Abstract
Objective
The aim of this study was to investigate educational differences in treatment responses to memory, reasoning, and speed of processing cognitive training relative to no-contact control.
Methods
Secondary analyses of the Advanced Cognitive Training for Independent and Vital Elderly trial were conducted. Two thousand eight hundred older adults were randomized to memory, reasoning, or speed of processing training or no-contact control. A repeated-measures mixed-effects model was used to investigate immediate post-training and 1-year outcomes with sensitivity analyses out to 10 years. Outcomes were as follows: (1) memory composite of Hopkins Verbal Learning Test, Rey Auditory Verbal Learning Test, and Rivermead Behavioral Memory Test; (2) reasoning composite of letter series, letter sets, and word series; and (3) speed of processing measured using three trials of useful field of view and the digit symbol substitution test.
Results
The effects of reasoning and memory training did not differ by educational attainment. The effect of speed of processing training did. Those with fewer than 12 years of education experienced a 50% greater effect on the useful field of view test compared with those with 16 or more years of education. The training advantage for those with fewer than 12 years of education was maintained to 3 years post-training.
Conclusion
Older adults with less than a secondary education are at elevated risk of dementia, including Alzheimer’s disease. The analyses here indicate that speed of processing training is effective in older adults with low educational attainment.
Keywords: older adults, cognitive training, education, cognition
Introduction
The 2015 Institute of Medicine Report Cognitive Aging recommended that research organizations “examine risk factors and interventions in under-studied and vulnerable populations” (Medicine, 2015). Recent estimates of population-attributable risks for Alzheimer’s disease (AD) identify low educational attainment as responsible for 19% of AD cases worldwide, the largest of seven modifiable risk factors (Norton et al., 2014). Thus, adults with low educational attainment represent a vulnerable population in terms of cognitive impairment and dementia risk.
Low educational attainment, however, may not be associated with a faster rate of cognitive decline. In analyses of six independent samples from four different countries, Piccinin et al. (2013) failed to find an association between education and cognitive decline in four of the six samples. Similarly, Zahodne et al. (2014) identified an effect of years of education on cognitive decline only among those with fewer than 10 years of education. Moreover, an imaging study found similar rates of cognitive decline across educational categories among older adults with normal brains and among older adults with underlying cerebrovascular or β-amyloid pathology (Vemuri et al., 2015). This evidence has led some to conclude that education improves cognitive reserve as measured by cognitive performance tests and that higher test performance provides some reserve against the insults of brain pathology (Vemuri et al., 2015).
Evidence of an effect of education on cognitive reserve (Larson et al., 2013; Meng and D’Arcy, 2012; Zahodne et al., 2014) raises the question of what interventions can be offered to the hundreds of millions of adults worldwide who have not achieved upper-level secondary education—an education threshold used in a recent analysis of global population-attributable risks for AD (Norton et al., 2014). Among the 32 relatively developed countries of the Organization of Economic Cooperation and Development (OECD), over one-third of adults over 55 years of age have not completed any secondary education (Sciences, 2015). According to the U.S. Census, seven million of today’s US older adults did not complete upper-level secondary education (i.e., no high school degree), and there are almost nine million US adults in the soon-to-be-older baby boom generation who did not complete secondary education (Census, 2015).
Emerging evidence points to some promising interventions for bolstering cognitive reserve in adults. For example, a recent analysis found that self-reported frequency of cognitive activity in adulthood was positively associated with cognitive function independent of age, neuropathology, or years of education (Wilson et al., 2013). The authors concluded that “more frequent cognitive activity can counterbalance the cognitive loss associated with neuropathology” (Wilson et al., 2013). Moreover, the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) randomized controlled trial (Willis et al., 2006) and other trials (Smith et al., 2009; Wolinsky et al., 2013) showed that short-term targeted cognitive training improves cognitive function in older adults.
What is not known is whether cognitive training in later adulthood is equally effective in improving cognitive function in those who did not complete upper-level secondary education. The three major cognitive training trials to date have enrolled very well-educated older adults with 80–95% having completed upper-level secondary education (Ball et al., 2002; Smith et al., 2009; Wolinsky et al., 2013). Five smaller trials had samples with the same restricted range of education (Ball et al., 2007).
