Significance
The current therapeutic approaches to the treatment of benign prostatic hyperplasia (BPH) do not take into consideration that inflammation is an important factor in the pathogenesis of this disease. We previously demonstrated that growth hormone-releasing hormone (GHRH) antagonists reduce prostatic weights and decrease the level of inflammatory cytokines in a testosterone-induced BPH model. This study sheds light on the paracrine roles of GHRH in prostatic inflammation and demonstrates that GHRH stimulates the growth of BPH-1 and primary prostate epithelial spheres and that GHRH antagonists reduce prostate volume in an experimental model of prostatic inflammation.
Keywords: chronic prostatic inflammation, neuropeptide, prostatic hyperplasia, targeted therapy, experimental autoimmune prostatitis
Abstract
The etiology of benign prostatic hyperplasia (BPH) is multifactorial, and chronic inflammation plays a pivotal role in its pathogenesis. Growth hormone-releasing hormone (GHRH) is a hypothalamic neuropeptide that has been shown to act as paracrine/autocrine factor in various malignancies including prostate cancer. GHRH and its receptors are expressed in experimental models of BPH, in which antagonists of GHRH suppressed the levels of proinflammatory cytokines and altered the expression of genes related to epithelial-to-mesenchymal transition (EMT). We investigated the effects of GHRH antagonist on prostatic enlargement induced by inflammation. Autoimmune prostatitis in Balb/C mice was induced by a homogenate of reproductive tissues of male rats. During the 8-wk induction of chronic prostatitis, we detected a progressive increase in prostatic volume reaching 92% at week 8 compared with control (P < 0.001). Daily treatment for 1 mo with GHRH antagonist MIA-690 caused a 30% reduction in prostate volume (P < 0.05). Conditioned medium derived from macrophages increased the average volume of spheres by 82.7% (P < 0.001) and elevated the expression of mRNA for N-cadherin, Snail, and GHRH. GHRH antagonist reduced the average volume of spheres stimulated by inflammation by 75.5% (P < 0.05), and TGF-β2 by 91.8% (P < 0.01). The proliferation of primary epithelial cells stimulated by IL-17A or TGF-β2 was also inhibited by 124.1% and 69.9%, respectively. GHRH stimulated the growth of BPH-1 and primary prostate spheres. This study provides evidence that GHRH plays important roles in prostatic inflammation and EMT and suggests the merit of further investigation to elucidate the effects of GHRH antagonists in prostatitis and BPH.
Benign prostatic hyperplasia (BPH) is an age-dependent condition with a prevalence of 50–60% in men in their 60s (1). The term BPH refers to the enlargement of the prostate caused by expansion epithelial and stromal cells appearing primarily in the transition zone of the prostate (2). Patients with BPH frequently develop bladder outlet obstruction, leading to an increased resistance of the urethra, followed by the occurrence of various symptoms, collectively known as lower urinary tract symptoms (LUTSs) (3, 4). Current therapies include 5α-reductase inhibitors, which reduce dihydrotestosterone levels, and α1-adrenergic blockers, which lower the adrenergic tone (5, 6). These medical modalities have low efficacy and require continuous long-term administration. The number of patients with symptomatic BPH is expected to increase from 8.1 million in 2010 to 10.3 million in 2020 (7). Minimally invasive surgical techniques such as transurethral needle ablation and microwave thermotherapy are also used in severe cases (8). Although these techniques cause less complications than the invasive transurethral resection of the prostate, they still carry significant risks. Consequently, the development of an efficient, noninvasive medical therapy is urgently needed.
The pathogenesis of BPH is not completely understood, and it has been linked to many factors, including age-dependent changes in estrogen/testosterone ratio (9–11), age-related tissue remodeling, elevated levels of growth factors, hypoxia, and metabolic disturbances (12–15). Most recently, research has been more focused on the role of chronic inflammation as a central factor in the development of BPH (16). A majority of BPH specimens contain some degree of leukocyte infiltration (17–20). In addition, elevated levels of lymphocyte-derived cytokines, such as IL-2, IL-4, and IFN-γ, have been found in resected BPH tissue and appear to be involved in the stimulation of fibromuscular growth of the prostate (21). Similarly, cytokines released by infiltrated macrophages have also been demonstrated to stimulate stromal cell proliferation (22). Experimental animal models of bacterial prostatitis present a significant increase in epithelial proliferation induced by inflammation (23).
An established connection exists between inflammation and epithelial-to-mesenchymal transition (EMT), as demonstrated by the ability of inflammatory cytokines to regulate the expression of key genes of EMT in cancer and fibrosis (24). The occurrence of EMT has been confirmed in human BPH tissue and in an in vitro inflammation model (25–27). However, little is known about the exact molecular mechanism how the chronic prostatitis/EMT/BPH transition may occur.
The classical role of the hypothalamic neurohormone growth hormone (GH)-releasing hormone (GHRH) is to regulate the production and secretion of GH in the pituitary. Since its discovery, mitogenic effects of autocrine/paracrine GHRH have been demonstrated in various types of cancers, including prostatic, which express GHRH and GHRH receptors (GHRHRs) (28–33). An autostimulatory loop, composed of tumor-derived GHRH and its tumoral receptors, can be disrupted by specific GHRH antagonists, resulting in inhibition of tumor growth in experimental models. GHRH antagonists induce marked reduction in the growth of prostate cancer cells in in vivo cancer xenograft models and in vitro (34).
The involvement of GHRH signaling in the pathogenesis of BPH has been studied by our group in a testosterone-induced rat BPH model (35). The expression of protein for GHRH and GHRHRs was markedly elevated in this model, indicating the involvement of the GHRH autostimulatory loop in the pathogenesis of hormone-induced prostatic enlargement (36). In this experimental model, antagonists of GHRH, synthesized in our laboratories, demonstrated beneficial effects on prostate size, mitotic index, and the expression of various growth factors, inflammation-related genes, and key regulators of EMT (36–38). Anti-inflammatory effects for GHRH antagonists were also demonstrated in experimental models of cancer and ocular inflammation (39, 40).
In this study, we investigated the effects of the GHRH antagonists MIA-690 and JV-I-38 in experimental autoimmune prostatitis (EAP). We also report antiproliferative effects of MIA-690 in in vitro 3D models of inflammation and EMT.
Results
Immunohistochemical and Immunocytochemical Confirmation of the Expression of GHRH and GHRHR Proteins.
As revealed by immunohistochemical analyses, GHRH and GHRHR are predominant in epithelial cells of the ventral prostates of mice (Fig. 1A). The expression of GHRH and GHRHR were also confirmed in human BPH-1 cells and primary prostate epithelial (PrEp) cells (Fig. 2A).
Fig. 1.
