Abstract
Introduction
During fetal development, sex steroids influence sexually dimorphic behaviors, such as visual-spatial abilities. Thus, endocrine disrupting chemicals that impact sex steroids during gestation may affect these behaviors.
Objective
We investigated the relationship between prenatal urinary phthalate metabolite, triclosan, and BPA concentrations and visual-spatial abilities in a prospective cohort of 198 mother-child dyads.
Methods
Data are from a prospective cohort in Cincinnati, OH (HOME Study). We measured nine phthalate metabolites, triclosan, and BPA in maternal urine samples collected at 16 and 26 weeks of gestation. We assessed children’s visual-spatial abilities at 8 years of age using the Virtual Morris Water Maze (VMWM), a computerized version of the rodent Morris Water Maze. We quantified the covariate-adjusted change in the time or distance to complete the VMWM and time spent in the correct quadrant during a probe trial with an interquartile range increase in chemical concentrations using linear mixed models and linear regression, respectively.
Results
Boys completed the VMWM faster (4.1 seconds; 95% CI:−7.1, −1.2) and in less distance (1.4 units; 95% CI:−2.8, 0) than girls. Overall, children with higher mono-n-butyl (MnBP), mono- benzyl (MBzP), and mono-carboxypropyl phthalate concentrations completed the VMWM in less time and distance than children with lower concentrations. For example, children with higher MnBP concentrations completed the VMWM in 0.9 less distance units (95% CI:−1.8, −0.0). Child sex modified the association between MnBP and VMWM performance. In girls, higher MnBP concentrations were associated with longer time (1.7 seconds; 95% CI: −0.7, 4.1) and shorter distance (−1.7 units; 95% CI: −2.8, −0.5), whereas in boys, it was associated with shorter time (−3.0 seconds; 95% CI:−5.6, −0.4), but not distance (−0.1 units; 95% CI:1.4, 1.0). Other phthalate metabolites, triclosan, and BPA were not associated with VMWM performance, and sex did not consistently modify these associations.
Conclusions
In this cohort, greater prenatal urinary concentrations of some phthalate metabolites were associated with improved VMWM performance, particularly among boys. Future studies should confirm these findings and determine if phthalates affect other hormonally sensitive aspects of child neurobehavior.
Keywords: Children, endocrine disrupting chemicals, epidemiology, prenatal, neurodevelopment
Introduction
Phthalates, triclosan, and bisphenol A (BPA) are endocrine disrupting chemicals used in a multitude of consumer products. Phthalates are a multifunctional class of chemicals used in some personal care and beauty products, polyvinyl chloride plastics, food processing or packaging, rainwear, adhesives, and flooring.1 Because phthalates are not covalently bound to the products in which they are used, they can leach out and be dermally absorbed, inhaled, or ingested. BPA is used to produce some canned food linings, epoxy resins, polycarbonate plastics, medical equipment, dental sealants, and thermal receipts.2 Diet is the predominant route of BPA exposure; dermal absorption from handling thermal receipts is another route of exposure. Triclosan is an antimicrobial compound used in personal care products, soaps, cleaning supplies, and medical devices. Triclosan exposure is predominately through oral routes, but dermal absorption may occur from use of soaps or personal care products.3 Exposure to phthalates, triclosan, and BPA is ubiquitous, including among pregnant women.4-7
In experimental studies, phthalates, triclosan, and BPA affect sex steroid hormone synthesis, metabolism, transport, or action. Prenatal exposure to di-2-ethylhexyl phthalate (DEHP), butyl-benzyl phthalate (BBzP), di-n-butyl phthalate (DnBP), and di-iso-butyl phthalate (DiBP) reduces Leydig cell testosterone production by decreasing the expression of genes involved in cholesterol biosynthesis and steroidogenic enzymatic pathways.8,9 Triclosan can also reduce testosterone production by disrupting cholesterol biosynthesis in Leydig cells.10,11 BPA is considered a weak environmental estrogen, but may also reduce testosterone levels and increase estrogen production by inhibiting enzymes involved in sex steroid synthesis and metabolism.12,13 Experimental studies also show that some aspects of brain development are dependent on the action of sex steroids, particularly testosterone.14 These hormones have organizational effects on the brain during fetal development that are partly responsible for sexually-dimorphic behaviors.15 In humans, sexual dimorphisms include differences in toy preference, play style, and visual-spatial abilities, as well as differences in the risk of autism and attention-deficit hyperactivity disorder.16,17 Studies of children with congenital adrenal hyperplasia (CAH), a genetic disorder that causes excess fetal androgen production, show that high testosterone exposure during the sensitive prenatal period of brain development can affect human neurodevelopment. Female children with CAH have behavioral profiles that are more “masculine” than unaffected females.17 For example, girls with the most severe form of CAH have visual-spatial abilities that are similar to those of unaffected males and better than those of unaffected girls.18
Sexually dimorphic behaviors, including visual spatial abilities, may be sensitive to endocrine disrupting chemical exposures that disrupt the organizational effects of sex steroids during fetal development. Thus, we investigated the relationship of prenatal exposures to phthalates, triclosan, and BPA with visual-spatial abilities at 8 years of age in a prospective birth cohort of 198 children from Cincinnati, OH. To facilitate comparisons to prior animal studies investigating the neurotoxicity of these chemicals, we assessed child visual-spatial abilities with a computerized version of the Morris Water Maze (MWM).
Materials and Methods
Study Participants
The Health Outcomes and Measures of the Environment (HOME) Study is a prospective pregnancy and birth cohort that has followed mothers and their children in the greater Cincinnati, OH metropolitan area from the 2nd trimester of pregnancy (March 2003-January 2006) until their singleton children were 7.5-10 years old (March 2012-July 2014).19 We designed the study to assess the relationship between low-level environmental chemical exposures and child development. Inclusion criteria at enrollment included: 1) 16±3 weeks gestation, 2) ≥18 years old, 3) living in a home built before 1978, 4) no history of HIV infection, and 5) not taking medications for seizure or thyroid disorders. All women provided informed consent for themselves and their child’s participation. The institutional review boards (IRBs) of Cincinnati Children’s Hospital Medical Center and the cooperating delivery hospitals approved this study. The Brown University relinquished IRB authority to Cincinnati Children’s Hospital Medical Center with an Interagency Agreement.
