Abstract
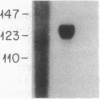
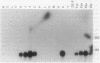
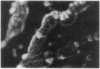
The trefoil peptide family encompasses a group of small proteins that appear to assume a distinctive secondary structure that leads to intrinsic resistance to protease digestion. Induction of these peptides has been associated with response to injury in the gastrointestinal tract and related organs. Using an oligonucleotide derived from N-terminal amino acid sequencing of a transformed growth-inhibiting protein, a cDNA was cloned from rat intestinal villus epithelial cells that encodes a protein 81 amino acids in length with the characteristic trefoil peptide cysteine residue motif. Northern blot analysis demonstrates specific expression of a single transcript of 0.43 kilobase in small and large intestinal epithelium in rat and man. Indirect immunofluorescent staining with antiserum raised using a synthetic peptide based on the predicted C-terminal sequence of this protein, designated intestinal trefoil factor, demonstrated that it is primarily expressed and secreted onto the intestinal surface by goblet cells, suggesting that it may be an important component of intrinsic mechanisms for defending mucosal integrity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnard J. A., Beauchamp R. D., Coffey R. J., Moses H. L. Regulation of intestinal epithelial cell growth by transforming growth factor type beta. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1578–1582. doi: 10.1073/pnas.86.5.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Goustin A. S., Soderquist A. M., Shipley G. D., Wolfshohl J., Carpenter G., Moses H. L. Transforming growth factor alpha and beta expression in human colon cancer lines: implications for an autocrine model. Cancer Res. 1987 Sep 1;47(17):4590–4594. [PubMed] [Google Scholar]
- Coffey R. J., Jr, Shipley G. D., Moses H. L. Production of transforming growth factors by human colon cancer lines. Cancer Res. 1986 Mar;46(3):1164–1169. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen E. K., Jørgensen K. H., Thim L. Receptor binding of pancreatic spasmolytic polypeptide (PSP) in rat intestinal mucosal cell membranes inhibits the adenylate cyclase activity. Regul Pept. 1986 Dec 30;16(3-4):291–297. doi: 10.1016/0167-0115(86)90028-5. [DOI] [PubMed] [Google Scholar]
- Gonnella P. A., Siminoski K., Murphy R. A., Neutra M. R. Transepithelial transport of epidermal growth factor by absorptive cells of suckling rat ileum. J Clin Invest. 1987 Jul;80(1):22–32. doi: 10.1172/JCI113051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hauser F., Gertzen E. M., Hoffmann W. Expression of spasmolysin (FIM-A.1): an integumentary mucin from Xenopus laevis. Exp Cell Res. 1990 Aug;189(2):157–162. doi: 10.1016/0014-4827(90)90230-8. [DOI] [PubMed] [Google Scholar]
- Hoffmann W. A new repetitive protein from Xenopus laevis skin highly homologous to pancreatic spasmolytic polypeptide. J Biol Chem. 1988 Jun 5;263(16):7686–7690. [PubMed] [Google Scholar]
- Hoosein N. M., Thim L., Jørgensen K. H., Brattain M. G. Growth stimulatory effect of pancreatic spasmolytic polypeptide on cultured colon and breast tumor cells. FEBS Lett. 1989 Apr 24;247(2):303–306. doi: 10.1016/0014-5793(89)81357-2. [DOI] [PubMed] [Google Scholar]
- Jeltsch J. M., Roberts M., Schatz C., Garnier J. M., Brown A. M., Chambon P. Structure of the human oestrogen-responsive gene pS2. Nucleic Acids Res. 1987 Feb 25;15(4):1401–1414. doi: 10.1093/nar/15.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen K. D., Diamant B., Jørgensen K. H., Thim L. Pancreatic spasmolytic polypeptide (PSP): III. Pharmacology of a new porcine pancreatic polypeptide with spasmolytic and gastric acid secretion inhibitory effects. Regul Pept. 1982 Mar;3(3-4):231–243. doi: 10.1016/0167-0115(82)90128-8. [DOI] [PubMed] [Google Scholar]
- Jørgensen K. H., Thim L., Jacobsen H. E. Pancreatic spasmolytic polypeptide (PSP): I. Preparation and initial chemical characterization of a new polypeptide from porcine pancreas. Regul Pept. 1982 Mar;3(3-4):207–219. doi: 10.1016/0167-0115(82)90126-4. [DOI] [PubMed] [Google Scholar]
