Abstract
Rationale
In heart failure, myofilament proteins display abnormal phosphorylation, which contributes to contractile dysfunction. The mechanisms underlying the dysregulation of protein phosphorylation on myofilaments is not clear.
Objective
This study aims to understand the mechanisms underlying altered phosphorylation of myofilament proteins in heart failure.
Methods and Results
We generate a novel genetically encoded protein kinase A (PKA) biosensor anchored onto the myofilaments in rabbit cardiac myocytes to examine PKA activity at the myofilaments in responses to adrenergic stimulation. We show that PKA activity is shifted from the sarcolemma to the myofilaments in hypertrophic failing rabbit myocytes. In particular, the increased PKA activity on the myofilaments is due to an enhanced β2 adrenergic receptor (β2AR) signal selectively directed to the myofilaments together with a reduced phosphodiesterase activity associated with the myofibrils. Mechanistically, the enhanced PKA activity on the myofilaments is associated with downregulation of caveolin-3 in the hypertrophic failing rabbit myocytes. Reintroduction of caveolin-3 in the failing myocytes is able to normalize the distribution of β2AR signal by preventing PKA signal access to the myofilaments, and to restore contractile response to adrenergic stimulation.
Conclusions
In hypertrophic rabbit myocytes, selectively enhanced β2AR signaling toward the myofilaments contributes to elevated PKA activity and PKA phosphorylation of myofilament proteins. Reintroduction of caveolin-3 is able to confine β2AR signaling and restore myocyte contractility in response to β-adrenergic stimulation.
Keywords: Heart failure, myofilament protein, PKA, adrenergic receptor, protein kinase A phosphorylation
Subject Terms: Heart Failure Basic, Science Research
INTRODUCTION
In animal hearts, elevated sympathetic activity stimulates the β-adrenergic signal to promote inotropy and lusitropy via the concerted PKA phosphorylation of substrates at multiple subcellular locations. PKA is spatially and temporally regulated through a set of A-kinase anchoring protein complexes.1 As a result, the precise PKA phosphorylation of myofilament proteins such as cardiac troponin I (TnI) and myosin binding protein C (MyBP-C) and Ca2+-handling proteins such as phospholamban (PLB) and ryanodine receptor on the sarcoplasmic reticulum (SR) is necessary to coordinate positive inotropic and lusitropic cardiac effects.2, 3
Chronic adrenergic signaling also promotes structural and functional remodeling in the myocardium, which contributes to cardiac hypertrophy and eventually heart failure in a variety of clinical conditions. A desensitized cardiac βAR signaling pathway,4 a hallmark of the failing heart, is associated with a reduced number of β1AR but not β2AR binding sites at the plasma membrane (PM). As a consequence, diminished βAR signaling may lead to lower PKA-mediated protein phosphorylation after adrenergic stimulation.5 The disturbed βAR signaling and reduced PKA phosphorylation result in blunted contractile response following administration of βAR agonists.6 Interestingly, recent progress suggests that βAR can continuously signal both at the cell surface7 and after catecholamine-induced endocytosis.8 These βAR signals may play a role in adaptive cardiac hypertrophic remodeling and promote compensatory contractility in the heart. Until now, it remains poorly understood how remodeling of βAR signaling affects PKA activity in different subcellular locations for PKA-dependent phosphorylation of substrates in failing myocytes.
Recent efforts have been put forward to understand β-adrenergic signaling at the sarcolemma and the SR due to their essential roles in calcium handling and arrhythmia in cardiac diseases. By using genetically encoded biosensors, studies show that adrenergic-induced PKA activity at the SR can be selectively inhibited by chronic insulin9 and the pro-inflammatory factor, prostaglandin E,10 both of which are elevated in clinical conditions associated with heart failure. Moreover, in hypertrophic failing myocytes isolated from a transaortic constriction mouse model, the adrenergic stimulation-induced cAMP activity is reduced at the PM11 and the SR,12 consistent with depressed PKA phosphorylation of PLB.13 In contrast, much less is known about the regulation of PKA activity and PKA phosphorylation of substrates on the myofilaments during cardiac remodeling and disease development. A disturbed PKA phosphorylation of myofilament proteins such as TnI and MyBP-C alters their calcium binding sensitivity, which directly contributes to inotropic and lusitropic dysfunction in the heart.14
Here, we develop a novel genetically encoded PKA biosensor that is anchored on the myofibrils to probe PKA activity in cardiac myocytes from a rabbit hypertrophic heart failure model.15 Our data show that while the β1AR-induced PKA signal is detected at the myofilaments as well as the sarcolemma and the SR in healthy myocytes, the β2AR-induced PKA signal is restricted along the sarcolemma and the SR, and does not have access to the myofilaments. However, in hypertrophic failing rabbit myocytes, stimulation of β2AR promotes a strong PKA activity at the myofilaments. The emerging β2AR signal is associated with down regulation of caveolin-3 in hypertrophic failing myocytes, a key structural protein of caveolae that confines the β2AR signal.16 Thus, in failing myocytes stimulation of the β2AR promotes strong PKA activity and PKA phosphorylation of TnI. Moreover, we demonstrate that re-introduction of caveolin-3 normalizes the distribution of the β-adrenergic signal, and restores contractile response to adrenergic stimulation.
METHODS
Experimental animals, cardiac myocyte isolation and culture
The animal care and experimental protocols followed US National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committees of the University of California at Davis. HF was induced in New Zealand White rabbits by combined aortic insufficiency and stenosis as previously described.15
Fluorescence resonance energy transfer (FRET) measurements
Images were acquired using a Leica DMI3000B inverted fluorescence microscope (Leica Biosystems, Buffalo Grove, IL) with a 40X oil-emersion objective lens and a charge-coupled device camera controlled by Metafluor software (Molecular Devices, Sunnyvale, CA). Fluorescence images for FRET analysis were carried out as described previously. 17
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 software (La Jolla, CA).
RESULTS
Novel biosensors reveal highly localized β2AR-induced PKA activity that does not reach the myofilaments in healthy rabbit cardiac myocytes
To analyze the dynamics of PKA activity on the myofilaments, we took a novel strategy to anchor FRET-based A-kinase activity reporter 3 (AKAR3)17 onto the myofilaments (MFs) by linking AKAR3 to the C-terminus of troponin T (MF-AKAR3, Figure 1A). MF-AKAR3 was used together with two other targeted PKA biosensors developed previously: PM-AKAR3 is linked to a PM targeting sequence from Kras and SR-AKAR3 is linked to the transmembrane domain of PLB.10, 18 Confocal analysis of rabbit adult cardiac myocytes shows that the biosensors display proper subcellular distribution, co-localizing with specific markers for subcellular organelles (Figure 1B). The introduction of biosensors does not affect adrenergic stimulation-induced PKA phosphorylation of endogenous troponin I (TnI, Figure 1C) or PLB.10, 18 These data indicate that the molecular pathways are preserved upon the expression of biosensors, and the targeted probes are suitable for analysis of PKA activity in the microdomains of adult cardiac myocytes.
