Abstract
Objective:
To determine the frequency of medication use in patients with dystonia enrolled in an international biorepository study.
Methods:
In a cross-sectional analysis, we included 2,026 participants enrolled at 37 sites in the United States, Canada, Europe, and Australia through Project 1 of the Dystonia Coalition, an international biorepository study. The primary aim was to assess the frequency of medication classes recommended for treating patients with dystonia, and the secondary aim was to compare characteristics (disease type, age, sex, duration of disease, comorbid conditions, severity).
Results:
Querying the database for the presence of any medication for dystonia used (includes both injectable and oral therapy), we found 73% using medications (n = 1,488) and 27% using no dystonia medications (n = 538). Furthermore, 61% of the total sample used botulinum toxin (BoNT) therapy alone or in combination. Differences were found in medication use patterns by dystonia type, with the lowest oral medication use in focal dystonia and highest use in generalized dystonia; by region, with highest BoNT therapy rate reported in Italy and the lowest in the Northeast region of the United States; and by focal dystonia subtype, with highest BoNT therapy alone in blepharospasm and spasmodic dysphonia (49%) and lowest in other cranial dystonia (32%).
Conclusions:
The majority of patients with dystonia enrolled in the Dystonia Coalition Project 1 were using medications to treat their dystonia. Overall, a complex picture of medication use patterns emerged, with factors such as region, disease duration, type of dystonia, disease severity, and psychiatric comorbidities all playing a significant role.
Although botulinum toxin (BoNT) injections are the gold standard therapy for patients with focal dystonia, oral medications such as anticholinergics and GABAergic classes are typically recommended in published treatment reviews on dystonia.1 We currently do not know the range or type of oral medications actually used in patients with dystonia (alone or combined with BoNT).
In a study performed as an online survey, most patients (86%) with a self-reported diagnosis of cervical dystonia had used BoNT, and the majority of patients (53%) also reported oral medication for their dystonia symptoms.2 This study supports that many patients with dystonia use oral medications, but we do not know the type or class of medication used in this population.
We conducted a cross-sectional study in an international cohort to better characterize and understand medication use in the dystonia population. This study provides an opportunity to explore the potential therapeutic gap in the treatment of dystonia and whether this gap might differ among the subtypes of dystonia, requiring a precision medicine approach.
METHODS
Study participants.
We reviewed data collected from 2,131 individuals across 37 enrolling sites in the United States, Canada, Europe, and Australia participating in Project 1 of the Dystonia Coalition (http://clinicaltrials.gov/show/NCT01373424). The full inclusion and exclusion criteria for the Dystonia Coalition Natural History and Biorepository studies can be found at http://www.rarediseasesnetwork.org/cms/dystonia/Get-Involved/Research-Studies/6301-Natural-History and http://www.rarediseasesnetwork.org/cms/dystonia/Get-Involved/Research-Studies/6301-Biorepository. In general, the inclusion criteria consisted of having isolated dystonia (cervical dystonia, blepharospasm or craniofacial dystonia, oromandibular dystonia, spasmodic dysphonia, limb dystonia, segmental dystonia, or generalized dystonia) and being ≥18 years of age. Of the 2,131 participant records provided, we maintained the same inclusion criteria but extended the exclusionary criteria to include participants with muscle tension dysphonia who were enrolled in the cohort with the intention of serving as controls in a completed project within the biorepository (n = 50); participants taking medications known to lead to dystonic symptoms (e.g., dopamine antagonists, n = 21); and participants with duplicate data entries, in which case we used the earliest initial encounter date (n = 34). After applying the exclusionary criteria, we included data collected from 2,026 individuals for analysis (figure e-1 at Neurology.org).
Standard protocol approvals, registrations, and patient consents.
This study was approved by the University of New Mexico Health Sciences Center Institutional Review Board, and the Dystonia Coalition Executive Committee provided access to the database for analysis. All patients were examined, videotaped, and enrolled in either the natural history arm (if disease onset was ≤5 years previously) or the biorepository arm (for disease duration >5 years) following a standardized protocol after written informed consent was obtained from all patients participating in the study.
Dystonia.
Data used in the analysis included dystonia type (e.g., focal, generalized, hemidystonia, multifocal, segmental), region where the patient participated (e.g., Australia, Canada, England, France, Germany, Italy, United States [Northeast, Midwest, South, West]), and severity of disease determined by total score of the Global Dystonia Rating Scale (GDRS).3
Medications.
Medication use was recorded by the enrolling site and entered into the database either by dropdown menu of frequently used medications or by free-text entry if “other” was selected. BoNT use was also noted by date of last injection given. Medications recorded included medications that could be used for the treatment of dystonia symptoms and medications used for other conditions.
