Abstract
Objective
Phosphatidylcholine transfer protein (PC-TP; synonym StarD2) is highly expressed in liver and oxidative tissues. PC-TP promotes hepatic glucose production during fasting and aggravates glucose intolerance in high fat fed mice. However, because PC-TP also suppresses thermogenesis in brown adipose tissue (BAT), its direct contribution to obesity-associated diabetes in mice remains unclear. Here we examined the effects of genetic PC-TP ablation on glucose homeostasis in leptin-deficient ob/ob mice, which exhibit both diabetes and altered thermoregulation.
Animals/methods
Mice lacking both PC-TP and leptin (Pctp−/−;ob/ob) were prepared by crossing Pctp−/− with ob/+ mice. Glucose homeostasis was assessed by standard assays, and energy expenditure was determined by indirect calorimetry using a comprehensive laboratory animal monitoring system, which also recorded physical activity and food intake. Body composition was determined by NMR and hepatic lipids by enzymatic assays. Core body temperature was measured using a rectal thermocouple probe.
Results
Pctp−/−;ob/ob mice demonstrated improved glucose homeostasis, as evidenced by markedly improved glucose and pyruvate tolerance tests, without changes in insulin tolerance. However, there were no differences in EE at any ambient temperature. There were also no effects of PC-TP expression on physical activity, food intake or core body temperature.
Conclusions
Improved glucose tolerance in Pctp−/−;ob/ob mice in the absence of increases in energy expenditure or core body temperature indicates a direct pathogenic role for PC-TP in diabetes in leptin deficient mice.
Keywords: diabetes, thermoregulation, insulin resistance, leptin
1. Introduction
Phosphatidylcholine transfer protein (PC-TP; synonym StarD2) is enriched in liver and oxidative tissues, and genetic polymorphisms suggest its pathogenic role in type 2 diabetes [1]. Pctp−/− mice exhibit marked reductions in hepatic glucose production when fed either chow [2] or high fat [3] diets, as well as enhanced adaptive thermogenesis and increased core body temperature [4]. Because these effects would each be expected to improve glucose homeostasis, their relative contributions remain unclear.
Leptin-deficient mice (ob/ob) become obese, developing insulin resistance [5] and increased hepatic glucose production [6], as well as impaired thermoregulation [7, 8]. To further elucidate the specific contribution of PC-TP to glucose homeostasis, we created mice lacking both PC-TP and leptin (Pctp−/−;ob/ob). The absence of PC-TP markedly improved glucose tolerance and reduced hepatic gluconeogenesis, without effects on energy expenditure (EE). These findings reveal that regulatory effects of PC-TP on glucose homeostasis can be dissociated from thermoregulation, and indicate that PC-TP contributes directly to the pathogenesis of diabetes in leptin-deficient mice.
2. Materials and methods
2.1 Mice
Male Pctp−/− mice [2] backcrossed 20 generations to a C57BL/6J genetic background were maintained on a 12-h light/dark cycle, fed a standard rodent diet 5053 (PicoLab Rodent Diet 20, 5053, LabDiets, St. Louis, MO) with ad libitum access to water. C57BL/6J mice heterozygous for leptin (ob/+) (The Jackson Laboratory, Bar Harbor, ME) were bred to Pctp−/− mice [9]. Experiments were performed on 5 successive cohorts of mice. Upon sacrifice, livers were harvested, snap frozen and stored at −80 °C. Protocols for animal use were approved by Harvard Medical School.
2.2 Glucose, pyruvate and insulin tolerance
Tolerance tests to glucose (GTT), pyruvate (PTT) and insulin (ITT) were performed after food withdrawal for 16 h (GTT and PTT) and 6 h (ITT). Plasma glucose concentrations were measured (OneTouch Ultra glucose monitor, LifeScan, Milpitas, CA) at baseline and periodically following intraperitoneal administration of 0.5 mg/g bw D-glucose for GTTs, 0.5 mg/g bw pyruvate for PTTs or 2 U/kg bw insulin (HumulinR, Eli Lilly, Indianapolis, IN) for ITTs.