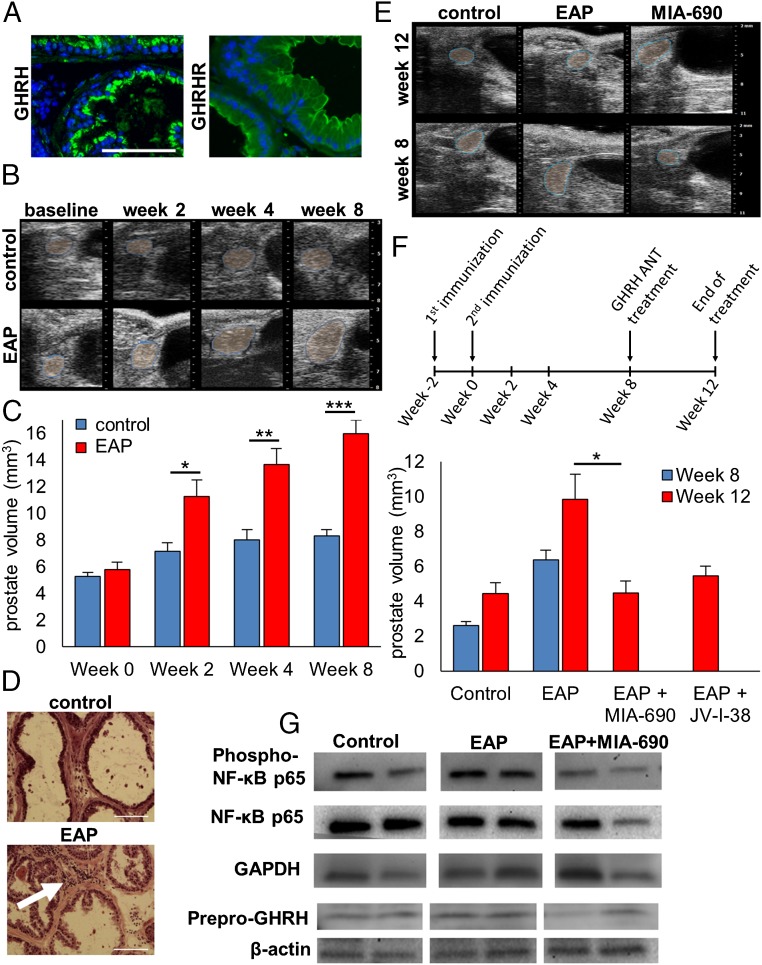
GHRH antagonists MIA-690 and JV-I-38 reduce prostate enlargement in EAP. (A) GHRH and GHRHRs are localized mainly in the epithelial cells of the ventral prostate of Balb/C mice, as shown by immunohistochemistry. (Scale bar: 100 µm.) (B) Representative images showing volumetric changes in the ventral prostate detected by VEVO 1100 US imaging system. EAP was induced by two immunizations with a homogenate of rat male tissue injected 2 wk apart. Ultrasound images were recorded before the first injection (baseline) and 2 and 8 wk after the second injection. (C) Average volume of ventral prostates in EAP and control animals before and 2, 4, and 8 wk after the induction. (D) H&E staining shows leukocyte infiltrate in the ventral prostate of the EAP model (arrow) and no signs of inflammation in the control tissue. (Scale bar: 100 µm.) (E and F) GHRH antagonists reduce inflammation-induced enlargement of the ventral prostate in EAP. As depicted by the scheme, mice were treated with GHRH antagonists MIA-690 or JV-I-38 at 5 µg/d for 4 wk starting 8 wk after the induction of EAP and compared with vehicle-treated animals. Representative ultrasound images (E) and average volumes of the ventral prostates (F) are shown 8 and 12 wk after the induction of EAP. (G) Western blot analysis of the expression of phospho-NF-κβ p65, NF-κβ p65, and prepro-GHRH in ventral prostates from control, EAP, and EAP treated with MIA-690. GAPDH or β-actin were used as standard genes (*P < 0.05, **P < 0.01, and ***P < 0.01 by Student’s t test).
Fig. 2.
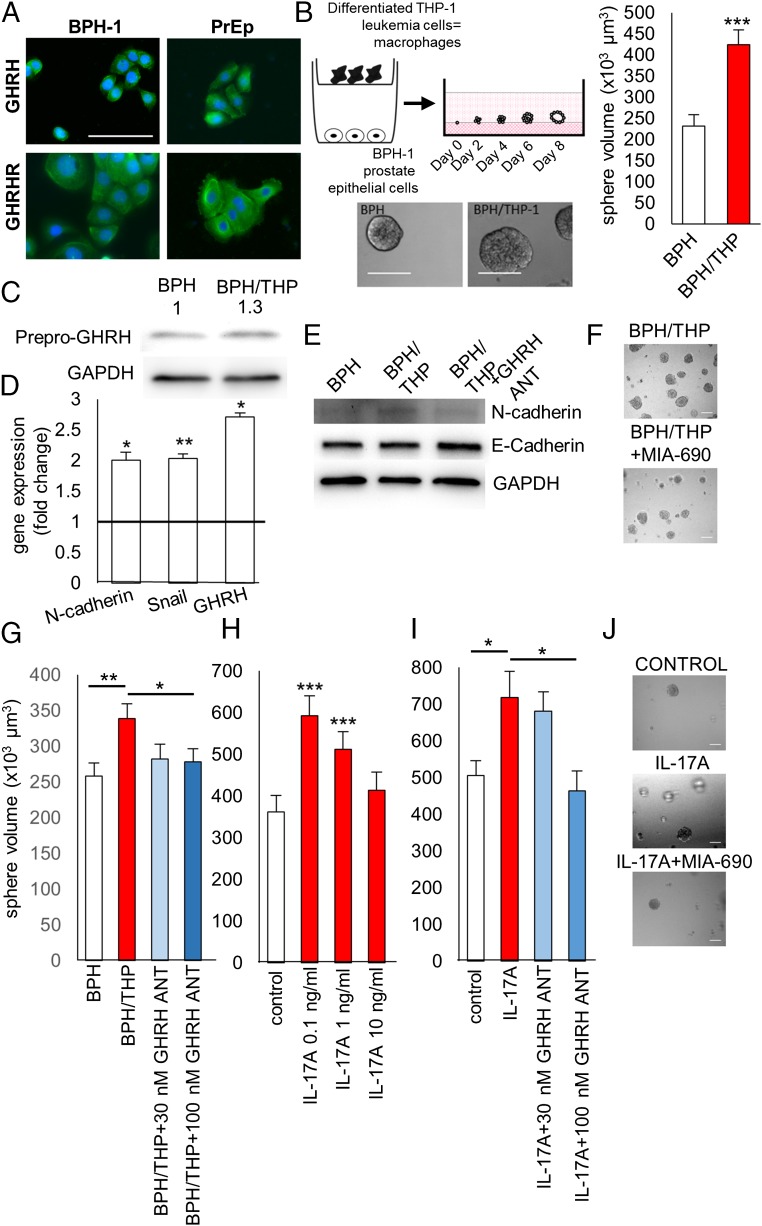
GHRH antagonists inhibit proliferation and EMT induced by inflammation in 3D cell culture in BPH-1 cells and in PrEp cells. (A) GHRH and GHRHRs are expressed in BPH-1 cells and in PrEp cells as detected by immunocytochemistry. (Scale bar: 100 µm.) (B) In vitro model of inflammation-induced proliferation: BPH-1 cells were treated with conditioned medium derived from differentiated THP-1 macrophages that are cocultured with BPH-1 cells (BPH/THP) for 8 d. Control cells were incubated in medium derived from BPH-1 cells only (BPH). Representative images of BPH-1 spheres are shown. (Scale bar: 100 µm.) The average volume of spheres was determined from three parallel experiments. (C) Expression of protein for GHRH and (D) the expression of mRNA for N-cadherin, Snail, and GHRH are increased in cells treated with BPH/THP medium compared with control as determined by Western blot and quantitative PCR, respectively. (E) Representative images of Western blot analysis of the expression of N-cadherin, E-cadherin, and GAPDH in 3D cultures from BPH, BPH/THP, or BPH/THP with 100 nM treatment. (F and G) GHRH antagonist MIA-690 added at 30 nM or 100 nM concentration to BPH/THP medium suppresses the stimulatory action of macrophage-conditioned medium on the growth of BPH-1 spheres. (Scale bar: 100 µm.) (H) The effect of IL-17A on the growth of PrEp cells was tested at 0.1 ng/mL, 1 ng/mL, and 10 ng/mL. (I and J) GHRH antagonist MIA-690 inhibits growth of PrEp spheres stimulated by IL-17A. Cultures were treated with 0.1 ng/mL IL-17A alone or with MIA-690 at 30-nM or 100-nM concentrations. (Scale bar 100 µm.) *P < 0.05, **P < 0.01, and ***P < 0.01 by Student’s t test.
