We examined the feasibility of using intraspinal microstimulation (ISMS) of the cervical spinal cord to evoke diaphragm activity ipsilateral to acute and subacute hemisection of the upper cervical spinal cord of the rat. This proof-of-concept study demonstrated the efficacy of diaphragm activation, using an upper airway respiratory EMG signal to trigger ISMS at the level of the ipsilesional phrenic nucleus during acute and advanced postinjury intervals.
Keywords: phrenic motor nucleus, rat, respiration, hypoglossal respiratory activity, diaphragm function
Abstract
Intraspinal microstimulation (ISMS) using implanted electrodes can evoke locomotor movements after spinal cord injury (SCI) but has not been explored in the context of respiratory motor output. An advantage over epidural and direct muscle stimulation is the potential of ISMS to selectively stimulate components of the spinal respiratory network. The present study tested the hypothesis that medullary respiratory activity could be used to trigger midcervical ISMS and diaphragm motor unit activation in rats with cervical SCI. Studies were conducted after acute (hours) and subacute (5–21 days) C2 hemisection (C2Hx) injury in adult rats. Inspiratory bursting in the genioglossus (tongue) muscle was used to trigger a 250-ms train stimulus (100 Hz, 100–200 μA) to the ventral C4 spinal cord, targeting the phrenic motor nucleus. After both acute and subacute injury, genioglossus EMG activity effectively triggered ISMS and activated diaphragm motor units during the inspiratory phase. The ISMS paradigm also evoked short-term potentiation of spontaneous inspiratory activity in the previously paralyzed hemidiaphragm (i.e., bursting persisting beyond the stimulus period) in ∼70% of the C2Hx animals. We conclude that medullary inspiratory output can be used to trigger cervical ISMS and diaphragm activity after SCI. Further refinement of this method may enable “closed-loop-like” ISMS approaches to sustain ventilation after severe SCI.
NEW & NOTEWORTHY We examined the feasibility of using intraspinal microstimulation (ISMS) of the cervical spinal cord to evoke diaphragm activity ipsilateral to acute and subacute hemisection of the upper cervical spinal cord of the rat. This proof-of-concept study demonstrated the efficacy of diaphragm activation, using an upper airway respiratory EMG signal to trigger ISMS at the level of the ipsilesional phrenic nucleus during acute and advanced postinjury intervals.
severe respiratory compromise often occurs after spinal cord injury (SCI) at upper cervical to midcervical spinal levels (Mansel and Norman 1990; Winslow and Rozovsky 2003). When independent breathing is not possible, positive-pressure mechanical ventilation is often used to sustain alveolar ventilation, but at the risk of potentially rapid diaphragm atrophy, atelectasis, and respiratory tract infections (Bezzant and Mortensen 1994; Laghi et al. 2003; Smuder et al. 2016). Diaphragm and phrenic nerve pacing options are clinically available (DiMarco et al. 2005; Glenn and Phelps 1985; Onders et al. 2009), and recent preclinical work has begun exploring high-frequency epidural stimulation of the spinal cord to activate the diaphragm (DiMarco and Kowalski 2013, 2015; Kowalski et al. 2013).
Another approach that may effectively activate respiratory muscles after SCI is intraspinal microstimulation (ISMS). Implementation of this method in motor systems has been demonstrated (Giszter 2015; Kasten et al. 2013; McPherson et al. 2015; Tator et al. 2012), but applications to respiratory motor output after cervical SCI have not been investigated. Potential advantages of ISMS include the ability to deliver currents over a relatively wide range of intensities and with high selectivity for specific motor systems (Mondello et al. 2015; Mushahwar et al. 2000; Sunshine et al. 2013).
The present study was therefore designed to obtain proof-of-concept evidence in support of cervical ISMS as a means of activating diaphragm motor units after cervical SCI. To move toward a “closed-loop-like” design for ISMS, we recorded the inspiratory output of an upper airway muscle (the genioglossus, innervated by cranial nerve XII) and used that signal to trigger stimulation. Thus cervical ISMS was activated by endogenous inspiratory drive. The data show that a physiologically relevant medullary inspiratory output can be used to trigger cervical ISMS and that this approach can effectively activate diaphragm motor units after acute and subacute SCI.
MATERIALS AND METHODS
Thirteen female Sprague-Dawley rats (255 ± 16 g; Harlan, Indianapolis, IN) were distributed between two experimental groups: 1) ISMS after acute SCI, tested before and immediately after lateral hemisection of the spinal cord at C2 (C2Hx) (n = 8), and 2) ISMS after subacute SCI, tested 5–21 days after C2Hx (n = 5). All data were collected in terminal procedures using protocols approved by the Institutional Animal Care and Use Committee at the University of Florida and the US Army Medical Research and Materiel Command Animal Care and Use Review Office.
C2Hx surgery.
For survival surgery, rats were initially anesthetized with isoflurane (3–5% in O2) in a closed chamber and then anesthesia was maintained via nosecone (1.5–3% in O2). A dorsal incision was made over the cervical spine, followed by a C2 laminectomy and durotomy. For acute C2Hx, resection lesions were performed with a needle-blade microknife (Fine Science Tools, Foster City, CA). Subacute C2Hx resection lesions were made with microscissors and fine forceps followed by gentle aspiration of tissue to complete the hemisection (Fuller et al. 2008). The dura and overlying muscles were closed and lactated Ringer solution (5 ml sc) and buprenorphine were administered (0.03 mg/kg sc.; Hospira, Lake Forest, IL). Postoperative care consisted of daily lactated Ringer solution (5 ml/day sc) and oral nutritional supplement (1–3 ml/day; Nutrical, Webster Veterinary). Buprenorphine was given at ∼12-h intervals for 2 days after surgery.
General neurophysiology protocols.
Rats were anesthetized as above. Rectal temperature was maintained at ∼37.5°C by a heating pad (CWE, Ardmore, PA). A femoral artery was catheterized (PE-50) for blood pressure measurements (Statham P-10EZ pressure transducer, CP122 AC/DC strain gauge amplifier; Grass Instruments, West Warwick, RI) and arterial blood samples. The femoral vein of the same hindlimb was also catheterized (PE-50) for supplemental fluid administration and conversion from isoflurane to urethane anesthesia (1.7 g/kg iv; Sigma, St. Louis, MO). Animals received a tracheotomy and were mechanically ventilated (50–65% O2, balance N2; 6–7 ml/kg volume; 70–72 bpm frequency; Harvard Apparatus, Holliston, MA) throughout the experimental procedures. Since spontaneously breathing anesthetized rats can rapidly become hypercapnic, we elected to employ mechanical ventilation for these initial experiments in order to keep blood gases stable and remove PaO2 and/or PaCO2 fluctuations as confounding variables.
End-tidal CO2 (Capnogard 1265; Respironics, Wallingford, CT) was continuously monitored, and arterial blood gases (iSTAT1; Abbott, Princeton, NJ) were periodically assessed from 0.1-ml arterial samples. On the basis of these measures, the inspired CO2 content was adjusted to maintain PaCO2 at 40 mmHg. If base excess was greater than −3 meq, this was corrected with intravenous administration of sodium bicarbonate solution [8.4%, Hospira; dose (ml) = 0.3·weight (kg)·standard base excess].
