Abstract
Natural killer T (NKT) cells recognize lipid antigens presented by a class I MHC-like molecule CD1d, a member of the CD1 family. While most of the initial studies on NKT cells focused on a subset with semi-invariant T cell receptor (TCR) termed iNKT cells, majority of CD1d-restricted lipid-reactive human T cells express diverse TCRs and are termed as type II NKT cells. These cells constitute a distinct population of circulating and tissue-resident effector T cells with immune-regulatory properties. They react to a growing list of self- as well as non-self lipid ligands, and share some properties with both iNKT as well as conventional T cells. Emerging body of evidence points to their role in the regulation of immunity to pathogens/tumors and in autoimmune/metabolic disorders. Improved understanding of the biology of these cells and the ability to manipulate their function may be of therapeutic benefit in diverse disease conditions.
Introduction
It is becoming clear that in addition to conventional MHC-restricted T cells, a diverse repertoire of unconventional T cells are present in both mice and humans and play an important role in immunity against infection, tumors and in autoimmunity. These cells are characterized by higher frequency, faster response and limited TCR diversity. They are often enriched in different tissues and can respond to a distinct molecular pattern or biochemical class of antigenic ligands. Some examples of such T cells include, CD1- and MHC class Ib--restricted T cells, γδ T cells and MR-1-restricted mucosal associated invariant T cells (MAIT)(1).
Natural killer T (NKT) cells are an important subgroup of such unconventional T cells that recognize lipid antigens presented by a class I MHC-like molecule CD1d, a member of the CD1 family. It is noteworthy that while mice only express CD1d, other members CD1a, CD1b and CD1c also bind lipid molecules and present them to human T cells(2). The remaining member CD1e remains intracellular and only contributes to antigen processing and loading. Two broad categories of CD1d-restricted NKT cells exist: type I or invariant iNKT cells that express an invariant TCRα chain (TRAV11 and TRAJ18 in mice and TRAV10 and TRAJ18 in humans) and a limited number of non-invariant TCRβ chains (Table 1). Type II NKT cells (also called diverse NKT or dNKT) do not use invariant TCRα chain and use diverse TCRα and β chains. Since type II NKT cells are reactive to diverse lipid antigens derived from self or microbes and are more abundant than type I NKT cells in humans(3), it is important to understand their physiological role. In this brief review we will primarily focus on lipid-reactive CD1d-restricted TCRαβ+ type II NKT cells and their emerging role in health and in disease.
Table 1.
Type I versus II NKT cells
| Type I NKT | Type II NKT | |
|---|---|---|
| Restriction Element | CD1d | CD1d |
| T Cell Receptor | Vα14-Ja18 with Vβ8,7 or 2 (mice) Vα24-Jα18 with Vβ11 (human) |
Diverse but oligoclonal |
| Transcription Factor | PLZF (high) | PLZF (low) |
| Reactive to α-GalCer | Yes | No |
| Ligands | α-GalCer | Sulfatide, Lyso-sulfatide, Lyso-PC, Lyso-GL1 |
| Prevalence | More prevalent than type II NKT in mice |
More prevalent than type I NKT in human |
| Subsets | NKT-1, NKT-2, NKT-17 | Subsets to be determined |
Antigenic targets of type II NKT cells
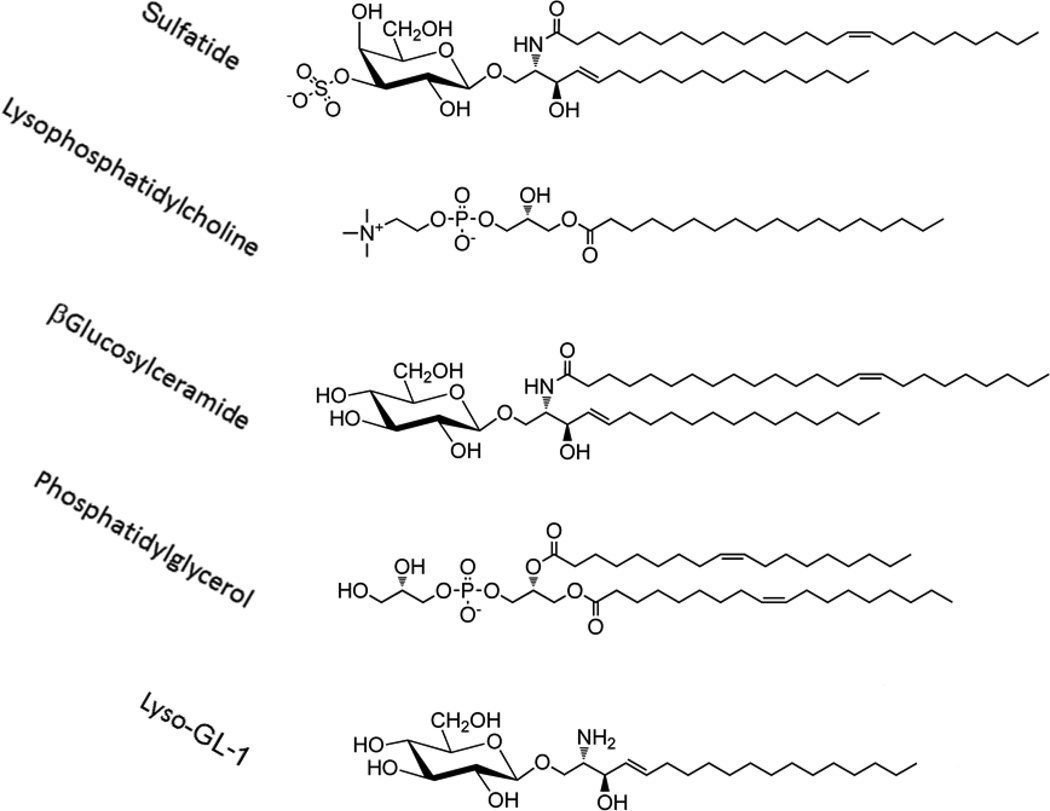
Type II NKT cells are reactive to both glycolipids and phospholipids derived from self as well as microbes (Figure 1). Mass spectrometry based approaches have identified diverse lipid species capable of binding to human CD1d (4, 5). However one of the major differences in the two NKT cell subsets is in the recognition of α– vs β-anomeric linkage of a carbohydrate moiety to a lipid tail in glycolipids. For example, while type I NKT cells recognize their prototypic ligand αGalCer, type II NKT cells are not reactive to αGalCer or other α-linked glycolipids examined (1, 2, 6–8). The first antigen defined for a subset of murine type II NKT cells was sulfatide, a sulfated glycolipid enriched in membranes of various tissues, e.g. myelin of central nervous system (CNS), pancreas, kidney and liver (9). Subsequently sulfatide and lysosulfatide-reactive CD1d-restricted NKT cells have been identified in humans as well (10, 11). Recently sulfatide reactive CD1d-restricted T cells in humans have been also shown to express γδ TCR (12, 13). Other self-glycolipids, including βGlcCer and βGalCer can also activate murine type II NKT cells (14, 15). Consistently, Nair and co-workers also showed that two major sphingolipids accumulating in Gaucher disease (GD), βglucosylceramide 22:0 and glucosylsphingosine, are recognized by human and murine type II NKT cells (16). The lysoforms of glycolipids lacking the fatty acid chain or with longer chain (C18–C24) are more potent in activating the type II NKT cells. Interestingly, glycosphingolipids derived from microbial sources have not yet been shown to activate type II NKT cells.
