Abstract
Background
Gastric pyloric gland adenomas (PGAs) are rare epithelial polyps that are more commonly found in autoimmune atrophic gastritis and familial adenomatous polyposis. Little is known about the morphology and genetics of PGAs in familial adenomatous polyposis.
Aims
PGAs in familial adenomatous polyposis are studied morphologically and genetically. Findings in FAP associated PGAs are compared to sporadic PGAs and related lesions such as oxyntic gland adenoma (OGA) to increase our understanding of these rare polyps.
Methods
7 PGAs and 18 FGPs from FAP patients were collected. KRAS and GNAS mutations we determined in 6 PGAs and 18 FGPs. Immunohistochemistry was applied on 5 PGAs to provide further confirmation of the histologic subtypes and genetic alterations. Morphology of all PGAs was studied and compared to literature on sporadic PGAs and related lesions.
Results
All successfully sequenced PGAs (6/6) carried a GNAS mutations and half of the successfully sequenced PGAs carried a KRAS mutation (3/6). Nuclear β-catenin was only seen in one PGS with focal high-grade dysplasia. Morphologically, PGAs in FAP showed overlapping features with OGA.
Conclusion
FAP associated PGAs have a similar genetic background, i.e. KRAS and GNAS mutation.
Based on morphological findings in FAP associated PGAs it is hypothesized that PGAs and OGAs are closely related lesions.
Keywords: Gastric adenoma, pyloric gland adenoma, oxyntic gland adenoma, fundic gland polyp, familial adenomatous polyposis
Introduction
Gastric pyloric gland adenomas (PGAs) are rare polyps composed of a proliferation of pyloric gland-like epithelium with low columnar cells with typical pale or “ground glass” cytoplasm. PGAs can be found throughout the gastro-intestinal tract, but are most often seen in the stomach. Sporadic gastric PGAs are associated with auto-immune atrophic gastritis1, and, as this is more prevalent in females, gastric PGAs are more frequent in elderly female patients. It has been estimated that 2.7% of gastric polyps are PGAs.2 In addition, gastric pyloric gland adenomas (PGAs) were recently described as a novel manifestation of Familial Adenomatous Polyposis (FAP), occurring in in 6% of these patients.3
Immunohistochemistry of sporadic PGAs shows mucin expression typical for pyloric gland differentiation, confirming the histological classification. The pyloric gland type mucin MUC6 was initially reported as positive in the deeper pyloric type glands, while MUC5AC – a surface gastric foveolar type mucin – was initially believed to be positive in the surface epithelium of these polyps4. More recently co-labeling of MUC6 and MUC5AC has been noted in some of these lesions5. Genetic analysis showed GNAS mutations in 63% of sporadic PGAs but not in sporadic gastric foveolar and intestinal adenomas6. In contrast to sporadic PGAs, PGAs in FAP patients arise in pristine oxyntic mucosa wholly lacking gastritis, at least in a North American population3. Little is known about the morphologic, immunohistochemical and genetic characteristics of PGAs in FAP. Only one previous study analyzed 6 FAP associated upper gastrointestinal PGAs concluding that FAP-associated and sporadic PGAs of the upper gastrointestinal tract show common genetic features including GNAS, KRAS and APC mutations7.
In this study we analyze 7 PGAs and 18 FGPs from FAP patients for mutations in KRAS and GNAS using a highly sensitive pyrosequencing technique. Also, immunohistochemistry is applied to provide further confirmation of the histologic subtypes and genetic alteration and FAP associated PGAs are compared to existing literature on sporadic PGAs and related lesions such as oxyntic gland adenoma (OGA).
Materials and Method
FAP polyp samples
A computer search of the Johns Hopkins Department of Pathology Archives for “Pyloric Gland Adenoma” and “Fundic Gland Polyp AND Polyposis” was performed and cross-referenced to the Johns Hopkins Polyposis Registry for FAP patients. From each patient with a PGA in this study at least one FGP was included. In addition, a number of FGPs from FAP patients without PGAs were obtained. In total 7 PGAs and 18 FGPs were included (Table 1). The corresponding H&E slides of the polyps/biopsies were histologically reviewed by expert gastrointestinal pathologists (EAM, GJAO, LDW and LAAB) to confirm the diagnosis and to mark the area for DNA extraction. The study was performed in accordance with local medical ethical guidelines.
Table 1.
GNAS and KRAS mutations in pyloric gland adenomas and fundic gland polyps from familial adenomatous polyposis patients.