Recordings of EMG activity from respiratory-related muscles (i.e., diaphragm, genioglossus, and intercostal) and an off-target, nonrespiratory muscle [i.e., extensor carpi radialis longus (ECR)] were obtained with pairs of Teflon-coated tungsten hooked wires (A-M Systems, Sequim, WA). Recordings of the genioglossus muscle were obtained at the base of the tongue (Fuller et al. 1999; Fuller and Fregosi 2000) and exhibited a centrally driven inspiratory rhythm that was used to trigger ISMS. For intercostal EMG recordings, a small incision lateral to the sternum was made at the T2 level and wires were placed 1–1.5 cm lateral to the midline.
The spinal cord was exposed via a cervical middorsal incision followed by laminectomy and durotomy from C2 to C5. Raw EMG signals were amplified at 100-10K, band-pass filtered at 100 Hz–10 kHz (A-M Systems, Carlsborg, WA), and, in the case of the genioglossus EMG recording, passed through a moving time averager (50 ms time constant; CWE, Ardmore, PA). The moving time average signal was used to trigger the stimulator for ISMS. All EMG signals were digitized at 25 kHz (CED Power 1401) and recorded (Spike2 v8; CED, Cambridge, UK) to a PC and then analyzed off-line.
ISMS at midcervical spinal cord.
A tungsten microwire electrode (FHC, Bowdoin, ME) was mounted in a stereotaxic micromanipulator (David Kopf Instruments, Tujunga, CA). The microwire had a 100-μm segment at the tip stripped of all insulation. The electrode was placed above the C4 segment with the dorsal root entry zone as the lateral anatomical landmark. Electrical activity was initially recorded via the stimulating electrode and assessed visually with Spike2 software and audibly with an AM8 Audio Monitor (Grass Technologies, Quincy, MA). The software program simultaneously displayed the inspiratory diaphragm EMG recording; these procedures enabled placement of the stimulating electrode tip in proximity to inspiratory neurons (i.e., targeting phrenic motoneurons). Inspiratory bursting was absent in rats with subacute C2Hx injury. Therefore, a stimulator and constant-current stimulus isolation unit (S88X and SIU-C; Grass Technologies, Warwick, RI) were used to deliver single pulses (0.3 ms duration) during electrode descent with gradually decreasing currents (200, 100, 50 μA). This approach allowed us to determine a location for ISMS that evoked left diaphragm activation.
Stimulation of the spinal cord was initiated (triggered) with the EMG signal recorded from the genioglossus muscle. Specifically, a “threshold crossing” was established such that when the inspiratory integrated EMG burst (50 ms time constant) reached a preset amplitude the spinal cord was stimulated. All stimulations during the inspiratory phase were made at the onset of the genioglossus inspiratory burst, while stimulations made during expiratory periods were accomplished by adding a 400-ms delay to the trigger.
Experimental group 1: ISMS before and after acute C2Hx.
The genioglossus EMG signal was used to trigger ISMS during two consecutive inspiratory cycles, followed by ISMS delivered during two consecutive expiratory cycles. In both cases, repeated 250-ms stimulus trains (200 μA) were delivered with 0.3 ms pulse duration and 100 Hz stimulus frequency. Once these initial stimulations were complete, ISMS was delivered during the inspiratory cycle for 1 min. All of the aforementioned ISMS was done with the spinal cord intact. After C2Hx, the ISMS protocol was repeated, as described above. Subsequent to protocols performed with spinal cord intact or after C2Hx, an additional bout of ISMS was administered after neuromuscular blockade via intravenous pancuronium bromide (2.5 mg/kg; Hospira) to confirm unequivocally that the evoked activity was not contaminated by a stimulus artifact.
Selection of stimulus parameters.
Pilot experiments were done with a manually triggered, open-loop approach in spinal-intact animals. Repeated 250-ms trains of ISMS were targeted to the left phrenic motor nucleus, and EMG activity was recorded in both hemidiaphragms, the genioglossus, and ipsilateral intercostal and forelimb (ECR) muscles (see, e.g., Fig. 1). A range of stimulation frequencies (50, 100, 200, 300 Hz) and currents (50–200 μA) were tested to optimize parameters for eliciting compound motor unit action potentials (MUAPs) in the left diaphragm. Activation of the ipsilateral hemidiaphragm was more pronounced when 100-Hz stimulation was used, with stimulation frequencies above 200 Hz producing large contractions of nonrespiratory muscles. Preliminary experiments also indicated that progressive attenuation of ISMS-induced phrenic MUAP amplitude occurred during sustained stimulation (e.g., 1 min) at 200 Hz but not at lower stimulus frequencies (50 Hz, 100 Hz). In the first series of experiments, a stimulus current of 200 μA was used to activate the ipsilateral diaphragm.
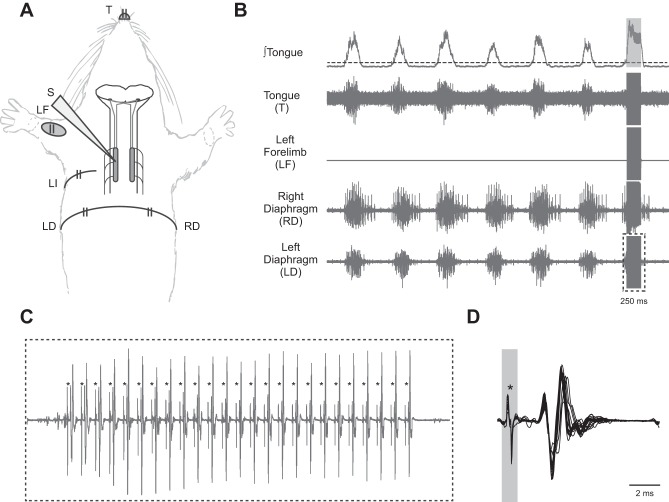
Fig. 1.
Cervical ISMS activates the ipsilateral diaphragm A: schematic diagram illustrates the EMG recording sites relative to the placement of the stimulating electrode (S). The stimulating electrode was placed in the immediate vicinity of the phrenic motor nucleus, and ISMS was initiated via a trigger signal based on inspiratory tongue (genioglossus) EMG activity. LI, left intercostal. B: pre-C2Hx representative EMG recordings from the genioglossus, extensor carpi radialis (ECR), and both sides of the diaphragm showing baseline (prestimulation) activity and a period of genioglossus-triggered cervical ISMS (100 Hz, 200 μA; 0.3-ms pulse duration, 250-ms train duration) represented by a gray box in the ∫tongue trace. C: expanded trace of the EMG recording from the left diaphragm during the period of ISMS shows each stimulus artifact, indicated by asterisks, and each subsequent MUAP. D: overlay of each elicited MUAP, aligned by the stimulus artifact, demonstrates constant latency and amplitude.
Experimental group 2: ISMS after subacute C2Hx.