Figure 1.
Antigenic targets for type II NKT cells. Sulfatide was the first and remains the best characterized ligand for type II NKT cells. More recently several other antigens including lysolipids such as lysophosphatidylcholine and Lyso-GL-1 have been shown to be recognized by type II NKT cells.
Among phospholipids, lysophosphatidylcholine (LPC) has been shown to be recognized by both human and murine type II NKT cells (17–20). Notably while LPC can be recognized by a few human type I NKT cell clones it is not recognized by murine type I NKT cells (20–23). Since the endogenous levels of lysophospholipids can be altered following phospholipid hydrolysis during inflammation (24, 25), it has been suggested that lysophospholipid-reactive type II NKT cells play a role in the regulation of inflammation-induced pathology or autoimmunity. Similarly, glycolipids from Mycobacterium tuberculosis or Corynebacterium glutamicum (26) and phosphatidylglycerol, from Listeria monocytogenes (27) have been shown to be ligands for type II NKT cells. This is consistent with the finding that phosphatidylglycerol, diphosphatidylglycerol and phosphatidylinositol from both microbial and mammalian sources can stimulate type II NKT cell hybridomas.
Antigen recognition mechanism of type II NKT cells use features of both conventional T cells and type I NKT cells
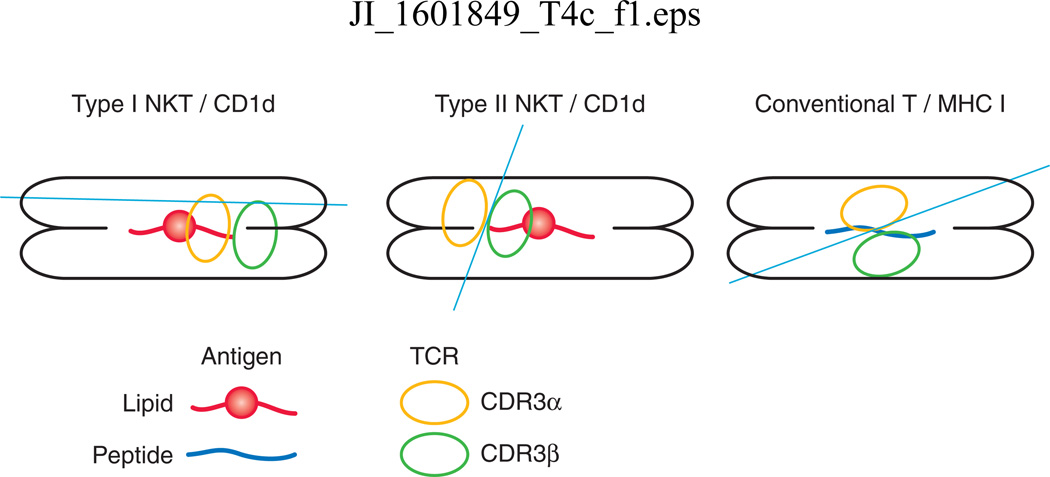
The CD1d binding groove consists of two deep binding pockets, the A’ and the F’, in which lipid antigens dock. The crystal structure of the cis-tetracosenoyl sulfatide/CD1d complex at 1.9 A0 resolution (28) clearly showed that while the fatty acid chain in sulfatide molecules occupy the large A’ pocket, the sphingosine chain was docked in the smaller F’ pocket. Interestingly and in contrast to the α-linked glycosyl head group in αGalCer, the β-linked head group in sulfatide was found to be projected away from the binding groove on CD1d. First clue that antigen-specific type II NKT cells may use TCR recognition mechanism different from type I NKT cells came from the analysis of the TCR repertoire of cis-tetracosenoylsulfatide/CD1d-tetramer+ cells from naïve B6 mice. Thus sulfatide-reactive type II NKT cells express an oligoclonal TCR repertoire predominantly using the Vα3/Vα1-Jα7/Jα9 and the Vβ8.1/Vβ3.1-Jβ2.7 TCR gene segments (29). Additionally, in contrast to type I NKT TCR, while the CDR3α regions were quite variable, the CDR3β region used conserved amino acid residues suggesting its binding to the antigen, similar to some of the conventional MHC-restricted T cells(29). Furthermore this enables TCRs from type I vs. type II NKT cells to dock differently using distinct antigen recognition mechanism as subsequently confirmed by the crystal structure of the trimolecular complex. Sulfatide-reactive TCR molecule was found to be docked over the A’ pocket of CD1d and primarily use the CDR3β loop to contact the antigen while the CDR3α loops associated with the CD1d (30–33). Thus the type II NKT-TCR, unlike the type I NKT-TCR, docks above the A’ pocket of CD1d in an anti-parallel fashion resembling the situation with conventional T cells (Figure 2). Also interesting is the fact that lysosulfatide lacking the fatty acyl chain still binds to the same A’ pocket of CD1d as occupied by the cis-tetracosenoyl sulfatide. It is not yet known whether all other type II NKT TCRs will dock onto CD1d in a similar fashion. It is noteworthy that a similar docking position has been found even for a γδ type II NKT TCR (13). An oligoclonal nature of antigen-reactive type II NKT cell subset has also recently been shown in case of sphingolipids accumulating in Gaucher disease (16). Thus TCR repertoire and antigen recognition mechanisms in type II NKT cells possess features of both conventional T cells and type I NKT cells.
Figure 2.
Differences in the mechanisms of TCR docking in CD1d-restricted lipid-reactive type I and II NKT cells and MHC I-restricted peptide-specific specific conventional T cells. Figure shows a top down view of the MHC-peptide or CD1d-lipid complex. Circles represent the orientation of the CDR3α and CDR3β region. In contrast to type I NKT cells, type II NKT sulfatide-reactive TCR uses the CDR3β to contact the antigen and docks in an anti-parallel fashion similar to the situation for conventional T cells.
Development of type II NKT cells
The development of both types of NKT cell subsets is dependent on the antigen-presenting molecule CD1d expressed on the cells of both non-hematopoietic and hematopoietic origin; although the developmental pathways in type II NKT cells are much less studied than for iNKT cells (34, 35). Immature CD1d+ thymocytes are likely major players in positive selection and CD1d+ antigen presenting cells likely contribute to negative selection. The identity of non-hematopoietic cells remain less defined. Unlike the conventional T cells, transcriptional program in NKT cells is driven by promyelocytic leukemia zinc finger (PLZF), and an adaptor molecule called SAP (signaling lymphocyte activation-molecule associated protein) and Gata-3 (36) (37). Jα18−/− mice lack type I NKT cells but develop type II NKT cells and have been extensively utilized to study these cell types. It is interesting to note that in the Jα18−/− IL-4 GFP reporter mice, TCRβ+GFP+ cells respond to βGlcCer but not to sulfatide or phospholipids. The Th1-like type I NKT cells, associated primarily with liver and spleen, also express T-bet, while the Th17-like type I NKT cells associated with lymph nodes, lungs and skin, express RORγt instead. Murine liver and splenic iNKT cells are also capable of making Th2 cytokines(38). The Th2-like type I NKT cells seem to operate in a Th2 like manner in the lungs and intestine because of a lack of co-expression of transcription factors T-bet or RORγt. It is not yet known whether type II NKT cells can also be Th1-, Th-17 or Th2-like with respect to cytokine secretion and the expression of specific transcription factors. Further studies are needed to test whether type II NKT cells in different tissues have distinct cytokine profiles, particularly in humans. It is noteworthy that the presence of sulfatide is not required for the development of type II NKT cells, as self-reactive NKT cells are present in CST−/− and CGT−/− mice, that are genetically deficient in the cerebroside sulfotransferase (CST) and UDP-galactose ceramide galactosyl transferase (CGT) respectively, key enzymes in the generation of the sulfatides (9, 29, 39).
