Abstract
OTX Homeobox genes are involved in embryonic morphogenesis and in the development of olfactory epithelium in adult. Mutations occurring in the OTX genes are reported to be associated to tumorigenisis in human. No reports correlate the expression of OTX genes and neoplasms of the nasal cavity. Thus, through immunohistochemical and Real-time PCR analysis we investigated OTX1 and OTX2 expression in the more frequent types of nasal and sinonasal tumours. Variable expression of both genes were found in normal sinonasal mucosa and in tumours. Interestingly, no expression of both OTX genes were detected in sinonasal intestinal-type adenocarcinomas; only OTX1 was found in non-intestinal-type adenocarcinomas and OTX2 was selectively expressed in olfactory neuroblastomas. In conclusion, OTX1 and OTX2 genes might have a role in the pathogenesis of different types of sinonasal neoplasms.
Key words: Homeobox genes, sinonasal neoplasms, immunohistochemistry, Real-time PCR, tumour marker
Introduction
OTX Homeobox (HB) genes are a family of regulatory genes encoding for transcription factors, which play a crucial role in embryonic morphogenesis. In particular, OTX2 is involved in rostral head development, including olfactory, auditory and visual systems1 whereas OTX1 is mainly involved in brain and sensory organ development, including the olfactory placode.2 Interestingly, in the adult, OTX1 is expressed in sense organs: it is involved in the formation of the ciliary body, in the anterior part of the retina, in the inner ear morphogenesis and it is expressed in the olfactory bulb. Due to crucial role of the OTX, mutations occurring in these genes are associated to human congenital, somatic or metabolic defects. In particular, the gain or loss of OTX genes promotes tumorigenisis as a consequence of their inappropriate effects on growth and differentiation. The deregulated expression of OTX genes has been described in leukemia and lymphomas3 and in many solid tumours, such as medulloblastomas,4 aggressive non Hodgkin lymphomas,2 breast carcinomas,5 colorectal cancers,6 and retinoblastoma.7 Recent advances in phenotypic and molecular markers in certain solid tumours have led to the introduction of novel new factors for the diagnosis, the biological classification and the treatment of several tumours including nasal and sinonasal neoplasms.8 Literature searches showed no reports of OTX1 and/or OTX2 expression in neoplasms of the nasal cavity, paranasal sinuses and nasopharynx. Although the nasal cavity and paranasal sinuses occupy a relatively small anatomical space, they are the site of origin of some of the most complex, histologically different group of tumours in the entire human body. These include neoplasms derived from mucosal epithelium, glands, soft tissues, bone, cartilage, neural/neuroectodermal tissue and hematolymphoid cells. Thus, we investigated OTX1 and OTX2 expression in the more frequent types of nasal and sinonasal tumours.
Materials and Methods
All analyses were performed on formalin-fixed paraffin-embedded archival samples collected from the Department of Pathology of the “Ospedale di Circolo” in Varese from 1985 to 2012. Normal sinonasal mucosae associated with pituitary adenomas were used as control samples. Analysed tumours were classified according to the WHO classification of Head and Neck Tumor9 in: inverted papilloma (IP), sinonasal intestinal-type adenocarcinoma (ITAC), non-intestinal-type adenocarcinoma (NITAC), adenoid cystic carcinoma (ACC), pleomorphic adenoma (PA), olfactory neuroblastoma (ON), poorly differentiated neuroendocrine carcinoma (PDNEC) and neuroendocrine tumour (NET). The whole study was carried out in accordance with the Declaration of Helsinki (1975).
Immunohistochemistry
Immunohistochemical studies were performed on 3 µm formalin-fixed, paraffinembedded sections deparaffinised and rehydrated through alcohol series to water. Endogenous activity was blocked with 3% aqueous hydrogen peroxide for 12 min. Antigen retrieval was performed with a 10 min microwave treatment in 10 mM Citrate Buffer (pH 6) and after washing in TBSTriton Buffer (pH 7.4). Sections were incubated overnight at 4°C with goat antihuman OTX2 antibody (R&D System, Minneapolis, MN, USA; 1:100 dilution) which recognizes also OTX1 due to the homology between the two proteins. The sections were then incubated with biotynilated rabbit anti-goat antibody for 1 h (Vector Laboratories, Burlingame, CA, USA) followed by ABC-peroxidase complex (Vector Laboratories) according to manufacturer’s protocol. The immunoreaction was developed with 3.3’-diaminobenzidine tetrahydrochloride (DAB) (Sigma Aldrich, St. Louis, MO, USA) as chromogen. Nuclei were counterstained with Harris hematoxylin and finally sections were dehydrated and embedded in Pertex (Kaltek Srl, Padua, Italy). Statistical analysis were performed by chi-square and/or Fisher’s exact test.
