Abstract
Metastases account for the great majority of cancer-associated deaths, yet this complex process remains the least understood aspect of cancer biology. As the body of research concerning metastasis continues to grow at a rapid rate, the biological programs that underlie the dissemination and metastatic outgrowth of cancer cells are beginning to come into view. In this review we summarize the cellular and molecular mechanisms involved in metastasis, with a focus on carcinomas where the most is known, and highlight the general principles of metastasis that have begun to emerge.
INTRODUCTION
The diversity of cancers that arise in humans exceeds 200 distinct disease entities – reflecting differences in the normal cells-of-origin, acquired somatic mutations, variably altered transcriptional networks, and influences of local tissue microenvironments. Attempts have been made to distill this complexity into a unifying set of organizing principles termed cancer hallmarks (Hanahan and Weinberg, 2000, 2011). In spite of significant advances in the study, diagnosis, and treatment of cancer, the vast majority of patients with advanced metastatic disease confront a terminal illness that is, with rare exception, incurable by current therapeutic regimens. Stated differently, the overwhelming majority of cancer-associated deaths (about 90%) are caused by metastatic disease rather than primary tumors.
The dissemination of cancer cells from primary tumors and their subsequent seeding of new tumor colonies in distant tissues involves a multi-step process known as the invasion-metastasis cascade (Fidler, 2003; Gupta and Massague, 2006; Talmadge and Fidler, 2010). This sequence of events involves the local invasion of primary tumor cells into surrounding tissues; intravasation of these cells into the circulatory system and survival during hematogenous transit; arrest and extravasation through vascular walls into the parenchyma of distant tissues; formation of micrometastatic colonies in this parenchyma; and the subsequent proliferation of microscopic colonies into overt, clinically detectable metastatic lesions, this last process being termed colonization.
In contrast to the large body of findings that have revealed the detailed pathogenetic mechanisms leading to primary tumor formation, the biological underpinnings of metastatic disease remain poorly understood. Furthermore, relatively few principles have emerged that would unify our understanding of how diverse types of metastases arise and how similar or different each may be relative to the behavior of its corresponding primary tumor. Nonetheless, over the past fifteen years significant progress has been made in elucidating various aspects of the metastatic program, particularly for carcinomas, which in aggregate account for ~80% of cancer cases and thus the majority of cancer deaths.
Here we summarize important advances that have revealed some of the mechanisms that underlie the dissemination and metastatic outgrowth of carcinoma cells. Drawing from this increasingly large and complex body of work, we suggest that a few key biological principles have begun to emerge for certain aspects of the metastatic cascade, while for other steps of the cascade a unifying conceptual framework remains more elusive.
DISSEMINATION OF CARCINOMA CELLS
The process of dissemination subsumes the initial steps of the invasion-metastasis cascade that enable malignant tumor cells to acquire traits that equip them with the ability to leave the primary site and travel to distant tissues (Figure 1A). As with almost all of the discussions in this review, we describe these processes in the context of the intensively studied carcinomas. One centrally important process enabling these steps is the cell-biological program termed the epithelial-mesenchymal transition (EMT), a developmental program that is normally employed during embryogenesis and, in adults, the healing of epithelial tissues, and is hijacked by carcinoma cells, endowing them with multiple malignant traits associated with the loss of epithelial properties and the acquisition of certain mesenchymal features in their stead (Thiery, 2002).
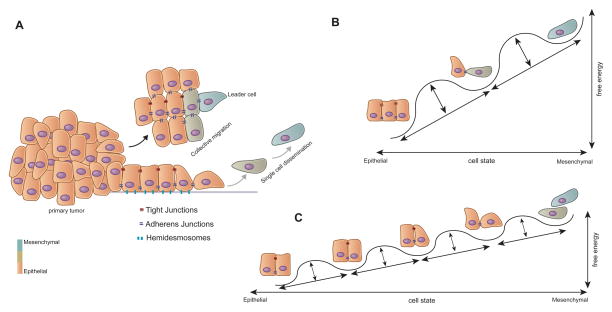
Figure 1. Dissemination of Carcinoma Cells.
(A) Carcinoma cell dissemination occurs via two mechanisms - single cell dissemination through an EMT (grey arrow) or the collective dissemination of tumor clusters (black arrow). Recent evidence suggests that the leader cells of tumor clusters also undergo certain phenotypic changes associated with the EMT.
(B) The epithelial state can be portrayed as the default state of residence; as cells undergo an EMT they enter into a succession of multiple epigenetic states, depicted here as free energy wells, with each state moving toward a more mesenchymal state representing a higher energy state.
(C) However, the barriers between states, depicted here again as free energy wells, may be relatively low, resulting in substantial spontaneous interconversion between them, this being manifested as phenotypic plasticity.
The Epithelial-Mesenchymal Transition
The EMT program confers on epithelial cells, both normal and neoplastic, properties that are critical to invasion and metastatic dissemination, notably increased motility, invasiveness and the ability to degrade components of the extracellular matrix (ECM) (Kalluri and Weinberg, 2009; Nieto et al., 2016; Thiery, 2002). In fact, the EMT is really a group of cell-biological programs that share features in common but differ in certain critical details, depending on the tissue site, the degree of malignancy, and the contextual signals experienced by individual neoplastic cells. These complex programs are orchestrated and coordinated by a series of master EMT-inducing transcription factors (EMT-TFs), notably Snail, Slug, Twist and Zeb1, which have been explored in great experimental detail (De Craene and Berx, 2013; Lamouille et al., 2014). Yet other TFs capable of inducing components of the EMT program have also been described (e.g., Zeb2, Foxc2, Prrx1, among others), but their roles in cancer pathogenesis remain less well documented. Although traditional models of tumorigenesis posit that metastasis is a late event during the course of multi-step tumor progression, some studies have shown that acquisition of EMT-associated traits and the process of dissemination can actually occur relatively early, being evident even in certain preneoplastic lesions (Husemann et al., 2008; Rhim et al., 2012).
Of additional relevance is the fact that several types of carcinoma cells have been found to acquire tumor-initiating capability following induction of EMT programs. These include breast (Mani et al., 2008; Morel et al., 2008), colorectal (Brabletz et al., 2005; Fan et al., 2012; Pang et al., 2010), ovarian (Long et al., 2015), pancreatic (Rasheed et al., 2010), prostate (Kong et al., 2010) and renal (Zhou et al., 2016), among other types of carcinomas. Tumor-initiating ability, usually depicted as the defining trait of cancer stem cells (CSCs), is generally gauged by implantation of populations of neoplastic cells in appropriate mouse hosts. Such tests indicate that CSCs are almost always present as relatively small subpopulations of neoplastic cells residing within individual tumors among larger populations of cancer cells that lack tumor-initiating powers. Residence of a disseminating carcinoma cell in the CSC state would seem to be critical for progression through the invasion-metastasis cascade, since disseminated tumor cells must presumably be endowed with tumor-initiating ability in order to function as the founders of new metastatic colonies. Moreover, acquisition of more mesenchymal traits, as driven by an EMT program, has been found to elevate the resistance of carcinoma cells to various types of cytotoxic treatments, including both radio- and chemotherapies (Gupta et al., 2009; Kurrey et al., 2009), providing one explanation of the often-observed phenomenon that CSCs tend to be more therapy-resistant than their non-CSC counterparts (Singh and Settleman, 2010).
While the EMT program might be depicted as operating much like a binary switch, in which cancer cells reside either in an epithelial or a mesenchymal state, the truth is more complex, in that EMT programs activated in carcinoma cells usually drive the acquisition of certain mesenchymal traits while permitting the retention of some epithelial traits, leaving carcinoma cells with mixed epithelial/mesenchymal phenotypes (Figure 1B and 1C).
EMT programs seem almost invariably to be triggered in carcinoma cells by heterotypic signals that these cells receive from the nearby tumor-associated stroma. Thus, during the course of tumor progression, the stroma – which is composed of a variety of fibroblasts, myofibroblasts, endothelial, myeloid and lymphoid cells recruited from host tissues – increasingly takes on the appearance of a stroma that typically forms during the healing of various wounded epithelial tissues. Such “reactive” stroma releases various signals, including TGF-βs, Wnts and certain interleukins that impinge on nearby carcinoma cells, inducing the latter to activate their previously silent EMT programs. This activation is generally reversible, and indeed carcinoma cells that have activated EMT programs may revert via a mesenchymal-epithelial transition (MET) to the phenotypic state in which their ancestors resided prior to induction of the EMT program.
While the EMT program appears to be critical to invasion and dissemination of most and possibly all carcinoma types (see below), to date there have been no rules formulated to predict expression of its various components in different tissue contexts. Among the unresolved fundamental issues are: (i) the nature of the heterotypic signals that converge on carcinoma cells and collaborate to activate previously silent EMT programs in these cells; (ii) the extent to which these programs are activated at various stages of carcinoma progression; (iii) the extent to which the differentiation programs of normal cells-of-origin influence the expression of various components of the EMT program; (iv) the respective roles of the various EMT-TFs cited above in collaborating with one another in choreographing various types of EMT programs; (v) the influence of somatic mutations sustained during primary tumor formation on the activation and expression of EMT programs; and (vi) the roles of intracellular and extracellular signaling pathways in sustaining the expression of already-activated EMT programs.
Invasion by Collective Migration
While the EMT is widely embraced as an important mode of carcinoma cell dissemination, its precise roles in primary tumor behavior remain unresolved. For example, invasion by primary tumor cells generally involves the collective migration of large, cohesive cohorts of cells into adjacent tissues rather than the dispersal of individual carcinoma cells (Friedl et al., 2012). The organization of these cohorts appears to conflict with the behavior of cells that have passed through an EMT and have lost cohesive cell-cell interactions, notably those mediated by adherens junctions. Thus, these cohorts provoke the question of whether EMT programs are indeed central to eventual carcinoma cell dissemination, as implied above, or instead represents only one of several alternative cell-biological programs that enable dissemination to occur.
Collective migration involving groups of cells, which is commonly seen at the borders of invasive carcinomas, is best documented in the case of carcinomas of the breast and lungs (Friedl et al., 2012); similar invasive cohorts undoubtedly participate in invasion by other types of carcinoma cells as well (Chung et al., 2016; Veracini et al., 2015). Cells residing within these invasive cell phalanxes continue to express key epithelial markers such as E-cadherin, which helps to sustain the cohesion between the individual epithelial cells within these cohorts. Moreover, the polyclonal nature of metastatic colonies of certain breast cancers raises the possibility that they arose from genetically heterogeneous clusters of disseminated cells, rather than arising clonally from single disseminated cells (Cheung et al., 2016). This raises the question of whether collective migration represents an alternative to EMT, and whether the two cell-biological programs are essentially mutually exclusive.
In fact, detailed histopathological analyses of invasive cohorts often suggest that the EMT does indeed participate in collective migration (Ye et al., 2015). Thus, these cohorts are themselves internally complex, with invading cells at the leading edges paving the way for large populations of followers to which they remain attached via cell-cell junctions (Cheung et al., 2013). In some cases, careful examination has revealed that certain mesenchymal traits are exhibited by the leading cells at the invasive fronts during collective migration (Revenu and Gilmour, 2009; Westcott et al., 2015; Ye et al., 2015). Such invading leaders are likely to release various proteases that degrade the extracellular matrix that would otherwise impede the forward progress of the cohort as a whole. Moreover, such leader cells may also possess the EMT-associated motility to enable forward motion of the cohort as a whole. Together, the cells at invasive edges may therefore pave the way for the followers that constitute the bulk of invasive cell phalanxes.
Unresolved is a key experimental test of this model: Can collective invasion occur if activation of EMT programs is totally blocked? Yet other studies report the presence of cancer-associated fibroblasts, rather than carcinoma cells that have undergone an EMT, as leaders cells at the invasive edges of carcinomas (Gaggioli et al., 2007). Thus, more experimental evidence is required to address and clarify more precisely the events occurring at the invasive edges of carcinomas and the nature of the normal and neoplastic cell types involved.
An Essential Role of the EMT Program in Metastasis
Two studies have recently undertaken to refute the essential role of the EMT program in the process of metastasis (Fischer et al., 2015; Zheng et al., 2015). In both instances, the proofs that EMTs did not occur while metastasis proceeded were not supported by the evidence presented, leaving open the continuing question of whether EMT is indeed critical to the metastatic ability of all types of carcinoma cells. Moreover, the reports of these findings coincide with a time when the definition of the EMT is undergoing re-evaluation, as suggested above. Thus, EMT programs are increasingly viewed as generating cells residing in a spectrum of multiple intermediate states lying in between epithelial and mesenchymal poles (Figure 1B and 1C), as suggested earlier (Bednarz-Knoll et al., 2012; Grosse-Wilde et al., 2015; Li and Kang, 2016; Nieto et al., 2016). It is therefore likely that in some cases, metastasizing carcinomas may exhibit overt mesenchymal properties that aid in metastatic spread (Bonnomet et al., 2012; Trimboli et al., 2008), whereas in other cases they may not require the same suite of EMT-associated traits (Celia-Terrassa et al., 2012).