Urinary Biomarkers of Phthalate Metabolites, Triclosan, and BPA
We assessed maternal exposure to phthalates, triclosan, and BPA by measuring total (conjugated+free) urinary concentrations of nine phthalate metabolites, triclosan, and BPA. Women provided up to two urine samples in polypropylene cups at prenatal care clinic visits at 16 and 26 weeks of pregnancy. All samples were refrigerated for <24 hours until they were processed, after which they were stored at or below −20°C until shipped on dry ice to the CDC for analysis. CDC staff measured phthalate metabolites, triclosan, and BPA concentrations using previously described analytic chemistry methods.20,21
We summed the molar concentrations of mono(2-ethylhexyl) phthalate (MEHP), mono(2- ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), and mono(2-ethyl-5-carboxypentyl) phthalate (MECPP) to create a summary measure of di(2- ethylhexyl phthalate (ΣDEHP) exposure. We expressed the ΣDEHP concentrations in μg/L of MECPP by multiplying the molar sum of the individual metabolites by 308 μg/μmol.
We measured urinary creatinine concentrations to account for urine dilution and creatinine-normalized urine concentrations of phthalate metabolites, triclosan, and BPA in units of μg/g creatinine and then log10-transformed concentrations before averaging the 16 and 26 week concentrations. One-hundred ninety one (96%) women provided urine samples at both timepoints.
We applied the methods of Varshavsky et al. (2016) to calculate the anti-androgenic weighted daily phthalate intake of BBzP, DnBP, DiBP, and DEHP.22 Briefly, we calculated the daily intakes of BBzP, DnBP, DiBP, and DEHP at 16 and 26 weeks gestation using urine concentrations of monobenzyl phthalate (MBzP), mono-n-butyl phthalate (MnBP), mono-iso-butyl phthalate (MiBP), and ΣDEHP, respectively. Then, we weighted each of these intakes by the potency of each phthalate, and took the average of the 16 and 26 week weighted intakes.23,24 The potencies used to weight each phthalate were based on benchmark doses associated with a 5% reduction in testosterone production in rat experiments.25,26
Child Visual-Spatial Ability Assessment
We assessed child visual-spatial abilities using the Virtual Morris Water Maze (VMWM). The VMWM is a computerized version of the MWM, a rodent test of learning and visual-spatial reference memory.27,28 On average, in humans, rats, and mice, males perform better on the MWM and VMWM than females.29,30 In experimental rat studies, female performance on this task is improved after prenatal exposure to androgens; epidemiological studies suggest that girls’ performance is positively related to in utero androgen exposure.18,31
We administered the VMWM to children on a laptop computer in a quiet room in our study clinic. We instructed children to navigate around a virtual pool (Supplemental Figure 1) and find a hidden platform as quickly as possible using a joystick to move forward and turn left or right; children were not allowed to move backwards. Children completed four practice trials in a room with no visual landmarks and a visible platform. After the practice trials, children completed four blocks of four trials in a room with visual landmarks and a platform hidden in a fixed location across trials. Within each block of four trials, children started from four different start locations (north, east, south, and west). Finally, children completed a 30-second probe trial in the same virtual room as the hidden platform trials, but with the platform removed. The program recorded the time children took to find the platform (latency) and their path length from the start location to the platform (distance).
Our primary outcomes were the latency and distance on the hidden platform trials and the time spent in the correct quadrant of the probe trial. To account for the different starting positions on each hidden platform trial, we calculated the average latency or distance for each block of hidden platform trials.
Covariates
We adjusted for potential confounding variables that might be associated with our exposures and VMWM performance based on biological plausibility and prior knowledge. Data on maternal demographic and perinatal factors, including maternal age at delivery, race, marital status, education, parity, employment, household income, and prenatal vitamin use were obtained using structured interviews and chart reviews conducted by trained research staff. Depressive symptoms during the 2nd trimester were measured with the Beck Depression Inventory-II.32 Maternal full scale IQ was measured using the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence.33 Cotinine, a tobacco smoke exposure biomarker, was measured in maternal serum samples.34 Child age and sex were examined as covariates because they are correlated with performance on virtual tests.35
Statistical Analyses
We began our statistical analyses by describing the characteristics of covariates, VMWM performance, and urinary concentrations of phthalate metabolites, triclosan, and BPA. We also calculated the Pearson correlation coefficient between log10-transformed creatinine-standardized urinary phthalate metabolite, triclosan, and BPA concentrations. Then, we used a linear mixed model with a random intercept and unstructured covariance matrix to estimate the difference in VMWM performance between boys and girls.
Next, we examined the relationship between urinary concentrations of phthalates, triclosan, or BPA and VMWM performance on the blocks of hidden platform trials using linear mixed models with a random intercept and unstructured covariance matrix to account for the correlation of repeated measures within a child. We characterized phthalate, triclosan, and BPA concentrations as continuous log10-transformed variables and estimated the mean difference in VMWM latency and distance for an interquartile range (IQR) increase in chemical concentrations. In addition to covariates, we adjusted for block in these models. We used multivariable linear regression to examine the time spent in the correct quadrant of the probe trial.
To investigate whether the associations between phthalate metabolites, triclosan, or BPA concentrations and VMWM performance were sexually-dimorphic, we used confounder adjusted models with a product interaction term between child sex and biomarker concentrations to produce sex-specific estimates and test whether the associations in boys differed from those in girls. We considered an association between the urinary concentration of a phthalate metabolite, triclosan, or BPA and VWMW performance to be sexually dimorphic if the p-value for this interaction term was <0.10.
Secondary Analysis
Because the effect of endocrine disrupting chemicals on the developing brain could depend on the timing of exposure, we conducted a secondary analysis examining the association between VMWM performance and urinary concentrations of phthalate metabolites, triclosan, or BPA separately in samples collected at 16 and 26 weeks.
