ABSTRACT
Microenvironment and stromal fibroblasts are able to inhibit tumor cell proliferation both through secreted signaling molecules and direct cell-cell interactions but molecular mechanisms of these effects remain unclear. In this study, we investigated a role of cell-cell contact-related molecules (protein ECM components, proteoglycans (PGs) and junction-related molecules) in intercellular communications between the human TERT immortalized fibroblasts (BjTERT fibroblasts) and normal (PNT2) or cancer (LNCaP, PC3, DU145) prostate epithelial cells. It was shown that BjTERT-PNT2 cell coculture resulted in significant decrease of both BjTERT and PNT2 proliferation rates and reorganization of transcriptional activity of cell-cell contact-related genes in both cell types. Immunocytochemical staining revealed redistribution of DCN and LUM in PNT2 cells and significant increase of SDC1 at the intercellular contact zones between BjTERT and PNT2 cells, suggesting active involvement of the PGs in cell-cell contacts and contact inhibition of cell proliferation. Unlike to PNT2 cells, PC3 cells did not respond to BjTERT in terms of PGs expression, moderately increased transcriptional activity of junctions-related genes (especially tight junction) and failed to establish PC3-BjTERT contacts. At the same time, PC3 cells significantly down-regulated junctions-related genes (especially focal adhesions and adherens junctions) in BjTERT fibroblasts resulting in visible preference for homotypic PC3-PC3 over heterotypic PC3-BjTERT contacts and autonomous growth of PC3 clones. Taken together, the results demonstrate that an instructing role of fibroblasts to normal prostate epithelial cells is revoked by cancer cells through deregulation of proteoglycans and junction molecules expression and overall disorganization of fibroblast-cancer cell communication.
KEYWORDS: extracellular matrix, fibroblast, junction molecules, microenvironment, prostate cancer, proteoglycan
Introduction
Tumor microenvironment (TME) has a crucial role in tumor initiation and progression.1-4 Complex relationship between epithelial cancer cells and the organ-specific microenvironment is well documented for prostate cancer5-8 and targeting the stroma has been suggested as a promising and attractive therapeutic option for the cancer treatment.9-11
The major cellular components of TME are composed of cancer-associated fibroblasts (CAFs) and cooperative interaction between heterotypic fibroblasts and tumor cells contribute to cancer progression.12-14 Molecular mechanisms of transition of normal fibroblasts to CAF during prostate carcinogenesis include changes in transcriptional activity of numerous genes. Comparative analysis of fetal human prostate, normal prostate human fibroblasts (NPFs) and CAFs identified 671 transcripts that were enriched in CAFs and 356 transcripts whose levels were decreased relative to normal fibroblasts.15 Twelve new proteins differentially expressed in cancer-associated fibroblasts versus normal fibroblasts were identified in our previous study and 4 of them were related to the Rho kinase signaling pathway suggesting a potential mechanism of fibroblast-CAF transition.16 Although the molecular changes in CAF phenotype are evidently associated with tumor growth, invasion, and metastasis,17 exact molecular mechanisms how CAF or normal fibroblast affect prostate cancer cells not clear. It is shown that normal human fibroblasts inhibit proliferation and motility of prostate cancer cells through both soluble factors and direct cell-cell interactions18,19 but involved genes are not identified yet as well as molecular mechanisms by which these changes actively induce or repress gene expression in normal and malignant cells.20
Among the numerous cell surface and extracellular matrix (ECM) molecules potentially involved in fibroblast-cancer cell interaction, proteoglycans and junction molecules as well as protein ECM components (collagens, fibronectin, elastin) look most perspective candidates responsible for normal or pathological cell-cell interactions.
Proteoglycans (PGs) are complex molecules consisting of protein core and covalently attached polysaccharide chains of glycosaminoglycans (GAG) which are present in every mammalian tissue.21 PGs are abundant at cell surface and extracellular matrix in any tissue and both core protein and polysaccharide chains contribute to functional activity of the molecules.22,23 Progressive changes in proteoglycans occur both in prostate cancer cells and tumor microenvironment, but neither the source nor consequences of those changes are well understood.24 As to individual PGs in prostate carcinogenesis, an important functional role is shown for syndecan-1,25-30 syndecan-2,29-31 perlecan,32-34 decorin,35,36 lumican,37 versican,38-40 CD44,41 glypican-1.42
Other key players responsible for intercellular contacts and communication are junctions pathways. Among them, adherens junction and tight junction are the most studied.
Adherens junctions are protein complexes in which cadherin adhesion receptors connect the actin cytoskeletons of neighboring animal cells mediating cell-cell adhesion and providing the tissue with mechanical continuity and barrier function.43 Perturbations with their main components (cadherins, NOTCH1-4, PVRL1-3) affects expression of numerous downstream genes and is associated with aggressive phenotype of prostate cancer cells.44-46
Tight junctions represent the closely associated areas of 2 cells whose membranes join together via special transmembrane proteins (claudins, occludines). They help to maintain the polarity of cells by preventing the lateral diffusion of integral membrane proteins between the apical and lateral/basal surfaces, prevent the passage of molecules and ions through the space between plasma membranes of adjacent cells and precisely control the transcellular transport. Tight junctions has a vital role in maintaining cell to cell integrity and the loss of cohesion of the structure can lead to invasion and metastasis of cancer cells.47,48 Structure and function of tight junctions are controled by the Rho GTPase family49 and CLDN3 (claudin 3) was identified as potential diagnostic and prognostic biomarker for prostate cancer.50
Overall, the extracellular molecules and cell surface junction molecules are considered as promising therapeutic targets in the treatment of prostate tumors.21,51,52
A main aim of the work was to perform a complex analysis of the main extracellular and cell surface molecules involved in direct cell-cell and cell-matrix interactions (collagens, fibronectin, proteoglycans and cell adhesion molecules) into crosstalk between fibroblasts and normal and cancer prostate epithelial cells.
Results
Fibroblasts inhibit proliferation of normal and cancer prostate epithelial cells in coculture system in vitro
Coculture model was used to study crosstalk between human fibroblasts and normal or cancer prostate epithelial cells in vitro. In the model, human recombinant telomerase-transfected immortalized human fibroblasts (BJhTERT) were cocultured with normal prostate epithelial cells (PNT2) and prostate cancer cell lines with different tumorigenic properties (hormone-dependent non-metastatic LNCaP cells and hormone-independent metastatic PC3 and DU145 cells) (Fig. 1). Upon coculture, different modes of interaction with fibroblasts were observed for normal (PNT2) and cancer (LNCaP, PC3, DU145) cells (Fig. 1A, B). No confrontation was observed between fibroblasts and normal cells, which formed tight intercellular contacts. However, adhesion of epithelial cancer cells to the surface was evidently impaired especially at the points of epithelial cell-fibroblast contacts.
Figure 1.
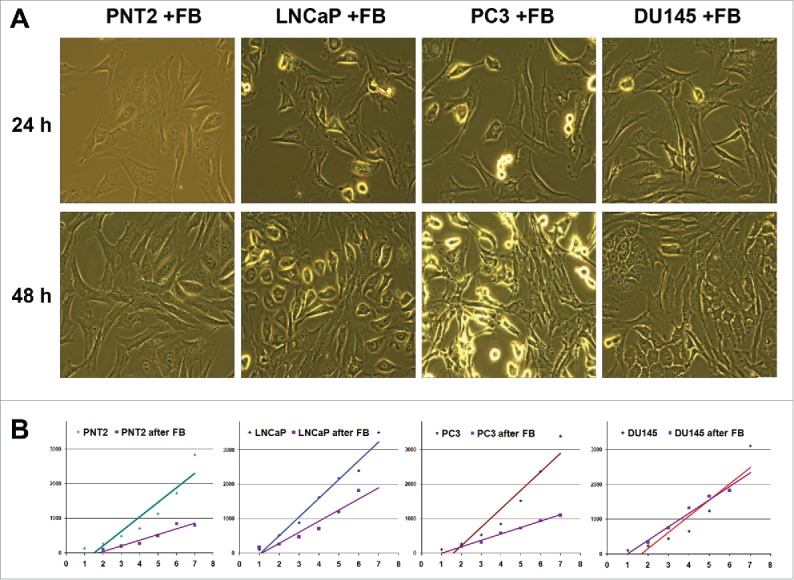
Effects of BJhTERT fibroblasts on proliferation of normal and cancer prostate epithelial cells in vitro. (A) Morphology of cocultures at day 1 and day 2 (light microscopy), magnification ×200. (B) Proliferation rates of normal (PNT2) and cancer epithelial prostate cells before and after coculture with fibroblasts (CyQUANT NF Cell proliferation assay).