Figure 1. Generation of cardiac myofilament-targeted FRET biosensors.
A) Schematic representation of subcellular-localized PKA AKAR3 biosensors. Yellow (YFP) and cyan (CFP) fluorescent proteins flank a PKA substrate and a forkhead-associated (FHA) domain that recognizes the phosphorylated PKA substrate. AKAR3 is linked to a Kras-derived sequence for plasma membrane (PM-AKAR3) localization, to a phospholamban (PLB)-derived sequence for sarcoplasmic reticulum (SR-AKAR3) localization, and to troponin T for myofilament (MF-AKAR3) localization. B) Representative confocal images of biosensor (green) expressed in young rabbit cardiac myocytes. Cells are immunostained with subcellular specific markers (red) for the SR (Ryanodine Receptor, RyR), the PM (Wheat Germ Agglutinin, WGA), and the MF (Phalloidin). Merge images confirm co-localization of fluorescent signals. C) Immunoblot analyses of PKA phosphorylation of troponin I (TnI) at serine 23/24 in response to isoproterenol (ISO) stimulation (1 or 100 nmol/L) in young rabbit myocytes infected with MF-AKAR3 probes. D) Young rabbit myocytes expressing PM-AKAR3, SR-AKAR3, and MF-AKAR3 are stimulated with a set of incremental doses of ISO. Time courses show AKAR3 FRET responses after stimulation with ISO. E) Normalized isoproterenol-induced dose response curves of AKAR3 biosensors (EC50 PM-AKAR3 at 2.16 × 10−10 mol/L, SR-AKAR3 at 2.57 × 10−9 mol/L, and MF-AKAR at 3.89 × 10−9 mol/L). F) Maximal increases in AKAR3 FRET ratio after stimulation with ISO (1 μM) or after co-treatment with forskolin (10 μM) and IBMX (100 μM). G) Normalized maximal FRET responses of individual AKAR3 biosensors against the increases induced by co-treatment with forskolin and IBMX, respectively.
We then further characterized the AKAR3 biosensors in response to a set of incremental doses of β-adrenergic agonist isoproterenol in adult rabbit myocytes. Stimulation of myocytes with isoproterenol induces dose-dependent increases in FRET ratio of the PM, SR and MF-anchored AKAR3 biosensors; and the maximal responses are higher at the PM than those at the SR and MF (Figure 1D and Supplemental Figure I). The responses of PM-AKAR3 are also more sensitive to isoproterenol stimulation than those of SR-AKAR3 and MF-AKAR3 (Figure 1D and 1E, and Supplemental Figure I). This data is consistent with previous studies showing that PKA phosphorylation of substrates at the PM is more sensitive to lower doses of isoproterenol stimulation than those at the intracellular compartments.19 The isoproterenol-induced maximal responses are not due to saturation of individual biosensors since co-treatment of myocytes with forskolin and IBMX induces higher increases in FRET ratios of biosensors than those induced by isoproterenol, respectively (Figure 1F). Notably, the isoproterenol-induced maximal responses in these localized biosensors are equivalent when normalized by those induced by co-treatment of forskolin and IBMX (Figure 1G), suggesting that these biosensors are functionally comparable in response to adrenergic stimulation in rabbit myocytes.
To analyze the alteration of local PKA dynamics at the myofilaments in failing heart, we used cardiac myocytes isolated from rabbits with hypertrophic heart failure (HF) induced by combined aortic insufficiency and stenosis.15 Myocytes from age-matched rabbits with sham surgery (SHAM) were used as controls. In age-matched SHAM myocytes, stimulation of the βAR with a saturated dose of isoproterenol (100 nmol/L) leads to an increase of PKA activity, which is completely blocked by β1AR selective antagonist CGP20712A, but not by β2AR selective antagonist ICI118551 (Figure 2A). Accordingly, stimulation of SHAM myocytes with isoproterenol significantly promotes PKA phosphorylation of troponin I (TnI) at serine 23/24, which is also blocked by CGP20712A, but not by ICI118551 (Figure 2B). However, stimulation with isoproterenol promotes a stronger PKA activity associated with the myofilaments in failing myocytes than SHAM cells (Figure 2C and 2D). Notably, stimulation of the β2AR also induces a strong increase in PKA activity in failing myocytes (Figure 2C and 2D). Consistent with the FRET measurement of PKA activity, stimulation of the β2AR promotes a strong increase in PKA phosphorylation of TnI in HF myocytes but not in SHAM controls (Figure 2E). These data indicate that in HF myocytes, the β2AR gains an ability to promote a strong PKA signal on the myofilaments.
Figure 2. A highly localized β2AR signal is not accessible to the myofilaments in SHAM rabbit cardiac myocytes.
A) SHAM rabbit cardiac myocytes expressing MF-AKAR3 are stimulated with 100 nmol/L ISO (total), or in the presence of 100 nmol/L β2AR blocker ICI118551 (β1AR) or 300 nmol/L of the β1AR blocker CGP20712A (β2AR). The maximal increases in MF-AKAR3 are plotted in bar graph. The dash line indicates the maximal increases induced by forskolin (10 μmol/L) and IBMX (100 μmol/L). *** p < 0.01 by one-way ANOVA followed by post hoc Bonferroni test. B) Stimulation of SHAM cardiac myocytes with ISO in the presence of CGP20712A or ICI118551 for 5 min. The PKA phosphorylation of TnI at Ser 23/24 is detected in western blot, and the increases in phosphorylation of TnI are plotted in bar graph. N = 6, ** p < 0.01 by one-way ANOVA followed by post hoc Bonferroni test). C) Time courses of FRET responses are from SHAM and HF cardiac myocytes expressing MF-AKAR3 after stimulation of βARs. D) Bar graph represents the maximal increases in MF-AKAR3 FRET ratio in panel C. * p < 0.05 and *** p < 0.001 by student t-test. E) Stimulation of SHAM and HF cardiac myocytes with ISO in the presence of CGP20712A or ICI118551 for 5 min. The PKA phosphorylation of TnI at Ser 23/24 is detected in western blot and quantified in bar graph. N = 6, ** p < 0.01 by one-way ANOVA followed by post hoc Bonferroni test).
