Abstract
Our objective was to determine how circulatory failure develops following systemic administration of potassium cyanide (KCN). We used a non-inhaled modality of intoxication, wherein the change in breathing pattern would not influence the diffusion of CN into the blood, akin to the effects of ingesting toxic levels of CN. In a group of 300–400 g rats, CN-induced coma (CN IP, 7 mg/kg) produced a central apnea within 2–3 minutes along with a potent and prolonged gasping pattern leading to auto-resuscitation in 38% of the animals. Motor deficits and neuronal necrosis were nevertheless observed in the surviving animals. To clarify the mechanisms leading to potential auto-resuscitation versus asystole, 12 urethane-anesthetized rats were then exposed to the lowest possible levels of CN exposure that would lead to breathing depression within 7–8 minutes; this dose averaged 0.375 mg/kg/min iv. At this level of intoxication, a cardiac depression developed several minutes only after the onset of the apnea, leading to cardiac asystole as PaO2 reached value around 15 Torr, unless breathing was maintained by mechanical ventilation or through spontaneous gasping. Higher levels of KCN exposure in 10 animals provoked a primary cardiac depression, which led to a rapid cardiac arrest by pulseless electrical activity despite the maintenance of PaO2 by mechanical ventilation. These effects were totally unrelated to the potassium contained in KCN.
It is concluded that circulatory failure can develop as a direct consequence of CN induced apnea but in a narrow range of exposure. In this “low” range, maintaining pulmonary gas exchange after exposure, through mechanical ventilation (or spontaneous gasping) can reverse cardiac depression and restore spontaneous breathing. At higher level of intoxication, cardiac depression is to be treated as a specific and spontaneously irreversible consequence of CN exposure, leading to a pulseless electrical activity.
Keywords: Cyanide, pulseless electrical activity, gasping, apnea
INTRODUCTION
Cyanide (CN) certainly represents the archetype of poison as it can kill, in very small amounts, within seconds or minutes. The mechanism of the rapid fatal outcome produced by CN is not always clear and even if the ultimate outcome is a profound cardiogenic shock leading to asystole, its mechanism of production remains equivocal. Indeed CN can produce 1- central apnea, due to an inhibition of the activity of medullary neurons (1, 2), which rapidly opposes the initial hyperpnea produced by CN, eventually leading to hypoxemia induced asystole; 2- on the other hand an acute primary depression in cardiac contractility leading to a pulseless electrical activity is a well described complication of acute CN poisoning (3–7). In contrast to the research done on the development on specific CN antidotes, aimed at decreasing the concentration of CN in the blood and tissues by either trapping CN or speeding up its elimination as thiocyanide, the symptomatic treatments of the respiratory and circulatory depression produced by CN is currently lacking a well accepted rational framework and standard of care.
Our specific objective was to determine 1- the temporal profile of CN induced apnea and CN induced circulatory failure to identify the interactions between the depression in breathing and in cardiac function in keeping with the severity of the intoxication; 2- the immediate outcome of a reduction in cardiac contractility resulting from CN induced apnea versus a primary cardiac failure produced by CN (8, 9). We used a systemic administration of CN, wherein CN rapidly diffuses to the blood stream, irrespective of the level of pulmonary ventilation as during CN ingestion (10, 11). These intoxications differ from those produced by the inhalation of CN during as CN diffusion into the blood does not increase during the period of hyperventilation or decrease during the period of CN-induced apnea. We first focused our attention on the response of unsedated rats receiving a unique lethal dose of intra-peritoneal (IP) CN salt (12–15); then we attempted to dissect the mechanisms leading to death or auto-resuscitation that we saw in the “IP model”, by using of intravenous infusion of different, but well controlled, concentrations of KCN (16–19) that would all potentially lead to cardiac asystole.
METHODS
Adult male Sprague-Dawley rats were used for this study. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, 8th Edition (National Research Council (US) Institute for Laboratory Animal Research). The study was approved by the Pennsylvania State University, College of Medicine Institutional Animal Care and Use Committee. All rats were housed in open top cages in a temperature-controlled conventional room (24 ± 1°C) with a 12-hour light/dark cycle, and were provided with standard laboratory diet and water ad libitum.
1 Cyanide-induced apnea in unanesthetized rats
Animal preparation and protocol (n=10)
Rats (336 ± 43 g) received an injection of CN IP at a dose of 7 mg/kg that was reported to be lethal in about 75% of animals (12, 20) and that we also found to be lethal within less than 20 minutes. KCN was diluted in sterile saline at a concentration of 6 mg/ml. Clinical examinations were performed before then every minute for 30 minutes following CN administration. This examination included the determination of presence of the righting reflex, the response to hand clapping and air puff, the grasping reflex, and the withdrawal reflex. Breathing pattern and the presence of a cardiac pulse were monitored. Three animals were placed in a plethysmographic chamber for recording of the breathing pattern, as soon as they presented an apnea. Coma was defined by the disappearance of response to clapping and air puff along with the loss of the righting reflex. Animals that displayed a phase of somnolence, while all the reflexes were still preserved were therefore not considered to present a coma. Breathing movements were monitored to identify tachypnea (rapid shallow breathing with a breathing frequency above 400/min), hypopnea (breathing frequency below 100/min) and production of abrupt and large breaths at a low frequency (gasping). During the first 48 hours following KCN injection, each surviving animal was observed carefully for signs of distress or discomfort. Animals showing signs of prostration, inability to walk, eat, or drink, paralysis or obvious visual deficit, were euthanized. The rats were anesthetized with 3.5% isoflurane in O2 followed by urethane (1.2 g/kg, IP). The animals were then perfused trans-cardially with phosphate buffered saline followed by 10% neutral buffered formalin (NBF). The head of the animal was skinned, decapitated and immersion fixed for 24 hours in 10% NBF before brain removal. The brains were fixed for an additional five days before trimming into nine 5-mm coronal slices using a brain-trimming matrix (Zivic). Tissues were processed in an automated Tissue-Tek VIP processor and paraffin-embedded with a Tissue-Tek TEC embedding station (Sakura Finetek USA, Torrance, CA). Sections were cut at 6 µm for routine hematoxylin and eosin (H&E) staining. All tissues were examined by an ACVP pathologist blinded to treatment. All images were obtained with an Olympus BX51 microscope and DP71 digital camera using cellSens Standard 1.6 imaging software (Olympus America, Center Valley, PA).
2 Cyanide-induced apnea in urethane anesthetized animals
Animal preparation (n=22)
Rats weighing 448 ± 55 g were anesthetized with inhalation of isoflurane 3.5% in O2, followed by IP injection of urethane (1.2 g/kg). They were tracheostomized (14G Surflo catheter). A catheter (PE-50 tubing) was inserted into the right femoral artery for continuous monitoring of systemic arterial blood pressure and arterial blood sampling. A similar catheter was inserted into the left ventricle through the right carotid artery for continuous monitoring of left ventricular pressure. Additional catheters were inserted into the right femoral vein for KCN infusion and in the right jugular vein for drug administration.
The tracheal catheter was connected to a low dead space two-way valve (Hans Rudolph, Shawnee, KS) and the inspiratory flow was measured using a pneumotachograph (1100 Series, Hans Rudolph, Shawnee, KS) (21, 22). The expiratory valve was connected to a series of two 5 ml mixing chambers. An additional source of air was introduced into the first mixing chamber in which flow was continuously measured using another pneumotachograph (1100 Series, Hans Rudolph, Shawnee, KS). Mixed-diluted expired O2 (FEO2) and CO2 fractions (FECO2) were measured continuously using O2 (Oxystar-100, CWE, Inc. Ardmore, PA) and CO2 (Model 17630, VacuMed, Ventura, CA) analyzers, as previously described (23, 24)
The arterial and ventricular catheters were connected to pressure transducers (BD DTXPlus Transducer, Becton Dickinson, Franklin Lakes, NJ; amplifier TA-100, CWE, Inc., Ardmore, PA) to monitor arterial blood pressure and left ventricular pressure. Aortic blood flow and femoral blood flow were measured using transonic flow probes (Transonic Systems, Inc., Ithaca, NY) connected to the transit-time flowmeter (TS420, Transonic Systems Inc., Ithaca, NY). Electrocardiogram was recorded by a bioelectric amplifier (differential AC amplifier, Model 1700, A-M Systems, Sequim, WA).
All signals were digitized at 400 Hz using an analog-to-digital data acquisition system (Power Lab 16/35, ADInstruments, Inc., Colorado Springs, CO) and were visualized online. All data were stored for further analysis by LabChart 7 (ADInstruments, Inc., Colorado Springs, CO).
Breathing frequency (f) and tidal volume (VT) were determined using peak detection and integration of the inspiratory flow signal, respectively, and minute ventilation (V̇E) was computed as f × VT in body temperature and pressure, saturated (BTPS) conditions. Oxygen uptake (V̇O2) was computed as previously described (23, 25). Left ventricular systolic pressure (LVSP) and heart rate (HR) were determined and dP/dt max was analyzed by derivative of the left ventricular pressure signal. Data were averaged over 10 seconds every 10 seconds.
Arterial blood gases, concentrations of lactate and potassium were determined using i-stat analyzer (Abaxis, Union City, CA)
Experimental protocol: intravenous potassium cyanide infusion
Cyanide solutions were prepared using potassium cyanide (KCN, Sigma, St. Louis, MO). KCN was diluted in sterile saline at a concentration of 0.5 mg/ml corresponding to a concentration of 7.7 mM of KCN.