2.3 Gene expression
Gene expression levels were determined by quantitative real-time PCR using RNA extracted from livers [2].
2.4 Metabolic monitoring
Measurements were performed using a Comprehensive Lab Animal Monitoring System (Columbus Instruments, Columbus, OH) [2]. Following 48 h of acclimation, rates of O2 consumption (VO2) and of CO2 production (VCO2) were recorded for 24 h at ambient temperatures of 30 °C, 22 °C and 15 °C. Respiratory exchange ratio (RER) was calculated as the ratio of VCO2/VO2. EE (kcal/h) was calculated from values of VO2 and VCO2 as EE = (3.82VO2 + 1.22VCO2) and was adjusted by ANCOVA for lean body mass as determined by NMR spectroscopy (EchoMRI, Houston TX) [10, 11]. Physical activity was recorded as beam breaks. Food consumption was measured gravimetrically. Core body temperature was measured by rectal thermocouple probe (Oakton Instruments, Vernon Hills, IL).
2.5 Hepatic lipid concentrations
Reagent kits (Wako Diagnostics, Richmond, VA) were used to determine hepatic concentrations of triglycerides (reagents 461-08992/461-09092), cholesterol (reagents 439-17501) and free fatty acids (reagents 999-34691/991-34891/995-34791/993-35191).
2.6 Statistical methods
Differences between groups were analyzed using a two-tailed unpaired Student’s t-test, with corrections for multiple comparisons (Prism v6.0h, GraphPad, La Jolla, CA).
3. Results
3.1 Improved glucose homeostasis in Pctp−/−;ob/ob mice
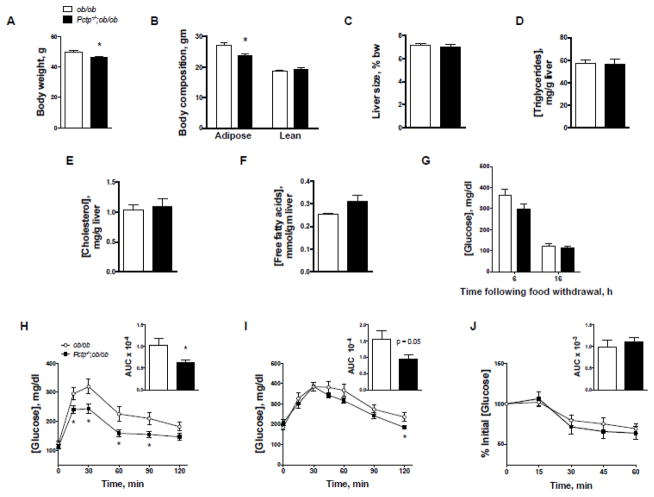
Pctp−/−;ob/ob mice were viable and without apparent developmental abnormalities. At 10 w of age Pctp−/−;ob/ob mice exhibited modest reductions in body weight (Fig. 1A). This was primarily attributable to reduced adipose tissue mass (Fig. 1B). Liver size was unaffected by PC-TP expression (Fig. 1C), as were hepatic concentrations of triglycerides (Fig. 1D), cholesterol (Fig. 1E) or free fatty acids (Fig. 1F).
Figure 1.
Genetic ablation of PC-TP decreases adiposity and improves glucose tolerance without altering hepatic lipid concentrations in ob/ob mice. (A) Body weights of male leptin-deficient mice at 10 w of age (ob/ob, n = 9; Pctp−/−;ob/ob, n = 20). (B) Body composition assessed by NMR spectroscopy at 9 w of age and displayed as tissue mass (ob/ob, n = 11; Pctp−/−;ob/ob, n = 11). (C) Liver size expressed as a percentage of bw. Hepatic concentrations of (D) triglycerides, (E), cholesterol and (F) free fatty acids. (G) Plasma glucose concentration following 6 or 16 h of food withdrawal (n = 12–13 per group). Tolerance tests to (H) glucose (7–8 w old mice), (I) pyruvate and (J) insulin (8–9 w old mice) were performed following 16 h (H, I) or 6 h (J) of food withdrawal, respectively, for mice housed at room temperature on chow diet (n = 12–13 for GTT and ITT; 6–8 for PTT). Values of area under the curve (AUC) are presented as inset barplots. Data are mean +/− SEM. *P < 0.05 Pctp−/−;ob/ob vs. ob/ob.