In fact, a large number of reports highlight the existence of the “partial EMT” state and its propensity to enhance tumor progression and metastasis (Bednarz-Knoll et al., 2012; Grosse-Wilde et al., 2015; Hong et al., 2015; Jordan et al., 2011; Lundgren et al., 2009; Sampson et al., 2014; Schliekelman et al., 2015). In contrast, induction of a fully mesenchymal state, as achieved experimentally through the actions of introduced, highly expressed EMT-TFs and resulting completion of an entire EMT program, yields cells that have lost tumor-initiating ability and thus the power to found metastatic colonies (Ocana et al., 2012; Tsai et al., 2012). Stated differently, the phenotypic plasticity associated with carcinoma cells inhabiting the middle of the epithelial-mesenchymal spectrum appears to be critical to the founding of metastatic colonies and their subsequent robust outgrowth. Unaddressed by this discussion is the behavior of ovarian carcinomas, whose spread through the peritoneal space operates through principles very different from those characteristic of most solid tumors.
Circulating Tumor Cells
Individual invasive carcinoma cells and invasive cohorts arising from primary tumors may, sooner or later, invade into the vasculature either of adjacent normal tissues or the neovasculature that has been assembled within the tumors themselves. The resulting intravasation provides access to an avenue for circulating tumor cells (CTCs) to travel to distant sites, where they may seed new metastatic colonies (Kang and Pantel, 2013). Such travelers may move as individual cells or as multi-cellular clumps that can persist in the circulation until they encounter the small-bore microvessels of distant tissues (which often possess luminal diameters as small as ~8 microns). The resulting physical trapping would seem to ensure that the vast majority of intravasated CTCs dwell in the general circulation for only seconds or minutes after their initial entry into the vasculature. While the vast majority of CTCs may be rapidly cleared, some have recently reported that even clusters of CTCs are capable of maneuvering through capillary-sized vessels, doing so as a single-cell chain still held together through adhesive interactions (Au et al., 2016). CTC clusters introduced experimentally into the venous circulation are far more efficient than individual carcinoma cells in seeding metastatic colonies (Aceto et al., 2014), ostensibly because they are shielded from various types of attacks, such as those launched in the circulation by natural killer (NK) cells; in addition, these clusters may have an advantage in physically lodging in the lumina of vessels and certain poorly understood advantages in post-extravasation proliferation that could contribute to their increased metastatic efficiency.
Nonetheless, single-cell CTCs have been extensively studied in recent years because of technical improvements in their isolation from the blood of cancer patients (Aceto et al., 2015). Implicit in these surveys is the notion that these cells represent intermediaries between primary tumors and eventually formed metastatic colonies. However, in light of the considerations discussed above, it remains unclear which types of CTCs (single-cell vs. clusters) are actually responsible for the lion’s share of metastasis formation. Indeed, the probability of a single CTC successfully founding a metastatic colony is vanishingly small (Baccelli et al., 2013). Independent of these considerations is the notion that single-cell and clustered CTCs released by primary tumors are often produced in a certain ratio, in which case the solitary CTCs may serve as surrogate markers of the cell clusters that may indeed be responsible for the formation of the great majority of metastatic colonies.
Of additional relevance here is the fact the CTCs, traveling either as individual cells or as clusters, often exhibit combinations of epithelial and mesenchymal traits, reinforcing the role of the EMT program in the process of intravasation and cancer cell dissemination (Yu et al., 2013). Moreover, in longitudinal studies of individual patients, the fraction of mesenchymal CTCs has been found to increase progressively with acquired treatment resistance and disease progression. One concern here derives from the fact that CTC enrichment methods that rely on the display by CTCs of cell-surface epithelial markers may well miss capturing a sizeable, clinically relevant portion of the CTCs that are responsible for seeding distant metastases but have shed the bulk of their epithelial cell-surface markers as a consequence of extensive progression through EMT programs.
All of these provisos do not detract from certain already-proven uses of CTC technology. Single-cell CTCs may indeed be useful for certain types of diagnosis, since the presence of CTCs has been repeatedly found in commonly occurring carcinomas, including those of the breast, prostate, lung and colon (Aceto et al., 2015). In particular, the longitudinal monitoring of CTC concentrations through “liquid biopsies” may provide highly useful information about the responses of a patient’s tumor to various types of therapies. Another clearly useful application is the measurement of CTCs in patients whose primary tumors have been removed in order to determine whether residual, occult metastatic deposits persist and continue to empty carcinoma cells into the circulation.
In addition, the isolation, ex vivo expansion, and analysis of viable CTCs can be used to profile genetic mutations and drug sensitivities of the cells residing within primary tumors (Yu et al., 2014). This may allow the prediction of patient responses to various types of therapy, especially when the lesions being treated are not readily biopsied, for example those in the brain. Indeed, one already-published report demonstrates that CTCs isolated from prostate cancer patients can be harbingers of eventually acquired drug resistance, such as those carrying molecular changes that can confer resistance to androgen receptor antagonists (Miyamoto et al., 2015). Ideally, early detection and characterization of CTCs prior to the appearance of clinically detectable metastatic growths could be used to initiate or switch treatment before the eruption of life-threatening metastases. At present, however, this seems to be impractical, given the fact that even actively growing, aggressive tumors tend to release relatively low numbers of detectable CTCs into the circulation.
INTERACTIONS IN TRANSIT: FATES OF INTRAVASATED CARCINOMA CELLS
In fact, carcinoma cells that have successfully invaded stromal environments surrounding primary tumors can intravasate either into blood or lymphatic vessels. The dissemination of cancer cells to draining lymph nodes represents an important clinical parameter that is incorporated into the histopathological staging of the disease and thus is associated with particular prognoses (de Boer et al., 2010). While carcinoma cells may promote the growth of lymphatic vessels through the process of lymphangiogenesis (Karaman and Detmar, 2014) – a process that is correlated with disease progression (Skobe et al., 2001) – there is scant evidence for the notion that the draining lymph nodes represent temporary staging areas that enable significant numbers of cancer cells to pause before proceeding further into the bloodstream and thereafter to distant sites in the body. Hence, these small metastatic deposits likely represent dead ends for cancer cells and primarily function as surrogate markers that reveal the extent of parallel, concomitant dissemination from the primary tumor into the general circulation. For this reason, the discussion below is focused on the hematogenous transport of carcinoma cells, as this is likely the main route that metastatic cancer cells traverse prior to entering and colonizing distant tissues.
The safe passage of intravasated cancer cells to distant anatomical sites is hardly guaranteed. While the transit time of a cancer cell through the bloodstream may only amount to a few minutes, CTCs encounter multiple obstacles en route to the parenchyma of distant tissues. Foremost here are the physical challenges associated with life in circulation, which include loss of attachment to a substrate, hydrodynamic flow, and shear stress (Headley et al., 2016). In addition, carcinoma cells in the circulation are vulnerable to an immune attack, notably by NK cells that target them for rapid elimination. However, certain interactions between circulating carcinoma cells and other cell types in the circulation can actually facilitate their passage to and extravasation at distant sites, notably those involving platelets, neutrophils, monocytes/macrophages, and endothelial cells (Figure 2).
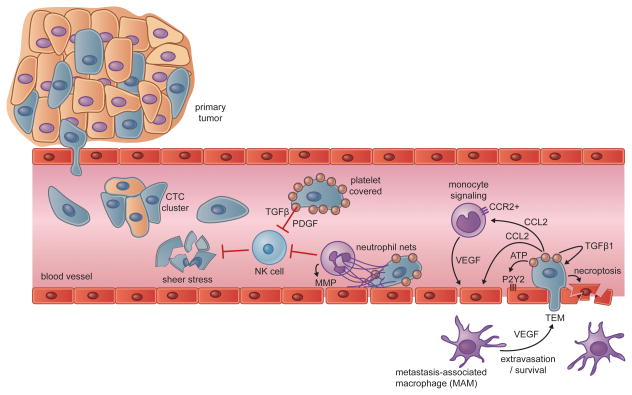
Figure 2. Interactions in Transit.
Carcinoma cells escaping from primary tumors can intravasate into the circulation, either as single circulating tumor cells (CTCs) or as multicellular CTC clusters. The bloodstream represents a hostile environment for CTCs, exposing them to rapid clearance by natural killer (NK) cells or fragmentation due to the physical stresses encountered in transit through the circulation. Carcinoma cells gain physical and immune protection through the actions of platelets, which coat CTCs shortly after their intravasation. Neutrophils can provide protection from NK cell attacks as well, while also contributing to the physical entrapment and extravasation of CTCs. Once lodged in a capillary, activated platelets and carcinoma cells secrete a number of bioactive factors that can act on monocytes, endothelial cells, and the carcinoma cells themselves. The collective effects of these interactions promote the transendothelial migration (TEM) of carcinoma cells, which can be aided by metastasis-associated macrophages (MAMs) in the target parenchyma. In lieu of TEM, arrested carcinoma cells may also proliferate intraluminally (not shown) or induce necroptosis in endothelial cells.
Interactions with Platelets
Once in the circulation, CTCs rapidly associate with platelets, an interaction that is triggered by Tissue Factor displayed on the surface of the carcinoma cells (Labelle and Hynes, 2012). Depending on the rate of CTC introduction into the circulation, this can lead to imbalances in the normal homeostatic controls on coagulation, which can result in certain clotting symptoms that are seen in patients with cancer, specifically microthrombi, disseminated intravascular coagulation, and even large pulmonary emboli (Gay and Felding-Habermann, 2011).
At the same time, platelets facilitate tumor metastasis. Indeed, the contribution of platelets to the metastatic process has been appreciated since the 1960s, when studies revealed that experimental induction of thrombocytopenia can exert an anti-metastatic effect (Gasic et al., 1968), while a high platelet count has for years been known to be associated with a poor clinical prognosis across diverse types of carcinomas (Gay and Felding-Habermann, 2011). Platelets contain a plethora of bioactive molecules that can potentially impact cancer progression and work in more recent years has revealed a number of mechanisms by which platelets can alter the fate of carcinoma cells in transit (Franco et al., 2015; Gay and Felding-Habermann, 2011).
Of relevance here is the fact that platelets can protect CTCs from elimination by cellular arms of the immune system. More specifically, adhered platelets can prevent tumor cell recognition and lysis by NK cells (Kopp et al., 2009; Nieswandt et al., 1999; Palumbo et al., 2005). This effect can be mediated by soluble factors derived from platelets, including TGF-β and PDGF that inhibit NK cell activity (Labelle and Hynes, 2012), and, quite possibly, by physically shielding cancer cells from NK cells through the formation of protective cloaks around CTCs and the deposition of fibrinogen on the cancer cells (Palumbo et al., 2007; Palumbo et al., 2005). Such protection specifically against NK cell-mediated attack may represent the most important benefit conferred on intravascular carcinoma cells by platelets, since the pro-metastatic effects of the thrombocytes are no longer apparent in mice depleted of NK cells (Palumbo et al., 2005).
In addition to protecting circulating tumor cells from external insults, platelets can also alter intracellular signaling pathways within carcinoma cells that ultimately affect the ability of the latter to establish metastatic growths. Notably, TGF-β secreted by degranulating platelets can act in coordination with contact-dependent signals that activate the NF-κB pathway in carcinoma cells, thereby inducing or sustaining the expression of EMT programs in the CTCs (Labelle et al., 2011). This direct signaling between platelets and carcinoma cells can presumably substitute for the absence of stroma-derived signals that previously led, in the context of the primary tumor, to the induction of an EMT. In the absence of such heterotypic interactions, CTCs may revert via an MET to the epithelial state of their ancestors in the primary tumor, thereby losing the invasive traits and tumor-initiating ability that would seem to be critical for subsequent successful extravasation and the founding of metastatic colonies.
Once activated by cancer cells, platelets can signal to nearby endothelial cells as well. Tumor cells elicit ATP secretion from activated platelets, which can proceed to render the vasculature more permeable by acting on P2Y2 receptors expressed by endothelial cells (Schumacher et al., 2013). Moreover, physical interactions between platelets and endothelial cells, for example those mediated by selectins, have been proposed to be important for the adhesion of platelet-cancer cell clusters to the walls of the vasculature (Kohler et al., 2010). It remains unclear, however, whether such adhesive interactions are actually critical to the eventual entrance by the neoplastic cells into the parenchyma of various tissues.