The effect could be accounted for the decreased proliferation activity of PC3 cells reported earlier,22 so proliferation rates of the normal and different cancer epithelial cells before and after coculture were determined using CyQuant proliferation assay (Fig. 1B). Fibroblast presence decreased the proliferation rates of normal PNT2 epithelial cells and 2 of 3 cancer cell lines under the study (LNCaP and PC3). Surprisingly, the most aggressive PC3 and DU145 cancer cell lines differentially responded to coculture with fibroblasts. The fibroblasts inhibited the proliferation of PC3 cells but were not able to decrease the proliferation rate of DU145 cells in vitro.
Prostate cancer cells lose the ability to respond to fibroblasts in terms of proteoglycan and ECM proteins expression
As shown previously for BJhTERT-PC3 cells confrontation,22 both soluble factors secreted by PC3-confronted fibroblasts and cell surface/ECM molecules (responsible for contact-dependent inhibition of tumor cell proliferation) contribute to inhibition of tumor cell proliferation upon confrontation. To identify important genes that might be involved in the control of prostate epithelial cells proliferation by BJhTERT fibroblasts, the expression of key cell surface and cell/tumor microenvironment macromolecules (collagen, fibronectin, proteoglycans) was investigated in the prostate cell lines before and after coculture with BJhTERT by real-time RT-PCR analysis (Fig. 2). To analyze an overall ability of the studied cells to express a pool of different PG core proteins, the obtained results were presented as a conventional parameter which compose of a sum of expression levels for all the proteoglycans under the study.
Figure 2.
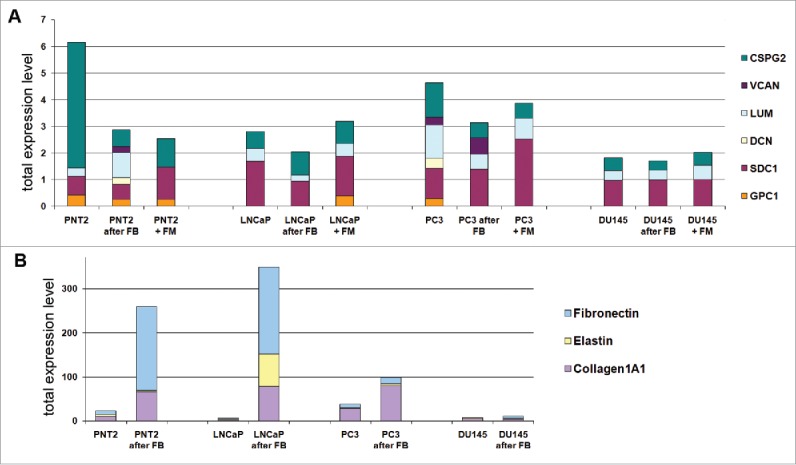
Fibroblast coculture effect on normal and cancer prostate epithelial cells. Changes in expression of proteoglycans (A) and ECM components (B) in normal and cancer prostate cells upon coculture with fibroblasts. Intensity of the amplified DNA fragments normalized to that of GAPDH.
For PNT2 cells, coculture with fibroblasts resulted in re-organization of the transcriptional activity of main microenvironment-coding genes - decrease of the overall transcriptional activity of PGs (mainly, due to NG2) and increase of collagen 1A1 and fibronectin expression (Fig. 2A and B). Similar effects were observed after culturing of PNT2 in fibroblast conditioned medium (Fig. 2 A), although patterns of the expressed proteoglycans were slightly different supporting both direct cell-cell contacts and soluble factors as necessary contributors to the microenvironmental regulation of PG expression in epithelial cells.
As to prostate cancer epithelial cell lines, they demonstrated 2–3-fold decreased transcriptional activity for PG genes compared to normal PNT2 cells and did not respond significantly to coculture with BJhTERT (Fig. 2A). Expression levels for protein ECM components (collagen, fibronectin, elastin) in the prostate cancer epithelial cells were similar to normal PNT2 cells when cultivated as monoculture but differentially affected by BJhTERT fibroblasts (Fig. 2B). Hormone-dependent non-metastatic LNCaP cells looked more similar to the normal PNT2 cells, responding to the fibroblast coculture by the significant increasing of ECM components expression although PGs expression was unaffected. In contrast, hormone-independent non-metastatic PC3 and DU145 cells were almost irresponsive to BJhTERT fibroblasts in terms of microenvironment-related genes expression in spite of different basic transcriptional activities of the PG- and ECM-coding genes. Possibly, retained expression of PGs and collagen 1A1 in PC3 cells could be related to their ability still to respond to BJhTERT fibroblasts by the decreasing of their cell proliferation rate, whereas DU145 cells almost did not expressed PGs and ECM components and did not respond to fibroblasts in terms of cell proliferation rate (Fig. 1B). The results support an important instructive role of fibroblasts in modulation of transcriptional activities of microenvironmental genes in surrounding epithelial cells.
Fibroblasts reveal heterogeneous changes in proteoglycan expression coordinated with proliferation rate inhibition upon coculture with different cancer cells lines
The next intriguing question was whether prostate epithelial cells (normal or cancer) affect fibroblasts in terms of their proliferation rate and ability to express key cell surface molecules and microenvironmental components (Fig. 3).
Figure 3.
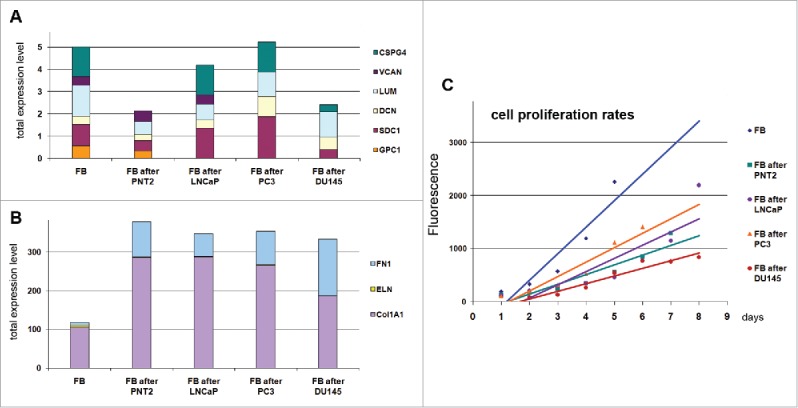
Effects of normal and cancer prostate epithelial cells to TERT-immortilized fibroblasts. (A) Proteoglycan expression. (B) ECM components expression. (C) Changes in fibroblast proliferation rate upon coculture with prostate epithelialcells.
It was shown that coculture of fibroblasts with normal PNT2 epithelial cells resulted in significant inhibition of their proliferation and re-organization of transcriptional activity of the studied genes (collagen, fibronectin and proteoglycans) toward a more active production of protein ECM components. Significant activation of fibronectin and collagen 1A1 expression and 2-fold down-regulation of proteoglycans (especially CSPG4/NG2) resulted in completely different expression pattern of the microenvironmental genes in PNT2-exposed BJhTERT fibroblasts compared with BjTERT in monoculture (Fig. 3A and B). The observed changes correspond the functional role of fibroblasts in normal tissue stroma as key producers of protein and glycan ECM components to create appropriate microenvironment for other cell types. The ability of fibroblasts to activate collagen and fibronectin expression and fine-tune proteoglycans expression in response to epithelial cells presence seems to provide a precise self-regulating system for creation of the optimal microenvironment for the cells and proper organization of normal tissue. Do inability of fibroblasts to properly respond to surrounding cells in terms of ECM production could be a key point of transformation of normal microenvironment into TME.
All studied prostate cancer cell lines affected the fibroblast proliferation rate and ability to express collagen 1A1 and fibronectin in the similar manner as normal cells, whereas their effects to the proteoglycan expression were various. The common trend was the decrease of the variety of the proteoglycans expressed by fibroblast - inactivation of glypican-1 expression upon coculture with LNCaP cells, and glypican-1 and versican after PC3 cells. Another hormone-independent metastatic DU145 cell line demonstrated additionally 2–4-fold downregulation of syndecan-1 and NG2 (Fig. 3A).
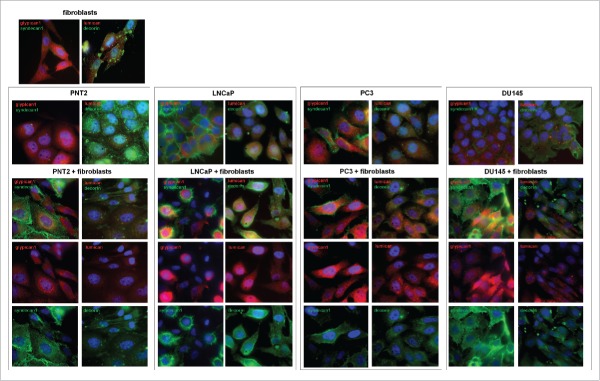
To test the effect at the protein level, immunostaining of the co-cultured cells for main PGs (members of the small leucine-rich proteoglycan (SLRP) family decorin and lumican, and cell surface heparansulfate proteoglycans syndecan-1 and glypican-1) was performed (Fig. 4). The presented results support transcriptional data on reorganization of PGs expression both in normal prostate epithelial PNT2 cells and BJhTERT fibroblasts upon their coculture. The changes were more pronounced in PNT2 cells where significant down-regulation of decorin and up-regulation of syndecan-1 and lumican occur (Fig. 4A and B). In their turn, BJhTERT fibroblasts were characterized by moderate redistribution of decorin and increase of syndecan-1 over their cell surface.