Plasma glucose concentrations tended to be lower in Pctp−/−;ob/ob mice following 6 h of food withdrawal, but this nonsignificant difference (P = 0.11) was diminished after 16 h (Fig. 1G). By contrast, plasma glucose concentrations during the GTT were uniformly lower (Fig. 1H) and there was a 39 % decrease in the AUC (inset to Fig. 1H). Similarly, Pctp−/− mice exhibited decreased hepatic gluconeogenesis, as evidence by reduced plasma glucose concentrations in response to pyruvate, with trend towards reduction in AUC by 39 % (Fig. 1I). The absence of PC-TP expression did not influence the sensitivity to insulin (Fig. 1J). These results did not differ for a weight-matched subset (data not shown). Additionally, there were no changes in the mRNA expression of the gluconeogenic enzymes phosphoenolpyruvate carboxykinase or glucose-6-phosphatase.
3.2 Thermoregulation in ob/ob mice is independent of PC-TP expression
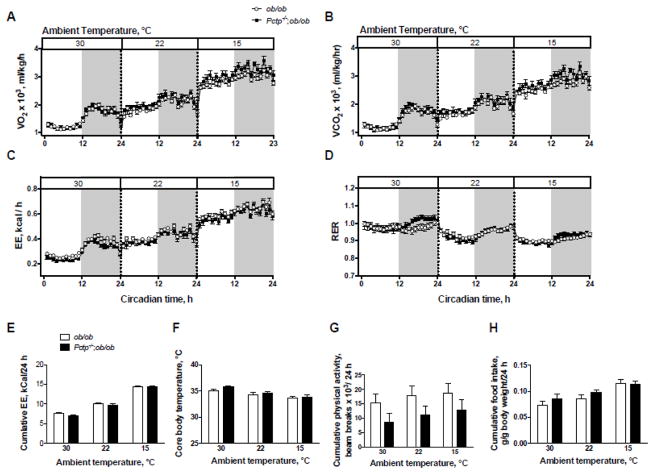
Ambient temperatures were varied to elicit potential effects of PC-TP on adaptive thermogenesis. Upon reducing ambient temperature from thermoneutrality (30 °C), VO2 values (Fig. 2A), VCO2 values (Fig. 2B), and rates of EE (Fig. 2C) all increased by approximately 3-fold. However, there were no effects of PC-TP expression, as evidenced by cumulative 24 h EE values (Fig. 2E), which were unaffected by Pctp genotype. RER values generally decreased as ambient temperature decreased, but there was no effect of PC-TP expression (Fig 2D). Core body temperatures were similarly unaffected by PC-TP (Fig. 2F). There was a trend towards decreased physical activity in the Pctp−/−;ob/ob mice, but this did not differ significantly either for cumulative values (P = 0.08–0.30) (Fig. 2G) or during the light or dark cycles (P = 0.13–0.25). Finally, food consumption normalized for bw was not influenced by PC-TP expression (Fig. 2H).
Figure 2.
PC-TP expression does not affect EE in absence of leptin. Values of (A) VO2 and (B) VCO2 were determined by indirect calorimetry (n = 11–14/group). Values of (C) EE and (D) RER were calculated from VO2 and VCO2 as described in section 2.3 of the text. Vertical dotted lines denote intervening 48 h equilibration periods. White background denotes light hours and gray denotes dark hours. (E) Cumulative EE values over 24 h were adjusted for lean body mass by ANCOVA. (F) Core body temperatures following 48 h acclimation. (G) Cumulative physical activity over 24 h. (H) Cumulative food intake. Data are mean +/− SEM.
4. Discussion
The genetic ablation of PC-TP improved glucose tolerance in ob/ob mice in the absence of increases in EE. This uncoupling of phenotypes suggests an important direct contribution of PC-TP to excess hepatic glucose production in the setting of obesity.