Interactions with Neutrophils
Neutrophils can exist in distinct and dynamically changing phenotypic states that can be shaped by the primary tumor as well as other host cells (Coffelt et al., 2016; Fridlender et al., 2009; Sagiv et al., 2015). We focus here on their actions in circulation, where evidence is beginning to clarify their role during this phase of the metastatic cascade. In certain instances neutrophils have been found to inhibit metastasis. For example, primary tumors can educate neutrophils via CCL2 secretion, giving rise to tumor-entrained neutrophils (TENs) (Granot et al., 2011). These cells appear to accumulate in the circulation and the lungs of tumor-bearing mice even prior to metastatic progression and have been found to prevent carcinoma cells from seeding the lungs. Neutrophils mobilized by G-CSF treatment lack this power (Granot et al., 2011), highlighting the fact that neutrophils can be primed to adopt different functional states.
In large part, however, the molecular and cellular physiology of neutrophils appears to dictate that their predominant role is one that favors metastatic seeding. For example, neutrophil extracellular traps (NETs), which are formed from released DNA molecules, are designed to entangle pathogens during a response to infection but can also be deployed by neutrophils to capture tumor cells in the circulation (Cools-Lartigue et al., 2013). Such entangled CTCs may be more apt to survive intraluminally, adhere to endothelial cells, and extravasate. Neutrophils can directly interact with tumor cells trapped in the vasculature, prolonging their retention in the lung after intravenous injection (Huh et al., 2010). In a similar manner, neutrophils can facilitate adhesive interactions within liver sinusoids, thereby serving as physical platforms on which CTCs can dock prior to extravasation (Spicer et al., 2012). Additionally, neutrophils enhance the extravasation of tumor cells after arrest, mainly through the secretion of various matrix metalloproteinases (MMPs) (Spiegel et al., 2016).
Neutrophils have also been shown to exert immunosuppressive functions. Often mobilized through systemic signaling by a primary tumor, neutrophils can inhibit both cytotoxic CD8+ T cell responses (Coffelt et al., 2015) and the intraluminal clearance of carcinoma cells by NK cells (Spiegel et al., 2016). Such protection from attack by arms of the innate and adaptive immune system offers a clear advantage to tumor cells in transit. Finally, some of the effects mediated by neutrophils may occur in response to the aggregation of platelets and tumor cells noted previously. Thus, the release of platelet-derived chemokines can recruit neutrophils, which can then, as described here, enhance the seeding and metastatic outgrowth of carcinoma cells in circulation (Labelle et al., 2014).
Extravasation
Many of the intravascular interactions described above influence the ability of CTCs to extravasate and thereby enter into the parenchyma of distant tissues. Extravasation requires carcinoma cells to traverse the endothelial wall through a process that is termed transendothelial migration (TEM) (Reymond et al., 2013). Earlier we cited the ability of ATP released by activated platelets to render the capillary walls more permeable; in more detail, this is achieved by causing endothelial cells to retract from one another. In addition, breast carcinoma cells primed by TGF-β in the primary tumor acquire the ability to produce angiopoietin-like 4 (ANGPTL4), which enhances the permeability of the lung vasculature, promotes TEM of carcinoma cells, and leads to an increased capacity for metastatic outgrowth (Padua et al., 2008). Several other proteins produced by carcinoma cells have been reported to function as disruptors of vascular integrity, including VEGF, MMPs and ADAM12; these secreted molecules seem to enhance both intravasation as well as extravasation (Gupta et al., 2007; Reymond et al., 2013), indicating that certain traits that were advantageous previously early in the course of primary tumor invasion may also prove useful at later steps in the invasion-metastasis cascade.
The recruitment of monocytes has also been demonstrated to play a functional role in tumor cell extravasation. In particular, the recruitment of CCR2+ inflammatory monocytes in response to CCL2 secretion by carcinoma or host cells can facilitate extravasation and subsequent metastatic growth in the lung parenchyma (Qian et al., 2011; Wolf et al., 2012). These inflammatory monocytes may differentiate into metastasis-associated macrophages, which similarly enhance the seeding of carcinoma cells in the lung but do so from an extravascular location (Qian et al., 2009; Qian et al., 2011). CCL2 can also act directly on endothelial cells to enhance vascular permeability (Wolf et al., 2012). While inhibition of the CCL2-CCR2 axis would seem to represent an ideal anti-metastatic therapy, the interruption of anti-CCL2 therapy actually results in an enhanced monocyte infiltration of tumors and lungs with a corresponding acceleration of disease progression (Bonapace et al., 2014), underscoring the dynamic and unpredictable nature of targeting such microenvironmental interactions.
Most experimental models of metastasis have, for various reasons, focused on the lungs as destination sites of disseminated tumor cells. However, the requirements for successful extravasation and the relevant interactions that facilitate this process are likely to be quite different in various tissue sites. For instance, the fenestrated sinusoids of the bone marrow and liver are more likely to permit the passive entry of CTCs, obviating many of the complex interactions and mechanisms enumerated above. In the case of the brain, the dissemination of carcinoma cells would seem to require passage through the blood-brain barrier, which may in fact necessitate the actions of a tissue-specific program for extravasation that is very different from those enabling metastatic seeding elsewhere in the body. Indeed, breast cancer cells selected for preferential metastasis to the brain express at high levels a number of genes that are known to facilitate passage through the blood-brain barrier (Bos et al., 2009; Sevenich et al., 2014).
In certain cases, TEM migration may not be required at all, as arrested carcinoma cells have been found to proliferate in the lumina of blood vessels, leading to the growth of large intraluminal tumor colonies that eventually rupture nearby endothelial walls, enabling direct access to the tissue parenchyma (Al-Mehdi et al., 2000). Finally, a novel mechanism has recently been described, in which tumor cells can extravasate and generate lung metastases via induction of programmed necrosis (necroptosis) in endothelial cells (Strilic et al., 2016).
METASTATIC COLONIZATION
The growth of an overt metastatic colony represents the final and most deadly phase in the malignant progression of a tumor. Still, the vast majority of carcinoma cells in circulation seem ill-prepared for growth in a distant organ environment; some experimental evidence has yielded estimates of the efficiency of metastasis after intravenous injection of tumor cells as low as 0.01% (Chambers et al., 2002). Even carcinoma cells that have managed to extravasate seem almost invariably destined to either be eliminated from the tissue parenchyma or to enter into a state of dormancy (Luzzi et al., 1998), in which they persist in an indolent state as single disseminated tumor cells (DTCs) or as small micrometastatic clusters – sometimes for weeks, months, even years.
Having traveled far from the primary tumor, DTCs find themselves in a new tissue microenvironment that is devoid of the familiar stromal cells, growth factors, and ECM constituents that previously sustained the lives of their predecessors in the primary site. Hence, their inability to continue proliferating and the resulting entrance into a prolonged growth-arrested state may often be attributable to a microenvironment to which these cells are poorly adapted when they first arrive following extravasation. When portrayed in this way, metastatic dormancy reflects a failure of DTCs to adapt to and colonize a given tissue. Importantly, a dormant state can also be actively imposed by certain anti-proliferative signals encountered by recently arrived cells in the parenchyma of foreign tissues. We first consider the programs operative in dormant DTCs before turning to those that enable colonization.
Dormancy Programs
The latent, clinically inapparent phase of metastasis might well be the result of factors beyond those cited here that render carcinoma cells unable to proliferate, such as an inability to induce angiogenesis or active suppression by the immune system (Aguirre-Ghiso, 2007). These two particular mechanisms are thought to permit a low level of proliferation that is counterbalanced by ongoing elimination, resulting in no net increase in the sizes of micrometastatic clusters.
From a clinical perspective, patients successfully treated for their primary tumors but potentially harboring such dormant metastatic cells are considered to have asymptomatic minimal residual disease (MRD) (Figure 3A). For certain carcinomas, such as those of the breast, prostate and kidney, this period of dormancy may last for many years, even decades after ostensibly successful courses of initial therapy. And while it is difficult to formally prove that a metastatic colony directly developed from a preexisting dormant DTC, the presence of DTCs in the bone marrow is clearly correlated with an increased risk of eventual clinical recurrence (Braun et al., 2005). This reveals why an understanding of the biologic bases of dormancy is of utmost clinical importance, if only because the period of dormancy represents a critical time window during which therapeutic interventions directed at DTCs – either targeting them for elimination or restraining their proliferation – may well succeed in preventing the eventual eruption of life-threatening metastatic disease.
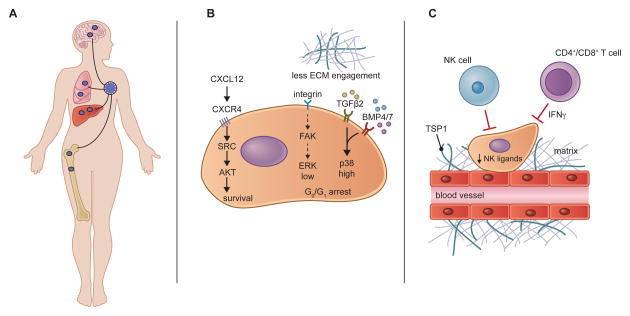
Figure 3. Dormancy Programs and Niches.
(A) Carcinoma cells that have disseminated prior to the surgical removal of the primary tumor may persist in distant tissue environments as dormant disseminated tumor cells (DTCs). Patients harboring such reservoirs of occult carcinoma cells are considered to have minimal residual disease and are at increased risk of eventual metastatic recurrence. Although DTCs are most frequently examined in the bone, the delayed outgrowth of metastases in other organs suggests that they, too, can harbor dormant DTCs.
(B) Dormant DTCs rely on unique biochemical signaling pathways that sustain their survival and impose programs of quiescence. Signals from the microenvironment, such as CXCL12, can activate SRC and AKT to promote DTC survival. Reduced integrin-mediated mitogenic signaling, coupled with the actions of certain dormancy-inducing cytokines, enacts a quiescent program in DTCs that is associated with an ERKlow/p38high signaling state.
(C) DTCs may reside in dormant niches such as the hematopoietic stem cell niche (not shown) or the perivascular niche illustrated here. Thrombospondin-1 (TSP1), present in the basement membrane surrounding mature blood vessels promotes dormancy. Dormant cells can evade detection by NK cells through the repression of NK-activating ligands and are likely subject to surveillance by the adaptive immune system, which may keep cancer cells in a dormant state through the actions of IFN γ.
Dormancy programs (Figure 3B) can be initiated from either an active response to signals encountered in the new tissue microenvironment or from an absence of contextual cues that carcinoma cells previously depended on while residing in their sites of origin within primary tumors (Giancotti, 2013; Sosa et al., 2014). As an example, DTCs that respond to survival signals present in the microenvironment can avoid destruction and persist for extended periods within a tissue parenchyma. In one well-studied case, breast cancer cells that have lodged in the bone marrow and possess high SRC activity and expression of CXCR4 are able to activate pro-survival pathways in response to bone-derived CXCL12 (Zhang et al., 2009). DTCs capable of sensing and responding to these survival cues are able to counteract TRAIL-induced apoptosis, a conserved tissue defense mechanism that can work in the opposite direction to eliminate DTCs. The survival of DTCs may also be related to their ability to withstand anoikis, for example through the expression of the tyrosine kinase receptor TrkB (Douma et al., 2004) or through non-canonical WNT signaling mediated by WNT2 (Yu et al., 2012).
Even if DTCs benefit from such survival signals in their new tissue environment, in the absence of additional mitogenic cues, including interactions with the extracellular matrix (ECM), these cells may languish in a dormant state. Thus, dormancy has been reported to ensue when disseminated carcinoma cells fail to engage integrin β1 and the downstream activation of focal adhesion kinase (FAK) (Aguirre Ghiso et al., 1999; Barkan et al., 2008; Shibue and Weinberg, 2009). The ability of DTCs to productively interact with the matrix, at least in the context of the lung, appears to be contingent upon the formation of filopodium-like protrusions (FLPs) that are coated with integrin β1 (Shibue et al., 2012; Shibue et al., 2013). DTCs that are unable to sense or respond to such adhesive signals fail to activate proliferative programs that are primarily driven by FAK, SRC and ERK signaling (Barkan et al., 2010; Shibue et al., 2012). Accordingly, combined inhibition of both the SRC and ERK pathways blocks the escape of DTCs from dormancy and thus prevents their subsequent success in metastatic colonization (El Touny et al., 2014).
