single crystals with good mechanical flexibility and stability, which makes them easy to survive in complex in vivo environments; (5) suitability for quantitative functionalization and labeling with biomolecules and ligands due to their atomically flat surface 267.
Various biomedical applications of AuNCs have been reviewed recently 9. AuNCs have attracted tremendous attention as a novel class of DDSs, due to their hollow interiors and good photothermal absorption properties, and particularly because of their inherent theranostic ability to combine optical imaging with targeted drug release. For a controllable drug delivery, the surface of AuNCs is generally functionalized before drug loading. A common method for functionalization is to employ thermally-responsive polymers combined with near-infrared (NIR) laser irradiation or high-intensity focused ultrasound (HIFU). Figure 10 shows a schematic illustration of how polymer-modified AuNCs perform in stimuli-responsive drug delivery. The surface of the AuNCs can be coated with smart polymers such as poly(N-isopropylacrylamide) (pNIPAAm) or its derivatives using Au-thiolate bond formation. The conformation of the polymer chains depends on temperature (whether the NIR or HIFU trigger is on/off) and abruptly changes at a point called the LCST. If irradiation with the NIR laser overlaps with the LSPR peak, the light will be absorbed and converted into heat through a photothermal effect. By increasing the temperature above the LCST (i.e. 39°C), the polymer chains will collapse and become hydrophobic and the pores will open and the preloaded drug will be released. When HIFU or NIR irradiation is turned off, the temperature will decrease and polymer chains will return to the extended state and regain their hydrophilicity, with the drug delivery having been accomplished. Therefore, the release of drug can be remotely controlled by adjusting the power and/or the time of NIR or HIFU irradiation 272. It was found that HIFU was more effective due to its deeper penetration into soft tissues than NIR radiation, and because the rapid local temperature rise within the focal volume achieved by the focused HIFU, produced highly localized drug release. The above system is limited to hydrophilic drugs, because polymers such as poly(vinyl pyrrolidone) (used for AuNC interior modification) are hydrophilic 128. Moon et al. 127 introduced a new method which allowed encapsulation and delivery of both hydrophilic and hydrophobic drugs. The method was based on loading the hollow interiors of AuNCs with drug-doped phase-change materials (PCM) such as 1-tetradecanol with a melting point of 38-39°C 127, 267. PCMs have a surfactant like structure and have good miscibility with drug molecules. By intermixing of drug and PCM, the drug could be loaded into the AuNCs via diffusion of the mixture. When the AuNC-PCM was heated to a temperature beyond the PCM melting point, the preloaded drug was released into the surrounding medium (Figure 11-a). Rhodamine R6G was used as a model drug in this study, and the results of thermal gravimetric analysis showed that 84% of the void space inside each AuNC was occupied with dye/PCM mixture. Figure 11-b shows controlled drug delivery by varying the power and exposure time of HIFU. At room temperature, R6G release was negligible, and at 37°C there was a slight release (less than 5% after 3 days), but, when temperature was increased beyond the melting point of PCM (38-39°C), 28% of the drug was released over 3 days 127, 267. In another study, photothermal activation was used to trigger drug release from an AuNC. In this study its pores were covered with a thermo-responsive moiety, poly(N-isopropylacrylamide) (PNIPAAm) conjugated onto the AuNC surface via Au-thiolate bonding. Then a NIR irradiation triggered LSPR absorption of AuNCs, thus increasing the temperature. This led toa LCST transition of the PNIPAAm, which became hydrophobic and shrank via conformational changes, resulting in release of the encapsulated cargos (Figure 11-c) 317. AuNCs have shown remarkable potential as a biomcomputing nanoplatform in logic-gate controlled DDSs. Shi et al. designed polymer shell-conjugated AuNCs loaded with an enzyme and also a prodrug. Here, the NIR-irradiation photothermal effect was employed to build an ON/OFF logic-gated release system with various functions such as AND, OR, or INHIBIT. As an example of logic gate operation, the AND logic state performed as follows. Two types of AuNCs were opened by a set of two NIR irradiations with different wavelengths 670 and 808 nm, through which enzymatic reactions were induced. This led to an AND gated operation in the form of fluorescence signals, and releasing of prodrug molecules (Figure 11-d) 318.
Figure 11.
a) Schematic illustration of loading the hollow interior of AuNCs with a drug-doped PCM and its release from the AuNCs by direct or ultrasonic heating. b) Release profiles of the dye exposed to HIFU at different applied powers and time points. Reprinted with permission from ref. 127 Copyright 2011 the American Chemical Society, c) photothermal triggered release of encapsulated cargos from PNIPAAm-conjugated AuNCs due to NIR-irradiation induced temperature increase. This allowed LCST transition of PNIPAAm moieties, leading to their hydrophobicity and shrinkage, which induced release of the cargos, d) logic-gated operation of polymer shell conjugated AuNCs , which can perform various logic-gates including AND, OR, and INHIBIT and lead to logic-gated cargo release. Reprinted with permission from ref. 318, Copyright 2014 John Wiley & Sons, Inc.
4-6 Carbon-based NCs
Carbon nanomaterials e.g. graphene, carbon nanotubes, and fullerenes have received much attention in nanotechnology due to their special properties and interesting structures. Their electrical and electrochemical properties and their interaction with biomolecules make them good candidate in a diverse range of biomedical applications such as drug delivery or bio-sensors 319.
Graphene is a single atomic layer of sp2 hybridized carbon atoms that has been much studied because of its novel mechanical and electrical properties. By overcoming the energy-barrier for folding graphene sheets, folded graphene sheets can be produced320. Zhang et al. reported a procedure to control the folding-morphology of graphene sheets by doping pristine graphene with hydrogen in a specific pattern. Molecular dynamics methods were used to simulate cross-shaped cubic graphene NC and encapsulation of biomolecules, i.e., drugs. The biomolecule encapsulation occurs when the top graphene sheet is “wrapped” downward, and the bottom graphene sheet is wrapped upward. This simulation opened up new options to control the 3D structure of graphene sheets that could be used for drug delivery purposes321. Carbon NCs can also be used in quantitative analysis, as Guo et al.322 showed with thin-walled graphitic nanostructures (“bucky-paper films”). Work on innovative closed structures starting from graphene nanoribbons is still in progress, and possible drug delivery applications for them have been suggested; however, currently there is no research that shows their direct application for drug delivery323.
Nanostructures (similar to those formed from carbon) but containing other elements have also been investigated. Ganji et al. 273 investigated the ability of NCs constructed from boron nitride B36N36 (a fullerene-like molecule) to bind amino acids like glycine. They calculated the binding energy and compared it with the binding energy of pure carbon fullerene (C60). The electronic structure and Mulliken charge population for most favorable complexes also analyzed, and the results showed that unlike C60 fullerene, B36N36 NC could form a stable bond with amino acids via their carbonyl oxygen. Therefore, B36N36 NCs could be used as a nanocarrier for DDSs.
In a study, mesoporous carbon nanospheres (MCN) were designed having a diameter ranging from 150 to 200 nm and capped with cupper sulfide (CuS) NPs. The MCNs due to their hollow and porous structure could encapsulate DOX efficiently with the help of π- π stacking interaction between DOX and MCN. CuS was used as the cap of the newly designed structure with NIR absorption and photothermal conversion ability. Therefore, this nanocomposite structure was a novel design of NCNCs showing low pre-release, efficient drug loading and because the cap was pH and NIR responsive, a combination antitumor activity was obtained 324.
4-7 Inorganic Oxide NCs
An important category of NCs belongs to oxide NCs such as mesoporous silica NPs (MSNs), SiO2, and iron oxide NCs. MSN-based NCs have a large surface area, high pore volume, good chemical and thermal stability and show versatile surface chemistry which makes them suitable materials as carriers in drug delivery 325-328. Wang and et al. 222 reported the synthesis of monodisperse hollow mesoporous silica (HMS) NCs with a uniform size possessing a hollow cubic core and a mesoporous shell with pore channels that could be tailored by a template-coating-etching process. HMS NCs had a higher loading capacity because of their larger cubic volume, and allowed a chemotherapeutic drug, DOX to be loaded and released. Successful in vitro fluorescence imaging indicated HMS NCs labeled with florescent dyes had good cellular uptake 87. In addition to the high storage capacity of NCs, MSNs have also shown sustained release properties. HMS NCs have demonstrated initial fast drug release followed by a longer sustained release, and also pH-triggered drug release can be used for anticancer targeting 87, 329. In addition to MSNs, iron oxide NCs have been also been used as potential cage-shaped structures. In a recent study, a drug (riluzole)-loaded iron oxide NC capped with a catechol-functionalized dextran was synthesized and used for targeted delivery against metastatic osteosarcoma cells 92.
5- Synthesis methods of NCs
5-1 Ferritin
Ferritins are self-assembled in living organisms 7. They are produced in almost all living organisms such as animals, higher plants, archaea, fungi and eubacteria, but not in yeast. Ferritin subunits self-assemble into spherical naocages with outer and inner diameters of 12 nm and 8 nm, respectively. Ferritin can be found in external and interacellular compartments such as cytosol, nucleus and mitochondria 84.
5-2 Small heat shock
The sHSP NCs are expressed in response to elevated temperatures and other types of cellular stress as infection, inflammation and hypoxia. sHSP has extracted and purified from organisms such as Methanococcus jannaschii.
5-3 Vault
Vault proteins are present in the cells of diverse eukaryotic cells 330. Organisms like protozoa, molluscs, the slime mold Dictyostelium discoideum, echinoderms, fish, amphibians, avians, and mammals331 contain vault proteins. Vault proteins self-assemble from subunits in living organisms and make spherical NCs of which the majority exist in the cytoplasm and they extracted and purified from cytoplasm.
Also according to the recent studies vaults can be produced with chemically active or fluorescent proteins sequestered within the particle cavity. The baculovirus system is robust leads to production and purification of 4-20 mg of vaults per liter of cells culture 332.
5-4 Virus-like NCs
VLPs can be purified after expression in yeast and insect cells using baculoviruses, Escherichia coli or mammalian cells 333. The producing selection method depends on the type of VLPs and the number of different proteins included in the specific VLP 334. Two of the most prominent HPV VLP vaccines, Gardasil and Cervarix™, are produced in yeast and baculovirus / insect cell expression system, respectively. Providing by baculovirus or in mammalin cells is often preferred when several different proteins are co-expressed in order to form the VLPs.
5-5- Supramolecular nanosystems
5-5-1 Cyclodextrins (CDs)
Several methods are known for the assembly of NCs from CDs 335. A straightforward method is covalently connecting several CD cavities to a linker molecule or grafting a number of CD cavities onto a polymeic molecule through nucleophilic displacement, condensation or acylation. Another method for CDs assembly is based on the combined contribtion of several non-covalent interactions which leads to highly ordered architectures with well-oganized topology. Gold nuclei and carbon nanotubes are widely used as templates in the construction of three-dimensional CD-based supramolecular assemblies 336.
5-5-2 Cucurbiturils (CBs)
CB –based supramolecular assemblies can be performed by the reaction of glycoluril with formaldehyde in mineral acids (H2SO4 and HCl) at 75-90 °C, produces a mixture of the CB[n] family. The lower reaction temperature leads to the remarkable amounts of CB homologues in addition to CB[6]. CB[n] (n=5, 7 and 8) can be separate and purify by fractional crystallization and dissolution, or chromatography 337. In another study, the new CB[6] derivative, (3-(6-hydroxyhexanethio)-propan -1-oxy)nCB[6] (average n=11.4), was synthesized by photoreaction between (allyloxy)12CB[6] and 6-mercaptohexanol in methanol 338.
5-5-3 Hybrid metal-organic NCs
General methods used for the hybrid metal-organic synthesis including; nanoprecipitation, solvothermal, reverse microemulsion, and surfactant-templated solvothermal reactions. First method leads to amorphous materials, while the latter three methods can afford crystalline materials 272. according to other research hybrid metal-organic NCs are synthesized under mild conditions via a coordination-directed self-assembly processes 339.
5-5-4 Calixarene
Calixarene – based NCs generally have been synthesized by the solvothermal method. For example, a family of high-nuclearity M4n (m=Ni or Co, n=2-6) coordination NCs constructed by M4-calix[4]arene molecular building blocks with inorganic phosphate or organic phosphonate ligands have been isolated after solvothermal syntheses 340. Calixarenes (including resorcinarenes and pyrogallolarenes) are one of the important categories of macrocyclic host molecules in supermolecular chemistry 340. Various calixarenes- and resorcinarenes–based NCs have been prepared through metal –mediated self–assembly 341. Most of the cages (especially capsules) are made by two metal-bridged calixarenes. Larger cages have been constructed from six calixarenes linked with 12 or 24 metal ions.
A series of MOF NCs was reported by Zhang and et. al. 342 by a solvothermal method without any template. A surface energy driven mechanism was suggested for this synthesis.
5-6 Carbon NCs
Different methods such as pyrolysis, carbon atom deposition and template methos have been developed for carbon NCs (CNCs) preparation. In one report an in situ MgO template method was developed to prepare CNCs from a benzene precursor 343. In another study CNCs were produced by pyrolysis of ethanol and ferrous oxalate 344. First, iron ions were converted to cubic Fe3O4 nanoparticles by a solvothermal reaction using the decomposition of ferrous oxalate. Then, cubic Fe3O4 nanoparticles catalyzed the carbonization of ethanol and the carbon atoms begin to deposit on the surface of Fe3O4 nanoparticles. CNCs could be obtained after washing the Fe3O4 nanoparticles with HCl. In an other research, iron/graphite core –shell NPs were produced by pyrolysis of a mixture of acetylene and iron carbonyl in order to form hollow CNCs 345. The core-shell NPs were heat-treated in the presence of iodine. The Fe particles entrapped by carbon shells could be removed with little damage of the graphitic structure and little loss of carbon material.