Activation of type II NKT cells
One of the important features of NKT cells is their ability to rapidly become effector cells and thereby producing cytokines and in some cases, cytotoxic activity within minutes to hours following antigen encounter on CD1d+ antigen-presenting cells. Accordingly, nature of the antigenic ligand, cytokine milieu, APC populations and tissue environment should play important role in their activation and function. Type I NKT cells can be activated either directly through TCR stimulation or indirectly without TCR signaling by cytokines (IL-12, IL-18, or type I IFN) produced through Toll-like receptor (TLR)-mediated signaling in DCs (40–42). It seems that the main pathway for type II NKT cell activation is via TCR signaling following recognition of lipid/CD1d complex (15, 26). Consistently in many experimental conditions in which type I NKT cells are activated by TLR signaling in APCs, type II NKT cells remain un-activated (43). Even during HBV infection, lysophospholipid-reactive type II NKT activation does not depend upon the presence of IL-12 (44). Interestingly, while IL-18R expression did not vary significantly in two subsets, IL-12rβ1 gene expression was several fold lower in type II NKT cells in comparison to that in type I NKT (45). Type II NKT cells also express lower levels of retinoic acid receptor γ (RAR γ), and accordingly are less susceptible to inhibition by RAR γ agonist (46). More critical studies are needed to examine whether lower expression of receptors for these molecules may explain a stricter requirement via TCR-signaling for the activation of type II NKT cells. It is also possible that the avidity of the lipid ligand for the type II NKT TCR and tissue microenvironment can also have a major impact on activation and cytokine secretion of type II NKT cells and may explain in general their pathogenic or protective role in inflammatory or autoimmune diseases (47).
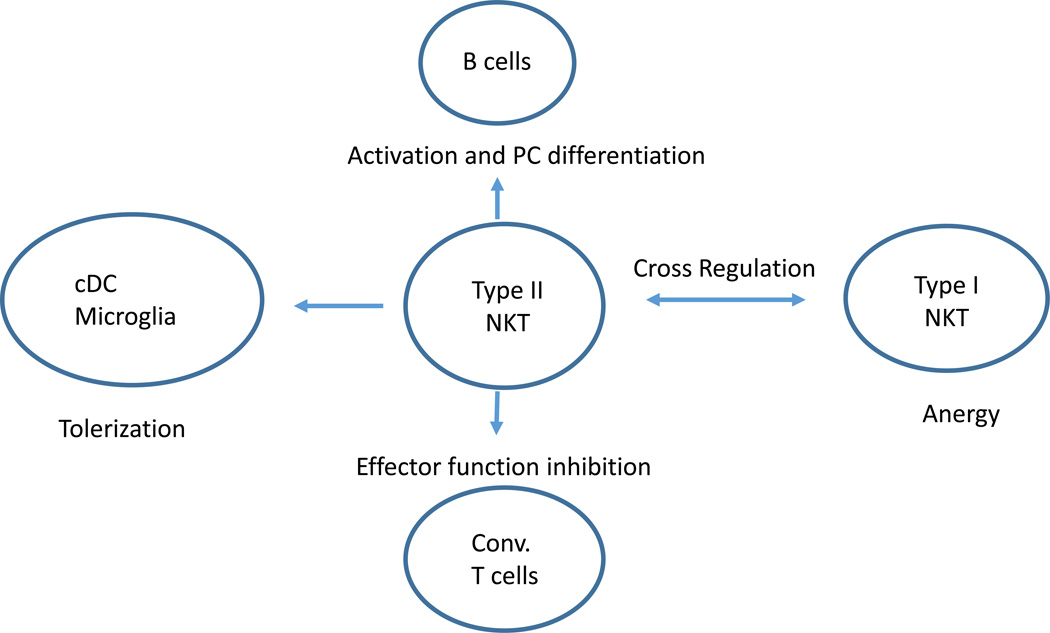
As with type I NKT cells, activation of murine type II NKT cells has a major impact on antigen-presenting cells, including dendritic cells and B cells (Figure 3). Type II NKT cells can leads to activation of pDC but the tolerization of mDC. Thus CD1d expression was significantly upregulated in liver CD11cintB220+/PDCA-1+ (pDC) but not of myeloid DCs (CD11chighCD11b+) following sulfatide-mediated activation of type II NKT cells(48). Although human pDCs express little CD1d and it will be interesting to investigate whether CD1d expression is upregulated following type II NKT activation. Myeloid DC tolerized following instruction by sulfatide-reactive NKT cells can adoptively transfer tolerance and protect recipients from inflammatory or autoimmune disease (49). It was concluded that type II NKT cells influence B cell responses as alum-induced antibody response were more compromised in CD1d-deficient mice as compared to Jα18-deficient mice, although this conclusion is limited as a role for type II NKT cells was only indirectly assessed (50).
Figure 3.
Cross-talk between type II NKT cells and other immune cells including DCs, conventional T cells, B cells and type I NKT cells. An important role of type II NKT cells in the immune system may be their ability to interact with several other cell types to modulate their function. Most of these interactions have to date only been studied in the context of murine type II NKT cells and sulfatide-reactive T cells in particular.
Immune-regulatory role of type II NKT cells
The enrichment of self-lipid ligands for type II NKT cells such as sulfatides or LPC in different tissues and during inflammation as well as the ability of type II NKT cells to influence other immune cells may have important consequences for immunity. Additionally, type II NKT cells have also been shown to be enriched in the target tissues—thus sulfatide/CD1d-tetramer+ cells are enriched in the CNS, pancreas and kidney during disease (9, 51). Broadly murine type II NKT cells inhibit the pro-inflammatory functions of type I NKT cells, conventional T cells and dendritic cells (8, 43, 52, 53). However, in gut immunity these cells may have a proinflammatory role in both in mice and in humans (10, 54).