Several dormancy-inducing signals found in the microenvironment of certain target tissues have been identified as well. For instance, TGFβ2, present in high concentrations in the bone marrow and acting through stimulation of TGF-β-RI and TGF-β-RIII displayed by DTCs, can impose a state of dormancy upon head-and-neck squamous carcinoma cells (Bragado et al., 2013). Members of the related BMP ligand family have also been linked to metastatic dormancy. BMP7, which can be produced by bone stromal cells, can induce dormancy in prostate cancer cells (Kobayashi et al., 2011). In the lung, too, a number of alternative BMP ligands are expressed, including BMP4, and these have been implicated as factors that maintain a state of dormancy in disseminated mammary carcinoma cells (Gao et al., 2012). Many of these dormancy-inducing cytokines lead to activation of the p38 MAPK pathway; coupled with the absence of mitogenic signals, this has the net effect of promoting an ERKlow/p38high state in DTCs, which leads in turn to arrest in the G0/G1 phases of the cell cycle and associated quiescence (Sosa et al., 2011).
The Dormant Niche
Dormant DTCs may reside in specialized niches (Figure 3C) that support their survival, restrain their proliferation, and, quite possibly, provide resistance to therapeutic agents (Ghajar, 2015). Of particular interest here is the idea that dormant DTCs can co-opt a niche that is otherwise reserved for tissue-resident stem cell populations. A compelling demonstration of this phenomenon is provided by the case of prostate cancer cells that metastasize to the bone, where these carcinoma cells have been found to compete with hematopoietic stem cells (HSC) for occupancy of sites in the endosteal niche; this occurs via the CXCL12-CXCR4 signaling axis that is normally reserved for the physiologic regulation of HSCs (Shiozawa et al., 2011). The fact that DTCs can specifically target a stem-cell niche suggests that they may be poised to respond to the quiescent and survival signals present within the HSC microenvironment.
In multiple organs – including the lung, bone, and brain – DTCs have been found to reside in the microenvironment surrounding the vasculature, a region known as the perivascular niche (Ghajar, 2015). Whether this represents their active retention in this niche or simply indicates an inability to move farther from the vasculature after initial extravasation is unclear. An alternative mechanism is suggested by the finding that factors present in the perivascular niche have been demonstrated to actively promote dormancy. Thus, thrombospondin-1, produced from mature endothelial cells and deposited in the microvascular basement membrane, is able to confine DTCs to residence in a quiescent state (Ghajar et al., 2013). Moreover, in a study using real-time imaging to examine the process of brain metastasis, the rare solitary DTCs that achieved long-term dormancy were invariably localized to the perivascular region (Kienast et al., 2010), suggesting a critical role for this niche in sustaining dormant DTCs in the brain as well.
DTCs must protect themselves from immune attack when dwelling as isolated as single cells lodged far from the confines of the immunosuppressive primary tumor microenvironment. Breast and lung carcinoma cells selected for their ability to persist in a latent state following seeding of distant organ sites succeed in evading clearance by NK cells through the repression of various NK cell-activating ligands, a program that appears to be tightly coupled with entrance into a quiescent state (Malladi et al., 2016). Indeed, these latency-competent cells have been observed to grow out when injected into mice that lack NK cells, indicating that the innate immune system is an important component of the dormant niche that effectively forces many cancer cells into a quiescent state. A quite different process is suggested by the observation that antigen-presenting dendritic cells can protect against metastasis (Headley et al., 2016), implying a role of the adaptive immune system in controlling the growth of metastatic deposits. Both CD4+ and CD8+ T cells have been implicated in the control of dormant primary tumor cells through the secretion of IFNγ (Koebel et al., 2007; Müller-Hermelink et al., 2008) and there is evidence that CD8+ T cells can hold disseminated uveal melanoma cells in a dormant state (Eyles et al., 2010). However, at present very little is known about such immune-mediated dormancy mechanisms in the context of DTCs originating from carcinomas.
Cancer Stem Cell Programs and the Initiation of Metastatic Colonization
As mentioned above, activation of the EMT program, which is capable of driving the physical dissemination of carcinoma cells to distant anatomical sites, can also confer upon these cells important stem cell traits (Mani et al., 2008; Morel et al., 2008) that would appear to be highly relevant to metastatic colonization. Thus, an apparent prerequisite to the successful formation of a metastatic colony is the property of tumor-initiation as embodied in CSCs. At least in principle, it is only those DTCs that reside in the CSC state that are qualified to serve as the founders of metastatic colonies.
Accumulating evidence, mostly from animal models, largely supports this notion. In the MMTV-PyMT mammary tumor model, a rare population of CSCs has been shown to be responsible for the initiation of metastatic growths in the lung and, accordingly, the ability of these tumors to metastasize is dependent on the maintenance of this stem cell population through enhanced Wnt signaling (Malanchi et al., 2012). In human breast cancer cells, the activation of key stem cells pathways, such as Wnt and Notch signaling, is also important for supporting their colonization in xenograft mouse models (Oskarsson et al., 2011). And mouse models of lung adenocarcinoma have revealed that metastatic progression is associated with a dedifferentiation program, mediated by loss of Nkx2-1 expression, which resembles programs operating in stem-like states (Li et al., 2015; Winslow et al., 2011). Thus, it appears that the metastatic potential of a carcinoma is closely related to its ability to dispatch populations of CSCs that can re-initiate tumor growth following arrival at distant sites (Oskarsson et al., 2014). This notion implies that cell state is a critical determinant of successful metastasis, more specifically residence in the epigenetic state associated with CSCs (see below).
As discussed extensively above, an alternative to metastatic outgrowth proceeding immediately after dissemination is the entrance of DTCs into an indolent state in which they may persist for extended periods of time before their progeny eventually erupt into readily detectable macroscopic metastases. Such persistence may be favored by the acquisition of stem cell characteristics. Thus, DTCs detected in the bone marrow of breast cancer patients exhibit features of CSCs (Balic et al., 2006). Consistent with this, cells that remain in a latent state in distant tissues also show CSC attributes, including expression of the SOX2 and SOX9 transcription factors (Malladi et al., 2016). In addition, single-cell expression analyses have been applied to DTCs isolated from the organs of patient-derived xenograft (PDX) models of breast cancer; some organs harbored low-burden metastatic disease due to the presence of small numbers of ostensibly dormant carcinoma cells (Lawson et al., 2015). These cells exhibited a distinctive gene expression profile, relative to carcinoma cells from advanced metastatic lesions, that was characterized by the expression of EMT, stem cell, and survival/dormancy genes. Most intriguingly, when neoplastic cells isolated from such low-burden tissues were implanted into new recipient animals, they retained their tumorigenic potential and could readily generate more differentiated carcinomas (Lawson et al., 2015). These studies provide further evidence in support of the notion that stem-like cancer cells often serve as the founders of metastatic colonies, even when such colonies appear only after great delay.
This scheme implicating the EMT and stem-cell programs as critical prerequisites to the successful founding of metastatic colonies must be reconciled with the commonly observed fact that carcinoma metastases tend to recapitulate key histopathological traits of their corresponding primary tumors. Among other traits, this usually includes significant epithelial features (Brabletz, 2012). On its surface, this notion this would seem incompatible with the proposition that EMT plays a central role in launching carcinoma metastases through its ability to impart mesenchymal and stem cell attributes to the disseminating cells. In fact, this paradox is resolved by numerous studies, some cited here in passing, that have found that the disseminated progeny of carcinoma cells appear to undergo the reverse of the EMT program at some point after dissemination, i.e., they pass through a MET. This reversion to an epithelial state should restore many of the cellular traits that were lost during the prior passage through an EMT (Brabletz, 2012) and enable reconstruction of hierarchical cell organizations similar to those present in the initial primary tumors. Indeed, such reversals by many cells within an early metastatic growth to a more epithelial state may actually be essential for metastatic colonization (Del Pozo Martin et al., 2015; Korpal et al., 2011; Ocana et al., 2012; Tsai et al., 2012). Of note, it remains unclear precisely why highly mesenchymal CSCs cannot generate robustly growing metastatic colonies in the absence of the epithelial progeny generated by such METs.
Mechanisms of Colonization
Metastatic colonization appears, at least as presently understood, to depend critically on two preconditions of the disseminated carcinomas cells: they must possess tumor-initiating ability, as argued above, and they must in some fashion contrive adaptive programs that enable them to thrive in the microenvironment present in the parenchyma of distant tissues. The ‘seed and soil’ hypothesis, put forth by Paget in the late 19th century, suggested a complementary notion – essentially, that certain types of carcinoma cells are more able to generate metastases in certain foreign tissue microenvironments than are others (Fidler, 2003). Unspoken by Paget was the notion that even in such favored metastatic sites, DTCs must still undergo certain forms of phenotypic adaptation in order to proliferate robustly in those sites. Thus, the proclivity of prostate and breast carcinomas to metastasize to the bone would seem to imply some preexisting ability of the corresponding DTCs to more readily assemble adaptive programs suited to that tissue, whereas other less favored tissue sites might require more elaborate, less readily assembled adaptive programs.
To be sure, in certain cases, the organ-specific tropism of metastatic cells is influenced by the design of the circulatory system. Colorectal carcinoma (CRC) metastasis to the liver is strongly favored simply because the portal vein draining the gut empties directly into the liver (Gupta and Massague, 2006). Hence, even if disseminated CRC cells were intrinsically poorly adaptable for liver colonization, the sheer numbers of these cells that are trapped in the liver following passage through the portal vein may, on its own, pre-ordain metastases eventually arising at this site.
Importantly, the layout of the circulatory system explains only a small proportion of the organ-specific metastases commonly observed in the oncology clinic. Often cited in this context is the proclivity of breast and prostate cancer cells, as mentioned above, to colonize the bone marrow, usually termed osteotropic metastasis. We highlight below specific examples that illustrate the nature of the adaptive programs that seem critical to successful metastatic outgrowth.
To begin, we note that some of these programs may act generally by conferring a survival advantage in a number of distinct target organs. For instance, cancer cells have been shown to experience higher levels of oxidative stress both in the circulation and in the parenchyma of a distant tissue (Piskounova et al., 2015). As a consequence, metabolic adaptations, including the synthesis of antioxidants, may promote the survival and eventual metastatic outgrowth in diverse sites. Adhesive interactions that substitute for those encountered in the primary tumor, such as homotypic cell-cell interactions in the case of disseminating CTC clusters (Aceto et al., 2014) or FLP-ECM interactions in the case of single DTCs (Shibue et al., 2012), may be capable of activating crucial survival pathways in a manner that could be independent of specific target organs and would thus qualify as more general adaptations promoting colonization.
These general adaptive programs may be nothing more than preludes to the challenging tasks of contriving more narrowly applicable, tissue-specific adaptations. Indeed, a diverse array of organ-specific metastatic programs that mediate colonization of the bone, lung, liver and brain have been reported and studied in mechanistic detail (Nguyen et al., 2009; Obenauf and Massague, 2015; Sethi and Kang, 2011). In the brain, for example, cancer cells encounter reactive astrocytes that produce plasminogen activator, leading to the production of plasmin that induces carcinoma cell death (Valiente et al., 2014). The ability of carcinoma cells to survive in this hostile environment is dependent upon the expression of serpins, which are typically produced by neurons and protect against plasminogen activator-mediated cell death. In the lung, VCAM-1-expressing carcinoma cells are able to activate their own AKT signaling by physically engaging with integrin α4 on macrophages that are particularly abundant in the pulmonary microenvironment (Chen et al., 2011). The survival of carcinoma cells in the liver has been linked to an ability to utilize creatine and ATP present in the extracellular microenvironment to generate and import phosphocreatine, which may confer a significant survival advantage on DTCs subject to metabolic stress (Loo et al., 2015). The diversity of these survival mechanisms is a clear reflection of the varied cellular and molecular determinants of successful colonization that operate within different target organs.
More generally, the mechanisms that permit and/or promote the proliferation of various types of cancer cells in diverse distant tissue microenvironments remain obscure. Arguably, the best-understood example to date involves the metastatic colonization of the bone, which has been documented in the case of the osteolytic metastases formed by breast cancers (Nguyen et al., 2009; Obenauf and Massague, 2015; Weilbaecher et al., 2011). Breast carcinoma cells produce a number of molecules, including parathyroid hormone-related protein (PTHrP), IL-11, and MMPs, that favor RANKL stimulation of osteoclast activity, which in turn liberates growth factors from the bone matrix that reciprocally promote tumor cell proliferation and the secretion of even more factors that enhance osteoclast activity. The resulting self-reinforcing positive-feedback loop has been termed the ‘vicious cycle’ of osteolytic metastasis (Mundy, 2002). In contrast, prostate carcinoma cells tend to spawn predominantly osteoblastic metastases that occur as a result of induced osteoblast differentiation (Weilbaecher et al., 2011). Presumably, the appearance of macroscopic metastases in other target organs is similarly dependent on the ability of carcinoma cells to subvert normal cell types residing within these organs, but the details of these heterotypic interactions remain to be defined. In one recent example, breast carcinoma cells that colonize the brain have been found to benefit from communication with astrocytes through the assembly of gap junctions established between cancer cells and astrocytes (Chen et al., 2016).
