Abstract
Background
P-selectin and thrombospondin1 (TSP1) have been suggested as counter ligands that may mediate GPIbα-dependent thrombus growth independently of VWF in vitro. However, residual thrombus formation still persists in Vwf −/−Tsp1−/− mice suggesting existence of other mechanisms that modulates thrombus propagation.
Objective
We determined whether P-selectin modulates thrombus propagation in injured arterioles independently of TSP1 and VWF.
Methods
CD-62P blocking antibody in Vwf −/−Tsp1−/− mice was used to inhibit P-selectin. We determined thrombus growth kinetics in two models of thrombosis: FeCl3 injury-induced and laser injury-induced thrombosis.
Results
In a 10% FeCl3 injury-induced thrombosis model, the initial platelet adhesion, time to form 1st thrombus, and non-occlusive residual thrombus growth kinetics were comparable between P-selectin-blocking antibody-treated Vwf −/−Tsp1−/− mice and control-IgG treated Vwf −/−Tsp1−/− mice. On the other hand, in a laser injury-induced thrombosis model, residual thrombus growth kinetics was significantly decreased in P-selectin-blocking antibody-treated Vwf −/−Tsp1−/− mice (P< 0.05 versus control-IgG treated Vwf −/−Tsp1−/− mice). Because P-selectin has been suggested as a counter ligand for platelet GPIbα, we determined the role of GPIbα in laser injury-induced thrombosis model. Surprisingly, in a laser injury model, unlike FeCl3 injury model, thrombus formation was not completely inhibited in IL4Rα/GPIbα-tg mice. Residual thrombus growth kinetics were comparable between P-selectin-blocking antibody-treated IL4Rα/GPIbα-tg mice and control-IgG-treated IL4Rα/GPIbα-tg mice. Comparison of slopes over time showed that residual thrombus growth kinetics were comparable in P-selectin-blocking antibody-treated Vwf −/−Tsp1−/− and control IgG-treated IL4Rα/GPIbα-tg mice
Conclusion
In laser injury-induced thrombosis model, P-selectin modulates thrombus propagation independently of VWF and TSP1.
Keywords: von Willebrand factor, Thrombospondin 1, P-selectin, arterial thrombosis, platelets
Introduction
The interaction between platelet GPIbα and its main ligand VWF plays an important role in mediating platelet adhesion/thrombus propagation at sites of vascular injury [1–4]. For several years the conventional view was that GPIbα binding to VWF in the extracellular matrix is the first step required for initial tethering of platelets to the injured vessel wall, particularly under arterial shear conditions [5, 6]. However, platelet adhesion under arterial shear conditions was completely absent in mice lacking platelet GPIbα, but not in mice lacking VWF in the FeCl3-injury induced thrombosis model, suggesting that other collagen receptors, such as GPVI, α2bβ1, cannot support platelet tethering/adhesion to the injured vessel wall in absence of GPIbα [3]. These findings suggest the existence of alternative ligands for GPIbα other than VWF that mediate initial platelet tethering/adhesion and subsequent thrombus formation. TSP1 was suggested as an adhesive substrate that may mediate GPIbα-dependent platelet adhesion/thrombus formation independently of VWF [7]. In contrast, utilizing Vwf −/−Tsp1−/− mice, we found that TSP1 does not contribute to initial platelet adhesion/thrombus formation independently of VWF [8]. It has been suggested that P-selectin, which is expressed on activated platelets and endothelial cells, can act as a counter ligand for GPIbα, and, thereby may contribute to platelet adhesion/thrombus formation independently of VWF [9, 10]. Herein, we determined whether P-selectin modulates thrombus propagation in injured arterioles independently of both VWF and TSP1.
Materials and Methods
Animals
Vwf −/− [1], Tsp1−/− [11], Vwf −/−/Tsp1−/− [8], IL4Rα/GPIbα-tg [12] and WT littermate mice (4–5 weeks in age) on the C57BL/6J background were used in the study. The University of Iowa Animal Care and Use Committee approved all experiments.