The intraspinal electrode placement was determined as described above. Triggered ISMS was then delivered during two consecutive expiratory periods, followed by one continuous minute of stimulation during the inspiratory phase. In both cases, a continuous 250-ms train was delivered with 0.3-ms pulses at 100 Hz and 100 μA. Stimulations also were repeated after neuromuscular blockade as described above.
Consistent with other reports, we used cessation of the ipsilateral diaphragm inspiratory EMG burst and histology as functional and anatomical verification, respectively, of the subacute C2Hx lesions (Goshgarian 1981). After the electrophysiology protocols, the animals were perfused with saline followed by 4% paraformaldehyde (Sigma). The cervical spinal cords from the subacute C2Hx animals were subsequently harvested, and a tissue block including the C2 region was paraffin embedded, sectioned (8 μm), and counterstained with cresyl violet.
Biomechanical impact of ISMS.
Changes in tracheal pressure were assessed as a crude indicator of the biomechanical impact of ISMS. It should be noted, however, that this was not considered to be a primary outcome variable but rather an accessory measurement intended to provide some insight regarding the functional impact of spinal stimulation. The pressures associated with lung inflation and deflation were measured at the tracheal cannula. Measurements were taken continuously and used to compare baseline conditions to stimulation periods. For each period of interest, tracheal pressure deflections for 20 consecutive breaths were averaged and presented in a box and whisker plot (SPSS; IBM, Armonk, NY). Passive tracheal pressure values during exhalation were obtained in each preparation after neuromuscular blockade and thus provided a baseline measurement that was devoid of any respiratory contribution from the animal (for additional details, see results).
Data acquisition and analyses.
For the first group of experiments, results from the spinal-intact condition were compared to data collected 10 min after acute C2Hx. In addition to the evoked responses (i.e., response during ISMS), we also evaluated the impact of the ISMS on spontaneous EMG activity (i.e., following the period of stimulation). To assess ISMS-entrained activation of motor units, the EMG signals were averaged with respect to stimulus pulses within each trial. The resulting stimulus-triggered average (McPherson et al. 2015; Moritz et al. 2007) served to minimize activity unrelated to ISMS. The resultant waveform averages represented ISMS-entrained MUAPs. All data were collected with a CED Spike2 data acquisition system and subsequently analyzed with Spike2 v8 software on a standard PC. Values are reported as means ± SD.
Statistics.
A one-way repeated-measures ANOVA followed by the Tukey post hoc test (SigmaPlot; Systat Software, San Jose, CA) was used to compare data collected during baseline vs. ISMS. Dependent variables included the amplitude of MUAPs and spontaneous EMG burst amplitude, blood pressure, tracheal pressure, heart rate, and blood gas data. A “detectability index” statistical test (Aertsen and Gerstein 1985) was used to compare ISMS-evoked MUAP amplitude to background activity in the EMG recordings. We used the modified version of this test with a more stringent value of D ≥ 3 to prevent “false positives” (Melssen and Epping 1987).
RESULTS
ISMS with spinal cord intact.
A representative example of triggered cervical ISMS during the inspiratory phase is provided in Fig. 1. Note that compound MUAPs in the left diaphragm are clearly discernible (Fig. 1C) and are entrained to each stimulation pulse (e.g., Fig. 1D). In this example, the average latency between stimulus artifact and MUAP peak is 2.14 ± 0.05 ms, which is consistent with a synaptic delay before motor unit activation. An example of ISMS delivered during the expiratory phase is provided in Fig. 2. In the absence of spontaneous inspiratory EMG activity, it can be appreciated that ISMS targeting the left phrenic motor nucleus evoked a marked response in both the ECR and diaphragm but had minimal impact on the left tongue or right diaphragm. To confirm that the very small-amplitude EMG signals in the tongue, diaphragm, and intercostals represented activation of motor units, we employed a previously published “detectability index” for evoked potentials (Aertsen and Gerstein 1985; Melssen and Epping 1987). This assessment indicated that ISMS evoked a small but statistically significant increase in entrained and averaged EMG activity in the tongue (5 of 8 animals), left intercostal (8 of 8 animals), and right diaphragm (8 of 8 animals). It should be noted, however, that the amplitudes of these off-target evoked potentials were modest compared with those of the left diaphragm and ECR muscles. Neuromuscular blockade eliminated evoked potentials, as expected (Fig. 2C).
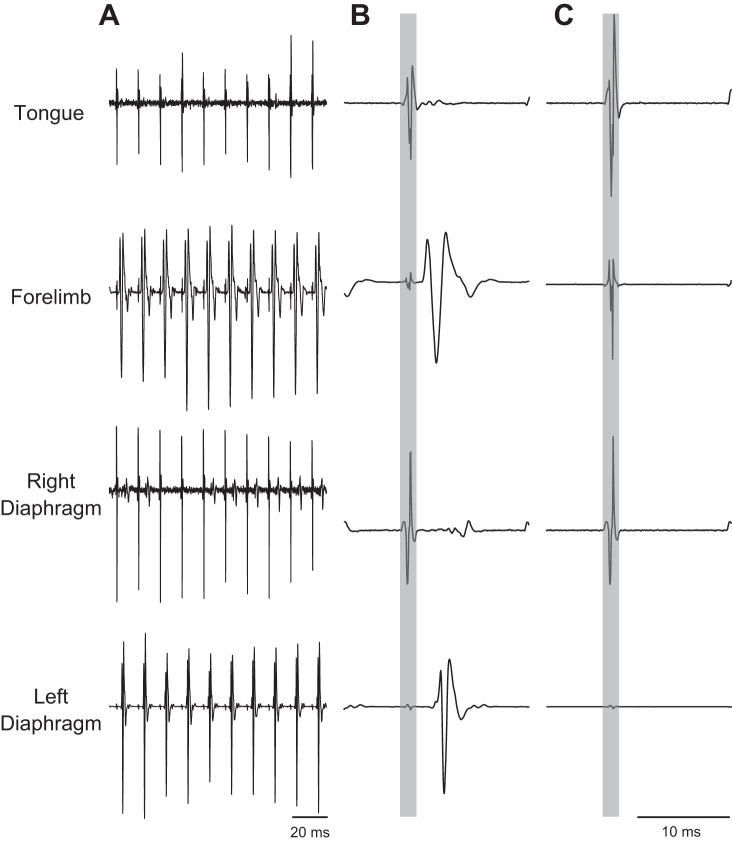
Fig. 2.
Examples of raw EMG activity recorded in the tongue (genioglossus), forelimb (ECR), and the left and right hemidiaphragm during ISMS. A: representative examples before C2Hx. B: stimulus-triggered averages of EMG activity show prominent MUAPs in the forelimb and ipsilateral diaphragm. C: stimulus-triggered averages of EMG activity after neuromuscular blockade. In B and C, the stimulus artifact is highlighted by the gray boxes. Stimulus-triggered averages were scaled to the same values and represent 25 stimulus triggers. In these examples, ISMS was delivered at 100 Hz and 200 μA during expiration.
ISMS after high cervical SCI.