| Patient | Dx | Grade of dysplasia | GNAS mutation | KRAS mutation | Type of sample | Sex | Age |
|---|---|---|---|---|---|---|---|
| 1 | PGA1 | LGD | failed | failed | Biopsy | F | 49 |
| 1 | PGA2 | LGD | R201S | G12V | Biopsy | F | 53 |
| 1 | FGP | ND | WT | WT | U | F | 49 |
| 1 | FGP | LGD | WT | WT | Biopsy | F | 53 |
| 1 | FGP | ND | WT | WT | Biopsy | F | 52 |
| 1 | FGP | ND | WT | WT | U | F | 50 |
| 2 | PGA3 | Focal HGD | R201H | G12V | Polypectomy | F | 24 |
| 2 | PGA4 | IGD | R201H | G12V | Polypectomy | F | 26 |
| 2 | FGP | ND | WT | WT | Biopsy | F | 29 |
| 2 | FGP | LGD | WT | WT | Biopsy | F | 21 |
| 3 | PGA5 | IGD | R201C | WT | Polypectomy | M | 61 |
| 3 | PGA6 | LGD | R201C | WT | biopsy | M | 66 |
| 3 | PGA7 | LGD | R201C | WT | biopsy | M | 65 |
| 3 | FGP | ND | WT | WT | biopsy | M | 66 |
| 4 | FGP | LGD | WT | WT | biopsy | F | 35 |
| 4 | FGP | LGD | WT | WT | biopsy | F | 34 |
| 4 | FGP | LGD | WT | WT | biopsy | F | 34 |
| 4 | FGP | LGD | WT | WT | biopsy | F | 34 |
| 4 | FGP | LGD | WT | WT | biopsy | F | 33 |
| 4 | FGP | LGD | WT | WT | biopsy | F | 33 |
| 4 | FGP | LGD | WT | WT | biopsy | F | 33 |
| 5 | FGP | LGD | WT | WT | EMR | F | 42 |
| 5 | FGP | LGD | WT | WT | biopsy | F | 42 |
| 5 | FGP | ND | WT | WT | biopsy | F | 34 |
| 6 | FGP | LGD | WT | WT | biopsy | M | 26 |
Dx: diagnosis; PGA: pyloric gland adenoma; FGP: fundic gland adenoma; ND: no dysplasia; LGD: low-grade dysplasia; IGD: intermediate grade dysplasia; HGD: high-grade dysplasia; WT: wilt-type; U: unknown; EMR: Endoscopic mucosal resection
DNA extraction
FFPE blocks of polyps and normal tissue were sectioned to slides (10μm thick). Tissue was deparaffinized and the region of interest scraped off with a fine sterilized needle. DNA was extracted using the QIAamp DNA FFPE Tissue Kit (Qiagen, MD, USA) and eluted in a final volume of 30ul TAE buffer. Extraction was performed in a UV hood to prevent DNA contamination.
Sequencing
Primers were designed on the Integrated DNA technologies (IDT, IA, USA) website and the reverse primers labeled with biotin on the 5′ end. The amplification primers for GNAS (forward: 5′-CCA GAC CTT TGC TTT AGA TTG G-3′, reverse: 5′-biotin-TCC ACC TGG AAC TTG GTC TC-3′) were used to sequence codon 201 of the GNAS gene. The amplification primers for KRAS (forward: 5′-AAG GCC TGC TGA AAA TGA CTG-3′, reverse: 5′-biotin-GGT CCT GCA CCA GTA ATA TGC-3′) were used to sequence codons 12 and 13 of the KRAS gene.
Polymerase Chain Reaction (PCR) was performed with a HotStarTaq DNA polymerase kit (Qiagen). PCR reactions of 25ul contained 1X reaction buffer, 0.8 units of HotStarTaq DNA Polymerase, 0.2 mM dNTP’s (dATP, dGTP, dCTP, dTTP), 0.2 mM of each primer, and 5 μl of genomic DNA. The initial denaturation at 95°C for 15 minutes was followed by DNA amplification cycles (60 cycles for KRAS, 80 cycles for GNAS; 95°C for 20 seconds, 53°C for 30 seconds, and 72°C for 20 seconds with a final extension step of 72°C for 5 min). A negative control was included in each run.
The GNAS and KRAS amplicons were sequenced on the Pyromark Q24 (Qiagen) and Pyromark Q24 Vacuum Prep Workstation with Pyromark Gold reagents (Qiagen), Streptavidin Sepharose High Performance (GE Healthcare Life Sciences, PA, USA), and the sequencing primer (GNAS 5′-TTT GTT TCA GGA CCT GCT TCG C-3′; KRAS 5′-TGT GGT AGT TGG AGC T) using the Pyromark Q24 user manual’s protocol. A negative control and water sample were included in each run.
Immunohistochemistry
Immunohistochemistry of MUC2, MUC5AC, MUC6, CDX2, Ki67, p53 and β-catenin was performed as previously described4, 8. The following antibodies were used: p53 (DO-7 + BP53-12; 1:2,000; Thermo scientific, PA, USA), β-catenin (Clone 14; 1:5,000 BD Transduction Laboratories, CA, USA), MUC2 (Ccp58; 1:500; Novocastra, Newcastle, UK), MUC5AC (CLH2; 1:100; Novocastra, Newcastle, UK), MUC6 (CLH5; 1:50; Novocastra, Newcastle, UK), CDX-2 (Clone EPR2764Y, Immunologic, Duiven, The Netherlands) Ki 67 (MIB-1; 1:100; Immunotech Marseille, France)
In brief, 4μm sections were deparaffinized in xylene and endogenous peroxidase was blocked in 0.3% H2O2 (Merck, NJ, USA) in methanol. Antigen retrieval was performed by cooking for 20 minutes in 10/mM Tris/EDTA buffer. A blocking step was performed with 5% normal goat serum in PBS for 10 minutes. Next, the primary antibody was incubated for 1 hour at room temperature (p53) or overnight at 4°C (MUC2, MUC5AC, MUC6, CDX2, Ki67, and β-catenin). Staining of the antibodies was done with the Poly-HRP-Goat α Mouse/Rabbit IgG (Immunologic, Duiven, The Netherlands) and 3,3-diamino-benzidine (DAB, Sigma Aldrich, MO, USA). Sections were lightly counterstained with hematoxylin.
