Abstract
Rationale and Objectives
To investigate for differences in metabolic concentrations and ratios between patients with systemic lupus erythematosus (SLE) without (group SLE) and those with neurological symptoms (group NPSLE) compared to a healthy control (group HC) in three normal-appearing brain regions: the frontal white matter, right insula (RI), and occipital gray matter and whether changes in any of the metabolites or metabolic ratios are correlated to disease activity and other clinical parameters.
Materials and Methods
Twenty patients with SLE (18 women and 2 men, age range 23.4–64.6 years, mean age 43.9 years), 23 NPSLE patients (23 women, age range 23.7–69.8 years, mean age 42.4 years), and 21 HC (19 women and 2 men, age range 21.0–65.7 years, mean age 43.4 years) were included. All subjects had conventional brain magnetic resonance imaging and 1H single-voxel spectroscopy, clinical assessment, and laboratory testing.
Results
NPSLE patients had significantly reduced N-acetylaspartate (NAA)/creatine compared to HC (P = .02) and SLE patients (P = .01) in the RI. Lower glutamine/creatine levels were also detected in RI in both patient groups and in frontal white matter in NPSLE patients compared to HC (P = .01, P = .02). NAA/Cr ratio in the RI was significantly negatively correlated with the Systemic Lupus Erythematosus Disease Activity Index (r = −0.41; P = .008), and patients with active SLE symptoms also had a trend toward lower NAA/creatine ratios (1.02 vs 1.12; P = .07).
Conclusions
The present data support previous findings of abnormal metabolic changes in normal-appearing regions in the brain of both SLE and NPSLE patients and raise the possibility that especially NAA, glutamine, and glutamate may be additional biomarkers for cerebral disease activity in SLE patients as these early metabolic changes occur in the brain of SLE patients before neurologic and imaging manifestations become apparent.
Keywords: NSPLE, SLE, MRI, MRS, metabolites
Systemic lupus erythematosus (SLE) is a chronic inflammatory, immune-mediated disease that affects 0.1% of the general population. Neuropsychiatric SLE (NPSLE) is a severe and life-threatening condition, reported to occur in 25% to 70% of patients with SLE (1,2) and associated with increased morbidity and mortality (3,4). Clinically, NPSLE can present with acute symptoms such as stroke, seizures, or movement disorders, which are usually diagnosed promptly and reliably. However, many individuals with SLE develop a variety of other symptoms, including headaches, psychosis, cognitive dysfunction, depression, or other less specific symptoms, and the diagnosis and attribution to NPSLE are much less certain. The wide variation in NSPLE incidence emphasizes the need for better diagnostic tests. Clinical manifestations of NPSLE include headaches, stroke, myelopathy, seizures, psychosis, confusional states, and cognitive impairment but also peripheral manifestations such as cranial neuropathies and inflammatory demyelinating polyradiculoneuropathies. Patients with concomitant antiphospholipid antibodies are at additional risk for neuropsychiatric events. Lupus patients are also at increased risk for a wide range of central nervous system (CNS) events related to immunosuppressive therapy, including infection and drug toxicity.
Magnetic resonance imaging (MRI) is frequently used to diagnose or exclude main brain alterations and has become part of the routine clinical workup of such alterations (5). Abnormal conventional MRI findings are common in both SLE and NPSLE patients (6,7) and range from nonspecific small punctate focal lesions in white matter (WM) (present in the majority of NPSLE patients but not specific) to more severe findings such as cortical atrophy, ventricular dilation, cerebral edema, cerebral infarctions, and intracranial hemorrhage (8). These findings are attributed to different mechanisms, including thrombosis, vasculopathy, and antibody-mediated neuronal injury (9).
Animal models suggest that there is evidence that inflammatory processes in the CNS might lead to neuronal loss (10), as well as impaired neurogenesis/neuronal migration (11) in SLE. Although clinical assessment is the cornerstone of the NPSLE diagnosis, this diagnosis can be difficult to make and is frequently presumptive. Previous MR spectroscopy (MRS) studies have demonstrated changes of the brain metabolites in SLE patients in both abnormal- and normal-appearing WM. Common metabolites previously evaluated are N-acetylaspartate (NAA), which is considered a marker for neuronal viability and function, creatine (Cr) a marker of cell energy and cell metabolism, and choline (Cho). Cho molecules detected in 1H-MR spectra are largely from phosphorylcholine and glycerophosphorylcholine, which are precursors to cell membrane biosynthesis and breakdown, respectively. Other MRS markers that are of interest in the SLE population include myoinositol as well as glutamate (Glu) and glutamine (Gln) and these combined (Glx). Most of these previous reports have showed a decline in the NAA/Cr ratio and an increase in the Cho/Cr ratio in periventricular WM and basal ganglia (12–14), while others have reported a more widespread significant decrease in the NAA/Cho and NAA/Cr ratios and a significant increase in the Cho/Cr ratio (12,13,15–18). A recent study has demonstrated changes in the Gln–Glu complex in the hippocampus correlating to memory dysfunction in SLE patients without neuropsychiatric symptoms (19). To our knowledge, there are no previous reports on different metabolic ratios or concentrations of these metabolites specifically in the insula region.
The purpose of this study was to investigate if differences in metabolic concentrations and ratios exist between patients with SLE and healthy controls (HC), as well as between SLE patients with neurologic symptoms (group NPSLE) and those without (group SLE), in three specific brain regions: the frontal WM (FWM), right insula (RI; a region included in the so-called pain matrix and commonly evaluated in fibromyalgia patients and other pain conditions) (20), and occipital gray matter (OGM; our control region), a good location for cortical gray matter evaluation (21). The SLE patients may, among their many symptoms, complain of diffuse pain (similar to fibromyalgia patients). Therefore, we decided to study the insula region to compare our findings to those previously found in fibromyalgia patients. Also, in this study we evaluate not only the basic metabolites NAA, Cho, myoinositol, and Cr but also Glu, a neurotransmitter, and Gln, a molecule used in Glu shuttle between neurons and glia. The metabolite Glu is often elevated in pain conditions like fibromyalgia and has shown to be positively correlated to the severity of pain (22). The Glu + Gln/Cr ratio has shown to be decreased in the right hippocampus in SLE patients and negatively correlated to memory function (19). The second aim was to investigate if any of the observed metabolite differences were correlated with disease activity and other clinical parameters. Our third aim was to follow the NPSLE patients longitudinally to see if any changes in conventional MRI and/or metabolic activity occurred following treatment.
MATERIALS AND METHODS
Participants
Twenty-three consecutive patients (23 women, age range 23.7–69.8 years, mean 42.4 years), with acute NPSLE, defined as one or more neuropsychiatric manifestations within 2 weeks before inclusion in the study and without a previous history of NPSLE; 20 consecutive patients with SLE (18 women and 2 men, age range 23.4–64.6 years, mean age 43.9 years), without history of or current neuropsychiatric symptoms; and 21 HC (19 women and 2 men, age range 21.0–65.7 years, mean age 43.4 years) were initially enrolled for this prospective 3-T MRI study. In addition, longitudinal MRI examinations were performed at 3 and 6 months after initial MRI examination in 13 of the 23 NSPLE patients. The classification of NPSLE was based on the 19 American College of Rheumatology (ACR) 1999 case definitions (23,24). To participate in the study, individuals needed to fulfill four or more of the ACR classification criteria and present within 2 weeks of the initial neuropsychiatric event. Informed consent was obtained for all participants, and the study was approved by the University of Michigan Institutional Review Board. Exclusion criteria included drug or alcohol abuse, diabetes, stroke, a diagnosis of fibromyalgia, and renal insufficiency.