Results
Among 389 singletons born to women in our cohort, 228 (59%) completed follow-up, and 199 (87%) successfully completed the VMWM between 7.5 and 10 years of age (mean: 8.1 years). Reasons for not completing the VMWM included insufficient time (n=8), child behavioral issues or refusals (n=11), out of town visits (n=7), illness (n=1), computer problems (n=2), or distractions during testing (n=1). One child had incomplete covariate data, leaving 198 children for our final analyses.
Mothers of children who completed the study visit were predominately non-Hispanic white (63%), college educated (47%), married (64%), and non-smokers (89%). Characteristics of women who completed this follow-up visit were similar to those who enrolled and delivered singleton infants (see reference 19).
Median urinary concentrations of phthalate metabolites during pregnancy ranged from 2.1 μg/g creatinine (mono-carboxypropyl phthalate) to 128 μg/g creatinine (mono-ethyl phthalate) (Supplemental Figure 2, Supplemental Table 1). Median maternal urinary BPA and triclosan concentrations during pregnancy were 2.0 and 18 μg/g creatinine, respectively. Urinary BPA and triclosan concentrations were weakly correlated (Pearson R=0.14), while some phthalate metabolites were weakly to moderately correlated (Pearson R’s=0.16 to 0.45) (Supplemental Table 2).
On average, children completed the VMWM in 48 seconds (standard deviation [SD]: 16) and in a distance of 18 pool units (SD: 7.6). Children’s performance on the VMWM improved over the four blocks (trend p-value<0.05). Children found the platform 3.1 seconds faster (95% CI: − 0.5, −5.7) and travelled 2.6 less units (95% CI: −1.3, −3.9) on the fourth block compared to the first. On average, boys completed the VMWM faster (−4.1 seconds; 95% CI: −7.1, −1.2) and in less distance (−1.4 units; 95% CI: −2.8, 0) than girls (Supplemental Figure 3).
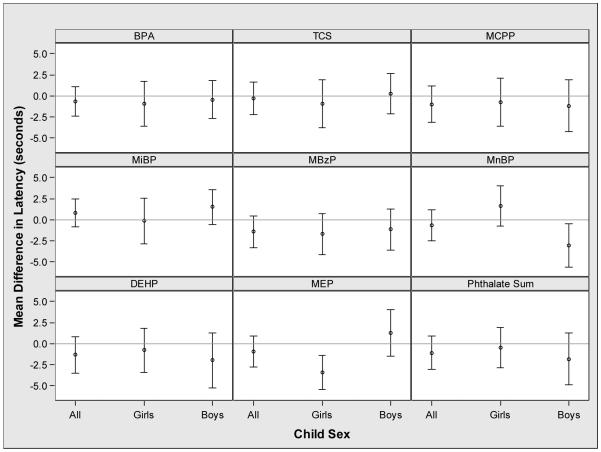
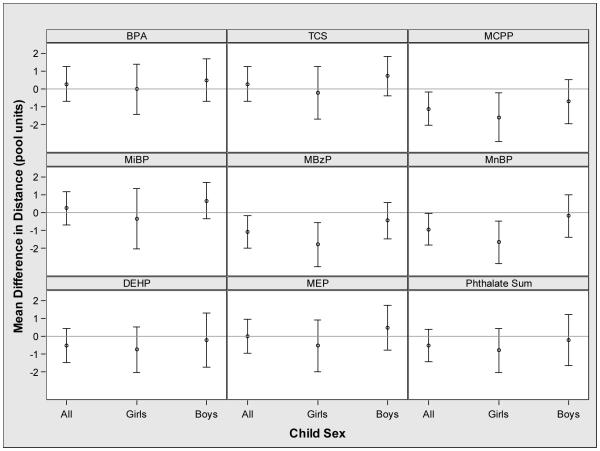
Increasing urinary MnBP, MBzP and MCPP concentrations were associated with reduced distance to complete the VMWM among all children, and there was a suggestive inverse association of urinary MBzP concentrations with VMWM latency (Figures 1 and 2). Sex modified the association between urinary MnBP concentrations and both VMWM latency and distance (latency EMM p-value=0.01 and distance EMM p-value=0.08) (Figures 1 and 2). An IQR increase in urinary MnBP concentration was associated with longer latency (1.7 seconds; 95% CI: −0.7, 4.1) and shorter distance (−1.7 units; 95% CI: −2.8, −0.5) among girls. In boys, an IQR increase in urinary MnBP concentration was associated with shorter latency (−3.0 seconds; 95% CI: −5.6, −0.4) and no association with distance (−0.2 units; 95% CI: −1.4, 1.0). We investigated the discrepant result of longer time and shorter distance with increasing urinary MnBP concentration among girls by examining the average speed during the hidden platform trials. Consistent with the latency and distance findings, an IQR increase in urinary MnBP concentration was associated with slower speed in girls (−0.04 units/second; 95% CI: −0.07, −0.02) and faster speed in boys (0.02 units/second; 95% CI: −0.00, 0.04) (EMM p-value<0.001).
Figure 1.
Adjusted mean difference in latency to complete the VMWM with an interquartile range increase in maternal concentrations of urinary phthalate metabolites, triclosan, or bisphenol A during pregnancya,b
*-Abbreviations: BPA: Bisphenol A, TCS: Triclosan, MCPP: mono-carboxypropyl phthalate, MiBP mono-iso-butyl phthalate, MBzP: mono-benzyl phthalate, MnBP: mono-n-butyl phthalate (MnBP), ΣDEHP: sum of di-2-ethylhexyl phthalate metabolites, and MEP: monoethyl phthalate.
a- Adjusted for maternal race, maternal age at delivery, marital status, maternal education, parity, household income, serum cotinine concentrations during pregnancy, binge drinking during pregnancy, depressive symptoms during pregnancy, prenatal vitamin use, maternal IQ, and child age at testing.
b- The p-values for the interaction terms between child sex and urinary BPA (p=0.74), MCPP (p=0.82), MiBP (p=0.31), MBzP (p=0.62), triclosan (p=0.46), ΣDEHP (p=0.50), and weighted phthalate sum (p=0.47) were not significant, while the p-value for the interaction term between child sex and urinary MnBP (p=0.01) and MEP (p<0.01) were significant.