Results
Histology
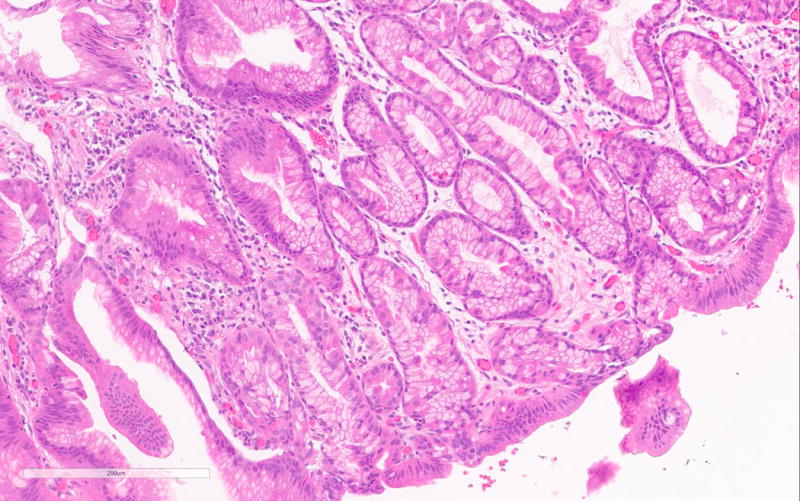
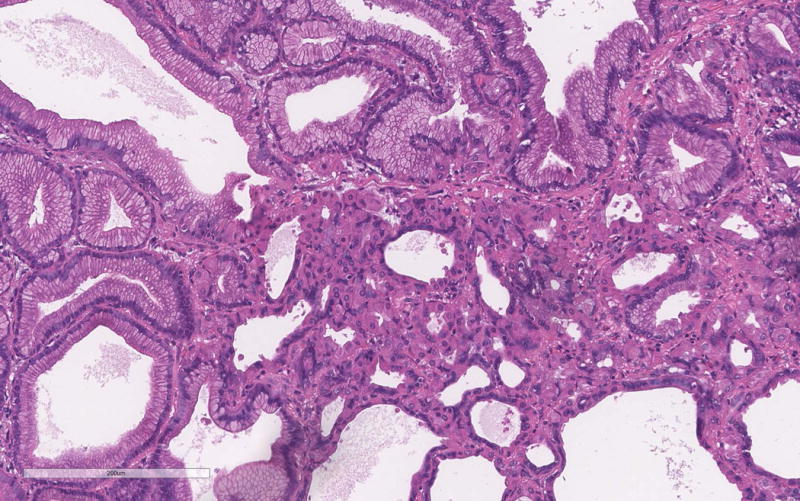
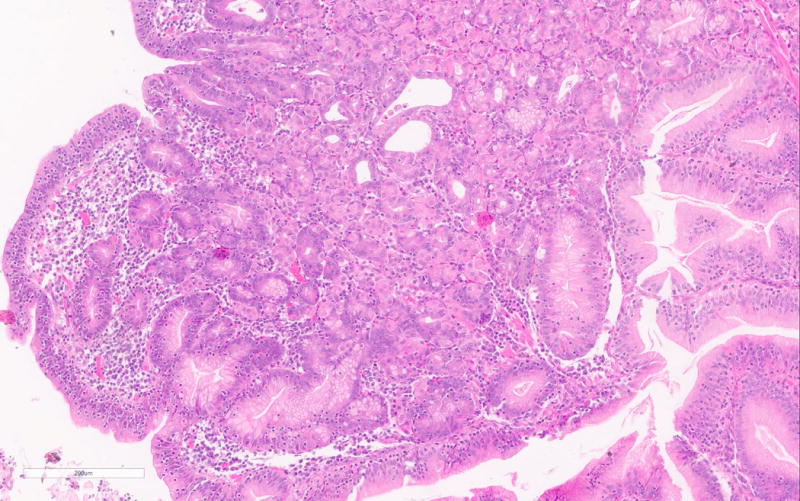
The PGA diagnosis was histologically confirmed by the presence of closely packed tubules of pyloric gland like epithelium composed of low columnar cells and the characteristic pale or “ground glass” cytoplasm (Figure 1a). Typically the nuclei of these cells were round and lacked prominent nucleoli. All but one case showed only low-grade dysplasia. Only PGA3 showed focal high-grade dysplasia. Parietal cells in between the mucous cells were noted in all PGAs varying from scattered to abundant and in some cases were localized very superficially (Figure 1b). PGA5 showed focal abundance of parietal cells in addition to scattered parietal cells (Figure 1c). Histology of PGA3 was particularly remarkable with innumerable parietal cells throughout the polyp, making distinction from oxyntic gland/chief cell adenoma difficult, with numerous intraepithelial lymphocytes and focal high-grade dysplasia (Figures 1d–e). Interestingly the second PGA from this patient (PGA4) only showed scattered parietal cells and had a more classic PGA histology (Figure 1b). None of the cases showed atrophy in the background mucosa.