βAR-induced PKA activity is diminished at the SR and the PM in failing rabbit myocytes
We also examined the alteration of local PKA dynamics at the SR in failing myocytes, an important intracellular organelle that regulates calcium signaling for myocyte contraction. In SHAM myocytes, stimulation of the βAR promotes a significant increase in PKA activity at the SR, which is inhibited by β1AR antagonist CGP20712A, but not by β2AR antagonist ICI118551 (Figure 3A). Consistent with the FRET-based measurement of PKA activity, stimulation of SHAM myocytes with isoproterenol promotes PKA phosphorylation of PLB (PLB) at serine 16, which is completely blocked by CGP20712A, but not by ICI118551 (Figure 3B). However, stimulation with isoproterenol promotes a weaker PKA activity at the SR in failing myocytes than SHAM cells (Figure 3C and 3D). Further analysis reveals that the β1AR-induced PKA activity at the SR is reduced whereas the β2AR-induced PKA activity at the SR is slightly increased (Figure 3C and 3D). Consistently, the adrenergic-induced PKA phosphorylation of PLB is diminished in HF myocytes compared to those in SHAM controls. In both SHAM and HF cells, the β1AR acts as the primary receptor subtype in adrenergic stimulation (Figure 3E). These data indicate that in HF myocytes, the βAR-induced PKA activity is diminished at the SR for substrate phosphorylation.
Figure 3. Diminished βAR-induced PKA activity at the SR in failing cardiac myocytes.
A) SHAM rabbit cardiac myocytes expressing SR-AKAR3 are stimulated with 100 nmol/L ISO (total), or in the presence of 100 nmol/L β2AR blocker ICI118551 (β1AR) or 300 nmol/L of the β1AR blocker CGP20712A (β2AR). The maximal increases in SR-AKAR3 are plotted in bar graph. The dash line indicates the maximal increases induced by forskolin (10 μmol/L) and IBMX (100 μmol/L). ** p < 0.01 when compared to total by one-way ANOVA followed by post hoc Bonferroni test. B) Stimulation of SHAM cardiac myocytes with ISO in the presence of CGP20712A or ICI118551 for 5 min. The PKA phosphorylation of PLB at serine 16 is detected in western blot, and plotted in bar graph. N = 6, ** p < 0.01 by one-way ANOVA followed by post hoc Bonferroni test). C) Time courses of FRET responses are from SHAM and HF cardiac myocytes expressing SR-AKAR3 after stimulation of βARs. D) Bar graph represents the maximal increases in SR-AKAR3 FRET ratio in panel C. * p < 0.05 by student t-test. E) Stimulation of SHAM and HF cardiac myocytes with ISO in the presence of CGP20712A or ICI118551 for 5 min. The PKA phosphorylation of PLB is detected in western blot and quantified in bar graph. N = 6, ** p < 0.01 and *** p < 0.001 by one-way ANOVA followed by post hoc Bonferroni test.
In comparison, stimulation of βAR promotes a robust increase in PKA activity at the PM in SHAM myocytes, which is significantly decreased in HF myocytes (Figure 4A–4C). Inhibition of either β1AR with CGP20712A or β2AR with ICI118551 attenuates PKA activity induced by isoproterenol, with a stronger inhibition by the β1AR blocker CGP20712A (Figure 4B). Further analysis reveals that the reduced adrenergic response at the PM in HF myocytes is primarily due to a diminished β1AR-stimulated PKA activity (Figure 4A and 4D). These data suggest that the βAR-induced PKA activity is diminished at the PM in failing myocytes.
Figure 4. Redistribution of βAR subtype activity at the PM in failing cardiac myocytes.
A) SHAM and HF rabbit cardiac myocytes expressing PM-AKAR3 are stimulated with 100 nmol/L ISO (total), in the presence of 100 nmol/L β2AR blocker ICI118551 (β1AR), or 300 nmol/L of the β1AR blocker CGP20712A (β2AR). Time courses show FRET responses from SHAM and HF cardiac myocytes expressing PM-AKAR3 after stimulation of βARs. B–D) The maximal increases in PM-AKAR3 in SHAM and HF myocytes are plotted in bar graph. In panel B, the dash line indicates the maximal increases induced by forskolin (10 μmol/L) and IBMX (100 μmol/L). # p < 0.05 and ## p < 0.01 by one-way ANOVA followed by post hoc Bonferroni test. * p < 0.05 by student t-test.
Redistribution of phosphodiesterase (PDE) activity contributes to elevated PKA activity on the myofilaments in failing heart
Recent studies have also revealed essential roles of alteration in PDE expression in remodeling of adrenergic signal in HF myocytes.11, 20 We next studied the involvement of various cAMP PDEs on the modification of local PKA activity in HF myocytes. In SHAM myocytes, inhibition of PDE3 with cilostamide induces a strong increase in PKA activity at the myofilaments and the SR, but not at the PM (Figure 5A–5C). In comparison, inhibition of PDE4 with rolipram induces a strong response in PKA activity at the PM, and to a lesser extent, at the myofilaments and the SR (Figure 5D–5F). Consistent with these observations, PDE3 displays co-localization with the myofilament binding protein actin whereas both PDE4D and PDE4B display co-localization with the plasma membrane marker caveolin-3 (Supplemental Figure II). In HF myocytes, the myofilament-associated PDE3 activity is decreased (Figure 5A and 5G) whereas the PM-associated PDE3 activity is increased relative to SHAM controls (Figure 5C and 5I), suggesting a shifted distribution of PDE3 activity from the myofilaments to the sarcolemma. In comparison, the PM-associated PDE4 activity in HF myocytes is decreased relative to SHAM controls (Figure 5F and 5I). Meanwhile, inhibition of PDE4 induces a small increase in PKA activity at the SR in SHAM myocytes, which is enhanced in HF cells (Figure 5E and 5H). However, the SR-associated PDE3 activity is not significantly altered in HF cells (Figure 5B and 5H). In addition, inhibition of PDE2 with erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA) induces a small increase in PKA activity at the PM and myofilaments in SHAM myocytes; the ENHA-induced response is increased at the PM in HF cell whereas the ENHA-induced response is reduced at the myofilaments in HF cells (Supplemental Figure III). While the PDE2 activity at the SR is minimal in SHAM myocytes, it is increased in HF cells (Supplemental Figure III).
Figure 5. Redistribution of PDE activity in failing cardiac myocytes.
A–F) SHAM and HF rabbit cardiac myocytes expressing MF-AKAR3, SR-AKAR3, or PM-AKAR3 are treated with 10 μmol/L rolipram (PDE4), or 1 μmol/L cilostamide (PDE3). Time courses of FRET traces from SHAM and HF cardiac myocytes are shown. G–I) The maximal increases in AKAR3 FRET ratio in SHAM and HF myocytes are plotted in bar graph. * p < 0.05 and *** p < 0.001 by student t-test.