Figure 2.
Adjusted mean difference in distance to complete the VMWM with an interquartile range increase in maternal urinary concentratios of phthalate metabolite, triclosan, or bisphenol A during pregnancya,b
*-Abbreviations: BPA: Bisphenol A, TCS: Triclosan, MCPP: mono-carboxypropyl phthalate, MiBP mono-iso-butyl phthalate, MBzP: mono-benzyl phthalate, MnBP: mono-n-butyl phthalate (MnBP), ΣDEHP: sum of di-2-ethylhexyl phthalate metabolites, and MEP: monoethyl phthalate.
a- Adjusted for maternal race, maternal age at delivery, marital status, maternal education, parity, household income, serum cotinine concentrations during pregnancy, binge drinking during pregnancy, depressive symptoms during pregnancy, prenatal vitamin use, maternal IQ, and child age at testing.
b- The p-values for the interaction terms between child sex and urinary BPA (p=0.57), MCPP (p=0.36), MiBP (p=0.28), triclosan (p=0.28), ΣDEHP (p=0.66), MEP (p=0.28), and weighted phthalate sum (p=0.55) were not significant, while the p-values for the interaction terms between child sex and urinary MBzP (p=0.05) and MnBP (p=0.07) were significant.
Neither maternal urinary triclosan nor BPA concentration during pregnancy was associated with VMWM latency or distance (Figures 1 and 2); child sex did not modify these associations.
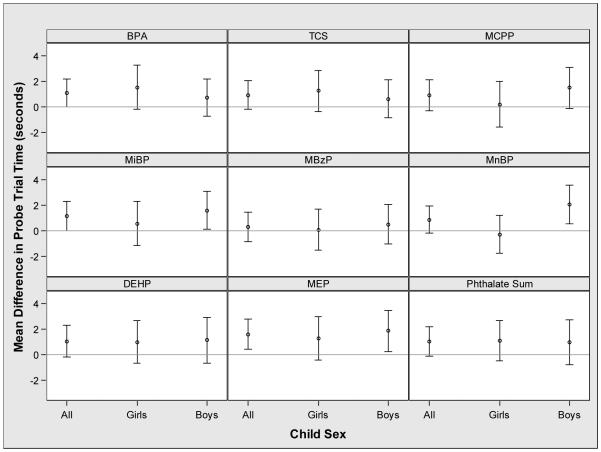
Among all children, increasing prenatal urinary phthalate metabolite, triclosan and BPA concentrations were generally associated with increased time in the correct quadrant during the probe trial, but the 95% CI of the point estimates included the null value (Figure 3). The relationship between urinary MnBP concentration and probe trial time was modified by child sex (EMM p-value=0.03). Increasing concentrations were associated with increased time in the correct quadrant in boys (2.1 seconds; 95% CI: 0.6, 3.6), but not girls (−0.3 seconds; 95% CI: −1.8, 1.2)
Figure 3.
Adjusted mean difference in probe trial time with an interquartile range increase in maternal urinary concentrations of phthalate metabolite, triclosan, or bisphenol A during pregnancya,b
*-Abbreviations: BPA: Bisphenol A, TCS: Triclosan, MCPP: mono-carboxypropyl phthalate, MiBP mono-iso-butyl phthalate, MBzP: mono-benzyl phthalate, MnBP: mono-n-butyl phthalate (MnBP), ΣDEHP: sum of di-2-ethylhexyl phthalate metabolites, and MEP: monoethyl phthalate.
a- Adjusted for maternal race, maternal age at delivery, marital status, maternal education, parity, household income, serum cotinine concentrations during pregnancy, binge drinking during pregnancy, depressive symptoms during pregnancy, prenatal vitamin use, maternal IQ, and child age at testing.
b- The p-values for the interaction terms between child sex and urinary BPA (p=0.44), MCPP (p=0.29), MiBP (p=0.44), MBzP (p=0.83) triclosan (p=0.49), ΣDEHP (p=0.82), MEP (p=0.62), and weighted phthalate sum (p=0.90) were not significant, while the p-value for the interaction term between child sex and urinary MnBP (p=0.04) was significant.
The median anti-androgenic weighted daily phthalate intake was 11 (range: 2.0-985) and 10 (range: 2.2-392) μg/kg/d at 16 and 26 weeks of gestation, respectively. An IQR increase in the anti-androgenic weighted phthalate summary measure was suggestively associated with decreased latency (−1.1 seconds; 95% CI: −3.1, 0.9), distance (−0.5 units; 95% CI: −1.4, 0.4), and increased time in the correct quadrant during the probe trial (1.1 seconds; 95% CI: −0.1, 2.2), but the 95% CIs of these point estimates include the null value. These associations were not modified by sex (EMM p-values >0.47).
Overall, we did not see consistent evidence that the association between VMWM performance and urinary concentrations of phthalate metabolites, triclosan or BPA varied by the timing of the urine measurement (Supplemental Figures 4, 5, and 6). An exception was that association between BPA concentrations at 16 and 26 weeks gestation and boy’s VMWM distance (Supplement Figure 5).
Discussion
Because sex steroids can influence the development of sexually dimorphic behaviors during fetal brain development, we hypothesized that prenatal endocrine disrupting chemical exposures may affect these behaviors in a sexually dimorphic manner. Based on previous toxicological studies, we hypothesized that BPA exposure would be associated with worse VMWM performance in girls and that TCS, DEHP, BBzP, MnBP, and MiBP would be associated with worse VMWM performance in boys.