Real-time PCR
RNA was isolated using the RecoverAll Total RNA Isol Kit FFPE (Applied Biosystems, Foster City, CA, USA) according to the protocol and cDNA was obtained by the High Capacity cDNA Kit (Applied Biosystems). Quantitative Real-time analysis (qRT-PCR) were performed by TaqMan technology using ABI Prism 7000 apparatus (Applied Biosystems). Gene expression analysis were carried on with TaqMan® Assays-on-Demand containing primers and fluorescent probe mix (Applied Biosystems). PCR reaction mix contained 12.5 µL of TaqMan Universal PCR Master Mix, no AmpErase UNG (Applied Biosystems), 1.25 µL Assays-on-Demand, 50 ng of cDNA and nuclease-free water up to 25 µL of total volume. Thermocycler program consisted of an initial hot start cycle at 50°C for 2 min and 90°C for 10 min, followed by 40 cycles at 95°C for 15 sec and a final cycle at 60°C for 1 min. For all tests, reactions were performed in triplicate. Human ACTB gene was used as endogenous control to normalize gene expression levels through comparative cycle threshold (ΔCt) method. Statistical analysis were performed by Student’s t-test. Results were statistically significant with (P<0.05).
Results
Here, we reported only preliminary data statistically significant. In normal mucosae, immunohistochemistry showed strong and homogeneous nuclear immunoreactivity for OTX both in the ciliated pseudostratified respiratory-type epithelium and in the underlying submucosal glandular cells (Figure 1A); at the same time, Real-time PCR analysis demonstrated the expression of both OTX genes (Figure 2 A,B). Different immunoreactivity and molecular expression of OTX were found in all cases of the different types of analysed tumours (data not showed). Of particular interest were results obtained comparing ITAC and NITAC and ON and PDNEC tumours. Homogeneously distributed nuclear OTX expression was present in all NITACs (Figure 1B), whereas no immunoreactivity or immunoreactivity restricted to very few scattered cells was observed in ITAC cases (Figure 1C). In fact, OTX1 gene was expressed in all NITACs but it was completely absent in ITACs (Figure 2A). No OTX2 expression was observed both in all ITACs and in all NITACs (Figure 2B). Contrarily, intense OTX immunoreactivity was present in all ONs (Figure 1D); in all these tumours OTX1 gene was completely absent (Figure 2A) whereas OTX2 was over-expressed (Figure 2B). Among PDNECs OTX expression varied in intensity and percentage of positive cells (Figure 1E). Also the expression of both the OTX genes varied in PDNECs. Immuno - histochemical statistical analysis demonstrated that OTX immunoreactivity was significantly absent in ITAC compared to all the tumour types (P<0.001). As regards the OTX genes expression, Student’s t-test confirmed data statistically significant (*) (P<0.05) for OTX1 in controls vs ITACs, controls vs ONs, controls vs PDNECs and for OTX2 in controls vs NITACs, controls vs ITACs, controls vs ONs, controls vs PDNECs.
Figure 1.