Figure 4.
Immunocytochemical analysis of proteoglycans expression in fibroblasts (FB) and normal (PNT2) and cancer prostate cells (LNCaP, PC3, DU145) before and after coculture with fibroblasts. Decorin (green), lumican (red), syndecan-1 (green), glypican-1 (red). Nuclear counterstain - DAPI (blue).
In contrast to PNT2 cells, neither LNCaP and PC3 prostate cancer cells nor BJhTERT did not demonstrated evident changes in decorin, lumican, syndecan-1 and glypican-1 expression upon coculture. Surprisingly, only aggressive prostate cancer DU145 cells responded to the BJhTERT coculture by activation of syndecan-1 expression both in themselves and surrounding fibroblasts (Fig. 4B). The results have something in common with similar morphology of the cocultures (Fig. 1A) and effects of PNT2 and DU145 to proteoglycan expression in BJhTERT fibroblasts (Fig. 3A) and BJhTERT proliferation rate (Fig. 3C). Possibly, retaining a specific proteoglycan expression pattern at their surface, DU145 cells escape a microenvironmental control (through some unknown molecular mechanisms related to cell-cell and cell-matrix interaction and recognition) and propagate and metastasize in vivo in spite of the not so high proliferation activity in vitro.
Cancer cells demonstrate increased expression of junction-related molecules and inhibit that in surrounding fibroblasts
Another interesting observation is related to the weak intercellular contacts between prostate cancer cells and BJhTERT fibroblasts upon their confrontation arguing for the disruption some important pathways for their communication. To study the matter further, we investigated a panel of genes involved in different types of intercellular contacts (PCR Array Human Cell Junction Pathway Finder array) in normal and cancer prostate epithelial cells and BJhTERT fibroblasts before and after coculture (Fig. 5, Table 1, Table S2).
Figure 5.
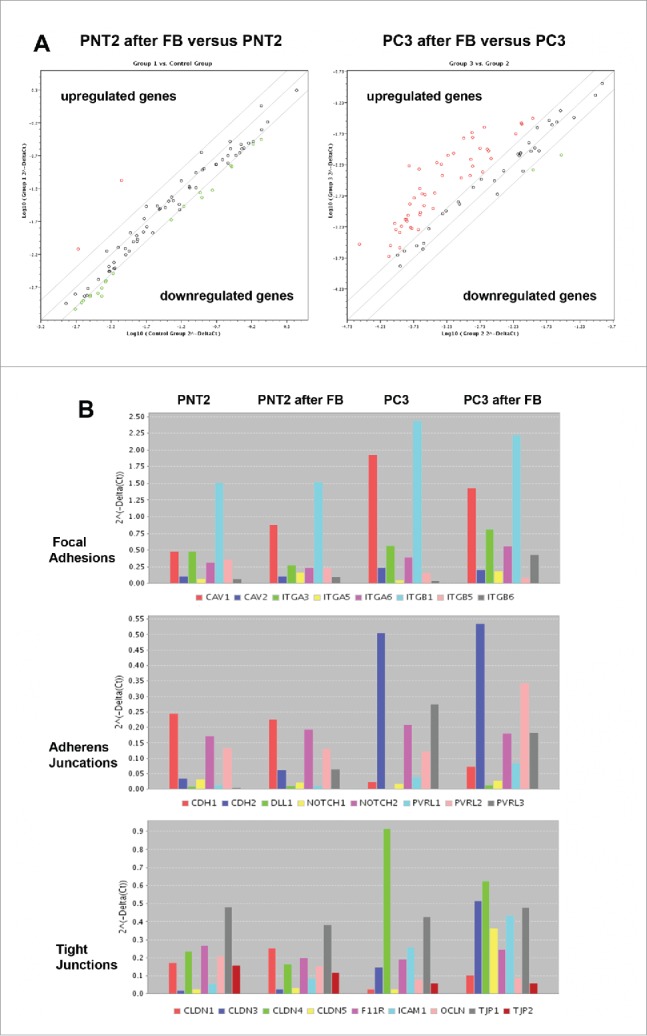
RT2 Profiler™ PCR Array Human Cell Junction Pathway Finder analysis of normal and cancer prostate epithelial cells before and after coculture with BJhTERT fibroblast. (A) The relative expression levels for each gene in PNT2 cells (Group 1) or PC3 cells (Group 3) after co-culture with fibroblasts are plotted against the same gene from the control PNT2 cells or PC3 cells (Control Group or Group 2, respectively). The middle line shows the similar expression in both groups with 2-fold change boundaries. Genes up-regulated >2-fold lie above the middle line and the down-regulated genes lie below the line. (B) Gene expression levels for PNT2 cells (Control group), BJhTERT-exposed PNT2 cells (Group 1), PC3 cells (Group 2) and BJhTERT-exposed PC3 cells (Group 3) (RT2 Profiler™ PCR Array Data Analysis Program version 3.5).
Table 1.
Changes in the expression of genes relevant to the Cell Junction pathway. Listed up- and down-regulated genes in the Test Sample (after coculture) divided by the normalized gene expression in the Control Sample (before coculture). Two-fold change is taken as significant.
| Cells | Genes Over-Expressed | Genes Under-Expressed |
|---|---|---|
| FB after PNT2 | CAV1,CAV2,CAV3,CDH1,CDH2,CLDN1 CLDN11,CLDN12,CLDN4,CLDN7,CLDN9 DLL1,DSC2,DSC3,DSG2,DSP,F11R,GJA1 GJA5,GJB1,GJB3,GJB4,GJB5,GJC2,ICAM1 ICAM2,ITGA1,ITGA4,ITGA5,ITGAV,ITGB1 ITGB3,ITGB4,ITGB5,ITGB6,JAM2,JUP,NOTCH1 NOTCH2,OCLN,PVRL2,PVRL3,TJP1,TJP3 |
|
| PNT2 after FB | GJA1,ITGA5,JAM3,PVRL3 | GJC3 |
| FB after PC3 | CDH1,CDH2,CLDN1,CLDN14,CLDN3,CLDN4 DSC2,DSC3,DSG2,ESAM,F11R,GJA1,GJB3 ICAM1,ITGB2,ITGB4,JUP,OCLN,PVRL1,TJP1 |
CAV1,CLDN10,CLDN11,CLDN16,CLDN17 CLDN18,CLDN2,CLDN6,DLL1,DSC1,DSG1 DSG3,DSG4,DST,GJA4,GJA5,GJA8,GJB6 GJD2,GJC3,ITGA2,ITGA4,ITGA7,ITGA8 ITGA9,JAM2,JAM3,PVRL3,TJP2,TJP3 |
| PC3 after FB | CAV3,CDH1,CLDN1,CLDN10,CLDN11,CLDN17 CLDN19,CLDN3,CLDN5,CLDN6,CLDN7,CLDN8 CLDN9,DLL1,DSC1,DSG1,DSG3,DSG4,DSP GJA1,GJA3,GJA4,GJA5,GJA8,GJB1,GJB2,GJB3 GJB4,GJD2,ICAM2,ITGA1,ITGA2,ITGA4,ITGA5 ITGA7,ITGA8,ITGA9,ITGAL,ITGAM,ITGB2 ITGB3,ITGB6,JAM2,JAM3,JUP,NOTCH3 PVRL1,PVRL2,TJP3,HGDC |
DSG2,ITGB4 |
Human Cell Junction Pathway Finder array let to determine the expression levels of 84 genes involved in different types of cell-cell and cell-matrix contacts (focal adhesions, tight junctions, gap junctions, adherens junctions, desmosomes, hemidesmosomes). Normal prostate epithelial PNT2 cells did not show significant changes in the transcriptional activity of the genes upon coculture with BJhTERT fibroblasts being consistent with the observation about no confrontation between the normal prostate epithelial cells and BJhTERT (Fig. 1A).
However, coculture of prostate cancer PC3 cells with BJhTERT resulted in upregulation of different junction-related genes in PC3 cells (especially Focal Adhesions, Tight Junctions and Adherens Junctions) (Fig. 5, Table 1).
