Abstract
Polychlorinated biphenyl (PCB) congeners with multiple ortho chlorine substituents and their metabolites exist as stable rotational isomers, or atropisomers, that are nonsuperimposable mirror images of each other. Additionally, the oxidation of certain axially prochiral PCBs, such as 2,2′,4,6′-tetrachlorobiphenyl (PCB 51) and 2,2′,4,5,6′-pentachlorobiphenyl (PCB 102), in the meta position of the symmetrically substituted phenyl ring is expected to form axially chiral hydroxylated metabolites (OH-PCBs); however, the formation of chiral OH-PCBs from prochiral PCBs has not been demonstrated experimentally. Here, we investigate if the oxidation of PCB 51 and PCB 102 by different microsomal preparations results in the formation of chiral OH-PCBs. Gas chromatographic analysis revealed that PCB 51 and PCB 102 were metabolized to 2,2′,4,6′-tetrachlorobiphenyl-3′-ol (OH-PCB 51) and 2,2′,4,5,6′-pentachlorobiphenyl-3′-ol (OH-PCB 102), respectively, by liver microsomes from male rats pretreated with different inducers; untreated male monkeys, guinea pigs, rabbits, and hamsters; and female dogs. The formation of both metabolites was inducer- and species-dependent. Both OH-PCB 51 and OH-PCB 102 were chiral and formed enantioselectively by all microsomal preparations investigated. These findings demonstrate that axially chiral PCB metabolites are formed from axially prochiral PCB congeners, a fact that should be considered when studying the environmental fate, transport, and toxicity of OH-PCBs.
Introduction
OH-PCBs are emerging environmental contaminants that represent a significant human health concern because of their presence in environmental and human samples.1 OH-PCBs are a group of structurally diverse compounds consisting of 837 theoretically possible individual congeners.2 Humans and laboratory studies have linked OH-PCBs to adverse outcomes, for example, developmental neurotoxicity, endocrine disruption, and cardiovascular effects.1,3 OH-PCBs are present in technical PCB mixtures and, together with the parent PCBs, have been released into the environment.4 In addition, PCBs can be transformed to OH-PCBs by both biotic and abiotic processes. Oxidation of PCBs by hydroxyl radicals in the atmosphere has been proposed as a major mechanism for the degradation of PCBs in the environment.5,6 There is also evidence that OH-PCBs are formed during wastewater treatment processes, either as a result of microbial oxidation or oxidation by hydroxyl radicals generated by advanced oxidation processes.7 In addition, PCBs undergo biotransformation to OH-PCBs in fungi,8 plants,9−11 fish,12 birds,13 and mammals,13−18 including humans,14,17 typically by reactions involving cytochrome P450 (P450) enzymes.
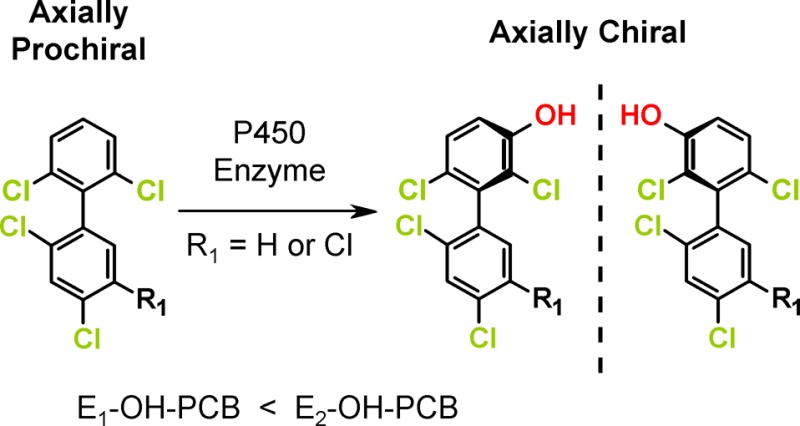
Like their parent compounds, OH-PCBs with three or four ortho chlorine substituents and an unsymmetrical substitution pattern in both phenyl rings relative to the phenyl–phenyl bond are axially chiral.19−21 These OH-PCBs and other PCB metabolites exist under ambient conditions as stable rotational isomers, or atropisomers, that are non-superimposable mirror images of each other.22 Chiral OH-PCBs can be formed by the oxidation of a chiral PCB congener. Considerable evidence demonstrates that plants, such as poplar,10,23 and mammals14,15,17 metabolize chiral PCBs in a congener and species-dependent manner to chiral OH-PCBs. P450 enzyme-mediated metabolism in mammals, such as rats and mice, results in nonracemic chiral signatures of these OH-PCB metabolites in vitro and in vivo.13,16,24,25 In addition, chiral OH-PCBs can theoretically be formed by oxidation of axially prochiral PCB congeners with three or four ortho chlorine substituents.21 Axially prochiral PCB congeners have one phenyl ring with an unsymmetrical chlorine substitution pattern and a second phenyl ring with a symmetrical chlorine substitution pattern (i.e., 2,6; 3,5; 2,4,6; 3,4,5; 2,3,5,6; and 2,4,3,4,5,6). Introduction of a hydroxyl group in the meta or ortho position breaks the symmetry of this ring, thus resulting in axially chiral molecules. However, the enantioselective formation of a chiral OH-PCB from an axially prochiral PCB congener has not been confirmed experimentally.
Here, we investigate the P450 isoform and species-dependent metabolism of two prochiral PCB congeners, PCB 51 and PCB 102, using liver microsomes. Both PCB congeners were selected because they are present at considerable levels in technical PCB mixtures. PCB 51 and PCB 102 are structurally related to 2,2′,3,4′,6-pentachlorobiphenyl (PCB 91) and 2,2′,3,4′,5′,6-hexachlorobiphenyl (PCB 132), two axially chiral PCB congeners that are enantioselectively oxidized by mammalian CYP2B enzymes in the meta position of the 2,3,6-trichloro-substituted phenyl ring.15,18,26 Based on the structural similarity between the axially prochiral vs axially chiral PCB congeners (i.e., 2,6- vs 2,3,6-substitution) and established structure–metabolism relationships,19,20 we investigated if PCB 51 and PCB 102 are oxidized by CYP2B enzymes in the meta position of the 2,6-dichloro substituted phenyl ring, thus resulting in novel, axially chiral PCB metabolites. Our results provide strong experimental evidence that both axially prochiral PCB congeners are enantioselectively metabolized by P450 enzymes to axially chiral OH-PCBs.
Experimental Section
Materials
PCB 51, PCB 102, 2,3,4′,5,6-pentachlorobiphenyl (PCB 117; recovery standard), 2,2′,3,4,4′,5,6,6′-octachlorobiphenyl (PCB 204; internal standard), and 2,3,3′,4,5,5′-hexachlorobiphenyl-4′-ol (4′-OH-PCB 159; recovery standard) were purchased from AccuStandard, Inc. (New Haven, CT). Solutions of diazomethane in diethyl ether for the derivatization of HO-PCBs to methylated OH-PCB derivatives were synthesized from N-methyl-N-nitroso-p-toluenesulfonamide (Diazald) using an Aldrich mini Diazald apparatus (Milwaukee, WI).27 β-Nicotinamide adenine dinucleotide 2′-phosphate reduced tetrasodium salt hydrate (NADPH) was purchased from Sigma-Aldrich, Inc. (Milwaukee, WI). Dimethyl sulfoxide (DMSO), sodium phosphate dibasic, sodium phosphate monobasic, magnesium chloride, tetrabutylammonium sulfite, sodium sulfite, and pesticide grade solvents were obtained from Fisher Scientific (Pittsburgh, PA). Liver microsomes prepared from male Sprague–Dawley rats pretreated with classical inducers of hepatic P450 enzyme activities, including β-naphthoflavone (BNF), clofibric acid (CFA), dexamethasone (DEX), isoniazid (INH), or phenobarbital (PB), were purchased from Xenotech (Lenexa, KS) to study the role of different P450 isoforms, in particular, CYP2Bs, in the enantioselective metabolism of PCBs.15,28,29 In addition, liver microsomes from untreated beagle dogs (female), Hartley albino guinea pigs (male), golden Syrian hamsters (male), Cynomolgus monkeys (male), and New Zealand rabbits (male) were obtained from Xenotech to study species differences in the metabolism of prochiral PCBs. The same microsomal preparations have been used in our previous study investigating the species-dependent metabolism of chiral 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB 136).17 Rat liver microsomes were prepared from corn oil (CO) treated rats and characterized as previously described.29 Additional details regarding the microsomal preparations are provided in Tables S1 and S2 (Supporting Information).