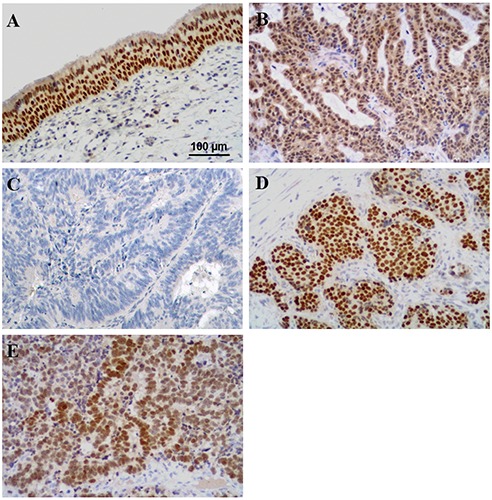
Representative images of OTX immunohistochemical expression in normal (5 analysed cases) (A) and neoplastic nasal tissues. B) Non-intestinal-type adenocarcinomas (7 analysed cases). C) Intestinal-type adenocarcinomas (23 analysed cases). D) Olfactory neuroblastomas (7 analysed cases). E) Poorly differentiated neuroendocrine carcinomas (11 analysed cases). DAB-Hematoxylin; original magnification: 200x.
Figure 2.
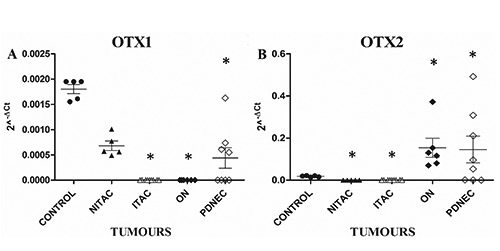
Molecular expression of OTX1 (A) and OTX2 (B) genes in normal and neoplastic nasal tissues. Normal mucosae (control) (5 analysed cases), non-intestinal-type adenocarcinomas (NITAC) (5 analysed cases), intestinal-type adenocarcinomas (ITAC) (9 analysed cases), olfactory neuroblastomas (ON) (6 analysed cases), poorly differentiated neuroendocrine carcinomas (PDNEC) (8 analysed cases). The X axis indicates the type of tumour, while the Y axis represents the 2^-ΔCt values. The asterisk indicates data statistically significant (P<0.05) between control and tumours obtained by Student’s t-test.
Discussion
For the first time, in this study, we demonstrated the presence of OTX1 and OTX2 proteins in normal sinonasal mucosa and in different epithelial and neuroectodermal nasal neoplasms of adult subjects. Although both OTX1 and OTX2 have been reported as expressed in the epithelia of the developing nasal cavities of mouse embryos,1 very few studies report localization of OTX proteins in human nasal mucosa. Here, variable expression of both genes was found in normal sinonasal mucosa, no expression of OTX were detected in ITACs, only OTX1 was found in NITACs, and OTX2 was selectively expressed in ONs and PDNECs. Concerning to nasal neoplasms, the absence of expression of both OTX1 and OTX2 genes in ITACs can be related to the loss of the respiratory type epithelial phenotype, with acquisition of histological and immunophenotypical similarity to colorectal carcinomas. On the other side, the overexpression of OTX2 in ONs is in line with the role played by OTX2 protein for the very existence of the olfactory placods, from which ON seem to be derived.10,11 OTX2 overexpression in ONs has not been previously reported. These results are in contrast with the accepted notion that OTX2 is mainly silenced in all adult tissues, including the central nervous system, with the exception of the retina (Cancer Genomic Anatomy Project data at http://cgap.nci.nih.gov/SAGE/Anatomic Viewer).12 The biological function of OTX2 is still unknown, but is particularly interesting because knockdown of OTX2 expression in medulloblastoma cell lines reduced cell proliferation and tumour progression.13,14 Moreover, overexpression of OTX2 in PDNECs, in some cases associated with OTX1 expression, indicates that OTX genes, and particularly OTX2, may be involved both in neuroendocrine (PDNECs) and neuroectodermal (ONs) differentiation of nasal cancers. In conclusion, the present study described the molecular and immunohistochemical expression of OTX1 and OTX2 in a wide-ranging series of epithelial and neuroectodermal sinonasal neoplasms, including all different histological subtypes. This provides an initial comprehensive survey comparing OTX1 and OTX2 expression in human primary sinonasal neoplasms and normal sinonasal mucosa. Taken together the findings of a significant modulation in the expression of OTX1 and/or OTX2 in neoplastic tissue, compared with normal tissue, suggest that the activation/inactivation of OTX factors is a significant event in the pathogenesis of different types of sinonasal neoplasms and may suggests that they could represent a potentially attractive diagnostic, prognostic and/or therapeutic targets.