Intravital microscopy of FeCl3 injury and laser injury-induced mesenteric thrombosis
Intravital microscopy was done as described [8, 13, 14]. Briefly, fluorescent platelets labeled with calcein green (1.25 X 109 platelets/kg) were infused in anaesthetized mice through the retro-orbital plexus. Platelets for infusion were isolated from adult (4–6 months) donor mice of the same genotype. Arterioles of diameter approximately 60–100 μm with shear rates of ~1300–1800 s−1 were used for the experiment. FeCl3 injury-induced thrombosis: Whatman paper saturated with FeCl3 (10%) solution was applied topically for 3 minutes and thrombus formation in the injured vessel was monitored in real time by using Nikon inverted microscope. Each injured vessel was recorded by using high-speed EM camera for 40 minutes or until occlusion. Laser injury-induced thrombosis: The injury was initiated in the arterioles using a micropoint laser ablation system. The precise illumination of the area of interest was done through the microscope eyepiece. The wavelength of light used for illumination was in the range of 365–400 nm with maximum output of 50–500 μJ. The power and frequency of bursts were controlled by software and empirically defined. Thrombus formation in the injured vessel (4) was monitored in real time by using a Nikon upright microscope with a Plan Fluor 10X/0.3 objective and thrombus growth was recorded using a high-speed EM camera for 4 minutes. In experimental conditions set up in our lab, in the laser injury-induced thrombosis model, thrombus grows to its maximum size in approximately 1 minute, and then gradually disintegrates in the following 3–4 minutes. Videos were evaluated offline using a Nikon computer-assisted image analysis program.
Statistics
For statistical analysis, Graph Pad Prism (version 7.0) was used. Statistical comparisons were performed using either unpaired, two-tailed, Student’s t test or non-linear regression analysis (comparison of two slopes). P<0.05 was considered statistically significant.
Results and Discussion
We utilized FeCl3 injury-induced thrombosis and laser injury-induced thrombosis models to determine whether P-selectin modulates thrombus propagation at arterial shear rates independently of both VWF and TSP1. P-selectin was inhibited in Vwf −/−Tsp1−/− mice by intravenous injection of anti-mouse CD-62P blocking antibody (RB40.34, no azide; 2 μg/g of body weight) as described [13, 15]. Identical dose of rat immunoglobulin (IgG) was used as control. Inhibition of P-selectin did not affect complete blood counts (not shown).
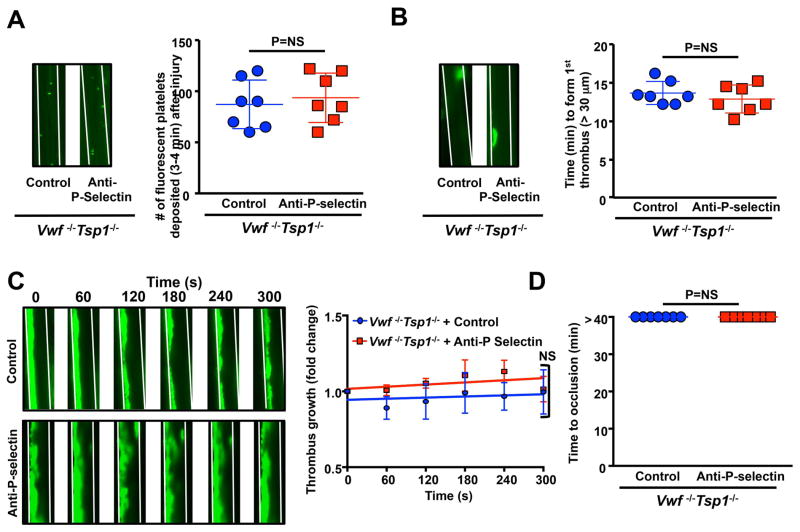
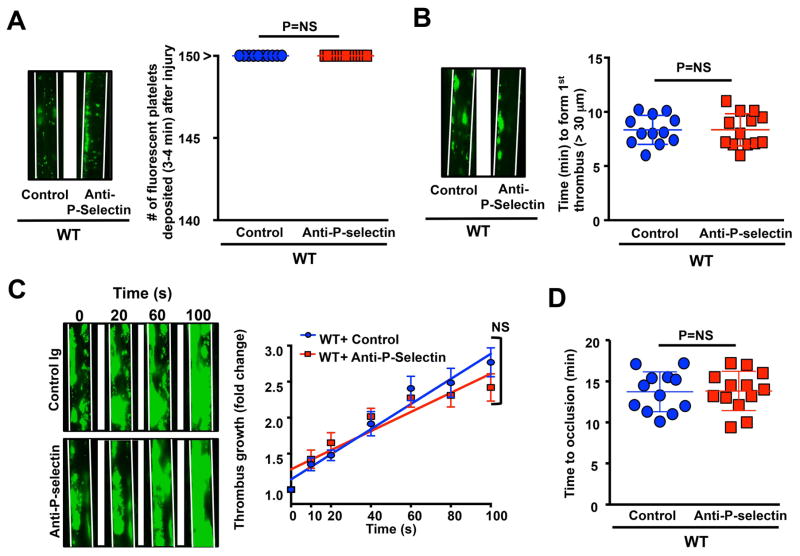