Together, in healthy rabbit myocytes, the PKA activity at the PM is under predominant control of PDE4 whereas PDE3 hydrolyzes preferentially a pool of cAMP in the vicinity of the myofilaments (Figure 5). In HF rabbit myocytes, the presence of PDE4 activity at the PM is reduced while the activity of PDE3 and PDE2 become more prominent. In contrast, the activity of PDE3 and PDE2 is decreased at the myofilaments (Figure 5 and Supplemental Figure III). These data indicate dysregulation of these hydrolytic enzymes in HF myocytes. Accordingly, western blotting analysis shows a reduction of PDE3 and an upregulation of PDE4 expression in left ventricles following HF (Figure 6A). Moreover, PDE4 is shifted to internal compartments whereas PDE3 is enriched at the sarcolemma region in HF myocytes relative to SHAM controls (Supplemental Figure IV). The membrane-enriched PDE3 does not co-localize with β2AR in HF myocytes (Supplemental Figure IV). Therefore, in HF myocytes, PDEs may not be effectively coupled to adrenergic receptors. Indeed, while inhibition of PDE4 and PDE3 potentiates adrenergic stimulation-induced PKA phosphorylation of TnI in SHAM myocytes, the effects of these PDE inhibitors are diminished in the HF cells (Supplemental Figure V).
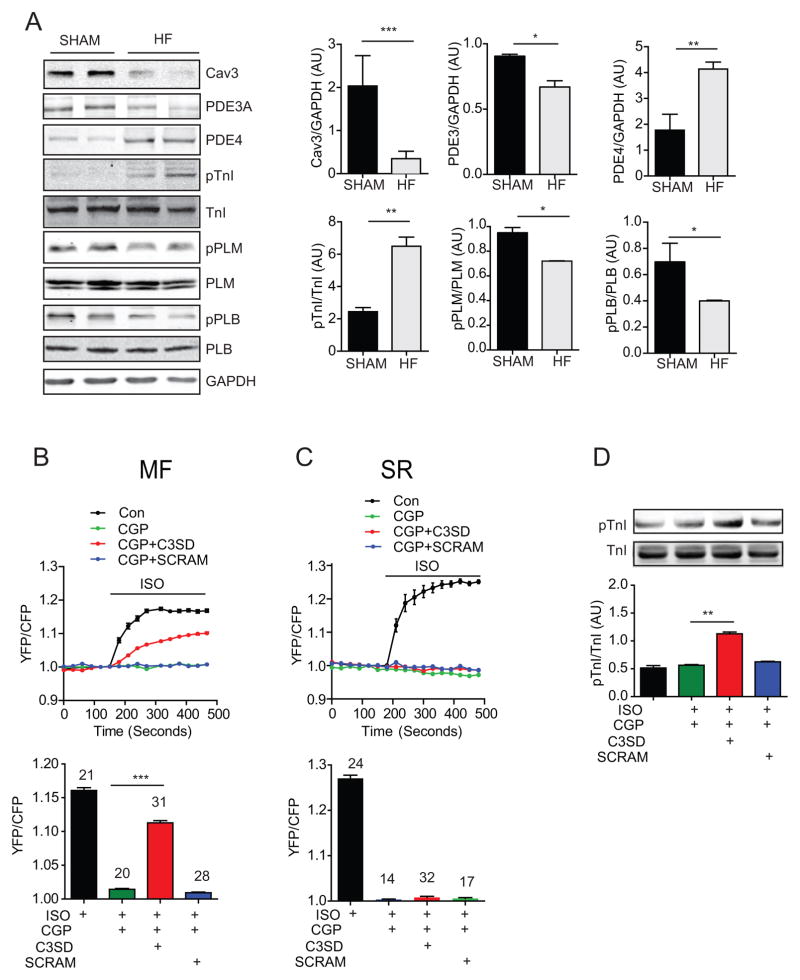
Figure 6. Inhibition of caveolin-3 promotes adrenergic signaling on the myofilaments in HF cardiac myocytes.
A) Immunoblots show expression of caveolin-3, PDE3A, PDE4, TnI, PLB, and phospholemman (PLM), as well as pTnI at serine 23/24, pPLB at serine 16, and pPLM at serine 68 in SHAM and HF heart lysates. Bar graphs represent mean ± SEM. N = 3; * p < 0.05, ** p < 0.01, and *** p < 0.01 by student’s t-test. B and C) Healthy young rabbit myocytes expressing SR-AKAR3 or MFAKAR3 are stimulated with 100nmol/L ISO and 300 nmol/L CGP20712A after incubation with 1 μmol/L C3SD or scrambled peptide for 1 hour. Time courses of changes in AKAR3 FRET ratio are shown, and the maximal increases in FRET ratio are plotted in bar graph. *** p < 0.001 by one-way ANOVA followed by post hoc Bonferroni test. D) Healthy young rabbit myocytes are stimulated with 100 nmol/L ISO in the presence of 1 μmol/L C3SD or scrambled peptide. PKA phosphorylation of troponin I at serine 23/24 is detected in western blots and quantified in bar graph. N = 3; ** p < 0.01 by one-way ANOVA followed by post hoc Bonferroni test.
In a separate set of experiments, we applied PKA inhibitors in the FRET measurement to assess the baseline subcellular PKA activity in HF and SHAM myocytes (Supplemental Figure VI). In SHAM myocytes, we detect a significant reduction of PKA activity by H89 at the myofilaments and the SR, but minimal change in PKA activity at the PM (Supplemental Figure VI). In HF myocytes, the H89-senstive PKA activity at the PM and the SR does not change (Supplemental Figure VI). However, the H89-senstive PKA activity at the myofilaments is increased in HF cells, indicating an elevation of baseline PKA activity associated with myofilaments in HF myocytes (Supplemental Figure VI).