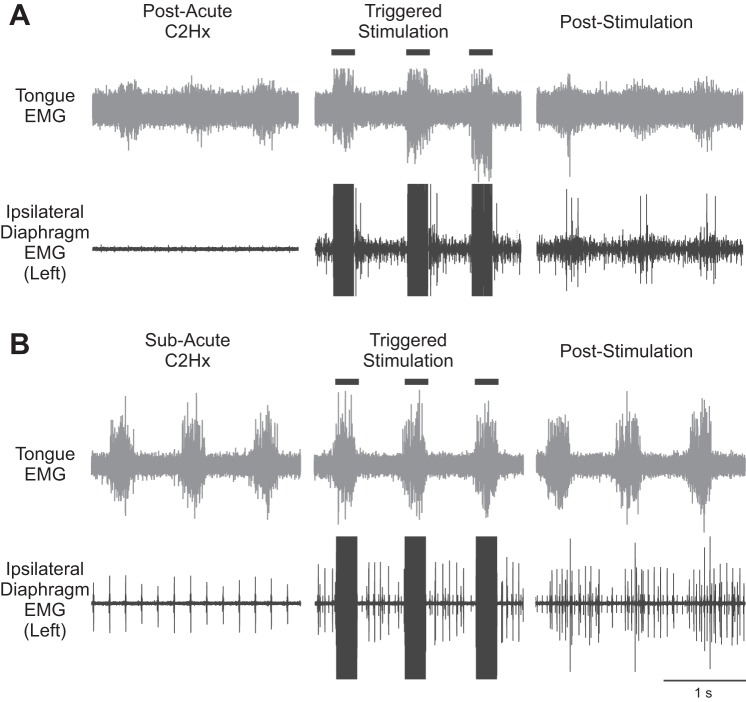
Experiments were performed immediately after C2Hx (acute injury) and also in rats that were 5–21 days post-C2Hx (subacute injury). These temporally advanced lesion experiments were performed to determine whether degenerative processes triggered by C2Hx (e.g., axonal retraction) prevented or mitigated the impact of ISMS on diaphragm activation. The general experimental paradigm is illustrated in Fig. 3A.
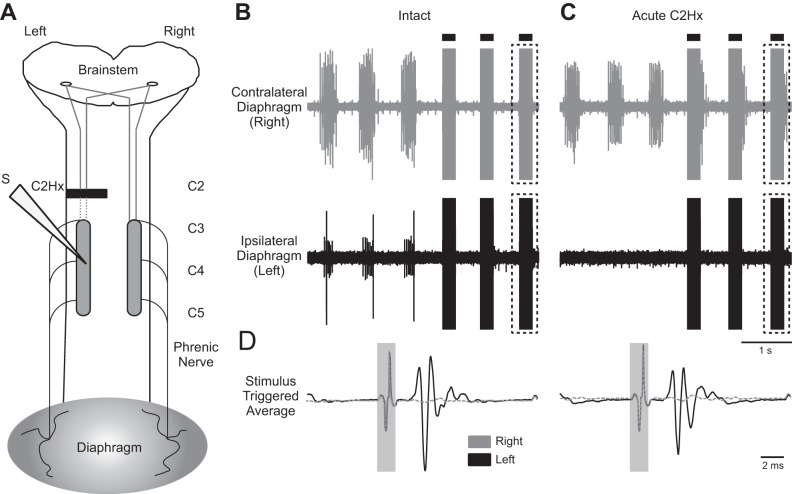
Fig. 3.
ISMS after acute C2Hx. A: illustration of the electrode placement relative to the C2Hx lesion. The solid dark bar represents the injury site; the dashed lines represent severed pathways. B: examples of EMG activity in the right and left diaphragm before C2Hx. C: examples of left and right diaphragm EMG activity after acute C2Hx. Note that activity is abolished in the left diaphragm. In B and C, 3 spontaneous breaths are shown followed by 3 breaths during triggered cervical ISMS (solid bars; 100 Hz, 200 μA). D: stimulus-triggered averages of right and left diaphragm EMG activity obtained from the period of ISMS highlighted by dashed boxes in B and C. Stimulus-triggered averages were scaled to the same values and represent 25 stimulus triggers. In D, the stimulus artifact is highlighted by the gray boxes.
In the acute C2Hx experiments, diaphragm MUAPs were first evaluated with the spinal cord intact (Fig. 3B). The ISMS-evoked potentials in the spinal-intact condition were similar to those described in the preceding section. Acute C2Hx abolished spontaneous inspiratory EMG activity in the ipsilateral hemidiaphragm in all animals (Fig. 3C). However, cervical ISMS still produced clear MUAPs in the ipsilateral diaphragm after the acute lesion (Fig. 3D). Before C2Hx, the average latency from stimulus to the peak of the MUAP was 1.99 ± 0.25 ms. After C2Hx the value tended to be reduced (1.69 ± 0.57 ms), but this did not approach statistical significance (P = 0.190). In seven of eight experiments, the amplitude of the ISMS-evoked MUAP remained relatively consistent after the acute C2Hx, ranging from 80% to 124% of the preinjury value, with a mean of 94 ± 15% (P = 0.065 vs. pre-C2Hx). There was one significant outlier rat (confirmed with Grubbs' outlier test, P < 0.05) that demonstrated a profound reduction in MUAP amplitude after acute C2Hx, with evoked responses reaching only 8% of the preinjury amplitude. If that particular data point is included in the calculation of the overall mean the values after acute C2Hx are 83 ± 35% of the preinjury condition, but the added variance actually produces a higher P value (P = 0.139 vs. preinjury). Collectively, the data are consistent with a slight reduction in cervical ISMS-evoked MUAP amplitude in most animals after acute C2Hx. The off-target impact of ISMS after C2Hx (i.e., evoked activity in ECR, tongue, and right diaphragm) was indistinguishable from that reported above for the spinal-intact condition (data not shown).
Tracheal pressures were evaluated to explore the potential of a biomechanical impact of ISMS-induced muscle contraction (Fig. 4, A and B). Negative pressure swings in tracheal pressure would be expected in this preparation if diaphragm contraction is altering the dimensions of the thoracic cavity. In the spinal-intact condition, genioglossus-triggered cervical ISMS caused a significant negative deflection in tracheal pressure (P = 0.02 vs. baseline; Fig. 4C), as expected. The acute C2Hx injury resulted in a small, but statistically significant, change in the tracheal pressures that were recorded during ventilator-induced lung inflation (i.e., independent of ISMS; P = 0.028; Fig. 4C). After acute C2Hx, ISMS also induced a change in tracheal pressure (P = 0.005 vs. baseline), thus suggesting a biomechanical impact of the stimulation. However, the relative magnitude of ISMS-induced changes in tracheal pressure were attenuated after C2Hx (P = 0.038; Fig. 4C).