The rational of the protocol used in this study was based on series of exploratory experiments: CN was infused at various higher rates (between 0.1 to 1.2 mg/min) in 3 rats (10 tests), not included in the current study. In keeping with these preliminary results, two levels of exposure were chosen: After 30 minutes of stable recording, KCN was infused at the rate of ~ 0.15 mg/min (0.37 mg/kg/min) in 12 rats. This rate produces a level of exposure that led to apnea within a few minutes according to our preliminary results and studies from Brierley et al. (18). CN infusion was interrupted upon the cessation of the respiratory movements. The time to cardiac arrest was computed. Pulseless electrical activity (PEA) was defined as the persistent electrocardiogram signal (sinus rhythm) but with no effective cardiac contractions. The latter was defined as the moment when left ventricular pulse pressure reached a value of less than 10 mmHg for more than 10 seconds. Out of these 12 rats, the natural history of the apnea was studied without any intervention or with mechanical ventilation initiated 90–120 seconds following the last spontaneous breathing cycle. Arterial Blood gases were determined before starting KCN infusion then about 100 seconds into apnea.
In a separate series of 10 rats, we studied the effects of infusing KCN at the rate of 0.3 mg/min (0.75 mg/kg/min, i.e. twice the rate producing an apnea), which would produce a very rapid drop in blood pressure sometimes even before apnea occurred. Infusion was performed while the animals were mechanically ventilated since they would all present an almost immediate apnea. Arterial blood gases, lactate and potassium concentrations were determined before starting KCN infusion then 3, 5 and 7 minutes into the infusion periods.
Intravenous infusion of potassium chloride
A solution of KCl was prepared at the same molar concentration than “high dose” KCN protocol (7.7 mM). This solution was infused in a group of 5 rats at the same rate as KCN (0.6 ml/min) for 7–8 minutes. Arterial blood gases, lactate and potassium concentrations were determined before starting KCl infusion then 7 minutes into the infusion period.
3 Statistical analysis
All results are presented as mean ± SD. All variables of interest (breathing pattern, blood pressure, dP/dt, ventricular pressure, cardiac output) were averaged every 10 seconds for comparison and analyzed over time using repeated-measured ANOVA followed by Bonferroni’s post-hoc comparisons. The changes from baseline level to the moment of apnea were compared using paired t test. All variables of interest were also compared between the KCl and KCN-intoxicated animals using an un-paired t test. The statistical analyses were conducted using GraphPad Prism 5 (Graphpad Software, La Jolla, CA). P<0.05 was regarded as significant for any of these comparisons.
RESULTS
1 Response to KCN IP in unsedated rats
In keeping with our preliminary data and data from the literature (12, 20), we used a dose of KCN, which averaged 2.3 ± 0.3 mg (7 mg/kg) IP to produce a fatal outcome within 8–10 minutes in 75% of the animals presenting a coma. Indeed, at this dose, 8 out of 10 intoxicated rats presented a coma (loss of righting reflex and disappearance of all other reflexes) within 2 minutes (88 ± 29 seconds) following the injection. The 2 rats that did not display a coma following CN administration presented a transient phase of somnolence and apathy, and for one rat a profound muscular weakness but did not display an apnea or a gasping pattern. The phase of coma was typically preceded by a brief period of tachypnea. Breathing depression was observed very rapidly into the period of coma (129 ± 32 seconds after CN administration). The period of apnea was interrupted after 35 ± 16 seconds in every animal by a long period of gasping for another 165 ± 37 seconds until the animal died or spontaneously recovered. The longest period of gasping was 15 minutes. This phase consisted in the production of large breathes at a very slow frequency (around 30 breath/min) and with a brief inspiratory time mimicking the production of a hiccup (Fig. 1). These large breaths were associated with a loud and brief inspiratory grunting. Out of the 8 rats that presented a coma and gasping, 3 survived. In these animals, the phase of gasping was progressively replaced by eupneic breathing, starting with the disappearance of the inspiratory grunting, followed by an increase in breathing frequency, and then a recovery of normal respiratory pattern. These 3 rats progressively recovered over a few hours but remained somnolent. The remaining 5 animals presented a total disappearance in cardiac pulsations 555 ± 75 seconds after CN injection; the duration of the period of gasping in these animals was twice as long as in the overall group, and, cardiac arrest occurred while the animals were still gasping.
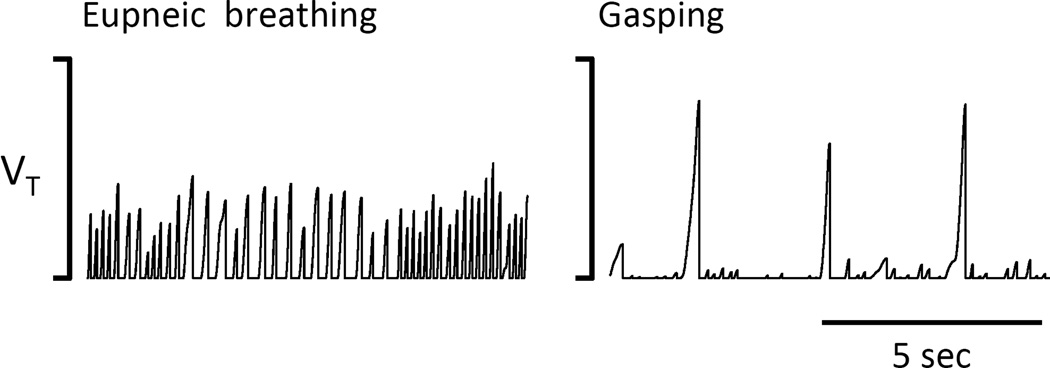
Figure 1.
Example of breathing recordings obtained in unanesthetized rats using our open flow plethysmograph before CN IP administration (left panel) then 2 minutes after the onset of the apnea (right panel), showing a production of large breaths associated with an inspiratory grunting, reflecting the production of gasps. This pattern was maintained until the animals fully recovered or died by cardiac arrest. Tidal volume (Vt) is expressed in arbitrary units.
Two animals that survived the phase of coma after presenting an apnea displayed severe motor deficits: one rat had a right hemiplegia that required its euthanasia within the first 12 hours, while the second rat was euthanized after 48 hours, as it was unable to eat and drink on its own. The histological examination of its brain (Figure 2 and 3) revealed: 1- diffuse bilateral and symmetric neuronal necrosis of the cortex affecting the superficial (outer) half of the frontoparietal (motor agranular) and infralimbic cerebral cortex. Necrosis spared the deepest 1/3 of the cortical grey. 2- necrosis of approximately 20% of neurons of the caudate putamen. Necrotic neurons were often surrounded by morphologically normal ones and were more frequent in the central portion. 3- bilateral and nearly diffuse neuronal necrosis of the mediodorsal nucleus of the thalamus, and bilateral neuronal necrosis and edema of the reticular substantia nigra, with minimal involvement of the compact substantia nigra. 4-bilateral and symmetric neuronal necrosis and edema of the hippocampal CA1 neurons immediately adjacent to the subiculum. The remainder of the hippocampus (CA1–3 and dentate gyrus) was unaffected, while the basolateral nucleus of the amygdala presented bilaterally symmetric neuronal necrosis; 5- multifocal to coalescing necrosis and neuropil edema in approximately 60% of the Purkinje cells in the cerebellum. There were no visible lesions of the medulla.
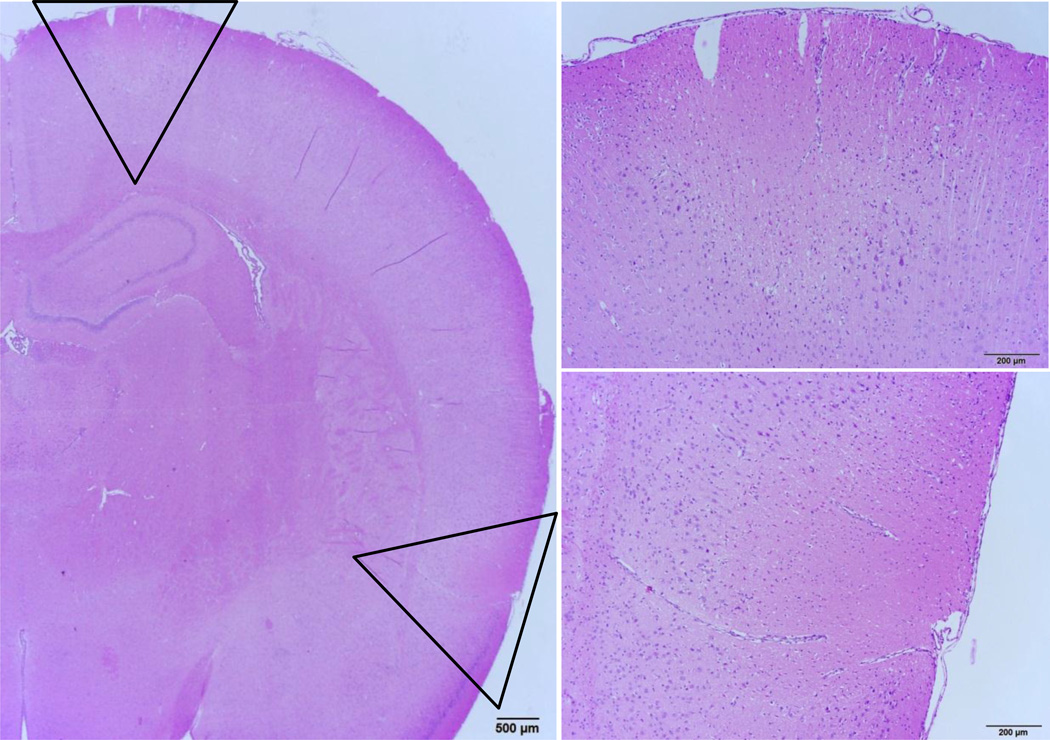
Figure 2.
