Abstract
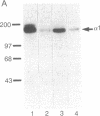
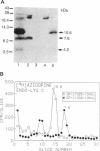
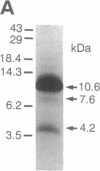
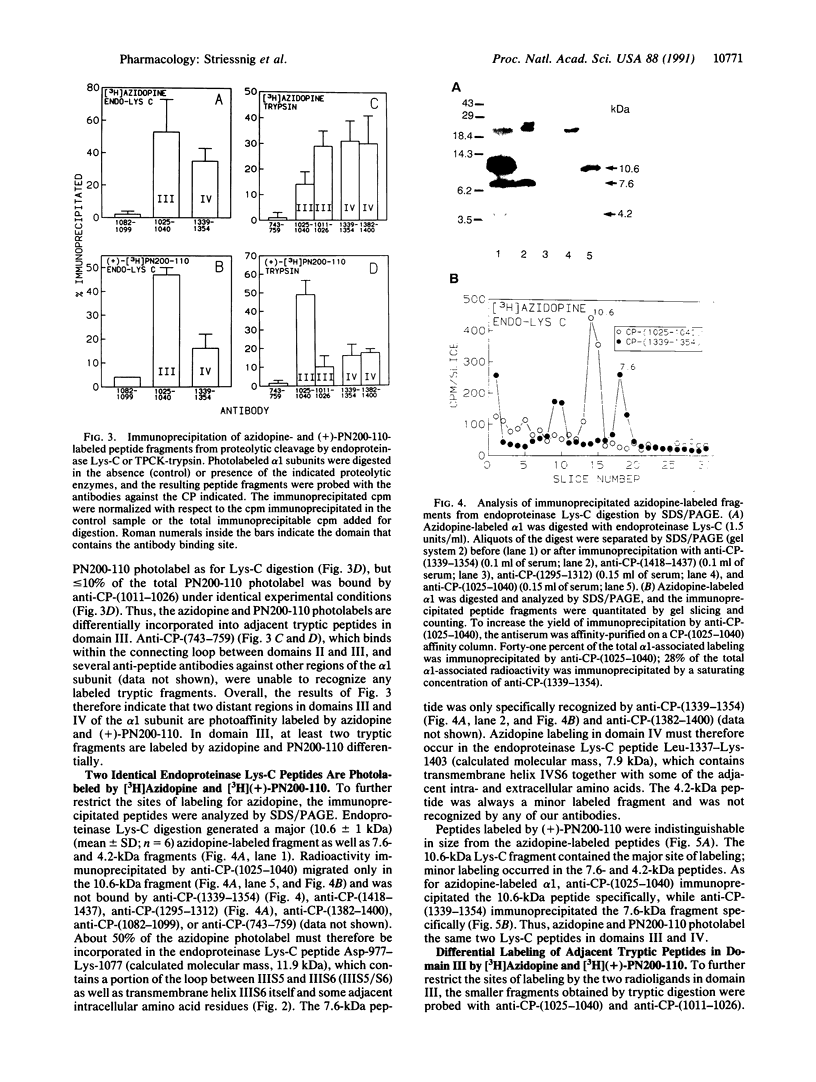
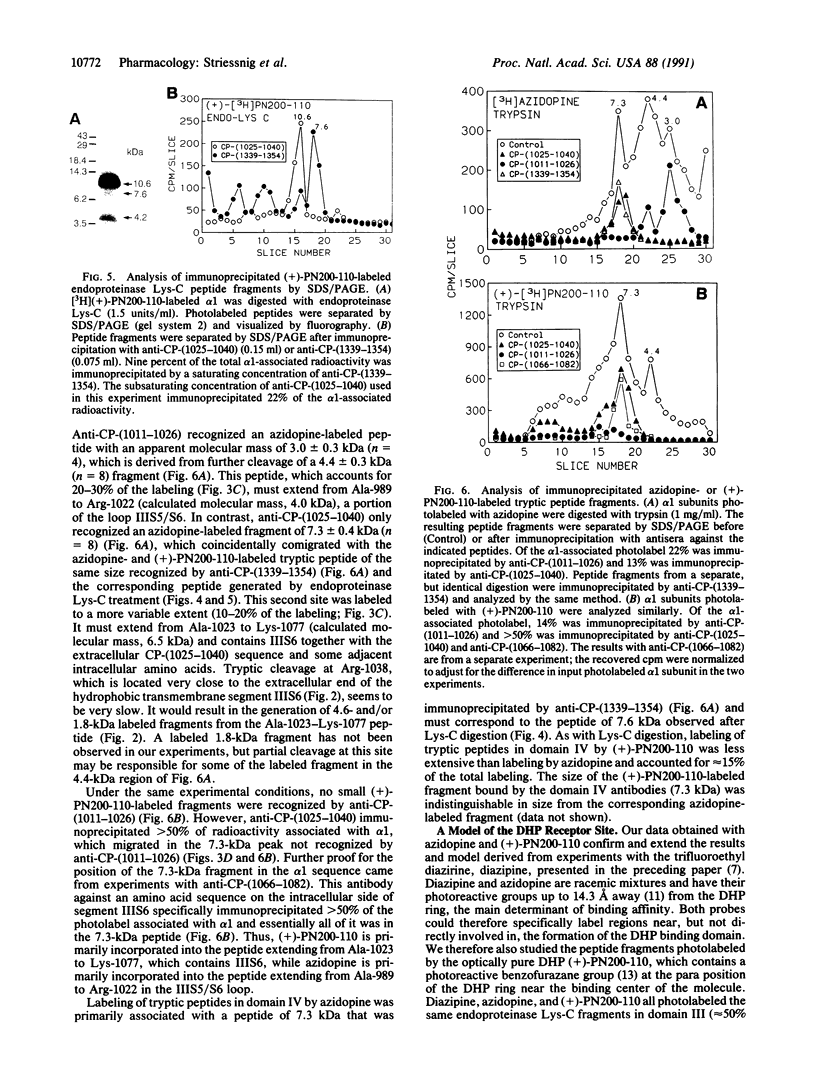
To identify the binding domain for dihydropyridine Ca2+ antagonists, skeletal muscle Ca2+ channels were photolabeled with [3H](+)-PN200-110 and [3H]azidopine. Regions of alpha 1 photolabeled by these ligands were then identified by antibody mapping of proteolytic fragments. Approximately 50% of the specific labeling by both ligands was incorporated in domain III. [3H]Azidopine labeled peptide Gln-989-Arg-1022, which contains a portion of the connecting loop between transmembrane segments IIIS5 and IIIS6 (IIIS5/S6), and peptide Ala-1023-Lys-1077, which contains IIIS6 itself and some adjacent amino acid residues. In contrast, [3H](+)-PN200-110 labeling occurred almost exclusively in the fragment containing IIIS6. A second site labeled by both ligands was identified in transmembrane segment S6 of domain IV and adjacent residues. In contrast to azidopine, the photoreactive benzofurazane group of (+)-PN200-110 is located in close proximity to the essential dihydropyridine ring. Therefore, the regions photolabeled by [3H](+)-PN200-110 within or adjacent to transmembrane segments IIIS6 and IVS6 must participate in the formation of the dihydropyridine binding site. As IIIS5/S6 is preferentially labeled by [3H]azidopine, it may contribute to drug binding by interaction with the long side chain of some dihydropyridines like azidopine. It is proposed, based on physiological studies, that these three peptide segments interact to form a receptor site accessible from the extracellular surface of the Ca2+ channel.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. doi: 10.1146/annurev.ph.51.030189.002055. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Seagar M. J., Takahashi M. Molecular properties of dihydropyridine-sensitive calcium channels in skeletal muscle. J Biol Chem. 1988 Mar 15;263(8):3535–3538. [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-gated sodium and calcium channels. Curr Opin Neurobiol. 1991 Jun;1(1):5–13. doi: 10.1016/0959-4388(91)90004-q. [DOI] [PubMed] [Google Scholar]