Immune regulatory mechanism involving cross-regulation of type I NKT cells by type II NKT cells
As mentioned above while studying sulfatide or LPC-mediated activation of type II NKT cells in mice, we found that there is a rapid IL-12 and MIP2-dependent accumulation of type I NKT cells into liver but these cells were anergized and accordingly treated mice were protected from Concanavalin A-induced liver injury, ischemic injury, alcoholic liver disease and CCL4-induced fibrosis (19, 48) (55) (46). Anergy induction in type I NKT cells induced following lipid-mediated activation of type II NKT cells is different than that following chronic administration of type I ligand, αGalCer (56). Thus αGalCer- but not sulfatide- or microbial-mediated anergy in type I NKT cells require programmed death-1 (PD-1)/ PD ligand (PDL)-1 signaling (57, 58). Another apparent difference is that type I NKT cells are activated before anergy induction with αGalCer but not with sulfatide which is not a ligand for them. Therefore type I and type II cross-regulatory interactions are important and may be crucial for their functional manipulation in disease. It is noteworthy that type II NKT-mediated inactivation of type I NKT cells eventually results in a significant decrease in the accumulation of pro-inflammatory myeloid cells and neutrophils, and consequently inhibited inflammation and liver injury. Sulfatide-mediated immune regulatory mechanism was also demonstrated in murine models of ischemic-reperfusion injury in kidney and in asthma (11, 59).
In addition to antigen-mediated activation of a specific subset of type II NKT cell population, several studies have used bulk population from Jα18−/− mice to indirectly examine their role in different experimental conditions. For example, in a murine model of graft versus host disease (GVHD) both IFN-γ and IL-4-producing type II NKT cells provide protection using different mechanisms (60). Thus, IFNγ producing type II NKT cells induced apoptosis of donor cells, while IL-4-secreting type II NKT cells skewed the response towards a Th2-phenotype. Interestingly, in another study it was found that IL-25 treatment had a beneficial effect in high fat diet-induced obesity and caused infiltration of innate cells into adipose tissue including type II NKT cells. Furthermore adoptive transfer of type II NKT cells (defined as NK1.1+ T cells from sulfatide injected mice) in obese mice improved weight loss and stabilized glucose homeostasis in recipients (61). Recent studies have shown that the defect in TCR repertoire in Jα18 −/− mice is broader than just iNKT cells(62), which may impact interpretation of studies relying simply on comparing the phenotype in CD1d −/− mice (that lack both type I and II NKT cells) and Jα18−/− mice and may benefit from utilization of newly developed strains to study iNKT cells(63).
Role in autoimmune and inflammatory diseases
The first demonstration of an immune regulatory role for a subset of sulfatide-reactive type II NKT cells was described in experimental autoimmune encephalomyelitis (EAE). Thus sulfatide/CD1d- but not aGalCer/CD1d-tetramer+ cells are enriched in the CNS during disease and their activation protected mice from EAE in a CD1d-dependent fashion (9). As mentioned above, type II NKT-mediated regulatory mechanism involves the tolerization of conventional DCs, microglia in the CNS, and the inhibition of the effector function of the encephalitogenic myelin protein reactive CD4 T cells (9, 49). Interestingly, ICOS (inducible co-stimulator) and PD-1 or IL-10 secretion by DC is involved in the type II NKT-mediated regulation of diabetes in NOD mice (64, 65). While still not clear, consistent with our preliminary data in case of murine EAE, IL-4 secretion by type II NKT cell has been shown to be protective (66). Since commensal microbiota has a major impact on NKT cells, it is likely that this may influence the activity of type II NKT cell-mediated protection of spontaneous diabetes (67). Recent studies in human Gaucher disease and its mouse model suggest a pathogenic role for type II NKT cells reactive to a novel ligand lyso-glucosylceramide (LGL-1) (16). Similarly, a colitogenic role for an autoreactive type II NKT cell population of yet unknown specificity has been suggested (54). Consistently, in patients with ulcerative colitis (UC), lysosulfatide/CD1d-tetramer+ and IL-13-secreting cells from the lamina propria were thought to have a proinflammatory or colitogenic role (10).
Role in microbial immunity
Type II NKT cells have also been implicated in regulating immunity to diverse viral, bacterial and parasitic infections. In a murine model of hepatitis B infection, type II NKT cells accumulated in the liver and mediated tissue injury in a CD1d and NKG2D-dependent manner(68). Sulfatide-reactive NKT cells were shown to inhibit HIV-1 replication and enhance hematopoiesis in a SCID-Hu HIV model(69). Zeissig et al. demonstrated a protective role for type II NKT cells in a mouse model of hepatitis B utilizing HBV-expressing adenoviral particles(44). Type II NKT cells were also shown to mediate protective immunity in a mouse model of diabetogenic encephalomyocarditis virus(70). Thus type II NKT cells can both mediate protective immunity as well as contribute to immune-mediated tissue damage. In humans, type II NKT cells expressing interferon-γ were identified in liver tissue infected with hepatitis C, although the impact of these cells in regulating viral immunity is not known(71).
Although bacterial lipids can serve as antigens for type II NKT cells, the role of these cells in host defense against bacterial pathogens remains to be clarified. NKT cells do not seem to be essential for host defense against Staphylococcus aureus, although activation of sulfatide-reactive type II NKT cells led to reduction in pathogen-induced cytokine storm and improved survival(72). Phosphatidylglycerol (PG) and diphosphatidylglycerol(DPG) derived from mycobacterium tuberculosis (MTb) were identified as ligands for a subset of type II NKT cells(26). Interestingly, bacterial lipids may be more potent antigens than mammalian counterparts. As an example, Listeria-derived phosphatidylglycerol led to greater activation of type II NKT cells compared to mammalian counterpart(27). Structurally, these lipids contain distinct short fully saturated fatty acid tails, which may enhance binding to CD1d. The nature of bacterial lipids that are recognized by human type II NKT cells needs further study and may provide insights into host response to bacteria.
The balance of type I and II NKT cells may also be important for regulating host defense against parasitic infections. For example, type II NKT cells were shown to promote inflammation and mortality in the setting of Trypanosoma cruzi infections(73). Similarly during murine Schistosoma mansoni infections, type II NKT cells promoted Th2 response, which is a prominent feature of disease-associated pathology(74, 75).
Collectively, these studies show that type II NKT cells can impact the biology of diverse viral, bacterial, or parasitic infections, and can either promote protective innate and adaptive immune responses or contribute to pathogen-mediated tissue injury. Most of these studies have only been performed in the setting of mouse models and therefore there is a need to better characterize human T cells against bacterial lipids and their alteration during bacterial infections and bacteria-mediated pathology.
Role in tumor immunity
In contrast to protective effect of type I NKT cells in most models of tumor immunity and immune-surveillance, several studies suggest a suppressive effect of type II NKT cells on tumor immunity. Initial studies by Terabe and colleagues provided indirect evidence that type II NKT cells were sufficient to suppress tumor immunity in several murine tumor models(76, 77). A suppressive role for sulfatide-reactive type II NKT cells was shown as administration of sulfatide led to increased tumor growth in a CD1d-dependent manner and thereby suggesting mutual antagonism between type I and II NKT cells in regulating tumor immunity (78). Type II NKT-mediated suppression of tumor immunity involved IL13-mediated signaling through the IL4R and STAT6 pathway, which together with TNF-α, led to increase in the production of TGF-β by CD11b+Gr1+ population of myeloid suppressor cells(79). MDSCs were also implicated in type II NKT-mediated suppression of immune surveillance in a lymphoma model(80). Thus type II NKT cells may suppress tumor immunity through several mechanisms that involve cross-talk with other immune-regulatory cells. The effects of type II NKT cells on tumor immunity may however be context dependent, as a recent study implicated type II NKT cells in contributing to tumor immunity in response to CpG in a B16 melanoma model(37).