EAP Induces Prostatic Enlargement.
Autoimmune prostatitis in Balb/C mice was induced by a previously established method (41). Mice were injected with two immunization injections of homogenate of prostate, seminal vesicle, and coagulating gland from rats that had been reported to induce a marked inflammation after 8 wk. Other models of experimental prostatitis showed an increase in the weights of the prostate induced by inflammation (42). Therefore, our first aim was to establish a detection method that enables the in situ detection of the growth of the prostate during the development of chronic prostatitis. Ventral prostates of mice were imaged by ultrasound by using a VEVO 1100 imaging system (FujiFilm VisualSonics), and volumes were calculated as described in Materials and Methods. Average volumes were not significantly different before the immunization (5.29 mm3 in control vs. 5.81 mm3 in EAP; Fig. 1 B and C). Two weeks after induction, EAP prostates were enlarged by 57% to 11.25 mm3 vs. 7.17 mm3 in the control group (P < 0.05). At week 4, prostates in the EAP group were 70% larger than controls (13.65 mm3 vs. 8.01 mm3; P < 0.01) and further increased to a final measurement of 92% at week 8 (15.99 mm3 vs. 8.33 mm3; P < 0.001). Prostatic inflammation was further confirmed by the transcriptional up-regulation of genes involved in inflammatory response such as bone morphogenic protein 3 (BMP3), Ctf2, growth differentiation factor 10 (Gdf10), Mstn, IFN-γ, IL-10, IL-13, and IL-7 (P < 0.05; Table S1). Inflammation was also confirmed histologically by the presence of leukocyte infiltrates 12 wk after the induction of inflammation by visual assessment of H&E staining of frozen sections of the ventral prostates (Fig. 1D).
Table S1.
Expression of genes in the ventral prostates of mice involved in inflammatory response in EAP
| Symbol | Description | Accession no. | Fold change |
| Bmp3 | Bone morphogenic protein 3 | NM_173404 | 2.66* |
| Ctf2 | Cardiotrophin 2 | NM_198858 | 2.3* |
| GDF-10 | Growth differentiation factor 10 | NM_145741 | 2.15* |
| Mstn | Myostatin | NM_010834 | 2.49* |
| Ifng | IFN-γ | NM_008337 | 2.51* |
| Il10 | IL-10 | NM_010548 | 4.09* |
| Il13 | IL-13 | NM_008355 | 1.77* |
| Il7 | IL-7 | NM_008371 | 3.42* |
| Cd70 | CD70 antigen | NM_011617 | 3.81 |
| Tnfsf8 | TNF (ligand) superfamily, member 8 | NM_009403 | 2.68 |
Multiple genes involved in inflammation were evaluated for expression using real-time PCR via the RT2 Profiler PCR Array system. The table lists the genes that are significantly up-regulated in the EAP group or increased nonsignificantly with at least a threefold elevation in ventral prostates obtained from the EAP model compared with controls. The data were evaluated by two-tailed Student’s t test.
P < 0.05.
GHRH Antagonists Reduce Prostatic Enlargement Induced by EAP.
Prostatic inflammation was induced by immunization with tissue of male rats, and animals were randomized into three groups (Fig. 1 E and F). Mice were treated with the GHRH antagonists MIA-690 or JV-I-38 at 5 µg/d for 4 wk, whereas control animals received vehicle treatment. The volumes of ventral prostates of control animals increased from 2.60 mm3 to 4.46 mm3, and there was also a further increase in the growth of EAP mice from 6.37 mm3 to 9.86 mm3 (Fig. 1F). Treatment with MIA-690 reduced the average volume of the ventral prostates in EAP mice by 30% to 4.48 mm3 (P < 0.05). JV-I-38 also decreased the average volume of the prostate, but this effect was not statistically significant (reduction of 14.3% to 5.46 mm3). In addition, MIA-690 reduced the phosphorylation of NF-κB and the elevation in the protein levels of prepro-GHRH induced by inflammation (Fig. 1G).
The Expression of GHRH Is Induced in an in Vitro Model of Inflammation.
An in vitro model of inflammation was generated according to a method published by Lu et al. (25). The average spherical volume increased by 82.7% in cultures treated with conditioned medium derived from BPH-1 and human acute monocytic leukemia cell lines (BPH/THP) compared with control (232,063.7 µm3 vs. 423,966.9 µm3; P < 0.001; Fig. 2B). The expression of mRNA for the mesenchymal markers N-cadherin and Snail was increased by the conditioned medium by one-fold (P < 0.05) and 1.03-fold (P < 0.01), respectively. The expression of mRNA and protein for GHRH was also up-regulated by 1.7-fold (P < 0.05) and 30%, respectively (Fig. 2 C and D).
GHRH Antagonists Inhibit Proliferation and EMT Induced by Inflammation.
The average volume of BPH-1 spheres that were stimulated by conditioned medium was reduced by GHRH antagonist MIA-690 at 30 nM and 100 nM by 56,332.9 µm3 and 60,658.2 µm3 (inhibition of 70.1% and 75.5%), respectively [not significant (NS) and P < 0.05, respectively; Fig. 2 F and G]. The protein levels for N-cadherin were also suppressed by 100 nM MIA-690 in cultures treated with BPH/THP medium (Fig. 2E).
GHRH Antagonists Reduce the Proliferation of Primary Epithelial Cells Stimulated by IL-17A.
IL-17A was tested at 0.1 ng/mL, 1 ng/mL, and 10 ng/mL to investigate its effect on the proliferation of PrEp cells grown in 3D culture and to establish an additional in vitro model of inflammation. The average sphere volume was significantly increased in cultures treated with 0.1 ng/mL and 1 ng/mL IL-17A (increase of 230,129.2 µm3 and 150,157.2 µm3 compared with control, respectively; P < 0.001), whereas, at the concentration of 10 ng/mL, IL-17A did not cause a significant change (Fig. 2H). The GHRH antagonist MIA-690 reduced the average volume of spheres by 191,458.2 µm3 (inhibition of 124.1%) at 100 nM concentration, but had no significant effect at 30 nM concentration (Fig. 2 I and J).
GHRH Antagonists Inhibit Proliferation and EMT Induced by TGF-β2.