Reintroduction of caveolin-3 restores distribution of βAR-induced PKA signal, PKA phosphorylation, and contractility in failing myocytes
β2AR is enriched in the caveolae of cardiac myocytes, which confines the receptor action to the local PM domains. Disruption of caveolae leads to enhanced β2AR stimulation of ion channels at the PM.16, 21 Previous studies have demonstrated that the expression of caveolin-3 is decreased in different mouse and human cardiac diseases including heart failure and hypertrophy.22–24 Loss of caveolin-3 is associated with redistribution of β2AR signal on the PM in failing myocytes.25 Here, we reveal an 80% reduction of protein expression of caveolin-3 in the failing rabbit myocytes. This reduction is associated with an increase of PKA phosphorylation of TnI (Figure 6A). Caveolin-3 has a scaffolding domain (C3SD) that is critical for the structure and function of caveolae. A membrane permeable peptide corresponding to C3SD modulates β2AR regulation of L-type calcium channel. 21 We applied the membrane permeable C3SD peptide to healthy rabbit myocytes to examine whether inhibition of caveolin-3 promotes β2AR signal to the myofilaments. Treatment with C3SD significantly enhances β2AR-induced PKA activity at the myofilaments but not at the SR (Figure 6B and 6C). In contrast, a scrambled peptide does not enhance β2AR-induced PKA activity at the MF. Consistent with the FRET-based measurement of PKA activity, C3SD significantly promotes β2AR-induced PKA phosphorylation of TnI at the myofilaments (Figure 6D). These data suggest that caveolin-3 plays an essential role in preventing the β2AR from signaling to the myofilaments; therefore, loss of caveolin-3 in HF myocytes may permit β2AR to signal to the myofilaments to increase PKA activity and substrate phosphorylation.
We sought to restore the expression of caveolin-3 in hypertrophic failing myocytes and to evaluate βAR signaling and PKA activity on the myofilaments. Reintroduction of caveolin-3 partially restores the distribution of PDEs (Supplemental Figure IV) and their activity in HF myocytes, including an increase in PDE4 activity at the PM and increases in PDE3 and PDE2 activity at the myofilaments (Supplemental Figure III and VII). Moreover, reintroduction of caveolin-3 partially normalizes the distribution of β2AR (Supplemental Figure VIII) and PKA activity at the subcellular compartments, including increases in PKA activity at the PM, and decreases in PKA activity at the SR and myofilaments after stimulation with isoproterenol (Figure 7A–7C). While the β1AR-induced PKA activity is increased at the PM, the β2AR-induced PKA activity is decreased throughout the myocytes. Notably, after reintroduction of caveolin-3, the β2AR-induced PKA activity does not reach the myofilaments; instead it is confined at the PM and the SR (Figure 7A–7C). Accordingly, the phosphorylation of TnI under β2AR stimulation in failing myocytes is significantly diminished after overexpression of caveolin-3 (Figure 7D). Finally, overexpression of caveolin-3 restores β-adrenergic-induced contractile shortening response in hypertrophic failing rabbit myocytes (Figure 7E).
Figure 7. Expression of caveolin-3 normalizes adrenergic signaling on the myofilaments in HF cardiac myocytes.
A–C) SHAM or HF myocytes expressing MF-AKAR3, PM-AKAR3 or SR-AKAR3 together with caveolin-3 as indicated. Myocytes are stimulated with 100 nmol/L ISO, or in the presence of 100 nmol/L ICI118551 or 300 nmol/L CGP20712A. The maximal increases in FRET ratio are plotted in bar graph. * p < 0.05, ** p < 0.01, and *** p < 0.001 by one-way ANOVA followed by post hoc Bonferroni test. D) HF myocytes expressing caveolin-3 are stimulated with 100 nmol/L ISO in the presence of 300 nmol/L CGP20712A. PKA phosphorylation of troponin I at serine 23/24 are detected in western blots, and quantified in bar graph. N = 5; * p < 0.01 by one-way ANOVA followed by post hoc Bonferroni test. E) SHAM and HF rabbit myocytes with or without expression of caveolin-3 are stimulated with ISO (100 nmol/L). Myocyte contractile shortening is plotted as bar graphs. * p < 0.05 by one-way ANOVA followed by post hoc Bonferroni test.
DISCUSSION
The cardiac PKA activity is fine-tuned to ensure coordinated phosphorylation of substrates in different compartments for excitation-contraction (E-C) coupling at baseline and after adrenergic stimulation. In this study, we have revealed a structural-functional remodeling in hypertrophic failing rabbit myocytes that links the redistribution of βAR signal to enhanced PKA phosphorylation of myofilament proteins (Figure 8). While the β2AR-induced signal is restricted along the sarcolemma and the SR in healthy myocytes, an augmented β2AR signal associated with loss of caveolin-3 is selectively directed to the myofilaments in hypertrophic failing cells. This augmented β2AR signal, together with reduced PDE activity associated with myofibrils, promotes strong PKA activity and PKA phosphorylation of the myofilament protein TnI in hypertrophic failing myocytes. Moreover, reintroduction of caveolin-3 is able to normalize the distribution of adrenergic signaling and restores contractile response to adrenergic stimulation in hypertrophic failing rabbit myocytes.
Figure 8. Schematic models on changes of local PKA signal in failing cardiac myocytes.
In SHAM cardiac myocytes, β1AR has a higher and further reaching response in all three subcellular compartments tested than β2AR. β2AR generates a highly localized response within the PM and the SR, but does not induce any significant PKA activity on the myofilaments. PKA activity at the PM microdomain is under predominant control of PDE4 whereas PDE3 preferentially hydrolyzes a pool of cAMP in the vicinity of myofilaments. In HF myocytes with reduced caveolin-3, the βAR-induced PKA activity at the PM and, to a lesser extent, at the SR is reduced. However, the βAR-induced PKA activity at the myofilament is increased due in part to augmented β2AR signal and miss-localization of PDE3 away from the myofilaments. Reintroduction of caveolin-3 normalizes the distribution of βAR-induced PKA activity at the subcellular compartments, including increases in PKA activity at the PM, and decreases in PKA activity at the myofilaments. In particular, the β2AR-induced PKA activity is confined at the PM and the SR, and does not access the myofilaments.
