Abstract
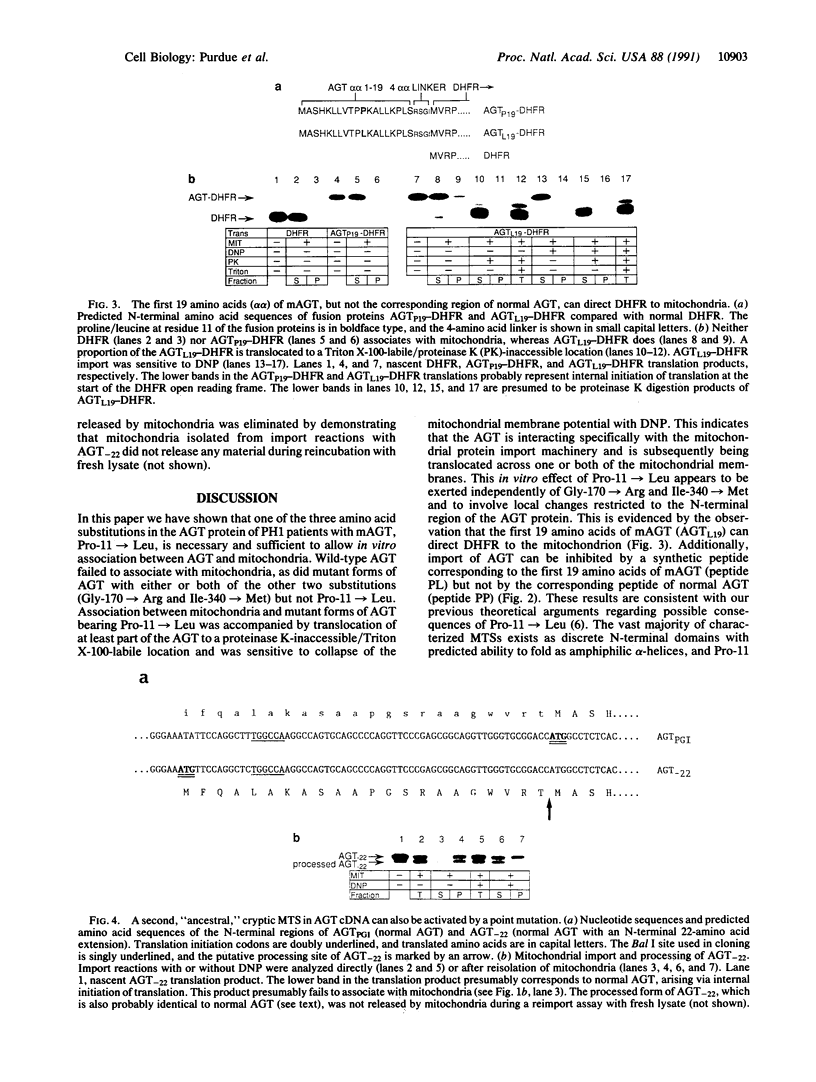
In approximately one-third of primary hyperoxaluria type 1 patients, disease is associated with a unique protein sorting defect in which hepatic L-alanine:glyoxylate aminotransferase (AGT; EC 2.6.1.44), which is normally peroxisomal, is mistargeted to mitochondria. In all such patients analyzed to date, the gene encoding the aberrantly targeted AGT carries three point mutations, each of which specifies an amino acid substitution. In this paper we show that one of these substitutions, a proline-to-leucine at residue 11, is necessary and sufficient for the generation of a mitochondrial targeting sequence in the AGT protein. AGT with this substitution appears to interact specifically with the mitochondrial protein import machinery, via a discrete N-terminal domain of the AGT protein. The N-terminal 19 amino acids of AGT with this substitution are sufficient to direct mouse cytosolic dihydrofolate reductase to mitochondria, and a synthetic peptide corresponding to this same 19-amino acid region reversibly inhibits mitochondrial protein import, not only of AGT but also of ornithine transcarbamoylase, a genuine cytoplasmically synthesized mitochondrial protein. We have extended these studies to analyze a region of normal human AGT cDNA directly upstream of the coding region. This sequence appears to correspond to an ancestral mitochondrial targeting sequence deleted from the human coding region by point mutation at the initiation codon. We show that reestablishment of this initiation codon produces an active mitochondrial targeting sequence that is different to that found in the hyperoxaluria patients. These results are discussed with reference to the AGT targeting defect in primary hyperoxaluria and also in relation to the highly unusual species specificity of subcellular distribution of AGT among mammals.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conboy J. G., Rosenberg L. E. Posttranslational uptake and processing of in vitro synthesized ornithine transcarbamoylase precursor by isolated rat liver mitochondria. Proc Natl Acad Sci U S A. 1981 May;78(5):3073–3077. doi: 10.1073/pnas.78.5.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danpure C. J., Cooper P. J., Wise P. J., Jennings P. R. An enzyme trafficking defect in two patients with primary hyperoxaluria type 1: peroxisomal alanine/glyoxylate aminotransferase rerouted to mitochondria. J Cell Biol. 1989 Apr;108(4):1345–1352. doi: 10.1083/jcb.108.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danpure C. J., Guttridge K. M., Fryer P., Jennings P. R., Allsop J., Purdue P. E. Subcellular distribution of hepatic alanine:glyoxylate aminotransferase in various mammalian species. J Cell Sci. 1990 Dec;97(Pt 4):669–678. doi: 10.1242/jcs.97.4.669. [DOI] [PubMed] [Google Scholar]
- Danpure C. J., Jennings P. R. Further studies on the activity and subcellular distribution of alanine:glyoxylate aminotransferase in the livers of patients with primary hyperoxaluria type 1. Clin Sci (Lond) 1988 Sep;75(3):315–322. doi: 10.1042/cs0750315. [DOI] [PubMed] [Google Scholar]
- Danpure C. J., Jennings P. R. Peroxisomal alanine:glyoxylate aminotransferase deficiency in primary hyperoxaluria type I. FEBS Lett. 1986 May 26;201(1):20–24. doi: 10.1016/0014-5793(86)80563-4. [DOI] [PubMed] [Google Scholar]