In parallel, similar experiments were performed to study expression of junctions-related genes in BJhTERT fibroblasts before and after coculture with normal or cancer prostate cells (Fig. 6, Table S1). The obtained results showed that BJhTERT cells responded to the coculture with normal (PNT2) or cancer (PC3) prostate cells in a different manner. PNT2-exposed BJhTERT cells demonstrated increased transcriptional activity of the genes involved in different types of cell-cell contacts (Fig. 6A), among which focal adhesions (CAV1, ITGA3, ITGA5, ITGB1, ITGB5), adherens junctions (CDH1, CDH2, Notch2, PVRL2, PVRL3) and tight junctions (Cldn1, Cldn4, Cldn12, F11R, ICAM1, JAM3, OCLN, TJP1, TJP2) were the main up-regulated pathways (Fig. 6B). On the contrary, PC3-confronted BJhTERT were characterized by selective inhibition of focal adhesion genes and re-organization of transcriptional patterns of adherens junctions and tight junctions-related genes with a common tendency to their downregulation (Fig. 6A, B). As the result, PC3-confronted fibroblasts demonstrate significantly decreased expression levels of the genes involved in different types of cell-cell contacts (especially focal adhesions and adherens junctions) compared with fibroblasts cocultured with normal PNT2 cells (Fig. 6A, right panel). Transcriptional inhibition of the junction pathways along with the down-regulation of some proteoglycans (glypican-1, versican) (Fig. 2) suppose that the ability of PC3-confronted fibroblasts to establish proper intercellular contacts with surrounding cancer cells and regulate their proliferation rate could be compromised. Taken together with significant up-regulation of the known CAF marker a-smooth muscle actin (SMA) in PC3-confronted fibroblasts (Fig. 6C), the obtained data suggest transcriptional deregulation of junction proteins and proteoglycans as a potential molecular mechanism contributing to reprogramming of normal fibroblast to cancer-associated fibroblast during malignant transformation.
Figure 6.
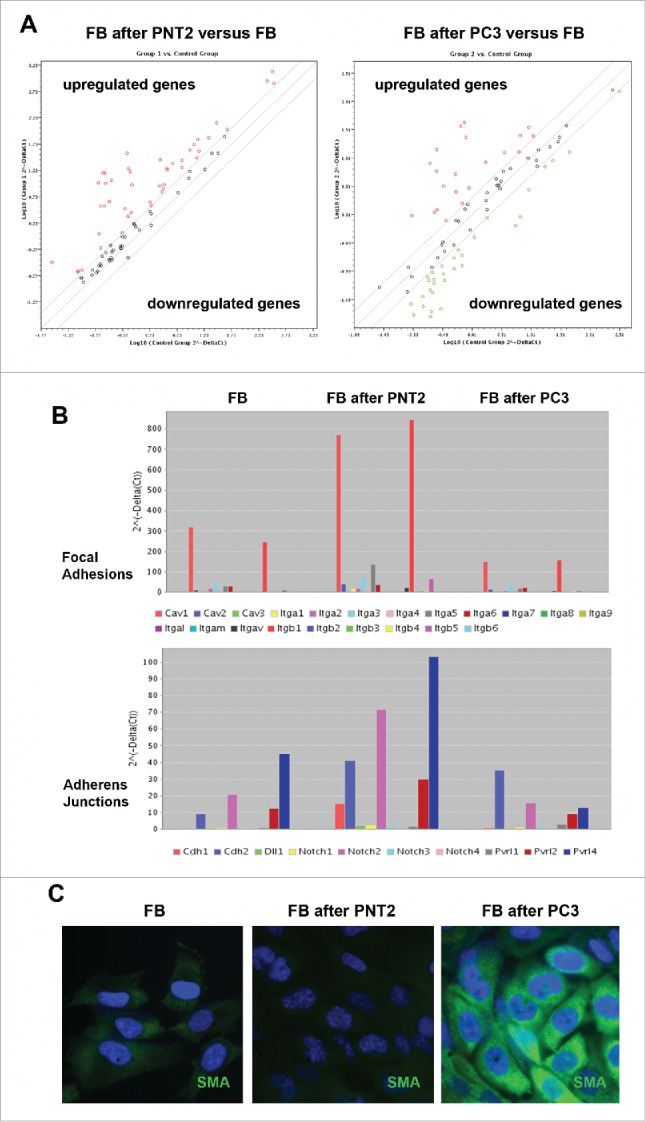
Expression profiling of cell junction-related genes of BJhTERT fibroblasts before and after coculture with normal and cancer prostate epithelial cells. (A) RT2 Profiler™ PCR Array Human Cell Junction Pathway Finder analysis. The relative expression levels for each gene in fibroblasts after co-culture with PNT2 cells (Group 1) or PC3 cells (Group 2) are plotted against the same gene from the control fibroblasts (Control Group). The middle line shows the similar expression in both groups with 2-fold change boundaries. Genes upregulated >2-fold lie above the middle line and the downregulated genes lie below the line. (B) Gene expression levels for fibroblasts, PNT2- and PC3-confronted fibroblasts for junctions pathways with highest expression levels. (C) Immunostaining for a-smooth muscle actin (SMA) (green). Nuclear counterstain - DAPI (blue).
Discussion
From the obtained results, we suggested a possible scheme for interference of human BjTERT fibroblasts and normal or cancer prostate epithelial cells (Fig. 7).
Figure 7.
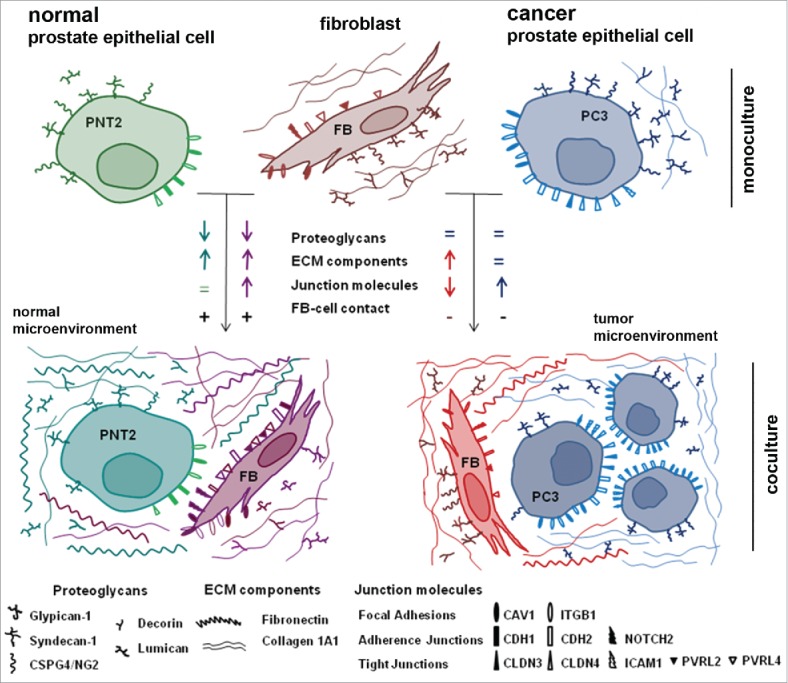
Schematic representation of a running hypothesis for fibroblast – prostate epithelial cells interactions.
Normal epithelial PNT2 cells and BjTERT fibroblasts establish tight heterotypic intercellular contacts (with no signs of confrontation) upon coculture in the experimental model in vitro. Complex changes in the expression of key contact-related ECM and cell surface molecules (COL1A1, FN1, PGs, junctions molecules) occur both in PNT2 cells and fibroblasts, and could be interpreted as a necessary adaptation of the cells to coexistence. Unfortunately, there are almost no literature data on normal prostate epithelial cell-fibroblast interactions to compare with. Anyway, this is a very important point to start with because it allows to outline the described expression changes as a normal physiological reaction to coculture and perform subsequent comparative analysis with prostate cancer cell-fibroblast interactions.
Prostate cancer cell lines (LNCaP, PC3, DU145) demonstrate completely different behavior in their interactions with BjTERT fibroblasts compared with normal PNT2 cells. Cancer cells seem to be independent from the regulating signals from fibroblasts in terms of proteoglycan and protein ECM components expression (Fig. 2) and fail to establish direct heterotypic intercellular contacts (LNCaP, PC3) (Fig. 1A). At the same time, cancer cells possess an ability to affect surrounding fibroblasts, different cancer cells lines induce heterogeneous changes in proteoglycan expression in cancer cell-exposed fibroblasts which correlate with the fibroblasts proliferation rate (Fig. 3A and C). Expression of junction molecules is simultaneously downregulated in BjTERT and upregulated in prostate cancer PC3 cells suggesting higher propensity of the cancer cells to aggregation and clonal growth with no or minimal involvement of BjTERT. The observation has something general with recently proposed 3D model for analysis of cancer cells aggregation which suggest that tumorigenesis in vitro is a developmental process involving coalescence of cancer cells in 3D facilitated by specialized cells (named “facilitators” and “probes”) that culminates in large hollow spheres with complex architecture.66
All the described effects result in completely different structure of fibroblast interactions with normal or cancer epithelial cells, where do failure to respond to stromal fibroblasts by physiological reorganization of expression of cell-cell contact-related molecules and establishment of heterotypic contacts could be a key point.