A tonic PKA activity is maintained by adenylyl cyclase-dependent cAMP production and phosphodiesterases-dependent cAMP hydrolysis.19, 26 Our results show that PDE4 is the major enzyme that controls local PKA activity along the sarcolemma whereas PDE3 mediates primarily cAMP hydrolysis on the myofilaments (Figure 5). In agreement, both PDE4D and PDE4B display overlap with the membrane marker caveolin-3 (Supplemental Figure II), consistent with a recent report showing that both enzymes have a binding motif to caveolin-3;27 loss of caveolin-3 may lead to loss of PDE4 activity at the sarcolemma. In contrast, PDE3 displays a co-localization with myofibrils (Supplemental Figure II), supporting its primary role in controlling local cAMP and PKA activity there (Figure 5). Meanwhile, we observe a high tonic PKA activity on the myofilaments, which is consistent with a relatively high PKA phosphorylation of MyBP-C and TnI in healthy cardiac tissues.28
While the PKA activity is usually depressed in the late stage of human heart failure,29, 30 there are reports showing preserved or elevated PKA phosphorylation on TnI and PLB.31, 32 It is not completely understood how the cellular PKA activity in myocytes evolves during the course of heart failure development. Here, we observe a redistribution of PKA activity in hypertrophic failing myocytes, including an increased PKA activity at the myofilaments. In agreement, a recent report also shows that PKA phosphorylation of myofilament proteins is elevated in the spontaneous hypertensive rat model.33 It is therefore possible that PKA activity is redistributed as part of the remodeling of βAR signaling during the compensatory stage before a general depression of PKA activity at the late stage of heart failure. In a classic view, βARs are desensitized in HF due in part to the reduced β1AR density at the cell surface,34 rendering the heart unable to respond to catecholamine stimulation 6. However, recent progress suggests that βARs can continuously signal at the cell surface7 and after catecholamine-induced endocytosis.8 Studies show that βARs are accumulated at the endosome under chronic elevation of sympathetic activity in HF.35 These βARs may signal from endosomes, offering a potential mechanism on redistribution of PKA activity from the PM to the myofilaments in failing myocytes (Figure 2 and 4). Further analysis reveals that the diminished β1AR signaling contributes to lower PKA activity at the sarcolemma (Figure 4); which is consistent with previous observations that β1AR density is downregulated in this rabbit HF model.36 In comparison, β2AR density is not reduced;36 rather an augmented β2AR signal may be preferentially directed to the myofilaments. Moreover, the shifted PKA activity can be further exacerbated by relocation of PDE3 and PDE2 activity away from the myofilaments to the sarcolemma (Figure 5 and Supplemental Figure III). Together, these observations suggest that the βAR signal undergoes relocation intracellularly during the development of heart failure. Further studies will likely yield novel information on βAR-PKA signal remodeling in subcellular compartments in models of cardiac disease, which may offer insight into cardiac hypertrophic remodeling as well as compensatory contractility in the heart.
Detubulation and loss of caveolae are two prominent features during cardiac structural remodeling; these alterations can in turn promote signal remodeling in cardiac myocytes. While the β2AR is confined in the tubular region in healthy myocytes, chemical disruption of caveolae with a cholesterol-depleting agent, methyl-β-cyclodestrin, results in > 60% increase in β2AR-dependent PKA phosphorylation of PLB and TnI, as well as lusitropic responses in adult myocytes.37 In addition, a membrane permeable peptide representing the scaffolding domain of caveolin-3 (C3SD) leads to reduction of β2AR-dependent PKA phosphorylation of L-type calcium channel at the PM.21 Detubulation and loss of caveolae in hypertrophic failing myocytes isolated from a mouse model yield broad distribution of β2AR signaling along the plasma membrane crest.25, 37 The loss of close proximity of β2AR to caveolin-3-associated PDE427 can also permit a much broader reach of receptor signaling outside of the T-tubular region in failing cells.25 In this study, we have observed an amplified β2AR signal to the myofilaments after treatment with C3SD to promote PKA phosphorylation of TnI. Together, these observations suggest that inhibition of caveolin-3 leads to a signal shift from the PM to intracellular compartments. Moreover, we observed an amplified β2AR signal associated with loss of caveolin-3 in failing rabbit myocytes, which preferentially propagates toward the myofilaments (Figure 2 and 6). These data, for the first time, link the redistribution of β2AR to amplified PKA signal at the myofilaments in failing myocytes. Furthermore, reintroduction of caveolin-3 in HF myocytes can increase caveolae density,38 which may facilitate the development and extension of T-tubules39 and enrichment of β2AR to the T-tubule (Supplemental Figure VIII). Reintroduction of caveolin-3 may also recruit PDE4 enzymes to the proximity of the β2AR local domain (27 and Supplemental Figure II and IV) to confine β2AR signals in local subcellular environments.25, 40 Consequently, reintroduction of caveolin-3 effectively prevents β2AR from sending a signal to the myofilaments in rabbit failing myocytes and from promoting PKA phosphorylation of TnI (Figure 8). Moreover, reintroduction of caveolin-3 could normalize the functional relationship between βAR and the L-type Ca2+ channel at both T-tubules and caveolae.16, 21 As a result, the caveolin-3-expressing myocytes become more responsive to adrenergic stimulation to enhance contractility (Figure 7). Together, these data support the notion that caveolin-3 is essential in regulation of compartmented cardiac βAR signaling.25, 40
Together, this study reveals that structural remodeling leads to βAR signal remodeling in failing myocytes, which yields a decrease in PKA activity at the PM, and an increase in β2AR-PKA activity and phosphorylation of substrates on the myofilaments in hypertrophic rabbit hearts. Our study suggests caveolin-3 as an essential regulator of subcellular adrenergic signal at the myofilaments in myocytes.
Supplementary Material
Novelty and Significance.
What Is Known?
Abnormal PKA phosphorylation of substrates on myofilaments directly contributes to inotropic and lusitropic dysfunction in heart failure.
Adrenergic signaling is altered in heart failure myocytes, including down regulation of β1 adrenergic receptor (AR)-induced signals and an increase in the β2AR-induced signal.
What New Information Does This Article Contribute?
In heart failure myocytes, β1AR-induced signaling is selectively down regulated at the sarcolemma whereas β2AR-induced signaling gains access to myofilament.
Reintroduction of caveolin 3 in heart failure myocytes prevents β2ARs from sending signal to myofilaments for substrate phosphorylation.
Reintroduction of caveolin 3 normalizes βAR-induced contractile responses in heart failure myocytes.
These studies reveal that βAR signaling undergoes remodeling in heart failure myocytes, which shifts the PKA activity from the cell surface to the intracellular compartments including the SR and myofilaments. In particular, β2AR-induced signals gain access to the myofilament, which contributes to abnormal PKA phosphorylation of TnI and contractile dysfunction. Preclinical studies to assess the effects of inhibition of β2AR signal on heart failure are indicated.
Acknowledgments
We thank Matt L. Stein, Linda Talken, C. Blake Nichols, Ian Palmer, Max Bergman, Lisa J. Gilardoni, Maura Ferrero, and Yan Jiang for rabbit cell isolation.
SOURCES OF FUNDING
This study is supported by NIH grant R01 HL082846 to YKX, P01 HL80101 to DB, RO1 HL091071 to HHP, Veteran Affairs Merit Award from the Department of Veterans Affairs BX001963 to HHP and BX002900 to YKX, and Italian grant FIRB 2010 RBFR10URHP to FB. TW is a recipient of AHA predoctoral fellow; QS is a recipient of AHA postdoctoral fellow; and YKX is an AHA established investigator of the American Heart Association and a Shanghai Eastern Scholar.
