Abstract
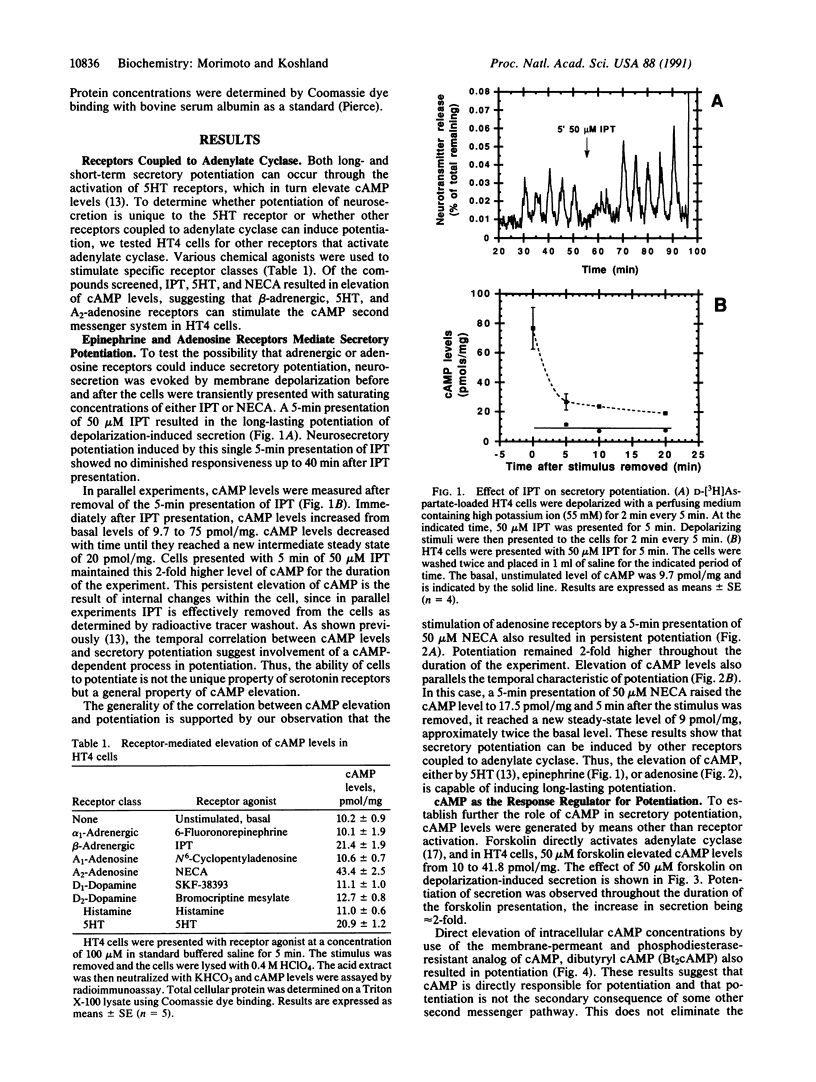
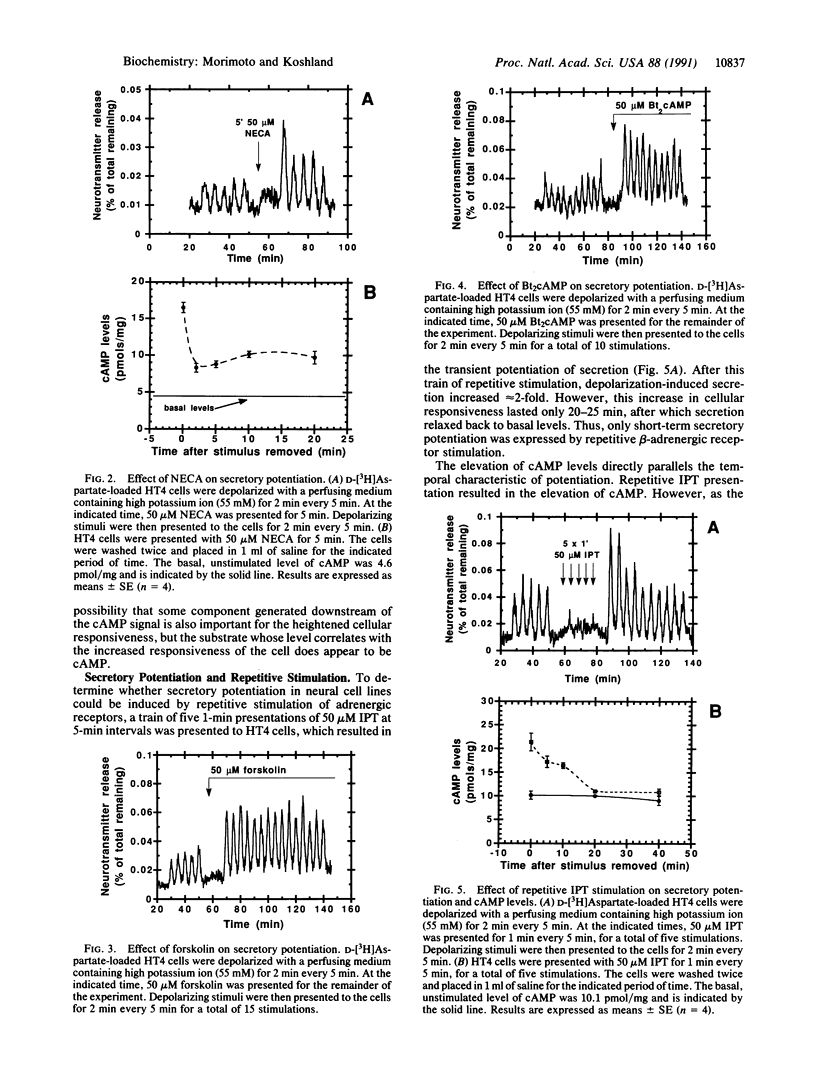
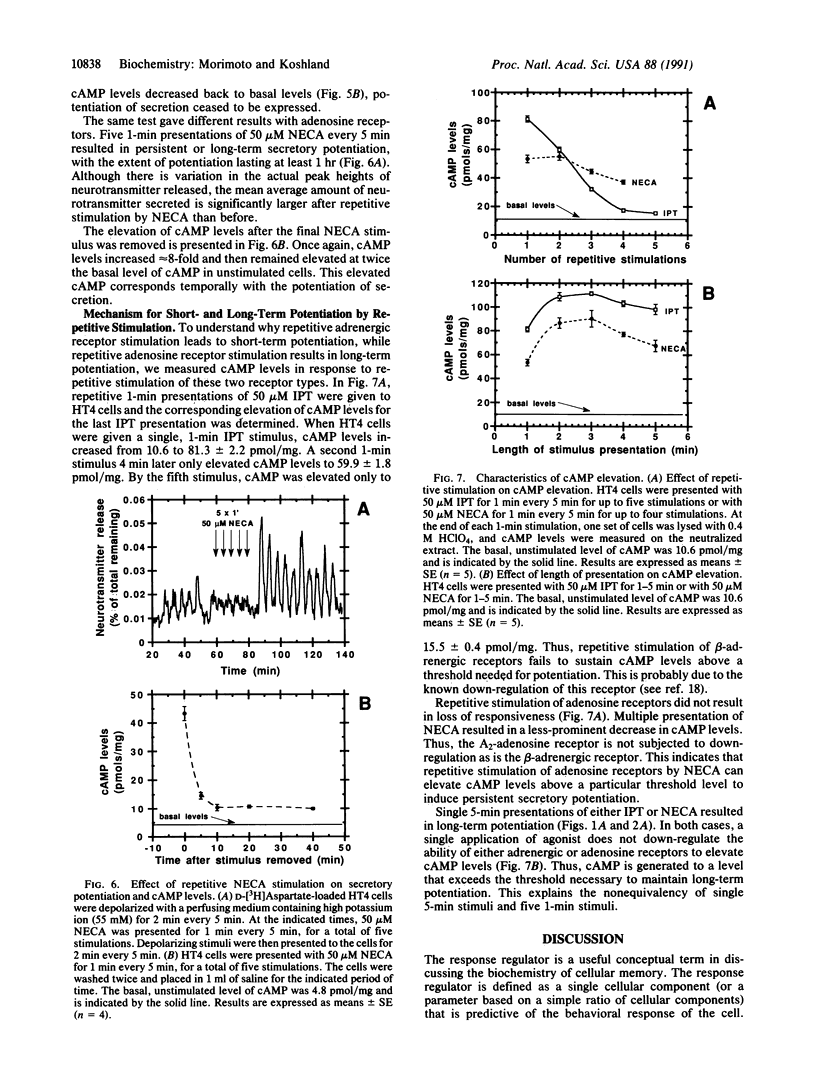
The neural cell line HT4 serves as a model for memory by exhibiting short- and long-term potentiation of neurotransmitter secretion. Previous studies showed that membrane depolarization elicits secretion and that serotonin and N-methyl-D-aspartate receptors are involved in potentiation of the response. Adrenergic and adenosine receptors, which are coupled to adenylate cyclase, are also found to induce potentiation. In addition, the direct evaluation of cAMP levels by forskolin, or by addition of dibutyryl cAMP, induces potentiation. In these different types of stimuli, it is the level of cAMP that is the common factor allowing prediction of whether potentiation will be observed or not. The cAMP level therefore qualifies as the response regulator for this phenomenon. Repetitive adrenergic receptor stimulation results in short-term potentiation, while repetitive adenosine stimulation results in long-term potentiation. This difference can be explained by assuming that some precursor that determines the cAMP level exceeds a threshold, to produce long-term potentiation. This threshold is exceeded by adenosine stimulation but not by stimulation of the beta-adrenergic receptor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourret R. B., Hess J. F., Borkovich K. A., Pakula A. A., Simon M. I. Protein phosphorylation in chemotaxis and two-component regulatory systems of bacteria. J Biol Chem. 1989 May 5;264(13):7085–7088. [PubMed] [Google Scholar]
- Cepko C. Retrovirus vectors and their applications in neurobiology. Neuron. 1988 Jul;1(5):345–353. doi: 10.1016/0896-6273(88)90184-5. [DOI] [PubMed] [Google Scholar]