(Left) Examples of lesions found in rats that spontaneously auto-resuscitated. Discrete, bilateral, wedge-shaped regions of cortical necrosis were observed in the dorsal frontal cortex and the ventral parietal cortex adjacent to the piriform lobe. Necrotic foci were found approximately at the level of the rhinal fissure extending from cortical layers into the middle grey and endopiriform neucleus, but sparing the remaining piriform cortex and amygdala (outlined wedge regions shown in panels to the right). In the cortical regions between the focal lesions, moderate necrosis was observed as both individual neurons and discrete, roughly circular geographic foci. Furthermore, nearly diffuse, severe, and acute bilaterally symmetric neuronal necrosis affects the superficial layers of the frontoparietal (motor agranular) and infralimbic cerebral cortex (not shown, additional description in results). The deeper cortex displays marked neuropil edema, with sparing of the deepest third of the cortical grey. Additionally, mild, acute neuronal necrosis is observed bilaterally and symmetrically in the medial anterior olfactory nucleus, while the piriform cortex was not affected (not shown).
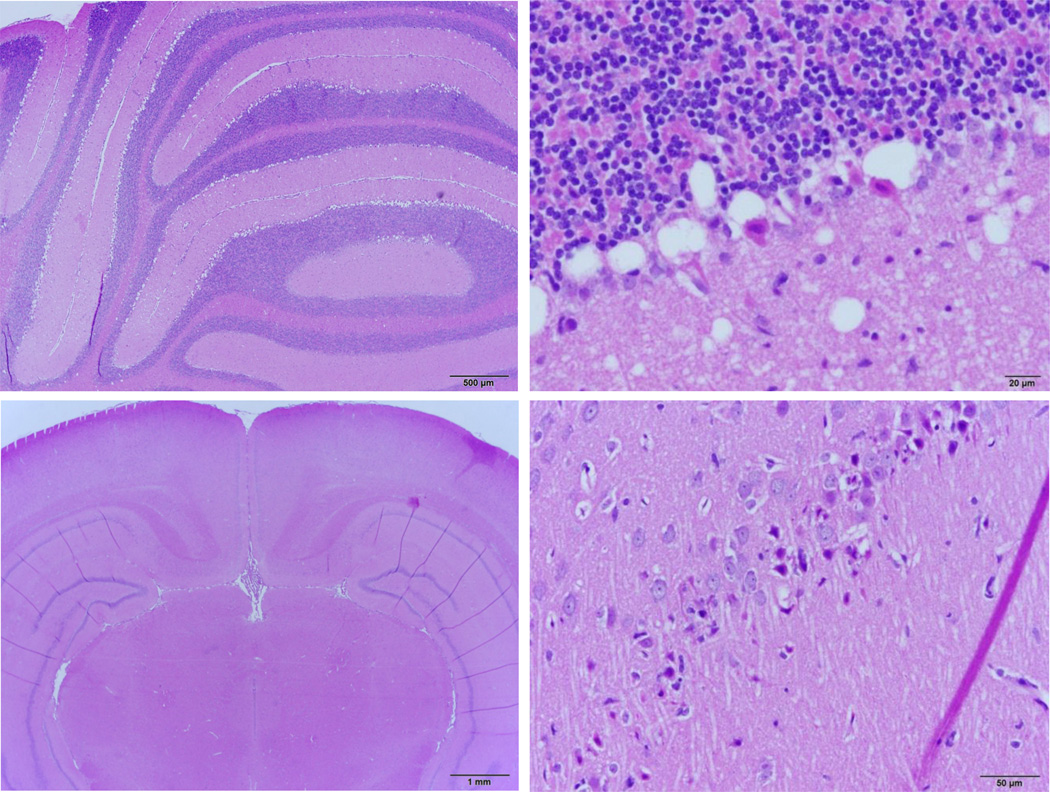
Figure 3.
Damage to the cerebellum and hippocampus after KCN (Top panels). Cerebellar Purkinje cell layer displayed severe regions of neuronal necrosis. The pattern of necrotic damage displayed is prototypical for ischemia of the brain. (Bottom panels) Bilaterally symmetric necrosis and edema was found in a small region of CA1 neurons immediately adjacent to the subiculum. Approximately 20% of neurons in the caudate putamen displayed necrosis (not shown). The central caudate putamen appeared most affected with a higher density of necrotic neurons. Interestingly, individual necrotic neurons were typically surrounded by morphologically normal cells.
2 Outcome of CN induced apnea in anesthetized animals
To dissect the mechanisms leading to auto-resuscitation or to death, we first determined the lowest levels of KCN infusion, which led within 8–10 minutes to a central apnea. We found that this level corresponded to 0.15 mg/min (0.375 mg/kg/min). A total of 12 rats were studied using this rate of infusion. In all rats, an apnea occurred within 551 ± 120 seconds, as illustrated on figure 4. The total amount of CN required to stop breathing with this protocol averaged of 1.5 ± 0.4 mg (3.4 ± 0.7 mg/kg). Of note, cyanide infusion initially stimulated breathing within the first 2–3 minutes, reaching its maximum 242 ± 157 seconds into KCN infusion; minute ventilation increased from 299 ± 53 ml/min to 565 ± 83 ml/min (P<0.01). Following this initial stimulation, breathing frequency and minute ventilation started to decrease during the subsequent 3 minutes, while tidal volume kept increasing until the animals presented few large breaths and then became apneic (Figure 4). Oxygen consumption significantly decreased by half throughout the period of CN exposure (P<0.05, Figure 5). Of note is that typical augmented breaths (sighs) were continuously produced during CN infusion, a likely marker of the stimulatin of the arterial chemoreceptors.
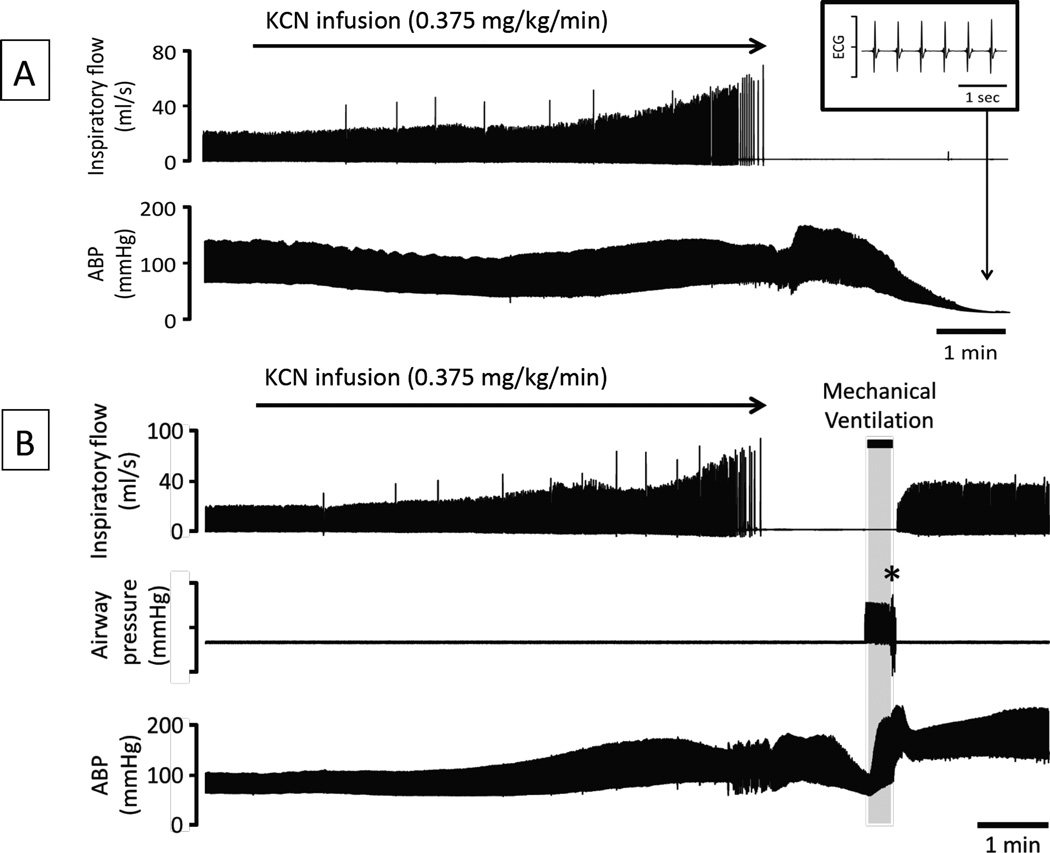
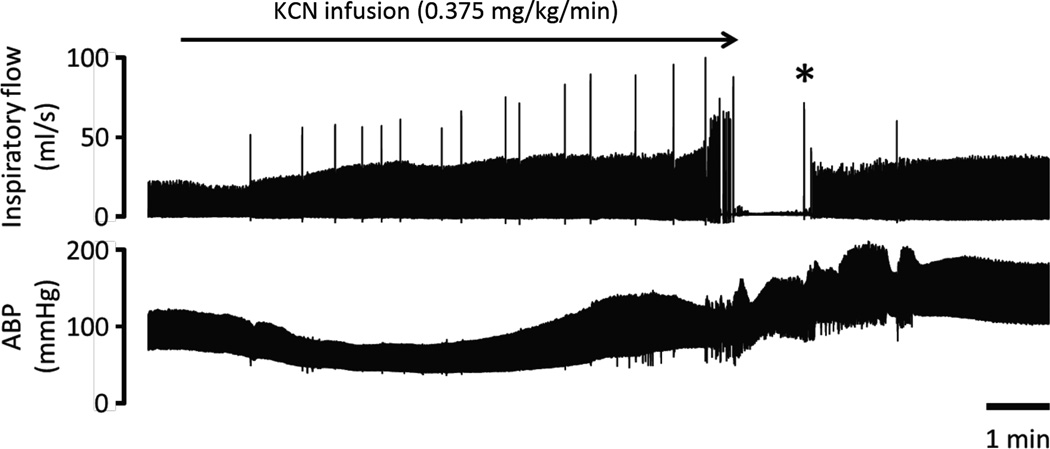
Figure 4.