We categorized the medications into classes typically recommended for treatment of dystonia based on expert review.4 The treatment classes included in the analysis as medications used to treat dystonia were the following: (1) muscle relaxant (cyclobenzaprine, tizanidine, carisoprodol, orphenadrine, metaxalone, dantrolene); (2) benzodiazepine (clonazepam, lorazepam, diazepam, alprazolam, temazepam, bromazepam); (3) dopaminergic (levodopa, pramipexole, amantadine [low-dopaminergic properties], bromocriptine); (4) anticholinergic (trihexyphenidyl, benztropine, diphenhydramine); (5) antidopaminergic (tetrabenazine, clozapine); (6) baclofen; (7) non–benzo-hypnotic (zolpidem); and (8) BoNT (onabotulinumtoxin A, abobotulinumtoxin A, incobotulinumtoxin A, rimabotulinumtoxin B). In the United States, the above oral agents do not have a Food and Drug Administration (FDA)–approved indication for dystonia and are used in an off-label manner. Because we used data collected at sites outside the United States, some of the medications listed have not received FDA approval for any indication.
Statistical analyses.
We performed a cross-sectional analysis using SAS (version 9.4; SAS Institute Inc, Cary, NC). We looked at prevalence of medication use by demographic characteristics (age, sex, region), by dystonia characteristics (type, severity, duration), and by comorbidities (e.g., anxiety/depression). We then looked further at the type of medication (oral vs BoNT vs both oral and BoNT vs none) using the same demographic and dystonia characteristics. Missing data elements (i.e., sex) were handled by excluding that participant for that particular analysis. For each result below, the total number included in the analysis is disclosed. We used χ2 and Fisher exact tests (for sample size <5) to analyze these associations, with values of p < 0.05 considered significant. Multivariate logistic regression was used to test for regional differences while controlling for dystonia type, presence of anxiety/depression, disease severity, and disease duration.
RESULTS
Study participants.
We studied a total of 2,026 participants (1,437 [71%] female and 587 [29%] male) who were enrolled into the Dystonia Coalition Biorepository and Natural History Projects from January 4, 2011, to July 22, 2015 (with 2 missing values for sex). Participant mean age was 59.4 years (SEM 0.3 year) with mean age at onset of 45.3 years (SEM 0.3 year) and mean disease duration of 14.1 years (SEM 0.3 year). Mean severity score on the GDRS was 9.0 (SEM 0.2). A total of 1,588 participants were enrolled in the United States (9.1% in the Northeast region, 30.0% in the Midwest region, 49.2% in the South region, and 11.6% in the West region). The international site enrollment consisted of 438 participants, with 16.4% in Canada, 4.3% in Australia, 0.7% in England, 29.7% in France, 36.1% in Germany, and 12.8% in Italy.
Dystonia characterization.
We determined the frequencies of dystonia syndrome by type in the database. Focal dystonia was the most common type of dystonia at 76.4% (n = 1,528) of the population, followed by segmental dystonia at 16.4% (n = 328), generalized dystonia at 3.8% (n = 76), multifocal dystonia at 3% (n = 61), and hemidystonia at 0.4% (n = 8). For the focal dystonia subtypes, cervical dystonia was the most prevalent at 60.9% (n = 930) of the focal dystonia sample, followed by laryngeal at 11.9% (n = 182), limb at 10.3% (n = 157), cranial (includes oromandibular, lingual, and Meige) at 8.7% (n = 132), and blepharospasm at 8.3% (n = 127) (total n = 2,001 with 25 missing values for dystonia type).
Medication use.
Querying the whole database for the presence of any medication for dystonia used (includes both injectable and oral therapy), we found 73% using medications (n = 1,488) and 27% using no dystonia medications (n = 538) (total n = 2,026).
By dystonia type.
The same trend continued for dystonia type. We found 74% using medications (n = 1,481) and 26% using no dystonia medications (n = 520). Of those using medications, 48% (n = 712) used BoNT therapy alone, 17% (n = 251) used oral medications alone, and 35% (n = 518) used both BoNT therapy and oral medications (total n = 2,001 with 25 missing values for dystonia type).
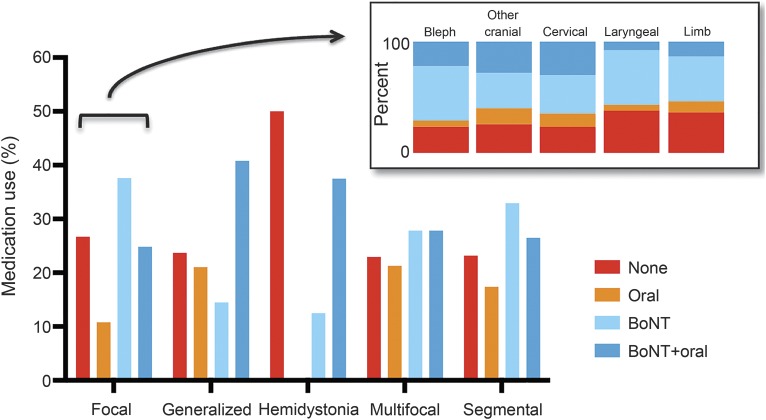
Analyzing by dystonia type showed that the frequencies of no medications, BoNT therapy, oral medications, and combination therapy were different among the dystonia types (p < 0.0001, figure 1). Hemidystonia had the highest rate of no medication use. Generalized dystonia and hemidystonia had the lowest rates of BoNT alone use but had BoNT and oral medication combination use at 41% and 38%, respectively. Oral medication use was highest in generalized dystonia.