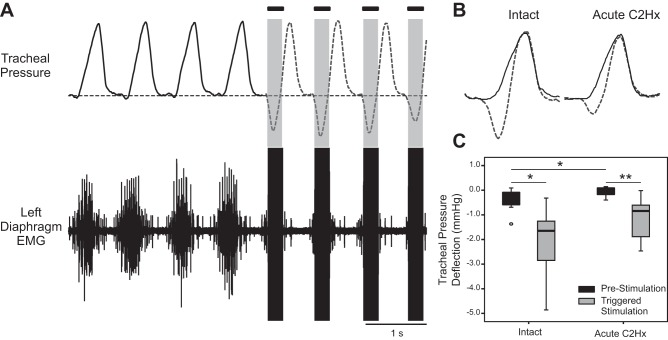
Fig. 4.
Impact of ISMS on tracheal pressure. A: representative examples of tracheal pressure and left diaphragm EMG activity before and during cervical ISMS (100 Hz, 200 μA). The period of ISMS is indicated by the solid bars. Note the negative deflection in tracheal pressure during ISMS. B: overlay plot further illustrates the tracheal pressure before stimulation (solid line) and during ISMS (dashed line). C: average change in tracheal pressure during lung inflation at prestimulation baseline and during ISMS. Data are shown for the spinal-intact condition (left) and after acute C2Hx (right). *P < 0.05, **P < 0.005.
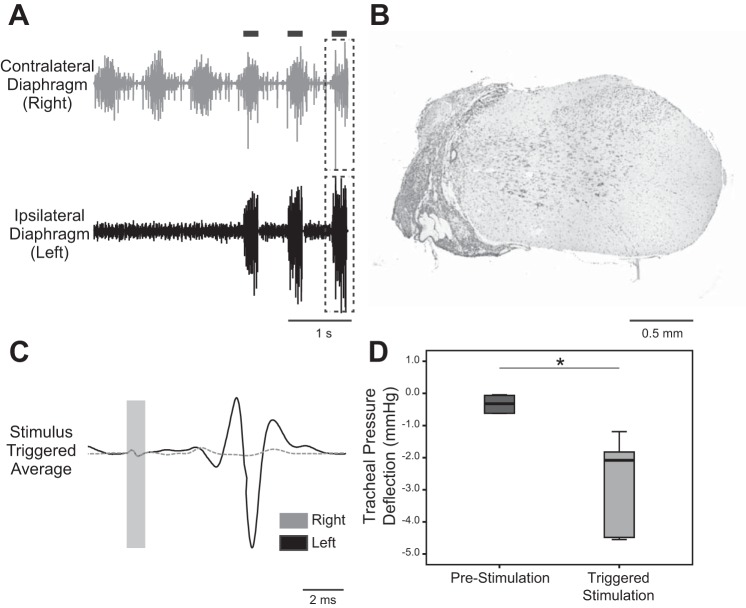
Spontaneous ipsilateral diaphragm EMG activity was absent in rats studied 5–21 days after C2Hx (Fig. 5A), and subsequent histological evaluation of the spinal cord indicated anatomically complete hemilesion in all animals (Fig. 5B). The ISMS procedure evoked clearly discernible MUAPs in the ipsilateral (paralyzed) hemidiaphragm (Fig. 5C). Compared with the acute injury group, the only apparent difference in the ISMS responses in the animals with the subacute lesions was a trend for more variable and longer latencies. On average, however, there were no statistical differences between the groups for latency (acute C2Hx: 1.69 ± 0.57 ms, subacute C2Hx: 2.50 ± 0.67 ms), but the increase in latency in the subacute group was close to threshold for significance (P = 0.065). The MUAP amplitude was variable, but with no evidence for a difference between the two groups (acute C2Hx: 1.62 ± 1.45 mV, subacute C2Hx: 1.76 ± 2.65 mV, P = 0.833). ISMS caused the expected negative deflection in tracheal pressure in the subacute C2Hx animals, thus confirming that there was a biomechanical impact of the stimulation (Fig. 5D). The off-target muscle activation was similar to what was obtained in the spinal-intact and acute C2Hx animals. Thus very small but statistically significant changes in EMG activity during ISMS were detected in the tongue (4/5 animals), intercostal (3/4 animals), and right diaphragm (4/5 animals). The left ECR showed greater EMG responses in five of five animals.
Fig. 5.
ISMS after subacute C2Hx. A: representative diaphragm EMG activity after subacute C2Hx. In the example traces, genioglossus-triggered ISMS was delivered during the breaths marked by the black bars. B: histological section of the C2 spinal cord stained with cresyl violet. The example demonstrates an anatomically complete hemilesion extending to the midline of cervical cord. C: stimulus-triggered averages from the ipsilesional (solid line) and contralesional diaphragm (dashed line); data were obtained from the period indicated by the dashed boxes in A. These traces illustrate activation of the diaphragm ipsilateral to the C2Hx lesion. D: average change in tracheal pressure during lung inflation at prestimulation baseline and during ISMS. *P < 0.05.
Inspiratory-related diaphragm EMG bursting is enhanced after ISMS.
While our primary intent was to determine whether ISMS evoked diaphragm activity during the period of stimulation, we noted that spontaneous diaphragm EMG activity often was present after the stimulus was turned off. Thus, in six of eight animals after acute C2Hx, the hemidiaphragm that was electrically silent before ISMS showed both tonic and inspiratory-related activity during and after the 1-min period of stimulation (Fig. 6A). In these experiments, “activity” was defined as one or more clearly discernible motor unit potentials that were discharging phasically during the inspiratory period. The duration of the effect was variable and did not persist beyond 2 min in any experiment. On average, ISMS-induced spontaneous diaphragm motor unit activity lasted for 43 ± 38 respiratory cycles after ISMS was terminated. The first breath immediately after cessation of ISMS typically demonstrated the greatest amount of motor unit recruitment, and this was followed by a gradual decrease in motor unit activity over subsequent respiratory cycles. In addition, we noted an increase in tonic activity (i.e., activity persisting across the entire respiratory cycle) after ISMS that followed a time course similar to the phasically active motor units. A similar response to ISMS was observed in three of five rats after subacute C2Hx injury (Fig. 6B).
Fig. 6.
Example recordings illustrate short-term potentiation of ipsilateral diaphragm EMG activity following ISMS. A: tongue and ipsilateral (left) diaphragm EMG activity after acute C2Hx, during ISMS, and immediately after cessation of ISMS. Note that clear phasic (inspiratory) activity can be seen after ISMS, whereas the baseline showed no such activity. B: a similar response can be observed in a subacute C2Hx animal. In these examples, the ISMS was triggered by the inspiratory genioglossus EMG signal and was delivered at 100 Hz for 1 min of respiratory efforts.
DISCUSSION
This study has demonstrated the feasibility of using endogenous medullary output to trigger ISMS to the midcervical spinal cord. The ISMS paradigm was successful in recruiting diaphragm motor units in spinal-intact animals and also after both acute and subacute interruption of bulbospinal inspiratory drive to the phrenic motoneuron pool following high cervical SCI. Further refinement of this approach may enable development of a “respiratory neuroprosthesis” that, by virtue of the endogenous trigger, could adapt to temporally changing metabolic demands.
Endogenously triggered ISMS induces diaphragm activation.