Nonstandard Abbreviations and Acronyms
- PDE
Phosphodiesterase
- AR
adrenergic receptor
- PKA
protein kinase A
- TnI
troponin I
- SR
sarcoplasmic reticulum
- PLB
phospholamban
- AKAR
A-kinase activity biosensor
- FRET
fluorescence resonant energy transfer
- Cav3
caveolin-3
- ISO
isoproterenol
Footnotes
DISCLOSURES
None.
References
- 1.Ruehr ML, Russell MA, Bond M. A-kinase anchoring protein targeting of protein kinase A in the heart. J Mol Cell Cardiol. 2004;37:653–65. doi: 10.1016/j.yjmcc.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Koss KL, Kranias EG. Phospholamban: a prominent regulator of myocardial contractility. Circ Res. 1996;79:1059–63. doi: 10.1161/01.res.79.6.1059. [DOI] [PubMed] [Google Scholar]
- 3.Stelzer JE, Patel JR, Walker JW, Moss RL. Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase A phosphorylation. Circ Res. 2007;101:503–11. doi: 10.1161/CIRCRESAHA.107.153650. [DOI] [PubMed] [Google Scholar]
- 4.Eschenhagen T. Beta-adrenergic signaling in heart failure-adapt or die. Nat Med. 2008;14:485–7. doi: 10.1038/nm0508-485. [DOI] [PubMed] [Google Scholar]
- 5.Post SR, Hammond HK, Insel PA. Beta-adrenergic receptors and receptor signaling in heart failure. Annu Rev Pharmacol Toxicol. 1999;39:343–60. doi: 10.1146/annurev.pharmtox.39.1.343. [DOI] [PubMed] [Google Scholar]
- 6.Freeman K, Colon-Rivera C, Olsson MC, Moore RL, Weinberger HD, Grupp IL, Vikstrom KL, Iaccarino G, Koch WJ, Leinwand LA. Progression from hypertrophic to dilated cardiomyopathy in mice that express a mutant myosin transgene. Am J Physiol Heart Circ Physiol. 2001;280:H151–9. doi: 10.1152/ajpheart.2001.280.1.H151. [DOI] [PubMed] [Google Scholar]
- 7.Fu Q, Kim S, Soto D, De Arcangelis V, DiPilato L, Liu S, Xu B, Shi Q, Zhang J, Xiang YK. A long lasting beta1 adrenergic receptor stimulation of cAMP/protein kinase A (PKA) signal in cardiac myocytes. J Biol Chem. 2014;289:14771–81. doi: 10.1074/jbc.M113.542589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsvetanova NG, von Zastrow M. Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nat Chem Biol. 2014;10:1061–5. doi: 10.1038/nchembio.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu Q, Xu B, Liu Y, Parikh D, Li J, Li Y, Zhang Y, Riehle C, Zhu Y, Rawlings T, Shi Q, Clark RB, Chen X, Abel ED, Xiang YK. Insulin inhibits cardiac contractility by inducing a Gi-biased beta2-adrenergic signaling in hearts. Diabetes. 2014;63:2676–89. doi: 10.2337/db13-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S, Li Y, Kim S, Fu Q, Parikh D, Sridhar B, Shi Q, Zhang X, Guan Y, Chen X, Xiang YK. Phosphodiesterases coordinate cAMP propagation induced by two stimulatory G protein-coupled receptors in hearts. Proc Natl Acad Sci U S A. 2012;109:6578–83. doi: 10.1073/pnas.1117862109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perera RK, Nikolaev VO. Compartmentation of cAMP signalling in cardiomyocytes in health and disease. Acta Physiol (Oxf) 2013;207:650–62. doi: 10.1111/apha.12077. [DOI] [PubMed] [Google Scholar]
- 12.Sprenger JU, Perera RK, Steinbrecher JH, Lehnart SE, Maier LS, Hasenfuss G, Nikolaev VO. In vivo model with targeted cAMP biosensor reveals changes in receptor-microdomain communication in cardiac disease. Nat Commun. 2015;6:6965. doi: 10.1038/ncomms7965. [DOI] [PubMed] [Google Scholar]
- 13.El-Armouche A, Pamminger T, Ditz D, Zolk O, Eschenhagen T. Decreased protein and phosphorylation level of the protein phosphatase inhibitor-1 in failing human hearts. Cardiovasc Res. 2004;61:87–93. doi: 10.1016/j.cardiores.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Messer AE, Marston SB. Investigating the role of uncoupling of troponin I phosphorylation from changes in myofibrillar Ca(2+)-sensitivity in the pathogenesis of cardiomyopathy. Front Physiol. 2014;5:315. doi: 10.3389/fphys.2014.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM. Upregulation of Na(+)/Ca(2+) exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res. 1999;85:1009–19. doi: 10.1161/01.res.85.11.1009. [DOI] [PubMed] [Google Scholar]
- 16.Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc Natl Acad Sci U S A. 2006;103:7500–5. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Ma Y, Taylor SS, Tsien RY. Genetically encoded reporters of protein kinase A activity reveal impact of substrate tethering. Proc Natl Acad Sci U S A. 2001;98:14997–5002. doi: 10.1073/pnas.211566798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, Zhang J, Xiang YK. FRET-based direct detection of dynamic protein kinase A activity on the sarcoplasmic reticulum in cardiomyocytes. Biochem Biophys Res Commun. 2011;404:581–6. doi: 10.1016/j.bbrc.2010.11.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Arcangelis V, Liu S, Zhang D, Soto D, Xiang YK. Equilibrium between adenylyl cyclase and phosphodiesterase patterns adrenergic agonist dose-dependent spatiotemporal cAMP/protein kinase A activities in cardiomyocytes. Mol Pharmacol. 2010;78:340–9. doi: 10.1124/mol.110.064444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehel H, Emons J, Vettel C, Wittkopper K, Seppelt D, Dewenter M, Lutz S, Sossalla S, Maier LS, Lechene P, Leroy J, Lefebvre F, Varin A, Eschenhagen T, Nattel S, Dobrev D, Zimmermann WH, Nikolaev VO, Vandecasteele G, Fischmeister R, El-Armouche A. Phosphodiesterase-2 is up-regulated in human failing hearts and blunts beta-adrenergic responses in cardiomyocytes. J Am Coll Cardiol. 2013;62:1596–606. doi: 10.1016/j.jacc.2013.05.057. [DOI] [PubMed] [Google Scholar]