- Dale N., Schacher S., Kandel E. R. Long-term facilitation in Aplysia involves increase in transmitter release. Science. 1988 Jan 15;239(4837):282–285. doi: 10.1126/science.2892269. [DOI] [PubMed] [Google Scholar]
- Goldbeter A., Koshland D. E., Jr An amplified sensitivity arising from covalent modification in biological systems. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6840–6844. doi: 10.1073/pnas.78.11.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Nairn A. C., McGuinness T. L., Huganir R. L., Greengard P. Role of protein phosphorylation in neuronal signal transduction. FASEB J. 1989 Mar;3(5):1583–1592. doi: 10.1096/fasebj.3.5.2493406. [DOI] [PubMed] [Google Scholar]
- Hess J. F., Oosawa K., Kaplan N., Simon M. I. Phosphorylation of three proteins in the signaling pathway of bacterial chemotaxis. Cell. 1988 Apr 8;53(1):79–87. doi: 10.1016/0092-8674(88)90489-8. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., Schwartz J. H. Molecular biology of learning: modulation of transmitter release. Science. 1982 Oct 29;218(4571):433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr A response regulator model in a simple sensory system. Science. 1977 Jun 3;196(4294):1055–1063. doi: 10.1126/science.870969. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr Chemotaxis as a model second-messenger system. Biochemistry. 1988 Aug 9;27(16):5829–5834. doi: 10.1021/bi00416a001. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Goldbeter A., Stock J. B. Amplification and adaptation in regulatory and sensory systems. Science. 1982 Jul 16;217(4556):220–225. doi: 10.1126/science.7089556. [DOI] [PubMed] [Google Scholar]