In 10% FeCl3 injury-induced thrombosis model, we found that initial platelet adhesion to injured arterioles was similar in P-selectin-blocking antibody-treated Vwf −/−Tsp1−/− mice compared with control-IgG-treated Vwf −/−Tsp1−/− mice (Figure 1A). We speculated that P-selectin might support thrombus propagation, but not platelet adhesion, via other mechanisms independently of VWF and TSP1 based on following studies. These include: 1) recruitment of tissue bearing microparticles in growing thrombus [16], 2) shear-dependent platelet aggregation [17], and 3) via its soluble form [18]. We found that time to form 1st thrombus, and non-occlusive thrombus growth kinetics were similar in P-selectin-blocking antibody-treated Vwf −/−Tsp1−/− mice when compared with control-IgG-treated Vwf −/−Tsp1−/− mice (Figure 1BC). As expected none of the vessels occluded in P-selectin-blocking antibody-treated Vwf −/−Tsp1−/− mice or control-IgG-treated Vwf −/−Tsp1−/− mice (Figure 1D) because it is known that VWF is required for occlusive thrombus growth under arterial and venous shear conditions [1, 2, 4, 19]. Similarly, P-selectin inhibition did not affect initial platelet adhesion and thrombus growth rate in WT mice (Figure 2). Together these results suggest that, in a 10% FeCl3 injury model, P-selectin is not essential for thrombus propagation.
Figure 1.
Figure 2.
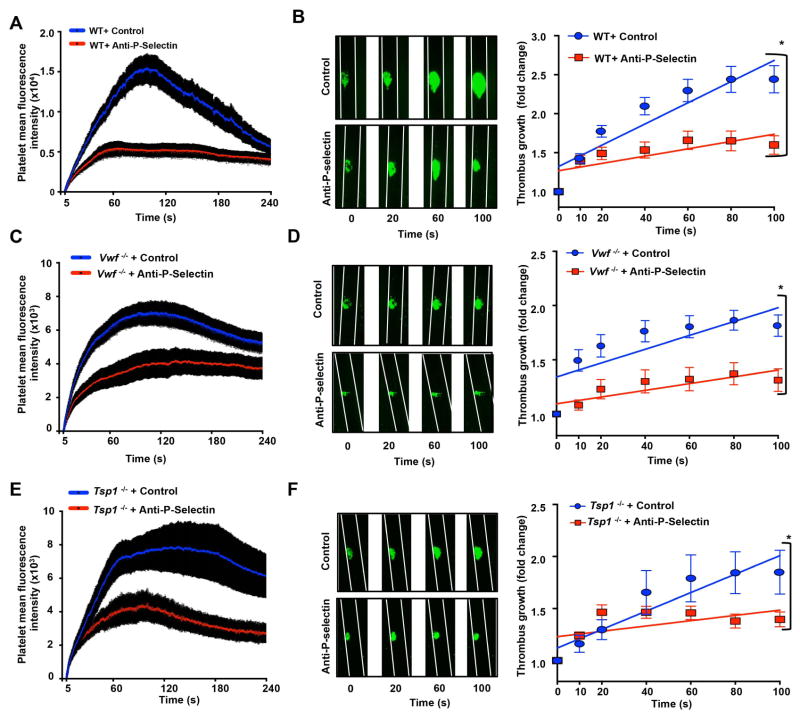
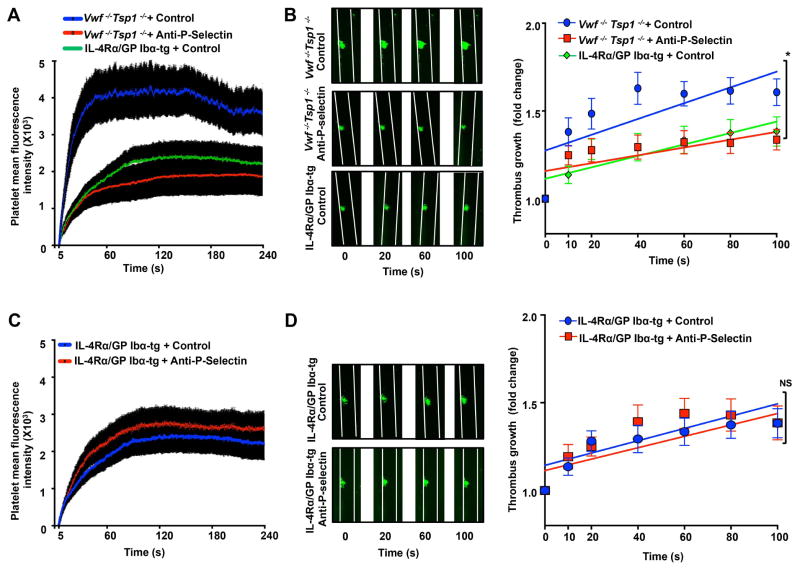
In laser injury-induced thrombosis model, we found that inhibition of P-selectin in WT mice significantly decreased platelet mean fluorescence intensity (MFI) and rate of thrombus growth (Figure 3AB). Our findings are consistent with previous studies that have suggested that P-selectin was incorporated within the thrombus and modulates thrombus propagation in laser injury-model [16]. Furthermore, in the Vwf −/−, Tsp1−/−, and Vwf −/−Tsp1−/− mice, we found that platelet MFI was markedly, but not completely, reduced when compared to WT mice (Figure 3) suggesting that both VWF and TSP1 contribute to thrombus propagation in laser injury-induced thrombosis model. Because residual thrombus was present in Vwf −/−, Tsp1−/−, and Vwf −/−Tsp1−/− mice, it was feasible to determine whether P-selectin modulates thrombus propagation independently of VWF or TSP1 or both. Platelet MFI and thrombus growth rate were decreased in P-selectin-blocking antibody-treated Vwf −/− mice (P<0.05 versus control-IgG-treated Vwf −/− mice; Figure 3CD), P-selectin-blocking antibody-treated Tsp1−/− mice (P<0.05 versus control-IgG-treated Tsp1−/− mice; Figure 3EF), and P-selectin-blocking antibody-treated Vwf −/−Tsp1−/− mice (P<0.05 vs. control-IgG-treated Vwf −/−Tsp1−/− mice; Figure 4AB). These findings suggest that, at arterial shear rate, P-selectin can contribute to thrombus propagation on the activated endothelium independently of VWF and TSP1.