Figure 1. Medication use by dystonia type and subtype.
Main graph shows medication use by dystonia type (n = 2,001 with 25 missing values) with percentage used along the y-axis. Inset shows percentage of no medication use and medication use by class for each subtype of focal dystonia (n = 1,528 with 473 nonfocal dystonia and 25 missing). Other cranial includes lower cranial dystonia and Meige syndrome. For each focal dystonia subtype, percentages for none, oral, botulinum toxin (BoNT), and BoNT plus oral: blepharospasm, 23.6%, 5.5%, 48.8%, and 22.1%, respectively; other cranial, 25.8%, 14.4%, 31.8%, and 28.0%; cervical, 23.4%, 12.2%, 34.3%, and 30.1%; laryngeal, 37.9%, 5.5%, 48.9%, and 7.7%; and limb, 36.3%, 10.2%, 40.1%, and 13.4%.
By focal dystonia subtype.
Dystonia medication use varied by focal dystonia subtype (p < 0.0001). The prevalence of no medication use, BoNT use, oral medication use only, and use of BoNT plus oral medications was analyzed for each focal dystonia subtype (figure 1). The highest prevalence for no dystonia medication use was seen in laryngeal dystonia (38%) and limb dystonia (36%) and the lowest in cervical dystonia (23%), blepharospasm (24%), and other cranial dystonia (26%). For BoNT alone, highest use was in laryngeal dystonia (49%) and blepharospasm (49%).
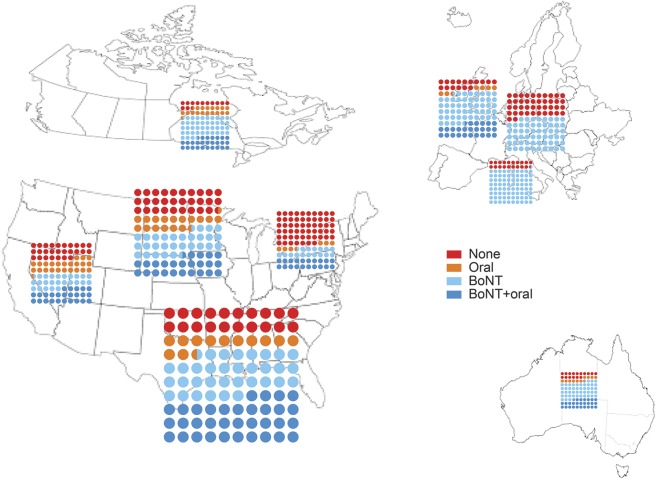
By region.
Looking at medication use by region, we found that the frequency of dystonia medication use varied by region (p < 0.0001). We examined this by no medication use, oral medication use only, BoNT use only, and use of BoNT plus oral medications (figure 2). No medication use ranged from a high of 57% in the Northeast to a low of 13% in Canada. This regional difference in medication use (i.e., taking dystonia medications vs not taking dystonia medications) persisted when adjusted for dystonia type, presence of anxiety/depression, disease severity, and disease duration (p < 0.0001, table e-1). Two hundred fourteen patients were excluded because of missing values for at least one variable used in the analysis (n = 1,812).
Figure 2. Dystonia medication use plotted by region.
The individual region plots are approximately scaled to reflect number of participants in each region (actual numbers: Northeast n = 145; Midwest n = 477; South n = 782; West n = 184; Canada n = 72; Australia n = 19; France n = 130; Germany n = 158; Italy n = 56; England excluded because of small number [n = 3]; p < 0.0001). BoNT = botulinum toxin.
By demographic and clinical variables.