- De Jongh K. S., Merrick D. K., Catterall W. A. Subunits of purified calcium channels: a 212-kDa form of alpha 1 and partial amino acid sequence of a phosphorylation site of an independent beta subunit. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8585–8589. doi: 10.1073/pnas.86.21.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry D. R., Kämpf K., Goll A., Glossmann H. Subunit composition of skeletal muscle transverse tubule calcium channels evaluated with the 1,4-dihydropyridine photoaffinity probe, [3H]azidopine. EMBO J. 1985 Aug;4(8):1933–1940. doi: 10.1002/j.1460-2075.1985.tb03873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizzi J. P., Borsotto M., Barhanin J., Fosset M., Lazdunski M. Characterization and photoaffinity labeling of receptor sites for the Ca2+ channel inhibitors d-cis-diltiazem, (+/-)-bepridil, desmethoxyverapamil, and (+)-PN 200-110 in skeletal muscle transverse tubule membranes. J Biol Chem. 1986 Jan 25;261(3):1393–1397. [PubMed] [Google Scholar]
- Glossmann H., Striessnig J. Molecular properties of calcium channels. Rev Physiol Biochem Pharmacol. 1990;114:1–105. doi: 10.1007/BFb0031018. [DOI] [PubMed] [Google Scholar]
- Guy H. R., Conti F. Pursuing the structure and function of voltage-gated channels. Trends Neurosci. 1990 Jun;13(6):201–206. doi: 10.1016/0166-2236(90)90160-c. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Arena J. P., Chin S. Block of L-type calcium channels by charged dihydropyridines. Sensitivity to side of application and calcium. J Gen Physiol. 1991 Jul;98(1):63–75. doi: 10.1085/jgp.98.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Arena J. P. Influence of pHo on calcium channel block by amlodipine, a charged dihydropyridine compound. Implications for location of the dihydropyridine receptor. J Gen Physiol. 1989 Jun;93(6):1109–1127. doi: 10.1085/jgp.93.6.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller C. 1990: annus mirabilis of potassium channels. Science. 1991 May 24;252(5009):1092–1096. doi: 10.1126/science.252.5009.1092. [DOI] [PubMed] [Google Scholar]