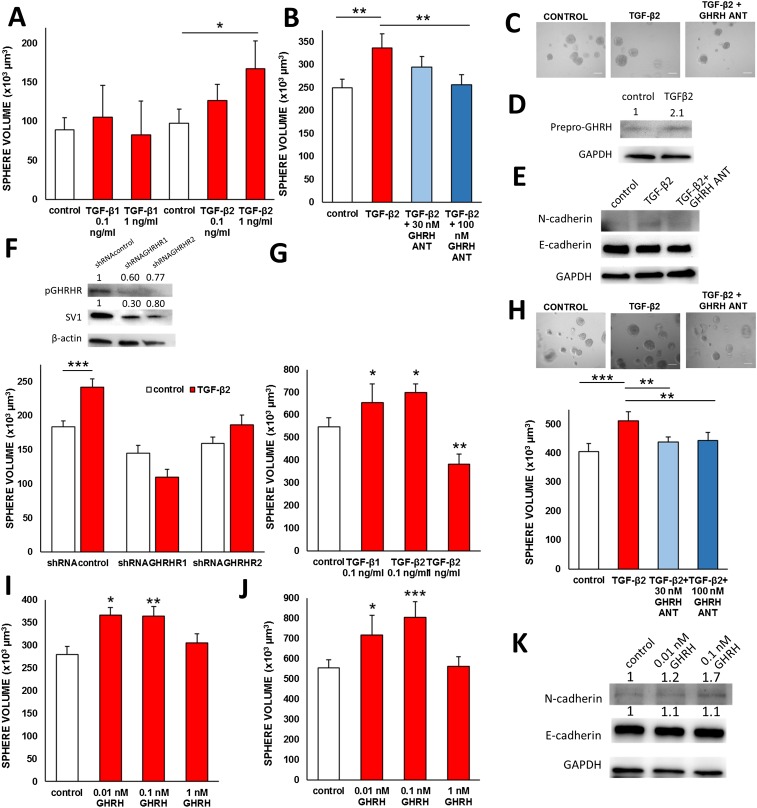
The increase in the expression of N-cadherin observed in BPH-1 cells stimulated with conditioned medium is dependent on TGF-β activity (25). To establish an EMT-induced model of proliferation, we tested the effect of TGF-β1 and TGF-β2 at 0.1 ng/mL and at 1 ng/mL concentrations in 3D cultures of BPH-1 cells. TGF-β1 did not affect the proliferation of BPH-1 cells. In contrast, TGF-β2 at 0.1 ng/mL increased average sphere volume by 29,210.9 µm3 (NS) and 69,816.78 µm3 at 1 ng/mL concentration (P < 0.05; Fig. S1A). Protein levels of GHRH were also increased by 1 ng/mL TGF-β2 (Fig. S1D). The GHRH antagonist MIA-690 at 100 nM reduced the average volume of spheres of BPH-1 cells stimulated by 1 ng/mL TGF-β2 (91.8% inhibition of TGF-β2 stimulatory activity; Fig. S1 B and C). The expression of protein for N-cadherin was also suppressed by MIA-690 in cells stimulated by TGF-β2, whereas the expression of E-cadherin was not presented with consistent changes (Fig. S1E). The stimulatory action of TGF-β2 was diminished by stable transfection of shRNA constructs that target the expression of GHRHR and its splice variant (SV1) as shown on the representative Western blot (Fig. S1F). The volume of BPH-1 spheres expressing scrambled control shRNA was increased by 31.4% upon stimulation with 1 ng/mL TGF-β2 (183,995.5 µm3 vs. 241,736.0 µm3 with stimulation; P < 0.001). In contrast, treatment with 1 ng/mL TGF-β2 decreased the volume of spheres composed of shRNAGHRHR1 expressing cells by 24.3% (144,882.4 µm3 vs. 109,625.5 µm3 with stimulation; NS). The stable transfection of another construct, shRNAGHRHR2, also decreased the stimulatory action of TGFβ-2 by 16.7% (159,700.5 µm3 vs. 186,341.1 µm3 with stimulation; NS). The effects of TGF-β1 and TGFβ-2 were also tested on the proliferation of PrEp cells in 3D cultures. At 0.1 ng/mL, TGF-β1 and TGF-β2 increased the average volume of spheres by 106,594.3 µm3 (P < 0.05) and 150,387.0 µm (P < 0.05), respectively, whereas TGF-β1 at 1 ng/mL had an inhibitory effect (Fig. S1G). The stimulatory effect of TGF-β2 was significantly inhibited by GHRH antagonist MIA-690 at 30-nM and 100-nM concentrations (reduction of 73,999.8 µm3/69.9% and 67,678.8 µm3/63.9%, respectively; P < 0.01 for both; Fig. S1H).
Fig. S1.
GHRH antagonists inhibit proliferation and EMT induced by TGF-β2 in prostate epithelial cells. (A) TGF-β2, but not TGF-β1, promotes the growth of BPH-l spheres. TGF-β1 and TGF-β2 were tested at 0.1 ng/mL and 1 ng/mL for 8 d. (B and C) GHRH antagonist MIA-690 at 30-nM or 100-nM concentrations suppressed the growth of BPH-1 spheres induced by 1 ng/mL TGF-β2. (Scale bar: 100 µm.) (D) The expression of GHRH protein is up-regulated by treatment with TGF-β2 at 1 ng/mL concentration in BPH-1 cells grown in 3D culture. (E) The expression of N-cadherin increased by TGF-β2 treatment is reduced by MIA-690 as determined by Western blot. E-cadherin levels were not affected by MIA-690. (F) The effect of TGF-β2 on the growth of BPH-1 spheres is reduced when the expression of GHRHR is silenced. The levels of pituitary-type GHRHR (pGHRH) and its splice variant 1 (SV1) are down-regulated by the stable transfection of shRNA in psi-H1 vector as determined by Western blot. (G) The growth of PrEp spheres is stimulated by TGF-β1 and TGF-β2. Cells were stimulated with 0.1 ng/mL TGF-β1 or 0.1 ng/mL or 1 ng/mL TGF-β2 for 8 d. (H) GHRH antagonist MIA-690 inhibits the effect of TGF-β2 on the growth of PrEp spheres. (I) GHRH(1-29)NH2 at concentrations of 0.01 nM and 0.1 nM significantly increased the average diameter of BPH-1 spheres, but had no effect at 1 nM. (J) The average diameter of PrEp spheres was stimulated by 0.1 nM GHRH(1-29)NH2. (K) GHRH(1-29)NH2 at 0.1 nM up-regulated the expression of N-cadherin, whereas it did not affect the levels of E-cadherin in BPH-1 cells. *P < 0.05, **P < 0.01, and ***P < 0.01 by Student's t test.
GHRH Stimulates the Proliferation and Expression of N-Cadherin in Prostate Epithelial Cells.
GHRH(1-29)NH2 at low concentrations of 0.01 nM and 0.1 nM increased the volume of BPH-1 spheres by 86,712.0 µm3 (P < 0.05) and 84,448.5 µm3 (P < 0.01), respectively (Fig. S1I). GHRH(1-29)NH2 at 0.1 nM concentration also increased the volume of PrEp spheres by 250,084.2 µm3 (P < 0.001; Fig. S1J). GHRH(1-29)NH2 at 0.1 nM increased the expression of N-cadherin protein but had no effect on the protein levels of E-cadherin (Fig. S1K).
Discussion
The clinical management of BPH shifted from surgical to medical care in the 1990s, decreasing risks related to surgery, but this converted the disease into a chronic condition, increasing the economic burden on the US healthcare system (43). Currently available treatments, 5-α reductase inhibitors and α-blockers, have been selected based on their beneficial action in alleviating symptoms, but these therapeutic medical modalities have low efficiency (43). The development of new therapies is challenging because the root cause of the condition is still unresolved. BPH is an age-related disease that is most likely caused by multiple factors (14). There is a growing body of evidence implying that inflammation is a causal factor in the pathogenesis of BPH, including a recently identified positive correlation between the presence/degree of inflammation and prostatic volume/weight (16, 44).