Effects of continuous infusion of KCN (0.375 mg/kg/min) on breathing (inspiratory flow) and arterial blood pressure (ABP) in one rat (panel A). Breathing was stimulated by the infusion of KCN before stopping after 8 minutes. ABP was sustained during the phase of infusion and the initial 2 minutes of apnea before dropping leading to cardiac arrest despite the cessation of the KCN infusion. The inset on the top-right corner shows the electrocardiogram (ECG) signal during the period of asystole, reflecting the presence of a pulseless electrical activity (PEA). Note that no gasping was produced during the phase of apnea in this rat. Panel B shows effects of CN as in panel A, but mechanical ventilation was initiated 90 seconds into the period of apnea, while blood pressure had already dropped by half. Note the restoration of hemodynamics followed by the recovery of a spontaneous eupneic breathing activity resulting from the mechanical ventilatory support that was maintained for 30 seconds only (gray area).
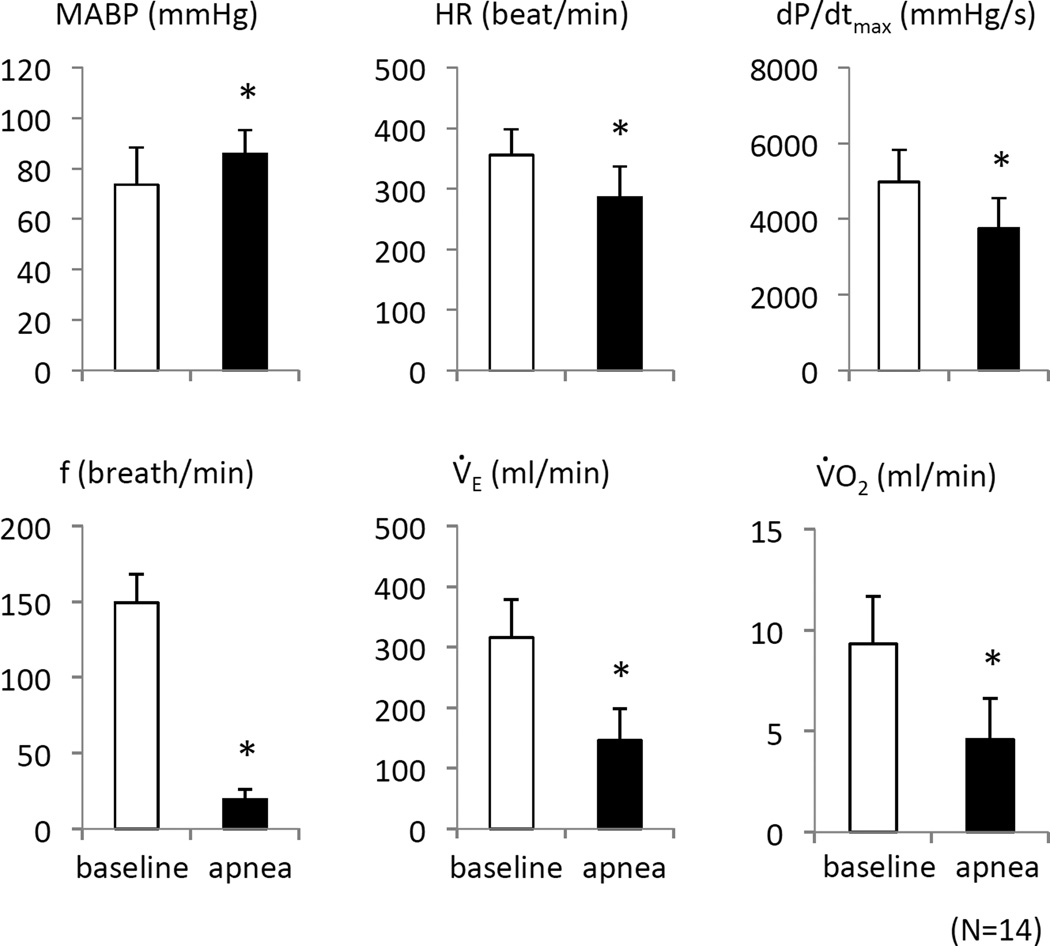
Figure 5.
Cardiovascular and ventilatory parameters in baseline conditions and during the last 30 seconds preceding the apnea while infusion KCN at 0.375 mg/kg/min in 12 rats. Data ± SD values are shown. Note that MABP was unchanged at the moment of apnea, while HR and dP/dt max were moderately decreased. *Significant difference between groups, P<0.05.
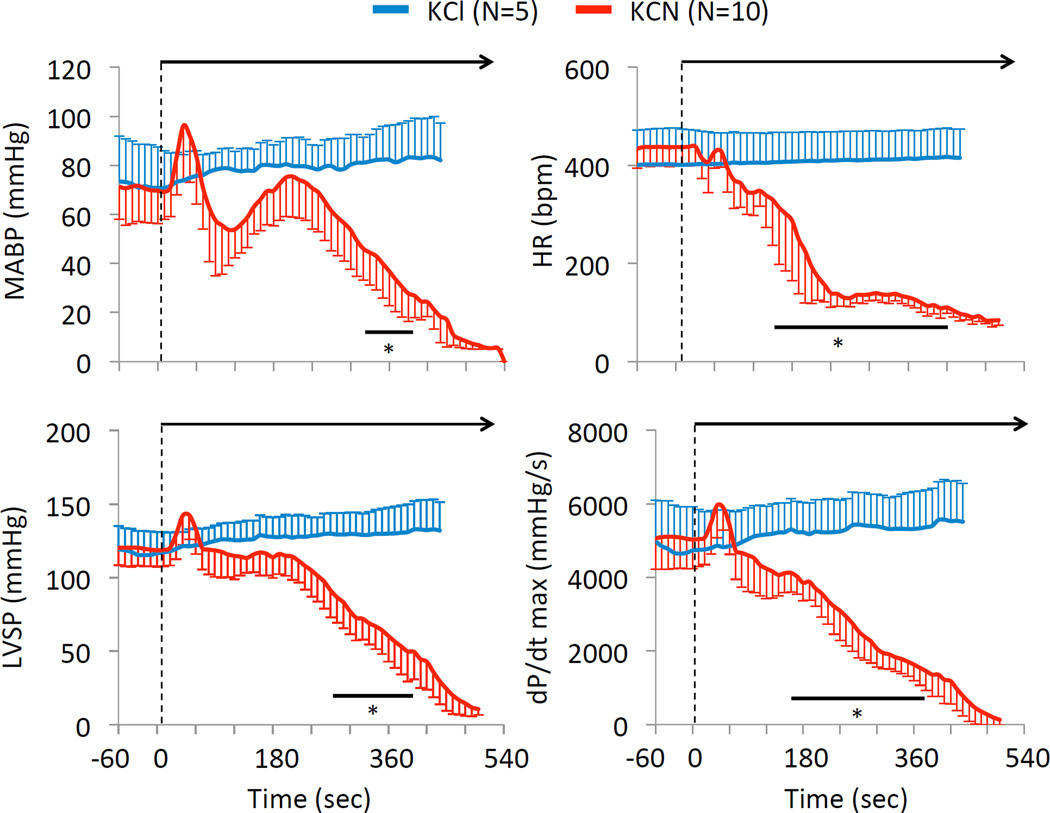
In contrast to breathing, mean arterial blood pressure (MABP) that displayed an initial drop, from 75 ± 14 mmHg to 55 ± 10 mmHg, reaching its nadir at 200 seconds (Figure 5, P<0.01) returned to baseline and remained around 86 ± 9 mmHg (P<0.01 versus baseline, Figure 5) at the time of apnea. Heart rate decreased from 355 ± 43 bpm to 285 ± 51 bpm at the moment of apnea (Figure 2, P<0.05). Finally, left ventricular dP/dt max was lower than baseline (4978 ± 849 mmHg/s versus 3752 ± 796 mmHg/s, P<0.01), just before breathing stopped, as illustrated on Figure 5.