Conventional MRI and Proton MRS
All subjects had conventional MRI pre– and post–intravenous (IV) contrast administration with following sequences: T1-weighted three-dimensional turbo field echo images pre– and post–IV contrast enhancement, diffusion-weighted imaging, axial T2-weighted, and FLAIR (fluid-attenuated inversion recovery) images. All subjects had single-voxel 1H-MRS (SVS), using the following parameters—pointed-resolved spectroscopy, repetition time (TR) 2000 ms, echo time (TE) 30 ms, voxel size 2 × 2.2 cm—performed at baseline and at 3 and 6 months after baseline for the NPSLE patients. In this study, we have chosen the SVS technique with short TE of 30 ms, as this technique result in a spectrum with more metabolite peaks, such as myoinositol and Gln-Glu, which are not detected with long TE, and also allow a more accurate quantification of the metabolites. The individual volumes were placed in the following regions of interest: FWM, RI, and OGM (Fig 1). All MRI and MRS examinations were performed on a Philips 3-T Achieva MR scanner (Philips MRI system, Best, Netherlands), with an eight-channel sensitivity encoding head coil. The metabolite concentrations of NAA, total Cho (tCho), total creatine (tCr), Glu, and Gln, and the presence of lactate (Lac) were analyzed. The ratios NAA/Cr, Cho/Cr, Gln/Cr, and myoinositol/Cr were calculated. The data from the SVS were analyzed on a computerized analysis system: the LC model (25). Metabolite concentrations were used only for statistical analysis from the LC model if the Cramér–Rao bounds were less than 20%.
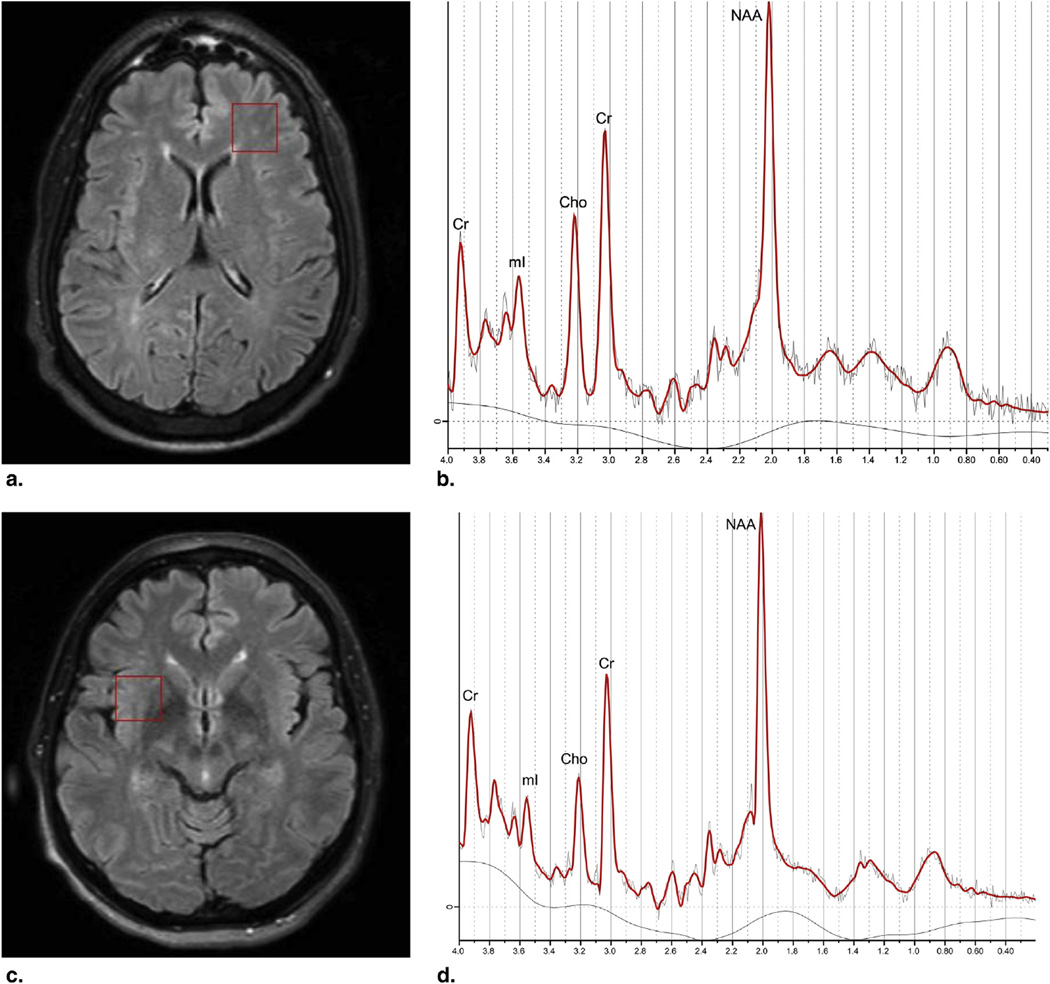
Figure 1.
Voxel placements (red box) and corresponding metabolic spectra in anterior frontal white matter (a,b) and right insula (c,d). Cho, choline; mI, myoinositol; Cr, creatine; NAA, N-acetylaspartate.
Clinical Outcomes
All the patients and the HC had standardized clinical and neurological examinations including medical history, demographics, physical exam, standard laboratory assessment, and a Mini-Mental State Examination (MMSE) (26). MMSE evaluation in NPSLE patients presenting with acute confusional state, who could not be evaluated at the time of admission, was delayed. However, all patients were able to perform the test within 96 hours of treatment. SLE and NPSLE patients underwent required laboratory tests to determine disease activity by the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) (27) as well as antiphospholipid antibody status. SLE was considered active with an SLEDAI score greater than 3. Disease duration was defined as the time between the diagnosis of SLE and the day of the MRI. CNS manifestations were divided into central and peripheral following ACR case definitions. Cumulative damage in both patient groups was assessed using the Systemic Lupus International Collaborating Clinics/ACR Damage Index (SLICC) (4) at the time of MRI acquisition.
MR Image Evaluation
The conventional MR images were evaluated by an experienced neuroradiologist (blinded to the patient history) for any signal abnormalities, hemorrhage, acute ischemic events, old infarcts, focal lesions, and focal or general atrophy and for any additional lesions not related to SLE. Lesion burden in form of WM T2/FLAIR hyperintensive lesions was defined as mild (1–5 WM lesions), moderate (5–10 WM lesions), or severe (>10 WM lesions). In addition to the conventional images, SVS MRS was analyzed as mentioned earlier. Any clinically relevant findings on structural MRI were reported to the patient’s primary physician.