The proinflammatory environment may stimulate prostatic growth by multiple ways depending on the origin of inflammation (autoimmune vs. bacterial prostatitis) and the duration of the inflammatory episode (acute vs. chronic). Inflammation and EMT have been linked in the context of carcinogenesis and metastatic potential (45), but little is known about their interplay in benign cells. The first evidence of the involvement of EMT in BPH was provided by Alonso-Magdalena et al., who reported the down-regulation of CK8 and E-cadherin and up-regulation of pSMAD, Snail, and Slug in multilayered BPH ducts (27). We used an in vitro model of inflammation that contains various factors secreted by macrophages originally reported by Lu et al. (25). Lu et al. reported that, in this system, cells undergo EMT characterized as up-regulation in the expression of N-cadherin and Snail with a down-regulation of E-cadherin. The authors identified this phenomenon as an enrichment of mesenchymal cells linked with the concept that BPH is primarily a proliferative stromal disease (25). In our study, we were unable to show the down-regulation of E-cadherin, but this might be the result of the small number of cells that underwent EMT. Interestingly, immortalized human mammary cells similarly undergo EMT upon TGF-β1 treatment, but they also gain stem cell properties (46). This is consistent with earlier findings reported by Wang et al. that showed an increased number of epithelial progenitor cells in the prostate in a bacterially induced mouse model of inflammation (47). In our study, the increase of N-cadherin–positive cells might indicate the appearance of stem-like cells that fuels the expansion of epithelial cells. Our finding that antagonists of GHRH disrupt the increased proliferative activity and decrease the expression of N-cadherin in prostatic epithelial cells triggered by the inflammatory environment was not previously described and is of great interest.
In the past few years, essential roles for fibrosis consequent to inflammation and aging in the development of LUTSs have been suggested (48). Fibrosis is defined as the progressive buildup of fibrotic connective tissue involving an increased number of myofibroblasts emerging through the transdifferentiation of epithelial cells and fibroblasts (49). The up-regulation of mesenchymal markers in BPH-1 cells triggered by the inflammatory environment in our study may mark the appearance of the mesenchymal phenotype and represent the very early stages of tissue fibrosis. Aging may contribute to the preservation of the fibrotic tissue by triggering changes in cell homeostasis and resistance to apoptosis (50). Further studies of this phenomenon would be of interest to determine how repeated activation of inflammatory pathways induces proliferation and/or mesenchymal transition in prostate epithelial cells.
In an effort to model inflammation in PrEp cells, we tested the effect of IL-17A at different concentrations on the growth of spheres. IL-17A is secreted by a subclass of T helper cells (TH17), linked to autoimmune prostatitis, and stimulates the expression of other cytokines and chemokines such as TNF-α, IL-6, and IL-8 (51, 52). An earlier study could not demonstrate stimulatory activity on the proliferation of prostatic epithelial cells at the concentration of 20 ng/mL (53). We were able to verify the stimulatory effects of IL-17A added at low concentrations; moreover, we showed that this effect was also inhibited by GHRH antagonists.
The up-regulation of N-cadherin induced by the inflammatory environment inspired our further tests with TGF-β peptides, which are the primary regulators of EMT but are known to inhibit the proliferation of noncancerous epithelial cells in various contexts (54). In our system, we used long-term administration of low concentrations that are rarely used in other studies, and we found that TGF-β2 in BPH-1 and PrEp cells, and TGF-β1 in PrEp cells, stimulate proliferative activity. Moreover, GHRH seems to be a key modulator of this effect given the following: (i) the expression of GHRH is stimulated by TGF-β2, (ii) GHRH antagonists decrease the volume of spheres induced by TGF-β2, and (iii) the effect of TGF-β2 on the growth of BPH-1 spheres is disrupted when the expression of GHRHRs is silenced by the stable transfection of shRNA. Our finding that the down-regulation of GHRH signaling counteracts the proliferative effects of TGF-β2 is particularly intriguing but requires further investigation to reveal the exact interaction between these pathways. Untreated cells in which GHRH receptors are silenced to a greater extent with shRNAGHRH1 form spheres with smaller volume. Therefore, it is a challenge to estimate the distinct impact of GHRHR signaling on the effects of TGF-β2; it is also not possible to identify if this interaction is direct or indirect. Nevertheless, these important findings stimulate further investigations of the interplay between GHRH and TGF-β2 in healthy and inflamed cells.
In our previous studies that used a testosterone-induced BPH model in rats, we found that GHRHRs in the prostate are up-regulated after treatment with testosterone, whereas treatment with GHRH antagonists decreased prostate weights and reduced the protein levels of IL-1β, NF-κβ/p65, and cyclooxygenase-2 (36). In the current study, we used a model of autoimmune prostatitis that has been previously shown to develop progressive and chronic inflammation (41). We demonstrated the up-regulation of pro- and anti-inflammatory genes 8 wk after the immunization by PCR array. These genes have been associated with the induction of proliferation (IL-7, IFN-γ) (21), interference with steroidogenesis (IL-13) (55), and inhibition of inflammation (IL-10) (56) or have currently unknown function in the prostate (cardiotrophin-2). We also detected an increase in the expression of members of the transforming growth factor-β superfamily such as myostatin, GDF-10, and BMP-3. We established an ultrasound method that enables the in situ detection of volumetric growth of the ventral prostate during the progression of prostatitis. Enlarged prostates were shrunk by a 1-mo treatment with GHRH antagonists, which could be the consequence of multiple actions of the blockade of GHRH in vivo. First, GHRH antagonists exert their effects by down-regulating the activity of the GHRH/GH/IGF1 axis (33). IGF1, in fact, has been shown to direct the effects of IL-1 during experimental prostatic hyperplasia (57). Second, GHRH antagonists potentially act on local receptors in the prostate. This is supported by our finding that the expression of GHRH is up-regulated in vivo and in the in vitro inflammation model, as well as by treatment with TGF-β2. This paracrine GHRH is most likely a growth factor based on our results demonstrating that low concentrations of exogenous GHRH stimulated the growth of prostatic spheres. In addition, GHRH also increased the expression of N-cadherin, which implies that GHRH may be a key regulator of EMT and/or stimulate the expansion of progenitor cells.
In summary, we demonstrated that GHRH antagonists reduce prostatic enlargement, proliferation of prostatic epithelial cells, and expression of N-cadherin induced by inflammation. This effect is exerted through at least in part by prostatic GHRHRs. GHRH antagonists also disrupt the effects of TGF-β on cell proliferation and EMT. Our findings also indicate that GHRH acts as a local growth factor that is induced by the inflammatory environment. Our current and previous findings strongly indicate that GHRH antagonists may be clinically useful in the treatment of chronic prostatitis and BPH.
Materials and Methods
Peptides and Reagents.
The GHRH antagonists MIA-690 and JV-I-38 were synthesized in our laboratory by solid-phase methods and purified by reversed-phase high-performance liquid chromatography as described previously (58). The chemical structure of MIA-690 is [(PhAc-Ada)0-Tyr1, d-Arg2, Cpa6, Ala8, Har9, Fpa510, His11, Orn12, Abu15, His20, Orn21, Nle27, d-Arg28, Har29]hGH-RH(1–29)NH2. The synthesis and structure of JV-I-38 was previously published (59). TGF-β1, TGF-β2, and IL-17A were purchased from Cell Signaling Technology.
Animals and EAP.
Eight-week-old BALB/c mice, purchased from Harlan Laboratories, were housed in a climate-controlled environment with a 12-h light/dark cycle and fed standard laboratory diet with water ad libitum. Animal care was in accordance with institutional guidelines and complied with National Institutes of Health policy. EAP was induced as previously described (41). Tissues of male rats (prostate, seminal vesicle, and coagulating gland) were homogenized in PBS solution with protease inhibitors, and homogenate was centrifuged at 10,000 × g for 30 min. Supernatant was collected and emulsified with an appropriate volume of complete Freund’s adjuvant by using opposed glass syringes. Mice were immunized with emulsion containing 1 mg protein in 150 µL volume administered twice, 2 wk apart, by s.c. injections into the flank. Control animals received no injections.
Study Design.