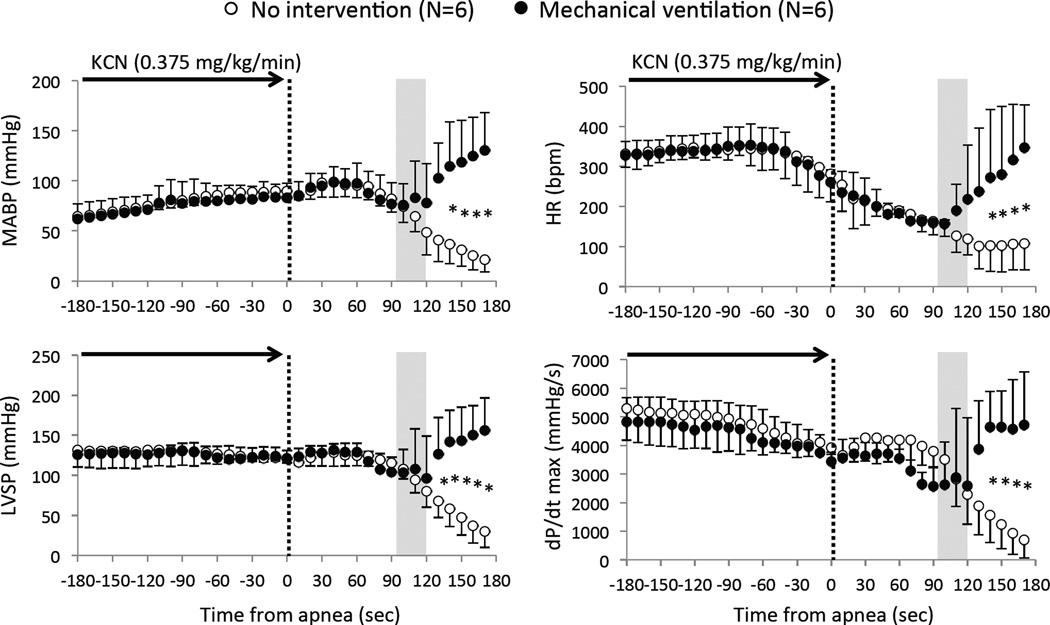
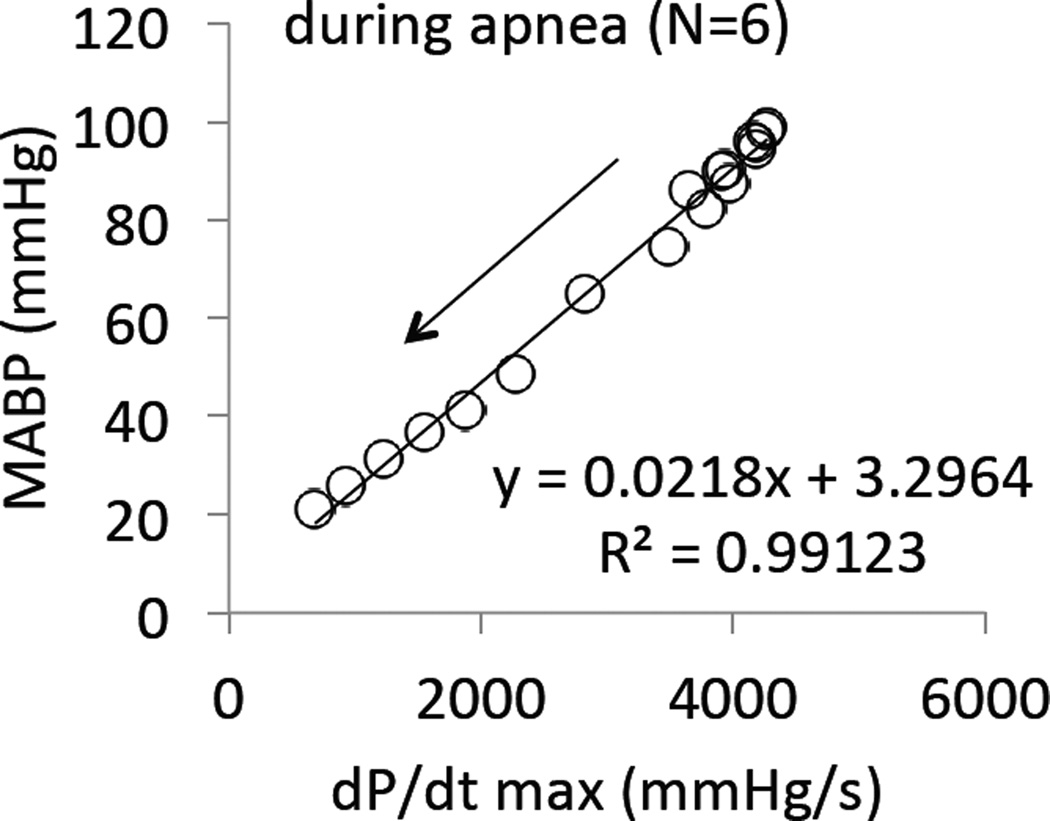
CN infusion was interrupted as soon as breathing stopped. Out of these 12 animals, 10 presented a similar evolution following apnea (Figure 4 and 6): blood pressure remained constant for about 1 to 2 minutes before decreasing very rapidly. The drop in MABP was linearly related to the decrease in cardiac contractility (dP/dt max) during this phase of apnea (Figure 7). In 6 out of these 10 animals, no intervention was applied and all the animals died by PEA, which occurred with a median of 208 sec after the onset of apnea. Of note, PaO2 reached 14±4 mmHg 100 seconds into apnea. In the 4 remaining animals, mechanical ventilation was initiated as MABP dropped to 76 ± 21 mmHg (by the end of the second minute of apnea): ABP and cardiac contractility returned to, or even increased above, baseline as shown in Figure 4 and 6. In these 4 animals, spontaneous breaths identified from the airway pressure signal resumed within 90 seconds, while the animal was still ventilated. In other words, mechanical ventilation counteracted the reduction in blood pressure, prevented PEA, and restored eupneic breathing. The same protocol was repeated in 2 of these animals 30 minutes after recovery and led to the exact same outcome. Figure 6 includes the responses obtained during these 6 trials.
Figure 6.
Mean ± SD values of mean arterial blood pressure (MABP) heart rate (HR), left ventricular systolic pressure (LVSP) and dP/dt max in all 10 rats (12 tests) during and following KCN-induced apnea (0.375 mg/kg/min). The time of apnea was considered as time zero (dashed line). In 4 rats (6 tests, closed-circles), ventilatory support was initiated 90 seconds into the period of apnea, while 6 rats (6 tests, open-circles) were not ventilated. Hemodynamics (MABP, LVSP, and dP/dt max) were maintained for about 60 sec after the onset of apnea, then MABP, LVSP, and dP/dt max started to decrease, until PEA occurred in the non-treated group. The circulatory parameters and cardiac function were restored by the mechanical ventilation, preventing PEA in all the treated rats. The shaded area shows the duration of ventilatory support (about 30 seconds). *Significant difference between groups, P<0.05.
Figure 7.
Average mean blood pressure (MABP) as a function of dP/dt max throughout the phase of apnea (after the cessation of KCN infusion) in the group of 6 rats that did not auto-resuscitate. The drop of MABP clearly paralleled the decrease in cardiac contractility during the period of apnea.
The 2 remaining animals (out of initial 12) survived with no intervention; they displayed a gasping pattern of breathing 42 and 66 seconds into the period of apnea (Fig. 8). The gasps consisted in few large breaths and resulted in auto-resuscitation, as eupneic breathing spontaneously resumed in both animals and PEA did not occur. As a result, spontaneous survival, i.e. without any intervention, occurs in 17% of intoxications, only when gasps were present, while 100% of the animals recovered using mechanical ventilation.
Figure 8.
Effects of continuous infusion of KCN (0.375 mg/kg/min) on breathing and arterial blood pressure (ABP) in one rat, same protocol and legends as in Figure 1. In contrast to the test displayed in figure 4, gasping was produced during the phase of apnea (*). This pattern was observed in 2 rats allowing spontaneous breathing to resume and blood pressure to rise (auto-resuscitation).
3 Effects of higher rate of CN infusion
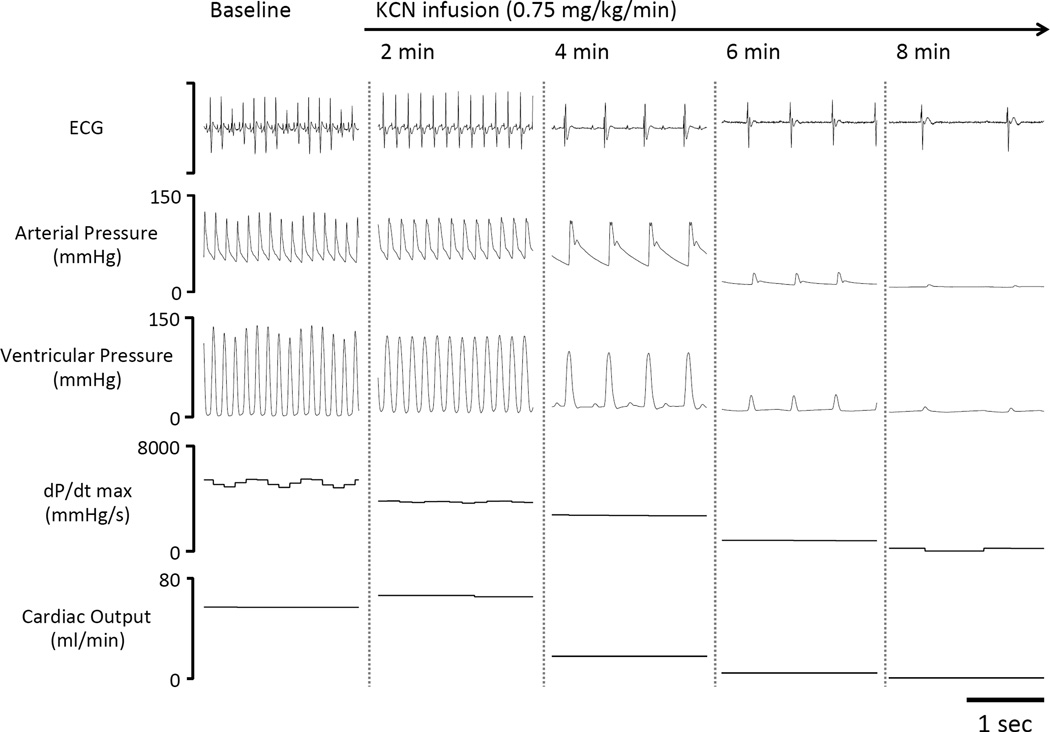
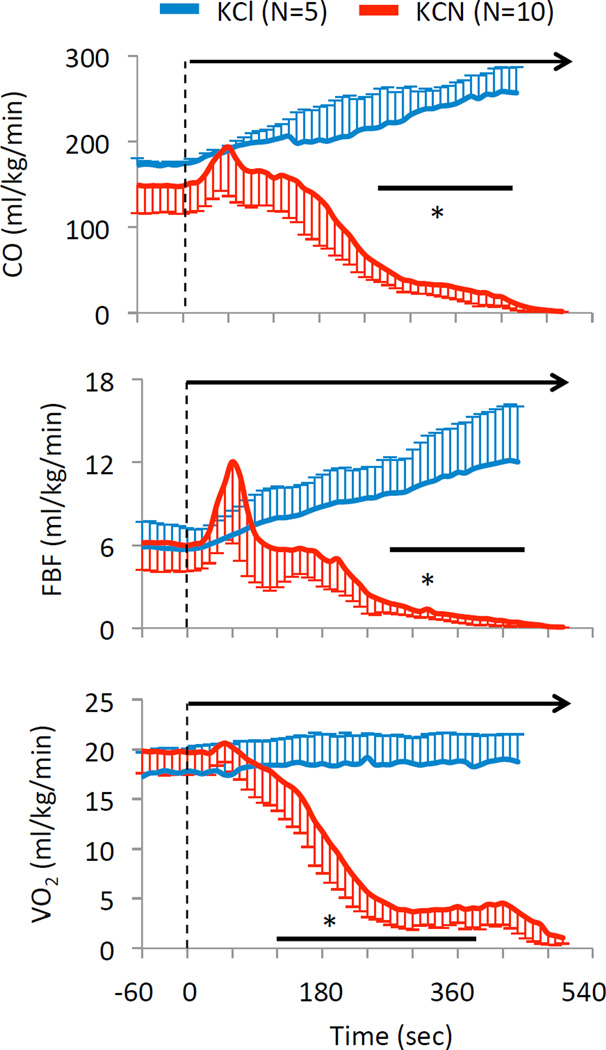