- Nakayama H., Taki M., Striessnig J., Glossmann H., Catterall W. A., Kanaoka Y. Identification of 1,4-dihydropyridine binding regions within the alpha 1 subunit of skeletal muscle Ca2+ channels by photoaffinity labeling with diazipine. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9203–9207. doi: 10.1073/pnas.88.20.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regulla S., Schneider T., Nastainczyk W., Meyer H. E., Hofmann F. Identification of the site of interaction of the dihydropyridine channel blockers nitrendipine and azidopine with the calcium-channel alpha 1 subunit. EMBO J. 1991 Jan;10(1):45–49. doi: 10.1002/j.1460-2075.1991.tb07919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Sieber M., Nastainczyk W., Zubor V., Wernet W., Hofmann F. The 165-kDa peptide of the purified skeletal muscle dihydropyridine receptor contains the known regulatory sites of the calcium channel. Eur J Biochem. 1987 Aug 17;167(1):117–122. doi: 10.1111/j.1432-1033.1987.tb13311.x. [DOI] [PubMed] [Google Scholar]
- Striessnig J., Glossmann H., Catterall W. A. Identification of a phenylalkylamine binding region within the alpha 1 subunit of skeletal muscle Ca2+ channels. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9108–9112. doi: 10.1073/pnas.87.23.9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striessnig J., Moosburger K., Goll A., Ferry D. R., Glossmann H. Stereoselective photoaffinity labelling of the purified 1,4-dihydropyridine receptor of the voltage-dependent calcium channel. Eur J Biochem. 1986 Dec 15;161(3):603–609. doi: 10.1111/j.1432-1033.1986.tb10484.x. [DOI] [PubMed] [Google Scholar]
- Taki M., Nakayama H., Kanaoka Y. Diazipine, a novel photoaffinity probe for dihydropyridine receptors of calcium channels. FEBS Lett. 1991 Jun 3;283(2):259–262. doi: 10.1016/0014-5793(91)80602-y. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]