Animals were randomized 8 wk after the second injection of immunization and were treated with MIA-690 or JV-I-38 at a dose of 5 µg/d for 4 wk. Control animals received 0.1% DMSO in 10% (vol/vol) aqueous propylene glycol solution. Mice were killed under anesthesia after 4 wk of treatment. Ventral prostates were collected and snap-frozen, preserved in optimal cutting temperature compound (OCT) or immersed in buffered 10% (vol/vol) formalin (pH 7.4), and embedded in paraffin for histological analysis.
Monolayer and 3D Culture of Cells.
The human prostate epithelial BPH-1 cell line was donated by Simon Hayward (Vanderbilt University Medical Center, Nashville, TN) and maintained in RPMI-1640 medium (Life Technologies) supplemented with 5% (vol/vol) FBS. THP-1 cells (human acute monocytic leukemia cell) were obtained from American Type Culture Collection and were maintained in RPMI-1640 with 10% (vol/vol) FBS. PrEp cells were purchased from Millipore and were cultured in EpiGRO Basal Medium (Millipore). Cells were kept at 37 °C in a humidified atmosphere containing 5% CO2. The 3D cultures were generated as described previously (25). Briefly, 45 μL of Matrigel was added to wells in an eight-well chamber slide and placed in a 37 °C incubator for 1 h. BPH-1 and PrEp cells were plated at 2,000 cells per well in medium containing 2% (vol/vol) Matrigel. Treatments (conditioned medium, TGF-β1, TGF-β2, or IL-17A) were added the next day for 8 d. Images were acquired on a Nikon Eclipse Ti fluorescence microscope at 10× magnification, and the diameters of spheres were measured by using ImageJ software (National Institutes of Health). The average diameter of cells was calculated from triplicates. Additional information is provided in SI Materials and Methods.
SI Materials and Methods
Prostate Echography and Calculation of Prostatic Volumes.
Mice were weighed and anesthetized with 5.0% isoflurane in pure oxygen (0.8 L/min) by using a 1.0-L induction chamber (VetEquip). Anesthesia was maintained by using 1.5% isoflurane delivered by a nose cone. Anesthetized mice were transferred to a heated monitoring platform (Indus Instruments) and placed in a supine position. Vital signs (heart and breath rates) were monitored during the echographic procedure. Prewarmed ultrasound coupling gel (Aquasonic 100; Parker Laboratories) was applied to the depilated skin before imaging. Echo images were acquired by using a VEVO 1100 imaging system (FujiFilm VisualSonics) with a MicroScan 30-MHz high-frequency linear-array transducer (model MS400). The 2D images of the prostate were used to determine prostate structure. Short-axis (transverse) and long-axis (sagittal) views of the prostate were obtained by using brightness mode (B-mode) imaging. Probe rotation of 90° allowed imaging of either orientation by using the bladder as a landmark. Serial echographic measurements were obtained at 0, 2, 4, and 8 wk after the experimental induction of EAP. Offline echo analysis was performed by a single observer by using Vevo Lab 1.7.0 postprocessing software (FujiFilm VisualSonics). The 2D B-mode ultrasound images were processed and analyzed to derive the prostate volume. The ventral prostate boundaries were manually delineated, and the volume was calculated by using the prolate ellipsoid formula (V = 4/3πab2).
Generation of Conditioned Medium.
THP-1 cells were differentiated into macrophages in T75 flasks containing 60 ng/mL phorbol myristate acetate in culture medium for 48 h. Flasks were then washed with PBS solution and incubated with normal medium for 48 h. Conditioned media was generated in six-well transwell plates in which differentiated THP-1 cells (3 × 105 cells) were seeded in the upper insert (0.4 μm) and BPH-1 cells in the lower part (3 × 105 cells). Medium was collected after 48 h and used for further experiments.
Stable Transfection of shRNA into BPH-1 Cells.
Constructs containing shRNA were designed to target the expression of pituitary type GHRHR and all its splice variants (sequences, shRNA GHRH1, GGA TCA TCA AAG GGC CCA TTG; shRNA GHRH2, GGC AAA GGT GCT GAC ATC TAT) and were obtained in psi-H1 vector with an additional scrambled control from GeneCopoeia. Constructs were transfected in BPH-1 cells by electroporation by using a Bio-Rad Gene Pulser at 230 V, 950 μF with 5 μg of DNA/0.4 cm cuvette. Stable clones were selected in medium containing 0.3 µg/mL puromycin for 2 wk, and individual clones were isolated with cloning rings.
Isolation of RNA, Quantitative Real-Time RT-PCR, and Real-Time PCR Array.
Total RNA was isolated from BPH-1 spheres and mouse prostates by using the RNA Spin Mini Kit (GE Healthcare). One microgram of RNA was reverse-transcribed with the RevertAid H minus First-Strand cDNA kit (Thermo Scientific). Primers used were as follows: N-cadherin sense, 5′-AGC CTG ACA CTG TGG AGC CT-3′; Snail sense, 5′GAG GCG GTG GCA GAC TAG AGT 3′; antisense, 5′CGG GCC CCC AGA ATA GTT C 3′, GHRH sense, 5′AAG ATT CCT CCT GTG ACC CG; antisense, 5′TTA AAT GCA CAC CGG TAT GGG G 3′; GAPDH sense, CTT CAC CAC CAT GGA GAA GGC 3′; antisense, 5′ CAT ACC AGG AAA TGA GCT TGA CAA 3′. Quantitative real-time PCR was conducted by using a Bio-Rad CFX96 system with SYBR Green to determine the level of mRNA expression of a gene of interest. Expression levels were normalized to the expression of GAPDH RNA. Mouse common cytokine arrays (PAMM-021A RT2 Profiler Real-Time PCR arrays; SABiosciences) were used to examine the mRNA levels of inflammatory cytokines in the ventral prostates of EAP mice compared with control animals. Fold changes in gene expression were calculated by ΔΔCt method. Normalization was performed by using five housekeeping genes on the arrays.
Immunohistochemistry, Immunocytochemistry, and H&E Staining of Frozen Sections.
Tissues embedded in OCT were cryosectioned at 5 µm, dried, and fixed in 4% buffered paraformaldehyde. H&E staining was performed with standard protocol. For immunohistochemistry, tissues were fixed in 4% paraformaldehyde, permeabilized in ice-cold methanol, and blocked with 5% goat serum. Primary antibody for GHRH was added overnight (1:50 dilution; ab187512; Abcam).
Paraffin sections were deparaffinized and rehydrated with xylene and graded series of ethanol. Antigen retrieval was performed in a pressure cooker with Trilogy antigen retrieval solution (Cell Marque), and tissues were blocked with Background Terminator (Biocare Medical). Tissues were incubated with GHRHR antibody overnight (1:200 dilution; ab76263; Abcam). For immunocytochemistry, cells were grown in chamber slides, washed with PBS solution three times, and fixed in 4% paraformaldehyde for 7 min. Permeabilization was performed by a 10-min incubation in PBS solution containing 0.2% Triton-X, and then cells were blocked with 5% goat serum in PBS solution for 1 h. Human-specific primary antibodies for GHRH (1:500 dilution; ab8899; Abcam) and GHRHR (1:150 dilution; ab28692; Abcam) were added for 1 h.
Anti-rabbit secondary antibody (Alexa Fluor 488; Life Technologies) was applied for 1 h in immunohistochemistry and immunocytochemistry protocols. Slides were counterstained with nuclear staining (DAPI; Sigma-Aldrich) and were mounted in SlowFade Diamond Antifade Mountant. Images were acquired on a Nikon Eclipse Ti fluorescence microscope.
Western Blot.