In literature, there are scattered data on expression changes for individual proteoglycans, protein ECM components or junctions molecules in prostate cancer cell-fibroblast model systems in vitro. It was shown that prostate tumor cells (LNCaP, PC3, DU145) induce fibroblasts to secrete increased versican levels via a paracrine mechanism mediated by transforming growth factor beta1;53 metastatic cancer cells PC3 and DU145 create a metastatic niche by altering the phenotype of local fibroblasts, leading to changes in the ECM (significant downregulation of collagens I, II, III, IV, decorin, biglycan, lumican, fibromodulin, TGF-β and upregulation of vimentin, α5β1 integrin, MT1-MMP).54 Cocultivation with PC3 cells make the cancerous and hyperplastic fibroblasts more alike each other (only 26 differentially expressed genes vs. 383 differentially expressed genes between fibroblasts from cancerous and hyperplasia areas before cocultivation with PC-3 cells);55 PC3 conditioned media increase α-smooth muscle actin (α-SMA) and vimentin expression in human prostate fibroblasts, and their differentiation into CAF-like phenotype through the TGFβ2-dependent mechanism.56 Transformation of normal fibroblasts into CAFs seems occur in a heterogeneous manner and might be strongly dependent on biochemical characteristics of adjacent cancer cells.57 All the data support our results on the ability of prostate cancer cells to affect stromal fibroblasts and transform them into a myofibroblastic phenotype similar to that found in CAFs.
Together with the induced morphological changes of fibroblasts, all the prostate cancer cell lines (LnCaP, PC3, DU145) decrease fibroblast proliferation rates in our coculture system in vitro, and the published data to compare with are controversial. It was shown that conditioned media from the cancer cell lines stimulate fibroblast proliferation58 possibly via down-regulation of cell adhesion, cell-cell contact, and cell cycle regulation proteins59 or inhibit it60 and the matter needs further investigation.
As to an ability of stromal fibroblasts to affect prostate cancer cells, there are much less data concerning cell-cell and cell-ECM contact molecules. It is shown that gingival fibroblasts do not express E-cadherin, while prostate cancer cells LNCaP, PC3, DU145 demonstrate differential E-cadherin staining and coculture of PC3 cells with fibroblasts results in a reduction in the nuclear E-cadherin reactivity in fibroblast-exposed PC3 cells.60 From the other side, P-cadherin but not E-cadherin was identified as important component for maintaining adherens junctions in DU145 cells, and depletion of any of the cadherin-associated proteins (p120ctn, β-catenin or α-catenin) was sufficient to disrupt adherens junctions in DU145 cells and increase migration and cancer cell invasion.61
Presented results on the ability of BjTERT fibroblasts to differentially affect proliferation rate of different prostate cancer cell lines upon coculture need to be analyzed in context of already published data. According Kaminski et al., fibroblast conditioned medium enhance proliferation and anchorage-independent growth of DU145 cells and increase migration of PC3 cells;58 fibroblasts of different origin and their conditioned media stimulate proliferation of normal or malignant prostate epithelial cell, while fixed fibroblast monolayers and extracellular matrix prepared from fibroblast cultures failed to stimulate prostatic epithelial growth.62 In our previous work, we showed that primary human fibroblasts inhibit proliferation of 6 human cancer cell lines18 and ECM/other surface proteins of the fibroblasts seems to be involved in contact-dependent inhibition of tumor cell proliferation.19 Possibly, the apparent contradictions depend on the experimental conditions such as an initial ratio of fibroblasts/cancer cells or analysis time-points. For example, it was demonstrated that conditioned medium from gingival fibroblasts inhibit proliferation of LNCaP cells, after 3 days by 33% and after 6 days by up to 82%, but has no effect on the PC3 and DU145 cell growth.60 Taking into account long duration of such coculture experiments (up to 6 days), one should keep in mind a process of potential transformation of the fibroblasts to CAFs during the experiment, which could significantly influence functional interactions between the cells. According Paland et al., whereas normal human fibroblasts inhibit the growth of immortalized prostate epithelial cells but promote the growth of metastatic PC3 cells, CAFs promote the growth of prostate epithelial cells but not of PC3. The results suggest that normal fibroblast cells have a protective function at very early stages of carcinogenesis by preventing immortalized epithelial cells from proliferating whereas CAFs aid immortalized epithelial cells to further develop.63 Finally, they co-evolute with cancer cells and affect every prostate cancer cell line in different ways, which may be because of their different origin.59
Our data stay in line with this hypothesis and support heterogeneous mechanisms for interaction of different prostate cancer cell subtypes (LNCaP, PC3, DU145) with BjTERT- fibroblasts.
In co-culture, androgen-sensitive non-metastatic LNCaP cells grow in a network on the top of the monolayer formed by fibroblasts and both cell types undergo morphological changes, while colonies of androgen-insensitive metastatic PC3 and DU145 cells were surrounded by fibroblasts.60 They respond to prostate fibroblasts by alterations in their cytogenetic and biologic profiles in xenograft model in vivo.64
Androgen-independent metastatic cell lines PC3 and DU145 demonstrate relative independence from the regulating signals from fibroblasts and molecular mechanisms of the effect seem to be different. Among them, DU145 cell line is the most intriguing, and paradoxical combination of different physiological and molecular parameters of DU145 cells takes a special attention to the cell line. DU145 cells look as a least sensitive to regulating effect by fibroblasts (Figs. 1 and 2) but are the most potent in their effects to surrounding fibroblasts among the studied prostate cancer cell lines (Fig. 3A and C) underlying their aggressive metastatic nature. Coculture with fibroblasts do not change DU145 proliferation rate (Fig. 1) and fibroblast conditioned medium results in an enhanced proliferation and anchorage-independent growth of this cell line in soft agar.59 At the same time, the cells demonstrate a lowest proliferation rate similar to that of PNT2 and retain the ability to respond to BjTERT by activation of syndecan-1 at their surface (Fig. 4). These observations suggest that do combination of evident oncogenic properties with unchanged cell surface architecture could be a favorable feature for successful dissemination of such cancer cells. Interestingly, a similar paradox was described for breast cancer cell subtype with high hyaluronan (HA) binding capacity. The HA(high) subpopulation exhibit lower proliferation activity along with significantly higher local invasion and lung micrometastases than either unsorted parental cells or the HA(-/low) subpopulation. The results revealed a previously undetected form of heterogeneity that predicts invasive/metastatic behavior.65 Possibly, DU145 could be an appropriate model to study molecular mechanisms of prostate cancer metastasis further.
Collectively, the obtained results suppose that
decrease of the proliferation rate and increase of ECM components expression could be a common reaction of fibroblasts to the presence of normal or cancer epithelial cells;
transcriptional patterns of junction-related molecules are associated with malignant transformation of cell and homotypic/heterotypic intercellular contacts;
normal or cancer epithelial cells specifically re-program PG expression in fibroblasts to provide themselves with a suitable microenvironment.
We hypothesized that proteoglycan patterns seem to be peculiar “fingerprints” in cell-cell communication and specific “sensors” to dynamically react into their microenvironment. However, a functional contribution of their protein cores and polysaccharide chains into this process is still unknown what can be a future step in the investigation of their involvement in cell-cell and cell-matrix interactions in normal or cancer tissues.
In summary, the presented data suggest deregulation of proteoglycans and junction molecules in prostate cancer cells and nearby fibroblasts as a potential molecular mechanism for autonomous growth of cancer cells in their microenvironment. The ability of different prostate cancer cell sub-types differentially affect their microenvironment lies within the context of our knowledge of the heterogenic structure of prostate tumors and contribute to the understanding of the biology of prostate cancer.
Materials and methods
Materials and antibodies
Mouse monoclonal anti-human Syndecan-1, rabbit polyclonal anti-human Glypican-1, mouse monoclonal anti-human β-actin antibodies (Abcam, UK); rabbit polyclonal anti-human Lumican, mouse monoclonal anti-human Decorin, mouse monoclonal anti-human HS antibodies (Abnova, USA); mouse monoclonal anti-human CS antibody (Sigma, USA), FITC-conjugated antibody against mouse IgGs, TexasRed-conjugated antibody against rabbit IgGs (Vector Laboratories, USA). Prolong Gold antifade reagent with DAPI (ThermoFisher Scientific, USA).
Cell lines and coculture assay
The human TERT-immortalized fibroblasts (BjTERT) and prostate cancer cell lines LNCaP, PC3, DU145 were obtained from MTC (Karolinska Institute, Stockholm, Sweden). The normal human prostate epithelial cell line PNT2 was obtained from the European Collection of Cell Cultures (ECACC, Salisbury, UK). All cell lines were maintained in RPMI medium supplemented with 2 mM L-glutamine, 100 units penicillin, 100 µg/ml streptomycin, and 10% (v/v) fetal bovine serum at 37°C in a humidified 5% CO2 incubator. Cells were harvested for analysis using trypsin/EDTA. For coculture experiments, fibroblasts and prostate epithelial cells were seeded with 1:1 ratio with 25–30% confluency at the start point and separated after 72h of incubation.