Direct control of glucose metabolism in the liver by PC-TP was initially demonstrated as reductions in hepatic glucose production in chow and high fat fed Pctp−/− mice [3, 12]. Mechanisms were elucidated using isolated cultured hepatocytes: Under simulated fasting conditions, PC-TP promotes fatty acid oxidation, which primes the TCA cycle for gluconeogenesis [13]. This occurs when PC-TP interacts with thioesterase superfamily member 2 (Them2) at the mitochondrial membrane to generate free fatty acids, which are subsequently taken up into mitochondria [14]. Studies in cell culture also revealed that the PC-TP-Them2 complex suppresses insulin signaling in hepatocytes by inhibiting insulin receptor substrate 2 phosphorylation and by stabilizing the tuberous sclerosis complex 1 (TSC1)-TSC2 heterodimer [15]. Because PC-TP-Them2 interactions are enhanced by fasting, this protein complex appears to promote the hepatic export of glucose [13]. In support of this possibility, both proteins are regulated by peroxisome proliferator activated receptor α [16, 17], which plays key roles in coordinating the transcriptional response to fasting [18]. However, these collective effects of PC-TP and Them2 become maladaptive under conditions of overnutrition, by supporting excess hepatic glucose production [3]. The current study indicates that PC-TP also promotes excess hepatic glucose production in ob/ob mice [6] in the absence of upregulation in gluconeogenic gene expression. Notwithstanding that the absence of leptin is associated with impaired glucose disposal [6], the absence of PC-TP did not improve in insulin tolerance of ob/ob mice. This is in keeping with prior studies that demonstrated no effects of PC-TP on glucose disposal in either chow or high fat fed mice [2, 3].
Pctp−/− mice also exhibit increases in EE and core body temperature, along with a shift in energy substrate utilization towards fatty acids [2, 4]. Studies using primary brown adipocytes suggest that the PC-TP-Them2 complex limits the flux of fatty acids into mitochondria [1], as evidence by increased fatty acid oxidation rates in BAT of mice lacking either protein [10, 19]. However, the absence of PC-TP did not increase EE or core body temperature in ob/ob mice at thermoneutrality (30 °C), room temperature or in response to a significant cold-challenge (15 °C). Unchanged RER values further argue against a substantial regulatory effect of PC-TP on fatty acid oxidation in BAT in the absence of leptin.
Although reductions in EE have been reported for ob/ob mice [7], a recent analysis has demonstrated that the absence of leptin does not diminish the thermogenic response [8]. Rather, it appears that ob/ob mice defend a reduced body temperature [8]. In Pctp−/− mice, alterations in adaptive thermogenesis, EE, and core body temperature [4] could reflect effects of PC-TP expression both in the periphery (i.e. in BAT) and the central nervous system via leptin-mediated signaling. However, in the absence of leptin, we observed no changes in EE attributable to PC-TP expression. Therefore, it is reasonable to infer that improved glucose metabolism in this model of obesity can be attributed principally to direct effects of PC-TP on hepatic glucose production.
5. Conclusion
The direct contribution of PC-TP to excess hepatic glucose production in ob/ob mice supports the development of small molecule inhibitors [3, 20] for the pharmacologic management of type 2 diabetes.
Acknowledgments
Funding: Supported by the National Institutes of Health, grant numbers R37 DK048872 and R01 DK056626.
Footnotes
Disclosure: The authors have nothing to disclose.