5-7 Au NCs
The easiest method to synthesize AuNCs is based on a simple galvanic replacement reaction between Ag nanocubes and gold salts such as HAuCl4 in aqueous media (equation 1) 346, 347. Four different Ag templates can be exploited including single-crystal cubes with and without truncated corners; single crystal octahedrons with truncated corners; and polycrystalline quasi-spheres for the synthesis of AuNCs 75. The truncated corners of the Ag nanocubes are used as templates, causing the NCs to have pores preferentially located on corners rather than the facets75. This makes AuNCs suitable for specific applications348, 349.
| (1) |
The production process of AuNCs begins by a reaction between Ag and HAuCl4 at the surface of the Ag cube 346 (sites of highest energy) using a syringe pump to control the rate of HAuCl4 addition while the temperature of the Ag nanocube suspension is also controlled 347. During this reaction, the silver dissolves and the released electrons migrate to the nanocube faces and are captured by AuCl4− generating Au atoms which form a thin layer of gold on the outer surface of the cube 350. As the reaction continues, more silver is removed from the interior wall of the cube and small pits are created in the wall, so a uniform, smooth and hollow gold-silver alloy nano-box is formed. The last step of the process includes removing of all the silver from the gold-silver alloy walls, while a highly porous material remains which forms the final NC 351. In this method, the wall-thickness can be controlled by adjusting the amount of HAuCl4. The process steps of the above-mentioned approach are summarized in Figure 12-a 347. Furthermore, Figure 12-b shows SEM images and a brief review of Au NCs formation process from Ag nanocubes, in a study conducted by Sun et al. 352.
Figure 12.
a) Schematic illustration of the major steps of the production of AuNCs from Ag nanocubes by the galvanic replacement method. (1) Starting reaction; (2) Production of initial hollow nanostructure; (3) Formation of nanoboxes; (4) Dealloying of the Au/Ag nanoboxes; (5) Formation of AuNC-nanobox with pores in the walls; (6) Production of AuNCs. Reprinted with permission from ref. 347 Copyright 2007 Nature Publishing group, b) SEM images (i, ii, iii and iv) (E) of morphological changes in formation of Au NCs: starting from conversion of Ag nanocubes into Au/Ag nanoboxes, and finaly into Au NCs. Reprinted with permission from ref. 352 Copyright 2004 the American Chemical Society, c) Schematic showing synthesis of hollow mesoporous silica NCs and their modification for drug delivery. [CTAB: cetyltrimethylammonium bromide; TEOS: tetraethyl orthosilicate; APTMS: 3-aminopropyltriethoxysilane; PEG: polyethylene glycol; DOX: doxorubicin]. Reprinted with permission from ref. 87 Copyright 2011 the Royal Society of Chemistry.
5-8 Oxide inorganic NCs
As previously stated, two important oxide NCs are MSNs, and iron oxide NCs. Monodisperse uniform HMS NCs with cubic hollow cores can be synthesized using a template–coating–etching technique. This approach utilizes cetyltrimethylammonium bromide (CTAB) as the template to define the morphology of lead sulfide (PbS) nanocubes that function as a structure-directing template for the formation of mesoporous silica NCs. PbS nanocubes are synthesized in aqueous solution using Pb(Ac)2 and thioacetamide. PbS nanocubes are a good choice as a template for production of silica NCs due to their simple and reproducible synthetic process and suitable CTAB–capped surface, which leads to the formation of mesoporous silica shells with a cubic shape. As illustrated in Figure 12-c, the CTAB solution was mixed with PbS nanocubes to adsorb CTAB molecules onto the surface. Then, the silica precursor, tetraethyl orthosilicate (TEOS), was added and TEOS was hydrolyzed occurred by base-catalysis, and the mesoporous silica shells were produced on the surface of PbS nanocubes (PbS@mSiO2) 87. The surface of the HMS NCs was then functionalized with amino groups using 3-aminopropyltriethoxysilane (APTMS). Finally, the surface of the HMS NCs was modified with PEG succinimidyl groups to provide long circulation times and colloidal stability under physiological conditions. The PEG-HMS NCs were loaded with DOX as a cytotoxic drug against liver cancer cells 87.
The monodisperse PbS@mSiO2 nanocubes were uniform (44 ± 2 nm in diameter with an outer shell thickness of 14 ± 2 nm) according to the TEM images. The HMS NCs were found to have a thicker outer shell of 24 ± 2 nm. The mesoporous structure of the silica NC enhances drug loading because the pore channels of the shells facilitate diffusion; moreover drug molecules can be stored in the large cubic cavities in the center as well as inside the mesoporous channels. A high cargo loading efficiency into HMS-NCs was reported as high as approximately 89.1% and the drug loading ratio of 57 μg per 1 mg of NCs 87, 329.
In regard to iron oxide NCs, they can be synthesized via etching of cubic nanocrystal seeds using galvanic exchange reactions, after which a cage shape with hollow cavity and single crystal nature can be obtained. 92. Moreover, the synthesized iron oxide NCs can be equipped with various cargos either encapsulated or conjugated, and besides, can be functionalized with capping agents.
6- Nanotoxicology
Nanocarriers in general, and more specifically NC drug carriers have demonstrated promising results that bring a future hope that drugs will have fewer side effects because the drug carriers can be made more selective and prevent many side effects. Nevertheless, nanocarriers should be biocompatible, or in other words the carriers themselves should not cause any off-target cytotoxicity. This is why after passing initial in vitro tests, the carriers should be closely examined in vivo. Carrier-mediated cytotoxicity is mainly due to interactions between NCs and body systems which can lead to immune activation, chronic toxicity, mutation. Moreover there is a concern about long-term environmental accumulation.
Recent studies have investigated the genotoxicity of silver and metal oxide nanocarriers in vitro using the alkaline comet assay353-355. Moreover, CNTs, silver and zinc NCs have caused apoptosis caused by DNA damage induced in cells, which were detected by the TUNEL (terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling) assay356-358. It has been also shown that silica NPs can cause lipid peroxidation, reactive oxygen species (ROS) generation and depletion of GSH. Therefore MSP may have the potential to induce oxidative effects in human liver cells359. Furthermore, depolarization of mitochondrial membrane, when exposed to NCs could be a sign of apoptosis initiation, which can be caused by nickel oxide and gold NCs360. Titanium dioxide NCs and CNTs can reduce mitochondrial metabolic activity, as they have photocatalytic properties361. Additional investigations also demonstrated that silver and gold NCs could cause reduction in metabolic activity 362, 363. Cadmium-containing silica NCs, at a shorter exposure time, showed down-regulation in renal gene expression, associated with acute inflammation, immunity and defense processes. After 30 days, down-regulation in renal gene expression was related to apoptosis induction and cell regulation364.
Immune responses, inflammatory reactions and phagocytosis have been demonstrated by NCs, such as iron oxide NCs365, 366. Additionally, cytotoxicity of graphene quantum dots (GQDs) has led to a decrease in expression of the anti-apoptosis protein Bcl-2 and an increase in expression of pro-apoptotic proteins Bax and Bad367. Accumulation in organs and histological abnormalities also can result from NCs, such as copper NCs368 and titanium dioxide NCs369. In addition, NC-treated animals may show changes in some blood constituents; these may be positive or negative changes, in comparison with the control group. In this case, these variations can be used as an indicator of nanotoxicity 370. However blood analysis of mice after 14 successive days of administration of gold-iron oxide NCs, showed no toxicity371.
Excretion routes of NCs from the body are another important issue in nanomaterial safety. Inductively coupled plasma (ICP) mass spectrometry tests on samples of urine and feces, collected at different times after administration, is one way to monitor NC excretion. Analyses of different excretion routes after silica NC administration, determined that feces (liver and bile) was the route by which highest percentage of silica NCs were excreted372, while after gold NC administration, the renal route into urine showed the highest percentage373.
Only a few of the virus-based protein cages have reached the stage of evaluating toxicity and immunological reactions using in vivo tests. These virus-based structures have mostly included CPMV and CCMV. One example is Texas Red (TR)-labeled CCMV, which was injected into mice and then the tissues were evaluated by fluorescence microscopy. The CCMV was rapidly (within 1hour after injection) found in the liver, lungs and kidneys; the presence in these organs declined 24 hours after injection. A high percentage (around 60%) of NCs was excreted in 24 hours. The fast excretion of CCMV from the human body can reduce the side effects of those molecules. However a severe IgM and IgG response indicated that CCMV was highly immunogenic175.
CPMV NCs were found to be non-toxic and safe, as was shown by the study of Finn and Manchester et al. 176 who investigated the toxicity and biodistribution of these particles in vivo. Different doses of CPMV VLP were injected into mice and no visibly concerning signs were observed. Within 30 minutes, these NCs were also cleared from the blood circulation, with an average half-life of 4–7 min in plasma. A mild leukopenia was observed, which could be due to the presence of viruses in the blood circulation374, 375. The cytotoxicity and cellular uptake of several VLPs have been measured in vitro. It was concluded from an MTT cytotoxicity assay that HCRSV is not cytotoxic against the CNE-1, CCL-186 and OVCAR-3 cell lines at concentrations up to 1 mg/mL 376. CPMV–APC interactions need to be studied further in order to develop CPMV based vaccine systems377.
The first idea that comes to mind for reducing the cytotoxicity of NCs is by formation of a protective coating (for instance PEGylation) around the NC to alter the interaction between the NCs and the biological system. There are some advantages and disadvantage of various materials and methods used in coating different NCs. For example, protein-based NCs and AuNCs have flexibility for surface modification (the latter one due to the ease of forming gold–thiolate linkages)88. Mesoporous silica NCs combine high chemical and thermal stability with versatile chemistry for surface functionalization69, 377. AuNCs can also encapsulate various bio-molecules378, and have a broad range of possibilities in theranostic applications75, 351. However AuNCs have issues with biocompatibility, and immunological issues could present a challenge because of B-cell activation, and high IgG titers especially for repeated applications 68. IgM antibody production and increased complement activation has been observed mainly in studies using multiple administrations 379, 380.
The toxicity of MOFs is challenging due to the incorporation of heavy metal ions (Cu2+, Gd3+, Fe3+, Mn2+. etc.)76 in their structures, which is similar to recent discussion on the toxicity of semiconductor quantum dots and gold nanostructures. Concerns exist about the generation of ROS, possible genotoxicity, mitochondrial damage and cell death by apoptosis and/or necrosis.
Although several studies have been done to investigate the toxicity of NPs and NCs, a categorical assessment and consensus regarding their toxicity is controversial. This is partly because measures of toxicity are affected not only be the assay chosen, but also by factors such as shape, surface charge, size, coating and surface functionalization, through which the interaction of NC with biological systems can be changed, resulting in changes in toxicity measured. Nanotoxicity is still an area that is under active investigation.
7- Translation into clinical applications
Despite all the fascinating features, the majority of innovative drug delivery vehicles are hampered by their toxicity, biodegradability or immunogenicity when it comes to clinical applications. Some NP-based DDSs that have been approved by FDA so far are: Doxil (liposomal-polyethylene glycol doxorubicin), DanoXome (liposomal daunorubicin), Oncaspar (Polyethylene glycol-L-Asparaginase), and Abraxane (albumin-bound paclitaxel)381. Natural polymeric vehicles, such as albumin, because of posing less biocompatibility concerns, have recently reached growing applications in clinical trials. Similarly, ferritin, the naturally occurring protein, having insignificant toxicity and immunogenicity along with several unique properties are highly potent for future clinical translation382. Ferritin NCs are being increasingly investigated for imaging and drug delivery to cancer cells. Photodynamic therapy is a highly promising treatment modality, which have been undergone intensive preclinical and clinical investigations for treatment of cancer as well as other diseases. In this regard, ferritin NCs have been designed for delivery of photosentisizers to tumors site-specifically and have proved the advantage of NCs over previously developed nanocarriers, such as liposomes, which are suffering from suboptimal PS loading rate and a large particle size82. Such novel ferritin based nanovehicles that have been explored for theransostic applications toward cancer treatment by loading NIR dyes (new cyanine green, IR820) into ferritin and developing simultaneous multimodal imaging-guided photothermal therapy in vivo, can be potentially used for future clinical applications upon applying modifications on the developed delivery system 83. Several studies on in-vivo drug delivery of modified ferritin NPs to tumor-bearing mice have approved high capability of these NPs. So far, only limited numbers of NCs have been reported to have actually undergone clinical trials. A nanovehicle system based on self-assembly of four components, cyclodextrin-containing polymer (CDP), a polythethylene glycol (PEG) steric stabilization agent, and human transferrin (Tf), was developed and loaded with siRNA toward ribonucleotide reductase subunit 2 and was utilized to treat patients in a phase 1 clinical trial, which indicated the high potential of cyclodextrin NCs for real tumor targeting applications108. Moreover, VLP NCs are also suggested to have potentials for clinical applications e.g. as enzyme prodrugs for cancer therapy 186. However, for further translational applications there are several issues that should be addressed, such as the possibility of inducing immunogenicity of NPs upon modification of the ferritin surface for improved targeting. Moreover, the fate of ferritin NCs after systematic injection, inhibiting ferritin uptake in off-target organs and possibility of modifying ferritin surface by incorporating stealth moieties to extend drug half-life should be further investigated. Some future outcomes are estimated to be nuclear translocation of ferritin NCs for developing Trojan horses for chemotherapy and translation of unfunctionalized DOX-loaded ferritin for clinical trials 84.
Apart from ferritin, new classes of natural-protein based NCs are arriving. In a recent study, HSPs have been developed for tumor targeting via genetic engineering and attaching a specific peptide to its C terminus, through which it can readily bind to the receptors expressed on pancreatic cancer cell. So, HSPs can improve drug targeting, which normally can be hardly uptaken by specific cells and organs, such as pancreatic cancer cells 61.