- 21.Bryant S, Kimura TE, Kong CH, Watson JJ, Chase A, Suleiman MS, James AF, Orchard CH. Stimulation of ICa by basal PKA activity is facilitated by caveolin-3 in cardiac ventricular myocytes. J Mol Cell Cardiol. 2014;68:47–55. doi: 10.1016/j.yjmcc.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markandeya YS, Phelan LJ, Woon MT, Keefe AM, Reynolds CR, August BK, Hacker TA, Roth DM, Patel HH, Balijepalli RC. Caveolin-3 Overexpression Attenuates Cardiac Hypertrophy via Inhibition of T-type Ca2+ Current Modulated by Protein Kinase Calpha in Cardiomyocytes. J Biol Chem. 2015;290:22085–100. doi: 10.1074/jbc.M115.674945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feiner EC, Chung P, Jasmin JF, Zhang J, Whitaker-Menezes D, Myers V, Song J, Feldman EW, Funakoshi H, Degeorge BR, Jr, Yelamarty RV, Koch WJ, Lisanti MP, McTiernan CF, Cheung JY, Bristow MR, Chan TO, Feldman AM. Left ventricular dysfunction in murine models of heart failure and in failing human heart is associated with a selective decrease in the expression of caveolin-3. J Card Fail. 2011;17:253–63. doi: 10.1016/j.cardfail.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horikawa YT, Panneerselvam M, Kawaraguchi Y, Tsutsumi YM, Ali SS, Balijepalli RC, Murray F, Head BP, Niesman IR, Rieg T, Vallon V, Insel PA, Patel HH, Roth DM. Cardiac-specific overexpression of caveolin-3 attenuates cardiac hypertrophy and increases natriuretic peptide expression and signaling. J Am Coll Cardiol. 2011;57:2273–83. doi: 10.1016/j.jacc.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright PT, Nikolaev VO, O’Hara T, Diakonov I, Bhargava A, Tokar S, Schobesberger S, Shevchuk AI, Sikkel MB, Wilkinson R, Trayanova NA, Lyon AR, Harding SE, Gorelik J. Caveolin-3 regulates compartmentation of cardiomyocyte beta2-adrenergic receptor-mediated cAMP signaling. J Mol Cell Cardiol. 2014;67:38–48. doi: 10.1016/j.yjmcc.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu Q, Chen X, Xiang YK. Compartmentalization of beta-adrenergic signals in cardiomyocytes. Trends Cardiovasc Med. 2013;23:250–6. doi: 10.1016/j.tcm.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timofeyev V, Myers RE, Kim HJ, Woltz RL, Sirish P, Heiserman JP, Li N, Singapuri A, Tang T, Yarov-Yarovoy V, Yamoah EN, Hammond HK, Chiamvimonvat N. Adenylyl cyclase subtype-specific compartmentalization: differential regulation of L-type Ca2+ current in ventricular myocytes. Circ Res. 2013;112:1567–76. doi: 10.1161/CIRCRESAHA.112.300370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gresham KS, Stelzer JE. The contributions of cardiac myosin binding protein C and troponin I phosphorylation to beta-adrenergic enhancement of in vivo cardiac function. J Physiol. 2016;594:669–86. doi: 10.1113/JP270959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Velden J, Narolska NA, Lamberts RR, Boontje NM, Borbely A, Zaremba R, Bronzwaer JG, Papp Z, Jaquet K, Paulus WJ, Stienen GJ. Functional effects of protein kinase C-mediated myofilament phosphorylation in human myocardium. Cardiovasc Res. 2006;69:876–87. doi: 10.1016/j.cardiores.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 30.Schulz EM, Wilder T, Chowdhury SA, Sheikh HN, Wolska BM, Solaro RJ, Wieczorek DF. Decreasing tropomyosin phosphorylation rescues tropomyosin-induced familial hypertrophic cardiomyopathy. J Biol Chem. 2013;288:28925–35. doi: 10.1074/jbc.M113.466466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraysse B, Weinberger F, Bardswell SC, Cuello F, Vignier N, Geertz B, Starbatty J, Kramer E, Coirault C, Eschenhagen T, Kentish JC, Avkiran M, Carrier L. Increased myofilament Ca2+ sensitivity and diastolic dysfunction as early consequences of Mybpc3 mutation in heterozygous knock-in mice. J Mol Cell Cardiol. 2012;52:1299–307. doi: 10.1016/j.yjmcc.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKee LA, Chen H, Regan JA, Behunin SM, Walker JW, Walker JS, Konhilas JP. Sexually dimorphic myofilament function and cardiac troponin I phosphospecies distribution in hypertrophic cardiomyopathy mice. Arch Biochem Biophys. 2013;535:39–48. doi: 10.1016/j.abb.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong X, Sumandea CA, Chen YC, Garcia-Cazarin ML, Zhang J, Balke CW, Sumandea MP, Ge Y. Augmented phosphorylation of cardiac troponin I in hypertensive heart failure. J Biol Chem. 2012;287:848–57. doi: 10.1074/jbc.M111.293258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–12. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 35.Perrino C, Naga Prasad SV, Schroder JN, Hata JA, Milano C, Rockman HA. Restoration of beta-adrenergic receptor signaling and contractile function in heart failure by disruption of the betaARK1/phosphoinositide 3-kinase complex. Circulation. 2005;111:2579–87. doi: 10.1161/CIRCULATIONAHA.104.508796. [DOI] [PubMed] [Google Scholar]
- 36.Desantiago J, Ai X, Islam M, Acuna G, Ziolo MT, Bers DM, Pogwizd SM. Arrhythmogenic effects of beta2-adrenergic stimulation in the failing heart are attributable to enhanced sarcoplasmic reticulum Ca load. Circ Res. 2008;102:1389–97. doi: 10.1161/CIRCRESAHA.107.169011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calaghan S, Kozera L, White E. Compartmentalisation of cAMP-dependent signalling by caveolae in the adult cardiac myocyte. J Mol Cell Cardiol. 2008;45:88–92. doi: 10.1016/j.yjmcc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Galbiati F, Volonte D, Chu JB, Li M, Fine SW, Fu M, Bermudez J, Pedemonte M, Weidenheim KM, Pestell RG, Minetti C, Lisanti MP. Transgenic overexpression of caveolin-3 in skeletal muscle fibers induces a Duchenne-like muscular dystrophy phenotype. Proc Natl Acad Sci U S A. 2000;97:9689–94. doi: 10.1073/pnas.160249097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parton RG, Way M, Zorzi N, Stang E. Caveolin-3 associates with developing T-tubules during muscle differentiation. J Cell Biol. 1997;136:137–54. doi: 10.1083/jcb.136.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macdougall DA, Agarwal SR, Stopford EA, Chu H, Collins JA, Longster AL, Colyer J, Harvey RD, Calaghan S. Caveolae compartmentalise beta2-adrenoceptor signals by curtailing cAMP production and maintaining phosphatase activity in the sarcoplasmic reticulum of the adult ventricular myocyte. J Mol Cell Cardiol. 2012;52:388–400. doi: 10.1016/j.yjmcc.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.