Statistical Evaluation
With respect to age, disease duration, and SLEDAI and SLICC scores, as well as most lab data (except from the lupus anticoagulant), groups were compared using an analysis of variance (for comparison of all three groups). Post-hoc t-tests were used to determine which group—SLE, NPSLE, or HC—displayed different values. For the longitudinal assessment of changes in metabolite levels, we used a repeated-measures general linear model for each metabolite and compared baseline, 3-month, and 6-month follow-up values. Post-hoc paired t-tests were performed to determine which of the three periods displayed statistically different values. Nonparametric tests (χ2) were applied to compare gender distribution, smoking history, cardiovascular risk factors, and the lupus anticoagulant confirmation measure (Lac). Evidence of any Lac resulted in a score of 1; the absence of Lac resulted in a score of 0.
RESULTS
Clinical Manifestations, Disease Severity, and Serological Data
Demographic data and disease activity are presented in Table 1. Disease duration was slightly longer in the NPSLE group (mean 10.5 years, range 2–25 years, SD 6.8 years) compared to the SLE group (9.4 years, range 3–19 years, SD 5.2 years); however, this difference did not achieve significance. Disease activity was significantly higher in the NPSLE group, by SLEDAI score (P < .001). No differences were found in SLICC scores or in MMSE scores between the groups (Table 1). NPSLE patients had higher levels of anti–β2-glycoprotein I immunoglobulin (Ig)A (P < .05) than SLE patients. NPSLE patients also showed a trend to have higher levels of double-stranded DNA antibodies (anti–double-stranded DNA; P < .06). Other antiphospholipid antibodies (anti–β2-glycoprotein I IgG/IgM, anticardiolipin antibody, and Lac) did not differ significantly between the two patient groups (Table 2). In 3 of the 23 NPSLE patients, only parts of the laboratory data could be reviewed, due to some data errors; the remaining 20 NPSLE patients had all their serology data reviewed. A total of 17 diffuse neuropsychiatric manifestations and 7 focal manifestations were present in the NPSLE group, with 18 central and 2 peripheral events. The following manifestations were seen: psychosis (3 patients), transverse myelitis (1 patient), acute confusional state (10 patients), headaches (3 patients), depression (1 patient), cerebrovascular disease (6 patients), and cranial nerve deficit (1 patient). A few NPSLE patients had several different events that were both focal and diffuse at the same time. Nineteen of 23 patients were acutely treated with IV and/or oral steroids. One patient was treated exclusively with selective serotonin reuptake inhibitors. Thirteen patients required the addition of immunosuppressive drugs as well.
TABLE 1.
Demographic and Disease Severity of the Study Subjects
| HC (n = 21) | SLE (n = 20) | NPSLE (n = 23) | P Value | |
|---|---|---|---|---|
| Age | 21–66 | 23–65 | 23–70 | .9 |
| 43.9 | 43.9 | 42.4 | ||
| Female (n) | 19 (90.5%) | 18 (90%) | 23 (100%) | .3 |
| Age at disease onset (yr) | N/A | 18–56 | 13–65 | .8 |
| 33.4 ± 10.9 | 32.7 ± 13.5 | |||
| Disease duration (yr) | N/A | 3–19 | 2–25 | 1.0 |
| 9.4 ± 5.2 | 10.5 ± 6.8 | |||
| SLEDAI | N/A | 0–8 | 1–25 | <.001 |
| 2.1 ± 2.5 | 11.3 ± 6.2 | |||
| SLICC | N/A | 0–3 | 0–4 | >.2 |
| 0.53 ± 1.01 | 0.95 ±1.12 | |||
| Mini-Mental State Examination score | 32–33 | 31–33 | 31–32 | >.2 |
HC, healthy controls; SLE, systemic lupus erythematosus; NPSLE, neuropsychiatric systemic lupus erythematosus; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; SLICC, Systemic Lupus Erythematosus International Collaborating Clinics; N/A, not applicable.
Values are range and mean ± SD unless otherwise noted.
TABLE 2.
Laboratory Results and Antibody Data for Study Subjects
| HC (n = 21) | SLE (n = 20) | NPSLE (n = 23) | P Value | |
|---|---|---|---|---|
| Hemoglobin | 11.6–15.5 | 10.9–15.8 | 9.5–14.7 | 1.162* |
| 13.51/1.066 | 13.44/1.084 | 12.92/1.522 | ||
| WBC | 3.9–8.4 | 2.6–9.2 | 2.9–17.3 | 1.868* |
| 5.9/1.272 | 5.847/1.99 | 7.25/3.64 | ||
| Platelet count | 214–365 | 152–405 | 176–683 | .598* |
| 268.6/46.54 | 265.74/56.58 | 289.9/110.58 | ||
| Creatinine | 0.6–1.2 | 0.6–1.50 | 0.5–1.0 | 1.961* |
| 0.778/0.157 | 0.823/0.206 | 0.755/0.147 | ||
| Double-stranded DNA | N/A | 0–28 | 0–126 | .069 |
| 9.535/10.22 | 26.44/35.95 | |||
| C3 | N/A | 85–145 | 65–189 | .375 |
| 114.94/18.22 | 122.43/30.15 | |||
| C4 | N/A | 7–40 | 11–39 | .880 |
| 23.53/8.156 | 23.95/8.513 | |||
| ESR | N/A | 4–27 | 2–43 | .546 |
| 14.65/7.566 | 17.056/14.53 | |||
| CRP | N/A | 0–1 | 0–3.3 | .161 |
| 0.212/0.237 | 1.021/2.317 | |||
| β2G-1 IgG | N/A | 0–25 | 0–110 | .399 |
| 2.267/6.703 | 7.8/24.34 | |||
| β2G-l IgM | N/A | 0–9 | 0–230 | .312 |
| 0.867/2.475 | 14.55/51.404 | |||
| β2G-l IgA | N/A | 0–9 | 0–18 | .043 |
| 1.0/2.7 | 4.05/5.094 | |||
| CAL IgG | N/A | 0–32 | 0–40 | .438 |
| 2.412/7.722 | 5.1/12.19 | |||
| CAL IgM | N/A | 0–13 | 0–36 | .491 |
| 1.06/3.172 | 2.5/7.997 | |||
| LAC | 0–1 | 0–1 | 0–1 | .078 |
| All negative | 4 of 20 positive | 1 of 20 positive | .239 |
HC, healthy controls; SLE, systemic lupus erythematosus; NPSLE, neuropsychiatric systemic lupus erythematosus; β2-GPI, β2-glycoprotein I; C3, C3 complement; C4, C4 complement; aCL, anticardiolipin antibody; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; LAC, lupus anticoagulant (binominal); WBC, white blood cell count; N/A, not applicable; IgG GPL, IgG phospholipid unit; IgM MPL, IgM phospholipid unit.
Values are range and mean ± SD unless otherwise noted.
The serological data are presented for the 20 patients NPSLE in whom data was available.
F-value instead of P-value because the test is ANCOVA (across all groups).
Conventional MRI
The conventional MRI demonstrated mild or moderate diffuse brain atrophy in 30% of the patients (21 of 64). Mild atrophy was seen in 10 SLE patients, 7 NPSLE patients, and 1 HC subject. Moderate atrophy was present in 2 SLE patients and in 1 NPSLE patient. One patient with SLE had a wedge-shaped area of encephalomalacia in the right frontal lobe, and 1 patient with NPSLE had an old infarct in the right frontal lobe. Mild lesion burden was seen in 70% of the SLE patients (14 of 20), moderate in 15% (3 of 20), and severe in 5% (1 of 20). Mild lesion burden was seen in 52.2% of the NPSLE patients (12 of 23), moderate in 21.7% (5 of 23), and severe in 13% (3 of 23). In the HC group, 6 patients had mild lesion burden, 1 patient had moderate lesion burden, and 1 patient had severe lesion burden. Incidental findings were noted of seven venous angiomas, three pineal cysts, two arachnoid cysts, and one aneurysm of the right internal carotid artery.