Prochiral PCB Biotransformation Experiments
An incubation system containing phosphate buffer (0.1 M, pH 7.4), magnesium chloride (3 mM), liver microsomes (0.1 mg/mL), and NADPH (0.5 mM) was preincubated for 5 min at 37 °C in a shaking water bath. PCB 51 or PCB 102 in DMSO (≤0.5% of the incubation volume) was added to give a final concentration of 50 μM in a 2 mL incubation system.29 The mixtures were incubated for 2 h at 37 °C with the intent to generate enough products for enantioselective analyses. Hence, relatively high concentrations of both PCB congeners were used, and the formation of major metabolites may not necessarily be linear over the entire period of the incubation.15 The reaction was stopped by adding ice-cold sodium hydroxide (2 mL, 0.5 M) to each sample. The incubation mixture was heated at 110 °C for 10 min to further denature the protein and solubilize the microsomes. Blank samples containing only phosphate buffer accompanied each experiment. In addition, control incubations without PCB were included for each microsomal preparation investigated to check for background contamination with PCBs or their hydroxylated metabolites. Large-scale incubations with a final incubation volume of 16 mL were performed analogously with liver microsomes prepared from DEX-pretreated male rats to allow the characterization of hydroxylated metabolites.
Extraction of PCBs and Metabolites
A published method was used to extract PCB 51 or PCB 102 and their hydroxylated metabolites from the incubation mixtures.24,29 Briefly, samples were spiked with surrogate recovery standards PCB 117 (200 ng) and 4′-OH-PCB 159 (68.5 ng). Hydrochloric acid (6 M, 1 mL) was added followed by 2-propanol (5 mL). The samples were extracted with hexane–MTBE (1:1 v/v, 5 mL) and re-extracted with hexane (3 mL). The combined organic layers were washed with an aqueous potassium chloride solution (1%, 4 mL). The organic phase was transferred to a new vial, and the KCl mixture was re-extracted with hexane (3 mL). The combined organic layers were evaporated to dryness under a gentle stream of nitrogen. The samples were reconstituted with hexane (1 mL), methanol (5 drops) was added and samples were derivatized with diazomethane in diethyl ether (0.5 mL) for approximately 16 h at 4 °C to allow complete derivatization. The samples then underwent sulfur and sulfuric acid cleanup steps as described previously.30,31 The organic top layer was transferred to new vials, PCB 204 (50 ng) was added as the internal standard (or volume corrector), and extracts were concentrated as needed for gas chromatographic analysis.
Racemization of OH-PCBs metabolites
To confirm that the major hydroxylated metabolites of PCB 51 and PCB 102 were formed atropselectively during microsomal metabolism, aliquots of representative hexane extracts were transferred into a 2 mL amber ampule. Each extract was evaporated to dryness, and the flame-sealed ampule was heated to 300 °C for 2 h. PCBs with three ortho chlorine substituents have been shown to completely racemize under these conditions.32 Subsequently, the samples were reconstituted in hexane and analyzed by enantioselective gas chromatography as described below.
Identification of PCB Metabolites
Full mass spectra with accurate mass determinations were used to identify the hydroxylated PCB metabolite (as the corresponding methylated OH-PCB derivatives) in representative extracts. Briefly, samples were analyzed on an Agilent 7890A gas chromatograph combined with a Waters GCT Premier time-of-flight mass spectrometer (Waters Corporation, Milford, MA) in the High Resolution Mass Spectrometry Facility of the University of Iowa (Iowa City, IA). Analytes were separated using a DB-5ms capillary column (30 m length, 250 μm inner diameter, 0.25 μm film thickness; Agilent, Santa Clara, CA). The oven temperature was initially held at 150 °C for 1 min, ramped at a rate of 30 °C/min to a final temperature of 240 °C, and held for 15 min at 240 °C. The injector temperature was 280 °C and operated in the splitless mode. The helium flow rate was 1.5 mL/min. The source and transfer line temperatures were both 250 °C, and a mass range of m/z 50 to 650 was collected. Heptacosafluorotributylamine was continuously introduced as internal standard (lock mass). To correct for shifts in retention times (RT) across analyses, relative retention times (RRTs) of all metabolites were determined against the internal standard (PCB 204). If not stated otherwise, metabolites were identified as follows: RRTs of metabolite peaks were within 0.5% of the average of RRT of the respective metabolite;33 the experimental accurate mass of [M]+ was within 0.003 Da of the theoretical mass of the predicted molecular formula; and the isotope patterns matched the theoretical model with 20% error (Figures S1–S22). All samples contained traces of a monohydroxylated PCB metabolite (Figures S23 and S24).
Quantification of Relative Metabolite Levels
Sample extracts were analyzed on an Agilent 7890A gas chromatograph with a 63Ni-micro electron capture detector (GC-μECD) and a SPB-1 capillary column (60 m length, 250 μm inner diameter, 0.25 μm film thickness; Supelco, St Louis, MO) as reported previously.28 Both the inlet and detector temperatures were set to 250 °C. The initial oven temperature was 50 °C, held for 1 min, and then increased by 30 °C/min until it reached 200 °C. The temperature increased by 1 °C/min until 250 °C, then by 10 °C/min to a final temperature of 280 °C. The injector was operated in the splitless mode. Since authentic standards of the metabolites were not available, relative metabolite levels are presented as area of the major metabolite relative to the area of the internal standard (PCB 204). The RRTs of all metabolites, calculated relative to PCB 204, were within 0.5% of the average RRT for the respective metabolite.33
Enantioselective Gas-Chromatographic Analyses
Extracts from all microsomal incubations were analyzed using an Agilent 6890 gas chromatograph equipped with a μECD and a Chirasil-Dex (CD) capillary column (25 m length, 250 μm inner diameter, 0.25 μm film thickness; Agilent, Santa Clara, CA). The oven temperature was as follows: initial temperature was 50 °C held for 1 min, ramped at 10 °C/min to 140 °C and held at 140 °C for 190 min (for PCB 51 extracts) or 350 min (for PCB 102 extracts), the temperature was then ramped at 20 °C/min to the final temperature of 225 °C and held for 10 min. The helium flow was 3 mL/min. Details regarding the optimization of the temperature for the enantioselective analyses can be found in Tables S3A and S3B. Enantiomeric fractions (EF) were calculated by the drop valley method as EF = area E1/(area E1 + area E2), where area E1 and area E2 denote the peak area of the first and second eluting atropisomer.34 Extracts from large-scale incubations of both congeners with microsomes prepared from dexamethasone-pretreated rats were also analyzed by gas chromatography–mass spectrometry (GC–MS) on the Chiral-Dex B-DM capillary column (30 m length, 250 μm inner diameter, 0.12 μm film thickness; Supelco, St. Louis, MO). These limited experiments provide additional evidence that, based on their m/z, the two peaks observed in the enantioselective analysis are indeed methylated OH-PCB atropisomers (see Figure S25).
Quality Assurance/Quality Control
The recoveries of PCB 117 and 4′-OH-PCB 159 were 96 ± 11% (range: 65–130) and 115 ± 7% (range: 90–133), respectively. The resolution29 of atropisomers of OH-PCB 51 and OH-PCB 102 analyzed by GC−μECD using the CD column was 1.19 and 0.84, respectively. The EF of OH-PCB 51 and OH-PCB 102 after racemization were 0.46 ± 0.01 (n = 8) and 0.49 ± 0.01 (n = 6) on the CD column, as determined by GC−μECD.