However, the ACTIVE study was a large multi-site randomized trial. The ACTIVE sample size is large enough to allow secondary analyses of training effects by educational attainment. No prior reports have compared educational differences in mean improvement from baseline for subjects in a cognitive training arm relative to mean improvement for subjects in a control arm. It was hypothesized that participants with less than an upper-level secondary education (i.e., fewer than 12 years) experienced larger training gains than did participants with an upper-level secondary education or more.
Methods
Advanced Cognitive Training for Independent and Vital Elderly study design
Advanced Cognitive Training for Independent and Vital Elderly was a multi-site, randomized, controlled clinical trial (Jobe et al., 2001; Ball et al., 2002). Participants were randomized to no-contact control, memory, reasoning, or speed of processing training. ACTIVE focused on these trainings because prior research indicated these abilities show early age-related decline and are related to activities of daily living. Interventions were conducted in small groups in ten 60- to 75-min sessions over 5–6 weeks. Memory training focused on improving verbal episodic memory through instruction and practice in strategy use. Reasoning training focused on improving the ability to solve problems that contained a serial pattern. Speed training focused on visual search and the ability to process increasingly more information presented in successively shorter inspection times. All groups showed declines in memory, speed of processing, and reasoning at the end of the trial, but those in the speed of processing and reasoning arms showed less decline than did those in the memory or control arm. The benefits of speed of processing and reasoning training were maintained up to a 10-year follow-up (Rebok et al., 2014).
Advanced Cognitive Training for Independent and Vital Elderly study sample
Recruitment occurred in six metropolitan areas using a variety of sampling strategies. Community-dwelling adults aged 65 years and older were eligible. Persons were excluded if they had significant cognitive dysfunction (score< 23 on the mini-mental state examination (MMSE)) (Folstein et al., 1975); functional impairment (dependence or regular assistance in activities of daily living on minimum data set home care) (Morris et al., 1997); self-reported diagnoses of AD, stroke within the last 12 months, or certain cancers; current chemotherapy or radiation therapy; or poor vision, hearing, or communicative ability that would have interfered with the interventions or outcome assessments. Enrollment resulted in a sample of 2802 individuals. Eligible participants were randomly assigned to one of three treatment arms (memory, reasoning, or speed training) or a no-contact control group. Screening and baseline assessment took place before randomization. Study procedures were approved by the institutional review boards at the collaborating institutions, and all subjects gave informed consent to participate.
Measures
Advanced Cognitive Training for Independent and Vital Elderly covariates were primarily limited to sociodemographic and health measures. These were included as covariates in the models reported on here to adjust for well-known educational differences. Eligibility and demographic data (age, gender, race, and marital status) were gathered in telephone and in-person screenings. Health history (self-report of type 2 diabetes, myocardial infarction, angina, congestive heart failure, stroke, hypertension, high cholesterol, and current alcohol use), measured height and weight, physical function status (Short-Form 36) (Ware and Sherbourne, 1992), MMSE (Folstein et al., 1975), and cognitive measures (see the succeeding texts) were gathered via in-person examinations in individual and small-group formats. Depressive symptoms were measured with a 12-item version of the Center for Epidemiologic Studies Depression scale (Radloff, 1977) via a self-report questionnaire.
Education was self-reported as years of completed schooling. Education was categorized into four categories: (1) did not complete upper secondary education (i.e., fewer than 12 years of education); (2) completed upper secondary education (i.e., 12 years); (3) completed some tertiary education (i.e., 13–15 years); and (4) completed tertiary education (i.e., 16 or more years).
Cognitive outcomes include four cognitive performance measures used in prior investigations of ACTIVE data (Kuo et al., 2006). These measures of basic mental ability were gathered at each occasion of measurement (baseline, immediate post-training, and 1-, 2-, 3-, 5-, and 10-year follow-up). First, memory ability was measured using the Hopkins Verbal Learning Test (a total of three learning trials) (Brandt, 1991), Rey Auditory Verbal Learning Test (a total of five learning trials) (Rey, 1941), and Rivermead Behavioral Memory Test (immediate recall) (Wilson et al., 1985). Second, reasoning ability was measured using letter series (total correct) (Thurstone and Thurstone, 1949), letter sets (total correct) (Ekstrom et al., 1976), and word series (total correct) (Gonda, 1985). Third, speed of processing ability was measured using three tasks of useful field of view (UFOV) (Owsley et al., 1998) and the digit symbol substitution (DSS) test (Wechsler, 1981). Scores of each test were transformed using the Blom transformation, and the composite scores were created by averaging the individual Blom-transformed test scores (Blom, 1958). The Blom transformation was used to standardize the individual tests in each cognitive domain to have equal weights on the composite score and to reduce the skewness in the measures in order for the scores to be more normally distributed. The UFOV cognitive outcome measure was scored based on the presentation time needed to correctly perform the task 75% of the time; a higher score indicates poorer cognitive performance. For memory and reasoning composite scores and the DSS, a higher score indicates better cognitive performance.