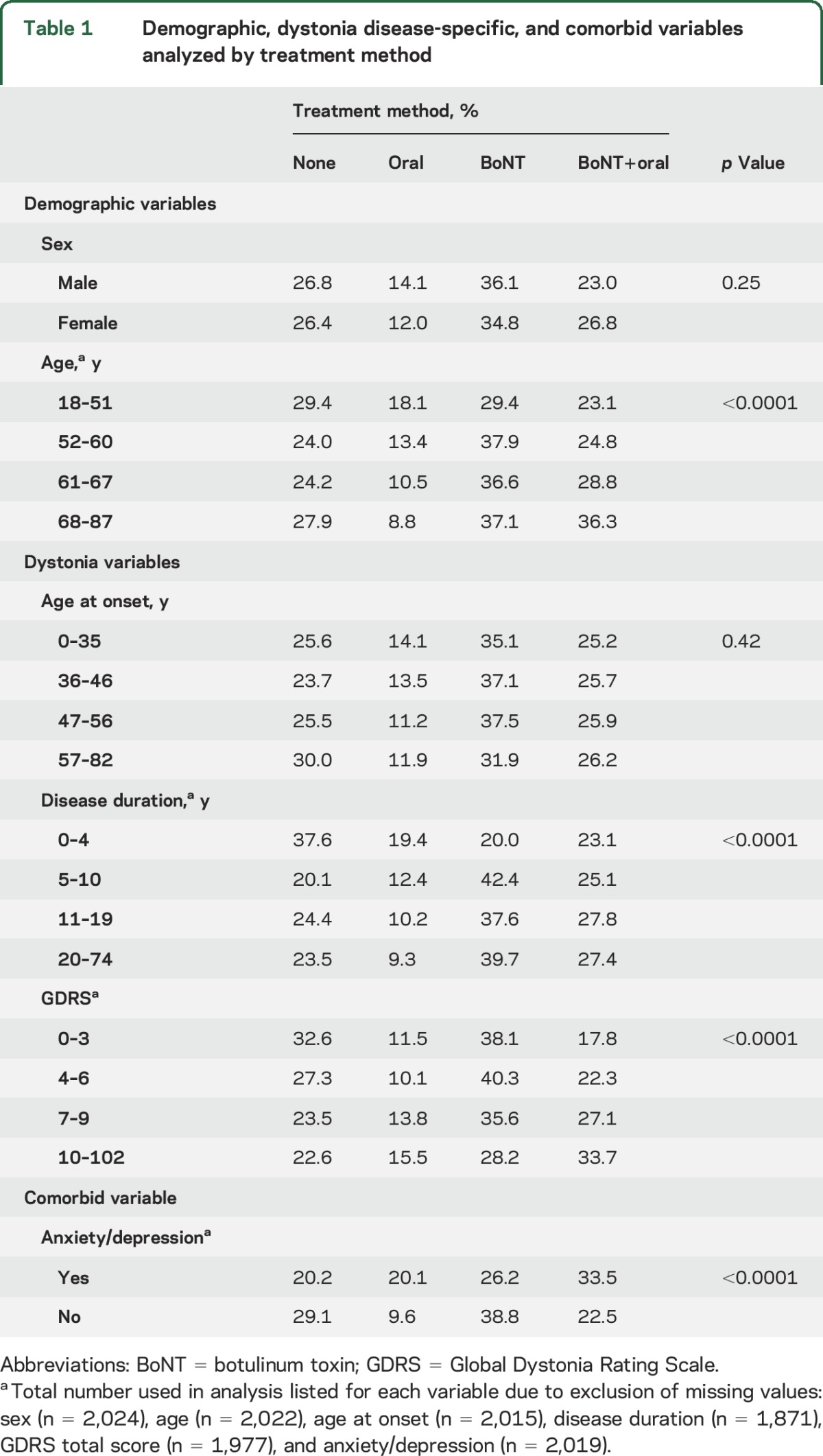
We analyzed the frequency of no medication use and medication use by class using demographic, dystonia disease–specific, and comorbid variables. To analyze age, age at onset, disease duration, and disease severity (measured by total GDRS score), we divided the population into quartiles. For age, there were differences in medication use patterns, with patients in the oldest quartile less likely to be using oral medications (p < 0.0001). Disease duration and severity varied both in frequency of medication use and by type of medication class (p < 0.0001). Longer disease duration was associated with lower oral medication use; however, higher disease severity scores were associated with higher rates of oral medication and combination therapy (BoNT plus oral) use. Finally, if anxiety/depression was present, patients were twice as likely to be taking oral medications. Complete results are presented in table 1.
Table 1.
Demographic, dystonia disease-specific, and comorbid variables analyzed by treatment method
By medication class.