Statistical Analyses
Unless stated otherwise, all data are reported as the mean of triplicate incubations ± one standard deviation. Differences of EF values of OH-PCB metabolites from microsomal incubations, and EF values of the racemic OH-PCBs were assessed with Student’s t test. The EF of OH-PCB metabolites from samples racemized for 2 h at 300 °C was considered racemic. All differences were considered statistically significant for p < 0.05.
Results and Discussion
Identification of PCB 51 Metabolites
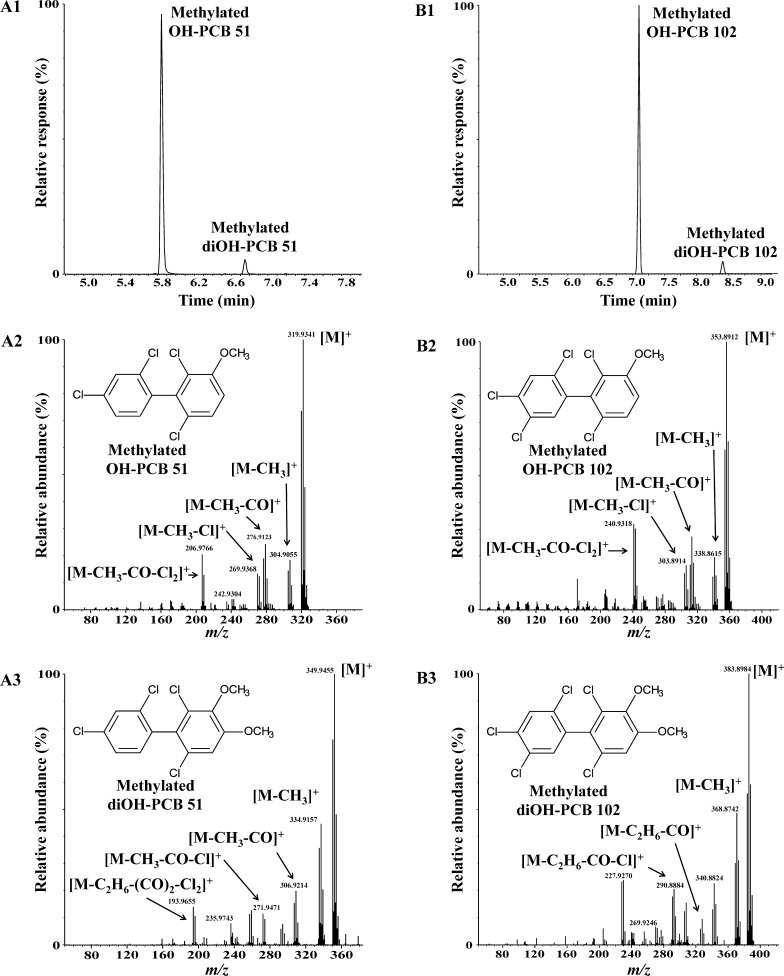
The oxidation of PCB 51 in incubations with liver microsomes prepared from male rats pretreated with PB, a potent inducer of hepatic CYP2B enzymes, resulted in the formation of two metabolites according to GC–MS analysis (Figure 1; panel A1). The molecular ion cluster of the major metabolite, OH-PCB 51 (RT = 5.84 min, RRT = 0.662), matched the theoretical m/z and abundance ratios of a methylated derivative of a monohydroxylated tetrachlorobiphenyl (calculated for C13H8O35Cl4m/z 319.9329, experimental m/z 319.9341; abundance ratio 1:1.4:0.6; Figure 1; panel A2). The fragmentation pattern was consistent with a methylated hydroxylated tetrachlorobiphenyl derivative, with characteristic fragments such as [M − CH3]+, [M − CH3 − CO]+, [M − CH3 − Cl]+, and [M − CH3 − CO − Cl2]+. The same fragmentation pattern has been reported previously for meta or para but not ortho-substituted derivatives of OH-PCBs.35−37 OH-PCB 51 was also formed in incubations with all other microsomal preparations investigated. We posit that OH-PCB 51 corresponds to a meta-hydroxylated metabolite, 2,2′,4,6′-tetrachlorobiphenyl-3′-ol, because CYP2B enzymes, which are present at high levels in microsomes from PB-treated rats, oxidize multiple-ortho substituted PCBs primarily in the meta position.14,15,38 Moreover, ortho-substituted PCBs with a p-chlorine substituent in one phenyl ring are preferentially hydroxylated in the non-para-substituted ring.39 Traces of other monohydroxylated PCB 51 metabolites (as methylated derivatives) were also detected by GC–MS in incubations with several microsomal preparations (see the Supporting Information). These metabolites were present as low levels and could not be further identified.
Figure 1.
Metabolism of prochiral PCB 51 or PCB 102 by rat liver microsomes results in the formation of one major monohydroxylated and a minor dihydroxylated metabolite (analyzed as the corresponding methylated derivatives). (A1) Gas chromatogram showing the presence of two metabolites, OH-PCB 51 and diOH-PCB 51, after incubation of PCB 51 with rat liver microsomes, and mass spectra of (A2) OH-PCB 51 and (A3) diOH-PCB 51 after derivatization with diazomethane. (B1) Chromatogram showing the presence of two metabolites, OH-PCB 102 and diOH-PCB 102, after incubation of PCB 102 with rat liver microsomes, and mass spectra of (B2) OH-PCB 102 and (B3) diOH-PCB 102 after derivatization with diazomethane. See text for additional details regarding the identification of these metabolites. Metabolism studies were performed for 2 h at 37 °C with rat liver microsomes from phenobarbital-pretreated male rats. Hexane/MTBE extracts of a representative incubation were analyzed after methylation of the OH-PCB metabolites with diazomethane by GC–MS as described in the Experimental Section.
The m/z of the monoisotopic molecular ion of the minor PCB 51 metabolite, diOH-PCB 51 (RT = 6.75 min, RRT = 0.765), corresponded to a dimethylated dihydroxylated PCB 51 derivative (calculated for C14H10O235Cl4m/z 349.9435, experimental m/z 349.9455; abundance ratio 1:1.3:0.6; Figure 1; panel A3). The mass spectrum of methylated diOH-PCB 51 showed characteristic fragments, including [M − CH3]+, [M − CH3 − CO]+, [M − C2H6 − CO]+, [M − CH3 − CO − Cl]+, [M − C2H6 − CO − Cl]+, and [M − C2H6 − (CO)2 − Cl2]+, which support the identification of this metabolite as a diOH-PCB 51 metabolite. Similar fragmentation patterns have been observed for other structurally related 4,5-dimethoxylated PCB derivatives.40,41 Based on the RRT, low levels of diOH-PCB 51 (as methylated derivative) were also detected by GC–MS in incubations with liver microsomal preparations prepared from CFA and INH pretreated rats (data not shown) but not in any other microsomal preparations investigated. Because diOH-PCB 51 is likely a secondary metabolite formed from OH-PCB 51, this dihydroxylated metabolite was tentatively identified as 3′,4′-dihydroxy-2,2′,4,6′-tetrachlorobiphenyl. The proposed catechol structure is consistent with in vitro metabolism studies demonstrating that meta-hydroxylated PCBs are metabolized to meta,para-dihydroxylated metabolites by recombinant rat CYP2B1,15 dog liver microsomes,42 and recombinant dog CYP2B11.43
Identification of PCB 102 Metabolites
The oxidation of PCB 102 by liver microsomes prepared from male PB-pretreated rats also resulted in the formation of two metabolites, OH-PCB 102 and diOH-PCB 102 (Figure 1; panel B1). The accurate mass determination and abundance ratios of the molecular ion cluster of the major metabolite, OH-PCB 102, matched a methylated derivative of a monohydroxylated pentachlorobiphenyl (calculated for C13H7O135Cl5m/z 353.8940, experimental m/z 353.8912; abundance ratio 1:1.7:1.1:0.3). The molecular ion cluster of the diOH-PCB 102 derivative was consistent with a dimethylated dihydroxylated PCB 102 metabolite (calculated for C14H9O235Cl5m/z 383.9045, experimental m/z 383.8984; abundance ratio 1:1.7:1.1:0.4). The fragmentation patterns of methylated derivatives of OH-PCB 102 and diOH-PCB 102 were identical to the fragmentation patterns of the PCB 51 metabolites discussed above (Figure 1; panels B2 and B3). OH-PCB 102 (as methylated derivative) was detected by GC–MS in incubations with all microsomal preparations investigated. diOH-PCB 102 was formed in incubations with microsomes prepared from CO, CFA, and INH pretreated rats as well as dogs and hamsters. We tentatively identified OH-PCB 102 as 2,2′,4,5,6′-pentachlorobiphenyl-3′-ol and diOH-PCB 102 as 3′,4′-dihydroxy-2,2′,4,5,6′-pentachlorobiphenyl based on the mass spectrometric evidence and established structure–activity relationships for the metabolism of ortho-substituted PCBs. GC–MS analysis suggested the formation of trace levels of other OH-PCB metabolites in several microsomal incubations that could not be further identified (see the Supporting Information).