Figure 1.
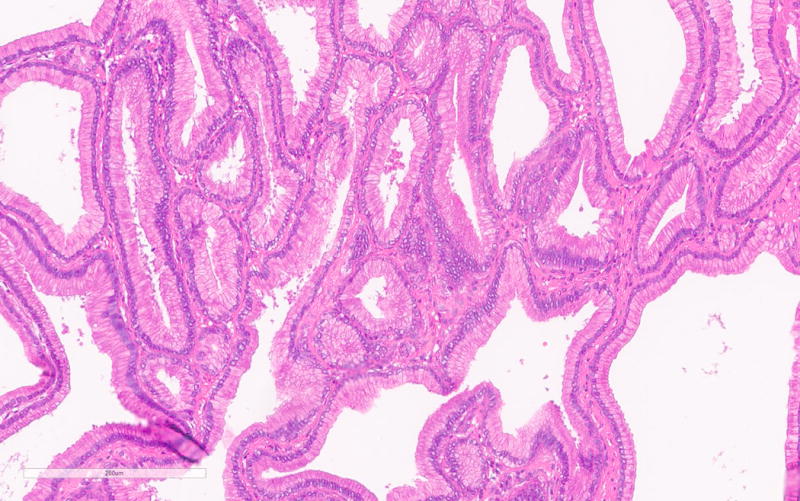
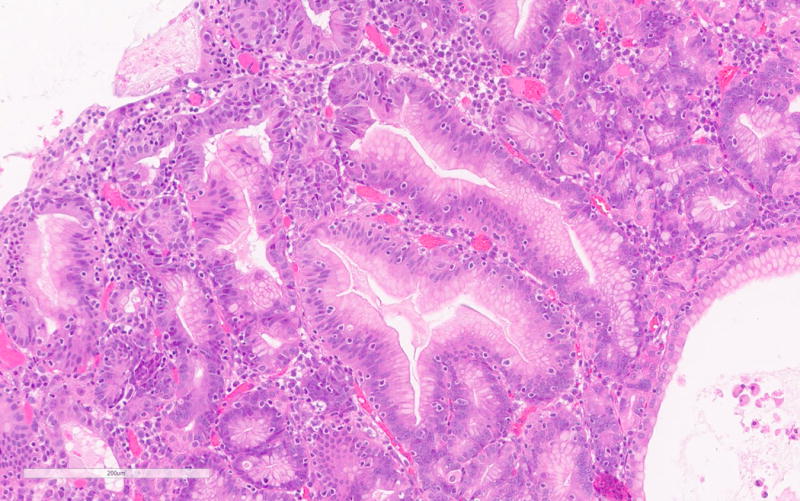
a. Typical histology of a PGA with pyloric type glands lined by low columnar cells with “ground glass” cytoplasm (PGA5).
b. Scattered presence and superficial localization of parietal cells in PGA4.
c. Focal abundant parietal cells in PGA5.
d. PGA3 with remarkable histology with numerous parietal cells and intraepithelial lymphocytes.
e. Focal high-grade dysplasia in PGA3
Immunohistochemistry
Immunohistochemistry for MUC2, MUC5, MUC6, CDX2, β-catenin and Ki-67 was done on 5 PGAs (Table 2). Consistent with existing literature, all PGAs showed MUC6 expression mostly confined to the glandular component (strongly positive in 2 and focally positive in 3 PGAs). MUC5A was positive in the foveolar surface epithelium of all polyps (Figure 2a–b). CDX2 was focally positive in 3 PGAs and intestinal type MUC2 was negative in all cases.
Table 2.
Histological and immunohistochemical characteristics of pyloric gland adenomas in familial adenomatous polyposis.
| Patient | Dx | Dysplasia | Size (mm) | location | Parietal cells | β-catenin | P53 | Ki67 | MUC2 | MUC5 | MUC6 | CDX2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PGA1* | LGD | U | U | None | M | np | mild increase | np | surface pos | failed | neg |
| 1 | PGA2 | LGD | 17 | corpus | scattered | M | np | not increased | neg | surface pos | focal pos | focal pos |
| 2 | PGA3 | Focal HGD | 15 | corpus or fundus | Many | Focal nuclear in HGD | WT | Increased in HGD | neg | surface pos | pos | focal pos |
| 2 | PGA4 | LGD | 8 | corpus | scattered | M | WT | Focally increased | neg | surface pos | pos | focal pos |
| 3 | PGA5 | LGD | 40 | corpus | Scattered and focally abundant | M | WT | not increased | neg | surface pos | focal pos | neg |
| 3 | PGA6 | LGD | 13 | corpus | scattered | np | np | np | np | surface pos | pos | np |
| 3 | PGA7 | LGD | U | corpus or fundus | scattered | np | np | np | np | surface pos | pos | np |
Dx: diagnosis; PGA: pyloric gland adenoma; LGD: low-grade dysplasia; IGD: intermediate grade dysplasia; HGD: high-grade dysplasia; M: membranous β-catenin expression; WT: wild-type expression of p53; neg: negative staining; pos: positive staining; np: not performed; U: unknown.