- Koyama S. Y., Podolsky D. K. Differential expression of transforming growth factors alpha and beta in rat intestinal epithelial cells. J Clin Invest. 1989 May;83(5):1768–1773. doi: 10.1172/JCI114080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokowa M., Lynch K., Podolsky D. K. Effects of growth factors on an intestinal epithelial cell line: transforming growth factor beta inhibits proliferation and stimulates differentiation. Biochem Biophys Res Commun. 1987 Feb 13;142(3):775–782. doi: 10.1016/0006-291x(87)91481-1. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Pleskow D. K., Jafari H. Latent transformed growth-inhibiting factor in human malignant effusions. Cancer Res. 1988 Jan 15;48(2):418–424. [PubMed] [Google Scholar]
- Quaroni A., Wands J., Trelstad R. L., Isselbacher K. J. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol. 1979 Feb;80(2):248–265. doi: 10.1083/jcb.80.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Daniel J. Y., Tomasetto C., Lathe R., Chenard M. P., Batzenschlager A., Chambon P. Breast cancer-associated pS2 protein: synthesis and secretion by normal stomach mucosa. Science. 1988 Aug 5;241(4866):705–708. doi: 10.1126/science.3041593. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Chambon P. The pS2 gene, mRNA, and protein: a potential marker for human breast cancer. Cancer Cells. 1990 Aug-Sep;2(8-9):269–274. [PubMed] [Google Scholar]
- Rio M. C., Chenard M. P., Wolf C., Marcellin L., Tomasetto C., Lathe R., Bellocq J. P., Chambon P. Induction of pS2 and hSP genes as markers of mucosal ulceration of the digestive tract. Gastroenterology. 1991 Feb;100(2):375–379. doi: 10.1016/0016-5085(91)90205-y. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheving L. A., Shiurba R. A., Nguyen T. D., Gray G. M. Epidermal growth factor receptor of the intestinal enterocyte. Localization to laterobasal but not brush border membrane. J Biol Chem. 1989 Jan 25;264(3):1735–1741. [PubMed] [Google Scholar]
- Seo M. K., Lynch K. E., Podolsky D. K. Multiplicity of transforming growth factors in human malignant effusions. Cancer Res. 1988 Apr 1;48(7):1792–1797. [PubMed] [Google Scholar]
- Suemori S., Ciacci C., Podolsky D. K. Regulation of transforming growth factor expression in rat intestinal epithelial cell lines. J Clin Invest. 1991 Jun;87(6):2216–2221. doi: 10.1172/JCI115256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thim L. A new family of growth factor-like peptides. 'Trefoil' disulphide loop structures as a common feature in breast cancer associated peptide (pS2), pancreatic spasmolytic polypeptide (PSP), and frog skin peptides (spasmolysins). FEBS Lett. 1989 Jun 19;250(1):85–90. doi: 10.1016/0014-5793(89)80690-8. [DOI] [PubMed] [Google Scholar]
- Thim L., Jørgensen K. H., Jørgensen K. D. Pancreatic spasmolytic polypeptide (PSP): II. Radioimmunological determination of PSP in porcine tissues, plasma and pancreatic juice. Regul Pept. 1982 Mar;3(3-4):221–230. doi: 10.1016/0167-0115(82)90127-6. [DOI] [PubMed] [Google Scholar]
- Thim L., Thomsen J., Christensen M., Jørgensen K. H. The amino acid sequence of pancreatic spasmolytic polypeptide. Biochim Biophys Acta. 1985 Mar 1;827(3):410–418. doi: 10.1016/0167-4838(85)90226-2. [DOI] [PubMed] [Google Scholar]
- Tomasetto C., Rio M. C., Gautier C., Wolf C., Hareuveni M., Chambon P., Lathe R. hSP, the domain-duplicated homolog of pS2 protein, is co-expressed with pS2 in stomach but not in breast carcinoma. EMBO J. 1990 Feb;9(2):407–414. doi: 10.1002/j.1460-2075.1990.tb08125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Wright N. A., Pike C., Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990 Jan 4;343(6253):82–85. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G. W., Hall P. A., Jeffery R. E., Longcroft J. M., Rio M. C., Tomasetto C., Chambon P. Epidermal growth factor (EGF/URO) induces expression of regulatory peptides in damaged human gastrointestinal tissues. J Pathol. 1990 Dec;162(4):279–284. doi: 10.1002/path.1711620402. [DOI] [PubMed] [Google Scholar]