Rat P450 Isoform-Dependent Metabolism of Prochiral PCBs
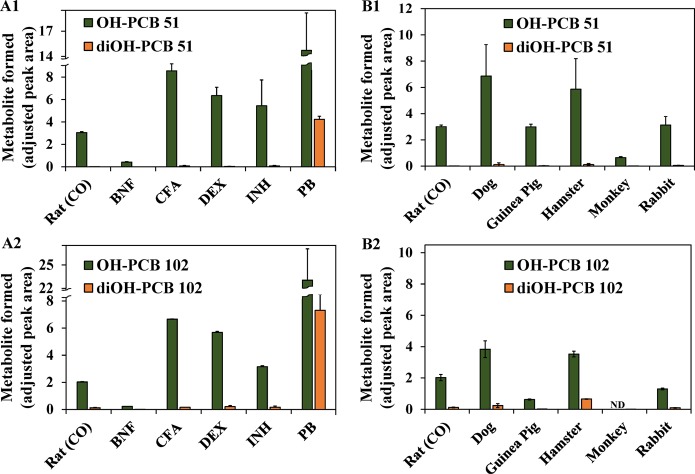
Similar to metabolism studies with PCB 3 (4-chlorobiphenyl)44 and several dichlorobiphenyls,39 we used rat liver microsomes from rats pretreated with the following classical inducers to assess the role of different P450 isoforms in the metabolism of prochiral PCBs: BNF (CYP1A inducer), CFA (CYP4A inducer), DEX (CYP3A inducer), INH (2E1 inducer), and PB (CYP2B inducer) (Figure 2).29,39,45−48 Extracts from microsomal incubations were analyzed using an established GC−μECD method.24,28,49 This method is more sensitive compared to the GC–MS analysis described above. Major metabolites were identified based on their RRTs and quantified relative to the internal standard (PCB 204). The highest levels of OH-PCB 51 were observed in incubations with liver microsomes prepared from male rats pretreated with PB (Figure 2; panel A1). The levels of OH-PCB 51 formed by different rat liver microsomal preparations followed the rank order PB ≫ CFA > DEX > INH ∼ CO ≫ BNF. Similarly, metabolism of PCB 102 resulted in the formation of only one monohydroxylated metabolite, OH-PCB 102 (Figure 2; panel A2). The rank order of the levels of OH-PCB 102 across the different rat liver microsomal preparations were the same as observed with OH-PCB 51. The observation that OH-PCB 51 and OH-PCB 102 are the major metabolites in incubations with microsomes from PB-pretreated rats is consistent with the well-documented CYP2B enzyme-mediated oxidation of ortho-substituted PCBs in the meta position.14,15,39,50
Figure 2.
Cytochrome P450-dependent metabolism of prochiral PCB 51 and PCB 102 to mono- and dihydroxylated metabolites is cytochrome P450 isoform and species dependent (analyzed as the corresponding methylated derivatives). The relative levels of hydroxylated metabolites of (A1) PCB 51 and (A2) PCB 102 differ between incubations with microsomal preparations from rats pretreated with inducers of different P450 enzymes. Moreover, relative levels of hydroxylated metabolites of (B1) PCB 51 and (B2) PCB 102 vary between incubations with liver microsomes obtained from different species. Levels of all OH-PCB metabolites were determined after methylation with diazomethane and are expressed relative to the internal standard because no authentic standards of the metabolites were available. Data are presented as mean ± standard deviation (n = 3 incubations per microsomal preparation; n = 2 for OH-PCB 102 hamster microsomal preparation incubations). Abbreviations of inducers: BNF, β-naphthoflavone; CFA, clofibric acid; DEX, dexamethasone; INH, isoniazid; PB, phenobarbital. ND: below the relative detection limit.
The rank order of OH-PCB 51 and OH-PCB 102 levels suggests that CYP1A, CYP2E, CYP3A, and CYP4A isoforms play only a minor if any role in the metabolism of both prochiral PCBs. Analogously, the profiles and levels of PCB 136 metabolites formed by hepatic microsomes obtained from 3,3′,4,4′,5- pentachlorobiphenyl (PCB 126) (CYP1A inducer) or DEX (CYP3A inducer) pretreated rats did not suggest their formation by CYP1A17 or CYP3A enzymes,29 respectively. Instead, the rank order observed in this study likely reflects the extent of CYP2B induction by different inducers. For example, inducer pretreatment decreases rat liver microsomal pentoxyresorufin-O-dealkylase (CYP2B) activity in the order PB ≫ DEX > CFA > CO.47 Similarly, both 7-(benzyloxy)resorufin-O-debenzylase (CYP2B) activity and PCB 136 biotransformation rates follow the rank order PB ≫ DEX > CO in liver microsomes prepared from male rats,29 which is in agreement with their formation by CYP2B1.14,15,43
diOH-PCB 51 and diOH-PCB 102 were minor metabolites in all rat liver microsomal preparations with the exception of liver microsomes from PB-pretreated rats. This observation suggests that both dihydroxylated metabolites are formed by the CYP2B-mediated oxidation of the corresponding monohydroxylated metabolite. Waller et al. found that a structurally related meta hydroxylated PCB 136 metabolite, 2,2′,3,3′,6,6′-hexachlorobiphenyl-5-ol (5-OH-PCB 136), is metabolized by recombinant rat CYP2B1 or dog CYP2B11 to the corresponding 4,5-dihydroxylated PCB 136 metabolite.43 We previously reported that 2,2′,3,5′,6-pentachlorobiphenyl-5-ol (5-OH-PCB 95) is enantioselectively metabolized by rat CYP2B1 to a 4,5-dihydroxylated metabolite of PCB 95.15 These in vitro metabolism studies did not detect the formation of dihydroxylated PCB metabolites with hydroxyl groups in separate phenyl rings. In contrast, Sundström et al. identified such dihydroxylated PCB metabolite in vivo in rats, mice, and quails,13 a finding that suggests more complex metabolism pathways in vivo.