- Larsen S. H., Reader R. W., Kort E. N., Tso W. W., Adler J. Change in direction of flagellar rotation is the basis of the chemotactic response in Escherichia coli. Nature. 1974 May 3;249(452):74–77. doi: 10.1038/249074a0. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Koshland D. E., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden P. N., Koshland D. E., Jr Habituation in the single cell: diminished secretion of norepinephrine with repetitive depolarization of PC12 cells. Proc Natl Acad Sci U S A. 1990 Mar;87(5):2031–2035. doi: 10.1073/pnas.87.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden P. N., Koshland D. E., Jr Parallel pathways for habituation in repetitively stimulated PC12 cells. Neuron. 1990 Apr;4(4):615–621. doi: 10.1016/0896-6273(90)90119-z. [DOI] [PubMed] [Google Scholar]
- Morimoto B. H., Koshland D. E., Jr Excitatory amino acid uptake and N-methyl-D-aspartate-mediated secretion in a neural cell line. Proc Natl Acad Sci U S A. 1990 May;87(9):3518–3521. doi: 10.1073/pnas.87.9.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto B. H., Koshland D. E., Jr Induction and expression of long- and short-term neurosecretory potentiation in a neural cell line. Neuron. 1990 Dec;5(6):875–880. doi: 10.1016/0896-6273(90)90347-i. [DOI] [PubMed] [Google Scholar]
- Morimoto B. H., Koshland D. E., Jr Short-term and long-term memory in single cells. FASEB J. 1991 Apr;5(7):2061–2067. doi: 10.1096/fasebj.5.7.2010059. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M., Simon M. Flagellar rotation and the mechanism of bacterial motility. Nature. 1974 May 3;249(452):73–74. doi: 10.1038/249073a0. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Lukat G. S., Stock A. M. Bacterial chemotaxis and the molecular logic of intracellular signal transduction networks. Annu Rev Biophys Biophys Chem. 1991;20:109–136. doi: 10.1146/annurev.bb.20.060191.000545. [DOI] [PubMed] [Google Scholar]