Author contributions: T.I.K. and D.E.C. conceived and designed the study; T.I.K. and K.B.L. acquired the data; T.I.K., K.B.L. and D.E.C. interpreted the data; T.I.K. and D.E.C. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kang HW, Wei J, Cohen DE. PC-TP/StARD2: Of membranes and metabolism. Trends Endocrinol Metab. 2010;21:449–56. doi: 10.1016/j.tem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scapa EF, Pocai A, Wu MK, Gutierrez-Juarez R, Glenz L, Kanno K, et al. Regulation of energy substrate utilization and hepatic insulin sensitivity by phosphatidylcholine transfer protein/StarD2. FASEB J. 2008;22:2579–90. doi: 10.1096/fj.07-105395. [DOI] [PubMed] [Google Scholar]
- 3.Shishova EY, Stoll JM, Ersoy BA, Shrestha S, Scapa EF, Li Y, et al. Genetic ablation or chemical inhibition of phosphatidylcholine transfer protein attenuates diet-induced hepatic glucose production. Hepatology. 2011;54:664–74. doi: 10.1002/hep.24393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang HW, Ribich S, Kim BW, Hagen SJ, Bianco AC, Cohen DE. Mice lacking Pctp/StarD2 exhibit increased adaptive thermogenesis and enlarged mitochondria in brown adipose tissue. J Lipid Res. 2009;50:2212–21. doi: 10.1194/jlr.M900013-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 6.Haluzik M, Colombo C, Gavrilova O, Chua S, Wolf N, Chen M, et al. Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinology. 2004;145:3258–64. doi: 10.1210/en.2004-0219. [DOI] [PubMed] [Google Scholar]
- 7.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 8.Fischer AW, Hoefig CS, Abreu-Vieira G, de Jong JM, Petrovic N, Mittag J, et al. Leptin Raises Defended Body Temperature without Activating Thermogenesis. Cell Rep. 2016;14:1621–31. doi: 10.1016/j.celrep.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 9.Sainsbury A, Schwarzer C, Couzens M, Jenkins A, Oakes SR, Ormandy CJ, et al. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev. 2002;16:1077–88. doi: 10.1101/gad.979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang HW, Ozdemir C, Kawano Y, LeClair KB, Vernochet C, Kahn CR, et al. Thioesterase superfamily member 2/Acyl-CoA thioesterase 13 (Them2/Acot13) regulates adaptive thermogenesis in mice. J Biol Chem. 2013;288:33376–86. doi: 10.1074/jbc.M113.481408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tschop MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, et al. A guide to analysis of mouse energy metabolism. Nat Methods. 2012;9:57–63. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–6. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawano Y, Ersoy BA, Li Y, Nishiumi S, Yoshida M, Cohen DE. Thioesterase superfamily member 2 (Them2) and phosphatidylcholine transfer protein (PC-TP) interact to promote fatty acid oxidation and control glucose utilization. Mol Cell Biol. 2014;34:2396–408. doi: 10.1128/MCB.01601-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerner J, Hoppel C. Fatty acid import into mitochondria. Biochim Biophys Acta. 2000;1486:1–17. doi: 10.1016/s1388-1981(00)00044-5. [DOI] [PubMed] [Google Scholar]
- 15.Ersoy BA, Tarun A, D’Aquino K, Hancer NJ, Ukomadu C, White MF, et al. Phosphatidylcholine transfer protein interacts with thioesterase superfamily member 2 to attenuate insulin signaling. Sci Signal. 2013;6:ra64. doi: 10.1126/scisignal.2004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang HW, Kanno K, Scapa EF, Cohen DE. Regulatory role for phosphatidylcholine transfer protein/StarD2 in the metabolic response to peroxisome proliferator activated receptor alpha (PPARalpha) Biochim Biophys Acta. 2010;1801:496–502. doi: 10.1016/j.bbalip.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei J, Kang HW, Cohen DE. Thioesterase superfamily member 2 (Them2)/acyl-CoA thioesterase 13 (Acot13): A homotetrameric hotdog fold thioesterase with selectivity for long-chain fatty acyl-CoAs. Biochem J. 2009;421:311–22. doi: 10.1042/BJ20090039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandard S, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci. 2004;61:393–416. doi: 10.1007/s00018-003-3216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang HW, Ribich S, Kim BW, Hagen SJ, Bianco AC, Cohen DE. Mice lacking phosphatidylcholine transfer protein/StarD2 exhibit increased adaptive thermogenesis and enlarged mitochondria in brown adipose tissue. J Lipid Res. 2009;50:2212–21. doi: 10.1194/jlr.M900013-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagle N, Xian J, Shishova EY, Wei J, Glicksman MA, Cuny GD, et al. Small-molecule inhibitors of phosphatidylcholine transfer protein/StarD2 identified by high-throughput screening. Anal Biochem. 2008;383:85–92. doi: 10.1016/j.ab.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]