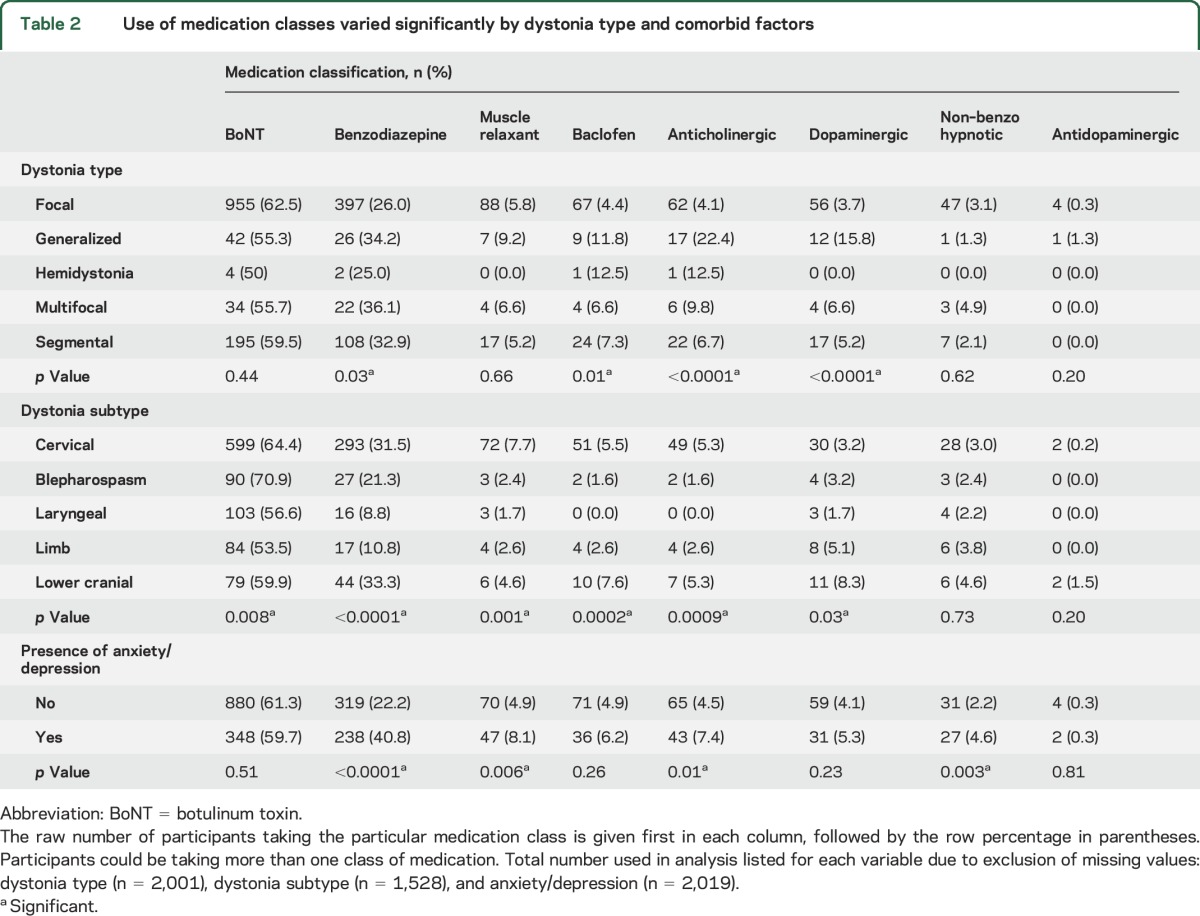
For the 8 classes of medication used to treat dystonia symptoms, we plotted each medication class individually (figure 3). Overall, the most frequently used medication was BoNT, followed by benzodiazepines and muscle relaxants (excluding baclofen). The least frequently used class was the antidopaminergic class. Of note, in this analysis, participants could be using more than one class of medication. The use of medication classes varied significantly by dystonia type, by focal dystonia subtype, and by the presence of anxiety/depression (figure 3 and table 2).
Figure 3. Medication use by class.
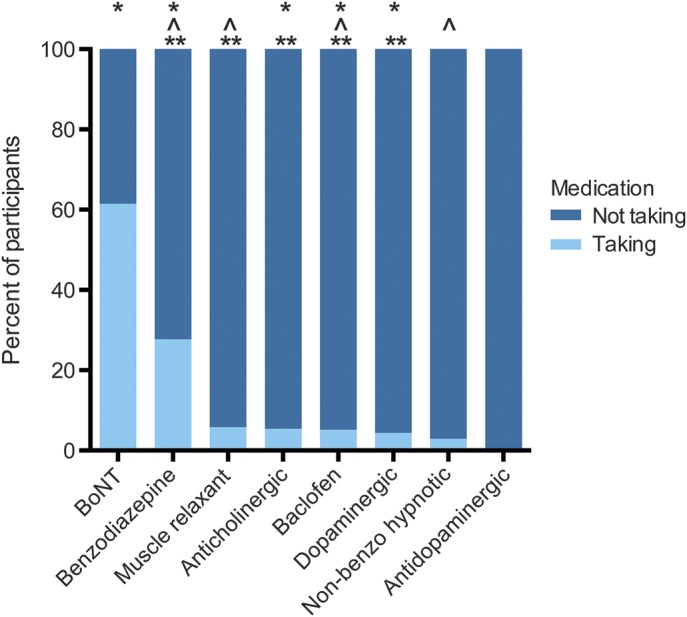
Percent of participants taking each class of medications. Participants may be taking more than one medication. Medication class use varied by dystonia type (*p < 0.05), by focal dystonia subtype (**p < 0.05), and by presence of anxiety/depression (^p < 0.05). BoNT = botulinum toxin.