Statistical analysis
Descriptive data are presented for each of the covariates and cognitive measures for each of the four education categories. Continuous variables are summarized using means and standard deviations. Differences across the education categories were evaluated using the nonparametric Kruskal–Wallis test. Categorical variables are presented using frequencies and proportions. Their association with education level was assessed using the Pearson chi-squared test.
A repeated-measures mixed-effects model was used to investigate immediate post-training and 1-year outcomes for each of the four cognitive outcomes. Fixed effects of the model included time (baseline, post-training, and 1 year post-training) treated as a categorical variable, education level, training group (memory, reasoning, speed, and control), and all of the two-way and three-way interaction terms between these three variables. The time variable was considered as a categorical variable because of the nonlinear nature of the cognitive trajectories. Random effect included subject-specific random intercept, which accounted for the correlation among repeatedly measured cognitive outcomes at multiple time points for a single subject. Because prior research has shown that cognitive training only improves the targeted cognitive ability, only the difference in the cognitive outcome targeted by the training was evaluated. That is, only the memory training effect on memory outcome, reasoning training effect on reasoning outcome, and speed training effect on UFOV and DSS were evaluated. The net training effects immediately post-training and at 1 year were defined as the mean improvement from baseline for subjects in a training arm relative to the mean improvement for subjects in the control arm and were estimated based on the mixed-effects model. Following prior research of the ACTIVE study, results are presented as effect sizes, defined as the net training effect divided by the intra-subject standard deviation (CDATA-Cohen, 1988), so that different cognitive outcomes could be compared. Baseline covariates included in the models to obtain adjusted training effects were age, female sex, minority race, married, body mass index, current smoker, alcohol use, Short-Form 36 physical functioning, Center for Epidemiologic Studies Depression score, hypertension, type 2 diabetes, stroke, congestive heart failure, ischemic heart disease, high cholesterol, myocardial infarction, MMSE score, visual acuity, and field site. Sensitivity analyses were conducted to determine the longevity of differential training gains (2, 3, 5, and 10 years post-training) and estimated in a model with education as a continuous variable. All analyses were conducted using SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
Analyses included 320 ACTIVE participants with fewer than 12 years of education, 797 with 12 years, 906 with 13–15 years, and 777 with 16 or more years of education. Table 1 compares baseline characteristics by these levels. The less-educated group was slightly older, was less likely married, and consisted of more minority adults, most all of whom were African American. The least educated also had a higher body mass index and were more likely to have hypertension or diabetes as well as heart disease. Baseline scores on the MMSE and visual acuity were lower in the less educated as were baseline scores for the four training outcome measures memory composite, reasoning composite, and speed of processing measures UFOV and DSS.
Table 1.