Studies in human myeloma and Gaucher disease (GD) patients also suggest a regulatory role for type II NKT cells. Chang et al observed an increase in LPC-reactive type II NKT cells in patients with advanced myeloma(81). Marked increase in LPC levels have been previously described in myeloma sera(82). As myeloma patients also have a decline in iNKT cell function(83), altered balance of type I versus II NKT cells may be common occurrence in human myeloma and a potential target for therapeutic manipulation(84, 85). GD is an inherited metabolic disorder characterized by marked alteration in βglucosylceramide and glucosylsphingosine (LGL1). Interestingly the risk of myeloma is markedly increased in GD patients and disease activity correlates with increased frequency of LGL1-reactive type II NKT cells (16, 86). Also both murine and human LGL1-specific type II NKT cells express markers of T follicular helper (TFH) cells. LGL1-specific human T cells also promote plasma cell differentiation in human T-B co-cultures(16). Clonal Ig in most patients with GD-associated monoclonal gammopathy and a subset of sporadic MM was found to be lipid-reactive, and the underlying plasma cell clone in GD models responded to reduction of underlying antigen(87). Progression to clinical myeloma is also associated with downregulation of CD1d(88). Together, these studies support a model wherein type II NKT cells likely activated due to abnormal accumulation of lipid antigens (as in GD) or other mechanisms provide help for chronic lipid-mediated B cell activation, and set the stage for the development of increased risk of plasma cell tumors observed in the setting of GD. It is notable that many of the lipid ligands (such as LPC) recognized by type II NKT cells are commonly increased in the setting of inflammation and cancer. Therefore systematic analysis of changes in type II NKT cells in the blood and tissues in different clinical conditions is needed.
Conclusions and Major unanswered questions
In this review, we have summarized current evidence supporting the emerging role for type II NKT cells in immune regulation of diverse states including immunity to pathogens, tumors and in metabolic disorders and autoimmune states. These cells not only biologically differ in several aspects from the better-studied subset of iNKT cells, they also constitute the majority of CD1d-restricted T cells in humans(3, 71, 81). Therefore there is an unmet need to better understand the biology of these cells and alterations of these cells in the setting of disease, particularly in human tissues (Table 2). Strategies to manipulate the levels of lipid ligands recognized by these cells are now entering the clinic(87, 89). Identification of antigenic ligands recognized by these cells will also enable new approaches to manipulate the level of these cells in vivo, with broad implications for regulation of immune responses.
Table 2.
Some examples of major unanswered questions relating to type II NKT cells and their role in health and disease
| • Is TCR recognition mechanism similar in murine and human type II NKT cells, including oligoclonality of the TCR repertoire and whether other antigen-specific type II NKT cells use the similar TCR docking mechanism to that of sulfatide- reactive NKT cells? |
| • Is there tissue specificity or bias in the cytokine secretion profiles or transcription factors involved in type II NKT cells similar to that associated with Th1/Th2/Th17? |
| • What are the factors critical for the physiological activation of type II NKT cells in inflammatory conditions in both mice and in humans? |
| • What are the conditions that favor type I versus type II NKT cell activation during disease and whether they can be selectively targeted to control autoimmunity or to enhance anti-tumor immunity? |
| • Do alterations in Type II NKT cells correlate with disease pathology? Do changes in the level of underlying lipid antigen lead to alteration in these cells in disease? |
| • Are microbial lipids recognized by human type II NKT cells? What are the functional consequences of recognition of self- versus microbial lipids by human CD1d-restricted T cells? Is there TCR degeneracy or TCR promiscuity in recognition of self vs. foreign lipids? |
| • Do all Type II NKT cells express NK markers? |
| • How self-lipids are transported in vivo to antigen-presenting cells and get presented? |
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 CA100660 and R01 AA020864; to V.K.) and CA106802 and CA197603 to M.D.)
References
- 1.Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. The burgeoning family of unconventional T cells. Nat Immunol. 2015;16:1114–1123. doi: 10.1038/ni.3298. [DOI] [PubMed] [Google Scholar]
- 2.Mori L, Lepore M, De Libero G. The Immunology of CD1- and MR1-Restricted T Cells. Annu Rev Immunol. 2016;34:479–510. doi: 10.1146/annurev-immunol-032414-112008. [DOI] [PubMed] [Google Scholar]
- 3.Exley MA, Tahir SM, Cheng O, Shaulov A, Joyce R, Avigan D, Sackstein R, Balk SP. A major fraction of human bone marrow lymphocytes are Th2-like CD1d-reactive T cells that can suppress mixed lymphocyte responses. J Immunol. 2001;167:5531–5534. doi: 10.4049/jimmunol.167.10.5531. [DOI] [PubMed] [Google Scholar]
- 4.Cox D, Fox L, Tian R, Bardet W, Skaley M, Mojsilovic D, Gumperz J, Hildebrand W. Determination of cellular lipids bound to human CD1d molecules. PLoS One. 2009;4:e5325. doi: 10.1371/journal.pone.0005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan W, Kang SJ, Evans JE, Cresswell P. Natural lipid ligands associated with human CD1d targeted to different subcellular compartments. J Immunol. 2009;182:4784–4791. doi: 10.4049/jimmunol.0803981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 7.Kain L, Webb B, Anderson BL, Deng S, Holt M, Costanzo A, Zhao M, Self K, Teyton A, Everett C, Kronenberg M, Zajonc DM, Bendelac A, Savage PB, Teyton L. The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian alpha-linked glycosylceramides. Immunity. 2014;41:543–554. doi: 10.1016/j.immuni.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasgupta S, Kumar V. Type II NKT cells: a distinct CD1d-restricted immune regulatory NKT cell subset. Immunogenetics. 2016 doi: 10.1007/s00251-016-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199:947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuss IJ, Joshi B, Yang Z, Degheidy H, Fichtner-Feigl S, de Souza H, Rieder F, Scaldaferri F, Schirbel A, Scarpa M, West G, Yi C, Xu L, Leland P, Yao M, Mannon P, Puri RK, Fiocchi C, Strober W. IL-13Ralpha2-bearing, type II NKT cells reactive to sulfatide self-antigen populate the mucosa of ulcerative colitis. Gut. 2014;63:1728–1736. doi: 10.1136/gutjnl-2013-305671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang SH, Lee JP, Jang HR, Cha RH, Han SS, Jeon US, Kim DK, Song J, Lee DS, Kim YS. Sulfatide-reactive natural killer T cells abrogate ischemia-reperfusion injury. J Am Soc Nephrol. 