Species-Dependent Hepatic Metabolism of Prochiral PCBs
Species differences in the metabolism of PCBs 51 and 102 were investigated using liver microsomes prepared from toxicologically and environmentally relevant mammalian species, such as dogs, guinea pigs, hamsters, monkeys, and rabbits.17 As with rat liver microsomes, OH-PCB 51 and OH-PCB 102 were the major metabolite in all incubations with PCB 51 and PCB 102, respectively (Figure 2; panels B1 and B2). Both OH-PCBs were formed in the rank order dog > hamster > rabbit ∼ rat ∼ guinea pig > monkey. A similar rank order for meta hydroxylation has been reported for the metabolism of chiral PCB 136.17 The finding that PCB 51 and PCB 102 are metabolized in the meta position by liver microsomes from different species is consistent with previous studies demonstrating that rat CYP2B1,14 human CYP2B6,51 dog CYP2B11,43 and guinea pig CYP2B18,52 but not rabbit CYP2B4,43 oxidize PCBs in the meta position. Although this has not been demonstrated previously, it is likely that fish, birds, and other animals metabolize multi-ortho-substituted PCBs because their liver microsomes display CYP2B-like activity (determined as 7-pentoxyresorufin-O-deethylase and/or 7-benzyloxyresorufin-O-deethylase activity). However, the respective P450 enzymes are not necessarily members of the CYP2B subfamily and may display a stereoselectivity different from mammalian CYP2B enzymes.12,13,53−55
Enantioselective Analyses
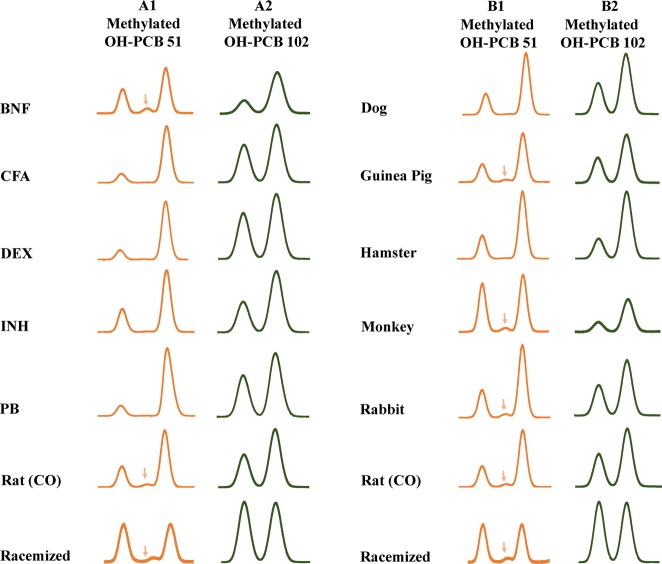
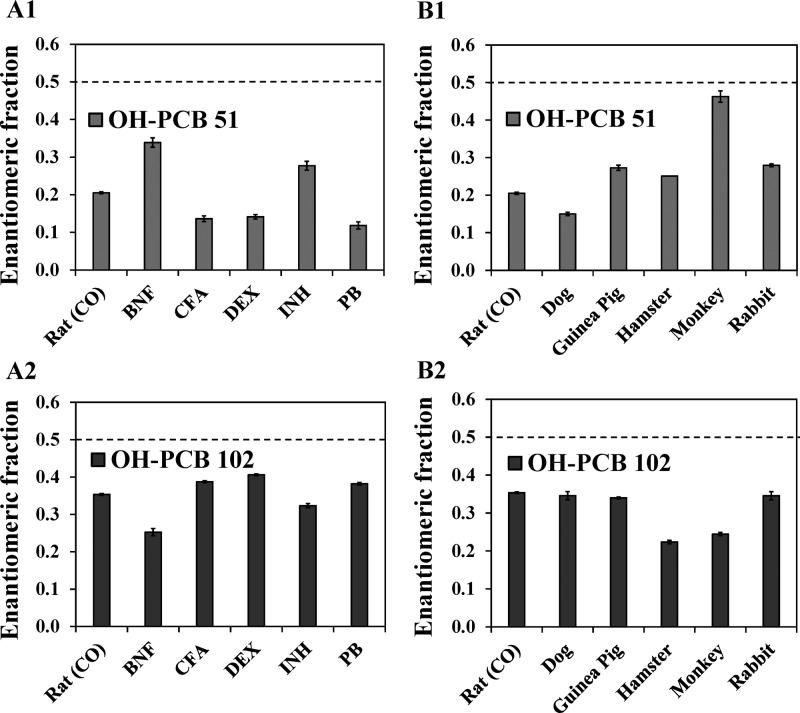
The introduction of a meta hydroxyl group in the 2,6-dichlorinated phenyl ring of PCB 51 and PCB 102 breaks its symmetry relative to the phenyl–phenyl bond, thus resulting in OH-PCB metabolites that are predicted to exist as stable atropisomers under ambient conditions.22 Enantioselective GC−μECD analysis of extracts from different rat liver microsomal incubations with PCB 51 and PCB 102 revealed an enrichment of E2-OH-PCB 51 and E2-OH-PCB 102, respectively (Figures 3; columns A1 and A2). The extent of the enantiomeric enrichment of the OH-PCB metabolites of PCB 51 and PCB 102 differed across these rat liver microsomal preparations (Figure 4). OH-PCB 51 displayed a more pronounced enantiomeric enrichment compared to OH-PCB 102 in incubations with rat liver microsomes. Similarly, the extent of the enantioselective metabolism of chiral PCBs to chiral hydroxylated metabolites is congener specific.28 Moreover, the EF of OH-PCB 51 and OH-PCB 102 varied between different rat liver microsomal preparations. In the case of PCB 51, incubations with rat liver microsomes from PB, CFA and DEX-treated rats resulted in the most pronounced enrichment of E2-OH-PCB 51, whereas the least pronounced enrichment was observed in incubations with microsomes from BNF and INH-treated rats (Figure 4; panel A1). In contrast, experiments using microsomes from BNF and INH-treated rats displayed a more pronounced atropisomeric enrichment of E2-OH-PCB 102 compared to the other rat liver microsomal preparations investigated (Figure 4; panel A2). The extent of the enantiomeric enrichment was also species dependent. The most pronounced atropisomeric enrichment of E2-OH-PCB 51 was observed with dog liver microsomes (Figure 4; panel B1), whereas a near racemic chiral signature was observed with monkey liver microsomes. EF values of OH-PCB 51 followed the rank order monkey > rabbit ∼ guinea pig > hamster > rat > dog. Metabolism of PCB 102 by hamster and monkey liver microsomes resulted in a more pronounced enrichment E2-OH-PCB 102 compared to the other liver microsomes investigated (Figure 4; panel B2). Additional metabolism studies are needed to determine if other, in particular nonmammalian, species also oxidize prochiral PCBs to axially chiral metabolites.
Figure 3.
Second eluting atropisomer (E2) of OH-PCB 51 and OH-PCB 102 is enriched in microsomal incubations irrespective of the inducer-pretreatment or the species (analyzed as the corresponding methylated derivatives). Representative gas chromatograms showing the enrichment of the second eluting atropisomer of OH-PCB 51 (column A1) and OH-PCB 102 (column A2) in incubations with liver microsomes obtained from rats pretreated with BNF, CFA, DEX, INH, PB, or corn oil (CO)-treated rats. The same direction of the atropisomeric enrichment was observed in incubations with liver microsomes prepared from dogs, guinea pigs, hamsters, monkeys, rabbits, and CO-treated rats for OH-PCB 51 (column B1) and OH-PCB 102 (column B2). Samples of OH-PCB 51 or OH-PCB 102 racemized by heating at 300 °C for 2 h are shown for comparison (bottom chromatograms). All extracts containing OH-PCB metabolites were analyzed after methylation with diazomethane and analyzed at 140 °C on a Chirasil-Dex column. An impurity present in control incubations is indicated by a downward arrow. Abbreviations of inducers: BNF, β-naphthoflavone; CFA, clofibric acid; DEX, dexamethasone; INH, isoniazid; PB, phenobarbital.
Figure 4.
Enantiomeric fraction (EF), a measure of the extent of the enantiomeric enrichment, of OH-PCB 51 and OH-PCB 102 depends of the composition of the hepatic cytochrome P450 system in rats and differs between mammalian species (analyzed as the corresponding methylated derivatives). EF values of (A1) OH-PCB 51 and (A2) OH-PCB 102 differ between incubations with microsomal preparations from rats pretreated with inducers of different P450 enzymes. Moreover, EF values (B1) OH-PCB 51 and (B2) OH-PCB 102 vary across incubations with liver microsomes obtained from different species. EF values were determined by valley drop method. The dotted line corresponds to the average EF value of representative samples after racemization at 300 °C for 2 h. Extracts from incubations with PCB 51 or PCB 102 were analyzed a Chirasil-Dex column at 140 °C after methylation of the OH-PCB metabolites with diazomethane. Data are presented as mean ± standard deviation (n = 3 incubations per microsomal preparation; n = 2 for OH-PCB 102 hamster microsomal preparation incubations). With the exception of monkey microsomal preparation, all EF values are significantly different from EF of corresponding racemic OH-PCBs (t test, p < 0.05). Abbreviations of inducers: BNF, β-naphthoflavone; CFA, clofibric acid; DEX, dexamethasone; INH, isoniazid; PB, phenobarbital.