The approach used in this study was a “closed-loop-like” strategy for activating phrenic motoneurons in the cervical spinal cord. As an initial proof of concept, cervical ISMS was triggered from EMG signals recorded from the base of the tongue. Such recordings consist largely of activity from the primary tongue protrudor muscle, the genioglossus (Fuller et al. 1998). Respiratory-related activation of the genioglossus occurs in humans (Mateika et al. 1999; Saboisky et al. 2007) and animal models (Bailey et al. 2005; Fregosi and Fuller 1997) and acts to stiffen and/or dilate the oropharyngeal airway and thus preserve upper airway patency during breathing. The respiratory-related discharge of genioglossus motor units increases during chemoreceptor stimulation in a manner similar to respiratory pump muscles such as the diaphragm (Bailey 2011; Fuller et al. 1998). Genioglossus and tongue muscle output is also subject to vagal modulation, with progressive inhibition occurring as the lung inflates (Bailey et al. 2001; Fukuda and Honda 1982). Collectively, the literature establishes that a complex but coordinated interaction between the genioglossus and the respiratory pump muscles is important for optimizing airway resistance across the respiratory cycle and maintaining upper airway patency. For the present study, the most salient point is that an inspiratory signal was recorded from the genioglossus that provided a marker for the endogenous respiratory rhythm. Thus the animals' own “decision to breathe” triggered the cervical ISMS.
The genioglossus EMG-triggered cervical ISMS approach resulted in diaphragm motor unit activation after both acute (i.e., minutes) and subacute (i.e., days-weeks) C2Hx injury. It was important to repeat the studies under these advanced lesion settings because severed descending pathways have not undergone degeneration after acute injury and therefore could still be capable of conducting action potentials during stimulation (Gandevia and Kirkwood 2011; Kowalski et al. 2013) as seen in other experimental conditions (Moldovan et al. 2009). Indeed, the latencies of the diaphragm MUAPs in the acute condition (i.e., >1.6 ms) were not consistent with direct phrenic motoneuron activation, and therefore it is likely that synaptic inputs to the phrenic motoneuron pool were being activated via ISMS. Accordingly, it was uncertain if the ISMS approach would be effective at later C2Hx postinjury intervals when degeneration of severed bulbospinal respiratory axons would be well underway. The results showed that diaphragm MUAPs could still be effectively evoked after subacute injury but with a trend toward longer and more variable diaphragm MUAP latencies compared with acute C2Hx animals. In addition, the biomechanical impact of the ISMS (indirectly inferred from changes in tracheal pressure) also appeared to be reduced after subacute vs. acute injury. Refinement of the ISMS method to enable direct phrenic motoneuron stimulation will likely be necessary to optimize diaphragm activation after long-term cervical SCI.
An interesting and potentially important observation that was not part of our original hypothesis was that ISMS-induced inspiratory bursting in the hemiparetic diaphragm persisted for several respiratory cycles after cessation of the stimulation. This occurred in the majority of acute and subacute C2Hx lesions and is similar to the previously described phenomenon of respiratory short-term potentiation (STP) (Lee et al. 2015). Exposure to brief periods of reduced oxygen (hypoxia) triggers phrenic STP, which is manifested as a progressive enhancement of activity followed by a slow decline to baseline levels after removal of the hypoxic stimulus (Lee et al. 2009; Powell et al. 1998). We previously demonstrated that hypoxia can induce STP of phrenic motor output in rats with subacute C2Hx, and the response is greater in the nerve ipsilateral vs. contralateral to the lesion (Lee et al. 2015). The STP in C2Hx rats appeared to reflect recruitment of a population of phrenic motoneurons that had been silenced by injury, which continued to burst beyond the period of hypoxic stimulation. It may be that ISMS can trigger similar STP-like mechanisms of short-term plasticity and ultimately have value in the context of neurorehabilitation. This increased phrenic bursting after cessation of ISMS also may be analogous to longer-duration functional recoveries others have observed with chronic ISMS in other motor systems (Kasten et al. 2013; McPherson et al. 2015).
Methodological caveats including off-target effects of cervical ISMS.
The method described here is not truly a “closed-loop” system because the ISMS intensity did not vary with the relative strength of endogenous “respiratory drive.” Varying the stimulus intensity or duration in proportion to the relative magnitude of the EMG burst used to trigger ISMS (vs. a simple “onset trigger”) could potentially address that issue, but this could also prove problematic since ISMS needs to occur at the onset (vs. peak) of the inspiratory effort. Another caveat is that rats were mechanically ventilated because in our experience spontaneously breathing and anesthetized rats rapidly develop arterial hypercapnia and respiratory acidosis. However, mechanical ventilation will impact endogenous respiratory drive and thereby alter the interactions between spontaneous and ISMS input to the phrenic region. It should also be noted that tongue EMG activity is unlikely to provide an effective ISMS trigger signal in the vagally intact and spontaneously breathing rodent or human. It is possible to record inspiratory-related discharge from the tongue muscles in the awake human and rodent, but available data indicate that tongue EMG may not provide a consistent inspiratory trigger signal (Bailey 2011; Sood et al. 2005). In the present experiments on anesthetized rats, however, the tongue EMG output enabled a rigorous test of the fundamental hypothesis, and we suggest that future work should focus on additional potential sources of respiratory output to serve as endogenous trigger signals or alternative detection algorithms (Dow et al. 2006).
Neural substrate considerations.
One of the more challenging issues related to electrical stimulation of the spinal cord is identification of the neural substrate being affected directly or indirectly. Here our immediate goal was to target ISMS of gray matter at the level of the phrenic motor nucleus. Whether phrenic motoneurons are being directly activated by ISMS cannot be determined from the present findings, although latencies in the range of 1.5–2.5 ms suggest indirect stimulation via polysynaptic pathways. In the subacute C2Hx group, stimulation of ipsilateral bulbospinal inputs to phrenic motoneurons can be effectively ruled out because these pathways will be undergoing degeneration. It is possible that ISMS activated commissural bulbospinal projections from the contralateral cord [i.e., pathways associated with the “crossed phrenic phenomenon” (Goshgarian 2003; Goshgarian et al. 1991)]. However, past studies in which intraspinal stimulation of the cervical cord was used to activate crossed phrenic pathways reported latencies of <1 ms (Fuller et al. 2003), and these are considerably shorter than the values reported here. Collectively, the evidence is most consistent with indirect activation of phrenic motoneurons via ISMS, possibly via spinal prephrenic interneurons (Lane 2011) or even ascending afferent projections originating below the injury (Decima and von Euler 1969).
ISMS is capable of discrete activation of circuitry with minimal undesired physiological effects (Pikov et al. 2007). Here we observed clear responses in the ipsilateral diaphragm, as intended, but also off-target motor unit recruitment. Very small and inconsistent activation of the contralateral diaphragm, intercostal muscles, and genioglossus were present during ISMS. This indicates current spread beyond the target region or activation of other circuits via interneurons (Perlmutter et al. 1998). For example, in the case of ISMS-evoked responses in the contralateral diaphragm and intercostal muscles, our previous transneuronal tracing studies provide an interneuronal basis for those off-target responses (Lane et al. 2008). Intercostal and/or contralateral diaphragm recruitment could in fact be advantageous when cervical SCIs involve much larger-scale compromise of diaphragm and inspiratory intercostal function compared with C2Hx. Activation of the genioglossus was barely above the threshold for detection and probably reflected activation of ascending projections to medullary respiratory control neurons. For example, we previously reported that some cervical interneurons associated with the phrenic motor circuit have ascending projections to the medulla (Lane et al. 2008).