2011;22:1305–1314. doi: 10.1681/ASN.2010080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai L, Picard D, Anderson B, Chaudhary V, Luoma A, Jabri B, Adams EJ, Savage PB, Bendelac A. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vdelta1 TCR. Eur J Immunol. 2012;42:2505–2510. doi: 10.1002/eji.201242531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luoma AM, Castro CD, Mayassi T, Bembinster LA, Bai L, Picard D, Anderson B, Scharf L, Kung JE, Sibener LV, Savage PB, Jabri B, Bendelac A, Adams EJ. Crystal structure of Vdelta1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human gammadelta T cells. Immunity. 2013;39:1032–1042. doi: 10.1016/j.immuni.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhost S, Lofbom L, Rynmark BM, Pei B, Mansson JE, Teneberg S, Blomqvist M, Cardell SL. Identification of novel glycolipid ligands activating a sulfatide-reactive, CD1d-restricted, type II natural killer T lymphocyte. Eur J Immunol. 2012;42:2851–2860. doi: 10.1002/eji.201142350. [DOI] [PubMed] [Google Scholar]
- 15.Roy KC, Maricic I, Khurana A, Smith TR, Halder RC, Kumar V. Involvement of secretory and endosomal compartments in presentation of an exogenous self-glycolipid to type II NKT cells. J Immunol. 2008;180:2942–2950. doi: 10.4049/jimmunol.180.5.2942. [DOI] [PubMed] [Google Scholar]
- 16.Nair S, Boddupalli CS, Verma R, Liu J, Yang R, Pastores GM, Mistry PK, Dhodapkar MV. Type II NKT-TFH cells against Gaucher lipids regulate B-cell immunity and inflammation. Blood. 2015;125:1256–1271. doi: 10.1182/blood-2014-09-600270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang DH, Deng H, Matthews P, Krasovsky J, Ragupathi G, Spisek R, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Blood. 2008;112:1308–1316. doi: 10.1182/blood-2008-04-149831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macho-Fernandez E, Brigl M. The Extended Family of CD1d-Restricted NKT Cells: Sifting through a Mixed Bag of TCRs, Antigens, and Functions. Front Immunol. 2015;6:362. doi: 10.3389/fimmu.2015.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maricic I, Girardi E, Zajonc DM, Kumar V. Recognition of lysophosphatidylcholine by type II NKT cells and protection from an inflammatory liver disease. J Immunol. 2014;193:4580–4589. doi: 10.4049/jimmunol.1400699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox LM, Cox DG, Lockridge JL, Wang X, Chen X, Scharf L, Trott DL, Ndonye RM, Veerapen N, Besra GS, Howell AR, Cook ME, Adams EJ, Hildebrand WH, Gumperz JE. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009;7:e1000228. doi: 10.1371/journal.pbio.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pei B, Speak AO, Shepherd D, Butters T, Cerundolo V, Platt FM, Kronenberg M. Diverse endogenous antigens for mouse NKT cells: self-antigens that are not glycosphingolipids. J Immunol. 2011;186:1348–1360. doi: 10.4049/jimmunol.1001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gapin L, Godfrey DI, Rossjohn J. Natural Killer T cell obsession with self-antigens. Curr Opin Immunol. 2013;25:168–173. doi: 10.1016/j.coi.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knowlden S, Georas SN. The Autotaxin-LPA Axis Emerges as a Novel Regulator of Lymphocyte Homing and Inflammation. J Immunol. 2014;192:851–857. doi: 10.4049/jimmunol.1302831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sevastou I, Kaffe E, Mouratis MA, Aidinis V. Lysoglycerophospholipids in chronic inflammatory disorders: the PLA(2)/LPC and ATX/LPA axes. Biochim Biophys Acta. 2013;1831:42–60. doi: 10.1016/j.bbalip.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Tatituri RV, Watts GF, Bhowruth V, Barton N, Rothchild A, Hsu FF, Almeida CF, Cox LR, Eggeling L, Cardell S, Rossjohn J, Godfrey DI, Behar SM, Besra GS, Brenner MB, Brigl M. Recognition of microbial and mammalian phospholipid antigens by NKT cells with diverse TCRs. Proc Natl Acad Sci U S A. 2013;110:1827–1832. doi: 10.1073/pnas.1220601110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf BJ, Tatituri RV, Almeida CF, Le Nours J, Bhowruth V, Johnson D, Uldrich AP, Hsu FF, Brigl M, Besra GS, Rossjohn J, Godfrey DI, Brenner MB. Identification of a Potent Microbial Lipid Antigen for Diverse NKT Cells. J Immunol. 2015;195:2540–2551. doi: 10.4049/jimmunol.1501019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zajonc DM, Maricic I, Wu D, Halder R, Roy K, Wong CH, Kumar V, Wilson IA. Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity. J Exp Med. 2005;202:1517–1526. doi: 10.1084/jem.20051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arrenberg P, Halder R, Dai Y, Maricic I, Kumar V. Oligoclonality and innate-like features in the TCR repertoire of type II NKT cells reactive to a beta-linked self-glycolipid. Proc Natl Acad Sci U S A. 2010;107:10984–10989. doi: 10.1073/pnas.1000576107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girardi E, Maricic I, Wang J, Mac TT, Iyer P, Kumar V, Zajonc DM. Type II natural killer T cells use features of both innate-like and conventional T cells to recognize sulfatide self antigens. Nat Immunol. 2012;13:851–856. doi: 10.1038/ni.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girardi E, Zajonc DM. Molecular basis of lipid antigen presentation by CD1d and recognition by natural killer T cells. Immunol Rev. 2012;250:167–179. doi: 10.1111/j.1600-065X.2012.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel O, Pellicci DG, Gras S, Sandoval-Romero ML, Uldrich AP, Mallevaey T, Clarke AJ, Le Nours J, Theodossis A, Cardell SL, Gapin L, Godfrey DI, Rossjohn J. Recognition of CD1d-sulfatide mediated by a type II natural killer T cell antigen receptor. Nat Immunol. 2012;13:857–863. doi: 10.1038/ni.2372. [DOI] [PubMed] [Google Scholar]
- 33.Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol. 2012;12:845–857. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chun T, Page MJ, Gapin L, Matsuda JL, Xu H, Nguyen H, Kang HS, Stanic AK, Joyce S, Koltun WA, Chorney MJ, Kronenberg M, Wang CR. CD1d-expressing dendritic cells but not thymic epithelial cells can mediate negative selection of NKT cells. J Exp Med. 2003;197:907–918. doi: 10.1084/jem.20021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schumann J, Pittoni P, Tonti E, Macdonald HR, Dellabona P, Casorati G. Targeted expression of human CD1d in transgenic mice reveals independent roles for thymocytes and thymic APCs in positive and negative selection of Valpha14i NKT cells. J Immunol. 2005;175:7303–7310. doi: 10.4049/jimmunol.175.11.7303. [DOI] [PubMed] [Google Scholar]