The conventional MRI demonstrated a few new lesions at the 3-month follow-up examination: new focal WM lesions in two NPSLE patients and acute ischemia in one NPSLE patient. No additional changes were seen at the 6-month follow-up.
MRS
Overall, the quality of the spectra in the three different locations was good. In the HC group, one of the spectra in the FWM, two in the RI, and two in the OGM were excluded due to poor quality of the spectra. In SLE patients, five of the spectra in the FWM and one in the RI were excluded due to poor quality of the spectra, and in the NPSLE patients, three of the spectra in the OGM were excluded due to poor quality of the spectra.
Metabolic Ratios
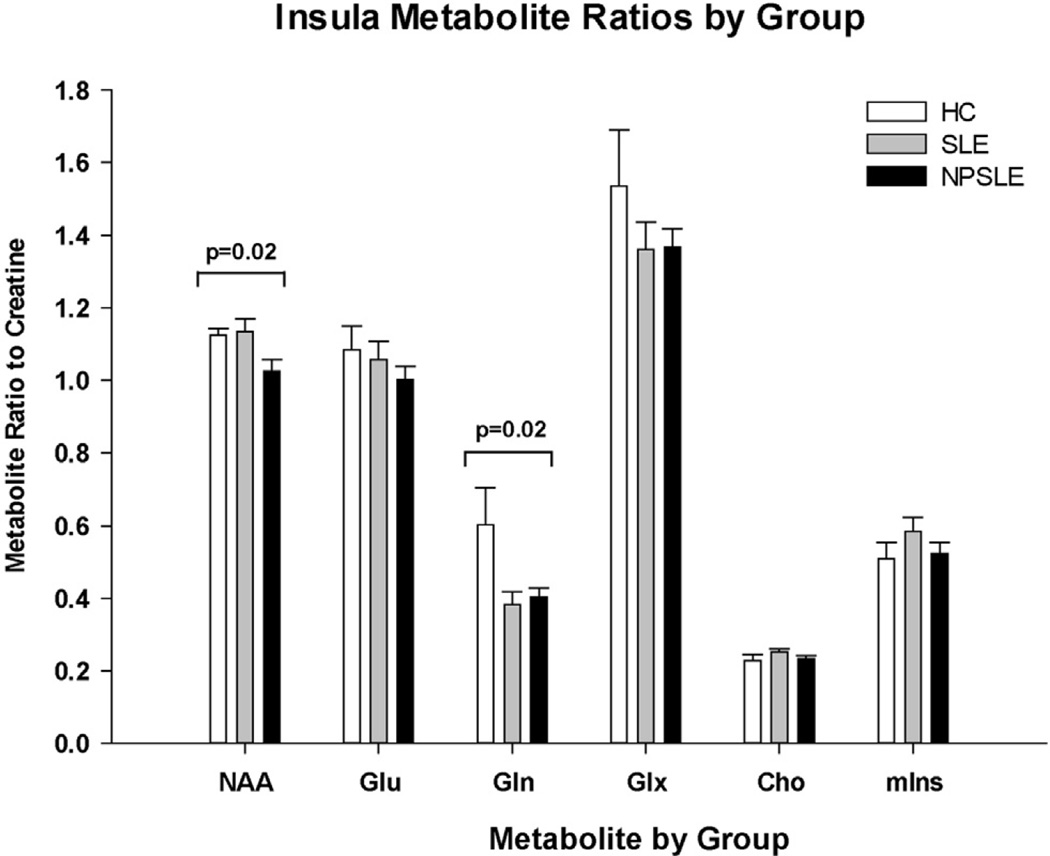
A one-way analysis of variance of the SVS MRS data revealed that the NAA/Cr ratio within the RI was significantly different across groups (mean [SD]: HC 1.12 [0.08], SLE 1.13 [0.16], NPSLE 1.03 [0.16]; P = .02). Post-hoc analyses indicated that this finding was due to lower NAA/Cr levels in the NPSLE patients. This group had significantly reduced NAA/ Cr compared to HC (P = .02) and SLE patients (P = .01). Gln/Cr levels within the RI were also found to differ across all three groups (mean [SD]: HC 0.60 [0.37], SLE 0.38 [0.13], NPSLE 0.40 [0.12]; P = .02). Post-hoc analysis revealed that this was due to lower Gln/Cr levels in both patient groups (SLE versus HC: P = .01, NSPLE versus HC: P = .01). No other metabolites in the insula, including myoinositol/Cr, showed differences across groups.
Within the FWM, Gln/Cr ratios differed across groups (mean [SD]: HC 0.64 [0.42], SLE 0.42 [0.31], NPSLE 0.36 [0.16]; P = .05). Post-hoc analyses indicated that this finding was due primarily to lower levels of Gln/Cr in the NPSLE group compared to HC (P = .02). The SLE group had a trend toward lower Gln/Cr in the FWM compared to controls (P = .08). Glu/Cr and Cho/Cr also had trends toward differing metabolite levels across group, but these were not significant (Cho/Cr: P = .07; Glu/Cr: P = .08). No other metabolites in the FWM regions, including myoinositol/Cr, showed differences across groups. Furthermore, no other metabolites showed significant differences across groups in the OGM. The different metabolic ratios from the three different regions were evaluated, and their P values are presented in Table 3. Figure 2 shows the alterations in metabolite ratios in the RI region among the groups.
TABLE 3.