Similar to protein NCs, Au NCs are not only highly promising for biomedical applications, but their translation also hinges on their biodistribution and pharmacokinetics in-vivo. Au NCs, with their unique features are the next candidates for clinical applications toward cancer diagnosis and therapy after introducing two clinically approved Au NP-based delivery systems, i.e. solid AuNPs for drug delivery and Au nanoshells for photothermal treatment 383. One promising advantage of such NCs is their maximum absorption in the NIR, which makes them suitable for PTT and as PEGylated Au nanotechnologies have been classified as “medical devices” by FDA for PTT applications, Au NCs can be developed and conjugated with PEG and readily undergo approval procedures 384. However, the accumulation of these NCs in healthy tissues and their clearance by microphysiological systems are some other challenges ahead. Their biodistribition can be improved by adjusting their surface characteristics and their size. The other challenging solution for the clearance of Au NCs from the body is fragmentation of these particles by developing particles with below 1 nm wall, so that they can be fragmented in-vivo into pieces less than 5 nm9.
Since some synthetic and natural nanomaterials have successfully passed clinical trials and received FDA approval, such as liposomes385 and poly (D,L-lactide-co-glycolide) (PLGA)386, NCs could be conjugated with these materials to improve their features and impart more biodegradablity and biocompatiblity to these materials for drug and gene delivery purposes.
In addition, another possibility for having clinically approvable and safe products in the near future would be extending already FDA-approved materials such as albumin, dextran-coated iron oxide NPs, etc. into caged structures in order to simultaneously benefiting from the properties of NCs; as an example was noted in this review by the Rampersaud group in 2016 92. Such strategies could open new horizons in developing efficient DDSs and nanotherapeutics with high safety and innovative approaches for improved treatment of diseases in clinical investigations.
8- Concluding remarks and future perspective
With the explosive growth of nanotechnology applied to drug delivery and allied medical fields, and an ever-increasing number of ingenious nano-delivery vehicles being reported, one relatively simple fact stands in danger of being forgotten. Namely, that not all nano-vehicles have the same size and loading capacity. The term “nano-cage” therefore applies to those nano-vehicles that are capacious enough to load a substantial amount of cargo or payload, yet have relatively strong bars to keep the cargo protected during the long and possible winding journey to its destination. Not only should the bars remain secure during the journey, but they should also be able to be easily opened, once the NC is ready to release its cargo. The extremely toxic nature of many cancer chemotherapeutic drugs can indeed be said to resemble a “man-eating tiger”, with the door of the nano-cage able to be remotely opened to set the ravenous beast free to devour the tumor, without any danger that bystanders (normal tissues) could be gobbled up as well. Therefore the ideal NC design could also incorporate a stimulus-responsive drug-release mechanism that can respond to or “sense” the tumor microenvironment in a “smart” or intelligent manner. Lower pH due to tumor glycolytic metabolism, a reducing environment provided by elevated glutathione fond in tumors, and raised temperature due to higher metabolic rate in tumors, are examples of these stimuli. However there is also the possibility of a remotely operated key that can be turned in the door of the cage from outside. Examples of these external “keys” could be light, ultrasound or magnetic fields. This rapidly growing area of research is expected to continue to grow in popularity, especially as fears of the predicted dire consequences of nanotoxiciology are shown to have been somewhat exaggerated. Moreover, NCs can be outfitted with many other desirable features such as super affinity targeting and bi/multi-specificity, e.g. specific targeting peptide ligand-incorporated NCs.
Moreover, applications of NCs are not only limited to the field of DDSs, but can also be utilized in diagnostic techniques. For instance, application of Au NCs is a fast-growing field approaches such as optical coherence tomography and photoacoustic tomography 84, 387. AuNCs have also been reported for photothermal therapies 388. Furthermore, the growth of publications on DNA NCs and developments in handling DNA structures through DNA origami techniques and other creative methods are considerable. There have also been efforts to combine the therapeutic and diagnostic approaches (theranostics) like combining DNA NCs and quantum dots 389.
Various goals in the design of state-of-the-art DDSs, are addressable including intracellular delivery, cell nucleus delivery, as well as targeting cell membranes and their components such as cell receptors and ion transport channels, NCs have great possibilities for the aforementioned goalss, as discussed in this review.
For future investigations, it is likely that new materials, or a novel combination of already-known materials will be possibly used to fabricate innovative NCs with multiple applications. Examples include, development of NCs with FDA-approved nanomaterials, or an interesting case reported by Tang et al in May 2016, where a water-dispersible Janus NC with platelet shape and a mesoporous silica shell was designed incorporating a graphene sheet in its interior and a PEG coating on its exterior surface, which was suggested for chemotherapy for platelet delivery of hydrophobic drugs along with having a photothermal effect in PTT 390.
However, despite many advantages of NC-based DDSs suggesting their potential applications in multimodal treatments, multi-responsive release systems and theranostic applications, the future clinical application of NC DDSs will depend on overcoming adverse immunological effects of the nanostructures, and optimizing their functionalization for in-vivo administrations.
Acknowledgements
MRH was supported by US NIH grant R01AI050875.
References
- 1.Sahandi Zangabad P, Khodabakhshi F, Simchi A, Kokabi AH. International Journal of Fatigue. 2016;87:266–278. [Google Scholar]
- 2.Mahmoudi N, Simchi A. Materials Science and Engineering: C. 2017;70:121–131. doi: 10.1016/j.msec.2016.08.063. [DOI] [PubMed] [Google Scholar]
- 3.Mahdi Karimi HZ, Sahandi Zangabad Parham, Bakhshian Nik Amirala, Yazdani Narges, Hamrang Mohammad, Mohamed Elmira, Masoud Moosavi Basri Seyed, Bakhtiari Leila, Hamblin MR. Nanomedicine, Future Medicine. 2016 doi: 10.2217/nnm.16.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang Y-Y, Choi H, Kushida Y, Bhayana B, Wang Y, Hamblin MR. Antimicrobial Agents and Chemotherapy. 2016;60:5445–5453. doi: 10.1128/AAC.00980-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirkin CA, Meade TJ, Petrosko SH, Stegh AH. Nanotechnology-Based Precision Tools for the Detection and Treatment of Cancer. Springer; 2015. [Google Scholar]
- 6.Karimi M, Ghasemi A, Sahandi Zangabad P, Rahighi R, Moosavi Basri SM, Mirshekari H, Amiri M, Shafaei Pishabad Z, Aslani A, Bozorgomid M, Ghosh D, Beyzavi A, Vaseghi A, Aref AR, Haghani L, Bahrami S, Hamblin MR. Chemical Society reviews. 2016 doi: 10.1039/c5cs00798d. DOI: 10.1039/C5CS00798D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee EJ, Lee NK, Kim I-S. Advanced Drug Delivery Reviews. 2016;106(Part A):157–171. doi: 10.1016/j.addr.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Karimi M, Eslami M, Sahandi-Zangabad P, Mirab F, Farajisafiloo N, Shafaei Z, Ghosh D, Bozorgomid M, Dashkhaneh F, Hamblin MR. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2016 doi: 10.1002/wnan.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pang B, Yang X, Xia Y. Nanomedicine. 2016;11:1715–1728. doi: 10.2217/nnm-2016-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen TM, Cullis PR. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 11.Fathi M, Entezami AA, Ebrahimi A, Safa KD. Macromolecular Research. 2013;21:17–26. [Google Scholar]
- 12.Eslahi N, Abdorahim M, Simchi AA. Biomacromolecules. 2016 doi: 10.1021/acs.biomac.6b01235. [DOI] [PubMed] [Google Scholar]
- 13.Coelho JF, Ferreira PC, Alves P, Cordeiro R, Fonseca AC, Góis JR, Gil MH. The EPMA journal. 2010;1:164–209. doi: 10.1007/s13167-010-0001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta A, Avci P, Sadasivam M, Chandran R, Parizotto N, Vecchio D, de Melo WC, Dai T, Chiang LY, Hamblin MR. Biotechnology advances. 2013;31:607–631. doi: 10.1016/j.biotechadv.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman-Amtenbrink M, Von Rechenberg B, Hofmann H. Superparamagnetic nanoparticles for biomedical applications. 2009 [Google Scholar]
- 16.Karimi M, Avci P, Ahi M, Gazori T, Hamblin MR, Naderi-Manesh H. Journal of Nanopharmaceutics and Drug Delivery. 2013;1:266–278. doi: 10.1166/jnd.2013.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karimi M, Mirshekari H, Basri SMM, Bahrami S, Moghoofei M, Hamblin MR. Advanced drug delivery reviews. 2016 doi: 10.1016/j.addr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mokhtarzadeh A, Alibakhshi A, Hejazi M, Omidi Y, Dolatabadi JEN. TrAC Trends in Analytical Chemistry. 2016;82:367–384. [Google Scholar]
- 19.Karimi M, Bahrami S, Ravari SB, Zangabad PS, Mirshekari H, Bozorgomid M, Shahreza S, Sori M, Hamblin MR. Expert opinion on drug delivery. 2016 doi: 10.1080/17425247.2016.1193149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massoumi B, Abdollahi M, Fathi M, Entezami AA, Hamidi S. Journal of Polymer Research. 2013;20:1–8. [Google Scholar]
- 21.Fathi M, Entezami AA, Arami S, Rashidi M-R. International Journal of Polymeric Materials and Polymeric Biomaterials. 2015;64:541–549. [Google Scholar]
- 22.Fathi M, Barar J, Aghanejad A, Omidi Y. BioImpacts: BI. 2015;5:159. doi: 10.15171/bi.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolatabadi JEN, Omidi Y. TrAC Trends in Analytical Chemistry. 2016;77:100–108. [Google Scholar]
- 24.Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, Katayama Y, Niidome Y. Journal of Controlled Release. 2006;114:343–347. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Li C, Huang S, Hou Z, Cheng Z, Yang P, Peng C, Lin J. Biomaterials. 2010;31:3374–3383. doi: 10.1016/j.biomaterials.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 26.Xiang H-J, Deng Q, An L, Guo M, Yang S-P, Liu J-G. Chemical Communications. 2016;52:148–151. doi: 10.1039/c5cc07006f. [DOI] [PubMed] [Google Scholar]
- 27.Chauhan G, Chopra V, Tyagi A, Rath G, Sharma RK, Goyal AK. European Journal of Pharmaceutical Sciences. 2017;96:351–361. doi: 10.1016/j.ejps.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Karimi M, Mirshekari H, Aliakbari M, Sahandi-Zangabad P, Hamblin MR. Nanotechnology Reviews. 2016;5:195–207. [Google Scholar]
- 29.Ozkan M. Drug Discovery Today. 2004;9:1065–1071. doi: 10.1016/S1359-6446(04)03291-X. [DOI] [PubMed] [Google Scholar]
- 30.Qi L, Gao X. 2008 [Google Scholar]
- 31.Karimi M, Solati N, Ghasemi A, Estiar MA, Hashemkhani M, Kiani P, Mohamed E, Saeidi A, Taheri M, Avci P. Expert opinion on drug delivery. 2015:1–17. doi: 10.1517/17425247.2015.1004309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen H, Zhang L, Liu M, Zhang Z. Theranostics. 2012;2:283–294. doi: 10.7150/thno.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ordikhani F, Farani MR, Dehghani M, Tamjid E, Simchi A. Carbon. 2015;84:91–102. [Google Scholar]