This was a very small biopsy of a PGA and therefore suboptimal for histopathological assessment.
Figure 2.
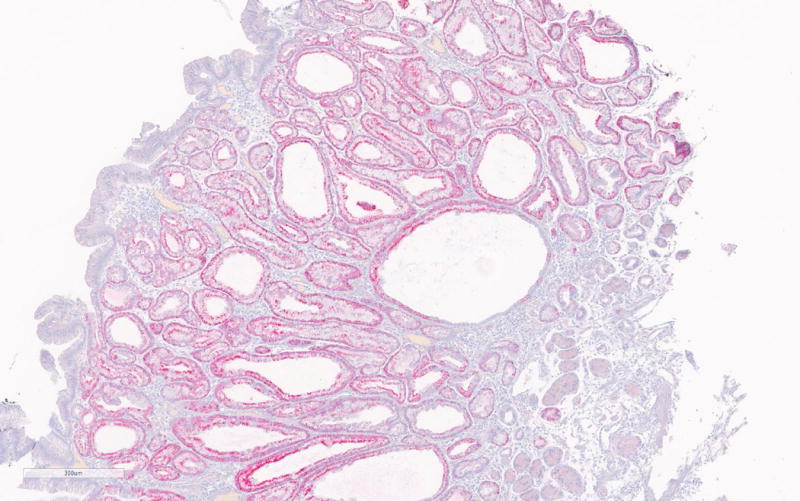
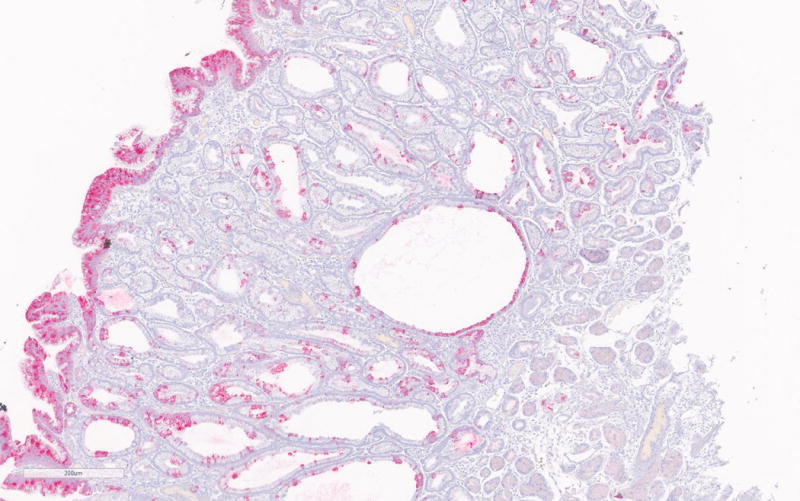
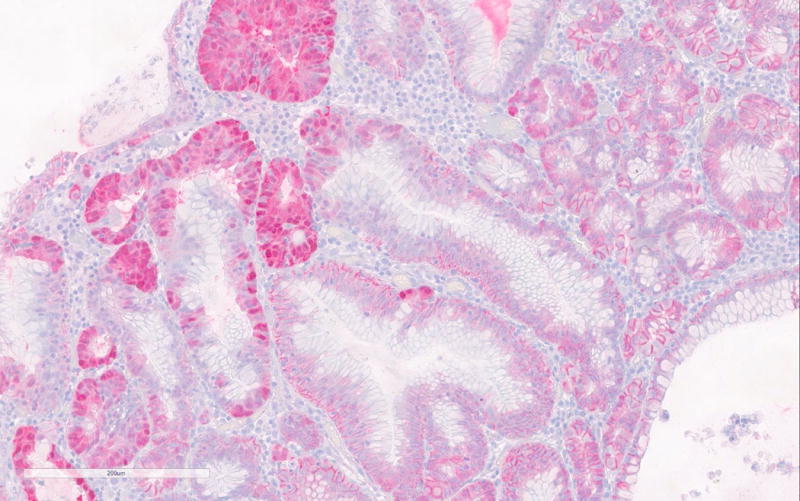
a. MUC6 immunohistochemistry of PGA showing strong positivity of the glandular compartment.
b. MUC5A immunohistochemistry showing positivity of the foveolar epithelium.
c. Nuclear β-catenin expression in areas with high-grade dysplasia in PGA3.
According to the literature, high-grade dysplasia is commonly encountered in sporadic PGAs, but was only seen in one case (PGA3) in the current series. (Figure 1b) Interestingly nuclear β-catenin expression was only seen in areas with HGD in PGA3. (Figure 2c) All other PGAs showed normal membranous β-catenin expression. Ki-67 was only significantly increased in the areas with high-grade dysplasia in PGA3 and focally in PGA4 of the same patient. The high-grade dysplasia showed positivity for both MUC5 and MUC6. The high-grade dysplasia seen in the first specimen of patient 2 (PGA3) was not seen in the second specimen of this patient (PGA4).
P53 immunohistochemistry was performed only in those PGA’s with moderate to high-grade dysplasia (PGA3, PGA4 and PGA5,) but showed normal wild-type expression in all cases.