Magnetic separation of different cell types upon coculture
After coculture, epithelial cells and BjTERT fibroblasts were separated on OctoMACS Separator using MS Columns and Anti-Fibroblast MicroBeads human (Miltenyi Biotec) according to the manufacturer's instructions. Briefly, cell suspension was mixed with Anti-Fibroblast MicroBeads conjugated to monoclonal mouse anti-fibroblast human antibodies and incubated for 30 minutes at room temperature. Cells suspension was applied onto the MACS column and flow-through, containing unlabeled prostate epithelial cells was collected. Magnetically labeled fibroblasts were eluted from the MACS column using elution buffer.
Total RNA isolation and reverse transcription
Total RNA was extracted from the cells using the RNeasy Plus Mini Kit (Qiagen) according to the manufacturer's instructions. The integrity and quality of the isolated RNA were checked by agarose gel electrophoresis and total RNA concentration was measured with Qubit–iT RNA Assays Kit (ThermoFisher Scientific, USA) according to the manufacturer's instructions. cDNA was synthesized from 1–2 µg of total RNA using a RevertAid First Strand cDNA Synthesis Kit (Fermentas, USA), and 1/10th of the product was subjected to PCR analysis.
Analysis of ECM molecules and proteoglycan genes expression by Real-Time PCR
Real-time PCR was performed using the CFX96 Real-Time PCR Detection System (BioRad, USA) under the following conditions: 95°C for 3 min, 95°C for 20 sec, 59°C for 15 sec and 72°C for 50 sec. The total reaction volume was 25 μl. GAPDH was used as the housekeeping gene. The PCR primers and conditions used are listed in Table S1.
In vitro cell proliferation assay
Cell proliferation rate was determined using the CyQUANT NF Cell Proliferation Assay (ThermoFisher Scientific, USA) according to the manufacturer's protocol. Briefly, cells were plated in a 96-well microplate at densities of 100–500 per well (8–12 identical wells in total) and the DNA content of the wells was measured every 24 h. This was achieved by removing the medium and adding 50 μl of fluorescent dye followed by incubation for 30 min at 37°C. The fluorescence intensity of each sample was measured at 485/530 nm using fluorescence microplate reader (SPECTRA max, Molecular Devices, Sunnyvale, CA, USA).
Human Cell Junctions PathwayFinder RT2 Profiler PCR array
The Cancer PathFinder RT2 Profiler PCR array (SABioscience, USA) was used to determine changes in the expression of 84 Junctions pathway-focused genes upon TSA treatment in fibroblasts and prostate epithelial cells after their coculture. Briefly, total RNA was isolated using a RNeasy Plus Mini Kit (Qiagen). The RNA concentration was determined using a Quant-iT Assay Kit for RNA quantification (ThermoFisher Scientific, USA) and was verified by electrophoresis. cDNA was synthesized from 1–2 μg of total RNA using a Maxima First Strand cDNA Synthesis Kit for RT-qPCR (ThermoFisher Scientific, USA). Real-Time PCR was performed using an RT2 Profiler PCR Array Human Cell Junctions PathwayFinder System (PAHS-213Z) with SYBR Green Fluor q-PCR Master Mix (Qiagen) and an CFX96 Real-Time PCR Detection System (Bio-Rad) according to the manufacturer's instructions. All data were analyzed using Excel-based RT2 PCR Array Data Analysis Software (SABioscience, USA). This integrated web-based software package automatically calculates ddCt-based fold changes in genes expression from the uploaded raw threshold cycle data. Each replicate cycle threshold (Ct) was normalized to the average Ct of 5 endogenous controls (B2M, HPRT1, RPL13A, GAPDH and ACTB) on a per plate basis.
Immunocytochemistry
For immunofluorescence analysis, cells were grown on glass coverslips and then fixed with phosphate-buffered 4% formaldehyde. Mouse monoclonal anti-syndecan-1 (Abcam; 1:150), rabbit polyclonal anti-glypican-1 (Abcam; 1:150), mouse monoclonal anti-decorin (Abnova; 1:150), rabbit polyclonal anti-lumican (Abnova; 1:150) were used for immunostaining. Staining patterns were visualized with Alexa 488-conjugated goat anti-mouse IgG (ThermoFisher Scientific; 1:1000) and Alexa 568-conjugated goat anti-rabbit IgG (ThermoFisher Scientific; 1:2000) antibodies. The cells were mounted and counterstained with DAPI using Prolong Gold SlowFade Gold with DAPI mounting medium (ThermoFisher Scientific, USA) and observed by fluorescent microscopy (LEICA DMRE).
Statistical analysis
Statistical analyses were performed using a computer program ORIGIN Pro 8.0; a value of p < 0.05 was considered to indicate a statistically significant difference. Data are expressed as the means ± SEM.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Authors thank Dr. L.A. Mostovich for technical assistance with immunostaining.
Funding
The work was supported by the research grant from Russian Foundation for Basic Research (RFBR 012-04-01657a); by the scholarship of Russian Federation President for young scientists (AVS, SP-2133.2015.4); by a FEBS Short-Term Fellowship (AVS), a UICC International Cancer Technology Transfer Fellowship (EVG, ICR/2015/353372); EVG was recipient of fellowship from the Concern Foundation in Los Angeles and the Cancer Research Institute in New York.
References
- [1].Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment Trends Genet. 2009; 25(1):30-8; PMID:19054589; http://dx.doi.org/ 10.1016/j.tig.2008.10.012 [DOI] [PubMed] [Google Scholar]
- [2].Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med 2011; 17(3):320-9; PMID:21383745; http://dx.doi.org/ 10.1038/nm.2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Goetz JG. Tumor microenvironment indoctrination: an emerging hallmark of cancer. Cell Adh Migr 2012; 6(3):190-2; PMID:22863738; http://dx.doi.org/ 10.4161/cam.20782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Werb Z, Lu P. The Role of Stroma in Tumor Development. Cancer J 2015; 21(4):250-3; PMID:26222075; http://dx.doi.org/ 10.1097/PPO.0000000000000127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chung LW, Baseman A, Assikis V, Zhau HE. Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol 2005; 173(1):10-20; PMID:15592017; http://dx.doi.org/ 10.1097/01.ju.0000141582.15218.10 [DOI] [PubMed] [Google Scholar]
- [6].Barron DA, Rowley DR. The reactive stroma microenvironment and prostate cancer progression. Endocr Relat Cancer 2012; 19(6):R187-204; PMID:22930558; http://dx.doi.org/ 10.1530/ERC-12-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chiarugi P, Paoli P, Cirri P. Tumor microenvironment and metabolism in prostate cancer. Semin Oncol 2014; 41(2):267-280; PMID:24787298; http://dx.doi.org/ 10.1053/j.seminoncol.2014.03.004 [DOI] [PubMed] [Google Scholar]
- [8].Krušlin B, Ulamec M, Tomas D. Prostate cancer stroma: an important factor in cancer growth and progression. Bosn J Basic Med Sci 2015; 15(2):1-8; http://dx.doi.org/ 10.17305/bjbms.2015.449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Karlou M, Tzelepi V, Efstathiou E. Therapeutic targeting of the prostate cancer microenvironment. Nat Rev Urol 2010; 7(9):494-509; PMID:20818327; http://dx.doi.org/ 10.1038/nrurol.2010.134 [DOI] [PubMed] [Google Scholar]
- [10].Corn PG. The tumor microenvironment in prostate cancer: elucidating molecular pathways for therapy development. Cancer Manag Res 2012; 4: 183-93; PMID:22904640; http://dx.doi.org/ 10.2147/CMAR.S32839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gururajan M, Posadas EM, Chung LW. Future perspectives of prostate cancer therapy. Transl Androl Urol 2012; 1(1):19-32; PMID:22773967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tripathi M, Billet S, Bhowmick NA. Understanding the role of stromal fibroblasts in cancer progression. Cell Adh Migr 2012; 6(3):231-5; PMID:22568983; http://dx.doi.org/ 10.4161/cam.20419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Franco OE, Hayward SW. Targeting the tumor stroma as a novel therapeutic approach for prostate cancer. Adv Pharmacol 2012; 65:267-313; PMID:22959029; http://dx.doi.org/ 10.1016/B978-0-12-397927-8.00009-9 [DOI] [PubMed] [Google Scholar]