Baseline covariate and cognition values by body mass index class
| Variable | <12 years (n = 320) |
12 years (n = 797) |
13–15 years (n = 906) |
16+ years (n = 777) |
p-value |
|---|---|---|---|---|---|
| Covariate
| |||||
| Age, mean (SD) | 74.5 (6.1) | 73.6 (5.9) | 73.6 (5.8) | 73.4 (5.9) | 0.045 |
| Female, n (%) | 258 (80.6%) | 659 (82.7%) | 736 (81.2%) | 472 (60.8%) | <0.001 |
| Minority race, n (%) | 144 (45%) | 203 (25.5%) | 253 (27.9%) | 148 (19.1%) | <0.001 |
| Married, n (%) | 73 (22.8%) | 249 (31.3%) | 306 (33.8%) | 378 (48.7%) | <0.001 |
| Body mass index, mean (SD) | 30.1 (5.9) | 28.8 (5.5) | 28.8 (5.7) | 27.4 (5) | <0.001 |
| Current smoker, n (%) | 31 (9.7%) | 62 (7.8%) | 74 (8.2%) | 41 (5.3%) | 0.037 |
| Alcohol consumption, n (%) | <0.001 | ||||
| Nondrinker | 197 (61.8%) | 407 (51.1%) | 395 (43.9%) | 245 (31.7%) | |
| Light drinker | 104 (32.6%) | 346 (43.4%) | 446 (49.6%) | 470 (60.8%) | |
| Heavy drinker | 18 (5.6%) | 44 (5.5%) | 58 (6.5%) | 58 (7.5%) | |
| Short-Form 36 physical function, mean (SD) | 59 (26.3) | 67.3 (24) | 68.8 (23.7) | 74.3 (22.2) | <0.001 |
| Center for Epidemiological Studies Depression Score, mean (SD) | 7.4 (5.6) | 5.7 (5.3) | 5 (5) | 4.1 (4.6) | <0.001 |
| Disease history, n (%) | |||||
| Hypertension | 188 (59.3%) | 415 (52.2%) | 466 (51.9%) | 358 (46.4%) | 0.001 |
| Diabetes mellitus | 61 (19.1%) | 98 (12.3%) | 127 (14.1%) | 72 (9.3%) | <0.001 |
| Stroke | 27 (8.5%) | 50 (6.3%) | 67 (7.5%) | 51 (6.6%) | 0.547 |
| Congestive heart failure | 27 (8.6%) | 32 (4%) | 53 (5.9%) | 26 (3.4%) | 0.001 |
| Ischemic heart disease | 69 (21.7%) | 122 (15.4%) | 133 (14.9%) | 97 (12.6%) | 0.002 |
| Myocardial infarction | 51 (16%) | 83 (10.5%) | 98 (10.8%) | 77 (10%) | 0.027 |
| High cholesterol | 146 (47.4%) | 363 (46.1%) | 385 (43.2%) | 331 (43.6%) | 0.432 |
| Mini-mental state exam, mean (SD) | 25.9 (2) | 27.2 (2) | 27.2 (1.9) | 28.1 (1.7) | <0.001 |
| Visual acuity, mean (SD) | 70.6 (11.9) | 72.6 (11.4) | 73 (11.7) | 74.8 (11) | <0.001 |
| Cognitive measures, Blom transformed
| |||||
| Memory composite, mean (SD) | − 0.6 (0.7) | − 0.1 (0.8) | 0 (0.8) | 0.3 (0.8) | <0.001 |
| Reasoning composite, mean (SD) | − 0.8 (0.7) | − 0.2 (0.8) | 0 (0.8) | 0.5 (0.8) | <0.001 |
| Useful field of view composite, mean (SD) | 0.4 (0.7) | 0 (0.8) | 0 (0.8) | −0.2 (0.8) | <0.001 |
| Digit symbol substitution, mean (SD) | − 0.9 (1) | −0.2 (0.9) | −0.1 (0.9) | 0.2 (0.9) | <0.001 |
The distributions of baseline scores on the four training measures are shown graphically in Fig. 1. What can be seen is that the least-educated group had lower mean scores as indicated in Table 1 but also a distribution of scores that was less dispersed, particularly for reasoning composite and UFOV. With the exception of one outlier on DSS, the least-educated group did not have high-performing members on any of the four measures. Each of the other three educational categories, on the other hand, did have high-performing members. The highest educational category had more high-performing members on memory composite and some higher performers on reasoning composite compared with the middle educational categories. The distribution of scores for UFOV or DSS did not differ much between the highest educational category and the two middle educational categories.
Figure 1.