Figure 3.
Figure 4.
Because P-selectin has been suggested as a counter ligand for platelet GPIbα[9], we next determined whether GPIbα is absolutely required to form thrombus in laser injury-induced thrombosis model. We utilized GPIbα/human IL4R transgenic (IL4Rα/GPIbα-tg) mice, which lack murine GPIbα, but express the extracellular domain of the human interleukin-4 (IL-4) receptor fused to the transmembrane and cytoplasmic domains of human GPIbα[12]. IL4Rα/GPIbα-tg mice have a 2-fold increase in circulating platelet count and a 50% reduction in platelet size when compared to platelets from GPIbα−/−, a mouse model of Bernard-Soulier syndrome [12]. Surprisingly, unlike FeCl3 injury model, in a laser injury model thrombus formation was not completely inhibited in IL4Rα-GPIbα-tg mice (Figure 4AB). Residual thrombus growth kinetics were comparable between P-selectin-blocking antibody-treated IL4Rα/GPIbα-tg mice and control-IgG-treated IL4Rα/GPIbα-tg mice (Figure 4CD). This result suggests that ligands other than P-selectin and GPIbα initiate thrombus formation in laser injury model (Figure 4CD). One of the possible mechanisms could be neutrophil interaction with activated endothelial cells, through LFA-1/ICAM1, independently of platelet GPIbα and P-selectin. Such a mechanism has been proposed as the first step that initiates fibrin generation before propagation of platelet thrombus in a laser injury model [20]. When we compared slopes over time, we found that thrombus growth kinetics were comparable in P-selectin-blocking antibody-treated Vwf −/−Tsp1−/− mice (Slope: 0.00223 ± 0.00064) and control IgG-treated IL4Rα/GPIbα-tg mice (Slope: 0.00246 ± 0.00062; Figure 4B). Previous studies suggest that platelets GPIbα-P-selectin interaction can support platelet interaction on activated endothelium at venous shear rate [9, 10]. Although not proved in this study, it is possible that GPIbα-P-selectin interaction may support thrombus propagation independently of both VWF and TSP1. Further studies using a specific inhibitor of P-selectin/GPIbα interaction or mutant mice are warranted to confirm these findings. The possibility that there may be additional mechanisms by which P-selectin may modulate thrombus propagation independently of VWF and TSP1 could not be ruled out. For example, a study in baboon has shown that blocking platelet-leukocyte interactions by anti P-selectin antibodies inhibits fibrin deposition in growing thrombus [21]. Another study showed that P-selectin interaction with an unknown ligand different from PSGL-1 or GPIbα could stabilize initial αIIβ3-fibrinogen interactions resulting in formation of stable platelet aggregates in vitro [22].