The circulatory effects of injection of 0.3 mg/min (twice the level leading to an “isolated” apnea) were studied while the animals were mechanically ventilated in 10 animals. Figure 9 illustrates the hemodynamic effects of the exposure, while the averaged values are shown in Figure 10 and 11. Baseline MABP and heart rate were 71 ± 13 mmHg and 434 ± 40 bpm, respectively. In major contrast to the low rate of 0.15 mg/min, infusion of KCN at 0.3 mg/min produced a rapid and large drop in heart rate by about 100 bpm (reaching 347 ± 47 bpm, 110 sec into infusion, as illustrated in figure 10), then both MABP and heart rate kept decreasing reaching 46 ± 13 mmHg and 137 ± 17 bpm, 4 minutes into exposure. The reduction in blood pressure was mainly the result of a drop in cardiac output (CO), which decreased from 149 ± 33 ml/kg/min at baseline to 99 ± 38 ml/kg/min (p<0.05) 210 seconds into infusion. CO kept decreasing until cardiac asystole occurred. In keeping with the drop of CO, femoral blood flow also decreased from 6.2 ± 2.0 ml/kg/min at baseline to 2.2 ± 1.2 ml/kg/min 6 minutes into CN infusion (Fig 10). The drop in cardiac output was related to a decrease in cardiac contractility, as left ventricular systolic pressure (LVSP) and left ventricular dP/dt max decreased throughout KCN infusion (Figure 10). A pulseless electrical activity (PEA) was observed in all rats at 457± 34 sec (median 455 sec), i.e. at a similar time than needed to produce an apnea at the lower dose. The rats received 2.5 ± 0.4 mg (5.6 ± 0.6 mg/kg) of cyanide to produce PEA. Until the last minute of shock, PaO2 remained higher than baseline reflecting the reduction in pulmonary blood flow in keeping with the level of ventilation that was maintained by the ventilator as shown in figure 12.
Figure 9.
Recording in one mechanically ventilated rat displaying the electrocardiogram signal (ECG), arterial pressure, ventricular pressure, dP/dt max and computed cardiac output at various times during KCN infusion at a higher dose (0.75 mg/kg/min). In this example, cardiac output (CO) started to decrease between 3 and 4 min into KCN infusion along with an abrupt drop in HR and dP/dt max, rapidly leading to a severe cardiogenic shock and a pulseless electrical activity, as demonstrated by the persistence of an ECG signal without effective cardiac contractions.
Figure 10.
Average ± SD mean arterial pressure (MAP), heart rate (HR), left ventricular systolic pressure (LVSP), and dP/dt max in the 10 rats exposed to 0.75 mg/kg/min KCN. Data have been compared to the effects of an infusion of KCl (equimolar infusion, 11.5 micromol/kg/min). In marked contrast to KCl infusion, all cardiac parameters significantly decreased within 330 sec into KCN infusion leading to PEA within 7–8 minutes. Note that the first rats died by PEA (LVSP < 10 mmHg) at 400 sec. * Significantly different from baseline, p<0.05.
Figure 11.
Mean ± SD values of cardiac output (CO), femoral blood flow (FBF) and oxygen consumption (V̇O2) in the 10 rats exposed to 0.75 mg/kg/min KCN. Data have been compared to the effects of an infusion of KCl (equimolar infusion, 11.5 micromol/kg/min). Same legend as in Figure 10. Again, note the very rapid decrease in cardiac output and VO2 despite mechanical ventilation unrelated to the effects of potassium per se.
Figure 12.
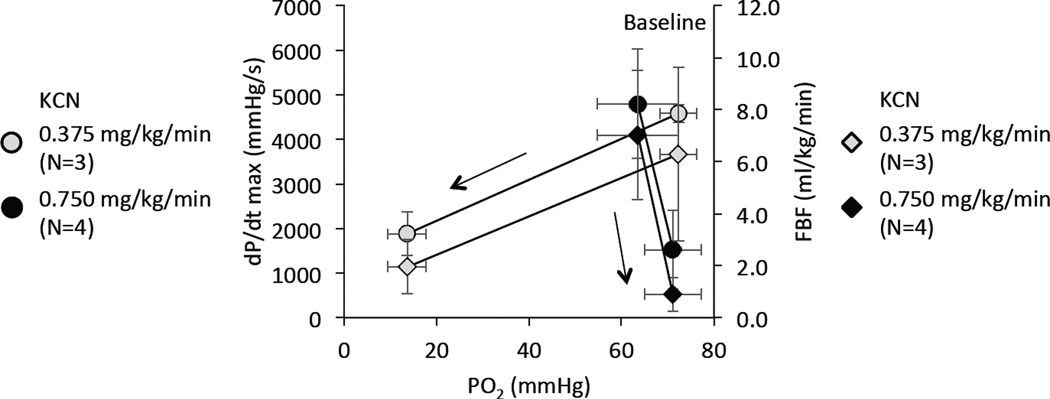
PaO2 (mean ± SD) versus dP/dt max and femoral blood flow (FBF) (mean ± SD) before and after intravenous administration of KCN at 2 different levels. At the low level of intoxication (0.375 mg/kg/min), data were obtained at baseline and 2 min after the apnea in the 6 animals that were not mechanically ventilated and did not present any spontaneous gasping. These data were compared to the effects of CN infusion at 0.75 mg/kg/min (n=10). In these animals, arterial blood gases were collected when dP/dtmax reached a level similar to that obtained 2 min into the apneic period following the low dose KCN infusion. PaO2 was drastically different between these conditions (#P<0.05) despite similar degree of cardiac depression; at the highest levels of CN infusion, the depression of cardiac function occurred in mechanically ventilated animals without any change in PaO2 level. Also note the severity of hypoxemia, which led to PEA in the group receiving 0.375 mg/kg/min.
4 Role of K in the effects of KCN infusion
Infusion of potassium chloride (KCl) was performed in 5 animals at a rate corresponding to that of KC during the high dose protocol. KCl infusion had no effect on the concentration of potassium in the blood (from 3.5 ± 0.4 meq/l to 3.8 ± 0.2 meq/, NS) and was unable to reproduce any of the effects of KCN on circulation or breathing (Fig. 13).
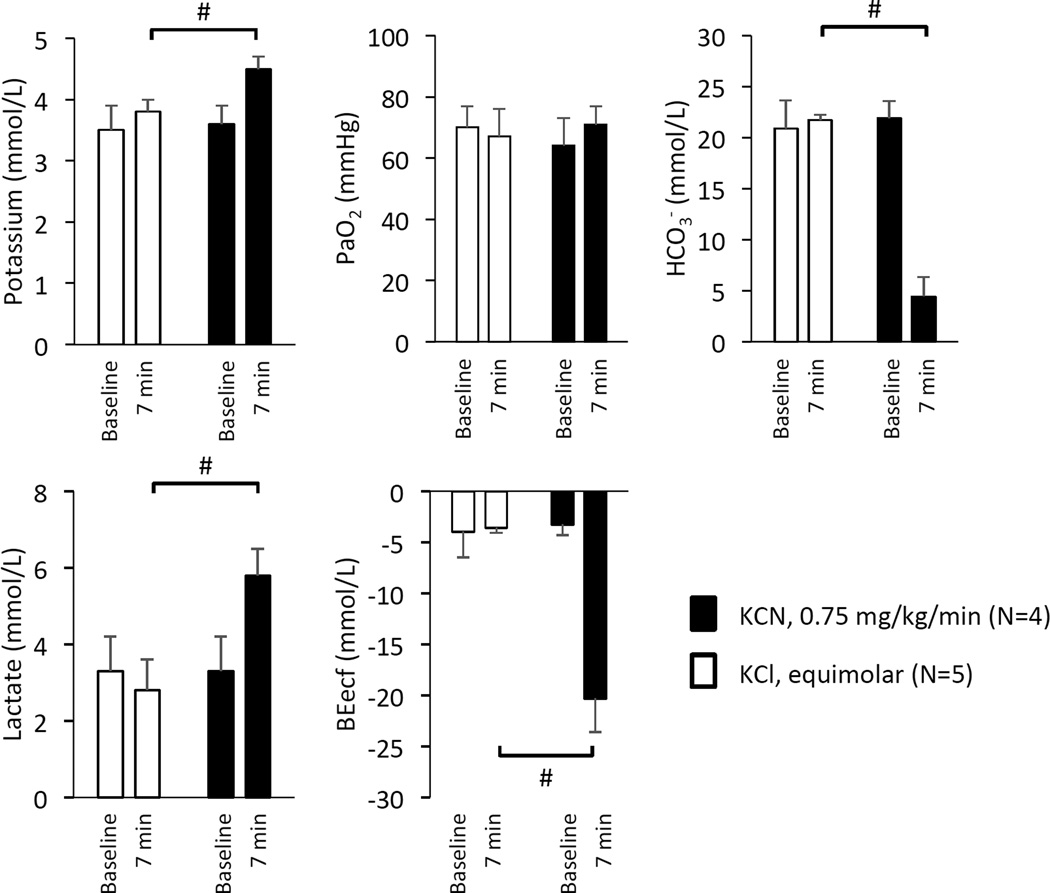
Figure 13.