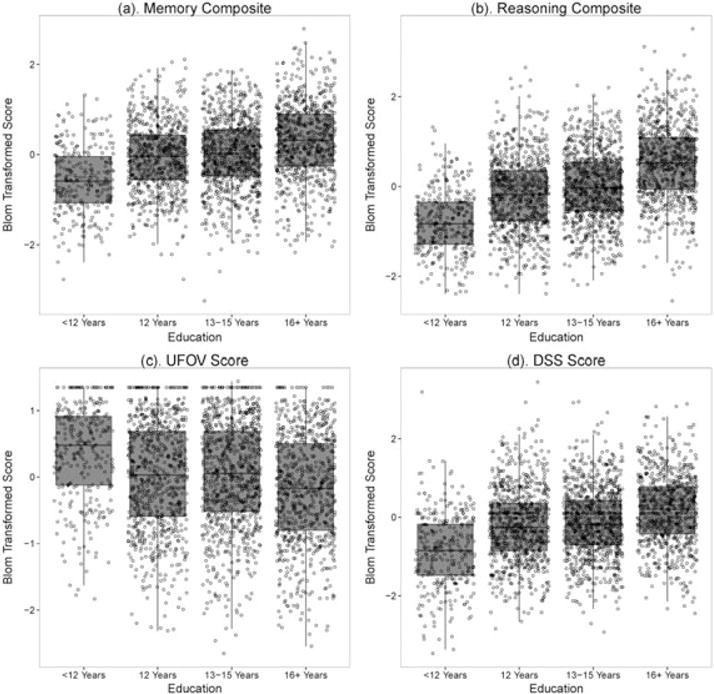
Advanced Cognitive Training for Independent and Vital Elderly and education baseline cognition. UFOV, useful field of view; DSS, digit symbol substitution.
Figure 2 shows the net effect of memory training on memory composite (panel a), reasoning training on reasoning composite (panel b), and speed of processing training on UFOV (panel c) and DSS (panel d) scores by educational category. The figures show the adjusted differences between groups immediately post-training and at 1 year. In the case of memory training (Figure 2(a)), there were no statistically significant differences on memory composite scores by educational attainment at immediate post-training or 1 year post-training. Similarly, as shown in Figure 2(b), educational differences in immediate reasoning training outcomes and 1-year post-training outcomes did not reach statistical significance. Educational differences in speed of processing were evident. Speed of processing training did not have a significant training effect on the DSS outcome, nor did it have a different training effect on DSS by education. However, for the UFOV outcome, educational differences of speed of processing training were highly significant. The least-educated group had a greater response to speed of processing training on UFOV compared with the most-educated group that was highly significant (p = 0.003).
Figure 2.
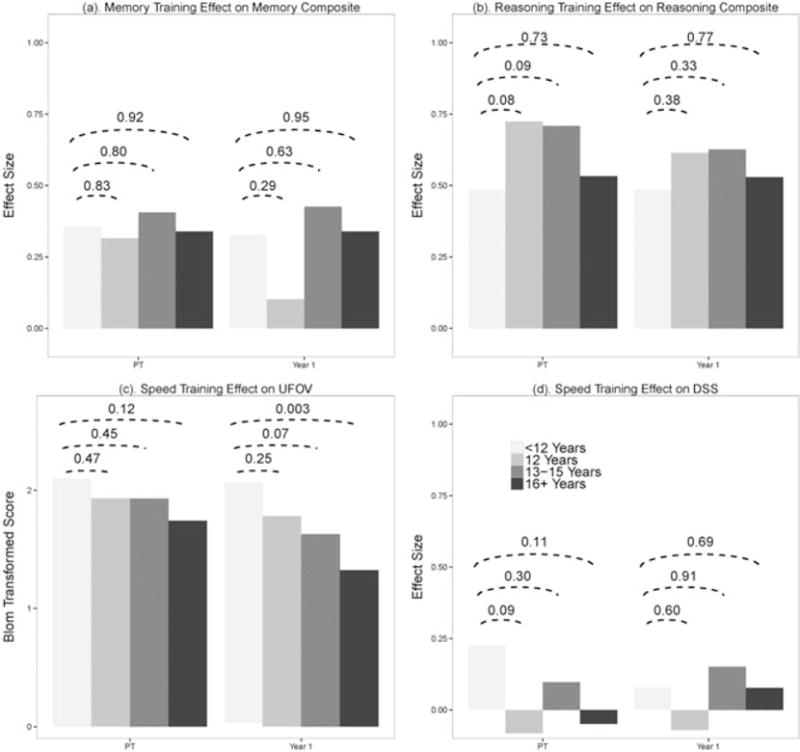
Advanced Cognitive Training for Independent and Vital Elderly and education training effect. UFOV, useful field of view; DSS, digit symbol substitution.
Sensitivity analyses indicated that the speed training advantage on UFOV scores in the least-educated group was maintained up to 3 years but not to 5 or 10 years. In other words, the differences in training effect between the least-educated and most-educated groups were significant at 1, 2, and 3 years post-training. A model that included MMSE score and visual acuity as additional baseline covariates resulted in p-values essentially the same as those reported previously. Finally, analyses in which education was modeled as a continuous variable showed that for every additional year of education, the treatment effect for speed of processing training on UFOV decreased by 0.075, which was highly significant (p = 0.002).