- 36.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 37.Zhao J, Weng X, Bagchi S, Wang CR. Polyclonal type II natural killer T cells require PLZF and SAP for their development and contribute to CpG-mediated antitumor response. Proc Natl Acad Sci U S A. 2014;111:2674–2679. doi: 10.1073/pnas.1323845111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen NR, Brennan PJ, Shay T, Watts GF, Brigl M, Kang J, Brenner MB C. ImmGen Project. Shared and distinct transcriptional programs underlie the hybrid nature of iNKT cells. Nat Immunol. 2013;14:90–99. doi: 10.1038/ni.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blomqvist M, Rhost S, Teneberg S, Lofbom L, Osterbye T, Brigl M, Mansson JE, Cardell SL. Multiple tissue-specific isoforms of sulfatide activate CD1d-restricted type II NKT cells. Eur J Immunol. 2009;39:1726–1735. doi: 10.1002/eji.200839001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N, Cohen NR, Hsu FF, Besra GS, Brenner MB. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med. 2011;208:1163–1177. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinjo Y, Kitano N, Kronenberg M. The role of invariant natural killer T cells in microbial immunity. J Infect Chemother. 2013;19:560–570. doi: 10.1007/s10156-013-0638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leite-De-Moraes MC, Hameg A, Arnould A, Machavoine F, Koezuka Y, Schneider E, Herbelin A, Dy M. A distinct IL-18-induced pathway to fully activate NK T lymphocytes independently from TCR engagement. J Immunol. 1999;163:5871–5876. [PubMed] [Google Scholar]
- 43.Marrero I, Ware R, Kumar V. Type II NKT Cells in Inflammation, Autoimmunity, Microbial Immunity, and Cancer. Frontiers in immunology. 2015;6:316. doi: 10.3389/fimmu.2015.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeissig S, Murata K, Sweet L, Publicover J, Hu Z, Kaser A, Bosse E, Iqbal J, Hussain MM, Balschun K, Rocken C, Arlt A, Gunther R, Hampe J, Schreiber S, Baron JL, Moody DB, Liang TJ, Blumberg RS. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat Med. 2012;18:1060–1068. doi: 10.1038/nm.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rolf J, Berntman E, Stenstrom M, Smith EM, Mansson R, Stenstad H, Yamagata T, Agace W, Sigvardsson M, Cardell SL. Molecular profiling reveals distinct functional attributes of CD1d-restricted natural killer (NK) T cell subsets. Mol Immunol. 2008;45:2607–2620. doi: 10.1016/j.molimm.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 46.Maricic I, Sheng H, Marrero I, Seki E, Kisseleva T, Chaturvedi S, Molle N, Mathews SA, Gao B, Kumar V. Inhibition of type I natural killer T cells by retinoids or following sulfatide-mediated activation of type II natural killer T cells attenuates alcoholic liver disease in mice. Hepatology. 2015;61:1357–1369. doi: 10.1002/hep.27632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar V, Delovitch TL. Different subsets of natural killer T cells may vary in their roles in health and disease. Immunology. 2014;142:321–336. doi: 10.1111/imm.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117:2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maricic I, Halder R, Bischof F, Kumar V. Dendritic cells and anergic type I NKT cells play a crucial role in sulfatide-mediated immune regulation in experimental autoimmune encephalomyelitis. J Immunol. 2014;193:1035–1046. doi: 10.4049/jimmunol.1302898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah HB, Devera TS, Rampuria P, Lang GA, Lang ML. Type II NKT cells facilitate Alum-sensing and humoral immunity. J Leukoc Biol. 2012;92:883–893. doi: 10.1189/jlb.0412177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Subramanian L, Blumenfeld H, Tohn R, Ly D, Aguilera C, Maricic I, Mansson JE, Buschard K, Kumar V, Delovitch TL. NKT cells stimulated by long Fatty acyl chain sulfatides significantly reduces the incidence of type 1 diabetes in nonobese diabetic mice. PLoS One. 2012;7:e37771. doi: 10.1371/journal.pone.0037771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bandyopadhyay K, Marrero I, Kumar V. NKT cell subsets as key participants in liver physiology and pathology. Cell Mol Immunol. 2016;13:337–346. doi: 10.1038/cmi.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar V. NKT-cell subsets: Promoters and protectors in inflammatory liver disease. J Hepatol. 2013;59:618–620. doi: 10.1016/j.jhep.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liao CM, Zimmer MI, Shanmuganad S, Yu HT, Cardell SL, Wang CR. dysregulation of CD1d-restricted type ii natural killer T cells leads to spontaneous development of colitis in mice. Gastroenterology. 2012;142:326–334. e321–e322. doi: 10.1053/j.gastro.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arrenberg P, Maricic I, Kumar V. Sulfatide-mediated activation of type II natural killer T cells prevents hepatic ischemic reperfusion injury in mice. Gastroenterology. 2011;140:646–655. doi: 10.1053/j.gastro.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang WS, Kim JY, Kim YJ, Kim YS, Lee JM, Azuma M, Yagita H, Kang CY. Cutting edge: Programmed death-1/programmed death ligand 1 interaction regulates the induction and maintenance of invariant NKT cell anergy. J Immunol. 2008;181:6707–6710. doi: 10.4049/jimmunol.181.10.6707. [DOI] [PubMed] [Google Scholar]
- 58.Parekh VV, Lalani S, Kim S, Halder R, Azuma M, Yagita H, Kumar V, Wu L, Kaer LV. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. J Immunol. 2009;182:2816–2826. doi: 10.4049/jimmunol.0803648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang G, Nie H, Yang J, Ding X, Huang Y, Yu H, Li R, Yuan Z, Hu S. Sulfatide-activated type II NKT cells prevent allergic airway inflammation by inhibiting type I NKT cell function in a mouse model of asthma. Am J Physiol Lung Cell Mol Physiol. 2011;301:L975–L984. doi: 10.1152/ajplung.00114.2011. [DOI] [PubMed] [Google Scholar]
- 60.Kim JH, Choi EY, Chung DH. Donor bone marrow type II (non-Valpha14Jalpha18 CD1d-restricted) NKT cells suppress graft-versus-host disease by producing IFN-gamma and IL-4. J Immunol. 2007;179:6579–6587. doi: 10.4049/jimmunol.179.10.6579. [DOI] [PubMed] [Google Scholar]
- 61.Hams E, Locksley RM, McKenzie AN, Fallon PG. Cutting Edge: IL-25 Elicits Innate Lymphoid Type 2 and Type II NKT Cells That Regulate Obesity in Mice. J Immunol. 2013;191:5349–5353. doi: 10.4049/jimmunol.1301176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bedel R, Matsuda JL, Brigl M, White J, Kappler J, Marrack P, Gapin L. Lower TCR repertoire diversity in Traj18-deficient mice. Nat Immunol. 2012;13:705–706. doi: 10.1038/ni.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chandra S, Zhao M, Budelsky A, de Mingo Pulido A, Day J, Fu Z, Siegel L, Smith D, Kronenberg M. A new mouse strain for the analysis of invariant NKT cell function. Nat Immunol. 2015;16:799–800. doi: 10.1038/ni.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duarte N, Stenstrom M, Campino S, Bergman ML, Lundholm M, Holmberg D, Cardell SL. Prevention of diabetes in nonobese diabetic mice mediated by CD1d-restricted nonclassical NKT cells. J Immunol. 2004;173:3112–3118. doi: 10.4049/jimmunol.173.5.3112. [DOI] [PubMed] [Google Scholar]