The Different Metabolic Ratios in the Three Evaluated Brain Regions Among the Three Groups
| HC |
SLE |
NPSLE |
||||||
|---|---|---|---|---|---|---|---|---|
| Metabolite | Mean | SD | Mean | SD | Mean | SD | F Value | P Value |
| Right insula | ||||||||
| NAA/Cr | 1.1241 | 0.07755 | 1.1342 | 0.15597 | 1.0250 | 0.15572 | 4.20 | .02 |
| Glu/Cr | 1.0838 | 0.28334 | 1.0577 | 0.21900 | 1.0009 | 0.18186 | 0.73 | .49 |
| Gln/Cr | 0.6029 | 0.3699 | 0.3823 | 0.12546 | 0.4023 | 0.11629 | 4.21 | .02 |
| Glx/Cr | 1.5355 | 0.6542 | 1.3609 | 0.32481 | 1.3672 | 0.24571 | 1.00 | .37 |
| Cho/Cr | 0.2282 | 0.0723 | 0.2517 | 0.04114 | 0.2336 | 0.04234 | 1.02 | .37 |
| Myoinositol/Cr | 0.5090 | 0.1874 | 0.5837 | 0.16625 | 0.5236 | 0.14535 | 1.09 | .34 |
| Frontal white matter | ||||||||
| NAA/Cr | 1.3331 | 0.12839 | 1.1948 | 0.35731 | 1.2287 | 0.11899 | 2.19 | .12 |
| glu/Cr | 1.0936 | 0.33804 | 1.0558 | 0.21130 | 0.9220 | 0.15644 | 2.68 | .08 |
| gln/Cr | 0.6384 | 0.41502 | 0.4193 | 0.31141 | 0.3628 | 0.15552 | 3.24 | .05 |
| Glx/Cr | 1.5770 | 0.68329 | 1.2759 | 0.73870 | 1.2056 | 0.23912 | 2.38 | .10 |
| Cho/Cr | 0.3089 | 0.08411 | 0.2509 | 0.10511 | 0.3098 | 0.05755 | 2.84 | .07 |
| Myoinositol/Cr | 0.7939 | 0.70784 | 0.6657 | 0.17832 | 0.6413 | 0.12802 | 0.73 | .49 |
| Occipital gray matter | ||||||||
| NAA/Cr | 1.4329 | 0.10830 | 1.4286 | 0.16598 | 1.3086 | 0.31613 | 2.12 | .13 |
| Glu/Cr | 0.9116 | 0.09624 | 0.9381 | 0.15049 | 0.8737 | 0.22014 | 0.76 | .47 |
| Gln/Cr | 0.2554 | 0.12949 | 0.2723 | 0.08932 | 0.2922 | 0.15251 | 0.23 | .79 |
| Glx/Cr | 1.1283 | 0.16053 | 1.1507 | 0.22297 | 1.1102 | 0.32390 | 0.13 | .88 |
| Cho/Cr | 0.1720 | 0.02321 | 0.1993 | 0.09818 | 0.1720 | 0.02737 | 1.36 | .26 |
| Myoinositol/Cr | 0.5772 | 0.08476 | 0.6547 | 0.14335 | 0.5840 | 0.09660 | 2.95 | .06 |
NAA, N-acetylaspartate; Cho, choline; Cr, creatine; Glu, glutamate; Gln, glutamine; Glx, combined Glu and Gln; HC, healthy controls; SLE, systemic lupus erythematosus; NPSLE, neuropsychiatric systemic lupus erytematosus.
Figure 2.
Bar graph demonstrating the different metabolic ratios in the insula in the systemic lupus erythematosus without neurological symptoms (SLE), NPSLE (neuropsychiatric systemic lupus erythematosus), and healthy control (HC) groups. NAA, N-acetylaspartate; Glu, glutamate; Gln, glutamine; Glx, Glu plus Gln; Cho, choline; mIns, myoinositol.
Metabolic Concentrations
The following metabolites were evaluated: NAA, tCho, tCr, Glu, and Gln. Metabolic concentration as given in institutional arbitrary values showed some significant differences between the groups. The tCho in the FWM is different across groups (P = .008). Post-hoc tests revealed that tCho is reduced in SLE (P = .004) compared to HC and reduced in SLE compared to NPSLE (P = .008). No significant differences in tCho were seen between NPSLE and HC (P > .10; mean [SD]: SLE 1.28 [0.578], NPSLE 1.63 [0.27], HC 1.68 [0.30]). NAA in the RI was different across groups (P = .009). Post-hoc test revealed that NAA was significantly reduced in NPSLE compared to HC (P = .003). No significant differences were seen between SLE and HC (P = .26); however, there was a trend for lower NAA in the NPSLE group compared to the SLE group (P = .06; mean [SD]: NPSLE 6.47 [0.63], SLE 6.80 [0.50], HC 6.99 [0.43]). The tCr levels had a trend to higher values for NPSLE vs. SLE (P = .09) but were not significantly different. There were no differences in tCr levels between NPSLE and HC in the insula.
Metabolite Ratios and SLEDAI
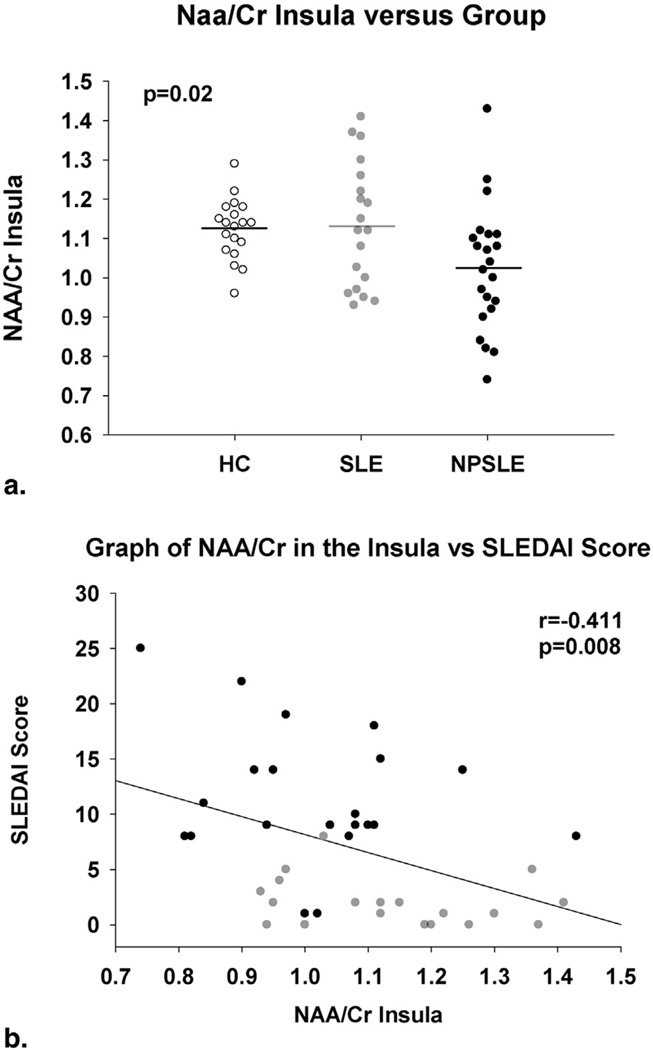
The difference between the SLEDAI scores for the SLE patients (2.1 ± 2.5) was significantly different from the NPSLE patients’ SLEDAI scores (11.3 ± 6.2; P < .01). When the SLE and NPSLE groups were combined, the NAA/Cr ratio in the RI was significantly negatively correlated with the SLEDAI score (r = −0.41, P = .008). Patients with lower NAA/Cr ratios had higher SLEDAI scores. This relationship was due in part to heavy weighting of neuropsychiatric symptoms in the SLEDAI since patients with SLEDAI scores reflecting neurological involvement had a trend toward lower NAA/Cr (mean [SD]: nonneurological 1.11 [0.16], neurological 1.01 [0.18]; P = .10). Furthermore, when SLE and NPSLE groups were analyzed on the basis of active SLE symptoms, patients with active symptoms also had a trend toward lower NAA/Cr ratios (mean [SD]: inactive 1.12 [0.15], active 1.02 [0.18]; P = .07). No significant relationships or trends were found between the RI Gln/Cr or the FWM NAA/Cr, Cho/Cr, or Gln/Cr ratios and other disease symptoms.
Longitudinal Follow-up of Metabolic Ratios
Fourteen of the 23 NPSLE patients completed the longitudinal follow-up and were scanned at 3 and 6 months following the baseline session. Over the course of these sessions, the NAA/ Cr level within the RI increased significantly (NAA/Cr mean [SD]: baseline 1.03 [0.17], 3 months 1.10 [0.12], 6 months 1.17 [0.16]; P = .035). Post-hoc analysis indicated a significant increase between baseline and 6 months (P = .008). No other significant changes in metabolic ratios occurred over time. The metabolic ratios in the RI region at the different time points in the NSPLE group are presented in Table 4. The longitudinal changes in the different metabolic ratios in the NPSLE group are presented in Figure 3.