In previous metabolism studies, the direction of the enantiomeric enrichment of OH-PCB 136 metabolites was also identical in incubations with rat liver microsomes and precision-cut tissue slices prepared from animals pretreated with PB or DEX.28,29 Specifically, E2-5-OH-PCB 136 and E1-4-OH-PCB 136 were enriched in microsomal incubations in the previous study, irrespective of inducer pretreatment. Incubations of PCB 51 and PCB 102 with liver microsomes from different species also resulted in an enrichment of E2-OH-PCB 51 and E2-OH-PCB 102, respectively (Figure 3; columns B1 and B2). A previously published metabolism study with PCB 136 similarly revealed a consistent enrichment of E2-5-OH-PCB 136 and E2-4-OH-PCB 136 in incubations with microsomes from dogs, guinea pigs, monkeys, and rabbits;17 however, there were differences in the direction of the enantiomeric enrichment of OH-PCBs for incubations with some microsomal preparations, such as liver microsomes obtained from mice. Such differences in the direction of the enantiomeric enrichment of PCB metabolites in our earlier study are likely due to congener- and species-specific differences in the enantioselectivity oxidation of PCBs by different P450 isoforms.
To demonstrate that the two peaks observed in the enantioselective analyses are indeed atropisomers of chiral PCB metabolites and not two different PCB metabolites, an aliquot of extracts containing OH-PCB 51 or OH-PCB 102, as methylated derivatives, was heated at 300 °C for 2 h. Subsequent enantioselective analysis revealed essentially a 1:1 peak ratio for the two OH-PCB atropisomer peaks due to racemization (for representative chromatograms showing the racemization of methylated derivatives of OH-PCB 51 and OH-PCB 102, see Figure 3). Similarly, heating pure PCB atropisomer above their rotational energy barrier results in their rapid racemization.32 In addition, selected metabolite extracts from incubations with microsomes from dexamethasone-pretreated rats were analyzed after derivatization by enantioselective GC–MS to confirm that the metabolites indeed have m/z’s of methylated OH-PCBs (Figure S25). Taken together, these results demonstrate that OH-PCB 51 and OH-PCB 102 are chiral compounds and that their atropisomers can be separated on conventional enantioselective columns.
Environmental Implications
The observation that axially chiral metabolites of environmental contaminants, such as PCBs, can be formed enantioselectively from prochiral parent compounds has broader implications for studies of their environmental fate and transport. It is well established that prochiral molecules, in particular, drugs, can undergo enantioselective biotransformation to metabolites containing a chiral center.56 For example, prochiral methyl groups or thioethers can be oxidized to chiral secondary alcohols or sulfoxides, respectively, and prochiral ketones can be reduced to secondary alcohols. Similarly, a variety of prochiral environmental contaminants, for example, the herbicide atrazine and the organophosphate insecticide fenamiphos, can form biotransformation products containing a chiral center. Oxidation of the isopropyl group of atrazine by cytochrome P450 enzymes results in the enantioselective formation of a chiral isopropyl-hydroxylated atrazine metabolite.57 The oxidation of prochiral thioethers, such as fenamiphos, results in the formation of chiral sulfoxides.58 In addition to PCBs, several other classes of environmental contaminants include axially prochiral compounds, such as polybrominated biphenyls, polychlorinated terphenyls, and acetamide pesticides (e.g., acetolachlor), and can theoretically be metabolized to axially chiral compounds. Moreover, many natural products and drug molecules contain biaryl or heterobiaryl moieties59 and, as the use of these chemicals increases, may become axially prochiral (or chiral) contaminants of environmental concern. Our study demonstrates that the biotransformation of these axially prochiral chemicals likely results in the enantioselective formation of axially chiral metabolites. In contrast, abiotic transformation processes are not enantioselective and should result in the formation of racemic transformation products. Therefore, chiral signatures of axially chiral transformation products of prochiral environmental contaminants, such as PCBs, represent an unexplored but potentially powerful tool to gain novel insights into the environmental fate and transport of their prochiral parent compounds.
Acknowledgments
We thank Dr. Izabela Kania-Korwel, Dr. Lynn Teesch, and Mr. Vic Parcell for help with chemical analysis. A.M.M. thanks the Interdisciplinary Summer Undergraduate Research Program for the opportunity to do research in Dr. Lehmler’s laboratory. This work was supported by grants ES05605, ES013661, and ES017425 from the National Institute of Environmental Health Sciences, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.6b05387.
Details of the liver microsomal preparations under investigation; effect of temperature on the atropselective separations; GC–MS data of OH-PCB metabolites formed in incubations with different microsomal preparations; atropselective GC–MS analyses showing OH-PCB 51 and OH-PCB 102 atropisomers (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Grimm F. A.; Hu D.; Kania-Korwel I.; Lehmler H. J.; Ludewig G.; Hornbuckle K. C.; Duffel M. W.; Bergman A.; Robertson L. W. Metabolism and metabolites of polychlorinated biphenyls. Crit. Rev. Toxicol. 2015, 45, 245–272. 10.3109/10408444.2014.999365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayne S.; Forest K. pK(a) values of the monohydroxylated polychlorinated biphenyls (OH-PCBs), polybrominated biphenyls (OH-PBBs), polychlorinated diphenyl ethers (OH-PCDEs), and polybrominated diphenyl ethers (OH-PBDEs). J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng. 2010, 45, 1322–1346. 10.1080/10934529.2010.500885. [DOI] [PubMed] [Google Scholar]
- Tehrani R.; Van Aken B. Hydroxylated polychlorinated biphenyls in the environment: sources, fate, and toxicities. Environ. Sci. Pollut. Res. 2014, 21, 6334–6345. 10.1007/s11356-013-1742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R. F.; Martinez A.; Hornbuckle K. C. Discovery of hydroxylated polychlorinated biphenyls (OH-PCBs) in sediment from a Lake Michigan waterway and original commercial Aroclors. Environ. Sci. Technol. 2013, 47, 8204–8210. 10.1021/es402323c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. N.; Hites R. A. OH radical reactions: The major removal pathway for polychlorinated biphenyls from the atmosphere. Environ. Sci. Technol. 1996, 30, 1756–1763. 10.1021/es950765k. [DOI] [Google Scholar]
- Mandalakis M.; Berresheim H.; Stephanou E. G. Direct evidence for destruction of polychlorobiphenyls by OH radicals in the subtropical troposphere. Environ. Sci. Technol. 2003, 37, 542–547. 10.1021/es020163i. [DOI] [PubMed] [Google Scholar]