- Furuya S., Okada M., Ito A., Aoyagi H., Kanmera T., Kato T., Sagara Y., Horiuchi T., Omura T. Synthetic partial extension peptides of P-450(SCC) and adrenodoxin precursors: effects on the import of mitochondrial enzyme precursors. J Biochem. 1987 Oct;102(4):821–832. doi: 10.1093/oxfordjournals.jbchem.a122121. [DOI] [PubMed] [Google Scholar]
- Glaser S. M., Cumsky M. G. A synthetic presequence reversibly inhibits protein import into yeast mitochondria. J Biol Chem. 1990 May 25;265(15):8808–8816. [PubMed] [Google Scholar]
- Horwich A. L., Fenton W. A., Williams K. R., Kalousek F., Kraus J. P., Doolittle R. F., Konigsberg W., Rosenberg L. E. Structure and expression of a complementary DNA for the nuclear coded precursor of human mitochondrial ornithine transcarbamylase. Science. 1984 Jun 8;224(4653):1068–1074. doi: 10.1126/science.6372096. [DOI] [PubMed] [Google Scholar]
- Isaya G., Kalousek F., Fenton W. A., Rosenberg L. E. Cleavage of precursors by the mitochondrial processing peptidase requires a compatible mature protein or an intermediate octapeptide. J Cell Biol. 1991 Apr;113(1):65–76. doi: 10.1083/jcb.113.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T., Takada Y. Peroxisomal localization of alanine: glyoxylate aminotransferase in human liver. Arch Biochem Biophys. 1979 Sep;196(2):645–647. doi: 10.1016/0003-9861(79)90319-9. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Takada Y. Purification and properties of peroxisomal pyruvate (glyoxylate) aminotransferase from rat liver. Biochem J. 1978 Nov 1;175(2):765–768. doi: 10.1042/bj1750765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda T., Miyajima H., Suzuki Y., Ichiyama A. Nucleotide sequence of the cDNA encoding the precursor for mitochondrial serine:pyruvate aminotransferase of rat liver. Eur J Biochem. 1987 Nov 2;168(3):537–542. doi: 10.1111/j.1432-1033.1987.tb13451.x. [DOI] [PubMed] [Google Scholar]
- Okuno E., Minatogawa Y., Nakanishi J., Nakamura M., Kamoda N., Makino M., Kido R. The subcellular distribution of alanine-glyoxylate aminotransferase and serine-pyruvate aminotransferase in dog liver. Biochem J. 1979 Sep 15;182(3):877–879. doi: 10.1042/bj1820877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdue P. E., Takada Y., Danpure C. J. Identification of mutations associated with peroxisome-to-mitochondrion mistargeting of alanine/glyoxylate aminotransferase in primary hyperoxaluria type 1. J Cell Biol. 1990 Dec;111(6 Pt 1):2341–2351. doi: 10.1083/jcb.111.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Kaneko N., Esumi H., Purdue P. E., Danpure C. J. Human peroxisomal L-alanine: glyoxylate aminotransferase. Evolutionary loss of a mitochondrial targeting signal by point mutation of the initiation codon. Biochem J. 1990 Jun 1;268(2):517–520. doi: 10.1042/bj2680517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Noguchi T. Subcellular distribution, and physical and immunological properties of hepatic alanine: glyoxylate aminotransferase isoenzymes in different mammalian species. Comp Biochem Physiol B. 1982;72(4):597–604. doi: 10.1016/0305-0491(82)90512-0. [DOI] [PubMed] [Google Scholar]
- Takada Y., Noguchi T. The evolution of peroxisomal and mitochondrial alanine: glyoxylate aminotransferase 1 in mammalian liver. Biochem Biophys Res Commun. 1982 Sep 16;108(1):153–157. doi: 10.1016/0006-291x(82)91844-7. [DOI] [PubMed] [Google Scholar]
- Wise P. J., Danpure C. J., Jennings P. R. Immunological heterogeneity of hepatic alanine:glyoxylate aminotransferase in primary hyperoxaluria type 1. FEBS Lett. 1987 Sep 28;222(1):17–20. doi: 10.1016/0014-5793(87)80183-7. [DOI] [PubMed] [Google Scholar]