- 34.Rahmanian N, Eskandani M, Barar J, Omidi Y. J Drug Target. 2016:20. doi: 10.1080/1061186X.2016.1238475. [DOI] [PubMed] [Google Scholar]
- 35.Giani G, Fedi S, Barbucci R. Polymers. 2012;4:1157–1169. [Google Scholar]
- 36.Uchida M, Terashima M, Cunningham CH, Suzuki Y, Willits DA, Willis AF, Yang PC, Tsao PS, McConnell MV, Young MJ. Magnetic Resonance in Medicine. 2008;60:1073–1081. doi: 10.1002/mrm.21761. [DOI] [PubMed] [Google Scholar]
- 37.Heywood BR. Nature. 1991:349. [Google Scholar]
- 38.Zafar N, Fessi H, Elaissari A. International journal of pharmaceutics. 2014;461:351–366. doi: 10.1016/j.ijpharm.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Corsi F, Mazzucchelli S. Therapeutic Delivery. 2016;7:149–151. doi: 10.4155/tde.15.95. [DOI] [PubMed] [Google Scholar]
- 40.Kedersha NL, Miquel M-C, Bittner D, Rome LH. The Journal of cell biology. 1990;110:895–901. doi: 10.1083/jcb.110.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka H, Kato K, Yamashita E, Sumizawa T, Zhou Y, Yao M, Iwasaki K, Yoshimura M, Tsukihara T. Science. 2009;323:384–388. doi: 10.1126/science.1164975. [DOI] [PubMed] [Google Scholar]
- 42.Hu Y, Wang J, Zhi Z, Jiang T, Wang S. Journal of colloid and interface science. 2011;363:410–417. doi: 10.1016/j.jcis.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 43.Hu Y, Zhi Z, Zhao Q, Wu C, Zhao P, Jiang H, Jiang T, Wang S. Microporous and Mesoporous Materials. 2012;147:94–101. [Google Scholar]
- 44.Nadrah P, Porta F, Planinšek O, Kros A, Gaberšček M. Physical Chemistry Chemical Physics. 2013;15:10740–10748. doi: 10.1039/c3cp44614j. [DOI] [PubMed] [Google Scholar]
- 45.Sun L, Wang Y, Jiang T, Zheng X, Zhang J, Sun J, Sun C, Wang S. ACS applied materials & interfaces. 2012;5:103–113. doi: 10.1021/am302246s. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Wang J, Bai X, Jiang T, Zhang Q, Wang S. Molecular pharmaceutics. 2012;9:505–513. doi: 10.1021/mp200287c. [DOI] [PubMed] [Google Scholar]
- 47.Hudson SP, Padera RF, Langer R, Kohane DS. Biomaterials. 2008;29:4045–4055. doi: 10.1016/j.biomaterials.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Zhao Q, Hu Y, Sun L, Bai L, Jiang T, Wang S. International journal of nanomedicine. 2013;8:4015. doi: 10.2147/IJN.S52605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.GarciÃÅa A, Colilla M, Izquierdo-Barba I, Vallet-RegiÃÅ M. Chemistry of Materials. 2009;21:4135–4145. [Google Scholar]
- 50.He Q, Shi J, Zhu M, Chen Y, Chen F. Microporous and Mesoporous Materials. 2010;131:314–320. [Google Scholar]
- 51.Colilla M, Manzano M, Izquierdo-Barba I, Vallet-RegiÃÅ M, Boissiére C. d., Sanchez C. m. Chemistry of Materials. 2009;22:1821–1830. [Google Scholar]
- 52.Lu J, Liong M, Li Z, Zink JI, Tamanoi F. Small. 2010;6:1794–1805. doi: 10.1002/smll.201000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Souris JS, Lee C-H, Cheng S-H, Chen C-T, Yang C-S, Ja-an AH, Mou C-Y, Lo L-W. Biomaterials. 2010;31:5564–5574. doi: 10.1016/j.biomaterials.2010.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J, Kim HS, Lee N, Kim T, Kim H, Yu T, Song IC, Moon WK, Hyeon T. Angewandte Chemie International Edition. 2008;47:8438–8441. doi: 10.1002/anie.200802469. [DOI] [PubMed] [Google Scholar]
- 55.Yang P, Gai S, Lin J. Chemical Society reviews. 2012;41:3679–3698. doi: 10.1039/c2cs15308d. [DOI] [PubMed] [Google Scholar]
- 56.Wu H, Zhang S, Zhang J, Liu G, Shi J, Zhang L, Cui X, Ruan M, He Q, Bu W. Advanced Functional Materials. 2011;21:1850–1862. [Google Scholar]
- 57.Doll TA, Raman S, Dey R, Burkhard P. Journal of The Royal Society Interface. 2013;10:20120740. doi: 10.1098/rsif.2012.0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang G, Gao W, Zhang X, Mei X. Scientific Reports. 2016:6. doi: 10.1038/srep28258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen J, Saeki F, Wiley BJ, Cang H, Cobb MJ, Li Z-Y, Au L, Zhang H, Kimmey MB, Li X. Nano letters. 2005;5:473–477. doi: 10.1021/nl047950t. [DOI] [PubMed] [Google Scholar]
- 60.Wang Z, Chen Z, Liu Z, Shi P, Dong K, Ju E, Ren J, Qu X. Biomaterials. 2014;35:9678–9688. doi: 10.1016/j.biomaterials.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 61.Murata M, Narahara S, Kawano T, Hamano N, Piao JS, Kang J-H, Ohuchida K, Murakami T, Hashizume M. Molecular pharmaceutics. 2015;12:1422–1430. doi: 10.1021/mp5007129. [DOI] [PubMed] [Google Scholar]
- 62.Fang J, Qin H, Nakamura H, Tsukigawa K, Shin T, Maeda H. Cancer science. 2012;103:535–541. doi: 10.1111/j.1349-7006.2011.02178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee EJ, Lee SJ, Kang YS, Ryu JH, Kwon KC, Jo E, Yhee JY, Kwon IC, Kim K, Lee J. Advanced Functional Materials. 2015;25:1279–1286. [Google Scholar]
- 64.Tosi G, Belletti D, Pederzoli F, Ruozi B. Taylor & Francis; 2016. [DOI] [PubMed] [Google Scholar]
- 65.Bertrand O, Poggi E, Gohy J.-F. o., Fustin C-A. Macromolecules. 2013;47:183–190. [Google Scholar]
- 66.Qie Y, Yuan H, von Roemeling CA, Chen Y, Liu X, Shih KD, Knight JA, Tun HW, Wharen RE, Jiang W. Scientific reports. 2016:6. doi: 10.1038/srep30663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liang L, Astruc D. Coordination Chemistry Reviews. 2011;255:2933–2945. [Google Scholar]
- 68.Molino NM, Wang S-W. Current opinion in biotechnology. 2014;28:75–82. doi: 10.1016/j.copbio.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li F, Chen Y, Chen H, He W, Zhang Z-P, Zhang X-E, Wang Q. Journal of the American Chemical Society. 2011;133:20040–20043. doi: 10.1021/ja207276g. [DOI] [PubMed] [Google Scholar]
- 70.Schoonen L, van Hest JC. Nanoscale. 2014;6:7124–7141. doi: 10.1039/c4nr00915k. [DOI] [PubMed] [Google Scholar]
- 71.Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Chemical reviews. 2005;105:1103–1170. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 72.Wang T, Chai F, Fu Q, Zhang L, Liu H, Li L, Liao Y, Su Z, Wang C, Duan B. Journal of Materials Chemistry. 2011;21:5299–5306. [Google Scholar]
- 73.Wang Y, Liu Y, Luehmann H, Xia X, Brown P, Jarreau C, Welch M, Xia Y. Acs Nano. 2012;6:5880–5888. doi: 10.1021/nn300464r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang W, Chen C, Li X, Wang S, Luo X. Chemical Communications. 2015;51:9109–9112. doi: 10.1039/c5cc02452h. [DOI] [PubMed] [Google Scholar]
- 75.Chen J, Yang M, Zhang Q, Cho EC, Cobley CM, Kim C, Glaus C, Wang LV, Welch MJ, Xia Y. Advanced Functional Materials. 2010;20:3684–3694. doi: 10.1002/adfm.201001329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao D, Tan S, Yuan D, Lu W, Rezenom YH, Jiang H, Wang LQ, Zhou HC. Advanced Materials. 2011;23:90–93. doi: 10.1002/adma.201003012. [DOI] [PubMed] [Google Scholar]
- 77.Yoo J-W, Irvine DJ, Discher DE, Mitragotri S. Nature reviews Drug discovery. 2011;10:521–535. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- 78.MaHam A, Tang Z, Wu H, Wang J, Lin Y. Small. 2009;5:1706–1721. doi: 10.1002/smll.200801602. [DOI] [PubMed] [Google Scholar]
- 79.Chang L, Wang G, Jia T, Zhang L, Li Y, Han Y, Zhang K, Lin G, Zhang R, Li J, Wang L. Armored long non-coding RNA MEG3 targeting EGFR based on recombinant MS2 bacteriophage virus-like particles against hepatocellular carcinoma. 2016 doi: 10.18632/oncotarget.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ashley CE, Carnes EC, Phillips GK, Durfee PN, Buley MD, Lino CA, Padilla DP, Phillips B, Carter MB, Willman CL. ACS nano. 2011;5:5729–5745. doi: 10.1021/nn201397z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhen Z, Tang W, Chen H, Lin X, Todd T, Wang G, Cowger T, Chen X, Xie J. ACS nano. 2013;7:4830–4837. doi: 10.1021/nn305791q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhen Z, Tang W, Guo C, Chen H, Lin X, Liu G, Fei B, Chen X, Xu B, Xie J. ACS nano. 2013;7:6988–6996. doi: 10.1021/nn402199g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang P, Rong P, Jin A, Yan X, Zhang MG, Lin J, Hu H, Wang Z, Yue X, Li W. Advanced Materials. 2014;26:6401–6408. doi: 10.1002/adma.201400914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Truffi M, Fiandra L, Sorrentino L, Monieri M, Corsi F, Mazzucchelli S. Pharmacological research. 2016;107:57–65. doi: 10.1016/j.phrs.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 85.Bova MP, Huang Q, Ding L, Horwitz J. Journal of Biological Chemistry. 2002;277:38468–38475. doi: 10.1074/jbc.M205594200. [DOI] [PubMed] [Google Scholar]
- 86.Toita R, Murata M, Tabata S, Abe K, Narahara S, Piao JS, Kang J-H, Hashizume M. Bioconjugate chemistry. 2012;23:1494–1501. doi: 10.1021/bc300015f. [DOI] [PubMed] [Google Scholar]
- 87.Wang T, Chai F, Fu Q, Zhang L, Liu H, Li L, Liao Y, Su Z, Wang C, Duan B, Ren D. Journal of Materials Chemistry. 2011;21:5299–5306. [Google Scholar]
- 88.Li W, Cai X, Kim C, Sun G, Zhang Y, Deng R, Yang M, Chen J, Achilefu S, Wang LV. Nanoscale. 2011;3:1724–1730. doi: 10.1039/c0nr00932f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Field LD, Delehanty JB, Chen Y, Medintz IL. Accounts of chemical research. 2015;48:1380–1390. doi: 10.1021/ar500449v. [DOI] [PubMed] [Google Scholar]
- 90.Steichen SD, Caldorera-Moore M, Peppas NA. European Journal of Pharmaceutical Sciences. 2013;48:416–427. doi: 10.1016/j.ejps.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Albanese A, Tang PS, Chan WC. Annual review of biomedical engineering. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 92.Rampersaud S, Fang J, Wei Z, Fabijanic K, Silver S, Jaikaran T, Ruiz Y, Houssou M, Yin Z, Zheng S. Nano Letters. 2016 doi: 10.1021/acs.nanolett.6b02577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Farokhzad O, Langer R. [Google Scholar]
- 94.Chen J, Glaus C, Laforest R, Zhang Q, Yang M, Gidding M, Welch MJ, Xia Y. Small. 2010;6:811–817. doi: 10.1002/smll.200902216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Steinmetz NF, Manchester M. Biomacromolecules. 2009;10:784–792. doi: 10.1021/bm8012742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sampath S, Carrico C, Janes J, Gurumoorthy S, Gibson C, Melcher M, Chitnis CE, Wang R, Schief WR, Smith JD. 2013 doi: 10.1371/journal.ppat.1003420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cho SK, Kwon YJ. Biomaterials. 2012;33:3316–3323. doi: 10.1016/j.biomaterials.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 98.Hu F, Zhang Y, Chen G, Li C, Wang Q. Small. 2015;11:985–993. doi: 10.1002/smll.201401360. [DOI] [PubMed] [Google Scholar]
- 99.Rao L, Xu J-H, Cai B, Liu H, Li M, Jia Y, Xiao L, Guo S-S, Liu W, Zhao X-Z. Nanotechnology. 2016;27:085106. doi: 10.1088/0957-4484/27/8/085106. [DOI] [PubMed] [Google Scholar]
- 100.Piao J-G, Wang L, Gao F, You Y-Z, Xiong Y, Yang L. ACS nano. 2014;8:10414–10425. doi: 10.1021/nn503779d. [DOI] [PubMed] [Google Scholar]
- 101.Vindigni G, Raniolo S, Ottaviani A, Falconi M, Franch O, Knudsen BR, Desideri A, Biocca S. ACS Nano. 2016;10:5971–5979. doi: 10.1021/acsnano.6b01402. [DOI] [PubMed] [Google Scholar]
- 102.Dong D-W, Xiang B, Gao W, Yang Z-Z, Li J-Q, Qi X-R. Biomaterials. 2013;34:4849–4859. doi: 10.1016/j.biomaterials.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 103.Flenniken ML, Willits DA, Harmsen AL, Liepold LO, Harmsen AG, Young MJ, Douglas T. Chemistry & biology. 2006;13:161–170. doi: 10.1016/j.chembiol.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 104.Yun Y, Cho YW, Park K. Advanced drug delivery reviews. 2013;65:822–832. doi: 10.1016/j.addr.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jeon JO, Kim S, Choi E, Shin K, Cha K, So I-S, Kim S-J, Jun E, Kim D, Ahn HJ. ACS nano. 2013;7:7462–7471. doi: 10.1021/nn403184u. [DOI] [PubMed] [Google Scholar]