Discussion
The analyses reported here sought to determine whether short-term, late-life cognitive training could reduce the cognitive reserve disadvantage of less-educated older adults. Based on post-intervention cognitive performance scores, we found some evidence that this may be the case. Our analyses indicate that the speed of processing training effect on the UFOV outcome was greatest among the least educated. In fact, the absolute difference in effect size at 1 year post-training between the most and least educated was greater than 0.5, suggesting a clinically meaningful training difference (Norman et al., 2003). Whether this greater improvement among the least educated served to lessen deficits in cognitive reserve is not known from our analyses, but existing literature indicating improved UFOV scores are associated with multiple downstream improvements gives hope that the clinical and public health significance of speed of processing training in less-educated adults is broad.
Speed of processing gains in the ACTIVE trial have been shown to have meaningfully improved locus of control (Ball et al., 2013), self-rated health (Wolinsky et al., 2010), depressive symptoms (Wolinsky et al., 2009), health-related quality of life (Wolinsky et al., 2006), and driving outcomes including longer time to driving cessation (Edwards et al., 2009) and fewer at-fault motor vehicle collisions (Ball et al., 2010). These beneficial downstream outcomes associated with speed of processing may operate through enhancement of cognitive resources necessary to successfully manage and cope with aging (Wolinsky et al., 2009). The mechanisms underlying the enhancement of cognitive reserve are unknown but may include training-dependent improvements in neural proliferation, inflammatory environment, gray matter density, and neural activation. Importantly, speed of processing training may build cognitive reserve and capacity for self-management. If confirmed, this would be important news for the over 15 million current and near-future older adults with low educational attainment.
Implementation of speed of processing training that reaches adults with low education could be achieved through existing Internet-based programs. Online and mobile training programs have emerged, some with an evidence base. The ACTIVE training modules, for example, were translated into online games now delivered by Posit Science. Posit’s BrainHQ program contains the original ACTIVE speed of processing training module and five additional programs designed to more broadly improve mental ability. With continued evidence of large effects on cognitive performance, depression, and driving performance, governments and health systems may find it cost-effective to distribute and subsidize or incentivize online training programs for vulnerable populations such as those with no secondary education.
As noted, no prior report has investigated net cognitive training effects by education. Prior ACTIVE reports have investigated educational differences in training within each training arm separately, that is, without respect to a control group or net training effects. In those single-arm analyses, authors found no effect of education on training (Ball et al., 2013), higher education to be associated with greater training gains (Rebok et al., 2013), and no effect of education on reasoning training gain (Willis and Caskie, 2013). We conclude that educational differences were evident in the analyses reported here because the control group was included (i.e., a net training effect was identified).
There are important limitations to our report. First, although ACTIVE is a very large cognitive training trial, it was not designed to test for educational differences in training outcomes. Potentially important covariates, income, for example, were not available. Second, there were only 66 people with fewer than 12 years of education who were assigned to the speed of processing training arm. However, the usual concern about small sample sizes is that they lack sufficient power because of larger variability. Third, there is one other ACTIVE report that found low education was associated with better training outcomes in the reasoning arm (Willis and Caskie, 2013). Our analysis did not show such effects. However, their treatment effect was defined as the difference between follow-up and baseline measurements with no comparison with the control arm. Our analyses were based on comparisons between the training arm and control arm participants. Fourth, education was presented categorically for ease of interpretation. Sensitivity analyses treating education as a continuous variable yielded similar results across all treatment arms and outcomes. Fifth, not all confounders can be controlled. For example, prior research showed that higher rates of dementia in African Americans were concentrated among those who spent their childhood, and presumably received their education, in the rural South (Hall and Hendrie, 2012). ACTIVE does not have data on region of childhood, but in analyses not shown, differential effects by minority status were not found (p-value=0.89). Sixth, a prior study of response to speed of processing training showed that randomization to booster sessions was associated with greater improvement (Ball et al., 2013). Data indicate that the same proportions of low-education and high-education participants were randomized to booster training (59%). Similarly, training adherence did not differ between the low-education and high-education categories (77% attended all 10 speed training sessions). Finally, the ACTIVE trial was restricted to cognitively normal adults who were aged 65 years or over in 1998 and 1999. Thus, ACTIVE participants were all born pre-1935. Post-1940, the USA achieved considerable gains in upper-level secondary education (Census, 2014). A few OECD nations experienced similar post-WWII educational gains, but many nations around the world still have limited educational opportunities. Also, educational disparities in life expectancy are substantial in OECD countries. In the USA, for example, life expectancy in those with fewer than 12 years of education is about 70 years compared with about 80 years for those with 13 or more years of education (Olshansky et al., 2012). The mean age of ACTIVE participants with fewer than 12 years of education was 74.5 years.