- Ueno D.; Darling C.; Alaee M.; Campbell L.; Pacepavicius G.; Teixeira C.; Muir D. Detection of hydroxylated polychlorinated biphenyls (OH-PCBs) in the abiotic environment: Surface water and precipitation from Ontario, Canada. Environ. Sci. Technol. 2007, 41, 1841–1848. 10.1021/es061539l. [DOI] [PubMed] [Google Scholar]
- Sietmann R.; Gesell M.; Hammer E.; Schauer F. Oxidative ring cleavage of low chlorinated biphenyl derivatives by fungi leads to the formation of chlorinated lactone derivatives. Chemosphere 2006, 64, 672–685. 10.1016/j.chemosphere.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Zhai G.; Lehmler H. J.; Schnoor J. L. Identification of hydroxylated metabolites of 3,3′,4,4′-tetrachlorobiphenyl and metabolic pathway in whole poplar plants. Chemosphere 2010, 81, 523–528. 10.1016/j.chemosphere.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C.; Zhai G.; Wu H.; Kania-Korwel I.; Lehmler H. J.; Schnoor J. L. Identification of a novel hydroxylated metabolite of 2,2′,3,5′,6-pentachlorobiphenyl formed in whole poplar plants. Environ. Sci. Pollut. Res. 2016, 23, 2089–2098. 10.1007/s11356-015-5939-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai G.; Lehmler H. J.; Schnoor J. L. Inhibition of cytochromes P450 and the hydroxylation of 4-monochlorobiphenyl in whole poplar. Environ. Sci. Technol. 2013, 47, 6829–6835. 10.1021/es304298m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman A. H.; Wong C. S.; Chow E. A.; Brown S. B.; Solomon K. R.; Fisk A. T. Biotransformation of polychlorinated biphenyls (PCBs) and bioformation of hydroxylated PCBs in fish. Aquat. Toxicol. 2006, 78, 176–185. 10.1016/j.aquatox.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Sundström G.; Jansson B. The metabolism of 2,2′,3,5′,6-pentachlorobiphenyl in rats, mice and quails. Chemosphere 1975, 4, 361–370. 10.1016/0045-6535(75)90032-6. [DOI] [Google Scholar]
- Warner N. A.; Martin J. W.; Wong C. S. Chiral polychlorinated biphenyls are biotransformed enantioselectively by mammalian cytochrome P-450 isozymes to form hydroxylated metabolites. Environ. Sci. Technol. 2009, 43, 114–121. 10.1021/es802237u. [DOI] [PubMed] [Google Scholar]
- Lu Z.; Kania-Korwel I.; Lehmler H. J.; Wong C. S. Stereoselective formation of mono- and dihydroxylated polychlorinated biphenyls by rat cytochrome P450 2B1. Environ. Sci. Technol. 2013, 47, 12184–12192. 10.1021/es402838f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.; Barnhart C.; Lein P. J.; Lehmler H. J. Hepatic metabolism affects the atropselective disposition of 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB 136) in mice. Environ. Sci. Technol. 2015, 49, 616–625. 10.1021/es504766p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.; Kammerer A.; Lehmler H. J. Microsomal oxidation of 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB 136) results in species-dependent chiral signatures of the hydroxylated metabolites. Environ. Sci. Technol. 2014, 48, 2436–2444. 10.1021/es405433t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I.; Duffel M. W.; Lehmler H.-J. Gas chromatographic analysis with chiral cyclodextrin phases reveals the enantioselective formation of hydroxylated polychlorinated biphenyls by rat liver microsomes. Environ. Sci. Technol. 2011, 45, 9590–9596. 10.1021/es2014727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I.; Lehmler H. J. Chiral polychlorinated biphenyls: absorption, metabolism and excretion-a review. Environ. Sci. Pollut. Res. 2016, 23, 2042–2057. 10.1007/s11356-015-4150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmler H.-J.; Harrad S. J.; Huhnerfuss H.; Kania-Korwel I.; Lee C. M.; Lu Z.; Wong C. S. Chiral polychlorinated biphenyl transport, metabolism, and distribution: A review. Environ. Sci. Technol. 2010, 44, 2757–2766. 10.1021/es902208u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter W. Gas chromatographic enantiomer separation of polychlorinated biphenyls (PCBs): Methods, metabolisms, enantiomeric composition in environmental samples and their interpretation. Isr. J. Chem. 2016, 56, 940–957. 10.1002/ijch.201600089. [DOI] [Google Scholar]
- Nezel T.; Müller-Plathe F. Theoretical considerations about chiral PCBs and their methylthio and methylsulfonyl metabolites being possibly present as stable enantiomers. Chemosphere 1997, 35, 1895–1906. 10.1016/S0045-6535(97)00229-4. [DOI] [Google Scholar]
- Zhai G.; Hu D.; Lehmler H.-J.; Schnoor J. L. Enantioselective biotransformation of chiral PCBs in whole poplar plants. Environ. Sci. Technol. 2011, 45, 2308–2316. 10.1021/es1033662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I.; Barnhart C. D.; Stamou M.; Truong K. M.; El-Komy M. H.; Lein P. J.; Veng-Pedersen P.; Lehmler H.-J. 2,2′,3,5′,6-Pentachlorobiphenyl (PCB 95) and its hydroxylated metabolites are enantiomerically enriched in female mice. Environ. Sci. Technol. 2012, 46, 11393–11401. 10.1021/es302810t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I.; Vyas S. M.; Song Y.; Lehmler H.-J. Gas chromatographic separation of methoxylated polychlorinated biphenyl atropisomers. J. Chromatogr. A 2008, 1207, 146–154. 10.1016/j.chroma.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.; Duffel M.; Lehmler H.-J. Oxidation of polychlorinated biphenyls by liver tissue slices from phenobarbital-pretreated mice is congener-specific and atropselective. Chem. Res. Toxicol. 2013, 26, 1642–1651. 10.1021/tx400229e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black T. H. The preparation and reactions of diazomethane. Aldrichimica Acta 1983, 16, 3–10. [Google Scholar]
- Wu X.; Kania-Korwel I.; Chen H.; Stamou M.; Dammanahalli K. J.; Duffel M.; Lein P. J.; Lehmler H.-J. Metabolism of 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB 136) atropisomers in tissue slices from phenobarbital or dexamethasone-induced rats is sex-dependent. Xenobiotica 2013, 43, 933–947. 10.3109/00498254.2013.785626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.; Pramanik A.; Duffel M. W.; Hrycay E. G.; Bandiera S. M.; Lehmler H.-J.; Kania-Korwel I. 2,2′,3,3′,6,6′-Hexachlorobiphenyl (PCB 136) is enantioselectively oxidized to hydroxylated metabolites by rat liver microsomes. Chem. Res. Toxicol. 2011, 24, 2249–2257. 10.1021/tx200360m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I.; Hornbuckle K. C.; Peck A.; Ludewig G.; Robertson L. W.; Sulkowski W. W.; Espandiari P.; Gairola C. G.; Lehmler H.-J. Congener specific tissue distribution of Aroclor 1254 and a highly chlorinated environmental PCB mixture in rats. Environ. Sci. Technol. 2005, 39, 3513–3520. 10.1021/es047987f. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I.; Shaikh N. S.; Hornbuckle K. C.; Robertson L. W.; Lehmler H.-J. Enantioselective disposition of PCB 136 (2,2′,3,3′,6,6′-hexachlorobiphenyl) in C57BL/6 mice after oral and intraperitoneal administration. Chirality 2007, 19, 56–66. 10.1002/chir.20342. [DOI] [PubMed] [Google Scholar]
- Harju M. T.; Haglund P. Determination of the rotational energy barriers of atropisomeric polychlorinated biphenyls. Fresenius' J. Anal. Chem. 1999, 364, 219–223. 10.1007/s002160051327. [DOI] [Google Scholar]