- 106.Steinmetz NF, Ablack AL, Hickey JL, Ablack J, Manocha B, Mymryk JS, Luyt LG, Lewis JD. Small. 2011;7:1664–1672. doi: 10.1002/smll.201000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wei B, Wei Y, Zhang K, Wang J, Xu R, Zhan S, Lin G, Wang W, Liu M, Wang L. Biomedicine & Pharmacotherapy. 2009;63:313–318. doi: 10.1016/j.biopha.2008.07.086. [DOI] [PubMed] [Google Scholar]
- 108.Deshayes S, Gref R. Nanomedicine. 2014;9:1545–1564. doi: 10.2217/nnm.14.67. [DOI] [PubMed] [Google Scholar]
- 109.Brunel FM, Lewis JD, Destito G, Steinmetz NF, Manchester M, Stuhlmann H, Dawson PE. Nano letters. 2010;10:1093–1097. doi: 10.1021/nl1002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ding M, Song N, He X, Li J, Zhou L, Tan H, Fu Q, Gu Q. Acs Nano. 2013;7:1918–1928. doi: 10.1021/nn4002769. [DOI] [PubMed] [Google Scholar]
- 111.Stephanopoulos N, Tong GJ, Hsiao SC, Francis MB. ACS nano. 2010;4:6014–6020. doi: 10.1021/nn1014769. [DOI] [PubMed] [Google Scholar]
- 112.Zhang Z, Lee SH, Feng S-S. Biomaterials. 2007;28:1889–1899. doi: 10.1016/j.biomaterials.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 113.Ulbrich K, Hekmatara T, Herbert E, Kreuter J. European Journal of Pharmaceutics and Biopharmaceutics. 2009;71:251–256. doi: 10.1016/j.ejpb.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 114.Shan L, Cui S, Du C, Wan S, Qian Z, Achilefu S, Gu Y. Biomaterials. 2012;33:146–162. doi: 10.1016/j.biomaterials.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 115.Destito G, Yeh R, Rae CS, Finn M, Manchester M. Chemistry & biology. 2007;14:1152–1162. doi: 10.1016/j.chembiol.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu M, Sherwin T, Brown WL, Stockley PG. Nanomedicine: Nanotechnology, Biology and Medicine. 2005;1:67–76. doi: 10.1016/j.nano.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 117.Li X, Qiu L, Zhu P, Tao X, Imanaka T, Zhao J, Huang Y, Tu Y, Cao X. Small. 2012;8:2505–2514. doi: 10.1002/smll.201200066. [DOI] [PubMed] [Google Scholar]
- 118.Wang Y, Xu J, Xia X, Yang M, Vangveravong S, Chen J, Mach RH, Xia Y. Nanoscale. 2012;4:421–424. doi: 10.1039/c1nr11469g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liang M, Fan K, Zhou M, Duan D, Zheng J, Yang D, Feng J, Yan X. Proceedings of the National Academy of Sciences. 2014;111:14900–14905. doi: 10.1073/pnas.1407808111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xia D, Tao J, He Y, Zhu Q, Chen D, Yu M, Cui F, Gan Y. Biomaterials. 2015;37:320–332. doi: 10.1016/j.biomaterials.2014.10.038. [DOI] [PubMed] [Google Scholar]
- 121.Wegner SV, Schenk FC, Witzel S, Bialas F, Spatz JP. Macromolecules. 2016 [Google Scholar]
- 122.Wegner SV, Sentürk OI, Spatz JP. Scientific reports. 2015:5. doi: 10.1038/srep18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Karimi M, Sahandi Zangabad P, Ghasemi A, Amiri M, Bahrami M, Malekzad H, Ghahramanzadeh Asl H, Mahdieh Z, Bozorgomid M, Ghasemi A, Rahmani Taji Boyuk MR, Hamblin MR. ACS applied materials & interfaces. 2016 doi: 10.1021/acsami.6b00371. DOI: 10.1021/acsami.6b00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karimi M, Zangabad PS, Ghasemi A, Hamblin MR. Smart Internal Stimulus-Responsive Nanocarriers for Drug and Gene Delivery. IOP Concise Physics. 2015 [Google Scholar]
- 125.Karimi M, Zangabad PS, Ghasemi A, Hamblin MR. Smart External Stimulus-Responsive Nanocarriers for Drug and Gene Delivery. Morgan & Claypool Publishers; 2015. [Google Scholar]
- 126.Karimi M, Zangabad PS, Ghasemi A, Hamblin MR. Smart Internal Stimulus-Responsive Nanocarriers for Drug and Gene Delivery. Morgan & Claypool Publishers; 2015. [Google Scholar]
- 127.Moon GD, Choi S-W, Cai X, Li W, Cho EC, Jeong U, Wang LV, Xia Y. Journal of the American Chemical Society. 2011;133:4762–4765. doi: 10.1021/ja200894u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Choi S-W, Zhang Y, Xia Y. Angewandte Chemie International Edition. 2010;49:7904–7908. doi: 10.1002/anie.201004057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lavelle L, Michel J-P, Gingery M. Journal of virological methods. 2007;146:311–316. doi: 10.1016/j.jviromet.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 130.Kim M, Rho Y, Jin KS, Ahn B, Jung S, Kim H, Ree M. Biomacromolecules. 2011;12:1629–1640. doi: 10.1021/bm200026v. [DOI] [PubMed] [Google Scholar]
- 131.Powell JJ, Bruggraber SFA, Faria N, Poots LK, Hondow N, Pennycook TJ, Latunde-Dada GO, Simpson RJ, Brown AP, Pereira DIA. Nanomedicine: Nanotechnology, Biology and Medicine. 2014;10:1529–1538. doi: 10.1016/j.nano.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fracasso G, Falvo E, Colotti G, Fazi F, Ingegnere T, Amalfitano A, Doglietto GB, Alfieri S, Boffi A, Morea V. Journal of Controlled Release. 2016;239:10–18. doi: 10.1016/j.jconrel.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 133.Li X, Zhang Y, Chen H, Sun J, Feng F. ACS applied materials & interfaces. 2016;8:22756–22761. doi: 10.1021/acsami.6b07038. [DOI] [PubMed] [Google Scholar]
- 134.Rahmanpour R, Bugg TDH. FEBS Journal. 2013;280:2097–2104. doi: 10.1111/febs.12234. [DOI] [PubMed] [Google Scholar]
- 135.Juul S, Iacovelli F, Falconi M, Kragh SL, Christensen B, Frøhlich R, Franch O, Kristoffersen EL, Stougaard M, Leong KW. ACS nano. 2013;7:9724–9734. doi: 10.1021/nn4030543. [DOI] [PubMed] [Google Scholar]
- 136.Liu Z, Tian C, Yu J, Li Y, Jiang W, Mao C. Journal of the American Chemical Society. 2015;137:1730–1733. doi: 10.1021/ja5101307. [DOI] [PubMed] [Google Scholar]
- 137.Zhao L, Peng J, Huang Q, Li C, Chen M, Sun Y, Lin Q, Zhu L, Li F. Advanced Functional Materials. 2014;24:363–371. [Google Scholar]
- 138.Peng T, Lee H, Lim S. Biomaterials Science. 2015;3:627–635. doi: 10.1039/c4bm00313f. [DOI] [PubMed] [Google Scholar]
- 139.Niikura K, Sugimura N, Musashi Y, Mikuni S, Matsuo Y, Kobayashi S, Nagakawa K, Takahara S, Takeuchi C, Sawa H. Molecular BioSystems. 2013;9:501–507. doi: 10.1039/c2mb25420d. [DOI] [PubMed] [Google Scholar]
- 140.Shi P, Li M, Ren J, Qu X. Advanced Functional Materials. 2013;23:5412–5419. doi: 10.1002/adfm.201202666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhou L, Liu J-H, Ma F, Wei S-H, Feng Y-Y, Zhou J-H, Yu B-Y, Shen J. Biomedical microdevices. 2010;12:655–663. doi: 10.1007/s10544-010-9418-1. [DOI] [PubMed] [Google Scholar]
- 142.Aljabali AA, Shukla S, Lomonossoff GP, Steinmetz NF, Evans DJ. Molecular pharmaceutics. 2012;10:3–10. doi: 10.1021/mp3002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu L, Zhang J, Watanabe W. Advanced drug delivery reviews. 2011;63:456–469. doi: 10.1016/j.addr.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 144.Xing R, Wang X, Zhang C, Zhang Y, Wang Q, Yang Z, Guo Z. Journal of inorganic biochemistry. 2009;103:1039–1044. doi: 10.1016/j.jinorgbio.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 145.Cutrin JC, Crich SG, Burghelea D, Dastrù W, Aime S. Molecular pharmaceutics. 2013;10:2079–2085. doi: 10.1021/mp3006177. [DOI] [PubMed] [Google Scholar]
- 146.Shariatinia Z, Shahidi S. Journal of Molecular Graphics and Modelling. 2014;52:71–81. doi: 10.1016/j.jmgm.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 147.Uchida M, Kang S, Reichhardt C, Harlen K, Douglas T. Biochimica et Biophysica Acta (BBA)-General Subjects. 2010;1800:834–845. doi: 10.1016/j.bbagen.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zeltins A. Molecular biotechnology. 2013;53:92–107. doi: 10.1007/s12033-012-9598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhao Q, Chen W, Chen Y, Zhang L, Zhang J, Zhang Z. Bioconjugate chemistry. 2011;22:346–352. doi: 10.1021/bc1002532. [DOI] [PubMed] [Google Scholar]
- 150.Li D, Zhou W, Landskron K, Sato S, Kiely CJ, Fujita M, Liu T. Angewandte Chemie International Edition. 2011;50:5182–5187. doi: 10.1002/anie.201007829. [DOI] [PubMed] [Google Scholar]
- 151.Ni R, Zhou J, Hossain N, Chau Y. Advanced Drug Delivery Reviews. 2016;106(Part A):3–26. doi: 10.1016/j.addr.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 152.Zdanowicz M, Chroboczek J. Acta Biochimica Polonica. 2016;3:469–473. doi: 10.18388/abp.2016_1275. [DOI] [PubMed] [Google Scholar]
- 153.Stanley S. Current opinion in biotechnology. 2014;28:69–74. doi: 10.1016/j.copbio.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 154.Mueller A, Kadri A, Jeske H, Wege C. Journal of virological methods. 2010;166:77–85. doi: 10.1016/j.jviromet.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 155.Li T, Johnson JE, Thomas G. Biophysical journal. 1993;65:1963–1972. doi: 10.1016/S0006-3495(93)81272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schickli JH, Whitacre DC, Tang RS, Kaur J, Lawlor H, Peters CJ, Jones JE, Peterson DL, McCarthy MP, Van Nest G, Milich DR. The Journal of Clinical Investigation. 2015;125:1637–1647. doi: 10.1172/JCI78450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee LA, Niu Z, Wang Q. Nano Research. 2009;2:349–364. [Google Scholar]
- 158.Uchida M, Klem MT, Allen M, Suci P, Flenniken M, Gillitzer E, Varpness Z, Liepold LO, Young M, Douglas T. Advanced Materials. 2007;19:1025–1042. [Google Scholar]
- 159.Kovacs J. M. H. Ernest W., Romanini Dante W., Holder Patrick G., Berry Katherine E., Francis Matthew B. 2007 doi: 10.1021/bc070006e. [DOI] [PubMed] [Google Scholar]
- 160.Stefania Galdiero AF, Vitiello Mariateresa, Grieco Paolo, Caraglia Michele, Morellia Giancarlo, Galdiero Massimiliano. 2014:20. doi: 10.1002/psc.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.P. I. Slovak Academy of Sciences, Institute of Measurement Science . Dúbravská cesta 9; Bratislava, Slovakia: 2014. [Google Scholar]
- 162.Schwarz B, Douglas T. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2015;7:722–735. doi: 10.1002/wnan.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Khudyakov Y, Pumpens P, Schwarz B, Patterson D, Douglas T. Viral Nanotechnology. CRC Press; 2015. pp. 371–382. [Google Scholar]
- 164.Loo L, Guenther RH, Basnayake VR, Lommel SA, Franzen S. Journal of the American Chemical Society. 2006;128:4502–4503. doi: 10.1021/ja057332u. [DOI] [PubMed] [Google Scholar]
- 165.Erdemci-Tandogan G, Wagner J, Van Der Schoot P, Podgornik R, Zandi R. Physical Review E. 2014;89:032707. doi: 10.1103/PhysRevE.89.032707. [DOI] [PubMed] [Google Scholar]
- 166.Hubbell JA, Chilkoti A. Science. 2012;337:303–305. doi: 10.1126/science.1219657. [DOI] [PubMed] [Google Scholar]
- 167.Lee LA, Wang Q. Nanomedicine: Nanotechnology, Biology and Medicine. 2006;2:137–149. doi: 10.1016/j.nano.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 168.Ramqvist T, Andreasson K, Dalianis T. 2007 [Google Scholar]
- 169.Douglas T, Strable E, Willits D, Aitouchen A, Libera M, Young M. Advanced materials. 2002;14:415–418. [Google Scholar]
- 170.Wang Q, Lin T, Tang L, Johnson JE, Finn M. Angewandte Chemie International Edition. 2002;41:459–462. doi: 10.1002/1521-3773(20020201)41:3<459::aid-anie459>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 171.Ren D, Kratz F, Wang SW. Small. 2011;7:1051–1060. doi: 10.1002/smll.201002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pan Y, Zhang Y, Jia T, Zhang K, Li J, Wang L. FEBS Journal. 2012;279:1198–1208. doi: 10.1111/j.1742-4658.2012.08512.x. [DOI] [PubMed] [Google Scholar]
- 173.Lockney DM, Guenther RN, Loo L, Overton W, Antonelli R, Clark J, Hu M, Luft C, Lommel SA, Franzen S. Bioconjugate chemistry. 2010;22:67–73. doi: 10.1021/bc100361z. [DOI] [PubMed] [Google Scholar]
- 174.Aanei IL, ElSohly AM, Farkas ME, Netirojjanakul C, Regan M, Taylor Murphy S, O'Neil JP, Seo Y, Francis MB. Molecular Pharmaceutics. 2016;13:3764–3772. doi: 10.1021/acs.molpharmaceut.6b00566. [DOI] [PubMed] [Google Scholar]