The growth of a metastatic colony may also ensue when dormant DTCs are awakened from their indolent state. For example, the awakening of previously dormant micrometastases may depend on the successful assembly of functional adaptive programs, which may be achieved only rarely per cell generation, explaining the extraordinary low efficiency of metastasis formation. Thus, we note that dormant micrometastases in the bone that somehow gain expression of VCAM-1 can transition to an active colonization phase through the recruitment of osteoclast progenitor cells expressing integrin α4β1, a receptor for VCAM-1, which enables bone resorption and initiation of the “vicious cycle” described above (Lu et al., 2011). Carcinoma cells in the lung are able escape dormancy through the production of Coco, a secreted inhibitor of BMP signaling that promotes colonization (Gao et al., 2012). Unspoken here are the mechanisms by which such adaptive programs are actually acquired. Thus, it seems likely that continuous, low level proliferation of the cells within individual micrometastatic deposits – this occurring over extended periods of time – is essential to the ability of DTCs to stumble through trial and error on highly effective gene expression programs and adaptive behaviors that enable them to thrive in the tissue microenvironment in which they happen to have landed.
Programs that confer multi-organ colonization potential may exist as well. Interestingly, the few examples of these programs that have been described center on interactions between DTCs and the ECM. For example, carcinoma cells selected in vivo for their ability to re-initiate tumor growth in subsequent xenotransplantation injections are also highly competent in establishing metastatic growths in multiple different organs (Ross et al., 2015). In this case, the capacity for multi-organ colonization has been traced to the production of the matrix protein laminin-a4 (LAMA4), which seems to be critical for the initial proliferation of DTCs. Similarly, the collagen receptor DDR1, in collaboration with the TM4SF1 adaptor protein, has recently been identified as a signaling axis that regulates CSCs and thereby enables the outgrowth of otherwise-dormant carcinoma cells in multiple organ sites (Gao et al., 2016). The activation of such programs could account for the apparently synchronous appearance of metastases in various organs – metastatic showers – that are occasionally observed in patients.
The Metastatic Microenvironment
The above discussions fail to address in any detail the nature of the resident cells within various types of normal tissues that sprout metastatic colonies. At least in the case of carcinomas, these residents are essentially the various types of more mesenchymal cells that constitute the tissue-associated stroma together with the ECM laid down by these cells. To begin, in the same way that primary tumors are highly dependent on their recruited stromal microenvironment, metastatic growths seem equally reliant on stromal support (Hanahan and Coussens, 2012; Quail and Joyce, 2013; Wan et al., 2013). Indeed, the transition of carcinoma cells from a dormant state to one of robust outgrowth may be provoked by changes in their local environment. For example, the apparent dormancy-inducing actions of the perivascular niche noted above seem to be reversed during neo-vascularization as sprouting endothelial tip cells secrete TGF-β1 and periostin (POSTN), which can break dormancy and promote tumor cell proliferation (Ghajar et al., 2013). Consistent with this idea, the outgrowth of dormant DTCs in the brain also seems to be dependent on angiogenesis (Kienast et al., 2010). Another recent report describes the outgrowth of DTCs in the lungs being provoked by inflammation (as mediated by pro-inflammatory cells) induced in this tissue (De Cock et al., 2016).
Other findings suggest that metastatic colonization requires, or at least can be aided by a supportive ECM. This idea is bolstered by the identification of specific ECM components, such as tenascin C (TNC) (Oskarsson et al., 2011) and POSTN (Malanchi et al., 2012) that drive colonization of the lung by breast carcinoma cells. Tumor cells may themselves produce these ECM components or, alternatively, they may evoke their secretion by resident stromal fibroblasts. In addition, separate but complementary lines of evidence have reported a connection between fibrosis and metastasis (Barkan et al., 2010; Cox and Erler, 2014), suggesting that the local fibroblast and ECM composition can influence the ability of carcinoma cells to colonize an organ. ECM stiffness (Levental et al., 2009; Mouw et al., 2014), which can be modulated by the collagen-crosslinking enzyme lysl oxidase (LOX), may also be important for the creation of pro-metastatic microenvironment (Erler et al., 2009; Erler et al., 2006). Indeed, the well-described contribution of hypoxia to metastasis may be substantially related to the production of LOX downstream of the transcription driven by hypoxia-inducible factor (HIF) (Rankin and Giaccia, 2016).
Metastatic colonization is also likely to be impacted by cells of both the innate and adaptive immune system (Kitamura et al., 2015; Quail and Joyce, 2013). Thus, both NK cells and CD8+ T cells have been implicated in the suppression of metastasis (Bidwell et al., 2012; Malladi et al., 2016). Conversely, in the lung, T cells sense oxygen, which physiologically suppresses inflammation and induces tolerance but in the context of cancer provides a more hospitable environment for metastatic colonization (Clever et al., 2016). Myeloid cells have also been identified as important contributors to the formation of a favorable metastatic microenvironment (Kitamura et al., 2015), where a unique population of metastasis-associated macrophages may be responsible for not only provoking, but also sustaining metastatic growth, perhaps by stimulation of angiogenesis (Qian et al., 2009). Finally, acute inflammatory responses have been found to trigger the outgrowth of previously-latent carcinoma cells, an effect that may be primarily driven by neutrophils (De Cock et al., 2016).
The establishment of a supportive metastatic environment may occur prior to the arrival of any carcinoma cells, through the formation of what has been termed a pre-metastatic niche. This niche formation may involve the actions of VEGFR+ bone marrow progenitors (Kaplan et al., 2005), myeloid-derived suppressor cells (MDSCs) (Psaila and Lyden, 2009), or neutrophils (Wculek and Malanchi, 2015). Some have also reported that tumor-derived exosomes – small tumor-derived vesicles that contain DNA, mRNAs, microRNAs, and protein – can re-shape the pre-metastatic environment in preparation for the arrival of carcinoma cells (Costa-Silva et al., 2015; Peinado et al., 2012). Thus, the formation of a pre-metastatic niche may represent one consequence of far-ranging systemic effects induced by primary tumors. More generally, the actions of a primary tumor can lead to the production of numerous systemic signaling factors that, by acting on distant tissues, can elicit responses that may thereafter affect primary tumor growth, pre-metastatic niches, and the outgrowth of previously latent micrometastases (McAllister and Weinberg, 2014).
Genetic and Epigenetic Drivers of Colonization
The classic description of multi-step tumorigenesis implies that the successive accumulation of genetic and/or epigenetic alterations drives primary tumor progression (Fearon and Vogelstein, 1990). A logical extension of this concept would suggest that the outgrowth of a metastatic colony depends on the acquisition of yet another somatic mutation or set of mutations that empower cancer cells to disseminate and thereafter proliferate in a distant organ. However, more than 25 years after the pioneering work on multi-step progression of colorectal carcinoma (Fearon and Vogelstein, 1990), no genetic mutations have been identified that are characteristically associated with progression to metastatic disease. Indeed, even large-scale genomic sequencing efforts have yet to uncover recurrent genetic mutations that can adequately explain the eruption of metastatic growths (Garraway and Lander, 2013; Vogelstein et al., 2013). This suggests that the development of metastasis is not contingent upon the accumulation of somatic driver mutations beyond those selected for during primary tumor formation.
In particular, these findings have focused attention on non-genetic mechanisms enabling colonization. According to one idea, colonization may depend on the amplification in metastatic cells of oncogenic signaling pathways that were previously activated in the cells of primary tumors (Vanharanta and Massague, 2013), for example, through the enrichment of existing clones with elevated signaling through the MAP kinase pathway (Campbell et al., 2010; Jacob et al., 2015). Metastatic carcinoma cells may also need to evade the actions of metastasis suppressor genes, which have been proposed to specifically block the later stages of the invasion-metastasis cascade (Steeg, 2003). Another alternative mechanism may involve defined epigenetic alterations that drive colonization, such as aberrant DNA methylation patterns (Ozturk et al., 2016).
In addition to the actions of individual genes, recent data suggest that metastatic carcinoma cells often exhibit global changes in the structure of their chromatin. Thus, in a mouse model of small cell lung cancer pathogenesis, carcinoma cells competent for metastasis displayed a distinct open chromatin configuration at distal regulatory regions, which were established and bound by the transcription factor Nfib; this change in chromatin structure, facilitated, in turn, a shift toward expression of a pro-metastatic neuronal gene expression program (Denny et al., 2016). Such altered epigenetic states may ease the adaptation of DTC to foreign microenvironments. These advances notwithstanding, the difficulties involved in the procurement and analysis of metastatic samples have led to a continued dearth of information concerning the genetic and epigenetic landscapes found within the neoplastic cells that form human metastases.
To summarize, in spite of the findings described above, metastatic colonization continues to represent the most puzzling phase of malignant progression and the most challenging to model experimentally. The physical dissemination of tumor cells from the primary tumor into the parenchyma of distant tissues can, at least in the context of many carcinomas, be largely understood through the actions of a single cell-biological program – the EMT. This contrasts starkly with the apparent extraordinary complexity of the last step of the invasion-metastasis cascade – colonization. This complexity, highlighted by the apparently myriad heterotypic interactions between populations of disseminated carcinoma cells and their newfound homes in distant tissues, has complicated attempts at deriving broadly applicable mechanistic principles underlying colonization. Nevertheless, we suggest that the weight of current evidence points to three main prerequisites that must be met in order for metastatic colonization to succeed (Figure 4): (i) the capacity to seed and maintain a population of tumor-initiating cancer stem cells; (ii) the ability to contrive adaptive, often organ-specific colonization programs; and (iii) the subsequent development of a supportive microenvironmental niche.
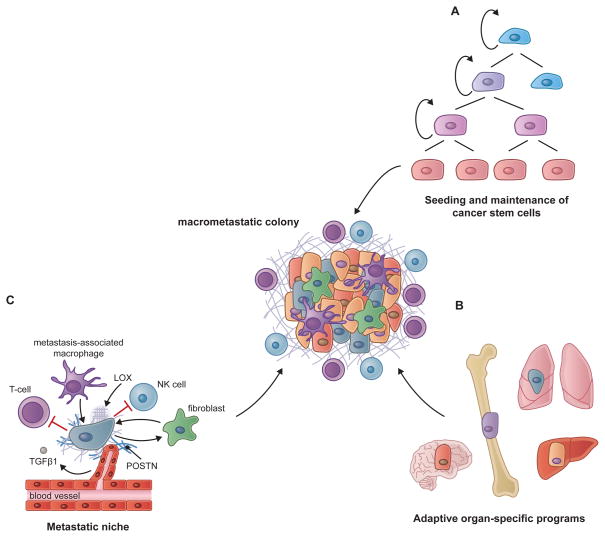
Figure 4. Prerequisites for Metastatic Colonization.
The ability of carcinoma cells to outgrow as lethal metastases appears to be dependent on three essential conditions.
(A) The capacity to seed and maintain a population of cancer stem cells, which are competent to re-initiate tumor growth, appears to be an initial prerequisite for metastatic growth. Dormant DTCs also exhibit key cancer stem cell attributes that likely contribute to their prolonged persistence in a quiescent state and their ability to eventually spawn a metastatic colony.
(B) Although cancer stem cells are endowed with the potential to re-initiate tumor growth, the proliferative expansion to an overt metastatic colony is dependent on the ability to contrive organ-specific colonization programs that allow these cells to thrive in a foreign tissue microenvironment. An array of organ-specific metastatic programs has been described in the literature but there is also evidence for the existence of colonization programs that confer multi-organ metastatic potential.
(C) During many stages of metastatic growth, cancer cells depend on interactions with their microenvironmental niche and cross talk with various stromal cells, including endothelial cells, fibroblasts and cells of the innate and adaptive immune system. The ECM is also an important component of the niche and can be modified in ways that support metastatic colonization. In some cases the formation of a metastatic niche may actually precede the arrival of cancer cells, in what is referred to as a pre-metastatic niche. Selected niche interactions discussed in the text are depicted here.