- [14].Augsten M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front Oncol 2014; 4:62; PMID:24734219; http://dx.doi.org/ 10.3389/fonc.2014.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Orr B, Riddick AC, Stewart GD, Anderson RA, Franco OE, Hayward SW, Thomson AA. Identification of stromally expressed molecules in the prostate by tag-profiling of cancer-associated fibroblasts, normal fibroblasts and fetal prostate. Oncogene 2012; 31(9):1130-42; PMID:21804603; http://dx.doi.org/ 10.1038/onc.2011.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bozóky B, Savchenko A, Csermely P, Korcsmáros T, Dúl Z, Pontén F, Székely L, Klein G. Novel signatures of cancer-associated fibroblasts. Int J Cancer 2013; 133(2):286-93; http://dx.doi.org/ 10.1002/ijc.28035 [DOI] [PubMed] [Google Scholar]
- [17].Han Y, Zhang Y, Jia T, Sun Y. Molecular mechanism underlying the tumor-promoting functions of carcinoma-associated fibroblasts. Tumour Biol 2015; 36(3):1385-94; PMID:25680413; http://dx.doi.org/ 10.1007/s13277-015-3230-8 [DOI] [PubMed] [Google Scholar]
- [18].Flaberg E, Markasz L, Petranyi G, Stuber G, Dicso F, Alchihabi N, Oláh È, Csízy I, Józsa T, Andrén O, et al.. High-throughput live-cell imaging reveals differential inhibition of tumor cell proliferation by human fibroblasts. Int J Cancer 2011; 128(12):2793-802; PMID:20715102; http://dx.doi.org/ 10.1002/ijc.25612 [DOI] [PubMed] [Google Scholar]
- [19].Alkasalias T, Flaberg E, Kashuba V, Alexeyenko A, Pavlova T, Savchenko A, Szekely L, Klein G, Guven H. Inhibition of tumor cell proliferation and motility by fibroblasts is both contact and soluble factor dependent. Proc Natl Acad Sci USA 2014; 111(48):17188-93; PMID:25404301; http://dx.doi.org/ 10.1073/pnas.1419554111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Spencer VA, Xu R, Bissell MJ. Extracellular matrix, nuclear and chromatin structure, and gene expression in normal tissues and malignant tumors: a work in progress. Adv Cancer Res 2007; 97:275-94; PMID:17419950; http://dx.doi.org/ 10.1016/S0065-230X(06)97012-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med 2011; 15(5):1013-31; PMID:21155971; http://dx.doi.org/ 10.1111/j.1582-4934.2010.01236.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Afratis N, Gialeli C, Nikitovic D, Tsegenidis T, Karousou E, Theocharis AD, Pavão MS, Tzanakakis GN, Karamanos NK. Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J 2012; 279(7):1177-97; PMID:22333131; http://dx.doi.org/ 10.1111/j.1742-4658.2012.08529.x [DOI] [PubMed] [Google Scholar]
- [23].Gallagher J. Fell-Muir Lecture: Heparan sulphate and the art of cell regulation: a polymer chain conducts the protein orchestra. Int J Exp Pathol 2015; 96(4):203-31; PMID:26173450; http://dx.doi.org/ 10.1111/iep.12135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Edwards IJ. Proteoglycans in prostate cancer. Nat Rev Urol 2012; 9(4):196-206; PMID:22349653; http://dx.doi.org/ 10.1038/nrurol.2012.19 [DOI] [PubMed] [Google Scholar]
- [25].Shimada K, Nakamura M, De Velasco MA, Tanaka M, Ouji Y, Konishi N. Syndecan-1, a new target molecule involved in progression of androgen-independent prostate cancer. Cancer Sci 2009; 100(7):1248-54; PMID:19432893; http://dx.doi.org/ 10.1111/j.1349-7006.2009.01174.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hu Y, Sun H, Owens RT, Gu Z, Wu J, Chen YQ, O'Flaherty JT, Edwards IJ. Syndecan-1-dependent suppression of PDK1/Akt/bad signaling by docosahexaenoic acid induces apoptosis in prostate cancer. Neoplasia 2010; 12(10):826-36; PMID:20927321; http://dx.doi.org/ 10.1593/neo.10586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Brimo F, Vollmer RT, Friszt M, Corcos J, Bismar TA. Syndecan-1 expression in prostate cancer and its value as biomarker for disease progression. BJU Int 2010; 106(3):418-23; PMID:20002675; http://dx.doi.org/ 10.1111/j.1464-410X.2009.09099.x [DOI] [PubMed] [Google Scholar]
- [28].Shimada K, Anai S, Fujii T, Tanaka N, Fujimoto K, Konishi N. Syndecan-1 (CD138) contributes to prostate cancer progression by stabilizing tumour-initiating cells. J Pathol 2013; 231(4):495-504; PMID:24549646; http://dx.doi.org/ 10.1002/path.4271 [DOI] [PubMed] [Google Scholar]
- [29].Contreras HR, Ledezma RA, Vergara J, Cifuentes F, Barra C, Cabello P, Gallegos I, Morales B, Huidobro C, Castellón EA. The expression of syndecan-1 and -2 is associated with Gleason score and epithelial-mesenchymal transition markers, E-cadherin and beta-catenin, in prostate cancer. Urol Oncol 2010; 28(5):534-40; PMID:19450993; http://dx.doi.org/ 10.1016/j.urolonc.2009.03.018 [DOI] [PubMed] [Google Scholar]
- [30].Ledezma R, Cifuentes F, Gallegos I, Fullá J, Ossandon E, Castellon EA, Contreras HR. Altered expression patterns of syndecan-1 and -2 predict biochemical recurrence in prostate cancer. Asian J Androl 2011; 13(3):476-80; PMID:21317913; http://dx.doi.org/ 10.1038/aja.2010.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Popović A, Demirović A, Spajić B, Stimac G, Kruslin B, Tomas D. Expression and prognostic role of syndecan-2 in prostate cancer. Prostate Cancer Prostatic Dis 2010; 13(1):78-82; PMID:19786981; http://dx.doi.org/ 10.1038/pcan.2009.43 [DOI] [PubMed] [Google Scholar]
- [32].Savorè C, Zhang C, Muir C, Liu R, Wyrwa J, Shu J, Zhau HE, Chung LW, Carson DD, Farach-Carson MC. Perlecan knockdown in metastatic prostate cancer cells reduces heparin-binding growth factor responses in vitro and tumor growth in vivo. Clin Exp Metastasis 2005; 22(5):377-390; http://dx.doi.org/ 10.1007/s10585-005-2339-3 [DOI] [PubMed] [Google Scholar]
- [33].Datta MW, Hernandez AM, Schlicht MJ, Kahler AJ, DeGueme AM, Dhir R, Shah RB, Farach-Carson C, Barrett A, Datta S. Perlecan, a candidate gene for the CAPB locus, regulates prostate cancer cell growth via the Sonic Hedgehog pathway. Mol Cancer 2006; 5:9; PMID:16507112; http://dx.doi.org/ 10.1186/1476-4598-5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Grindel BJ, Martinez JR, Pennington CL, Muldoon M, Stave J, Chung LW, Farach-Carson MC. Matrilysin/matrix metalloproteinase-7(MMP7) cleavage of perlecan/HSPG2 creates a molecular switch to alter prostate cancer cell behavior. Matrix Biol 2014; 36:64-76; PMID:24833109; http://dx.doi.org/ 10.1016/j.matbio.2014.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ricciardelli C, Mayne K, Sykes PJ, Raymond WA, McCaul K, Marshall VR, Horsfall DJ. Elevated levels of versican but not decorin predict disease progression in early-stage prostate cancer. Clin Cancer Res 1998; 4(4):963-71; PMID:9563891. [PubMed] [Google Scholar]
- [36].Hu Y, Sun H, Owens RT, Wu J, Chen YQ, Berquin IM, Perry D, O'Flaherty JT, Edwards IJ. Decorin suppresses prostate tumor growth through inhibition of epidermal growth factor and androgen receptor pathways. Neoplasia 2009; 11(10):1042-53; PMID:19794963; http://dx.doi.org/ 10.1593/neo.09760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Coulson-Thomas VJ, Coulson-Thomas YM, Gesteira TF, Andrade de Paula CA, Carneiro CR, Ortiz V, Toma L, Kao WW, Nader HB. Lumican expression, localization and antitumor activity in prostate cancer. Exp Cell Res 2013; 319(7):967-81; PMID:23399832; http://dx.doi.org/ 10.1016/j.yexcr.2013.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ricciardelli C, Russell DL, Ween MP, Mayne K, Suwiwat S, Byers S, Marshall VR, Tilley WD, Horsfall DJ. Formation of hyaluronan- and versican-rich pericellular matrix by prostate cancer cells promotes cell motility. J Biol Chem 2007; 282(14):10814-25; PMID:17293599; http://dx.doi.org/ 10.1074/jbc.M606991200 [DOI] [PubMed] [Google Scholar]