- 175.Kaiser CR, Flenniken ML, Gillitzer E, Harmsen AL, Harmsen AG, Jutila MA, Douglas T, Young MJ. International journal of nanomedicine. 2007;2:715. [PMC free article] [PubMed] [Google Scholar]
- 176.Singh P, Prasuhn D, Yeh RM, Destito G, Rae CS, Osborn K, Finn M, Manchester M. Journal of controlled release. 2007;120:41–50. doi: 10.1016/j.jconrel.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shoji Y, Prokhnevsky A, Leffet B, Vetter N, Tottey S, Satinover S, Musiychuk K, Shamloul M, Norikane J, Jones RM. Human vaccines & immunotherapeutics. 2015;11:118–123. doi: 10.4161/hv.34365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Klem MT, Willits D, Young M, Douglas T. Journal of the American Chemical Society. 2003;125:10806–10807. doi: 10.1021/ja0363718. [DOI] [PubMed] [Google Scholar]
- 179.Kwak M, Herrmann A. Chemical Society Reviews. 2011;40:5745–5755. doi: 10.1039/c1cs15138j. [DOI] [PubMed] [Google Scholar]
- 180.Kwak M, Minten IJ, Anaya D-M, Musser AJ, Brasch M, Nolte RJ, Müllen K, Cornelissen JJ, Herrmann A. Journal of the American Chemical Society. 2010;132:7834–7835. doi: 10.1021/ja101444j. [DOI] [PubMed] [Google Scholar]
- 181.Schwarz B, Madden P, Avera J, Gordon B, Larson K, Miettinen HM, Uchida M, LaFrance B, Basu G, Rynda-Apple A. ACS Nano. 2015;9:9134–9147. doi: 10.1021/acsnano.5b03360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ko E-J, Kwon Y-M, Lee JS, Hwang HS, Yoo S-E, Lee Y-N, Lee Y-T, Kim M-C, Cho MK, Lee YR. Nanomedicine: Nanotechnology, Biology and Medicine. 2015;11:99–108. doi: 10.1016/j.nano.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lua LH, Connors NK, Sainsbury F, Chuan YP, Wibowo N, Middelberg AP. Biotechnology and bioengineering. 2014;111:425–440. doi: 10.1002/bit.25159. [DOI] [PubMed] [Google Scholar]
- 184.Zhu F-C, Zhang J, Zhang X-F, Zhou C, Wang Z-Z, Huang S-J, Wang H, Yang C-L, Jiang H-M, Cai J-P. The Lancet. 2010;376:895–902. doi: 10.1016/S0140-6736(10)61030-6. [DOI] [PubMed] [Google Scholar]
- 185.Wen AM, Ryan MJ, Yang AC, Breitenkamp K, Pokorski JK, Steinmetz NF. Chemical Communications. 2012;48:9044–9046. doi: 10.1039/c2cc34695h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sánchez-Sánchez L, Tapia-Moreno A, Juarez-Moreno K, Patterson DP, Cadena-Nava RD, Douglas T, Vazquez-Duhalt R. Journal of nanobiotechnology. 2015;13:1. doi: 10.1186/s12951-015-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Biochimica et Biophysica Acta (BBA)-General Subjects. 2010;1800:760–769. doi: 10.1016/j.bbagen.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Harrison PM, Arosio P. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 189.Meyron-Holtz EG, Moshe-Belizowski S, Cohen LA. Journal of neural transmission. 2011;118:337–347. doi: 10.1007/s00702-011-0582-0. [DOI] [PubMed] [Google Scholar]
- 190.Belletti D, Pederzoli F, Forni F, Vandelli MA, Tosi G, Ruozi B. Expert Opinion on Drug Delivery. 2016 doi: 10.1080/17425247.2017.1243528. [DOI] [PubMed] [Google Scholar]
- 191.Theil EC. Annual review of biochemistry. 1987;56:289–315. doi: 10.1146/annurev.bi.56.070187.001445. [DOI] [PubMed] [Google Scholar]
- 192.Zhen Z, Tang W, Todd T, Xie J. Expert opinion on drug delivery. 2014;11:1913–1922. doi: 10.1517/17425247.2014.941354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chen H, Zhang S, Xu C, Zhao G. Chemical Communications. 2016;52:7402–7405. doi: 10.1039/c6cc03108k. [DOI] [PubMed] [Google Scholar]
- 194.Yang R, Gao Y, Zhou Z, Strappe P, Blanchard C. RSC Advances. 2016;6:35267–35279. [Google Scholar]
- 195.Powell JJ, Bruggraber SF, Faria N, Poots LK, Hondow N, Pennycook TJ, Latunde - Dada GO, Simpson RJ, Brown AP, Pereira DI. Nanomedicine: Nanotechnology, Biology and Medicine. 2014;10:1529–1538. doi: 10.1016/j.nano.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Han J-A, Kang YJ, Shin C, Ra J-S, Shin H-H, Hong SY, Do Y, Kang S. Nanomedicine: Nanotechnology, Biology and Medicine. 2014;10:561–569. doi: 10.1016/j.nano.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 197.Lei Y, Hamada Y, Li J, Cong L, Wang N, Li Y, Zheng W, Jiang X. Journal of Controlled Release. 2016;232:131–142. doi: 10.1016/j.jconrel.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 198.Kuruppu AI, Zhang L, Collins H, Turyanska L, Thomas NR, Bradshaw TD. Advanced healthcare materials. 2015;4:2816–2821. doi: 10.1002/adhm.201500389. [DOI] [PubMed] [Google Scholar]
- 199.Luo Y, Wang X, Du D, Lin Y. Biomaterials science. 2015;3:1386–1394. doi: 10.1039/c5bm00067j. [DOI] [PubMed] [Google Scholar]
- 200.Bellini M, Mazzucchelli S, Galbiati E, Sommaruga S, Fiandra L, Truffi M, Rizzuto MA, Colombo M, Tortora P, Corsi F, Prosperi D. Journal of Controlled Release. 2014;196:184–196. doi: 10.1016/j.jconrel.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 201.Zhang L, Li L, Di Penta A, Carmona U, Yang F, Schöps R, Brandsch M, Zugaza JL, Knez M. Advanced Healthcare Materials. 2015;4:1305–1310. doi: 10.1002/adhm.201500226. [DOI] [PubMed] [Google Scholar]
- 202.Fan K, Cao C, Pan Y, Lu D, Yang D, Feng J, Song L, Liang M, Yan X. Nature nanotechnology. 2012;7:459–464. doi: 10.1038/nnano.2012.90. [DOI] [PubMed] [Google Scholar]
- 203.Bennett KM, Bertram JF, Beeman SC, Gretz N. American Journal of Physiology-Renal Physiology. 2013;304:F1252–F1257. doi: 10.1152/ajprenal.00714.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cao C, Wang X, Cai Y, Sun L, Tian L, Wu H, He X, Lei H, Liu W, Chen G. Advanced Materials. 2014;26:2566–2571. doi: 10.1002/adma.201304544. [DOI] [PubMed] [Google Scholar]
- 205.Wang Z, Huang P, Jacobson O, Wang Z, Liu Y, Lin L, Lin J, Lu N, Zhang H, Tian R. ACS nano. 2016;10:3453–3460. doi: 10.1021/acsnano.5b07521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yang M, Fan Q, Zhang R, Cheng K, Yan J, Pan D, Ma X, Lu A, Cheng Z. Biomaterials. 2015;69:30–37. doi: 10.1016/j.biomaterials.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hainfeld JF. Proceedings of the National Academy of Sciences. 1992;89:11064–11068. doi: 10.1073/pnas.89.22.11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yao H, Su L, Zeng M, Cao L, Zhao W, Chen C, Du B, Zhou J. International Journal of Nanomedicine. 2016;11:4423. doi: 10.2147/IJN.S108039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mymrikov EV, Seit-Nebi AS, Gusev NB. Physiological reviews. 2011;91:1123–1159. doi: 10.1152/physrev.00023.2010. [DOI] [PubMed] [Google Scholar]
- 210.Deka K, Singh A, Chakraborty S, Mukhopadhyay R, Saha S. Cell Death Discovery. 2016:2. doi: 10.1038/cddiscovery.2016.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Basha E, O'Neill H, Vierling E. Trends in biochemical sciences. 2012;37:106–117. doi: 10.1016/j.tibs.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ehrnsperger M, Gräber S, Gaestel M, Buchner J. The EMBO Journal. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lee GJ, Roseman AM, Saibil HR, Vierling E. The EMBO Journal. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fernández-Fernández MR, Sot B, Valpuesta JM. Nanotechnology. 2016;27:324004. doi: 10.1088/0957-4484/27/32/324004. [DOI] [PubMed] [Google Scholar]
- 215.Sun Y, MacRae TH. Cellular and Molecular Life Sciences CMLS. 2005;62:2460–2476. doi: 10.1007/s00018-005-5190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Benjamin IJ, McMillan DR. Circulation research. 1998;83:117–132. doi: 10.1161/01.res.83.2.117. [DOI] [PubMed] [Google Scholar]
- 217.Haslbeck M, Braun N, Stromer T, Richter B, Model N, Weinkauf S, Buchner J. The EMBO journal. 2004;23:638–649. doi: 10.1038/sj.emboj.7600080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kappé G, Franck E, Verschuure P, Boelens WC, Leunissen JA, de Jong WW. Cell stress & chaperones. 2003;8:53–61. doi: 10.1379/1466-1268(2003)8<53:thgecs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Elicker KS, Hutson LD. Gene. 2007;403:60–69. doi: 10.1016/j.gene.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Siddique M, Gernhard S, von Koskull-Döring P, Vierling E, Scharf K-D. Cell Stress and Chaperones. 2008;13:183–197. doi: 10.1007/s12192-008-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Horwitz J. Experimental eye research. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 222.Kim KK, Kim R, Kim S-H. Nature. 1998;394:595–599. doi: 10.1038/29106. [DOI] [PubMed] [Google Scholar]
- 223.Flenniken ML, Willits DA, Brumfield S, Young MJ, Douglas T. Nano letters. 2003;3:1573–1576. [Google Scholar]
- 224.Ciocca DR, Calderwood SK. Cell stress & chaperones. 2005;10:86. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Park TS, Kim H-R, Koh JS, Jang SH, Hwang YI, Yoon HI, Chung J-H, Kim CH, Kim S-S, Kim WS. Lung Cancer. 2014;86:262–267. doi: 10.1016/j.lungcan.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 226.Chen S-F, Nieh S, Jao S-W, Liu C-L, Wu C-H, Chang Y-C, Yang C-Y, Lin Y-S. PLoS One. 2012;7:e49275. doi: 10.1371/journal.pone.0049275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Brayden DJ. Drug discovery today. 2003;8:976–978. doi: 10.1016/s1359-6446(03)02874-5. [DOI] [PubMed] [Google Scholar]
- 228.Murata M, Narahara S, Umezaki K, Toita R, Tabata S, Piao JS, Abe K, Kang J-H, Ohuchida K, Cui L. International journal of nanomedicine. 2012;7:4353. doi: 10.2147/IJN.S31365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kim DR, Lee I, Ha SC, Kim KK. Biochemical and biophysical research communications. 2003;307:991–998. doi: 10.1016/s0006-291x(03)01302-0. [DOI] [PubMed] [Google Scholar]
- 230.Kim R, Kim KK, Yokota H, Kim S-H. Proceedings of the national academy of sciences. 1998;95:9129–9133. doi: 10.1073/pnas.95.16.9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nakamoto H, Vigh L. Cellular and Molecular Life Sciences. 2007;64:294–306. doi: 10.1007/s00018-006-6321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Barrera A, Guerra B, Notvall L, Lanford RE. Journal of virology. 2005;79:9786–9798. doi: 10.1128/JVI.79.15.9786-9798.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Glebe D, Urban S. World Journal of gastroenterology. 2007;13:22. doi: 10.3748/wjg.v13.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dash S, Rao KV, Panda SK. Journal of medical virology. 1992;37:116–121. doi: 10.1002/jmv.1890370208. [DOI] [PubMed] [Google Scholar]
- 235.Paran N, Cooper A, Shaul Y. Reviews in medical virology. 2003;13:137–143. doi: 10.1002/rmv.376. [DOI] [PubMed] [Google Scholar]
- 236.Moon H, Lee J, Min J, Kang S. Biomacromolecules. 2014;15:3794–3801. doi: 10.1021/bm501066m. [DOI] [PubMed] [Google Scholar]
- 237.ShuáPiao J. Chemical Communications. 2013;49:7442–7444. doi: 10.1039/c3cc44508a. [DOI] [PubMed] [Google Scholar]
- 238.Snijder J, Kononova O, Barbu IM, Uetrecht C, Rurup WF, Burnley RJ, Koay MS, Cornelissen JJ, Roos WH, Barsegov V. Biomacromolecules. 2016;17:2522–2529. doi: 10.1021/acs.biomac.6b00469. [DOI] [PubMed] [Google Scholar]
- 239.Rurup WF, Snijder J, Koay MS, Heck AJ, Cornelissen JJ. Journal of the American Chemical Society. 2014;136:3828–3832. doi: 10.1021/ja410891c. [DOI] [PubMed] [Google Scholar]
- 240.Giessen TW. Current Opinion in Chemical Biology. 2016;34:1–10. doi: 10.1016/j.cbpa.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 241.Katsonis N, Putri R, Fredy JW, Cornelissen J, Koay M. ChemPhysChem. 2016 doi: 10.1002/cphc.201600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tamura A, Fukutani Y, Takami T, Fujii M, Nakaguchi Y, Murakami Y, Noguchi K, Yohda M, Odaka M. Biotechnology and bioengineering. 2015;112:13–20. doi: 10.1002/bit.25322. [DOI] [PubMed] [Google Scholar]