Ventral prostates of mice were homogenized in RIPA buffer containing protease and phosphatase inhibitors, and debris was removed by centrifugation at 14,000 × g for 10 min. BPH-1 and PrEp spheres were released from Matrigel by a 20-min incubation in Cell Recovery Solution (Corning) on ice and harvested by centrifugation. Proteins were isolated in RIPA buffer. Protein concentrations were determined by BCA protein assay (Thermo Fisher Scientific). Equal amounts of proteins were mixed with Laemmli buffer (Bio-Rad Laboratories) and were incubated at 99 °C for 5 min on a heat block. Proteins were separated on 4–15% PAGE and were transferred onto PVDF membrane. Blocking was performed in 5% milk. Membranes were incubated with primary antibodies for phosphorylated NF-κB (1:500, ab13110), total NF-κB (1:1,000, 8242; Cell Signaling), GHRH (1:250, ab187512; Abcam), N-cadherin (1:100, ab66025; Abcam), E-cadherin (1:1,000, 3195; Cell Signaling), GHRHR (1:2,000, ab28692; Abcam), GAPDH (1:10000, G8795; Sigma-Aldrich), and β-actin (1:10000; A-5441; Sigma-Aldrich) overnight. Species-specific HRP-conjugated secondary antibodies (Thermo Fisher Scientific) were left on the membranes for 1.5 h, and the signal was developed with ECL reagent (Thermo Fisher Scientific) and then imaged with a Bio-Rad ChemiDoc System. Densitometry was performed by using ImageJ software (National Institutes of Health).
Statistical Analysis.
For statistical evaluation, SigmaPlot 13 software was used (Systat Software) with a two-tailed Student’s t test, and significance was accepted at P < 0.05.
Acknowledgments
This work was supported in part by the Urology Care Foundation Research Scholar Award Program and the American Urological Association Southeastern Section (to P.P.), Medical Research Service of the Veterans Affairs Department (A.V.S.), Departments of Pathology and Medicine, Division of Hematology/Oncology, and Sylvester Comprehensive Cancer Center of the Miller Medical School, University of Miami (A.V.S.), and South Florida Veterans Affairs Foundation for Research and Education (A.V.S.). A.V.S. is listed as coinventor on patents for GHRH antagonists, which have been assigned to the University of Miami and the Veterans Affairs Department; however, this study was purely experimental.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620884114/-/DCSupplemental.
References
- 1.Roehrborn CG. Benign prostatic hyperplasia: An overview. Rev Urol. 2005;7(suppl 9):S3–S14. [PMC free article] [PubMed] [Google Scholar]
- 2.McNeal JE. Origin and evolution of benign prostatic enlargement. Invest Urol. 1978;15(4):340–345. [PubMed] [Google Scholar]
- 3.Levin RM, et al. Genetic and cellular characteristics of bladder outlet obstruction. Urol Clin North Am. 1995;22(2):263–283. [PubMed] [Google Scholar]
- 4.Levin RM, et al. Obstructive response of human bladder to BPH vs. rabbit bladder response to partial outlet obstruction: A direct comparison. Neurourol Urodyn. 2000;19(5):609–629. doi: 10.1002/1520-6777(2000)19:5<609::aid-nau7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Slater S, Dumas C, Bubley G. Dutasteride for the treatment of prostate-related conditions. Expert Opin Drug Saf. 2012;11(2):325–330. doi: 10.1517/14740338.2012.658040. [DOI] [PubMed] [Google Scholar]
- 6.Lepor H, Kazzazi A, Djavan B. α-Blockers for benign prostatic hyperplasia: The new era. Curr Opin Urol. 2012;22(1):7–15. doi: 10.1097/MOU.0b013e32834d9bfd. [DOI] [PubMed] [Google Scholar]
- 7.Woo HH, et al. Safety and feasibility of the prostatic urethral lift: A novel, minimally invasive treatment for lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH) BJU Int. 2011;108(1):82–88. doi: 10.1111/j.1464-410X.2011.10342.x. [DOI] [PubMed] [Google Scholar]
- 8.Berardinelli F, Hinh P, Wang R. Minimally invasive surgery in the management of benign prostatic hyperplasia. Minerva Urol Nefrol. 2009;61(3):269–289. [PubMed] [Google Scholar]
- 9.Tan MO, Karabiyik I, Uygur MC, Diker Y, Erol D. Serum concentrations of sex hormones in men with severe lower urinary tract symptoms and benign prostatic hyperplasia. Int Urol Nephrol. 2003;35(3):357–363. doi: 10.1023/b:urol.0000022909.57112.3e. [DOI] [PubMed] [Google Scholar]
- 10.Hammarsten J, et al. Insulin and free oestradiol are independent risk factors for benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2009;12(2):160–165. doi: 10.1038/pcan.2008.50. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: Past, present and future. Differentiation. 2011;82(4-5):184–199. doi: 10.1016/j.diff.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucia MS, Lambert JR. Growth factors in benign prostatic hyperplasia: Basic science implications. Curr Urol Rep. 2008;9(4):272–278. doi: 10.1007/s11934-008-0048-6. [DOI] [PubMed] [Google Scholar]
- 13.Berger AP, et al. Increased growth factor production in a human prostatic stromal cell culture model caused by hypoxia. Prostate. 2003;57(1):57–65. doi: 10.1002/pros.10279. [DOI] [PubMed] [Google Scholar]
- 14.Untergasser G, Madersbacher S, Berger P. Benign prostatic hyperplasia: Age-related tissue-remodeling. Exp Gerontol. 2005;40(3):121–128. doi: 10.1016/j.exger.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Hammarsten J, Högstedt B, Holthuis N, Mellström D. Components of the metabolic syndrome-risk factors for the development of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 1998;1(3):157–162. doi: 10.1038/sj.pcan.4500221. [DOI] [PubMed] [Google Scholar]
- 16.Elkahwaji JE. The role of inflammatory mediators in the development of prostatic hyperplasia and prostate cancer. Res Rep Urol. 2012;5:1–10. doi: 10.2147/RRU.S23386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohnen PW, Drach GW. Patterns of inflammation in prostatic hyperplasia: A histologic and bacteriologic study. J Urol. 1979;121(6):755–760. doi: 10.1016/s0022-5347(17)56980-3. [DOI] [PubMed] [Google Scholar]
- 18.Delongchamps NB, et al. Evaluation of prostatitis in autopsied prostates--is chronic inflammation more associated with benign prostatic hyperplasia or cancer? J Urol. 2008;179(5):1736–1740. doi: 10.1016/j.juro.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandaglia G, et al. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH) BJU Int. 2013;112(4):432–441. doi: 10.1111/bju.12118. [DOI] [PubMed] [Google Scholar]
- 20.Chughtai B, Lee R, Te A, Kaplan S. Role of inflammation in benign prostatic hyperplasia. Rev Urol. 2011;13(3):147–150. [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer G, et al. Increased expression of lymphocyte-derived cytokines in benign hyperplastic prostate tissue, identification of the producing cell types, and effect of differentially expressed cytokines on stromal cell proliferation. Prostate. 2002;52(1):43–58. doi: 10.1002/pros.10084. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, et al. Increased infiltrated macrophages in benign prostatic hyperplasia (BPH): Role of stromal androgen receptor in macrophage-induced prostate stromal cell proliferation. J Biol Chem. 2012;287(22):18376–18385. doi: 10.1074/jbc.M112.355164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boehm BJ, Colopy SA, Jerde TJ, Loftus CJ, Bushman W. Acute bacterial inflammation of the mouse prostate. Prostate. 2012;72(3):307–317. doi: 10.1002/pros.21433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.López-Novoa JM, Nieto MA. Inflammation and EMT: An alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1(6-7):303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu T, et al. Targeting androgen receptor to suppress macrophage-induced EMT and benign prostatic hyperplasia (BPH) development. Mol Endocrinol. 2012;26(10):1707–1715. doi: 10.1210/me.2012-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao R, et al. Epithelial-to-mesenchymal transition and estrogen receptor α mediated epithelial dedifferentiation mark the development of benign prostatic hyperplasia. Prostate. 2014;74(9):970–982. doi: 10.1002/pros.22814. [DOI] [PubMed] [Google Scholar]