GNAS and KRAS mutation analysis
Sequencing results are summarized in Table 1. In all 6 of 6 successfully sequenced PGAs, a GNAS mutation was confirmed (100%). A different GNAS mutation was identified in each patient (R201S, R201H, and R201C), but patients 2 and 3 showed the same GNAS mutation in PGAs from different time points. All KRAS mutations were G12V and were seen in 3 out of 6 PGAs (50%), representing 2 out of 3 patients (67%). KRAS and GNAS sequencing of PGA1 failed because the lesion was exceedingly small. All FGPs were wild type for KRAS and GNAS.
Discussion
Pyloric gland adenomas are rare gastric polyps, even in auto-immune gastritis9. Recent studies show GNAS and KRAS mutations in sporadic PGAs, but also in FAP associated PGAs despite the different background in which these lesions arise7. With highly sensitive pyrosequencing this study confirms that FAP-associated PGAs share these distinguishing GNAS mutations with sporadic PGAs but that FAP-associated FGPs lack these mutations. Nuclear expression of β-catenin was seen in only one case with high-grade dysplasia. TP53 showed normal expression in all stained cases, consistent with previous findings4. Our results agree with a previous study of FAP associated PGAs showing that sporadic and FAP-associated PGAs have a similar genetic background7.
Interestingly, over several years, patients 2 and 3 were diagnosed with 2 and 3 gastric PGAs, respectively. The first PGA in patient 2 (PGA3) was a 1.5 cm polyp removed with polypectomy. About 2.5 years later a 0.8 cm PGA was snared (PGA4). The first PGA in patient 3 (PGA5) was a 4 cm PGA removed by polypectomy. About 3.5 years (PGA7) and 5 years (PGA6) after the first polypectomy, gastric biopsies again showed PGAs. Identical GNAS and KRAS mutations were found in the different PGAs from each patient, while the mutations differed between these two patients. Although identical GNAS or KRAS mutations do not provide definitive proof of clonality, it could suggest that these polyps recurred after initial snare polypectomy. Unfortunately, the endoscopy notes do not state whether these polyps arose at the same gastric location as the initial PGAs in these patients. Alternatively, identical mutations can result from clonal expansion of a single transformed cell throughout the stomach (i.e. field cancerization), as previously suggested in a patient with three gastric hyperplastic polyps with identical KRAS mutations10. Either way, more radical techniques to remove PGAs and/or close surveillance may be warranted. In this regard, submucosal dissection has been applied successfully to remove a sporadic PGA11. However, despite the few cases studied, the rarity of high-grade dysplasia and lack of carcinoma in FAP-associated PGAs, as well as the fact that Western FAP patients are not at increased risk of gastric cancer, argue against aggressive treatment of gastric polyps in FAP12. In contrast, sporadic PGAs had high-grade dysplasia in 51.2% of cases of which about 25% also had an associated invasive cancer. This suggests that sporadic lesions may require more aggressive treatment1.
In contrast to Western FAP patients, FAP patients in high-risk areas for gastric cancer such as Japan carry an increased risk of gastric cancer13, 14. This increased risk is likely caused by environmental factors adding an extra trigger for tumorigenesis by inflammation, genetic mutations and/or epigenetic alterations.15 Similarly, the higher incidence of high-grade dysplasia and carcinoma in sporadic PGAs compared to FAP associated PGAs may be explained by the association of sporadic PGAs with auto-immune atrophic gastritis versus the normal gastric mucosa seen in the background of PGAs in FAP patients16.
Although, we confirmed that sporadic and FAP-associated PGAs have a similar genetic background, we noticed differences in morphology between FAP-associated PGAs and sporadic PGAs. Unfortunately, a direct morphological comparison of sporadic and FAP associated PGAs could not be performed. However, it is felt that our group is well equipped to compare our findings in PGAs in FAP to the existing literature on sporadic pyloric and oxyntic gland adenomas since several authors of the current study significantly contributed to this field1, 3–5, 9, 15, 17, 18. Unfortunately previous studies on PGAs in FAP did provide extensive morphological assessment of these polyps3, 7
Histopathological examination of FAP associated PGAs in the current series revealed remarkable presence of parietal cells in all PGAs. The presence of parietal cells varied from scattered to abundant (Figure 1 d and e). Parietal cells have not been previously reported in PGAs1, 5 likely since sporadic PGAs typically arise in the background of autoimmune atrophic gastritis with parietal cells lacking in this condition. In contrast, pyloric gland adenomas in FAP may have a morphology that more closely represents the background mucosa/glandular compartment where the lesion arises. Thus when a PGA arises in the antrum the polyp shows predominantly a proliferation of glands lined by mucous cells. However, when a polyp arises in oxyntic mucosa of a FAP patient, the lesion contains glands lined by a mixture of cells normally present at this location, including chief cells and parietal cells.