- 65.Kadri N, Korpos E, Gupta S, Briet C, Lofbom L, Yagita H, Lehuen A, Boitard C, Holmberg D, Sorokin L, Cardell SL. CD4(+) type II NKT cells mediate ICOS and programmed death-1-dependent regulation of type 1 diabetes. J Immunol. 2012;188:3138–3149. doi: 10.4049/jimmunol.1101390. [DOI] [PubMed] [Google Scholar]
- 66.Zeng D, Dick M, Cheng L, Amano M, Dejbakhsh-Jones S, Huie P, Sibley R, Strober S. Subsets of transgenic T cells that recognize CD1 induce or prevent murine lupus: role of cytokines. J Exp Med. 1998;187:525–536. doi: 10.1084/jem.187.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rhost S, Lofbom L, Mansson J, Lehuen A, Blomqvist M, Cardell SL. Administration of sulfatide to ameliorate type I diabetes in non-obese diabetic mice. Scand J Immunol. 2014;79:260–266. doi: 10.1111/sji.12157. [DOI] [PubMed] [Google Scholar]
- 68.Baron JL, Gardiner L, Nishimura S, Shinkai K, Locksley R, Ganem D. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immun. 2002;16:583–594. doi: 10.1016/s1074-7613(02)00305-9. [DOI] [PubMed] [Google Scholar]
- 69.Sundell IB, Halder R, Zhang M, Maricic I, Koka PS, Kumar V. Sulfatide administration leads to inhibition of HIV-1 replication and enhanced hematopoeisis. J Stem Cells. 2010;5:33–42. [PubMed] [Google Scholar]
- 70.Exley MA, Bigley NJ, Cheng O, Tahir SM, Smiley ST, Carter QL, Stills HF, Grusby MJ, Koezuka Y, Taniguchi M, Balk SP. CD1d-reactive T-cell activation leads to amelioration of disease caused by diabetogenic encephalomyocarditis virus. J Leukoc Biol. 2001;69:713–718. [PubMed] [Google Scholar]
- 71.Exley MA, He Q, Cheng O, Wang RJ, Cheney CP, Balk SP, Koziel MJ. Cutting edge: Compartmentalization of Th1-like noninvariant CD1d-reactive T cells in hepatitis C virus-infected liver. J Immunol. 2002;168:1519–1523. doi: 10.4049/jimmunol.168.4.1519. [DOI] [PubMed] [Google Scholar]
- 72.Kwiecinski J, Rhost S, Lofbom L, Blomqvist M, Mansson JE, Cardell SL, Jin T. Sulfatide attenuates experimental Staphylococcus aureus sepsis through a CD1d-dependent pathway. Infect Immun. 2013;81:1114–1120. doi: 10.1128/IAI.01334-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duthie MS, Kahn M, White M, Kapur RP, Kahn SJ. Critical proinflammatory and anti-inflammatory functions of different subsets of CD1d-restricted natural killer T cells during Trypanosoma cruzi infection. Infect Immun. 2005;73:181–192. doi: 10.1128/IAI.73.1.181-192.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mallevaey T, Fontaine J, Breuilh L, Paget C, Castro-Keller A, Vendeville C, Capron M, Leite-de-Moraes M, Trottein F, Faveeuw C. Invariant and noninvariant natural killer T cells exert opposite regulatory functions on the immune response during murine schistosomiasis. Infect Immun. 2007;75:2171–2180. doi: 10.1128/IAI.01178-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mallevaey T, Zanetta JP, Faveeuw C, Fontaine J, Maes E, Platt F, Capron M, de-Moraes ML, Trottein F. Activation of invariant NKT cells by the helminth parasite schistosoma mansoni. J Immunol. 2006;176:2476–2485. doi: 10.4049/jimmunol.176.4.2476. [DOI] [PubMed] [Google Scholar]
- 76.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 77.Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, Godfrey DI, Ostrand-Rosenberg S, Smyth MJ, Berzofsky JA. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202:1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ambrosino E, Terabe M, Halder RC, Peng J, Takaku S, Miyake S, Yamamura T, Kumar V, Berzofsky JA. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J Immunol. 2007;179:5126–5136. doi: 10.4049/jimmunol.179.8.5126. [DOI] [PubMed] [Google Scholar]
- 79.Terabe M, Khanna C, Bose S, Melchionda F, Mendoza A, Mackall CL, Helman LJ, Berzofsky JA. CD1d-restricted natural killer T cells can down-regulate tumor immunosurveillance independent of interleukin-4 receptor-signal transducer and activator of transcription 6 or transforming growth factor-beta. Cancer Res. 2006;66:3869–3875. doi: 10.1158/0008-5472.CAN-05-3421. [DOI] [PubMed] [Google Scholar]
- 80.Renukaradhya GJ, Khan MA, Vieira M, Du W, Gervay-Hague J, Brutkiewicz RR. Type I NKT cells protect (and type II NKT cells suppress) the host's innate antitumor immune response to a B-cell lymphoma. Blood. 2008;111:5637–5645. doi: 10.1182/blood-2007-05-092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang DH, Deng H, Matthews P, Krasovsky J, Ragupathi G, Spisek R, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Inflammation associated lysophospholipids as ligands for CD1d restricted T cells in human cancer. Blood. 2008;112:1308–1316. doi: 10.1182/blood-2008-04-149831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sasagawa T, Okita M, Murakami J, Kato T, Watanabe A. Abnormal serum lysophospholipids in multiple myeloma patients. Lipids. 1999;34:17–21. doi: 10.1007/s11745-999-332-5. [DOI] [PubMed] [Google Scholar]
- 83.Dhodapkar MV, Geller MD, Chang D, Shimizu K, Fujii SI, Dhodapkar K, Krasovsky J. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J. Exp. Med. 2003;197:1667–1676. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Richter J, Neparidze N, Zhang L, Nair S, Monesmith T, Sundaram R, Miesowicz F, Dhodapkar KM, Dhodapkar MV. Clinical regressions and broad immune activation following combination therapy targeting human NKT cells in myeloma. Blood. 2013;121:423–430. doi: 10.1182/blood-2012-06-435503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, Hutchinson A, Geller M, Liu N, Annable R, Shay J, Kirchhoff K, Nishi N, Ando Y, Hayashi K, Hassoun H, Steinman RM, Dhodapkar MV. Sustained expansion of NKT cells and antigen-specific T cells after injection of {alpha}-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mistry PK, Taddei T, vom Dahl S, Rosenbloom BE. Gaucher disease and malignancy: a model for cancer pathogenesis in an inborn error of metabolism. Crit Rev Oncog. 2013;18:235–246. doi: 10.1615/critrevoncog.2013006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nair S, Branagan AR, Liu J, Boddupalli CS, Mistry PK, Dhodapkar MV. Clonal Immunoglobulin against Lysolipids in the Origin of Myeloma. N Engl J Med. 2016;374:555–561. doi: 10.1056/NEJMoa1508808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spanoudakis E, Hu M, Naresh K, Terpos E, Melo V, Reid A, Kotsianidis I, Abdalla S, Rahemtulla A, Karadimitris A. Regulation of multiple myeloma survival and progression by CD1d. Blood. 2009;113:2498–2507. doi: 10.1182/blood-2008-06-161281. [DOI] [PubMed] [Google Scholar]
- 89.Mistry PK, Lukina E, Ben Turkia H, Amato D, Baris H, Dasouki M, Ghosn M, Mehta A, Packman S, Pastores G, Petakov M, Assouline S, Balwani M, Danda S, Hadjiev E, Ortega A, Shankar S, Solano MH, Ross L, Angell J, Peterschmitt MJ. Effect of oral eliglustat on splenomegaly in patients with Gaucher disease type 1: the ENGAGE randomized clinical trial. JAMA. 2015;313:695–706. doi: 10.1001/jama.2015.459. [DOI] [PMC free article] [PubMed] [Google Scholar]