Our studies have few limitations. First, our conclusions that P-selectin modulates thrombus propagation independently of VWF and TSP1 are based on laser injury-induced thrombosis model. Second, we did not define the role of endothelial P-selectin versus platelet P-selectin in thrombus propagation independently of VWF and TSP1. Further studies utilizing bone-marrow experiments from P-sel−/−Vwf −/−Tsp1−/− are warranted. Third, under what pathological conditions, such mechanism exists remain unclear. It is possible that P-selectin may contribute to platelet aggregation independently of VWF and TSP1 in certain conditions such as Helicobacter pylori infection [23].
In conclusion, our studies provide the first in vivo evidence that P-selectin on activated platelets and/or endothelial cells can provide a backup mechanism to VWF and TSP1 in recruiting and activating quiescent platelets, thereby promoting thrombus propagation.
Essentials.
The main receptor for platelet glycoprotein (GP) Ibα is von Willebrand factor (VWF).
P-selectin and thrombospondin1 (TSP1) have been suggested as counter receptors for GPIbα.
In laser injury model, P-selectin promotes thrombus propagation independently of VWF and TSP1.
In laser injury model, thrombus persists in interleukin-4 receptor α (IL4α)/GPIbα-transgenic mice.
Acknowledgments
A. Chauhan laboratory is supported by the following grants-AHA 16IRG27490003 and NHLBI-NIH R01 HL118246 and R01 HL118742.
Footnotes
Addendum
P. Prakash and M. Nayak performed experiments, analyzed, interpreted results and co-wrote the manuscript. A. Chauhan designed the study, interpreted results, and co-wrote the manuscript.
Disclosures of Conflicts of Interest
The authors state that they have no conflict of interest.
References
- 1.Denis C, Methia N, Frenette PS, Rayburn H, Ullman-Cullere M, Hynes RO, Wagner DD. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc Natl Acad Sci USA. 1998;95:9524–9. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ni H, Denis CV, Subbarao S, Degen JL, Sato TN, Hynes RO, Wagner DD. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J Clin Invest. 2000;106:385–92. doi: 10.1172/JCI9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergmeier W, Piffath CL, Goerge T, Cifuni SM, Ruggeri ZM, Ware J, Wagner DD. The role of platelet adhesion receptor GPIbalpha far exceeds that of its main ligand, von Willebrand factor, in arterial thrombosis. Proc Natl Acad Sci USA. 2006;103:16900–5. doi: 10.1073/pnas.0608207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chauhan AK, Kisucka J, Lamb CB, Bergmeier W, Wagner DD. von Willebrand factor and factor VIII are independentlyly required to form stable occlusive thrombi in injured veins. Blood. 2007;109:2424–9. doi: 10.1182/blood-2006-06-028241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007;100:1673–85. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 6.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–97. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 7.Jurk K, Clemetson KJ, de Groot PG, Brodde MF, Steiner M, Savion N, Varon D, Sixma JJ, Van Aken H, Kehrel BE. Thrombospondin-1 mediates platelet adhesion at high shear via glycoprotein Ib (GPIb): an alternative/backup mechanism to von Willebrand factor. FASEB J. 2003;17:1490–2. doi: 10.1096/fj.02-0830fje. [DOI] [PubMed] [Google Scholar]