- [39].Read JT, Rahmani M, Boroomand S, Allahverdian S, McManus BM, Rennie PS. Androgen receptor regulation of the versican gene through an androgen response element in the proximal promoter. J Biol Chem 2007; 282(44):31954-63; PMID:17728259; http://dx.doi.org/ 10.1074/jbc.M702099200 [DOI] [PubMed] [Google Scholar]
- [40].Du WW, Yang W, Yee AJ. Roles of versican in cancer biology–tumorigenesis, progression and metastasis. Histol Histopathol 2013; 28(6):701-13; PMID:23519970. [DOI] [PubMed] [Google Scholar]
- [41].Spaeth EL, Labaff AM, Toole BP, Klopp A, Andreeff M, Marini FC. Mesenchymal CD44 Expression Contributes to the Acquisition of an Activated Fibroblast Phenotype via TWIST Activation in the Tumor Microenvironment. Cancer Res 2013; 73(17):5347-59; PMID:23838935; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Suhovskih AV, Mostovich LA, Kunin IS, Boboev MM, Nepomnyashchikh GI, Aidagulova SV, Grigorieva EV. Proteoglycan expression in normal human prostate tissue and prostate cancer. ISRN Oncol 2013:680136; PMID:23691363; http://dx.doi.org/ 10.1155/2013/680136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Padmanabhan A, Rao MV, Wu Y, Zaidel-Bar R. Jack of all trades: functional modularity in the adherens junction. Curr Opin Cell Biol 2015; 36:32-40; PMID:26189061; http://dx.doi.org/ 10.1016/j.ceb.2015.06.008 [DOI] [PubMed] [Google Scholar]
- [44].Canel M, Serrels A, Frame MC, Brunton VG. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J Cell Sci 2013; 126(Pt 2):393-401; PMID:23525005; http://dx.doi.org/ 10.1242/jcs.100115 [DOI] [PubMed] [Google Scholar]
- [45].Deep G, Jain AK, Ramteke A, Ting H, Vijendra KC, Gangar SC, Agarwal C, Agarwal R. SNAI1 is critical for the aggressiveness of prostate cancer cells with low E-cadherin. Mol Cancer 2014; 13:37; PMID:24565133; http://dx.doi.org/ 10.1186/1476-4598-13-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vieira AF, Paredes J. P-cadherin and the journey to cancer metastasis. Mol Cancer 2015; 14(1):178; PMID:26438065; http://dx.doi.org/ 10.1186/s12943-015-0448-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Martin TA, Mason MD, Jiang WG. Tight junctions in cancer metastasis. Front Biosci (Landmark Ed) 2011; 16:898-936; PMID:21196209; http://dx.doi.org/ 10.2741/3726 [DOI] [PubMed] [Google Scholar]
- [48].Ding L, Lu Z, Lu Q, Chen YH. The claudin family of proteins in human malignancy: a clinical perspective. Cancer Manag Res 2013; 5:367-75; PMID:24232410; http://dx.doi.org/ 10.2147/CMAR.S38294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hopkins AM, Walsh SV, Verkade P, Boquet P, Nusrat A. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J Cell Sci 2003; 116(Pt 4):725-42; PMID:12538773; http://dx.doi.org/ 10.1242/jcs.00300 [DOI] [PubMed] [Google Scholar]
- [50].Amaro A, Esposito AI, Gallina A, Nees M, Angelini G, Albini A, Pfeffer U. Validation of proposed prostate cancer biomarkers with gene expression data: a long road to travel. Cancer Metastasis Rev 2014; 33(2–3):657-71; PMID:24477410; http://dx.doi.org/ 10.1007/s10555-013-9470-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hensley PJ, Desiniotis A, Wang C, Stromberg A, Chen CS, Kyprianou N. Novel pharmacologic targeting of tight junctions and focal adhesions in prostate cancer cells. PLoS One 2014; 9(1):e86238; PMID:24497940; http://dx.doi.org/ 10.1371/journal.pone.0086238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Harisi R, Jeney A. Extracellular matrix as target for antitumor therapy. Onco Targets Ther 2015; 8:1387-98; PMID:26089687; http://dx.doi.org/ 10.2147/OTT.S48883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sakko AJ, Ricciardelli C, Mayne K, Tilley WD, Lebaron RG, Horsfall DJ. Versican accumulation in human prostatic fibroblast cultures is enhanced by prostate cancer cell-derived transforming growth factor beta1. Cancer Res 2001; 61(3):926-30; PMID:11221884. [PubMed] [Google Scholar]
- [54].Coulson-Thomas VJ, Gesteira TF, Coulson-Thomas YM, Vicente CM, Tersariol IL, Nader HB, Toma L. Fibroblast and prostate tumor cell cross-talk: fibroblast differentiation, TGF-β, and extracellular matrix down-regulation. Exp Cell Res 2010; 316(19):3207-26; PMID:20727350; http://dx.doi.org/ 10.1016/j.yexcr.2010.08.005 [DOI] [PubMed] [Google Scholar]
- [55].Reinertsen T, Halgunset J, Viset T, Flatberg A, Haugsmoen LL, Skogseth H. Gene expressional changes in prostate fibroblasts from cancerous tissue. APMIS 2012; 120(7):558-71; PMID:22716211; http://dx.doi.org/ 10.1111/j.1600-0463.2011.02865.x [DOI] [PubMed] [Google Scholar]
- [56].Ting HJ, Deep G, Jain AK, Cimic A, Sirintrapun J, Romero LM, Cramer SD, Agarwal C, Agarwal R. Silibinin prevents prostate cancer cell-mediated differentiation of naïve fibroblasts into cancer-associated fibroblast phenotype by targeting TGF β2. Mol Carcinog 2015; 54(9):730-41; PMID:24615813; http://dx.doi.org/ 10.1002/mc.22135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ishii K, Mizokami A, Tsunoda T, Iguchi K, Kato M, Hori Y, Arima K, Namiki M, Sugimura Y. Heterogenous induction of carcinoma-associated fibroblast-like differentiation in normal human prostatic fibroblasts by co-culturing with prostate cancer cells. J Cell Biochem 2011; 112(12):3604-11; PMID:21809373; http://dx.doi.org/ 10.1002/jcb.23291 [DOI] [PubMed] [Google Scholar]
- [58].Kaminski A, Hahne JC, Haddouti el-M, Florin A, Wellmann A, Wernert N. Tumour-stroma interactions between metastatic prostate cancer cells and fibroblasts. Int J Mol Med 2006; 18(5):941-50; PMID:17016625. [PubMed] [Google Scholar]
- [59].Wernert N, Kaminski A, Haddouti el-M, Hahne JC. Tumor-stroma interactions of metastatic prostate cancer cell lines: analyses using microarrays. Methods Mol Biol 2007; 382:223-37; PMID:18220234; http://dx.doi.org/ 10.1007/978-1-59745-304-2_14 [DOI] [PubMed] [Google Scholar]
- [60].Iacopino F, Angelucci C, Sica G. Interactions between normal human fibroblasts and human prostate cancer cells in a co-culture system. Anticancer Res 2012; 32(5):1579-88; PMID:22593435. [PubMed] [Google Scholar]
- [61].Kümper S, Ridley AJ. p120ctn and P-cadherin but not E-cadherin regulate cell motility and invasion of DU145 prostate cancer cells. PLoS One 2010; 5(7):e11801; PMID:20668551; http://dx.doi.org/ 10.1371/journal.pone.0011801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kabalin JN, Peehl DM, Stamey TA. Clonal growth of human prostatic epithelial cells is stimulated by fibroblasts. Prostate 1989; 14(3):251-63; PMID:2499876. [DOI] [PubMed] [Google Scholar]
- [63].Paland N, Kamer I, Kogan-Sakin I, Madar S, Goldfinger N, Rotter V. Differential influence of normal and cancer-associated fibroblasts on the growth of human epithelial cells in an in vitro cocultivation model of prostate cancer. Mol Cancer Res 2009; 7(8):1212-23; PMID:19671672; http://dx.doi.org/ 10.1158/1541-7786.MCR-09-0073 [DOI] [PubMed] [Google Scholar]
- [64].Thalmann GN, Rhee H, Sikes RA, Pathak S, Multani A, Zhau HE, Marshall FF, Chung LW. Human prostate fibroblasts induce growth and confer castration resistance and metastatic potential in LNCaP Cells. Eur Urol 2010; 58(1):162-171; PMID:19747763; http://dx.doi.org/ 10.1016/j.eururo.2009.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Veiseh M, Kwon DH, Borowsky AD, Tolg C, Leong HS, Lewis JD, Turley EA, Bissell MJ. Cellular heterogeneity profiling by hyaluronan probes reveals an invasive but slow-growing breast tumor subset. Proc Natl Acad Sci USA 2014; 111(17):E1731-1739; PMID:24733940; http://dx.doi.org/ 10.1073/pnas.1402383111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Scherer A, Kuhl S, Wessels D, Lusche DF, Hanson B, Ambrose J, Voss E, Fletcher E, Goldman C, Soll DR. A computer-assisted 3D model for analyzing the aggregation of tumorigenic cells reveals specialized behaviors and unique cell types that facilitate aggregate coalescence. PLoS One 2015; 10(3):e0118628; PMID:25790299; http://dx.doi.org/ 10.1371/journal.pone.0118628 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.