TABLE 4.
Longitudinal Follow-up of the Different Metabolic Ratios in the Insula in the NPSLE Patients
| Baseline |
3 Months |
6 Months |
||||||
|---|---|---|---|---|---|---|---|---|
| Metabolite | Mean | SD | Mean | SD | Mean | SD | F Value | P Value |
| Insula | ||||||||
| NAA/Cr | 1.03071 | 0.173151 | 1.09793 | 0.117026 | 1.16893 | 0.159068 | 4.52 | .035 |
| Glu/Cr | 0.99243 | 0.169869 | 1.06321 | 0.160445 | 1.07129 | 0.198004 | 1.55 | .25 |
| Gln/Cr | 0.39169 | 0.113944 | 0.39282 | 0.082682 | 0.44621 | 0.288669 | 0.38 | .69 |
| Glx/Cr | 1.36129 | 0.185026 | 1.41314 | 0.209649 | 1.51743 | 0.427220 | 0.77 | .48 |
| Cho/Cr | 0.23843 | 0.047144 | 0.26179 | 0.031737 | 0.2559 | 0.04290 | 1.25 | .32 |
| Myoinositol/Cr | 0.51264 | 0.161923 | 0.57471 | 0.101972 | 0.5911 | 0.14314 | 1.29 | .31 |
NAA, N-acetylaspartate; Cho, choline; Cr, creatine; Glu, glutamate; Gln, glutamine; Glx, combined Glu and Gln; NPSLE, neuropsychiatric systemic lupus erytematosus.
Figure 3.
Bar graph demonstrating the longitudinal changes of the different metabolic ratios in the insula in the neuropsychiatric systemic lupus erythematosus (NPSLE) group. NAA, N-acetylaspartate; Glu, glutamate; Gln, glutamine; Glx, Glu plus Gln; Cho, choline; mIns, myoinositol.
The SLEDAI score significantly decreased over the course of 6 months (13.07 [6.20]; 3.80 [2.86]; P = .001); however, the change in SLEDAI was not correlated with changes in NAA/Cr (r = 0.12; P = .68) (Fig 4).
Figure 4.
Scatterplot demonstrating the N-acetylaspartate (NAA)/creatine (Cr) ratios in the insula between the three groups (a) and the correlation between the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score and the NAA/Cr ratios in the insular region neuropsychiatric systemic lupus erythematosus (NPSLE) group (b).
DISCUSSION
NPSLE is a spectrum of disorders that may be severe and life-threatening and that occur in some SLE patients. The pathogenesis of NPSLE is poorly understood with a wide range of clinical symptoms and various patterns on conventional MRI. Recently, an immune-mediated pathogenesis has been suggested (28). Clinically, NPSLE can present as acute symptoms such as stroke, seizures, or movement disorders, which are usually diagnosed promptly and reliably. However, many individuals with SLE develop a variety of other symptoms including headaches, psychosis, cognitive dysfunction, depression, or other less specific symptoms, and the diagnosis and attribution to NPSLE are much less certain. MRI has been used extensively in the evaluation of neuropsychiatric NPSLE and has proved to be more sensitive than computed tomography (29).
Previous 1H-MRS studies performed on 1.5-T scanner in SLE and NPSLE patients have reported decreased NAA/Cr and increased Cho/Cr in the periventricular WM and basal ganglia (5,13,15,16,30,31), while a few others (14) reported a significant decrease in the NAA concentration and increase in the Cho concentration. None of these previous studies have demonstrated these findings specifically in normal-appearing WM in the anterior FWM or in the RI region on a 3-T scanner. Therefore, the present study is focused, in contrast to the previous SVS MRS studies, on metabolic changes in areas of the brain that appear normal on conventional MRI with no signal abnormalities on T2 and FLAIR images or focal lesions in the analyzed volume of interest. In contradiction to previous reports, the present study showed a decline in tCho in normal-appearing anterior FWM in the SLE and NPSLE patients compared to HC (13–15). More interestingly, tCho was significantly reduced in SLE patients compared to NPSLE patients (P = .008) and there was also a trend toward reduction of the Cho/Cr between the groups even if not significant (P = .07). This may indicate that early metabolic changes occur in the brain of SLE patients before neurological and imaging manifestations become apparent. This assumption is also supported by our recent findings of significant decreased fraction anisotropy in several regions in the prefrontal WM in both SLE and NPSLE patients (32).
When neurological manifestations are finally present, an increase in Cho might be expected as Cho is suggested to represent a cell membrane turnover marker that can be seen in demyelization, remyelination, inflammation, and gliosis (33). Gln/Cr was also found to be decreased in both SLE and NPSLE patients compared to HC with a steeper decline in Gln/Cr in the NPSLE group. Significantly lower Gln/Cr levels within the RI were seen in both SLE and NPLSE patients compared to HC. In addition, NPSLE patients demonstrated significantly lower Gln/Cr ratios in the anterior FWM compared to HC (P = .02), and the SLE patients had a strong trend toward lower Gln/Cr in the FWM compared to HC. Also, Glu/Cr showed a trend to be lower in SLE and NPSLE patients, but these findings were not significant. These changes could be explained as indicators of the acute changes in brain tissue on a microscopic level, as no obvious abnormalities in the evaluated reviewed regions were seen on conventional MRI, at the time patients present with clinical neurological manifestations. The decline in Gln/Cr in the SLE group, less pronounced, could be explained as subclinical changes that may or may not fully manifest clinically. The present finding of decreased Gln and the trend to lower Glu in both SLE and NPSLE patients in the insula and frontal WM regions have not been demonstrated previously in SLE and NPSLE patients. However, previous studies have demonstrated changes in Glu and Gln in patients with, for example, fibromyalgia (20,22) and in patients with myofascial pain (34). Our present findings in the SLE population are in contrast to the findings seen in patients with fibromyalgia. These studies demonstrated an increase in Glu and Gln in the insular region in fibromyalgia patients, and the levels were correlated to the patient complaints of pain (20,22). A decrease in Glu was seen in the insular region in patients with myofascial pain after pain testing and the Gln levels were related to reported pain (34). In the present study, our initial thought was to examine the patients for pain sensitivity, but this was found to be logistically impossible and only the first few patients had pain testing. However, none of the included patients complained of pain, and we excluded patients who fulfilled the criteria for fibromyalgia or had chronic pain disorders. Our findings of decrease in Glu, a neurotransmitter, and Gln, a molecule used in Glu shuttle between neurons and glia, could potentially be explained by the neuronal loss with a decrease of synapses and neurons. Our findings of changes in Gln and Glu in SLE and NPSLE patients are not unique. Previous studies have shown a correlation between the gln–glu complex (Glx)/Cr ratio in the hippocampus and higher memory functions. They found that lower Glx/Cr ratios and NAA/Cr ratios were associated with lower visual memory in SLE patients without neuropsychiatric symptoms (19). In the present study, we did not specifically evaluate cognitive performance to the Gln and Glu levels. Certainly, additional studies on the roles of Glu and Gln and of cognitive performance in SLE and NPSLE patients are needed.