Mean ± SD values of blood concentration of potassium, PaO2, HCO3−, lactate, and base excess before and during the 7th minutes of either KCN infusion 0.75 mg/kg/min (black-bars, n=4) or infusion of an equimolar solution of KCl (11.5 µmol/kg/min, gray bars, n=5). #, significant difference between KCl and KCN at 7 min, P<0.05. Note that changes in blood concentration of potassium were trivial during KCN and that KCN infusion was associated with a decrease in HCO3- along with a base deficit averaging −20 mmol/l reflecting the profound circulatory shock and possibly impediment of mitochondrial respiration.
In addition, as shown on figure 13, in contrast to the effects of KCl infusion, KCN infusion changed the level of potassium from 3.6 ± 0.3 to 4.5 ± 0.2 meq/l (p<0.05). This increase in potassium remained however trivial (0.9 meq/l) and was associated with a severe base deficit which decreased form −3.3± 1.0 meq/l to −20.3 ± 3.3 meq/l, while lactate concentration increased from 3.3 ± 0.9 meq/l to 5.8± 0.7 meq/l. In other words, we did not find any evidence that the potassium contained in KCN contributed to any of the effects of CN infusion nor did it produce measurable effects on respiration of circulation.
DISCUSSION
The mechanisms leading to death versus auto-resuscitation (survival) during acute CN intoxication appear to differ according to the level of exposure. This difference has important clinical implications when considering and designing appropriate therapies for acute life threatening CN intoxication. Although we found that the direct cause of death during CN infusion was typically a pulseless electrical activity (PEA), the mechanisms leading to PEA clearly depends on the level of exposure. Indeed, as developed in the following paragraphs, the deterioration in cardiac function (reduction in cardiac output and contractility) could result from a primary apnea, at a lowest lethal range of CN exposure. The production of gasps can, in this situation, lead to spontaneous recovery, but death can also be prevented by mechanical ventilation. At higher levels of intoxication, a primary and rapidly developing cardiac depression led to a cardiac arrest by PEA even if mechanical ventilation was initiated and PaO2 maintained constant.
Non-inhaled forms of CN intoxication
The debate over the models to be used to study CN intoxication is not new and any reader interested in the question of CN poisoning could be rapidly lost amid the studies using inhaled, IP, oral or IV administration of CN -not to mention the almost infinite combinations of protocols that can be offered, from short, repetitive or prolonged exposures at low or high levels. Of note, the systemic administration of a solution of CN has been widely used because it is a convenient and safe way to study CN poisoning, even though IV or IP administration of KCN or NaCN do not correspond to a clinically relevant scenario of intoxication. Yet, these systemic administrations of a CN salt can provide precious and clinically relevant information. In other words, a physiological study does not have to be totally faithful to real life modalities of exposure to be relevant, if one wants, for instance, to dissect or understand the natural history of breathing and circulatory depression.
The experimental approaches we used produced effects that are extremely close to those produced by an acute CN intoxication by ingestion (10, 11). These forms of poisoning are regarded as a potential source of intoxication during a criminal act or a potential terrorist attack. Recent studies in rodents (10, 11) have established how rapidly lethal can oral intoxication be (within minutes). These effects are very well mimicked by an IP injection since during both oral or IP administration, the diffusion of CN into the blood does not depend on the level of breathing and CN will continue to diffuse at the same rate regardless of the levels of minute ventilation. The lack of effects of potassium in KCN was also important to clarify in our model and we found that potassium did not contribute at all to the response to KCN.
Circulatory failure during CN intoxication
At a rate of 0.15 mg/min IV, CN provoked a central terminal apnea within 7–8 minutes followed by a depression in circulation leading to PEA in all animals except if gasping was produced. At this level of exposure, central apnea was consistently observed at a time when blood pressure was still maintained around or even above baseline levels. In most cases, breathing remained inhibited until death, while blood pressure and cardiac contractility decreased rapidly leading to cardiac arrest (see Figures 4 and 7) when PaO2 reached values below 15 mmHg. Of note, this value of 15 mmHg corresponded to a level of hypoxemia which we previously found to be able, to stop ventilation per se (26), preventing therefore breathing to spontaneously recover during CN intoxication. Each time mechanical ventilation was initiated, when the blood pressure was already decreased but still maintained above 30–40 mmHg, cardiac contractility and circulation could be restored and eupneic breathing resumed, although no antidote was administered. The same effect was observed when gasping was produced - a rare event in our anesthetized model- in contrast to the effects of CN in unsedated animals, wherein gasping and auto-resuscitation was much more frequent (figure 1). Resuscitation, following CN intoxication, seems therefore to 1) depend on the partial restoration in alveolar ventilation (whether produced by gasps or mechanical ventilation) (27) via an increase in PaO2 (26) and possibly the beneficial effects of mechanical ventilation or gasping on cerebral blood flow (26, 28–30). This observation also suggests that the effects of CN on the brainstem respiratory neurons can be rapidly reversed.
For higher levels of intoxication, blood pressure and cardiac contractility are affected despite constant ventilatory support. In these conditions, the depressive effect of CN on cardiac contractility was the primary mechanism leading to death, which also develops within 7–8 minutes.
The determination of the precise mechanisms leading to central apnea and cardiogenic shock is beyond the scope of this study. It has been shown that the effect of blocking the mitochondrial complex IV by CN is an immediate impediment of ATP formation (31), which closely mimics the effects of acute anoxia produced, for instance, by breathing pure nitrogen or during cardiac arrest-induced brain stem ischemia (26, 27). During acute anoxia, the production of gasps following the inhibition of the central pattern generator, (32) has long been described (33, 34) and the sequence of events leading to auto-resuscitation mimics the effects of CN poisoning we are reporting in the unsedated rat model. The depression in cardiac contractility is obviously potentiated by the effects of apnea-induced hypoxemia which comprises the direct effects on cardiac contractions of the reduction in ATP, local acidosis (4, 7) and possibly non-ATP related effects on calcium transients (35).
It should be pointed out that the production of gasps, which appears to be extremely favorable during ingestion or systemic administration of CN, would certainly become deleterious during CN inhalation if exposure to CN persists. Gasps are large and deep breaths that allows the transport of CN to the alveolar regions and its diffusion to the pulmonary circulation to resume, resulting in cyclic periods of auto-resuscitation and apnea until a final cardiac arrest occurs. A similar reasoning can be applied following CN ingestion about the amount of CN that might be inhaled in the form of HCN released from the stomach into the upper-airways.
Clinical implications of these observations for the treatment of CN poisoning
If the lethality and morbidity of CN poisoning result from a primary depression in breathing, cardiac contractility or a combination of both, a given antidote may have a different outcome in keeping with the kinetics of its impact on CN metabolism and on the activity of medullary neurons or cardiomyocytes. Conversely, in conditions wherein the anoxic/ischemic or post-ischemic injuries secondary to an acute respiratory failure are dictating the short- and long-term neurological outcome- rather than the effects of CN per se, the acute circulatory failure as well as neurological deficits might not be efficiently counteracted by CN antidotes, which delay of action may be within minutes. Our present study suggests that the level of oxygenation of the cardiac cells and of the medulla contributes to the fatal outcome of CN poisoning at least in the less “severe” lethal forms. Any antidote administered during this period must take into account whether gasps can be produced, as the improvement of circulation will likely improve the diffusion of drugs if non-IV routes are to be used.
As a consequence, the lack of efficacy of any non-IV antidote in an animal model, in which gasping is blunted, should be challenged and retested using awake animal models. Perhaps more importantly, as the level of oxygenation becomes critical for the cardiac function and the rapid correction of hypoxia/anoxia becomes necessary, administration of antidotes without initiating mechanical ventilation may incorrectly lead to the rejection of candidate antidotes potentially very effective on cardiac contractility.
Finally, immediate recovery is not the only predictor of efficacy of an antidote, as dreadful neurological necrosis develops even after spontaneous auto-resuscitation from CN poisoning (figure 2 and 3). This illustrates that auto-resuscitation should not always be the defining marker of a benign form of intoxication or success of an antidote.
In conclusion, 1- the mechanisms leading to cardiac arrest during CN intoxication are primarily related to a reduction in cardiac contractility resulting in PEA; 2- apnea induced hypoxia can create a vicious circle maintaining the depression in respiratory medullary neurons produced by CN leading to a depression in cardiac contractility; 3- implementing mechanical ventilation when apnea is present, while circulatory failure is predominantly the consequence of apnea induced hypoxemia, has striking effects on circulation and can allow eupneic breathing to resume. This effect is, however, present only in a narrow range of CN exposure and as long as circulation is maintained; 4- whenever blood pressure is rapidly affected, a cardiac asystole develops due to a primary depression in cardiac contractility by CN; 5- when developing new antidotes against CN, long-term effects are essential to evaluate, as severe neuronal necrosis can develop even in animals presenting auto-resuscitation; and 6- finally, the present results suggest that CN intoxication by non inhalation exposure represents a form of CN poisoning wherein gasp-induced auto-resuscitation is an important mechanism of survival.
Acknowledgments
The animal studies conform to the requirements of the US Department of Agriculture and the Department of Health, Education and Welfare. The Penn State College of Medicine is accredited by the American Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). The study was approved by the Pennsylvania State University, College of Medicine Institutional Animal Care and Use Committee.
Support
This work has been supported in part by the National Institutes of Health Office of the Director (NIH OD), and the National Institute of Neurological Disorders and Stroke (NINDS), Grant Number R21 NS098991.