In general, we observed that increasing maternal urinary concentrations of some phthalate metabolites during pregnancy were associated with better VMWM performance among 8-year old children in this cohort. Few of these associations consistently differed by child sex, with the exception of the sexually dimorphic association between MnBP concentrations and VMWM performance. Specifically, we observed that higher maternal urinary MnBP concentrations during pregnancy were associated with shorter distance, longer time, and slower speed to complete the VMWM among girls, but not boys. Maternal urinary triclosan and BPA concentrations during pregnancy were not associated with VMWM performance, and these associations were not modified by child sex.
VMWM performance was sexually-dimorphic, with boys completing the test faster and more efficiently than girls, on average. This is consistent with other studies in rodents and humans.18,29-31 While this suggests that prenatal androgen exposure plays a role in the development of visual-spatial abilities, the magnitude of the differences was modest and may be attributable to other non-biological postnatal factors.
A strength of our study is the ability to compare our results to those of animal studies that have examined the neurotoxicity of these chemicals using the rodent MWM. We are aware of 13 studies examining the effect of prenatal or lactational exposure to BPA, DnBP (parent diester of MnBP), DiBP (parent diester of MiBP), BBzP (parent diester of MBzP), or DEHP on rodent MWM performance.36-48
Studies of prenatal phthalate exposure and MWM performance have been heterogeneous. Prenatal DnBP exposure caused poorer,43 better,44 or no change 45 in MWM performance among males rodents. One study reported that prenatal DEHP exposure caused deficits in MWM performance in males during puberty, but these effects did not persist into adulthood;47 while another study found no effect.48 Finally, one study reported that prenatal BBzP exposure caused poorer MWM performance in male rats.46 Our findings of better VMWM performance with increasing urinary MnBP concentrations are consistent with one prior study, but not two others. Our findings for the other phthalate metabolites we examined are inconsistent with prior animal studies. We can only speculate as to why we saw associations between urinary MnBP concentrations and VMWM performance in girls, but not boys. One possibility is that the male gonad is able to compensate for the anti-androgenic effect of phthalate exposure at the levels our participants were exposed to, whereas females cannot compensate due to their lower testosterone production during fetal development.49,50 However, this hypothesis is not consistent with epidemiological studies showing that some anti-androgenic phthalates are associated with reductions in masculine traits (e.g., anogenital distance, play behaviors) in boys, but not girls.51,52
We are not aware of any rodent studies examining early life exposure to triclosan and MWM performance or other neurodevelopmental endpoints.
Rodent studies examining the neurotoxicity of BPA using the MWM have observed mixed results, with some studies reporting no effect of prenatal BPA exposure.38,40,41 Other studies report inconsistent deficits, including sex-specific and BPA-induced differences in MWM learning and acquisition.36,37 Two studies reported that prenatal BPA exposure caused increased latency and distance to complete the task and decreased time in the correct quadrant of the probe trial.39,42 Thus, our null findings for BPA are consistent with some prior studies in rodents, but not others.
While using the VMWM may help facilitate comparisons to rodent studies assessing the neurotoxicity of these chemicals, it is not without limitations. The MWM is an aversive task and rodents are motivated to perform well by their instinct to reach the safety of the hidden platform. In contrast, children’s VMWM performance is not motivated by an aversive stimulus and may be influenced by many factors, including their desire to perform well or their interest (or lack thereof) in the test. Future epidemiological studies could consider using analogues of non-aversive rodent tests like the radial arm maze, which has been used to examine the neurotoxicity of BPA,53 and also has a computerized human version.35 In addition, it is important to consider that performance on the VMWM is related to multiple neuropsychological domains, including visual-spatial abilities, executive functions, and motor function, which in turn are related to multiple molecular pathways (e.g., endocrine and neurotransmitter systems), as well as brain structures, organization, and function.54 Thus, it is likely that VMWM performance is influenced by factors acting on a variety of mechanisms, and not solely by early life alterations in gonadal hormone homeostasis.
When comparing our results to rodent studies, rodent neurotoxicity studies typically expose animals to BPA or phthalate at doses several orders of magnitude higher than those experienced by the general population, including the HOME Study participants. Thus, the absence of significant associations in our study may be because children’s exposures were not high enough or spanning enough range to affect their VMWM scores. In addition, rodent studies have administered exposure at varying times and durations during gestation, which likely does not accurately reflect chronic, low-level human exposure. Finally, differences in the biological mechanism of these chemicals’ action across species might explain the discrepancy of our results with those of rodent studies.
Almost all women (96%) in this study had two urinary measurements of phthalates, triclosan, and BPA during the 2nd and 3rd trimesters of pregnancy. However, there is potential for exposure misclassification. Urine concentrations of these biomarkers vary over time because parent exposures are episodic and these chemicals have short biological half-lives.55-57 It is important to note that the degree of exposure misclassification may vary depending on the primary source of exposure, with more misclassification of chemical exposures coming predominately from dietary sources (e.g., BPA and DEHP) than those coming primarily from personal care products (e.g., triclosan). A simulation study found that the high within-person variation of a urinary biomarker like BPA leads to associations being biased towards the null, and that it might be necessary to obtain ≥10 repeated urine biomarkers from an individual to adequately characterize exposure over pregnancy.58 Thus, within-subject pooling and regression calibration techniques could be used to account for non-differential exposure measurement error in future studies.
While we were able to assess exposure across the latter two-thirds of pregnancy, we were unable to examine the associations between VMWM performance and exposure to phthalates, triclosan, or BPA during the 1st trimester, which may be another sensitive period of development for sexually dimorphic behaviors like visual-spatial abilities.14
Another strength of this study is that we used a biologically-weighted summary of exposure to DEHP, BBzP, DnBP, and DiBP.59 This allowed us to evaluate whether aggregate prenatal exposure to these anti-androgenic phthalates was associated with VMWM performance. The associations between VMWM performance and this summary measure were generally consistent in terms of direction and magnitude compared to the associations between VMWM performance and the individual metabolites making up this summary measure. Future studies would benefit from considering similar summary measures or others derived from other biologically relevant pathways that are important for neurodevelopment (e.g., thyroid disruption). One limitation of our biologically-weighted summary phthalate measure is the assumption that the potencies derived from rodent models are applicable to humans, including pregnant women.