- 27.Alonso-Magdalena P, et al. A role for epithelial-mesenchymal transition in the etiology of benign prostatic hyperplasia. Proc Natl Acad Sci USA. 2009;106(8):2859–2863. doi: 10.1073/pnas.0812666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiaris H, Chatzistamou I, Papavassiliou AG, Schally AV. Growth hormone-releasing hormone: Not only a neurohormone. Trends Endocrinol Metab. 2011;22(8):311–317. doi: 10.1016/j.tem.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Rick FG, et al. LHRH antagonist Cetrorelix reduces prostate size and gene expression of proinflammatory cytokines and growth factors in a rat model of benign prostatic hyperplasia. Prostate. 2011;71(7):736–747. doi: 10.1002/pros.21289. [DOI] [PubMed] [Google Scholar]
- 30.Rick FG, et al. Antagonists of growth hormone-releasing hormone inhibit growth of androgen-independent prostate cancer through inactivation of ERK and Akt kinases. Proc Natl Acad Sci USA. 2012;109(5):1655–1660. doi: 10.1073/pnas.1120588109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stangelberger A, et al. Inhibitory effects of antagonists of growth hormone releasing hormone on experimental prostate cancers are associated with upregulation of wild-type p53 and decrease in p21 and mutant p53 proteins. Prostate. 2012;72(5):555–565. doi: 10.1002/pros.21458. [DOI] [PubMed] [Google Scholar]
- 32.Schally AV, et al. Peptide analogs in the therapy of prostate cancer. Prostate. 2000;45(2):158–166. doi: 10.1002/1097-0045(20001001)45:2<158::aid-pros10>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 33.Schally AV, Varga JL, Engel JB. Antagonists of growth-hormone-releasing hormone: An emerging new therapy for cancer. Nat Clin Pract Endocrinol Metab. 2008;4(1):33–43. doi: 10.1038/ncpendmet0677. [DOI] [PubMed] [Google Scholar]
- 34.Fahrenholtz CD, et al. Preclinical efficacy of growth hormone-releasing hormone antagonists for androgen-dependent and castration-resistant human prostate cancer. Proc Natl Acad Sci USA. 2014;111(3):1084–1089. doi: 10.1073/pnas.1323102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Popovics P, Schally AV, Block NL, Rick FG. Preclinical therapy of benign prostatic hyperplasia with neuropeptide hormone antagonists. World J Clinic Urol. 2014;3(3):184–194. [Google Scholar]
- 36.Rick FG, et al. Antagonists of growth hormone-releasing hormone (GHRH) reduce prostate size in experimental benign prostatic hyperplasia. Proc Natl Acad Sci USA. 2011;108(9):3755–3760. doi: 10.1073/pnas.1018086108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rick FG, et al. Combining growth hormone-releasing hormone antagonist with luteinizing hormone-releasing hormone antagonist greatly augments benign prostatic hyperplasia shrinkage. J Urol. 2012;187(4):1498–1504. doi: 10.1016/j.juro.2011.11.081. [DOI] [PubMed] [Google Scholar]
- 38.Rick FG, et al. Mechanisms of synergism between antagonists of growth hormone-releasing hormone and antagonists of luteinizing hormone-releasing hormone in shrinking experimental benign prostatic hyperplasia. Prostate. 2013;73(8):873–883. doi: 10.1002/pros.22633. [DOI] [PubMed] [Google Scholar]
- 39.Qin YJ, et al. Antagonist of GH-releasing hormone receptors alleviates experimental ocular inflammation. Proc Natl Acad Sci USA. 2014;111(51):18303–18308. doi: 10.1073/pnas.1421815112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez R, et al. Antagonists of growth hormone-releasing hormone suppress in vivo tumor growth and gene expression in triple negative breast cancers. Oncotarget. 2012;3(9):988–997. doi: 10.18632/oncotarget.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson CM, et al. Strain-specific induction of experimental autoimmune prostatitis (EAP) in mice. Prostate. 2013;73(6):651–656. doi: 10.1002/pros.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng F, et al. Development and validation of an animal model of prostate inflammation-induced chronic pelvic pain: Evaluating from inflammation of the prostate to pain behavioral modifications. PLoS One. 2014;9(5):e96824. doi: 10.1371/journal.pone.0096824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hollingsworth JM, Wei JT. Economic impact of surgical intervention in the treatment of benign prostatic hyperplasia. Rev Urol. 2006;8(suppl 3):S9–S15. [PMC free article] [PubMed] [Google Scholar]
- 44.Bostanci Y, Kazzazi A, Momtahen S, Laze J, Djavan B. Correlation between benign prostatic hyperplasia and inflammation. Curr Opin Urol. 2013;23(1):5–10. doi: 10.1097/MOU.0b013e32835abd4a. [DOI] [PubMed] [Google Scholar]
- 45.Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, et al. Expansion of prostate epithelial progenitor cells after inflammation of the mouse prostate. Am J Physiol Renal Physiol. 2015;308(12):F1421–F1430. doi: 10.1152/ajprenal.00488.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma J, et al. Prostatic fibrosis is associated with lower urinary tract symptoms. J Urol. 2012;188(4):1375–1381. doi: 10.1016/j.juro.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hecker L, et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014;6(231):231ra47. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steiner GE, et al. Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab Invest. 2003;83(8):1131–1146. doi: 10.1097/01.lab.0000081388.40145.65. [DOI] [PubMed] [Google Scholar]
- 52.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.You Z, et al. Interleukin-17 induces expression of chemokines and cytokines in prostatic epithelial cells but does not stimulate cell growth in vitro. Int J Med Biol Front. 2012;18(8):629–644. [PMC free article] [PubMed] [Google Scholar]
- 54.Ten Dijke P, Goumans MJ, Itoh F, Itoh S. Regulation of cell proliferation by Smad proteins. J Cell Physiol. 2002;191(1):1–16. doi: 10.1002/jcp.10066. [DOI] [PubMed] [Google Scholar]
- 55.Gingras S, Côté S, Simard J. Multiple signaling pathways mediate interleukin-4-induced 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase type 1 gene expression in human breast cancer cells. Mol Endocrinol. 2000;14(2):229–240. doi: 10.1210/mend.14.2.0416. [DOI] [PubMed] [Google Scholar]
- 56.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 57.Hahn AM, Myers JD, McFarland EK, Lee S, Jerde TJ. Interleukin-driven insulin-like growth factor promotes prostatic inflammatory hyperplasia. J Pharmacol Exp Ther. 2014;351(3):605–615. doi: 10.1124/jpet.114.218693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zarandi M, et al. Synthesis and biological activities of highly potent antagonists of growth hormone-releasing hormone. Proc Natl Acad Sci USA. 1994;91(25):12298–12302. doi: 10.1073/pnas.91.25.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varga JL, et al. Synthesis and biological evaluation of antagonists of growth hormone-releasing hormone with high and protracted in vivo activities. Proc Natl Acad Sci USA. 1999;96(2):692–697. doi: 10.1073/pnas.96.2.692. [DOI] [PMC free article] [PubMed] [Google Scholar]