Despite these limitations, the findings reported here are encouraging and potentially highly significant. Future work to evaluate cognitive training, particularly speed of processing training, in younger, less-educated adults is needed, particularly those with comorbid risk factors (Katon, 2015). Such work could show that less-educated adults experience significant gains in speed of processing via short-term training and that those gains improve not only cognitive reserve but also downstream factors such as depression. An open question, but one worth pursuing, is how speed of processing training interacts with individual differences in brain pathology (e.g., protein-based degenerations versus cerebrovascular disease) to produce its outcomes.
Given the substantially elevated risk of dementia associated with low educational attainment (Norton et al., 2014), confirmation of the effects found here would have very high significance. With a relatively low cost and high potential reach and scalability, short-term cognitive training in less-educated adults could have worldwide significance and public health impact.
Key Points.
Speed of processing training has large effects on a broad range of outcomes.
Adults with less than secondary education are at high risk of dementia.
Adults with low education experience large speed of processing training effects.
Speed of processing training has potential to reduce educational disparities in dementia risk.
Acknowledgments
This work was supported by grants from the National Institute of Digestive and Diabetes and Kidney Diseases (R01DK092377) and National Institute on Aging (R01AG045157, P30AG10133, and U01NR04508).
Footnotes
Conflict of interest
None declared.
References
- Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Edwards JD, Ross LA. The impact of speed of processing training on cognitive and everyday functions. J Gerontol B Psychol Sci Soc Sci. 2007;62(1):19–31. doi: 10.1093/geronb/62.special_issue_1.19. [DOI] [PubMed] [Google Scholar]
- Ball K, Edwards JD, Ross LA, McGwin G., Jr Cognitive training decreases motor vehicle collision involvement of older drivers. J Am Geriatr Soc. 2010;58:2107–2113. doi: 10.1111/j.1532-5415.2010.03138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KK, Ross LA, Roth DL, Edwards JD. Speed of processing training in the ACTIVE study: how much is needed and who benefits? J Aging Health. 2013;25:65S–84S. doi: 10.1177/0898264312470167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom G. Statistical Estimates and Transformed Beta-Variables 1958 [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychol. 1991;5:125–142. [Google Scholar]
- CDATA-Cohen, J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Census USBOT. Population age 25 and over by educational attainment: 1940–2014. 2014 Figure 1 (ed.) [Google Scholar]
- Census USBOT. Educational attainment in the United States: 2014—detailed tables. 2015 Available: http://www.census.gov/hhes/socdemo/education/data/cps/2014/tables.html.