While we did not observe strong evidence that prenatal urinary concentrations of phthalate, triclosan, or BPA biomarkers were associated with VMWM performance at 8 years of age, prenatal exposure to these chemicals may adversely affect other neurodevelopmental domains not assessed by the VMWM, which assesses spatial and working memory, but not domains such as impulsivity. Prior studies have observed that prenatal phthalate exposure is associated with behavior problems, deficits in social behavior, executive function impairments, reduced intelligence, and reduced masculine play behavior in boys.51,60-62 In a previous study from this cohort, we did not observe associations between prenatal urinary concentrations of phthalates and children’s social behavior.63 However, we previously found that prenatal urinary BPA concentrations were associated with behavior problems and executive function impairments among girls in this cohort,64 and other studies have reported sexually dimorphic associations between prenatal urinary BPA concentrations and child behavior.65,66
In this cohort, children’s VMWM performance was sexually-dimorphic; on average, boys completed the test faster and in less distance than girls. We observed few notable associations between maternal urinary concentrations of phthalate metabolites, triclosan, or BPA during pregnancy and children’s visual-spatial abilities at 8 years of age. Phthalates with previously documented anti-androgenic activity were not associated with worse VMWM performance in boys or girls. The sexually-dimorphic association we observed between prenatal urinary MnBP concentrations and VMWM performance should be confirmed in other studies. Future studies should consider examining the relationship between prenatal exposure to these chemicals and other sexually dimorphic and hormonally sensitive neurodevelopmental outcomes using multiple and serial measures of phthalate, triclosan, and BPA biomarkers collected across the entire course of pregnancy.
Supplementary Material
Table 1.
Descriptive characteristics of women and their children from the HOME Study who completed the Virtual Morris Water Maze at 7.5 to 10 years of age (n=198)
| N (%) or Mean [SD] | |
|---|---|
| Maternal Age at Delivery (years) | 29 [6] |
| Maternal Race | |
| Non-Hispanic White | 124 (63) |
| Non-White | 74 (37) |
| Maternal Education | |
| ≥Bachelor’s Degree | 94 (47) |
| Some College | 58 (29) |
| ≤High School | 46 (23) |
| Marital Status | |
| Married | 126 (64) |
| Single-Living Together | 23 (12) |
| Single-Living Alone | 49 (25) |
| Maternal Depressive Symptoms | |
| Minimal | 159 (80) |
| Mild | 22 (11) |
| Moderate/Severe | 17 (9) |
| Prenatal Tobacco Smoke Exposure | |
| None | 69 (35) |
| SHS | 108 (55) |
| Active | 21 (11) |
| Prenatal Vitamin | |
| Never/Rarely | 28 (14) |
| Weekly/Daily | 170 (86) |
| Parity | |
| Nulliparous | 91 (46) |
| 1 | 60 (30) |
| 2+ | 47 (24) |
| Maternal IQ | 105 [16] |
| Household Income ($/year) | 57,222 [42,607] |
| Child Age at Testing (years) | 8.1 [0.6] |
| Child Sex | |
| Female | 107 (54) |
| Male | 91 (46) |
Highlights.
We administered the Virtual Morris Water Maze (VMWM) to 198 8-year old children.
On average, male children performed better than female children.
In boys, VMWM exposure was associated with prenatal exposure to some phthalates.
Prenatal bisphenol A or triclosan exposure was unassociated with VMWM performance.
Acknowledgement
We acknowledge the technical assistance of X. Ye, A. Bishop, X. Zhou, R. Hennings, M. Silva, E. Samandar, J. Preau, and T. Jia (CDC, Atlanta, GA) in measuring the urinary concentrations of phthalate metabolites, triclosan, and bisphenol A. We appreciate Dr. Robert Astur for providing us with the Virtual Morris Water Maze software.
Funding: This work was supported by NIEHS grants R00 ES020346, R01 ES024381, PO1 ES11261, R01 ES014575, R01 ES013744, P30 ES023515, and R01 ES020349.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare no conflict of interest.
References
- 1.Braun JM, Sathyanarayana S, Hauser R. Phthalate exposure and children's health. Curr Opin Pediatr. 2013;25(2):247–254. doi: 10.1097/MOP.0b013e32835e1eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Goetz N, Wormuth M, Scheringer M, Hungerbuhler K. Bisphenol a: how the most relevant exposure sources contribute to total consumer exposure. Risk Anal. 2010;30(3):473–487. doi: 10.1111/j.1539-6924.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- 3.Rodricks JV, Swenberg JA, Borzelleca JF, Maronpot RR, Shipp AM. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Critical reviews in toxicology. 2010;40(5):422–484. doi: 10.3109/10408441003667514. [DOI] [PubMed] [Google Scholar]
- 4.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environmental health perspectives. 2011;119(6):878–885. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environmental health perspectives. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Urinary concentrations of triclosan in the U.S. population: 2003-2004. Environmental health perspectives. 2008;116(3):303–307. doi: 10.1289/ehp.10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva MJ, Barr DB, Reidy JA, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environmental health perspectives. 2004;112(3):331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hannas BR, Lambright CS, Furr J, Howdeshell KL, Wilson VS, Gray LE., Jr. Dose-response assessment of fetal testosterone production and gene expression levels in rat testes following in utero exposure to diethylhexyl phthalate, diisobutyl phthalate, diisoheptyl phthalate and diisononyl phthalate. Toxicol Sci. 2011 doi: 10.1093/toxsci/kfr146. [DOI] [PubMed] [Google Scholar]
- 9.Howdeshell KL, Furr J, Lambright CR, Rider CV, Wilson VS, Gray LE., Jr. Cumulative effects of dibutyl phthalate and diethylhexyl phthalate on male rat reproductive tract development: altered fetal steroid hormones and genes. Toxicol Sci. 2007;99(1):190–202. doi: 10.1093/toxsci/kfm069. [DOI] [PubMed] [Google Scholar]
- 10.Kumar V, Balomajumder C, Roy P. Disruption of LH-induced testosterone biosynthesis in testicular Leydig cells by triclosan: probable mechanism of action. Toxicology. 2008;250(2-3):124–131. doi: 10.1016/j.tox.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Kumar V, Chakraborty A, Kural MR, Roy P. Alteration of testicular steroidogenesis and histopathology of reproductive system in male rats treated with triclosan. Reproductive toxicology (Elmsford, NY. 2009;27(2):177–185. doi: 10.1016/j.reprotox.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Chang H, Wiseman S, et al. Bisphenol A Disrupts Steroidogenesis in Human H295R Cells. Toxicol Sci. doi: 10.1093/toxsci/kfr061. [DOI] [PubMed] [Google Scholar]