Some of the PGAs in the current study showed overlapping features with so called oxyntic gland adenoma (OGA). OGA is another rare gastric polyp characterized by an admixture of gastric cell types with “predominance of chief cells and scattered parietal cells”17. Other terms used previously to address OGA are gastric adenocarcinoma with chief cell differentiation (GA-CCD) or gastric adenocarcinoma of fundic gland type (GAFG). Ueyama et al. subclassified GAFG/OGA into 3 categories: chief cell predominant type, parietal predominant type and mixed type19. A fourth category with predominantly mucous neck cells was suggested by Singhi et al.17. Indeed, OGA and PGAs are both characterized by MUC6 expression, and GNAS mutations are found in both lesions17, 20–22. Moreover, focal expression of chief cell markers has been shown in 8 of 12 PGAs21, and we note a variable presence of parietal cells in all PGAs from FAP patients making differentiation from OGA sometimes very difficult or even impossible (for example PGA3). Based on these observations and the common GNAS mutations in OGA and PGA, we believe that PGAs may be the fourth category proposed by Singhi et al.17. OGA and PGA, therefore, seem closely related and are likely the same lesions within a spectrum with subtle histological differences depending on the background mucosa in which they arise.
In contrast to foveolar or intestinal type adenomas23, PGA and OGA are caused by a proliferation of the specialized glandular gastric mucosa. To emphasize this key difference in the histogenesis and to uniform terminologies, gastric epithelial polyps may be classified according to the epithelial layer from which the polyp arises (Table 3). The most frequent gastric polyps are fundic gland polyps, arising from the glandular compartment, and hyperplastic polyps arising from the foveolar compartment2, 24. Adenomatous polyps arising from the foveolar compartment may or may not have intestinal metaplasia (intestinal type adenoma and gastric foveolar adenoma). The other category of adenomas arises from the glandular compartment and includes PGAs and OGAs. The common denominator is that OGA and PGA are subtypes for which the histology and presence of different specialized glandular cells depends on the background mucosa wherein the polyp arises. As opposed to gastric foveolar and intestinal type adenomas, “gastric glandular adenomas” could be an appropriate diagnostic term for such lesions (Table 3). This may clarify the diversity of terminologies currently used in the literature for such polyps. In hypertrophic gastritis a similar subdivision depending on the compartment that has expanded is common practice, with Zollinger-Ellison on the one end of the spectrum and Ménétrier disease on the other end.24
Table 3.
Suggested classification of gastric epithelial polyps and alternative terms used in the literature
| Polyps arising from the foveolar compartment | ||
| Hyperplastic polyp | ||
| Adenomas arising from foveolar compartment with or without intestinal metaplasia | ||
| • Foveolar adenoma | ||
| • Intestinal type adenoma | ||
| Polyps arising from glandular compartment | ||
| Fundic gland polyp | ||
| Gastric glandular adenoma (with GNAS mutation) | ||
| • chief cell predominant (also known as OGA, GA-CCD or GAFGs) | ||
| • parietal cell predominant | ||
| • mucous neck cell predominant (PGA) | ||
| • mixed type | ||
OGA: oxyntic gland adenoma; GA-CCD: gastric adenocarcinoma with chief cell differentiation; GAFG: gastric adenocarcinoma fundic gland type; PGA: pyloric gland adenoma
To conclude, we confirmed the presence of GNAS and KRAS mutations in pyloric gland adenomas in FAP. Based on morphological findings in FAP associated PGAs and on existing literature17 it is hypothesized that PGAs and OGAs are closely related lesions. Gastric glandular adenomas could be an appropriate unifying diagnostic term for such lesions which may clarify current diversity of terminologies for rare gastric polyps.
Acknowledgments
Supported by The John G Rangos Sr. Charitable Foundation; The Clayton Fund; NIH grant P50 CA62924;
WMH performed immunohistochemical and genetic analysis. MD and JE contributed essential reagents and assisted in performing genetic analyses. LAAB, LDW, EAM, GJAO, MV collected tissue samples and performed histopathological analyses. LAAB, LDW and EAM designed the study. LAAB wrote the paper. LDW, EAM, GJAO, FMG, ADS and MV critically reviewed the manuscript and contributed intellectually.
Footnotes
Disclosure/Conflict of Interest: The authors have nothing to disclose.