- Edwards JD, Delahunt PB, Mahncke HW. Cognitive speed of processing training delays driving cessation. J Gerontol A Biol Sci Med Sci. 2009:glp131. doi: 10.1093/gerona/glp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman H, Derman D. Kit of Factor Referenced Cognitive Tests. Educational Testing Service; Princeton, NJ: 1976. [Google Scholar]
- Folstein MF, Folstein SE, Mchugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gonda J. Schaie–Thurstone Mental Abilities Test: Word Series Test. Consulting Psychologists Press. F. W Unverzagt et al; Palo Alto. CA: 1985. [Google Scholar]
- Hall KS, Hendrie HC. Early childhood environment and dementia. Lancet. 2012;380:11–12. doi: 10.1016/S0140-6736(12)60767-3. [DOI] [PubMed] [Google Scholar]
- Jobe JB, Smith DM, Ball K, et al. ACTIVE: a cognitive intervention trial to promote independence in older adults. Control Clin Trials. 2001;22:453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon W, et al. Effect of depression and diabetes mellitus on the risk for dementia. JAMA. 2015 doi: 10.1001/jamapsychiatry.2015.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo HK, Jones RN, Milberg WP, et al. Cognitive function in normal-weight, overweight, and obese older adults: an analysis of the advanced cognitive training for independent and vital elderly cohort. J Am Geriatr Soc. 2006;54:97–103. doi: 10.1111/j.1532-5415.2005.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicine IO. Cognitive aging: progress in understanding and opportunities for action. 2015. p. 420. [DOI] [PubMed] [Google Scholar]
- Morris JN, Fries BE, Steel K, et al. Comprehensive clinical assessment in community setting: applicability of the MDS-HC. J Am Geriatr Soc. 1997;45:1017–1024. doi: 10.1111/j.1532-5415.1997.tb02975.x. [DOI] [PubMed] [Google Scholar]
- Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Antonucci T, Berkman L, et al. Differences in life expectancy due to race and educational differences are widening, and many may not catch up. Health Aff (Millwood) 2012;31:1803–1813. doi: 10.1377/hlthaff.2011.0746. [DOI] [PubMed] [Google Scholar]
- Owsley C, Ball K, McGwin G, et al. Visual processing impairment and risk of motor vehicle crash among older adults. JAMA. 1998;279:1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- Piccinin AM, Muniz-Terrera G, Clouston S, et al. Coordinated analysis of age, sex, and education effects on change in MMSE scores. J Gerontol B Psychol Sci Soc Sci. 2013;68:374–390. doi: 10.1093/geronb/gbs077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Rebok GW, Langbaum JB, Jones RN, et al. Memory training in the ACTIVE study: how much is needed and who benefits? J Aging Health. 2013;25:21S–42S. doi: 10.1177/0898264312461937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebok GW, Ball K, Guey LT, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62:16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique. Arch Psychol. 1941;28:286–340. [Google Scholar]
- Sciences, IOE. International Educational Attainment. 2015 Available: http://nces.ed.gov/programs/coe/indicator_cac.asp.
- Smith GE, Housen P, Yaffe K, et al. A cognitive training program based on principles of brain plasticity: results from the improvement in memory with plasticity-based adaptive cognitive training (IMPACT) study. J Am Geriatr Soc. 2009;57:594–603. doi: 10.1111/j.1532-5415.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurstone L, Thurstone T. Examiner Manual for the SRA Primary Mental Abilities Test (Form 10–14) Science Research Associates; Chicago, III: 1949. [Google Scholar]
- Vemuri P, Lesnick TG, Przybelski SA, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain. 2015:awu393. doi: 10.1093/brain/awu393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Wechsler D. Manual for the Adult Intelligence Scale-Revised. Psychological Corporation; New York: 1981. [Google Scholar]
- Willis SL, Caskie GI. Reasoning training in the ACTIVE study: how much is needed and who benefits? J Aging Health. 2013;25:43S–64S. doi: 10.1177/0898264313503987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B, Cockburn J, Baddeley A. The Rivermead Behavioral Memory Test. Thames Valley Test Co; Reading, England: 1985. [Google Scholar]
- Wilson RS, Boyle PA, Yu L, et al. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology. 2013;81:314–321. doi: 10.1212/WNL.0b013e31829c5e8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Unverzagt FW, Smith DM, et al. The effects of the ACTIVE cognitive training trial on clinically relevant declines in health-related quality of life. J Gerontol B Psychol Sci Soc Sci. 2006;61:S281–S287. doi: 10.1093/geronb/61.5.s281. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Vander Weg MW, Martin R, et al. The effect of speed-of-processing training on depressive symptoms in ACTIVE. J Gerontol A Biol Sci Med Sci. 2009:gln044. doi: 10.1093/gerona/gln044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Mahncke H, Vander Weg MW, et al. Speed of processing training protects self-rated health in older adults: enduring effects observed in the multisite ACTIVE randomized controlled trial. Int Psychogeriatr. 2010;22:470–478. doi: 10.1017/S1041610209991281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Dotson MM. A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PLoS One. 2013;8:e61624. doi: 10.1371/journal.pone.0061624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Stern Y, Manly JJ. Differing effects of education on cognitive decline in diverse elders with low versus high educational attainment. 2014 doi: 10.1037/neu0000141. [DOI] [PMC free article] [PubMed] [Google Scholar]