Table 2.
Use of medication classes varied significantly by dystonia type and comorbid factors
Surgical therapy.
One hundred eighteen participants reported prior surgical therapy for dystonia symptoms. Of those, 67 patients reported non–deep brain stimulation (DBS) surgery, with selective denervation for cervical dystonia being the most common at 31%, followed by myectomy for blepharospasm at 18%.
Fifty-one patients had DBS: 34 (83%) in the globus pallidus interna, 3 (7%) in the subthalamic nucleus, and 2 (5%) in the thalamus and unknown categories each. Half of the DBS patients had generalized dystonia (51%), and 27% had focal dystonia (primarily cervical dystonia). Most patients who had DBS had a younger age at onset (<35 years of age, 53%), longer disease duration (20–74 years, 55%), and more severe disease (GDRS total 10–102, 75%; table e-2). Forty-four of the 51 patients reported current dystonia medication use, with 28 patients (55%) using BoNT alone or in combination with oral medications (table e-2).
DISCUSSION
In the largest cross-sectional study ever undertaken in patients with dystonia, we showed that 73% of patients with dystonia reported using medications that have been recommended for the treatment of dystonia. The lowest oral medication use was found in focal dystonia and the highest in generalized dystonia, and within focal dystonia subtype, the BoNT alone rate was highest in blepharospasm and spasmodic dysphonia (49%) and lowest in other cranial dystonia (32%). Although BoNT has FDA approval for dystonia symptoms, many patients were using off-label oral medications, highlighting potential limitations to our gold standard therapy for complete symptom control. However, even more interesting is how many patients were not on medications used for dystonia. We looked at the effect of surgical treatment, specifically DBS, on medication use patterns in this population. Despite receiving DBS, these patients were more likely to be using medications (both BoNT and oral medications) than the population as a whole, with only 14% of DBS patients reporting no medication use. Other factors such as the cost of medications, especially of BoNT, and the side effects of oral medications (e.g., the anticholinergics) may play a role in the results seen in this study. These results raise many questions about gaps in our current treatment portfolio and future opportunities to close this treatment gap.
Multiple publications summarize and list treatment options for dystonia.4–6 Generally, the most common oral medication classes identified in these reviews are anticholinergics, dopaminergics, GABAergics, and muscle relaxants, as included in our study. We acknowledge the limitations and lack of evidence-based prescribing strategies due to an absence of large, controlled studies, highlighted in systematic evidence-based reviews.7,8
There is no clear order for the introduction of medications in an individual patient with dystonia because of a lack of substantial comparative-effectiveness studies due to small population size (i.e., 4 patients) or to study design issues.9,10 For anticholinergics, however, the recommendation for use comes from a double-blind placebo-controlled trial, which showed benefit of high-dose anticholinergic medication in improving generalized dystonia.11 However, the practical use is limited by side effects such as impaired recall and slowing of cognition, especially in the older patient population.12 In a prospective, randomized, blinded trial, BoNT vs the anticholinergic medication trihexyphenidyl was tested, with blinding achieved by saline injection.13 BoNT was shown to be more effective than trihexyphenidyl in this trial and had fewer adverse effects than the anticholinergic medication. In our population, we found that overall only 5% of patients reported anticholinergic use, primarily in the generalized dystonia group representing 15.7% of the total anticholinergic use.
BoNT is the gold standard for the treatment of focal dystonia.14 Although a clear breakthrough in the treatment of focal dystonia, it has limitations, including that the injections themselves are painful. Expected adverse events include weakness and swallowing difficulty with neck injections. Finally, many patients report a decline in effect at 9.5 weeks after injection despite a typical 12-week-long injection cycle.15 Despite these limitations, BoNT was the most common medication used in this population, either alone or in combination with oral medications. For the subtypes of focal dystonia, BoNT use (either alone or in combination with oral medications) varied on the basis of the particular subtype, ranging from a high of 71% of patients with blepharospasm to 54% of patients with limb dystonia. No differences were seen in BoNT use by sex or by age at onset. Younger age was associated with more oral medication use, and conversely, older age was associated with higher oral medication plus BoNT use. Patients with disease duration <4 years were more likely to not be using any medications than those with longer disease duration or to be using oral medications alone than those with disease duration >4 years. Disease severity was related to medication use most clearly in the most severely affected patients, who were more likely to be using BoNT in combination with oral medications. The least severely affected patients more commonly reported BoNT use by itself or no medications at all. Overall, we found a large percentage of patients (39%) not using BoNT, the FDA-approved medication for most focal dystonias. This finding was unexpected and varied from the online survey results showing the majority of patients using BoNT in cervical dystonia.2 A follow-up study may be required to understand the reasons for the lower BoNT use reported in this large, multicenter, international cohort.