- 8.Prakash P, Kulkarni PP, Chauhan AK. Thrombospondin 1 requires von Willebrand factor to modulate arterial thrombosis in mice. Blood. 2015;125:399–406. doi: 10.1182/blood-2014-06-581942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romo GM, Dong JF, Schade AJ, Gardiner EE, Kansas GS, Li CQ, McIntire LV, Berndt MC, Lopez JA. The glycoprotein Ib-IX-V complex is a platelet counterreceptor for P-selectin. J Exp Med. 1999;190:803–14. doi: 10.1084/jem.190.6.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katayama T, Ikeda Y, Handa M, Tamatani T, Sakamoto S, Ito M, Ishimura Y, Suematsu M. Immunoneutralization of glycoprotein Ibalpha attenuates endotoxin-induced interactions of platelets and leukocytes with rat venular endothelium in vivo. Circ Res. 2000;86:1031–7. doi: 10.1161/01.res.86.10.1031. [DOI] [PubMed] [Google Scholar]
- 11.Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest. 1998;101:982–92. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanaji T, Russell S, Ware J. Amelioration of the macrothrombocytopenia associated with the murine Bernard-Soulier syndrome. Blood. 2002;100:2102–7. doi: 10.1182/blood-2002-03-0997. [DOI] [PubMed] [Google Scholar]
- 13.Chauhan AK, Kisucka J, Brill A, Walsh MT, Scheiflinger F, Wagner DD. ADAMTS13: a new link between thrombosis and inflammation. J Exp Med. 2008;205:2065–74. doi: 10.1084/jem.20080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prakash P, Kulkarni PP, Lentz SR, Chauhan AK. Cellular fibronectin containing extra domain A promotes arterial thrombosis in mice through platelet Toll-like receptor 4. Blood. 2015;125:3164–72. doi: 10.1182/blood-2014-10-608653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin H, Gebska MA, Blokhin IO, Wilson KM, Ketsawatsomkron P, Chauhan AK, Keen HL, Sigmund CD, Lentz SR. Endothelial PPAR-gamma protects against vascular thrombosis by downregulating P-selectin expression. Arterioscler Thromb Vasc Biol. 2015;35:838–44. doi: 10.1161/ATVBAHA.115.305378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falati S, Liu Q, Gross P, Merrill-Skoloff G, Chou J, Vandendries E, Celi A, Croce K, Furie BC, Furie B. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197:1585–98. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merten M, Chow T, Hellums JD, Thiagarajan P. A new role for P-selectin in shear-induced platelet aggregation. Circulation. 2000;102:2045–50. doi: 10.1161/01.cir.102.17.2045. [DOI] [PubMed] [Google Scholar]
- 18.Andre P, Hartwell D, Hrachovinova I, Saffaripour S, Wagner DD. Pro-coagulant state resulting from high levels of soluble P-selectin in blood. Proc Natl Acad Sci USA. 2000;97:13835–40. doi: 10.1073/pnas.250475997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Kollnberger M, Wakefield TW, Lammle B, Massberg S, Wagner DD. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400–7. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darbousset R, Thomas GM, Mezouar S, Frere C, Bonier R, Mackman N, Renne T, Dignat-George F, Dubois C, Panicot-Dubois L. Tissue factor-positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood. 2012;120:2133–43. doi: 10.1182/blood-2012-06-437772. [DOI] [PubMed] [Google Scholar]
- 21.Palabrica T, Lobb R, Furie BC, Aronovitz M, Benjamin C, Hsu YM, Sajer SA, Furie B. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature. 1992;359:848–51. doi: 10.1038/359848a0. [DOI] [PubMed] [Google Scholar]
- 22.Merten M, Thiagarajan P. P-selectin expression on platelets determines size and stability of platelet aggregates. Circulation. 2000;102:1931–6. doi: 10.1161/01.cir.102.16.1931. [DOI] [PubMed] [Google Scholar]
- 23.Yeh JJ, Tsai S, Wu DC, Wu JY, Liu TC, Chen A. P-selectin-dependent platelet aggregation and apoptosis may explain the decrease in platelet count during Helicobacter pylori infection. Blood. 2010;115:4247–53. doi: 10.1182/blood-2009-09-241166. [DOI] [PubMed] [Google Scholar]