The relatively stronger coactivation of forelimb muscles induced by ISMS presents a more challenging technical issue. As with the ipsilateral diaphragm, constant-latency entrainment occurred in the forelimb, and this may reflect overlap of phrenic and forelimb circuits along the rostro-caudal axis (Gonzalez-Rothi et al. 2015). ECR motoneurons, however, appear to occupy a more dorso-lateral position than the phrenic motoneuron pool (Tosolini and Morris 2012). This raises the possibility that even if ISMS was precisely restricted to phrenic motoneurons, concomitant excitation of ECR axons as they course toward their ventral roots could still occur. Further investigations are needed to sort out the neuroanatomical basis for direct vs. indirect effects of ISMS. The latter may be especially beneficial by activating desired motoneuron pools in a more natural order than achieved with direct stimulation.
Conclusions.
Previous studies directed at spinal cord stimulation and the respiratory system after SCI have focused on epidural stimulation as a means of enhancing abdominal or phrenic motor output. Epidural stimulation has been successful at recruiting diaphragm motor units in animal studies (DiMarco and Kowalski 2009, 2011; Kowalski et al. 2013) and has been used to enhance cough in spinally injured humans (DiMarco et al. 2014). Epidural stimulation also shows promise in the context of locomotor function after SCI in both animals and humans (Edgerton and Harkema 2011; Rejc et al. 2015). Despite these successes, there is unquestionably a need for improvement and refinement of spinal cord stimulation methods. In that regard, the initial findings from our study raise the possibility of utilizing endogenous, physiologically relevant respiratory signals, which entail peripheral and chemosensory feedback, for triggering neuromodulation of the phrenic motor circuit and alleviation of ventilatory insufficiency following mid- to high-cervical SCI. Many ISMS parameters (Bamford and Mushahwar 2011; Giszter 2015; Tator et al. 2012) must be refined, however, before the benefits of invasive ISMS protocols to promote respiratory improvements after cervical SCI can be fully evaluated or compared with other approaches, high-frequency epidural stimulation (Kowalski et al. 2013) in particular. It also is possible that a given spinal stimulation approach will be better suited for some SCI cases than another. Future investigations involving awake, spontaneously breathing animals will be crucial for determining whether chronic ISMS delivery will function solely as a neuroprosthetic or can be used short term to promote functional and anatomical neuroplasticity (Moritz et al. 2007) alone or in combination with other therapeutic approaches leading to long-lasting improvement in respiratory function.
GRANTS
This work was funded by awards from the Department of Defense (W81XWH-14-1-0625; P. J. Reier), the National Institutes of Health (NIH) [R01 NS-054025 (P. J. Reier), R01 NS-080180 (D. D. Fuller)], and the State of Florida Brain and Spinal Cord Injury Research Trust Fund (P. J. Reier, D. D. Fuller, D. M. Baekey). The authors also express their appreciation for the generous support received from the Fraternal Order of Eagles Aerie #3496. L. M. Mercier was supported by a NIH T32 training grant (HD-043730).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
L.M.M. and E.J.G.-R. performed experiments; L.M.M., K.A.S., S.S.P., and A.S.P. analyzed data; L.M.M., D.D.F., P.J.R., and D.M.B. interpreted results of experiments; L.M.M. prepared figures; L.M.M. and D.M.B. drafted manuscript; L.M.M., E.J.G.-R., K.A.S., S.S.P., A.S.P., D.D.F., P.J.R., and D.M.B. approved final version of manuscript; D.D.F., P.J.R., and D.M.B. edited and revised manuscript; D.D.F., P.J.R., and D.M.B. conceived and designed research.
ACKNOWLEDGMENTS
We thank Dr. Danielle Meola, L. Emma Denholtz, Amy Poirier, Sarah El-Azab, and Alexis Caballero for their technical contributions.
REFERENCES
- Aertsen AM, Gerstein GL. Evaluation of neuronal connectivity: sensitivity of cross-correlation. Brain Res 340: 341–354, 1985. [DOI] [PubMed] [Google Scholar]
- Bailey EF. Activities of human genioglossus motor units. Respir Physiol Neurobiol 179: 14–22, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey EF, Janssen PL, Fregosi RF. PO2-dependent changes in intrinsic and extrinsic tongue muscle activities in the rat. Am J Respir Crit Care Med 171: 1403–1407, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey EF, Jones CL, Reeder JC, Fuller DD, Fregosi RF. Effect of pulmonary stretch receptor feedback and CO2 on upper airway and respiratory pump muscle activity in the rat. J Physiol 532: 525–534, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford JA, Mushahwar VK. Intraspinal microstimulation for the recovery of function following spinal cord injury. Prog Brain Res 194: 227–239, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzant TB, Mortensen JD. Risks and hazards of mechanical ventilation: a collective review of published literature. Dis Mon 40: 581–638, 1994. [DOI] [PubMed] [Google Scholar]
- Decima EE, von Euler C. Excitability of phrenic motoneurones to afferent input from lower intercostal nerves in the spinal cat. Acta Physiol Scand 75: 580–591, 1969. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. High-frequency spinal cord stimulation of inspiratory muscles in dogs: a new method of inspiratory muscle pacing. J Appl Physiol (1985) 107: 662–669, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. Distribution of electrical activation to the external intercostal muscles during high frequency spinal cord stimulation in dogs. J Physiol 589: 1383–1395, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. Spinal pathways mediating phrenic activation during high frequency spinal cord stimulation. Respir Physiol Neurobiol 186: 1–6, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE. Electrical activation to the parasternal intercostal muscles during high-frequency spinal cord stimulation in dogs. J Appl Physiol (1985) 118: 148–155, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Kowalski KE, Hromyak DR, Geertman RT. Long-term follow-up of spinal cord stimulation to restore cough in subjects with spinal cord injury. J Spinal Cord Med 37: 380–388, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF, Onders RP, Ignagni A, Kowalski KE, Mortimer JT. Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest 127: 671–678, 2005. [DOI] [PubMed] [Google Scholar]
- Dow DE, Mantilla CB, Zhan WZ, Sieck GC. EMG-based detection of inspiration in the rat diaphragm muscle. Conf Proc IEEE Eng Med Biol Soc 1: 1204–1207, 2006. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Harkema S. Epidural stimulation of the spinal cord in spinal cord injury: current status and future challenges. Expert Rev Neurother 11: 1351–1353, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregosi RF, Fuller DD. Respiratory-related control of extrinsic tongue muscle activity. Respir Physiol 110: 295–306, 1997. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Honda Y. Roles of vagal afferents on discharge patterns and CO2-responsiveness of efferent superior laryngeal, hypoglossal, and phrenic respiratory activities in anesthetized rats. Jpn J Physiol 32: 689–698, 1982. [DOI] [PubMed] [Google Scholar]