References
- 1.Chen ZM, Scudiere JR, Abraham SC, Montgomery E. Pyloric gland adenoma: an entity distinct from gastric foveolar type adenoma. Am J Surg Pathol. 2009;33:186–93. doi: 10.1097/PAS.0b013e31817d7ff4. [DOI] [PubMed] [Google Scholar]
- 2.Oberhuber G, Stolte M. Gastric polyps: an update of their pathology and biological significance. Virchows Arch. 2000;437:581–90. doi: 10.1007/s004280000330. [DOI] [PubMed] [Google Scholar]
- 3.Wood LD, Salaria SN, Cruise MW, Giardiello FM, Montgomery EA. Upper GI tract lesions in familial adenomatous polyposis (FAP): enrichment of pyloric gland adenomas and other gastric and duodenal neoplasms. Am J Surg Pathol. 2014;38:389–93. doi: 10.1097/PAS.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vieth M, Kushima R, Mukaisho K, et al. Immunohistochemical analysis of pyloric gland adenomas using a series of Mucin 2, Mucin 5AC, Mucin 6, CD10, Ki67 and p53. Virchows Arch. 2010;457:529–36. doi: 10.1007/s00428-010-0968-7. [DOI] [PubMed] [Google Scholar]
- 5.Vieth M, Montgomery EA. Some observations on pyloric gland adenoma: an uncommon and long ignored entity! J Clin Pathol. 2014;67:883–90. doi: 10.1136/jclinpath-2014-202553. [DOI] [PubMed] [Google Scholar]
- 6.Matsubara A, Sekine S, Kushima R, et al. Frequent GNAS and KRAS mutations in pyloric gland adenoma of the stomach and duodenum. J Pathol. 2013;229:579–87. doi: 10.1002/path.4153. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto T, Ogawa R, Matsubara A, et al. Familial adenomatous polyposis-associated and sporadic pyloric gland adenomas of the upper gastrointestinal tract share common genetic features. Histopathology. 2015;67:689–98. doi: 10.1111/his.12705. [DOI] [PubMed] [Google Scholar]
- 8.Brosens LA, Tytgat KM, Morsink FH, et al. Multiple colorectal neoplasms in X-linked agammaglobulinemia. Clin Gastroenterol Hepatol. 2008;6:115–9. doi: 10.1016/j.cgh.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Park JY, Cornish TC, Lam-Himlin D, Shi C, Montgomery E. Gastric lesions in patients with autoimmune metaplastic atrophic gastritis (AMAG) in a tertiary care setting. Am J Surg Pathol. 2010;34:1591–8. doi: 10.1097/PAS.0b013e3181f623af. [DOI] [PubMed] [Google Scholar]
- 10.Dijkhuizen SM, Entius MM, Clement MJ, et al. Multiple hyperplastic polyps in the stomach: evidence for clonality and neoplastic potential. Gastroenterology. 1997;112:561–6. doi: 10.1053/gast.1997.v112.pm9024310. [DOI] [PubMed] [Google Scholar]
- 11.Golger D, Probst A, Wagner T, Messmann H. Pyloric-gland adenoma of the stomach: case report of a rare tumor successfully treated with endoscopic submucosal dissection. Endoscopy. 2008;40(Suppl 2):E110–1. doi: 10.1055/s-2007-995554. [DOI] [PubMed] [Google Scholar]
- 12.Ngamruengphong S, Boardman LA, Heigh RI, et al. Gastric adenomas in familial adenomatous polyposis are common, but subtle, and have a benign course. Hered Cancer Clin Pract. 2014;12:4. doi: 10.1186/1897-4287-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iida M, Yao T, Itoh H, et al. Natural history of gastric adenomas in patients with familial adenomatosis coli/Gardner’s syndrome. Cancer. 1988;61:605–11. doi: 10.1002/1097-0142(19880201)61:3<605::aid-cncr2820610331>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Offerhaus GJ, Giardiello FM, Krush AJ, et al. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology. 1992;102:1980–2. doi: 10.1016/0016-5085(92)90322-p. [DOI] [PubMed] [Google Scholar]
- 15.Brosens LA, Wood LD, Offerhaus GJ, et al. Pathology and Genetics of Syndromic Gastric Polyps. Int J Surg Pathol. 2016;24:185–99. doi: 10.1177/1066896915620013. [DOI] [PubMed] [Google Scholar]
- 16.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–40. [PubMed] [Google Scholar]
- 17.Singhi AD, Lazenby AJ, Montgomery EA. Gastric adenocarcinoma with chief cell differentiation: a proposal for reclassification as oxyntic gland polyp/adenoma. Am J Surg Pathol. 2012;36:1030–5. doi: 10.1097/PAS.0b013e31825033e7. [DOI] [PubMed] [Google Scholar]
- 18.Vieth M, Kushima R, Borchard F, Stolte M. Pyloric gland adenoma: a clinico-pathological analysis of 90 cases. Virchows Arch. 2003;442:317–21. doi: 10.1007/s00428-002-0750-6. [DOI] [PubMed] [Google Scholar]
- 19.Ueyama H, Yao T, Nakashima Y, et al. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol. 2010;34:609–19. doi: 10.1097/PAS.0b013e3181d94d53. [DOI] [PubMed] [Google Scholar]
- 20.Lee TI, Jang JY, Kim S, et al. Oxyntic gland adenoma endoscopically mimicking a gastric neuroendocrine tumor: A case report. World J Gastroenterol. 2015;21:5099–104. doi: 10.3748/wjg.v21.i16.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kushima R, Sekine S, Matsubara A, et al. Gastric adenocarcinoma of the fundic gland type shares common genetic and phenotypic features with pyloric gland adenoma. Pathol Int. 2013;63:318–25. doi: 10.1111/pin.12070. [DOI] [PubMed] [Google Scholar]
- 22.Nomura R, Saito T, Mitomi H, et al. GNAS mutation as an alternative mechanism of activation of the Wnt/beta-catenin signaling pathway in gastric adenocarcinoma of the fundic gland type. Hum Pathol. 2014;45:2488–96. doi: 10.1016/j.humpath.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Abraham SC, Montgomery EA, Singh VK, Yardley JH, Wu TT. Gastric adenomas: intestinal-type and gastric-type adenomas differ in the risk of adenocarcinoma and presence of background mucosal pathology. Am J Surg Pathol. 2002;26:1276–85. doi: 10.1097/00000478-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Appelman HD. Localized and Extensive Expansions of the Gastric Mucosa: Mucosal Polyps and Giant Folds. In: Appelman HD, editor. Pathology of the esophagus, stomach and duodenum. New York: Churchill Livingstone Inc; 1984. pp. 79–119. [Google Scholar]