- 243.Choi B, Moon H, Hong SJ, Shin C, Do Y, Ryu S, Kang S. ACS Nano. 2016;10:7339–7350. doi: 10.1021/acsnano.5b08084. [DOI] [PubMed] [Google Scholar]
- 244.Rome L, Kedersha N, Chugani D. Trends in cell biology. 1991;1:47–50. doi: 10.1016/0962-8924(91)90088-q. [DOI] [PubMed] [Google Scholar]
- 245.Kedersha NL, Rome LH. The Journal of Cell Biology. 1986;103:699–709. doi: 10.1083/jcb.103.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Fauman EB, Rai BK, Huang ES. Current opinion in chemical biology. 2011;15:463–468. doi: 10.1016/j.cbpa.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 247.Uskoković V. Journal of biomedical nanotechnology. 2013;9:1441. doi: 10.1166/jbn.2013.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Buehler DC, Marsden MD, Shen S, Toso DB, Wu X, Loo JA, Zhou ZH, Kickhoefer VA, Wender PA, Zack JA. ACS nano. 2014;8:7723–7732. doi: 10.1021/nn5002694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Buehler DC, Toso DB, Kickhoefer VA, Zhou ZH, Rome LH. Small. 2011;7:1432–1439. doi: 10.1002/smll.201002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Åkerman ME, Chan WC, Laakkonen P, Bhatia SN, Ruoslahti E. Proceedings of the National Academy of Sciences. 2002;99:12617–12621. doi: 10.1073/pnas.152463399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Brigger I, Dubernet C, Couvreur P. Advanced drug delivery reviews. 2002;54:631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 252.Barratt G. Cellular and Molecular Life Sciences CMLS. 2003;60:21–37. doi: 10.1007/s000180300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Manchester M, Singh P. Advanced drug delivery reviews. 2006;58:1505–1522. doi: 10.1016/j.addr.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 254.Kickhoefer VA, Han M, Raval-Fernandes S, Poderycki MJ, Moniz RJ, Vaccari D, Silvestry M, Stewart PL, Kelly KA, Rome LH. Acs Nano. 2008;3:27–36. doi: 10.1021/nn800638x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Matsumoto NM, Buchman GW, Rome LH, Maynard HD. European Polymer Journal. 2015;69:532–539. doi: 10.1016/j.eurpolymj.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Llauró A, Guerra P, Kant R, Bothner B, Verdaguer N, de Pablo PJ. Scientific Reports. 2016:6. doi: 10.1038/srep34143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Yu M, Ng BC, Rome LH, Tolbert SH, Monbouquette HG. Nano letters. 2008;8:3510–3515. doi: 10.1021/nl080536z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Querol-Audí J, Casañas A, Usón I, Luque D, Castón JR, Fita I, Verdaguer N. The EMBO journal. 2009;28:3450–3457. doi: 10.1038/emboj.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Goldsmith LE, Yu M, Rome LH, Monbouquette HG. Biochemistry. 2007;46:2865–2875. doi: 10.1021/bi0606243. [DOI] [PubMed] [Google Scholar]
- 260.Gangemi CM, Pappalardo A, Sfrazzetto GT. RSC Advances. 2015;5:51919–51933. [Google Scholar]
- 261.Ahmad N, Younus HA, Chughtai AH, Verpoort F. Chemical Society Reviews. 2015;44:9–25. doi: 10.1039/c4cs00222a. [DOI] [PubMed] [Google Scholar]
- 262.Ahmad N, Younus HA, Chughtai AH, Verpoort F. Chemical Society Reviews. 2015;44:9–25. doi: 10.1039/c4cs00222a. [DOI] [PubMed] [Google Scholar]
- 263.Nimse SB, Kim T. Chemical Society Reviews. 2013;42:366–386. doi: 10.1039/c2cs35233h. [DOI] [PubMed] [Google Scholar]
- 264.Dan Z, Cao H, He X, Zeng L, Zou L, Shen Q, Zhang Z. International journal of pharmaceutics. 2015;483:63–68. doi: 10.1016/j.ijpharm.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 265.Zhang J, Ma PX. Advanced drug delivery reviews. 2013;65:1215–1233. doi: 10.1016/j.addr.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Loftsson T, Duchêne D. International journal of pharmaceutics. 2007;329:1–11. doi: 10.1016/j.ijpharm.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 267.Xia X, Xia Y. Front. Phys. 2014;9:378–384. [Google Scholar]
- 268.Namgung R, Lee YM, Kim J, Jang Y, Lee B-H, Kim I-S, Sokkar P, Rhee YM, Hoffman AS, Kim WJ. Nature communications. 2014:5. doi: 10.1038/ncomms4702. [DOI] [PubMed] [Google Scholar]
- 269.Králová J, Kejík Z. k., Bříza T. s., Poucková P, Král A, Martásek P, Král V. r. Journal of medicinal chemistry. 2009;53:128–138. doi: 10.1021/jm9007278. [DOI] [PubMed] [Google Scholar]
- 270.Swiech O, Mieczkowska A, Chmurski K, Bilewicz R. The Journal of Physical Chemistry B. 2012;116:1765–1771. doi: 10.1021/jp2091363. [DOI] [PubMed] [Google Scholar]
- 271.Moya-Ortega MD, Alvarez-Lorenzo C, Sigurdsson HH, Concheiro A, Loftsson T. Carbohydrate Polymers. 2012;87:2344–2351. [Google Scholar]
- 272.Della Rocca J, Liu D, Lin W. Accounts of chemical research. 2011;44:957–968. doi: 10.1021/ar200028a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.McKinlay A, Eubank J, Wuttke S, Xiao B, Wheatley P, Bazin P, Lavalley J-C, Daturi M, Vimont A, De Weireld G. Chemistry of Materials. 2013;25:1592–1599. [Google Scholar]
- 274.Miller SR, Alvarez E, Fradcourt L, Devic T, Wuttke S, Wheatley PS, Steunou N, Bonhomme C, Gervais C, Laurencin D. Chemical Communications. 2013;49:7773–7775. doi: 10.1039/c3cc41987h. [DOI] [PubMed] [Google Scholar]
- 275.Yi JW, Barry NP, Furrer MA, Zava O, Dyson PJ, Therrien B, Kim BH. Bioconjugate chemistry. 2012;23:461–471. doi: 10.1021/bc200472n. [DOI] [PubMed] [Google Scholar]
- 276.Mattsson J, Zava O, Renfrew AK, Sei Y, Yamaguchi K, Dyson PJ, Therrien B. Dalton Transactions. 2010;39:8248–8255. doi: 10.1039/c0dt00436g. [DOI] [PubMed] [Google Scholar]
- 277.Zhang Z, Chen Y, Xu X, Zhang J, Xiang G, He W, Wang X. Angewandte Chemie. 2014;126:439–443. doi: 10.1002/anie.201308589. [DOI] [PubMed] [Google Scholar]
- 278.Behrend R, Meyer E, Rusche F. Justus Liebig's Annalen der Chemie. 1905;339:1–37. [Google Scholar]
- 279.Freeman WA, Mock WL, Shih N-Y. J Am Chem Soc. 1981;103:7367–7368. [Google Scholar]
- 280.Pemberton BC, Raghunathan R, Volla S, Sivaguru J. Chemistry-A European Journal. 2012;18:12178–12190. doi: 10.1002/chem.201202083. [DOI] [PubMed] [Google Scholar]
- 281.Ma W-J, Chen J-M, Jiang L, Yao J, Lu T-B. Molecular pharmaceutics. 2013;10:4698–4705. doi: 10.1021/mp400529m. [DOI] [PubMed] [Google Scholar]
- 282.Wheate NJ, Buck DP, Day AI, Collins JG. Dalton Transactions. 2006:451–458. doi: 10.1039/b513197a. [DOI] [PubMed] [Google Scholar]
- 283.Zhao Y, Bali MS, Cullinane C, Day AI, Collins JG. Dalton Transactions. 2009:5190–5198. doi: 10.1039/b905112k. [DOI] [PubMed] [Google Scholar]
- 284.Montes-Navajas P, González-Béjar M, Scaiano J, García H. Photochemical & Photobiological Sciences. 2009;8:1743–1747. doi: 10.1039/b9pp00041k. [DOI] [PubMed] [Google Scholar]
- 285.Gürbüz S, Idris M, Tuncel D. Organic & biomolecular chemistry. 2015;13:330–347. doi: 10.1039/c4ob02065k. [DOI] [PubMed] [Google Scholar]
- 286.Ludwig R. Fresenius J Anal Chem. 2000;367:103–128. doi: 10.1007/s002160051611. [DOI] [PubMed] [Google Scholar]
- 287.Ukhatskaya EV, Kurkov SV, Matthews SE, Loftsson T. Journal of pharmaceutical sciences. 2013;102:3485–3512. doi: 10.1002/jps.23681. [DOI] [PubMed] [Google Scholar]
- 288.Su K, Jiang F, Qian J, Wu M, Gai Y, Pan J, Yuan D, Hong M. Inorganic Chemistry. 2014;53:18–20. doi: 10.1021/ic4024184. [DOI] [PubMed] [Google Scholar]
- 289.Bi Y, Du S, Liao W. Coordination Chemistry Reviews. 2014;276:61–72. [Google Scholar]
- 290.Su K, Jiang F, Qian J, Gai Y, Wu M, Bawaked SM, Mokhtar M, AL-Thabaiti SA, Hong M. Crystal Growth & Design. 2014;14:3116–3123. [Google Scholar]
- 291.Su K, Jiang F, Qian J, Chen L, Pang J, Bawaked SM, Mokhtar M, Al-Thabaiti SA, Hong M. Inorganic Chemistry. 2015;54:3183–3188. doi: 10.1021/ic502677g. [DOI] [PubMed] [Google Scholar]
- 292.Nasuhi Pur F, Dilmaghani KA. Journal of Coordination Chemistry. 2014;67:440–448. [Google Scholar]
- 293.Salem AB, Sautrey G, Fontanay S, Duval RE, Regnouf-de-Vains J-B. Bioorganic & medicinal chemistry. 2011;19:7534–7540. doi: 10.1016/j.bmc.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Gallego-Yerga L, Lomazzi M, Sansone F, Mellet CO, Casnati A, Fernández JMG. Chemical Communications. 2014;50:7440–7443. doi: 10.1039/c4cc02703e. [DOI] [PubMed] [Google Scholar]
- 295.Drakalska E, Momekova D, Manolova Y, Budurova D, Momekov G, Genova M, Antonov L, Lambov N, Rangelov S. International journal of pharmaceutics. 2014;472:165–174. doi: 10.1016/j.ijpharm.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 296.Zhang M, Yan X, Huang F, Niu Z, Gibson HW. Accounts of chemical research. 2014;47:1995–2005. doi: 10.1021/ar500046r. [DOI] [PubMed] [Google Scholar]
- 297.Torrens F, Castellano G. Current topics in medicinal chemistry. 2015 doi: 10.2174/1568026615666150506145619. [DOI] [PubMed] [Google Scholar]
- 298.Yu G, Yu W, Mao Z, Gao C, Huang F. Small. 2015;11:919–925. doi: 10.1002/smll.201402236. [DOI] [PubMed] [Google Scholar]
- 299.Zhang C, He Y, Su M, Ko SH, Ye T, Leng Y, Sun X, Ribbe AE, Jiang W, Mao C. Faraday discussions. 2009;143:221–233. doi: 10.1039/b905313c. [DOI] [PubMed] [Google Scholar]
- 300.Zhang C, Su M, He Y, Leng Y, Ribbe AE, Wang G, Jiang W, Mao C. Chem Commun (Camb) 2010;46:6792–6794. doi: 10.1039/c0cc02363a. [DOI] [PubMed] [Google Scholar]
- 301.Tian C, Li X, Liu Z, Jiang W, Wang G, Mao C. Angew Chem Int Ed Engl. 2014;53:8041–8044. doi: 10.1002/anie.201400377. [DOI] [PubMed] [Google Scholar]
- 302.Jiang D, England CG, Cai W. Journal of Controlled Release. 2016;239:27–38. doi: 10.1016/j.jconrel.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Chandrasekaran AR, Levchenko O. Chemistry of Materials. 2016;28:5569–5581. [Google Scholar]
- 304.Jiang Q, Song C, Nangreave J, Liu X, Lin L, Qiu D, Wang ZG, Zou G, Liang X, Yan H, Ding B. J Am Chem Soc. 2012;134:13396–13403. doi: 10.1021/ja304263n. [DOI] [PubMed] [Google Scholar]
- 305.Walsh AS, Yin H, Erben CM, Wood MJ, Turberfield AJ. ACS nano. 2011;5:5427–5432. doi: 10.1021/nn2005574. [DOI] [PubMed] [Google Scholar]
- 306.Li J, Pei H, Zhu B, Liang L, Wei M, He Y, Chen N, Li D, Huang Q, Fan C. ACS nano. 2011;5:8783–8789. doi: 10.1021/nn202774x. [DOI] [PubMed] [Google Scholar]
- 307.Chan MS, Lo PK. Small. 2014;10:1255–1260. doi: 10.1002/smll.201302993. [DOI] [PubMed] [Google Scholar]
- 308.Zhang C, Tian C, Li X, Qian H, Hao C, Jiang W, Mao C. J Am Chem Soc. 2012;134:11998–12001. doi: 10.1021/ja305969c. [DOI] [PubMed] [Google Scholar]
- 309.Liu Z, Li Y, Tian C, Mao C. Biomacromolecules. 2013;14:1711–1714. doi: 10.1021/bm400426f. [DOI] [PubMed] [Google Scholar]
- 310.Zhang ZM, Gao PC, Wang ZF, Sun BW, Jiang Y. Chem Commun (Camb) 2015;51:12996–12999. doi: 10.1039/c5cc05164a. [DOI] [PubMed] [Google Scholar]
- 311.Setyawati MI, Kutty RV, Tay CY, Yuan X, Xie J, Leong DT. ACS Appl Mater Interfaces. 2014;6:21822–21831. doi: 10.1021/am502591c. [DOI] [PubMed] [Google Scholar]
- 312.Jiang Q, Shi Y, Zhang Q, Li N, Zhan P, Song L, Dai L, Tian J, Du Y, Cheng Z, Ding B. Small. 2015;11:5134–5141. doi: 10.1002/smll.201501266. [DOI] [PubMed] [Google Scholar]
- 313.Boisselier E, Astruc D. Chemical Society Reviews. 2009;38:1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 314.Ghosh P, Han G, De M, Kim CK, Rotello VM. Advanced Drug Delivery Reviews. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 315.Kumar A, Zhang X, Liang X-J. Biotechnology Advances. 2013;31:593–606. doi: 10.1016/j.biotechadv.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 316.Yugang S, Younan X. Science. 2002;298:2176–2179. [Google Scholar]
- 317.Yavuz MS, Cheng Y, Chen J, Cobley CM, Zhang Q, Rycenga M, Xie J, Kim C, Song KH, Schwartz AG. Nature materials. 2009;8:935–939. doi: 10.1038/nmat2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Shi P, Ju E, Ren J, Qu X. Advanced Functional Materials. 2014;24:826–834. [Google Scholar]