- Fuller D, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol 507: 265–276, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol 211: 97–106, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Fregosi RF. Fatiguing contractions of tongue protrudor and retractor muscles: influence of systemic hypoxia. J Appl Physiol 88: 2123–2130, 2000. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci 23: 2993–3000, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol 519: 601–613, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Kirkwood PA. Spinal breathing: stimulation and surprises. J Neurotrauma 589: 2661–2662, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter SF. Spinal primitives and intra-spinal micro-stimulation (ISMS) based prostheses: a neurobiological perspective on the “known unknowns” in ISMS and future prospects. Front Neurosci 9: 72, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn WW, Phelps ML. Diaphragm pacing by electrical stimulation of the phrenic nerve. Neurosurgery 17: 974–984, 1985. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rothi EJ, Rombola AM, Rousseau CA, Mercier LM, Fitzpatrick GM, Reier PJ, Fuller DD, Lane MA. Spinal interneurons and forelimb plasticity after incomplete cervical spinal cord injury in adult rats. J Neurotrauma 32: 893–907, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG. The role of cervical afferent nerve fiber inhibition of the crossed phrenic phenomenon. Exp Neurol 72: 211–225, 1981. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol 94: 795–810, 2003. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Ellenberger HH, Feldman JL. Decussation of bulbospinal respiratory axons at the level of the phrenic nuclei in adult rats: a possible substrate for the crossed phrenic phenomenon. Exp Neurol 111: 135–139, 1991. [DOI] [PubMed] [Google Scholar]
- Kasten MR, Sunshine MD, Secrist ES, Horner PJ, Moritz CT. Therapeutic intraspinal microstimulation improves forelimb function after cervical contusion injury. J Neural Eng 10: 044001, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski KE, Hsieh YH, Dick TE, DiMarco AF. Diaphragm activation via high frequency spinal cord stimulation in a rodent model of spinal cord injury. Exp Neurol 247: 689–693, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi YS, Tobin MJ. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med 167: 120–127, 2003. [DOI] [PubMed] [Google Scholar]
- Lane MA. Spinal respiratory motoneurons and interneurons. Respir Physiol Neurobiol 179: 3–13, 2011. [DOI] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol 511: 692–709, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Reier PJ, Fuller DD. Phrenic motoneuron discharge patterns during hypoxia-induced short-term potentiation in rats. J Neurophysiol 102: 2184–2193, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KZ, Sandhu MS, Dougherty BJ, Reier PJ, Fuller DD. Hypoxia triggers short term potentiation of phrenic motoneuron discharge after chronic cervical spinal cord injury. Exp Neurol 263: 314–324, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansel JK, Norman JR. Respiratory complications and management of spinal cord injuries. Chest 97: 1446–1452, 1990. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Millrood DL, Kim J, Rodriguez HP, Samara GJ. Response of human tongue protrudor and retractors to hypoxia and hypercapnia. Am J Respir Crit Care Med 160: 1976–1982, 1999. [DOI] [PubMed] [Google Scholar]
- McPherson JG, Miller RR, Perlmutter SI. Targeted, activity-dependent spinal stimulation produces long-lasting motor recovery in chronic cervical spinal cord injury. Proc Natl Acad Sci USA 112: 12193–12198, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melssen WJ, Epping WJ. Detection and estimation of neural connectivity based on crosscorrelation analysis. Biol Cybern 57: 403–414, 1987. [DOI] [PubMed] [Google Scholar]
- Moldovan M, Alvarez S, Krarup C. Motor axon excitability during Wallerian degeneration. Brain 132: 511–523, 2009. [DOI] [PubMed] [Google Scholar]
- Mondello SE, Sunshine MD, Fischedick AE, Moritz CT, Horner PJ. A cervical hemi-contusion spinal cord injury model for the investigation of novel therapeutics targeting proximal and distal forelimb functional recovery. J Neurotrauma 32: 1994–2007, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz CT, Lucas TH, Perlmutter SI, Fetz EE. Forelimb movements and muscle responses evoked by microstimulation of cervical spinal cord in sedated monkeys. J Neurophysiol 97: 110–120, 2007. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Collins DF, Prochazka A. Spinal cord microstimulation generates functional limb movements in chronically implanted cats. Exp Neurol 163: 422–429, 2000. [DOI] [PubMed] [Google Scholar]
- Onders RP, Elmo M, Khansarinia S, Bowman B, Yee J, Road J, Bass B, Dunkin B, Ingvarsson PE, Oddsdottir M. Complete worldwide operative experience in laparoscopic diaphragm pacing: results and differences in spinal cord injured patients and amyotrophic lateral sclerosis patients. Surg Endosc 23: 1433–1440, 2009. [DOI] [PubMed] [Google Scholar]
- Perlmutter SI, Maier MA, Fetz EE. Activity of spinal interneurons and their effects on forearm muscles during voluntary wrist movements in the monkey. J Neurophysiol 80: 2475–2494, 1998. [DOI] [PubMed] [Google Scholar]
- Pikov V, Bullara L, McCreery DB. Intraspinal stimulation for bladder voiding in cats before and after chronic spinal cord injury. J Neural Eng 4: 356–368, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998. [DOI] [PubMed] [Google Scholar]
- Rejc E, Angeli C, Harkema S. Effects of lumbosacral spinal cord epidural stimulation for standing after chronic complete paralysis in humans. PLoS One 10: e0133998, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboisky JP, Gorman RB, De Troyer A, Gandevia SC, Butler JE. Differential activation among five human inspiratory motoneuron pools during tidal breathing. J Appl Physiol 102: 772–780, 2007. [DOI] [PubMed] [Google Scholar]
- Smuder AJ, Gonzalez-Rothi EJ, Kwon OS, Morton AB, Sollanek KJ, Powers SK, Fuller DD. Cervical spinal cord injury exacerbates ventilator-induced diaphragm dysfunction. J Appl Physiol (1985) 120: 166–177, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood S, Morrison JL, Liu H, Horner RL. Role of endogenous serotonin in modulating genioglossus muscle activity in awake and sleeping rats. Am J Respir Crit Care Med 172: 1338–1347, 2005. [DOI] [PubMed] [Google Scholar]
- Sunshine MD, Cho FS, Lockwood DR, Fechko AS, Kasten MR, Moritz CT. Cervical intraspinal microstimulation evokes robust forelimb movements before and after injury. J Neural Eng 10: 036001, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tator CH, Minassian K, Mushahwar VK. Spinal cord stimulation: therapeutic benefits and movement generation after spinal cord injury. Handb Clin Neurol 109: 283–296, 2012. [DOI] [PubMed] [Google Scholar]
- Tosolini AP, Morris R. Spatial characterization of the motor neuron columns supplying the rat forelimb. Neuroscience 200: 19–30, 2012. [DOI] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil 82: 803–814, 2003. [DOI] [PubMed] [Google Scholar]