Acknowledgments
This work was supported by Centro Grandi Strumenti Università dell’Insubria, Fondazione Comunitaria del Varesotto, Fondazione del Monte di Lombardia, and Fondazione Anna Villa e Felice Rusconi. AR and AC are Ph.D. students of the School in Biological and Medical Sciences, Program in Biotechnology, University of Insubria, Varese, Italy.
References
- 1.Boncinelli E, Simeone A, Acampora D, Gulisano M. Homeobox genes in the developing central nervous system. Ann Genet 1993;36:30-7. [PubMed] [Google Scholar]
- 2.Omodei D, Acampora D, Russo F, De Filippi R, Severino V, Di Francia R, et al. Expression of the brain transcription factor OTX1 occurs in a subset of normal germinal-center B cells and in aggressive Non-Hodgkin lymphoma. American J Pathol 2009;175:2609-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coletta RD, Jedlicka P, Gutierrez-Hartmann A, Ford HL. Transcriptional control of the cell cyclein mammary gland development and tunorigenesis. J Mammary Gland Biol Neoplasia 2004; 9:39-53. [DOI] [PubMed] [Google Scholar]
- 4.De Haas T, Oussoren E, Grajkowska W, Perek-Polnik M, Popovic M, Zadravec-Zaletel L, et al. OTX1 and OTX2 expression correlates with the clinicopathologic classification of medulloblastoma. J Neuropathol Exp Neurol 2006;65:176-86. [DOI] [PubMed] [Google Scholar]
- 5.Terrinoni A, Pagani IS, Zucchi I, Chiaravalli AM, Serra V, Rovera F, et al. OTX1 expression in breast cancer is regulated by p53. Oncogene 2001; 30:3096-03. [DOI] [PubMed] [Google Scholar]
- 6.Yu K, Cai XY, Li Q, Yang ZB, Xiong W, Shen T, et al. OTX1 promotes colorectal cancer progression through epithelial-mesenchymal transition. Biochem Biophys Res Commun 2014;44:1-5. [DOI] [PubMed] [Google Scholar]
- 7.Glubrecht DD, Kim JH, Russell L, Bamforth JS, Godbout R. Differential CRX and OTX2 expression in human retina and retinoblastoma. J Neurochem 2009;111:250-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordes B, Williams MD, Tirado Y, Bell D, Rosenthal DI, Al-Dhahri SF, et al. Molecular and phenotypic analysis of poorly differentiated sinonasal neoplasms: an integrated approach for early diagnosis and classification. Hum Pathol 2009;40:283-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes L, Eveson JW, Reichart P, Sidransky D. World health organization classification of tumors. Pathology and genetics of head and neck tumors, vol. 9. WHO Press, 2005. [Google Scholar]
- 10.Utsuki S, Kawano N, Oka H, Shimizu S, Sagiuchi T, Saegusa H, et al. Olfactory neuroepithelioma arising from the olfactory placode. Clin Neuropathol 2000;19:7-12. [PubMed] [Google Scholar]
- 11.Takahashi H, Ohara S, Yamada M, Ikuta F, Tanimura K, Honda Y. Estesioneu - roepithelioma: a tumor of true olfactory epithelium origin. An ultrastructural and immunohistochemical study. Acta Neuropathol 1987; 75:147-55. [DOI] [PubMed] [Google Scholar]
- 12.Boon K, Eberhart CG, Riggins GJ. Genomic amplification of orthodenticle homologue 2 in medulloblastomas. Cancer Res 2005;65:703-7. [PubMed] [Google Scholar]
- 13.Di C, Liao S, Adamson DC, Parrett TJ. Identification of OTX2 as a medulloblastoma oncogene whose product can be targeted by all-trans retinoic acid. Cancer Res 2005;65:919-24. [PubMed] [Google Scholar]
- 14.Adamson DC, Shi Q, Wortham M, Northcott PA, Di C, Duncan CG, et al. OTX2 is critical for the maintenance and progression of Shh-independent medulloblastomas. Cancer Res 2010; 70:181-91. [DOI] [PMC free article] [PubMed] [Google Scholar]