- 13.Matthews JB, Twomey K, Zacharewski TR. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors alpha and beta. Chemical research in toxicology. 2001;14(2):149–157. doi: 10.1021/tx0001833. [DOI] [PubMed] [Google Scholar]
- 14.Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Hormones and behavior. 2009;55(5):570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Hormones and behavior. 2009;55(5):597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyle CA, Boulet S, Schieve LA, et al. Trends in the prevalence of developmental disabilities in US children, 1997-2008. Pediatrics. 2011;127(6):1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 17.Cohen-Bendahan CC, van de Beek C, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neuroscience and biobehavioral reviews. 2005;29(2):353–384. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Mueller SC, Temple V, Oh E, et al. Early androgen exposure modulates spatial cognition in congenital adrenal hyperplasia (CAH) Psychoneuroendocrinology. 2008;33(7):973–980. doi: 10.1016/j.psyneuen.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun JM, Kalloo G, Chen A, et al. Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study. International journal of epidemiology. 2016 doi: 10.1093/ije/dyw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye X, Bishop AM, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for measuring parabens, triclosan, and other environmental phenols in human milk. Anal Chim Acta. 2008;622(1-2):150–156. doi: 10.1016/j.aca.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 21.Silva MJ, Samandar E, Preau JL, Jr., Reidy JA, Needham LL, Calafat AM. Quantification of 22 phthalate metabolites in human urine. Journal of chromatography. 2007;860(1):106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Varshavsky JR, Zota AR, Woodruff TJ. A novel method for calculating potency-weighted cumulative phthalates exposure with implications for identifying racial/ethnic disparities among U.S. reproductive-aged women in NHANES 2001-2012. Environmental science & technology. 2016 doi: 10.1021/acs.est.6b00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mage DT, Allen RH, Kodali A. Creatinine corrections for estimating children's and adult's pesticide intake doses in equilibrium with urinary pesticide and creatinine concentrations. Journal of exposure science & environmental epidemiology. 2008;18(4):360–368. doi: 10.1038/sj.jes.7500614. [DOI] [PubMed] [Google Scholar]
- 24.Qian H, Chen M, Kransler KM, Zaleski RT. Assessment of chemical coexposure patterns based upon phthalate biomonitoring data within the 2007/2008 National Health and Nutrition Examination Survey. Journal of exposure science & environmental epidemiology. 2015;25(3):249–255. doi: 10.1038/jes.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howdeshell KL, Wilson VS, Furr J, et al. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicol Sci. 2008;105(1):153–165. doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- 26.NRC . Phthalates and Cumulative Risk Assessment The Task Ahead. National Academies Press; Washington, DC: 2008. [PubMed] [Google Scholar]
- 27.Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water task: a large and reliable sex difference. Behavioural brain research. 1998;93(1-2):185–190. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 28.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of neuroscience methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 29.Newhouse P, Newhouse C, Astur RS. Sex differences in visual-spatial learning using a virtual water maze in pre-pubertal children. Behavioural brain research. 2007;183(1):1–7. doi: 10.1016/j.bbr.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neuroscience and biobehavioral reviews. 2005;28(8):811–825. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Roof RL. Neonatal exogenous testosterone modifies sex difference in radial arm and Morris water maze performance in prepubescent and adult rats. Behavioural brain research. 1993;53(1-2):1–10. doi: 10.1016/s0166-4328(05)80261-x. [DOI] [PubMed] [Google Scholar]
- 32.Beck AT, Steer RA, Brown GK. Beck Depression Inventory - 2nd Edition (BDI-II) The Psychological Corporation; San Antonio: 1996. [Google Scholar]
- 33.Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 34.Braun JM, Daniels JL, Poole C, et al. A prospective cohort study of biomarkers of prenatal tobacco smoke exposure: the correlation between serum and meconium and their association with infant birth weight. Environ Health. 2010;9:53. doi: 10.1186/1476-069X-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braun JM, Lucchini R, Bellinger DC, et al. Predictors of virtual radial arm maze performance in adolescent Italian children. Neurotoxicology. 2012;33(5):1203–1211. doi: 10.1016/j.neuro.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carr R, Bertasi F, Betancourt A, et al. Effect of neonatal rat bisphenol a exposure on performance in the Morris water maze. Journal of toxicology and environmental health. 2003;66(21):2077–2088. doi: 10.1080/713853983. [DOI] [PubMed] [Google Scholar]
- 37.Goncalves CR, Cunha RW, Barros DM, Martinez PE. Effects of prenatal and postnatal exposure to a low dose of bisphenol A on behavior and memory in rats. Environmental toxicology and pharmacology. 2010;30(2):195–201. doi: 10.1016/j.etap.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Jones BA, Watson NV. Perinatal BPA exposure demasculinizes males in measures of affect but has no effect on water maze learning in adulthood. Hormones and behavior. 2012;61(4):605–610. doi: 10.1016/j.yhbeh.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 39.Kumar D, Thakur MK. Perinatal exposure to bisphenol-A impairs spatial memory through upregulation of neurexin1 and neuroligin3 expression in male mouse brain. PloS one. 2014;9(10):e110482. doi: 10.1371/journal.pone.0110482. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Nakamura K, Itoh K, Dai H, et al. Prenatal and lactational exposure to low-doses of bisphenol A alters adult mice behavior. Brain Dev. 2011 doi: 10.1016/j.braindev.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Viberg H, Fredriksson A, Buratovic S, Eriksson P. Dose-dependent behavioral disturbances after a single neonatal Bisphenol A dose. Toxicology. 