A significant decrease in the NAA/Cr ratio and in the NAA concentration were present in the NPSLE patients compared to HC and SLE patients in a previously not evaluated region of the brain in SLE patients—the right insular region. Our findings of decreased NAA/Cr and NAA concentrations are in concordance with previous studies (12–15). Decline in NAA concentration can be seen as evidence of neuronal loss and neuronal damage. However, several recent studies indicate that these decreases in regional NAA levels can also represent reversible neuronal or mitochondrial dysfunction (35). Therefore, quantification of NAA or calculation of NAA/Cr ratio by MRS might be a valuable tool to assess neuronal dysfunction and the effects of potential neuroprotective therapies (ie, the persistence over time of low NAA versus normalization of levels after treatment can be used as a marker for treatment response).
When the SLE and NPSLE groups were combined, the NAA/Cr ratio in the RI was significantly negatively correlated with the SLEDAI score, so patients with lower NAA/ Cr ratios had higher SLEDAI scores. This relationship was due in part to neurobiological symptoms since patients with SLEDAI scores reflecting high neurobiological involvement had a trend toward lower NAA/Cr. As could be expected, our study demonstrates that patients with active SLE disease and active symptoms had a trend toward lower NAA/Cr ratios (P = .07) and significantly lower NAA concentration indicating axonal dysfunction. Similar findings of reduced NAA in SLE patients with active disease have been seen in other regions of the brain (15,36). They have also demonstrated that the relative reduction in NAA in SLE patients might be transient, and it is probably dependent on disease activity, as NAA will increase after treatment and will change in patients going from having active to inactive disease (15,36). The role that disease activity might have on cerebral metabolic activity is supported by previous studies that have demonstrated changes in cerebral vascularity in SLE patients with active disease with elevated cerebral blood flow, cerebral blood volume, and mean transit time compared to HC subjects (37–39). Interestingly, despite a correlation between SLEDAI and the NAA at baseline, there were no correlation between the SLEDAI score improvement and the increase in NAA/Cr over time. A possible explanation is that the SLEDAI measures are different parameters of lupus disease activity—not only CNS manifestations but also other organ and system involvement, as well as laboratory parameters to express the individual patient’s lupus activity 10 days prior to the MRS examination. Whereas the NAA represents only the metabolic changes due to neural or mitochondrial dysfunction in the brain over time.
Our study has limitations such as the limited patient cohort, the lack of some laboratory data in three of the 23 NPSLE patients, and the limited number of SVS voxels placed. Notably, the main goal was to evaluate a previously nonimaged region—the insula. Another possible limitation is that we used, as in so many other studies in this topic, the LC model software for analysis of the spectra. The LC model fitting algorithm uses the multiple peaks contributing to an individual metabolite spectrum to estimate the tissue content of each metabolite. In our study, all metabolites showed good reliability of fit as judged from the average Cramer-Rao lower bounds (<20%). Since previous studies have demonstrated certain variability in the NAA/Cr ratios, varying between 3.5% and 8% in different studies of healthy individuals and MS patients (40,41), another possible way of measuring could have been using coefficient of variance (COV). The benefit of the COV as a measure is that the SDs of such metabolites generally increase or decrease proportionally with changes in the mean, so that division by the mean removes it as a factor in the variability. The COV is therefore a standardization of the SD that allows comparison of variability estimates regardless of the overall magnitude of metabolite concentration. In our study, we used the SVS technique at one time point for the evaluation of the ratios between the different group. In addition, the SDs were similar for the different groups, and we judge that our results and significant differences presented would not have been changed using another method of analysis.
Another limitation is that we, like many others, use the Cr as denominator in our ratio calculation despite our study demonstrating that the tCr levels had a trend to higher values for NPSLE patients compared to SLE. In this study, we used the MMSE, a method not considered optimal for evaluation of cognitive performance in SLE patients, as mentioned in the ACR case definition (24), even if MMSE has been used in SLE studies by other authors.
CONCLUSIONS
These findings again support the role of MRS used with conventional MRI in the evaluation of SLE and NPSLE patients. Abnormalities reflecting metabolic changes can be demonstrated in both SLE and NPSLE patients even in the absence of morphological lesions detectable by conventional MRI. Notably, these data, as well as similar findings reported previously, suggest that NAA, Cho, Gln, and Glu may be useful biomarkers for cerebral disease activity in SLE patients, as these early metabolic changes occur in the brain of SLE patients before neurological and imaging manifestations become apparent, and therefore may predict future parenchymal damage.
Acknowledgments
Funding: This study was supported by the Department of Radiology, University of Michigan, Seed Grant U008039, and by MICHR UL1RR024986.
REFERENCES
- 1.Brey RL, Holliday SL, Saklad AR, et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology. 2002;58(8):1214–1220. doi: 10.1212/wnl.58.8.1214. [DOI] [PubMed] [Google Scholar]
- 2.Monastero R, Bettini R, Del Zotto, et al. Prevalence and pattern of cognitive impairment in systemic lupus erythematosus patients with and without overt neuropsychiatric manifestations. J Neurol Sci. 2001;184(1):33–39. doi: 10.1016/s0022-510x(00)00492-5. [DOI] [PubMed] [Google Scholar]
- 3.Sibley JT, Olszynski WP, Decoteau WE, et al. The incidence and prognosis of central nervous system disease in systemic lupus erythematosus. J Rheumatol. 1992;19(1):47–52. [PubMed] [Google Scholar]
- 4.Gladman D, Ginzler E, Godlsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39(3):363–369. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 5.Appenzeller S, Pike GB, Clarke AE. Magnetic resonance imaging in the evaluation of central nervous system manifestations in systemic lupus erythematosus. Clin Rev Allergy Immunol. 2008;34(3):361–366. doi: 10.1007/s12016-007-8060-z. [DOI] [PubMed] [Google Scholar]
- 6.Cotton F, Bouffard-Vercelli J, Hermier M, et al. MRI of central nervous system in a series of 58 systemic lupus erythematosus (SLE) patients with or without overt neuropsychiatric manifestations. Rev Med Interne. 2004;25(1):8–15. doi: 10.1016/s0248-8663(03)00265-0. [DOI] [PubMed] [Google Scholar]
- 7.Petri M, Naqibuddin M, Carson KA, et al. Brain magnetic resonance imaging in newly diagnosed systemic lupus erythematosus. J Rheumatol. 2008;35(12):2348–2354. doi: 10.3899/jrheum.071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sibbitt WL, Jr, Brooks WM, Kornfeld M, et al. Magnetic resonance imaging and brain histopathology in neuropsychiatric systemic lupus erythematosus. Semin Arthritis Rheum. 2010;40(1):32–52. doi: 10.1016/j.semarthrit.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fragoso-Loyo H, RRRichaud-Patin Y, Orozco-Narváez A, et al. Interleukin-6 and chemokines in the neuropsychiatric manifestations of systemic lupus erythematosus. Arthritis Rheum. 2007;56(4):1242–1250. doi: 10.1002/art.22451. [DOI] [PubMed] [Google Scholar]