- 319.Presser V, Heon M, Gogotsi Y. Advanced Functional Materials. 2011;21:810–833. [Google Scholar]
- 320.Oshima C, Nagashima A. Journal of Physics: Condensed Matter. 1997;9:1. [Google Scholar]
- 321.Zhang L, Zeng X, Wang X. Scientific reports. 2013:3. doi: 10.1038/srep03162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Guo J, Lan M, Wang S, He Y, Zhang S, Xiang G, Boi FS. Phys Chem Chem Phys. 2015;17:18159–18166. doi: 10.1039/c5cp02425k. [DOI] [PubMed] [Google Scholar]
- 323.Sgouros A, Kalosakas G, Sigalas M, Papagelis K. RSC Advances. 2015;5:39930–39937. [Google Scholar]
- 324.Zhang L, Li Y, Jin Z, Chan KM, Jimmy CY. RSC Advances. 2015;5:93226–93233. [Google Scholar]
- 325.Lee JE, Lee N, Kim H, Kim J, Choi SH, Kim JH, Kim T, Song IC, Park SP, Moon WK, Hyeon T. Journal of the American Chemical Society. 2009;132:552–557. doi: 10.1021/ja905793q. [DOI] [PubMed] [Google Scholar]
- 326.Park J-H, Gu L, von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. Nat Mater. 2009;8:331–336. doi: 10.1038/nmat2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Mamaeva V, Rosenholm JM, Bate-Eya LT, Bergman L, Peuhu E, Duchanoy A, Fortelius LE, Landor S, Toivola DM, Linden M, Sahlgren C. Mol Ther. 2011;19:1538–1546. doi: 10.1038/mt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Luo G-F, Chen W-H, Liu Y, Lei Q, Zhuo R-X, Zhang X-Z. Sci. Rep. 2014:4. doi: 10.1038/srep06064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Wang T, Zhang L, Su Z, Wang C, Liao Y, Fu Q. ACS Applied Materials & Interfaces. 2011;3:2479–2486. doi: 10.1021/am200364e. [DOI] [PubMed] [Google Scholar]
- 330.Lee LA, Wang Q. Nanomedicine: Nanotechnology, Biology and Medicine. 2:137–149. doi: 10.1016/j.nano.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 331.Mossink MH, van Zon A, Scheper RJ, Sonneveld P, Wiemer EAC. Oncogene. 22:7458–7467. doi: 10.1038/sj.onc.1206947. 0000. [DOI] [PubMed] [Google Scholar]
- 332.Kickhoefer VA, Garcia Y, Mikyas Y, Johansson E, Zhou JC, Raval-Fernandes S, Minoofar P, Zink JI, Dunn B, Stewart PL, Rome LH. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4348–4352. doi: 10.1073/pnas.0500929102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Ramqvist T, Andreasson K, Dalianis T. Expert Opinion on Biological Therapy. 2007;7:997–1007. doi: 10.1517/14712598.7.7.997. [DOI] [PubMed] [Google Scholar]
- 334.Szécsi J, Boson B, Johnsson P, Dupeyrot-Lacas P, Matrosovich M, Klenk H-D, Klatzmann D, Volchkov V, Cosset F-L. Virology Journal. 2006;3:70. doi: 10.1186/1743-422X-3-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Challa R, Ahuja A, Ali J, Khar RK. AAPS PharmSciTech. 2005;6:E329–E357. doi: 10.1208/pt060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Chen Y, Liu Y. Chemical Society Reviews. 2010;39:495–505. doi: 10.1039/b816354p. [DOI] [PubMed] [Google Scholar]
- 337.Kim K, Selvapalam N, Ko YH, Park KM, Kim D, Kim J. Chemical Society Reviews. 2007;36:267–279. doi: 10.1039/b603088m. [DOI] [PubMed] [Google Scholar]
- 338.Park KM, Suh K, Jung H, Lee D-W, Ahn Y, Kim J, Baek K, Kim K. Chemical Communications. 2009:71–73. doi: 10.1039/b815009e. DOI: 10.1039/b815009e. [DOI] [PubMed] [Google Scholar]
- 339.Huxford RC, Rocca JD, Lin W. Current opinion in chemical biology. 2010;14:262–268. doi: 10.1016/j.cbpa.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Su K, Jiang F, Qian J, Gai Y, Wu M, Bawaked SM, Mokhtar M, Al-Thabaiti SA, Hong M. Crystal Growth & Design. 2014;14:3116–3123. [Google Scholar]
- 341.Liu M, Liao W, Hu C, Du S, Zhang H. Angewandte Chemie International Edition. 2012;51:1585–1588. doi: 10.1002/anie.201106732. [DOI] [PubMed] [Google Scholar]
- 342.Zhang Z, Chen Y, Xu X, Zhang J, Xiang G, He W, Wang X. Angewandte Chemie International Edition. 2014;53:429–433. doi: 10.1002/anie.201308589. [DOI] [PubMed] [Google Scholar]
- 343.Xie K, Qin X, Wang X, Wang Y, Tao H, Wu Q, Yang L, Hu Z. Advanced Materials. 2012;24:347–352. doi: 10.1002/adma.201103872. [DOI] [PubMed] [Google Scholar]
- 344.Li G, Yu H, Xu L, Ma Q, Chen C, Hao Q, Qian Y. Nanoscale. 2011;3:3251–3257. doi: 10.1039/c1nr10284b. [DOI] [PubMed] [Google Scholar]
- 345.Teng SJ, Wang XX, Xia BY, Wang JN. Journal of Power Sources. 2010;195:1065–1070. [Google Scholar]
- 346.Siekkinen AR, McLellan JM, Chen J, Xia Y. Chemical physics letters. 2006;432:491–496. doi: 10.1016/j.cplett.2006.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Skrabalak SE, Au L, Li X, Xia Y. Nat. Protocols. 2007;2:2182–2190. doi: 10.1038/nprot.2007.326. [DOI] [PubMed] [Google Scholar]
- 348.Zhang Q, Huang CZ, Ling J, Li YF. The Journal of Physical Chemistry B. 2008;112:16990–16994. doi: 10.1021/jp8081535. [DOI] [PubMed] [Google Scholar]
- 349.Chen J, McLellan JM, Siekkinen A, Xiong Y, Li Z-Y, Xia Y. Journal of the American Chemical Society. 2006;128:14776–14777. doi: 10.1021/ja066023g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Skrabalak SE, Chen J, Sun Y, Lu X, Au L, Cobley CM, Xia Y. Accounts of Chemical Research. 2008;41:1587–1595. doi: 10.1021/ar800018v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Xia Y, Li W, Cobley CM, Chen J, Xia X, Zhang Q, Yang M, Cho EC, Brown PK. Accounts of chemical research. 2011;44:914–924. doi: 10.1021/ar200061q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Sun Y, Xia Y. Journal of the American Chemical Society. 2004;126:3892–3901. doi: 10.1021/ja039734c. [DOI] [PubMed] [Google Scholar]
- 353.Awasthi KK, Verma R, Awasthi A, Awasthi K, Soni I, John P. Adv Mater Lett. 2015;6:187–193. [Google Scholar]
- 354.Khan M, Naqvi AH, Ahmad M. Toxicology Reports. 2015;2:765–774. doi: 10.1016/j.toxrep.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Awasthi KK, Awasthi A, Verma R, Kumar N, Roy P, Awasthi K, John P. RSC Advances. 2015;5:34927–34935. [Google Scholar]
- 356.Montes-Fonseca SL, Orrantia-Borunda E, Aguilar-Elguezabal A, Horta CG, Talamás-Rohana P, Sánchez-Ramírez B. Nanomedicine: Nanotechnology, Biology and Medicine. 2012;8:853–859. doi: 10.1016/j.nano.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 357.Sharma V, Singh P, Pandey AK, Dhawan A. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2012;745:84–91. doi: 10.1016/j.mrgentox.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 358.Huo L, Chen R, Zhao L, Shi X, Bai R, Long D, Chen F, Zhao Y, Chang Y-Z, Chen C. Biomaterials. 2015;61:307–315. doi: 10.1016/j.biomaterials.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 359.Ahmad J, Ahamed M, Akhtar MJ, Alrokayan SA, Siddiqui MA, Musarrat J, Al-Khedhairy AA. Toxicology and applied pharmacology. 2012;259:160–168. doi: 10.1016/j.taap.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 360.Sathishkumar M, Pavagadhi S, Mahadevan A, Balasubramanian R. Ecotoxicology and environmental safety. 2015;114:232–240. doi: 10.1016/j.ecoenv.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 361.Kroll A, Pillukat MH, Hahn D, Schnekenburger J. Archives of toxicology. 2012;86:1123–1136. doi: 10.1007/s00204-012-0837-z. [DOI] [PubMed] [Google Scholar]
- 362.Gliga AR, Skoglund S, Wallinder IO, Fadeel B, Karlsson HL. Particle and fibre toxicology. 2014;11:1. doi: 10.1186/1743-8977-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Huang Y, Lü X, Qu Y, Yang Y, Wu S. Biomaterials. 2015;37:13–24. doi: 10.1016/j.biomaterials.2014.10.042. [DOI] [PubMed] [Google Scholar]
- 364.Coccini T, Roda E, Fabbri M, Sacco MG, Gribaldo L, Manzo L. Journal of Nanoparticle Research. 2012;14:1–10. [Google Scholar]
- 365.Mostaghaci B, Susewind J, Kickelbick G, Lehr C-M, Loretz B. ACS applied materials & interfaces. 2015;7:5124–5133. doi: 10.1021/am507193a. [DOI] [PubMed] [Google Scholar]
- 366.Escamilla-Rivera V, Uribe-Ramirez M, González-Pozos S, Lozano O, Lucas S, De Vizcaya-Ruiz A. Toxicology letters. 2016;240:172–184. doi: 10.1016/j.toxlet.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 367.Qin Y, Zhou Z-W, Pan S-T, He Z-X, Zhang X, Qiu J-X, Duan W, Yang T, Zhou S-F. Toxicology. 2015;327:62–76. doi: 10.1016/j.tox.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 368.Song L, Vijver MG, Peijnenburg WJ, Galloway TS, Tyler CR. Chemosphere. 2015;139:181–189. doi: 10.1016/j.chemosphere.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 369.Zhang J, Li B, Zhang Y, Li A, Yu X, Huang Q, Fan C, Cai X. Analyst. 2013;138:6511–6516. doi: 10.1039/c3an01267k. [DOI] [PubMed] [Google Scholar]
- 370.Marquis BJ, Love SA, Braun KL, Haynes CL. Analyst. 2009;134:425–439. doi: 10.1039/b818082b. [DOI] [PubMed] [Google Scholar]
- 371.Jenkins JT, Halaney DL, Sokolov KV, Ma LL, Shipley HJ, Mahajan S, Louden CL, Asmis R, Milner TE, Johnston KP. Nanomedicine: Nanotechnology, Biology and Medicine. 2013;9:356–365. doi: 10.1016/j.nano.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 372.Fu C, Liu T, Li L, Liu H, Chen D, Tang F. Biomaterials. 2013;34:2565–2575. doi: 10.1016/j.biomaterials.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 373.Yang H, Du L, Tian X, Fan Z, Sun C, Liu Y, Keelan JA, Nie G. Toxicology letters. 2014;230:10–18. doi: 10.1016/j.toxlet.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 374.Raja KS, Wang Q, Gonzalez MJ, Manchester M, Johnson JE, Finn M. Biomacromolecules. 2003;4:472–476. doi: 10.1021/bm025740+. [DOI] [PubMed] [Google Scholar]
- 375.Lewis JD, Destito G, Zijlstra A, Gonzalez MJ, Quigley JP, Manchester M, Stuhlmann H. Nature medicine. 2006;12:354–360. doi: 10.1038/nm1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Ren Y, Wong SM, Lim L-Y. Bioconjugate chemistry. 2007;18:836–843. doi: 10.1021/bc060361p. [DOI] [PubMed] [Google Scholar]
- 377.Gonzalez MJ, Plummer EM, Rae CS, Manchester M. PloS one. 2009;4:e7981. doi: 10.1371/journal.pone.0007981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Skrabalak SE, Au L, Lu X, Li X, Xia Y. 2007 [Google Scholar]
- 379.Molino NM, Bilotkach K, Fraser DA, Ren D, Wang S-W. Biomacromolecules. 2012;13:974–981. doi: 10.1021/bm300083e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Lila ASA, Kiwada H, Ishida T. Journal of Controlled Release. 2013;172:38–47. doi: 10.1016/j.jconrel.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 381.Agostinelli E, Vianello F, Magliulo G, Thomas T, Thomas T. Int J Oncol. 2015;46:5–16. doi: 10.3892/ijo.2014.2706. [DOI] [PubMed] [Google Scholar]
- 382.Todd TJ, Zhen Z, Xie J. Nanomedicine. 2013;8:1555–1557. doi: 10.2217/nnm.13.141. [DOI] [PubMed] [Google Scholar]
- 383.Cobley CM, Au L, Chen J, Xia Y. Expert opinion on drug delivery. 2010;7:577–587. doi: 10.1517/17425240903571614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Dreaden EC, Austin LA, Mackey MA, El-Sayed MA. Therapeutic delivery. 2012;3:457–478. doi: 10.4155/tde.12.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Rwei AY, Wang W, Kohane DS. Nano today. 2015;10:451–467. doi: 10.1016/j.nantod.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Panyam J, Labhasetwar V. Advanced drug delivery reviews. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 387.Weber J, Beard PC, Bohndiek SE. Nature Methods. 2016;13:639–650. doi: 10.1038/nmeth.3929. [DOI] [PubMed] [Google Scholar]
- 388.Huang S, Duan S, Wang J, Bao S, Qiu X, Li C, Liu Y, Yan L, Zhang Z, Hu Y. Advanced Functional Materials. 2016 [Google Scholar]
- 389.Wang Z, Yan TD, Susha AS, Chan MS, Kershaw SV, Lo PK, Rogach AL. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2016;495:62–67. [Google Scholar]
- 390.Tang L, Yang S, Liang F, Wang Q, Qu X, Yang Z. ACS applied materials & interfaces. 2016;8:12056–12062. doi: 10.1021/acsami.6b03208. [DOI] [PubMed] [Google Scholar]