- Commission Decision EC 2002/657 of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Communities Legis. 2002, 221, 0008–0036. [Google Scholar]
- Harner T.; Wiberg K.; Norstrom R. Enantiomer fractions are preferred to enantiomer ratios for describing chiral signatures in environmental analysis. Environ. Sci. Technol. 2000, 34, 218–220. 10.1021/es9906958. [DOI] [Google Scholar]
- Li X.; Robertson L. W.; Lehmler H.-J. Electron ionization mass spectral fragmentation study of sulfation derivatives of polychlorinated biphenyls. Chem. Cent. J. 2009, 3, 5. 10.1186/1752-153X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman Å.; Klasson Wehler E.; Kuroki H.; Nilsson A. Synthesis and mass spectrometry of some methoxylated PCB. Chemosphere 1995, 30, 1921–1938. 10.1016/0045-6535(95)00073-H. [DOI] [Google Scholar]
- Jansson B.; Sundström G. Mass spectrometry of the methyl ethers of isomeric hydroxychlorobiphenyls—potential metabolites of chlorobiphenyls. Biol. Mass Spectrom. 1974, 1, 386–392. 10.1002/bms.1200010605. [DOI] [PubMed] [Google Scholar]
- Ishida C.; Koga N.; Hanioka N.; Saeki H. K.; Yoshimura H. Metabolism in vitro of 3,4,3′,4′- and 2,5,2′,5′-tetrachlorobiphenyl by rat liver microsomes and highly purified cytochrome P-450. J. Pharmacobio-Dyn. 1991, 14, 276–284. 10.1248/bpb1978.14.276. [DOI] [PubMed] [Google Scholar]
- Kennedy M. W.; Carpentier N. K.; Dymerski P. P.; Kaminsky L. S. Metabolism of dichlorobiphenyls by hepatic microsomal cytochrome P-450. Biochem. Pharmacol. 1981, 30, 577–588. 10.1016/0006-2952(81)90129-5. [DOI] [PubMed] [Google Scholar]
- Haraguchi K.; Kato Y.; Koga N.; Degawa M. Metabolism of polychlorinated biphenyls by Gunn rats: Identification and serum retention of catechol metabolites. Chem. Res. Toxicol. 2004, 17, 1684–1691. 10.1021/tx0498096. [DOI] [PubMed] [Google Scholar]
- Joshi S. N.; Vyas S. M.; Duffel M. W.; Parkin S.; Lehmler H.-J. Synthesis of sterically hindered polychlorinated biphenyl derivatives. Synthesis 2011, 2011, 1045–1054. 10.1055/s-0030-1258454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyoshi N.; Koga N.; Oguri K.; Yoshimura H. Metabolism of 2,4,5,2',4',5'-hexachlorobiphenyl with liver microsomes of phenobarbital-treated dog; the possible formation of PCB 2, 3-arene oxide intermediate. Xenobiotica 1992, 22, 1275–1290. 10.3109/00498259209053156. [DOI] [PubMed] [Google Scholar]
- Waller S. C.; He Y. A.; Harlow G. R.; He Y. Q.; Mash E. A.; Halpert J. R. 2,2′,3,3′,6,6′-Hexachlorobiphenyl hydroxylation by active site mutants of cytochrome P450 2B1 and 2B11. Chem. Res. Toxicol. 1999, 12, 690–699. 10.1021/tx990030j. [DOI] [PubMed] [Google Scholar]
- McLean M. R.; Bauer U.; Amaro A. R.; Robertson L. W. Identification of catechol and hydroquinone metabolites of 4-monochlorobiphenyl. Chem. Res. Toxicol. 1996, 9, 158–164. 10.1021/tx950083a. [DOI] [PubMed] [Google Scholar]
- Hrycay E. G.; Bandiera S. M. Spectral interactions of tetrachlorobiphenyls with hepatic microsomal cytochrome P-450 enzymes. Chem.-Biol. Interact. 2003, 146, 285–296. 10.1016/j.cbi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I.; Hrycay E. G.; Bandiera S. M.; Lehmler H.-J. 2,2′,3,3′,6,6′-Hexachlorobiphenyl (PCB 136) atropisomers interact enantioselectively with hepatic microsomal cytochrome P450 enzymes. Chem. Res. Toxicol. 2008, 21, 1295–1303. 10.1021/tx800059j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortelboer H. M.; Dekruif C. A.; Vaniersel A. A. J.; Falke H. E.; Noordhoek J.; Blaauboer B. J. Comparison of cytochrome P450 isoenzyme profiles in rat-liver and hepatocyte cultures - the effects of model inducers on apoproteins and biotransformation activities. Biochem. Pharmacol. 1991, 42, 381–390. 10.1016/0006-2952(91)90726-L. [DOI] [PubMed] [Google Scholar]
- Madan A.; DeHaan R.; Mudra D.; Carroll K.; LeCluyse E.; Parkinson A. Effect of cryopreservation on cytochrome P-450 enzyme induction in cultured rat hepatocytes. Drug Metab. Dispos. 1999, 27, 327–335. [PubMed] [Google Scholar]
- Stamou M.; Uwimana E.; Flannery B. M.; Kania-Korwel I.; Lehmler H. J.; Lein P. J. Subacute nicotine co-exposure has no effect on 2,2′,3,5′,6-pentachlorobiphenyl disposition but alters hepatic cytochrome P450 expression in the male rat. Toxicology 2015, 338, 59–68. 10.1016/j.tox.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky L. S.; Kennedy M. W.; Adams S. M.; Guengerich F. P. Metabolism of dichlorobiphenyls by highly purified isozymes of rat liver cytochrome P-450. Biochemistry 1981, 20, 7379–7384. 10.1021/bi00529a009. [DOI] [PubMed] [Google Scholar]
- Ariyoshi N.; Oguri K.; Koga N.; Yoshimura H.; Funae Y. Metabolism of highly persistent PCB congener, 2,4,5,2′,4′,5′-hexachlorobiphenyl, by human CYP2B6. Biochem. Biophys. Res. Commun. 1995, 212, 455–460. 10.1006/bbrc.1995.1991. [DOI] [PubMed] [Google Scholar]
- Koga N.; Kikuichi N.; Kanamaru T.; Kuroki H.; Matsusue K.; Ishida C.; Ariyoshi N.; Oguri K.; Yoshimura H. Metabolism of 2,3′,4′,5-tetrachlorobiphenyl by cytochrome P450 from rats, guinea pigs and hamsters. Chemosphere 1998, 37, 1895–1904. 10.1016/S0045-6535(98)00256-2. [DOI] [PubMed] [Google Scholar]
- Buckman A. H.; Brown S. B.; Small J.; Muir D. C. G.; Parrott J.; Solomon K. R.; Fisk A. T. Role of temperature and enzyme induction in the biotransformation of polychlorinated biphenyls and bioformation of hydroxylated polychlorinated biphenyls by rainbow trout (Oncorhynchus mykiss). Environ. Sci. Technol. 2007, 41, 3856–3863. 10.1021/es062437y. [DOI] [PubMed] [Google Scholar]
- Borlakoglu J. T.; Wilkins J. P. G. Metabolism of di-, tri-, tetra-, penta- and hexachlorobiphenyls by hepatic microsomes isolated from control animals and animals treated with Aroclor 1254, a commercial mixture of polychlorinated biphenyls (PCBs). Comp. Biochem. Physiol., C: Comp. Pharmacol. 1993, 105, 95–106. 10.1016/0742-8413(93)90064-R. [DOI] [PubMed] [Google Scholar]
- Borlakoglu J. T.; Wilkins J. P.; Quick M. P.; Walker C. H.; Dils R. R. Metabolism of [14C]4-chlorobiphenyl by hepatic microsomes isolated from razorbills, pigeons and rats. Comp. Biochem. Physiol., C: Comp. Pharmacol. 1991, 99, 287–291. 10.1016/0742-8413(91)90243-M. [DOI] [PubMed] [Google Scholar]
- Caldwell J. Stereochemical determinants of the nature and consequences of drug-metabolism. J. Chromatogr. A 1995, 694, 39–48. 10.1016/0021-9673(94)00812-N. [DOI] [PubMed] [Google Scholar]
- Lang D.; Criegee D.; Grothusen A.; Saalfrank R. W.; Bocker R. H. In vitro metabolism of atrazine, terbuthylazine, ametryne, and terbutryne in rats, pigs, and humans. Drug Metab. Dispos. 1996, 24, 859–865. [PubMed] [Google Scholar]
- Cai X. Y.; Xiong W. N.; Xia T. L.; Chen J. W. Probing the stereochemistry of successive sulfoxidation of the insecticide fenamiphos in soils. Environ. Sci. Technol. 2014, 48, 11277–11285. 10.1021/es502834v. [DOI] [PubMed] [Google Scholar]
- Bringmann G.; Price Mortimer A. J.; Keller P. A.; Gresser M. J.; Garner J.; Breuning M. Atroposelective synthesis of axially chiral biaryl compounds. Angew. Chem., Int. Ed. 2005, 44, 5384–5427. 10.1002/anie.200462661. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.