Footnotes
This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Honda Y. Control of breathing and its modeling perspective. New York: Plenum Press; 1992. [DOI] [PubMed] [Google Scholar]
- 2.Carroll JL, Gozal D, Rector DM, Aljadeff G, Harper RM. Ventral medullary neuronal responses to peripheral chemoreceptor stimulation. Neuroscience. 1996;73(4):989–998. doi: 10.1016/0306-4522(96)00112-1. [DOI] [PubMed] [Google Scholar]
- 3.Allen DG, Orchard CH. Intracellular calcium concentration during hypoxia and metabolic inhibition in mammalian ventricular muscle. J Physiol. 1983;339:107–122. doi: 10.1113/jphysiol.1983.sp014706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisner DA, Nichols CG, O’Neill SC, Smith GL, Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J Physiol. 1989;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jose AD, Stitt F. Effects of hypoxia and metabolic inhibitors on the intrinsic heart rate and myocardial contractility in dogs. Circ Res. 1969;25(1):53–66. doi: 10.1161/01.res.25.1.53. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki T. Ultrastructural changes of heart muscle in cyanide poisoning. Tohoku J Exp Med. 1968;95(3):271–287. doi: 10.1620/tjem.95.271. [DOI] [PubMed] [Google Scholar]
- 7.Elliott AC, Smith GL, Eisner DA, Allen DG. Metabolic changes during ischaemia and their role in contractile failure in isolated ferret hearts. J Physiol. 1992;454:467–490. doi: 10.1113/jphysiol.1992.sp019274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortin JL, Desmettre T, Manzon C, Judic-Peureux V, Peugeot-Mortier C, Giocanti JP, Hachelaf M, Grangeon M, Hostalek U, Crouzet J, Capellier G. Cyanide poisoning and cardiac disorders: 161 cases. J Emerg Med. 2010;38(4):467–476. doi: 10.1016/j.jemermed.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 9.Bebarta VS, Pitotti RL, Dixon PS, Valtier S, Esquivel L, Bush A, Little CM. Hydroxocobalamin and epinephrine both improve survival in a swine model of cyanide-induced cardiac arrest. Ann Emerg Med. 2012;60(4):415–422. doi: 10.1016/j.annemergmed.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Hawk MA, Ritchie GD, Henderson KA, Knostman KA, Roche BM, Ma ZJ, Matthews CM, Sabourin CL, Wakayama EJ, Sabourin PJ. Neurobehavioral and Cardiovascular Effects of Potassium Cyanide Administered Orally to Mice. IntJ Toxicol. 2016 doi: 10.1177/1091581816646974. [DOI] [PubMed] [Google Scholar]
- 11.Sabourin PJ, Kobs CL, Gibbs ST, Hong P, Matthews CM, Patton KM, Sabourin CL, Wakayama EJ. Characterization of a Mouse Model of Oral Potassium Cyanide Intoxication. IntJ Toxicol. 2016 doi: 10.1177/1091581816646973. [DOI] [PubMed] [Google Scholar]
- 12.Boswell GW, Brooks DE, Murray AJ, Doye AA, Disselhorst DJ, Chin CL, Clifford CB. Exogenous methemoglobin as a cyanide antidote in rats. Pharm Res. 1988;5(12):749–752. doi: 10.1023/a:1015928432494. [DOI] [PubMed] [Google Scholar]
- 13.Mills EM, Gunasekar PG, Li L, Borowitz JL, Isom GE. Differential susceptibility of brain areas to cyanide involves different modes of cell death. Toxicol Appl Pharmacol. 1999;156(1):6–16. doi: 10.1006/taap.1999.8630. [DOI] [PubMed] [Google Scholar]
- 14.Zhang D, Lee B, Nutter A, Song P, Dolatabadi N, Parker J, Sanz-Blasco S, Newmeyer T, Ambasudhan R, McKercher SR, Masliah E, Lipton SA. Protection from cyanide-induced brain injury by the Nrf2 transcriptional activator carnosic acid. J Neurochem. 2015;133(6):898–908. doi: 10.1111/jnc.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan A, Balasubramanian M, Blackledge W, Mohammad OM, Alvarez L, Boss GR, Bigby TD. Cobinamide is superior to other treatments in a mouse model of cyanide poisoning. Clin Toxicol (Phila) 2010;48(7):709–717. doi: 10.3109/15563650.2010.505197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenner M, Mahon SB, Lee J, Kim J, Mukai D, Goodman S, Kreuter KA, Ahdout R, Mohammad O, Sharma VS, Blackledge W, Boss GR. Comparison of cobinamide to hydroxocobalamin in reversing cyanide physiologic effects in rabbits using diffuse optical spectroscopy monitoring. J Biomed Opt. 2010;15(1):017001. doi: 10.1117/1.3290816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavon O. Early administration of isosorbide dinitrate improves survival of cyanide-poisoned rabbits. Clin Toxicol (Phila) 2015;53(1):22–27. doi: 10.3109/15563650.2014.990564. [DOI] [PubMed] [Google Scholar]
- 18.Brierley JB, Brown AW, Calverley J. Cyanide intoxication in the rat: physiological and neuropathological aspects. J Neurol Neurosurg Psychiatry. 1976;39(2):129–140. doi: 10.1136/jnnp.39.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bebarta VS, Tanen DA, Boudreau S, Castaneda M, Zarzabal LA, Vargas T, Boss GR. Intravenous cobinamide versus hydroxocobalamin for acute treatment of severe cyanide poisoning in a swine (Sus scrofa) model. Ann Emerg Med. 2014;64(6):612–619. doi: 10.1016/j.annemergmed.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crankshaw DL, Goon DJ, Briggs JE, DeLong D, Kuskowski M, Patterson SE, Nagasawa HT. A novel paradigm for assessing efficacies of potential antidotes against neurotoxins in mice. Toxicol Lett. 2007;175(1–3):111–117. doi: 10.1016/j.toxlet.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haouzi P, Sonobe T, Torsell-Tubbs N, Prokopczyk B, Chenuel B, Klingerman CM. In vivo interactions between cobalt or ferric compounds and the pools of sulphide in the blood during and after H2S poisoning. ToxicolSci. 2014;141(2):493–504. doi: 10.1093/toxsci/kfu140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klingerman CM, Trushin N, Prokopczyk B, Haouzi P. H2S concentrations in the arterial blood during H2S administration in relation to its toxicity and effects on breathing. Am J Physiol Regul Integr Comp Physiol. 2013;305(6):R630–R638. doi: 10.1152/ajpregu.00218.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonobe T, Haouzi P. H2S induced coma and cardiogenic shock in the rat: Effects of phenothiazinium chromophores. Clin Toxicol (Phila) 2015;53(6):525–539. doi: 10.3109/15563650.2015.1043440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonobe T, Haouzi P. Sulfide Intoxication-Induced Circulatory Failure is Mediated by a Depression in Cardiac Contractility. Cardiovasc Toxicol. 2016;16(1):67–78. doi: 10.1007/s12012-015-9309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chenuel B, Sonobe T, Haouzi P. Effects of infusion of human methemoglobin solution following hydrogen sulfide poisoning. Clin Toxicol (Phila) 2015;53(2):93–101. doi: 10.3109/15563650.2014.996570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haouzi P, Ahmadpour N, Bell HJ, Artman S, Banchs J, Samii S, Gonzalez M, Gleeson K. Breathing patterns during cardiac arrest. J Appl Physiol (1985) 2010;109(2):405–411. doi: 10.1152/japplphysiol.00093.2010. [DOI] [PubMed] [Google Scholar]
- 27.Guntheroth WG, Kawabori I. Hypoxic apnea and gasping. J Clin Invest. 1975;56(6):1371–1377. doi: 10.1172/JCI108217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bobrow BJ, Zuercher M, Ewy GA, Clark L, Chikani V, Donahue D, Sanders AB, Hilwig RW, Berg RA, Kern KB. Gasping during cardiac arrest in humans is frequent and associated with improved survival. Circulation. 2008;118(24):2550–2554. doi: 10.1161/CIRCULATIONAHA.108.799940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie J, Weil MH, Sun S, Yu T, Tang W. Spontaneous gasping generates cardiac output during cardiac arrest. Crit Care Med. 2004;32(1):238–240. doi: 10.1097/01.CCM.0000105042.52059.5A. [DOI] [PubMed] [Google Scholar]
- 30.Ristagno G, Tang W, Sun S, Weil MH. Spontaneous gasping produces carotid blood flow during untreated cardiac arrest. Resuscitation. 2007;75(2):366–371. doi: 10.1016/j.resuscitation.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 31.Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr. 2008;40(5):533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- 32.Ramirez JM, Lieske SP. Commentary on the definition of eupnea and gasping. Respir PhysiolNeurobiol. 2003;139(1):113–119. doi: 10.1016/s1569-9048(03)00195-2. [DOI] [PubMed] [Google Scholar]
- 33.Gozal D, Torres JE. Maturation of anoxia-induced gasping in the rat: potential role for N-methyl-D-aspartate glutamate receptors. Pediatr Res. 1997;42(6):872–877. doi: 10.1203/00006450-199712000-00025. [DOI] [PubMed] [Google Scholar]
- 34.St John WM. Neurogenesis, control, and functional significance of gasping. J Appl Physiol (1985) 1990;68(4):1305–1315. doi: 10.1152/jappl.1990.68.4.1305. [DOI] [PubMed] [Google Scholar]
- 35.Kondo RP, Apstein CS, Eberli FR, Tillotson DL, Suter TM. Increased calcium loading and inotropy without greater cell death in hypoxic rat cardiomyocytes. Am J Physiol. 1998;275(6 Pt 2):H2272–H2282. doi: 10.1152/ajpheart.1998.275.6.H2272. [DOI] [PubMed] [Google Scholar]