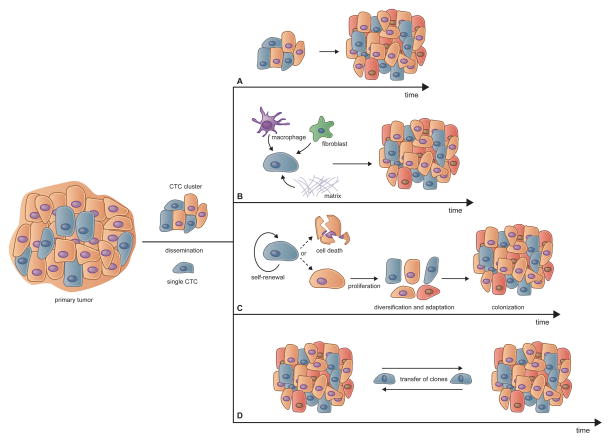
DYNAMICS OF METASTATIC EVOLUTION
The process of multi-step tumor progression and the subsequent seeding of metastases appears, at least superficially, to operate as a linear path beginning in the primary tumor and ending in macroscopic metastatic colonies. In truth, however, each of the intervening steps is confounded by multiple factors, as discussed above. Similarly, the processes that occur subsequent to the establishment of metastatic colonies and the mechanisms by which they evolve have been a subject of research and discussion over the past few decades. The notion that tumor progression operates according to the Darwinian model of evolutionary growth has become widely accepted and influential in our thinking about metastatic progression (Cairns, 1975; Nowell, 1976). Recent genomic studies have often revealed close genetic relationships between primary tumors and metastases in a variety of cancer types, implying that, at least in certain cases, the cells forming a metastatic colony derive from a dominant clonal subpopulation within the primary tumor that managed to complete all of the steps required both for primary tumor formation and the subsequent multi-step invasion-metastasis cascade (Naxerova and Jain, 2015). Implicit in this depiction once again is the notion that the genetic alterations required for completion of the invasion-metastasis cascade are already present in the genomes of disseminating tumor cells, and that completion of this cascade depends only on non-genetic changes, specifically epigenetically organized programs that complement the previously acquired genetic mutations.
Unanswered by such a scheme is the nature of the genetic and epigenetic alterations that render neoplastic cells especially fit to thrive within the context of the primary tumors, and how such alterations affect the proclivity of primary tumor cells to disseminate. Thus, it may be that phenotypic changes (of genetic and epigenetic origin) that are selectively advantageous within the context of primary tumor formation may, through happenstance, also make primary carcinoma cells more capable of disseminating. If so, the resulting metastases may arise as incidental side-products of primary tumor progression. Alternatively, many of the traits selected during primary tumor formation may prove irrelevant to the success of metastasis formation.
Such logic forces consideration of the genetic and non-genetic factors operating within primary tumors that favor the process of metastatic dissemination. To date, little attention has been placed on these factors. As a specific mechanistic example: What combination of epigenetic programs and somatic mutations render a primary carcinoma cell especially responsive to EMT-inducing heterotypic signals, enabling it to advance to a state of high-grade malignancy? Among important non-genetic factors may be the nature of the normal cells-of-origin and the differentiation programs that they bequeath to their neoplastic progeny. At present, we possess relatively little information on the fidelity with which preexisting differentiation programs operating in cells-of-origin are transmitted in a cell-heritable fashion to the distant descendants of the founders of neoplastic cell clones. Such programs could well represent the dominant determinants of metastatic dissemination, and may explain why certain subtypes of human cancers disseminate characteristically with predictable frequency to specific sites of metastatic colony formation. (Gupta et al., 2005; Ince et al., 2007; Lim et al., 2009; Molyneux et al., 2010; Proia et al., 2011). Unanswered by all of this is another question of great interest: Is metastatic ability a trait that is selected during multi-step primary tumor evolution, or is it nothing more than an unselected, incidental consequence of primary tumor progression?.
Dynamics of Tumor Progression and Metastasis
The development of metastasis has traditionally been considered as a relatively late event in multi-step tumor progression. More recent reports, however, suggest that dissemination can often occur early during the process of neoplastic transformation, perhaps even before departing cells are fully transformed (Husemann et al., 2008; Podsypanina et al., 2008; Rhim et al., 2012). At least in certain cases, this has been attributed to the presence of pre-neoplastic cells residing within inflammatory microenvironments that are able, via heterotypic signaling, to activate EMT programs, resulting in expression of invasive phenotypes (Rhim et al., 2012). Embedded in this thinking is the notion that EMTs operate both in fully normal epithelial cells and in neoplastic epithelial cells, suggesting that EMTs may also function in all of the intermediate cell states that define the multi-step progression of primary tumors.
Additionally, the kinetics of metastasis formation in certain mouse models of breast cancer are in line with the idea that dissemination, and hence metastasis, are early events during tumor progression (Weng et al., 2012). However, other studies have shown that the actual formation of distant metastases is a late event, taking place many years or decades following initial neoplastic transformation (Yachida et al., 2010). While physical dissemination itself could be an early event, it may have little bearing on the remaining steps of the cascade that result in the generation of macrometastatic foci. Stated differently, it is unclear whether early-disseminated carcinoma cells are ever able to evolve at distant anatomical sites to states of high-grade malignancy and spawn metastatic colonies, this situation representing the “parallel progression” model of metastasis formation.
Actually, two general models of metastatic dissemination have been proposed: the parallel progression model and the linear progression model. According to the latter, clones capable of spawning metastases arise at the later stages of tumorigenesis with a small degree of genetic divergence between those cells in the primary tumor that actually spawned a metastasis and the cells in the metastasis itself (Turajlic and Swanton, 2016). However, such genetic divergence may, in real life, be very difficult to gauge, given the clonal diversity that may have arisen within a primary tumor (Gerlinger et al., 2012) and the fact that various genetically distinct clonal subpopulations may be represented within the primary tumor in dramatically different sizes. Given the possibility that a minor subpopulation within a primary tumor can serve as the source of a metastasis (Haffner et al., 2013), how can one know with any certainty that a sampling of the genomes of primary tumor cells has been able to detect and gauge the genome of this minority population responsible for metastasis and its somatic mutations?
Yet another confounding factor when assessing the linear progression of metastasis is the difference in time between resection and sampling of the primary tumor and that of the metastasis. In fact, a majority of studies have carried out comparisons between primary and secondary (metastatic) tissues that were resected synchronously, while others have compared metastases sampled up to 17 years after resection of the corresponding primary tumors; both have found genetic similarities between the two tissues (Campbell et al., 2010; Ding et al., 2010; Haffner et al., 2013; Liu et al., 2009). These studies favor the linear progression model rather than the parallel progression model, which posits that metastasis occurs as an early event during tumorigenesis, after which the primary tumor and disseminated colonies evolve independently at sites far removed from one another (Klein, 2009).
The parallel progression model, for its part, is encumbered with its own complications. It assumes that the cells disseminating early from the primary tumor are able to proliferate sufficiently to allow for the acquisition of additional mutations that would render them fully transformed and thus capable of forming significant tumor masses. Given that metastatic colonization is a highly inefficient process, and given the complexity of essential adaptive programs, it seems unlikely that disseminated preneoplastic cells will actually continuously proliferate after their arrival in distant tissue microenvironments; in the absence of ongoing proliferation, it seems implausible that such cells can acquire, via stochastically occurring mutations, the complex repertoire of mutant alleles that are needed, in aggregate, for continuous growth and clonal expansion. Resolving between these models of metastatic progression may be further complicated by the fact that metastases have been reported to result from polyclonal populations ostensibly derived from clustered CTCs (Cheung et al., 2016), and by the observation that metastatic clones may be transferred between different metastatic lesions in the same patient (Gundem et al., 2015).
TREATMENT AND RESISTANCE
Metastatic cancer most often represents a terminal illness and patients eventually succumb to the disease or from complications that result from their course of treatment, indicating the current dearth of effective therapies (Steeg, 2016). Moreover, it remains unclear precisely whether the cells within metastases are intrinsically more resistant to therapy or whether they respond to therapies at rates comparable to the cells in their corresponding primary tumors. Comparable rates of responsiveness of metastases would certainly be compatible with the known genetic similarities between primary tumors and their derived metastases. Any heightened resistance might be explained by the fact that metastases derive from especially aggressive subpopulations of cells that resided within primary tumors or, alternatively, from further evolution to higher grades of malignancy following dissemination to distant sites.
Treatment of Primary Tumors and Metastatic Growths
Current therapeutic strategies for eliminating metastases are essentially the same as those directed at the corresponding primary tumors, the exception being surgery, which is infrequently employed to remove metastatic deposits. While cells that have succeeded in colonizing distant tissue microenvironments have often and perhaps always evolved adaptive programs that enable their robust proliferation at these secondary sites, it remains unclear whether this additional evolution, much of it achieved through epigenetic reprogramming, confers elevated therapeutic resistance. The alternative is that successful colonization of distant sites depends on the acquisition of adaptive traits that ultimately have no direct effect on therapeutic resistance.
The fact that metastatic lesions represent the progeny of minority subpopulations of the neoplastic cells present in a primary tumor (Ding et al., 2010; Yachida et al., 2010; Yates et al., 2015), suggests that metastatic colonies could be quite different from the primary tumor in terms of their clonal architecture and biology. And while numerous studies have examined the genetic and phenotypic diversity of the neoplastic cells that comprise primary tumors (Marusyk et al., 2012), the level of genetic and epigenetic heterogeneity and phenotypic plasticity that operates in metastatic growths is still in question.
A formidable obstacle to treating the minimal residual disease (MRD) that may remain after initial chemo- or radiotherapy derives from the fact that dormant carcinoma cells appear to perpetuate this disease and form the precursors of eventual metastatic relapses. Unfortunately, almost all currently deployed cytotoxic therapies preferentially kill proliferating cells rather than those that have exited the active cell cycle, rendering dormant cells intrinsically more resistant to almost all currently available therapies (Ghajar, 2015; Goss and Chambers, 2010). This stark contrast in the behavior of these dormant DTCs and the actively cycling cells of the primary tumor may ultimately prove to be far more critical in determining susceptibility to therapeutic elimination than any genetic or epigenetic differences distinguishing MRD from the corresponding primary tumors. Further complicating the development of novel agents directed at dormant metastatic deposits is the fact that, for various types of cancer, true efficacy can only be judged after extremely long follow-up periods when the much-feared relapses may appear (Steeg, 2016). Still, preventative adjuvant therapies directed at dormant DTCs and their ability to spawn clinical relapses arguably offer the best opportunity to prevent these outcomes.
Therapeutic Resistance
The mechanisms of therapeutic resistance acquired by metastatic growths may closely parallel those operating within corresponding tumors. In the context of targeted adjuvant therapy, drug resistant clones may emerge in primary tumors and metastatic lesions, as has been observed, for example, in ER+ breast cancer patients receiving hormone therapy (Alluri et al., 2014) and patients with EGFR-mutant non-small-cell lung cancer treated with targeted kinase inhibitors (Gazdar, 2009). Of special interest to the present discussion are resistance mechanisms that are particular to the sites of dissemination. One possibility is that the metastatic microenvironment favors the induction of biological programs that confer drug resistance. For example, it has been reported that CXCL1/2, which actively supports the establishment of metastases through the recruitment of myeloid cells, can also mediate resistance to chemotherapy (Acharyya et al., 2012), providing support for the idea that certain traits involved in metastatic dissemination may also contribute to therapeutic resistance.
More generally, the effect of chemotherapy on either primary or metastatic growths may elicit the secretion of various paracrine mediators from the surrounding stromal cells that can promote resistance, including CXCL1/2 (Acharyya et al., 2012), IL-6 and Timp1 (Gilbert and Hemann, 2010), WNT16B (Sun et al., 2012), and HGF (Straussman et al., 2012). Resistance to targeted kinase inhibitors can also be conferred by a host of secreted factors that are produced by carcinoma cells following exposure to a drug (Lee et al., 2014; Obenauf et al., 2015). While such effects may indeed promote the emergence of drug-resistant cell clones within in primary tumors, they may operate even more strongly in sites of metastasis.
According to an alternative view, the cells forming metastases are intrinsically no more or less resistant to therapies than their counterparts in primary tumors. Hence, if drug-resistant metastatic clones were present in the original neoplasm, then such cells would render this tumor as well as its derived metastases equally resistant to therapy. Following such thinking, a major benefit of surgically eliminating primary tumors derives from reducing the sheer number and diversity of neoplastic cells, thereby increasing the chance that any therapy-resistant variant clones are removed from the body of a patient. In the context of metastatic disease this is, it seems, often not possible.
CONCLUSION: PRINCIPLES AND OUTLOOK
As the preceding discussions have indicated, significant progress has been made over the past decade in elucidating the cellular and molecular programs that drive cancer metastasis. While our understanding of metastasis remains quite incomplete, we see a number of common biological principles beginning to emerge. Thus, we suggest that one can take stock of the information that is currently at hand and conclude that:
Metastasis occurs mainly through a sequential, multi-step process that can be conceptualized as invasion-metastasis cascade.