2011 doi: 10.1016/j.tox.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Xu XH, Zhang J, Wang YM, Ye YP, Luo QQ. Perinatal exposure to bisphenol-A impairs learning-memory by concomitant down-regulation of N-methyl-D-aspartate receptors of hippocampus in male offspring mice. Hormones and behavior. 2010;58(2):326–333. doi: 10.1016/j.yhbeh.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 43.Li XJ, Jiang L, Chen L, Chen HS, Li X. Neurotoxicity of dibutyl phthalate in brain development following perinatal exposure: A study in rats. Environmental toxicology and pharmacology. 2013;36(2):392–402. doi: 10.1016/j.etap.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Li T, Zhuang M, Wang K, Zhang J, Shi N. High-dose dibutyl phthalate improves performance of F1 generation male rats in spatial learning and increases hippocampal BDNF expression independent on p-CREB immunocontent. Environmental toxicology and pharmacology. 2010;29(1):32–38. doi: 10.1016/j.etap.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Zhuang M, Li T, Shi N. Neurobehavioral toxicity study of dibutyl phthalate on rats following in utero and lactational exposure. J Appl Toxicol. 2009;29(7):603–611. doi: 10.1002/jat.1447. [DOI] [PubMed] [Google Scholar]
- 46.Zhuang MZ, Li YF, Li T. Effects of butyl benzyl phthalate on neurobehavioral development of rats. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2008;26(5):285–288. [PubMed] [Google Scholar]
- 47.Dai Y, Yang Y, Xu X, Hu Y. Effects of uterine and lactational exposure to di-(2-ethylhexyl) phthalate on spatial memory and NMDA receptor of hippocampus in mice. Hormones and behavior. 2015;71:41–48. doi: 10.1016/j.yhbeh.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 48.Lee KI, Chiang CW, Lin HC, et al. Maternal exposure to di-(2-ethylhexyl) phthalate exposure deregulates blood pressure, adiposity, cholesterol metabolism and social interaction in mouse offspring. Archives of toxicology. 2015 doi: 10.1007/s00204-015-1539-0. [DOI] [PubMed] [Google Scholar]
- 49.Barry JA, Hardiman PJ, Siddiqui MR, Thomas M. Meta-analysis of sex difference in testosterone levels in umbilical cord blood. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2011;31(8):697–702. doi: 10.3109/01443615.2011.614971. [DOI] [PubMed] [Google Scholar]
- 50.Kuijper EA, Ket JC, Caanen MR, Lambalk CB. Reproductive hormone concentrations in pregnancy and neonates: a systematic review. Reprod Biomed Online. 2013;27(1):33–63. doi: 10.1016/j.rbmo.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Swan SH, Liu F, Hines M, et al. Prenatal phthalate exposure and reduced masculine play in boys. International journal of andrology. 2010;33(2):259–269. doi: 10.1111/j.1365-2605.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swan SH, Sathyanarayana S, Barrett ES, et al. First trimester phthalate exposure and anogenital distance in newborns. Human reproduction (Oxford, England) 2015 doi: 10.1093/humrep/deu363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sadowski RN, Park P, Neese SL, Ferguson DC, Schantz SL, Juraska JM. Effects of perinatal bisphenol A exposure during early development on radial arm maze behavior in adult male and female rats. Neurotoxicology and teratology. 2014;42C:17–24. doi: 10.1016/j.ntt.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Korthauer LE, Nowak NT, Frahmand M, Driscoll I. Cognitive correlates of spatial navigation: Associations between executive functioning and the virtual Morris Water Task. Behavioural brain research. 2016;5(317):470–478. doi: 10.1016/j.bbr.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Johns LE, Cooper GS, Galizia A, Meeker JD. Exposure assessment issues in epidemiology studies of phthalates. Environment international. 2015;85:27–39. doi: 10.1016/j.envint.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ye X, Wong LY, Bishop AM, Calafat AM. Variability of Urinary Concentrations of Bisphenol A in Spot Samples, First-morning Voids, and 24-Hour Collections. Environmental health perspectives. 2011 doi: 10.1289/ehp.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meeker JD, Cantonwine D, Rivera-Gonzalez LO, et al. Distribution, variability and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environmental science & technology. 2013 doi: 10.1021/es400510g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perrier F, Giorgis-Allemand L, Slama R, Philippat C. Within-subject Pooling of Biological Samples to Reduce Exposure Misclassification in Biomarker-based Studies. Epidemiology (Cambridge, Mass. 2016;27(3):378–388. doi: 10.1097/EDE.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varshavsky JR, Zota AR, Woodruff TJ. A novel method for calculating potency-weighted cuulative phthalates exposure with implications for identifying racial/ethnic disparities among U.S. reproductive-aged women in NHANES 2001-2012. Environ Sci Technol. 2016 doi: 10.1021/acs.est.6b00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Factor-Litvak P, Insel B, Calafat AM, et al. Persistent Associations between Maternal Prenatal Exposure to Phthalates on Child IQ at Age 7 Years. PloS one. 2014;9(12):e114003. doi: 10.1371/journal.pone.0114003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Engel SM, Miodovnik A, Canfield RL, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environmental health perspectives. 2010;118(4):565–571. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miodovnik A, Engel SM, Zhu C, et al. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32(2):261–267. doi: 10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Braun JM, Kalkbrenner AE, Just AC, et al. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environmental health perspectives. 2014;122(5):513–520. doi: 10.1289/ehp.1307261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Braun JM, Kalkbrenner AE, Calafat AM, et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128(5):873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roen EL, Wang Y, Calafat AM, et al. Bisphenol A exposure and behavioral problems among inner city children at 7-9 years of age. Environmental research. 2015 doi: 10.1016/j.envres.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harley KG, Gunier RB, Kogut K, et al. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environmental research. 2013 doi: 10.1016/j.envres.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.