There are limitations to these data. First, the majority of patients enrolling in the Dystonia Coalition studies are going to tertiary care medical centers for treatment, which may bias toward an overreporting of medication use in comparison to the dystonia population as a whole because our study population may be actively seeking out therapeutic options. However, an international web-based survey in patients with cervical dystonia reported a higher use of BoNT at 86% of respondents and oral medication use at 53% than we found in our study.2 The true prevalence of dystonia by type and subtype may not be reflected in our data (i.e., a relatively higher rate of laryngeal dystonia than reported in the general population) because specific projects within the Dystonia Coalition seek out these patients to develop diagnostic and measurement tools for spasmodic dystonia. The dystonia disease severity may be greater in our study than in the dystonia population as a whole, again because they are coming to specialty centers, and we cannot know the characteristics of patients who were not contacted to enroll in the Dystonia Coalition or patients who declined to enroll. We broke down the analysis by severity to try to identify whether this might be related to medication use, and this is described above. In terms of medication use patterns by region or by subtype, there were small samples for some of the regions and subtypes, so we tempered our conclusions because without a larger population size it is difficult to generalize. Finally, in a cross-sectional analysis, we cannot determine what medications patients might have tried in the past, nor can we determine reasons for discontinuation of medications tried. This would be an important next step.
Overall, we found that most patients with dystonia are using medications, both oral and injectable, to treat dystonic symptoms. Medication use patterns varied by region, dystonia type, focal subtype, disease severity, and the presence of comorbid conditions. We also found that a significant number of patients are not reporting current medication use for their dystonia. This persisted across demographic variables, dystonia variables, and comorbid variables, with more than a quarter of all patients not using medications. This clearly points to a potential therapeutic gap. Future directions in clinical therapeutic research in dystonia range from innovative drug therapy to neurorestorative approaches (both noninvasive and invasive). In addition, the development of coherent symptomatic treatment strategies and the identification of biologic markers to monitor treatment response are needed.16 These future approaches should include patients with dystonia across the age span, across the spectrum of disease localization, and across the range of disease severity.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Jo Wright for her helpful assistance with the database extraction from the Dystonia Coalition database. They also thank Andra Kiscaden for her help with the figures.
GLOSSARY
- BoNT
botulinum toxin
- DBS
deep brain stimulation
- GDRS
Global Dystonia Rating Scale
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: Dystonia Coalition Investigators, Ami R Rosen, Mark Hallett, Vesper Fe Marie, Jung Park, Karin Mente, Hyunjoo Cho, Elaine Considine, Alfredo Berardelli, Gina Gerrazzano, Scott A Norris, Joel Perlmutter, Laura Wright, Anja Pogarcic, Joseph Jankovic, Laura Marsh, Farah Ismail, Lawrence Severt, Emily Muller, Ludy C Shih, Christine Ashton, Pinky Agarwal, Carey Gonzales, Zoltan Mari, Becky Dunlop, Julie Leegwater-Kim, Caitlin Scopa, Ron Alterman, Steve Frucht, Laurie Ozelius, Kristina Simonyan, Miodrag Velickovic, Ruth Walker, Joan Bratton, Daniel Troung, Trong-Tuong Binh Nguyen, Cynthia Comella, Tracy Waliczek, Tanya Harlow, Stephanie Gonzales, Destini Spaeth, Fatta Nahab, Lissette Moreno, Tao Xie, Joan Young, Alberto J. Espay, Jaya Mishra, Ben Wissel, Brian Berman, Erika Shelton, Irene Malaty, Ramon L. Rodriguez, Kyle Rizer, Amanda Elers, Ergun Uc, Jeri Sieren, Stephen Reich, Katherine Holmes, Richard L. Barbano, Michael Bull, Mark LeDoux, Misty Thompson, Claudia M Testa, Virginia Norris, Daniel Demers, Alison Brashear, Charlotte Miller, Victor SC Fung, Florence Chang, Jane M Griffith, Sylvian Chouinard, Monica Beland, Oksana Suchowersky, Paul McCann, Susan Fox, Brandon Rothberg, Marie Vidailhet, Emmanuel Roze, Cecilia Bonnet, Marta Ruiz, Bertrand Degos, Jean Michel Mayer, Kailash Bhatia, Bettina Balint, Christine Klein, Norbert Brüggemann, Sylwia Dankert, Johanna Junker, Anne Weibach, Natividad Stover, Ashlee Brooke Rawlins, Charles Alder, Amy Duffy, Stephen Grill, Erica Stacy, Joel Blumin, Lynn Wheeler, William Ondo, and Chia Arif
AUTHOR CONTRIBUTIONS
Dr. Pirio Richardson participated in the conception, study design, data analysis, interpretation of the results, and drafting of the manuscript and made a critical revision of the manuscript. A.R. Wegele participated in the conception, study design, data analysis, interpretation of the results, drafting of the manuscript, and revising the manuscript. Dr. Skipper contributed to the data analysis, interpretation of results, and drafting of the manuscript. Dr. Deligtisch and Dr. Jinnah contributed to the interpretation of the results and made a critical revision of the manuscript.