In the case of carcinomas, the EMT program enables primary tumor cells to accomplish most, if not all of the steps involved in the physical dissemination of tumor cells to a distant site.
The fate of disseminating carcinoma cells is strongly influenced by interactions that they experience during transit through the circulatory system.
Disseminated carcinoma cells must escape clearance by the arms of the immune system and subvert the cellular programs that impose a state of dormancy.
The process of active metastatic colonization is contingent upon the dissemination of cancer stem cells that can re-initiate tumor growth; the ability of their progeny to assemble adaptive, organ-specific colonization programs; and the establishment of a microenvironment conducive to metastasis.
The processes that enable the physical translocation of cancer cells from primary tumors to the parenchyma of distant tissues are within sight and relatively small in number; in contrast the adaptive programs allowing cancer cells arising from diverse primary tumors to thrive in various tissue microenvironments may be large in number and not readily reducible to a common set of underlying mechanistic principles.
While these principles articulate general concepts, a number of key mechanistic details related to these ideas remain to be established. For example, we are beginning to appreciate that the EMT program is capable of generating a wide spectrum of carcinoma cells with various complements of mesenchymal traits, but there is little information on the functional role of these different phenotypic states in the metastatic process. Yet other critical questions about metastasis fall outside the bounds of the points outlined above. For one, it is not yet clear what specific factors determine the efficiency of clinical metastatic disease and why some patients present with metastatic cancer, while in other patients many years may lapse before the disease advances to this stage. The literature holds some provocative hints that could account for this variability, such as different cells-of-origin whose differentiation programs strongly predispose to an aggressive malignancy or to the dissemination of CTC clusters that may more readily establish a metastatic colony (Figure 5). Additionally, the fact that many patients experience metastatic spread to multiple organs suggests the existence of more universal, multi-organ metastatic programs, but the extent to which such programs operate is unclear and their biological details have just begun to be described. Finally, the clinical and biological impact of various immunotherapies, particularly checkpoint inhibitors (Sharma and Allison, 2015), on metastases is sure to be a continued area of active research, even offering the hope of seeking out and eliminating metastatic deposits.
Figure 5. Dynamics of Metastatic Evolution.
The progression and evolution of metastatic disease is highly variable, manifesting in ways that must affect the kinetics of metastatic colonization. Four hypothetical alternative outcomes are presented here:
(A) The dissemination of CTC clusters to distant sites may generate overt metastases with a relatively short latency, since such clusters are highly efficient at spawning metastatic growths. Their efficiency in forming metastases may derive from advantages during transit in the circulation or because they benefit from homotypic cell-cell interactions in a foreign tissue environment.
(B) Solitary disseminated carcinoma cells that are adept at recruiting and establishing a supportive metastatic niche, or that are able to generate a microenvironmental niche themselves, may be able to better survive and initiate programs of proliferation.
(C) While the dissemination of tumor-initiating cancer stem cells may be a prerequisite for metastasis, the generation and evolution of progeny that are well adapted to the local microenvironment could take many months or years.
(D) At later stages of metastatic progression, other dynamics come into play, such as the exchange of metastatic cells clones between different metastatic lesions in the same patient. The biological and clinical impact of such transfer, however, remains to be firmly established.
Perhaps most pressing is a better understanding of the biological similarities and differences between primary tumors and their metastatic descendants, especially in regard to the extent of heterogeneity, plasticity, and resistance that they exhibit. We believe that an accurate comparison of the principles that govern primary tumor growth with those that govern the dissemination and outgrowth of metastases will be essential in order to enable the development of new approaches and therapies that are specifically designed to prevent or treat metastatic disease.
Acknowledgments
We would like to thank all members of the Weinberg laboratory for fruitful discussions and especially Tsukasa Shibue for critical review of the manuscript. We would also like to thank Meredith Leffler for preparation of the figures. A.W.L. is supported by an American Cancer Society – New England Division – Ellison Foundation Postdoctoral Fellowship (PF-15-131-01-CSM). D.R.P was supported by a C. J. Martin Overseas Biomedical Fellowship from the National Health and Medical Research Council of Australia (NHMRC APP1071853) and is currently supported by a K99/R00 Pathway to Independence Award (NIH/NCI 1K99CA201574-01A1). Work in the Weinberg Laboratory is supported by grants from the National Institutes of Health (R01-CA078461), the Breast Cancer Research Foundation, the Advanced Medical Research Foundation and the Ludwig Center for Molecular Oncology. R.A.W. is an American Cancer Society Research Professor and a Daniel K. Ludwig Cancer Research Professor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, Brannigan BW, Kapur R, Stott SL, Shioda T, Ramaswamy S, Ting DT, Lin CP, Toner M, Haber DA, Maheswaran S. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158:1110–1122. doi: 10.1016/j.cell.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aceto N, Toner M, Maheswaran S, Haber DA. En Route to Metastasis: Circulating Tumor Cell Clusters and Epithelial-to-Mesenchymal Transition. Trends in cancer. 2015;1:44–52. doi: 10.1016/j.trecan.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, Norton L, Brogi E, Massague J. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol. 1999;147:89–104. doi: 10.1083/jcb.147.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nature reviews Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nature medicine. 2000;6:100–102. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- Alluri PG, Speers C, Chinnaiyan AM. Estrogen receptor mutations and their role in breast cancer progression. Breast cancer research : BCR. 2014;16:494. doi: 10.1186/s13058-014-0494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au SH, Storey BD, Moore JC, Tang Q, Chen YL, Javaid S, Sarioglu AF, Sullivan R, Madden MW, O’Keefe R, Haber DA, Maheswaran S, Langenau DM, Stott SL, Toner M. Clusters of circulating tumor cells traverse capillary-sized vessels. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:4947–4952. doi: 10.1073/pnas.1524448113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bauerle T, Wallwiener M, Holland-Letz T, Hofner T, Sprick M, Scharpff M, Marme F, Sinn HP, Pantel K, Weichert W, Trumpp A. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31:539–544. doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- Balic M, Lin H, Young L, Hawes D, Giuliano A, McNamara G, Datar RH, Cote RJ. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12:5615–5621. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- Barkan D, El Touny LH, Michalowski AM, Smith JA, Chu I, Davis AS, Webster JD, Hoover S, Simpson RM, Gauldie J, Green JE. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer research. 2010;70:5706–5716. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan D, Kleinman H, Simmons JL, Asmussen H, Kamaraju AK, Hoenorhoff MJ, Liu ZY, Costes SV, Cho EH, Lockett S, Khanna C, Chambers AF, Green JE. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer research. 2008;68:6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarz-Knoll N, Alix-Panabieres C, Pantel K. Plasticity of disseminating cancer cells in patients with epithelial malignancies. Cancer metastasis reviews. 2012;31:673–687. doi: 10.1007/s10555-012-9370-z. [DOI] [PubMed] [Google Scholar]
- Bidwell BN, Slaney CY, Withana NP, Forster S, Cao Y, Loi S, Andrews D, Mikeska T, Mangan NE, Samarajiwa SA, de Weerd NA, Gould J, Argani P, Moller A, Smyth MJ, Anderson RL, Hertzog PJ, Parker BS. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nature medicine. 2012;18:1224–1231. doi: 10.1038/nm.2830. [DOI] [PubMed] [Google Scholar]
- Bonapace L, Coissieux MM, Wyckoff J, Mertz KD, Varga Z, Junt T, Bentires-Alj M. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515:130–133. doi: 10.1038/nature13862. [DOI] [PubMed] [Google Scholar]
- Bonnomet A, Syne L, Brysse A, Feyereisen E, Thompson EW, Noel A, Foidart JM, Birembaut P, Polette M, Gilles C. A dynamic in vivo model of epithelial-to-mesenchymal transitions in circulating tumor cells and metastases of breast cancer. Oncogene. 2012;31:3741–3753. doi: 10.1038/onc.2011.540. [DOI] [PubMed] [Google Scholar]
- Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, Massague J. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabletz T. To differentiate or not--routes towards metastasis. Nature reviews Cancer. 2012;12:425–436. doi: 10.1038/nrc3265. [DOI] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nature reviews Cancer. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- Bragado P, Estrada Y, Parikh F, Krause S, Capobianco C, Farina HG, Schewe DM, Aguirre-Ghiso JA. TGF-beta2 dictates disseminated tumour cell fate in target organs through TGF-beta-RIII and p38alpha/beta signalling. Nature cell biology. 2013;15:1351–1361. doi: 10.1038/ncb2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G, Pierga JY, Marth C, Oruzio D, Wiedswang G, Solomayer EF, Kundt G, Strobl B, Fehm T, Wong GY, Bliss J, Vincent-Salomon A, Pantel K. A pooled analysis of bone marrow micrometastasis in breast cancer. The New England journal of medicine. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, Morsberger LA, Latimer C, McLaren S, Lin ML, McBride DJ, Varela I, Nik-Zainal SA, Leroy C, Jia M, Menzies A, Butler AP, Teague JW, Griffin CA, Burton J, Swerdlow H, Quail MA, Stratton MR, Iacobuzio-Donahue C, Futreal PA. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celia-Terrassa T, Meca-Cortes O, Mateo F, Martinez de Paz A, Rubio N, Arnal-Estape A, Ell BJ, Bermudo R, Diaz A, Guerra-Rebollo M, Lozano JJ, Estaras C, Ulloa C, Alvarez-Simon D, Mila J, Vilella R, Paciucci R, Martinez-Balbas M, de Herreros AG, Gomis RR, Kang Y, Blanco J, Fernandez PL, Thomson TM. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. The Journal of clinical investigation. 2012;122:1849–1868. doi: 10.1172/JCI59218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nature reviews Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, Jacob LS, Patwa R, Shah H, Xu K, Cross JR, Massague J. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533:493–498. doi: 10.1038/nature18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhang XH, Massague J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer cell. 2011;20:538–549. doi: 10.1016/j.ccr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KJ, Gabrielson E, Werb Z, Ewald AJ. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell. 2013;155:1639–1651. doi: 10.1016/j.cell.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, Fairchild AN, Gorin MA, Verdone JE, Pienta KJ, Bader JS, Ewald AJ. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E854–863. doi: 10.1073/pnas.1508541113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YC, Wei WC, Hung CN, Kuo JF, Hsu CP, Chang KJ, Chao WT. Rab11 collaborates E-cadherin to promote collective cell migration and indicates a poor prognosis in colorectal carcinoma. European journal of clinical investigation. 2016 doi: 10.1111/eci.12683. [DOI] [PubMed] [Google Scholar]
- Clever D, Roychoudhuri R, Constantinides MG, Askenase MH, Sukumar M, Klebanoff CA, Eil RL, Hickman HD, Yu Z, Pan JH, Palmer DC, Phan AT, Goulding J, Gattinoni L, Goldrath AW, Belkaid Y, Restifo NP. Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche. Cell. 2016;166:1117–1131 e1114. doi: 10.1016/j.cell.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJ, Ciampricotti M, Hawinkels LJ, Jonkers J, de Visser KE. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nature reviews Cancer. 2016;16:431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P, Ferri L. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. The Journal of clinical investigation. 2013 doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, Garcia-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nature cell biology. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TR, Erler JT. Molecular pathways: connecting fibrosis and solid tumor metastasis. Clin Cancer Res. 2014;20:3637–3643. doi: 10.1158/1078-0432.CCR-13-1059. [DOI] [PubMed] [Google Scholar]
- de Boer M, van Dijck JA, Bult P, Borm GF, Tjan-Heijnen VC. Breast cancer prognosis and occult lymph node metastases, isolated tumor cells, and micrometastases. Journal of the National Cancer Institute. 2010;102:410–425. doi: 10.1093/jnci/djq008. [DOI] [PubMed] [Google Scholar]