- 10.Ballok DA, Woulfe J, Sur M, et al. Hippocampal damage in mouse and human forms of systemic autoimmune disease. Hippocampus. 2004;14(5):649–661. doi: 10.1002/hipo.10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanojcic M, Burstyn-Cohen T, Nashi N, et al. Disturbed distribution of proliferative brain cells during lupus-like disease. Brain Behav Immun. 2009;23(7):1003–1013. doi: 10.1016/j.bbi.2009.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sibbitt WL, Jr, Sibbitt RR, Griffey RH, et al. Magnetic resonance and computed tomographic imaging in the evaluation of acute neuropsychiatric disease in systemic lupus erythematosus. Ann Rheum Dis. 1989;12:1014–1022. doi: 10.1136/ard.48.12.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim MK, Suh CH, Kim HJ, et al. Systemic lupus erythematosus: brain MR imaging and single-voxel hydrogen1 MR spectroscopy. Radiology. 2000;217:43–49. doi: 10.1148/radiology.217.1.r00oc1543. [DOI] [PubMed] [Google Scholar]
- 14.Axford JS, Howe FA, Heron C, et al. Sensitivity of quantitative 1H magnetic resonance spectroscopy of the brain in detecting early neuronal damage in systemic lupus erythematosus. Ann Rheum Dis. 2001;60:106–111. doi: 10.1136/ard.60.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundgren PC, Jennings J, Attwood TJ, et al. MRI and 2D–MR CSI spectroscopy of the brain in the evaluation of patients with acute onset of neuro-psychiatric systemic lupus erythematosus. Neuroradiology. 2005;47:576–585. doi: 10.1007/s00234-005-1371-y. [DOI] [PubMed] [Google Scholar]
- 16.Sibbitt WL, Jr, Haseler LJ, Griffey RR, et al. Neurometabolism of active neuropsychiatric lupus determined with proton MR spectroscopy. AJNR Am J Neuroradiol. 1997;18:1271–1277. [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks WM, Sabet A, Sibbitt WL, Jr, et al. Neurochemistry of brain lesions determined by spectroscopic imaging in systemic lupus erythematosus. J Rheumatol. 1997;24:2323–2329. [PubMed] [Google Scholar]
- 18.Chinn RJS, Wilkinson ID, Hall-Craggs MA, et al. Magnetic resonance imaging of the brain and cerebral proton spectros copy in patients with systemic lupus erythematosus. Arthritis Rheum. 1997;40:36–46. doi: 10.1002/art.1780400107. [DOI] [PubMed] [Google Scholar]
- 19.Kozora E, Brown MS, Filley CM, et al. Memory impairment associated with neurometabolic abnormalities of the hippocampus in patients with non-neuropsychiatric systemic lupus erythematosus. Lupus. 2011;20(6):598–606. doi: 10.1177/0961203310392425. http://dx.doi.org/10.1177/0961203310392425. Epub 2011 Feb 18. [DOI] [PubMed] [Google Scholar]
- 20.Harris RE, Sundgren PC, Pang Y, et al. Dynamic levels of glutamate within the insula are associated with improvements in multiple pain domains in fibromyalgia. Arthritis Rheum. 2008r;58(3):903–907. doi: 10.1002/art.23223. http://dx.doi.org/10.1002/art.23223. [DOI] [PubMed] [Google Scholar]
- 21.Bracken BK, Jensen JE, Prescot AP, et al. Brain metabolite concentrations across cortical regions in healthy adults. Brain Res. 2011;1369:89–94. doi: 10.1016/j.brainres.2010.11.036. http://dx.doi.org/10.1016/j.brainres.2010.11.036. Epub 2010 Dec 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris RE, Sundgren PC, Sen A, et al. Elevated insular glutamate (glu) in fibromyalgia (FM) is associated with experimental pain. Arthritis Rheum. 2009;60(10):3146–3152. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 24.The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42(4):599–608. doi: 10.1002/1529-0131(199904)42:4<599::AID-ANR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 25.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Bombardier C, Gladman DD, Urowitz MB, et al. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 28.Steup-Beekman GM, Zirkzee EJM, Cohen D, et al. Neuropsychiatric manifestations in patients with systemic lupus erythematosus: epidemiology and radiology pointing to an immune-mediated cause. Ann Rheum Dis. 2013;72:i76–ii79. doi: 10.1136/annrheumdis-2012-202369. http://dx.doi.org/10.1136/annrheumdis-2012-202369. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs L, Kinkel P, Costello PB, et al. Central nervous system lupus erythematosus: the value of magnetic resonance imaging. J Rheumatol. 1988;15:601–606. [PubMed] [Google Scholar]
- 30.Bosma GPTh, Steens SCA, Petropoulos H, et al. Multisequence magnetic resonance imaging study of neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2004;50(10):3195–3202. doi: 10.1002/art.20512. [DOI] [PubMed] [Google Scholar]
- 31.Appenzeller S, Li LM, Costallat LYL, et al. Neurometabolic changes in normal white matter may predict appearance of hyperintense lesions in systemic lupus erythematosus. Lupus. 2007;16:963–971. doi: 10.1177/0961203307084723. [DOI] [PubMed] [Google Scholar]
- 32.Sundgren PC, Cagnoli P, Wang P, et al. Changes in regional white matter integrity in the prefrontal cortex in patients with systemic lupus erythematosus; Proccedings Oral presentation #40, page 20, American Society of Neuroradiology Annual meeting; May 18–23, 2013; San Diego, CA, USA. [Google Scholar]
- 33.Narayana PA. Magnetic resonance spectroscopy in the monitoring of multiple sclerosis. J Neuroimaging. 2005;15:46S–57S. doi: 10.1177/1051228405284200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerstner GE, Gracely RH, Deebajah A, et al. Posterior insular molecular changes in myofascial pain. Dent Res. 2012;91(5):485–490. doi: 10.1177/0022034512443366. http://dx.doi.org/10.1177/0022034512443366. Epub 2012 Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moffett JR, Ross B, Arun P, et al. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Progr Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appenzeller S, Li LM, Costallat LTL, et al. Evidence of reversible axonal dysfunction in systemic lupus erythematosus: a proton MRS study. Brain. 2005;128:2933–2940. doi: 10.1093/brain/awh646. [DOI] [PubMed] [Google Scholar]
- 37.Wang P, Cagnoli PC, McCune WJ, et al. Perfusion weighted MR imaging in cerebral lupus erythematosus. Acad Radiol. 2012 Aug;19(8):965–970. doi: 10.1016/j.acra.2012.03.023. Epub 2012 May 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gasparovic C, Roldan C, Sibbitt W, et al. Elevated cerebral blood flow and volume in systemic lupus measured by dynamic susceptibility contrast magnetic resonance imaging. J Rheumatol. 2010;37(9):1834–1843. doi: 10.3899/jrheum.091276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emmer B, van Osch M, Wu O, et al. Perfusion MRI in neuro-psychiatric systemic lupus erthemathosus. J Magn Reson Imaging. 2010;32(2):283–288. doi: 10.1002/jmri.22251. [DOI] [PubMed] [Google Scholar]
- 40.Rotondo E, Bruschetta G, Sacca A, et al. Straightforward relative quantitation and age-related human standards of N-acetylaspartate at the centrum semiovale level by CSI (1)H-MRS. Magn Reson Imaging. 2003;21:1055–1060. doi: 10.1016/s0730-725x(03)00211-x. [DOI] [PubMed] [Google Scholar]
- 41.Mosteret JP, Blaauw Y, Koch MW, et al. Reproducibility over a 1-month period of 1H-MR spectroscopic imaging NAA/Cr ratios in clinically stable multiple sclerosis patients. Eur Radiol. 2008;18(8):1736–1740. doi: 10.1007/s00330-008-0925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]