STUDY FUNDING
This study was supported in part by a grant to the Dystonia Coalition (U54 TR001456 and NS065701) from the Office of Rare Diseases Research of the National Center for Advancing Translational Sciences and the National Institute of Neurological Disorders and Stroke. The project was also supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the NIH through grants KL2 1TR001448-01 and UL1 TR000041.
DISCLOSURE
S. Pirio Richardson is supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the NIH through grants KL2 1TR001448-01 and UL1 TR000041. A. Wegele, B. Skipper, and A. Deligtisch report no disclosures relevant to the manuscript. H. Jinnah reports support in part by a grant to the Dystonia Coalition (U54 TR001456 and NS065701) from the Office of Rare Diseases Research of the National Center for Advancing Translational Sciences and the National Institute of Neurological Disorders and Stroke. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Balint B, Bhatia KP. Dystonia: an update on phenomenology, classification, pathogenesis and treatment. Curr Opin Neurol 2014;27:468–476. [DOI] [PubMed] [Google Scholar]
- 2.Comella C, Bhatia K. An international survey of patients with cervical dystonia. J Neurol 2015;262:837–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Comella CL, Leurgans S, Wuu J, Stebbins GT, Chmura T; Dystonia Study Group. Rating scales for dystonia: a multicenter assessment. Mov Disord 2003;18:303–312. [DOI] [PubMed] [Google Scholar]
- 4.Jankovic J. Medical treatment of dystonia. Mov Disord 2013;28:1001–1012. [DOI] [PubMed] [Google Scholar]
- 5.Dressler D, Altenmueller E, Bhidayasiri R, et al. Strategies for treatment of dystonia. J Neural Transm (Vienna) 2016;123:251–258. [DOI] [PubMed] [Google Scholar]
- 6.Jinnah HA, Factor SA. Diagnosis and treatment of dystonia. Neurol Clin 2015;33:77–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albanese A, Barnes MP, Bhatia KP, et al. A systematic review on the diagnosis and treatment of primary (idiopathic) dystonia and dystonia plus syndromes: report of an EFNS/MDS-ES Task Force. Eur J Neurol 2006;13:433–444. [DOI] [PubMed] [Google Scholar]
- 8.Balash Y, Giladi N. Efficacy of pharmacological treatment of dystonia: evidence-based review including meta-analysis of the effect of botulinum toxin and other cure options. Eur J Neurol 2004;11:361–370. [DOI] [PubMed] [Google Scholar]
- 9.Smania N, Corato E, Tinazzi M, Montagnana B, Fiaschi A, Aglioti SM. The effect of two different rehabilitation treatments in cervical dystonia: preliminary results in four patients. Funct Neurol 2003;18:219–225. [PubMed] [Google Scholar]
- 10.Yoshor D, Hamilton WJ, Ondo W, Jankovic J, Grossman RG. Comparison of thalamotomy and pallidotomy for the treatment of dystonia. Neurosurgery 2001;48:818–824. [DOI] [PubMed] [Google Scholar]
- 11.Burke RE, Fahn S, Marsden CD. Torsion dystonia: a double-blind, prospective trial of high-dosage trihexyphenidyl. Neurology 1986;36:160–164. [DOI] [PubMed] [Google Scholar]
- 12.Taylor AE, Lang AE, Saint-Cyr JA, Riley DE, Ranawaya R. Cognitive processes in idiopathic dystonia treated with high-dose anticholinergic therapy: implications for treatment strategies. Clin Neuropharmacol 1991;14:62–77. [DOI] [PubMed] [Google Scholar]
- 13.Brans JA, Lindeboom R, Snoek JW, et al. Botulinum toxin versus trihexyphenidyl in cervical dystonia: a prospective, randomized, double-blind controlled trial. Neurology 1996;46:1066–1072. [DOI] [PubMed] [Google Scholar]
- 14.Brashear A. Botulinum toxin type A in the treatment of patients with cervical dystonia. Biologics 2009;3:1–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Sethi KD, Rodriguez R, Olayinka B. Satisfaction with botulinum toxin treatment: a cross-sectional survey of patients with cervical dystonia. J Med Econ 2012;15:419–423. [DOI] [PubMed] [Google Scholar]
- 16.Albanese A, Romito LM, Calandrella D. Therapeutic advances in dystonia. Mov Disord 2015;30:1547–1556. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.