- De Cock JM, Shibue T, Dongre A, Keckesova Z, Reinhardt F, Weinberg RA. Inflammation triggers Zeb1-dependent escape from tumor latency. Cancer research. 2016 doi: 10.1158/0008-5472.CAN-16-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nature reviews Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- Del Pozo Martin Y, Park D, Ramachandran A, Ombrato L, Calvo F, Chakravarty P, Spencer-Dene B, Derzsi S, Hill CS, Sahai E, Malanchi I. Mesenchymal Cancer Cell-Stroma Crosstalk Promotes Niche Activation, Epithelial Reversion, and Metastatic Colonization. Cell Rep. 2015;13:2456–2469. doi: 10.1016/j.celrep.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny SK, Yang D, Chuang CH, Brady JJ, Lim JS, Gruner BM, Chiou SH, Schep AN, Baral J, Hamard C, Antoine M, Wislez M, Kong CS, Connolly AJ, Park KS, Sage J, Greenleaf WJ, Winslow MM. Nfib Promotes Metastasis through a Widespread Increase in Chromatin Accessibility. Cell. 2016;166:328–342. doi: 10.1016/j.cell.2016.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Ellis MJ, Li S, Larson DE, Chen K, Wallis JW, Harris CC, McLellan MD, Fulton RS, Fulton LL, Abbott RM, Hoog J, Dooling DJ, Koboldt DC, Schmidt H, Kalicki J, Zhang Q, Chen L, Lin L, Wendl MC, McMichael JF, Magrini VJ, Cook L, McGrath SD, Vickery TL, Appelbaum E, Deschryver K, Davies S, Guintoli T, Lin L, Crowder R, Tao Y, Snider JE, Smith SM, Dukes AF, Sanderson GE, Pohl CS, Delehaunty KD, Fronick CC, Pape KA, Reed JS, Robinson JS, Hodges JS, Schierding W, Dees ND, Shen D, Locke DP, Wiechert ME, Eldred JM, Peck JB, Oberkfell BJ, Lolofie JT, Du F, Hawkins AE, O’Laughlin MD, Bernard KE, Cunningham M, Elliott G, Mason MD, Thompson DM, Jr, Ivanovich JL, Goodfellow PJ, Perou CM, Weinstock GM, Aft R, Watson M, Ley TJ, Wilson RK, Mardis ER. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douma S, van Laar T, Zevenhoven J, Meuwissen R, van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–1039. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- El Touny LH, Vieira A, Mendoza A, Khanna C, Hoenerhoff MJ, Green JE. Combined SFK/MEK inhibition prevents metastatic outgrowth of dormant tumor cells. The Journal of clinical investigation. 2014;124:156–168. doi: 10.1172/JCI70259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- Eyles J, Puaux A-L, Wang X, Toh B, Prakash C, Hong M, Tan TG, Zheng L, Ong LC, Jin Y, Kato M, Prévost-Blondel A, Chow P, Yang H, Abastado J-P. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. The Journal of clinical investigation. 2010;120:2030–2039. doi: 10.1172/JCI42002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Samuel S, Evans KW, Lu J, Xia L, Zhou Y, Sceusi E, Tozzi F, Ye XC, Mani SA, Ellis LM. Overexpression of snail induces epithelial-mesenchymal transition and a cancer stem cell-like phenotype in human colorectal cancer cells. Cancer medicine. 2012;1:5–16. doi: 10.1002/cam4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature reviews Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–476. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126:582–588. doi: 10.1182/blood-2014-08-531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Locker J, Sahai E, Segall JE. Classifying collective cancer cell invasion. Nature cell biology. 2012;14:777–783. doi: 10.1038/ncb2548. [DOI] [PubMed] [Google Scholar]
- Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nature cell biology. 2007;9:1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- Gao H, Chakraborty G, Lee-Lim AP, Mo Q, Decker M, Vonica A, Shen R, Brogi E, Brivanlou AH, Giancotti FG. The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell. 2012;150:764–779. doi: 10.1016/j.cell.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Chakraborty G, Zhang Z, Akalay I, Gadiya M, Gao Y, Sinha S, Hu J, Jiang C, Akram M, Brogi E, Leitinger B, Giancotti FG. Multi-organ Site Metastatic Reactivation Mediated by Non-canonical Discoidin Domain Receptor 1 Signaling. Cell. 2016;166:47–62. doi: 10.1016/j.cell.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway LA, Lander ES. Lessons from the cancer genome. Cell. 2013;153:17–37. doi: 10.1016/j.cell.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Gasic GJ, Gasic TB, Stewart CC. Antimetastatic effects associated with platelet reduction. Proceedings of the National Academy of Sciences of the United States of America. 1968;61:46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nature reviews Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar AF. Activating and resistance mutations of EGFR in non-small-cell lung cancer: role in clinical response to EGFR tyrosine kinase inhibitors. Oncogene. 2009;28(Suppl 1):S24–31. doi: 10.1038/onc.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar CM. Metastasis prevention by targeting the dormant niche. Nature reviews Cancer. 2015;15:238–247. doi: 10.1038/nrc3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, Chen EI, Lyden D, Bissell MJ. The perivascular niche regulates breast tumour dormancy. Nature cell biology. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155:750–764. doi: 10.1016/j.cell.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert LA, Hemann MT. DNA damage-mediated induction of a chemoresistant niche. Cell. 2010;143:355–366. doi: 10.1016/j.cell.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss PE, Chambers AF. Does tumour dormancy offer a therapeutic target? Nature reviews Cancer. 2010;10:871–877. doi: 10.1038/nrc2933. [DOI] [PubMed] [Google Scholar]
- Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer cell. 2011;20:300–314. doi: 10.1016/j.ccr.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse-Wilde A, Fouquier d’Herouel A, McIntosh E, Ertaylan G, Skupin A, Kuestner RE, del Sol A, Walters KA, Huang S. Stemness of the hybrid Epithelial/Mesenchymal State in Breast Cancer and Its Association with Poor Survival. PloS one. 2015;10:e0126522. doi: 10.1371/journal.pone.0126522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JM, Papaemmanuil E, Brewer DS, Kallio HM, Hognas G, Annala M, Kivinummi K, Goody V, Latimer C, O’Meara S, Dawson KJ, Isaacs W, Emmert-Buck MR, Nykter M, Foster C, Kote-Jarai Z, Easton D, Whitaker HC, Group IPU, Neal DE, Cooper CS, Eeles RA, Visakorpi T, Campbell PJ, McDermott U, Wedge DC, Bova GS. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520:353–357. doi: 10.1038/nature14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, Gomis RR, Manova-Todorova K, Massague J. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765–770. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- Gupta PB, Kuperwasser C, Brunet JP, Ramaswamy S, Kuo WL, Gray JW, Naber SP, Weinberg RA. The melanocyte differentiation program predisposes to metastasis after neoplastic transformation. Nature genetics. 2005;37:1047–1054. doi: 10.1038/ng1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner MC, Mosbruger T, Esopi DM, Fedor H, Heaphy CM, Walker DA, Adejola N, Gurel M, Hicks J, Meeker AK, Halushka MK, Simons JW, Isaacs WB, De Marzo AM, Nelson WG, Yegnasubramanian S. Tracking the clonal origin of lethal prostate cancer. The Journal of clinical investigation. 2013;123:4918–4922. doi: 10.1172/JCI70354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Headley MB, Bins A, Nip A, Roberts EW, Looney MR, Gerard A, Krummel MF. Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature. 2016;531:513–517. doi: 10.1038/nature16985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong T, Watanabe K, Ta CH, Villarreal-Ponce A, Nie Q, Dai X. An Ovol2-Zeb1 Mutual Inhibitory Circuit Governs Bidirectional and Multi-step Transition between Epithelial and Mesenchymal States. PLoS computational biology. 2015;11:e1004569. doi: 10.1371/journal.pcbi.1004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh SJ, Liang S, Sharma A, Dong C, Robertson GP. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer research. 2010;70:6071–6082. doi: 10.1158/0008-5472.CAN-09-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmuller G, Klein CA. Systemic spread is an early step in breast cancer. Cancer cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Ince TA, Richardson AL, Bell GW, Saitoh M, Godar S, Karnoub AE, Iglehart JD, Weinberg RA. Transformation of different human breast epithelial cell types leads to distinct tumor phenotypes. Cancer cell. 2007;12:160–170. doi: 10.1016/j.ccr.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Jacob LS, Vanharanta S, Obenauf AC, Pirun M, Viale A, Socci ND, Massague J. Metastatic Competence Can Emerge with Selection of Preexisting Oncogenic Alleles without a Need of New Mutations. Cancer research. 2015;75:3713–3719. doi: 10.1158/0008-5472.CAN-15-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan NV, Johnson GL, Abell AN. Tracking the intermediate stages of epithelial-mesenchymal transition in epithelial stem cells and cancer. Cell cycle. 2011;10:2865–2873. doi: 10.4161/cc.10.17.17188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. The Journal of clinical investigation. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Pantel K. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer cell. 2013;23:573–581. doi: 10.1016/j.ccr.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaman S, Detmar M. Mechanisms of lymphatic metastasis. The Journal of clinical investigation. 2014;124:922–928. doi: 10.1172/JCI71606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkler F. Real-time imaging reveals the single steps of brain metastasis formation. Nature medicine. 2010;16:116–122. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15:73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein CA. Parallel progression of primary tumours and metastases. Nature reviews Cancer. 2009;9:302–312. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C, Wilber A, Watabe K. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med. 2011;208:2641–2655. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, Smyth MJ, Schreiber RD. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- Kohler S, Ullrich S, Richter U, Schumacher U. E-/P-selectins and colon carcinoma metastasis: first in vivo evidence for their crucial role in a clinically relevant model of spontaneous metastasis formation in the lung. British journal of cancer. 2010;102:602–609. doi: 10.1038/sj.bjc.6605492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D, Banerjee S, Ahmad A, Li Y, Wang Z, Sethi S, Sarkar FH. Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells. PloS one. 2010;5:e12445. doi: 10.1371/journal.pone.0012445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer research. 2009;69:7775–7783. doi: 10.1158/0008-5472.CAN-09-2123. [DOI] [PubMed] [Google Scholar]
- Korpal M, Ell BJ, Buffa FM, Ibrahim T, Blanco MA, Celia-Terrassa T, Mercatali L, Khan Z, Goodarzi H, Hua Y, Wei Y, Hu G, Garcia BA, Ragoussis J, Amadori D, Harris AL, Kang Y. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nature medicine. 2011;17:1101–1108. doi: 10.1038/nm.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, Bapat SA. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem cells. 2009;27:2059–2068. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E3053–3061. doi: 10.1073/pnas.1411082111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labelle M, Hynes RO. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012;2:1091–1099. doi: 10.1158/2159-8290.CD-12-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nature reviews Molecular cell biology. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson DA, Bhakta NR, Kessenbrock K, Prummel KD, Yu Y, Takai K, Zhou A, Eyob H, Balakrishnan S, Wang CY, Yaswen P, Goga A, Werb Z. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526:131–135. doi: 10.1038/nature15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Zhuang G, Cao Y, Du P, Kim HJ, Settleman J. Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer cell. 2014;26:207–221. doi: 10.1016/j.ccr.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CM, Gocheva V, Oudin MJ, Bhutkar A, Wang SY, Date SR, Ng SR, Whittaker CA, Bronson RT, Snyder EL, Gertler FB, Jacks T. Foxa2 and Cdx2 cooperate with Nkx2-1 to inhibit lung adenocarcinoma metastasis. Genes Dev. 2015;29:1850–1862. doi: 10.1101/gad.267393.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Kang Y. Probing the Fifty Shades of EMT in Metastasis. Trends in cancer. 2016;2:65–67. doi: 10.1016/j.trecan.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat ML, Gyorki DE, Ward T, Partanen A, Feleppa F, Huschtscha LI, Thorne HJ, kConFab, Fox SB, Yan M, French JD, Brown MA, Smyth GK, Visvader JE, Lindeman GJ. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nature medicine. 2009;15:907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- Liu W, Laitinen S, Khan S, Vihinen M, Kowalski J, Yu G, Chen L, Ewing CM, Eisenberger MA, Carducci MA, Nelson WG, Yegnasubramanian S, Luo J, Wang Y, Xu J, Isaacs WB, Visakorpi T, Bova GS. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nature medicine. 2009;15:559–565. doi: 10.1038/nm.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H, Xiang T, Qi W, Huang J, Chen J, He L, Liang Z, Guo B, Li Y, Xie R, Zhu B. CD133+ ovarian cancer stem-like cells promote non-stem cancer cell metastasis via CCL5 induced epithelial-mesenchymal transition. Oncotarget. 2015;6:5846–5859. doi: 10.18632/oncotarget.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo JM, Scherl A, Nguyen A, Man FY, Weinberg E, Zeng Z, Saltz L, Paty PB, Tavazoie SF. Extracellular metabolic energetics can promote cancer progression. Cell. 2015;160:393–406. doi: 10.1016/j.cell.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, Yan J, Hua Y, Tiede BJ, Lu X, Haffty BG, Pantel K, Massague J, Kang Y. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer cell. 2011;20:701–714. doi: 10.1016/j.ccr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren K, Nordenskjold B, Landberg G. Hypoxia, Snail and incomplete epithelial-mesenchymal transition in breast cancer. British journal of cancer. 2009;101:1769–1781. doi: 10.1038/sj.bjc.6605369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, Groom AC. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. 1998;153:865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–89. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- Malladi S, Macalinao DG, Jin X, He L, Basnet H, Zou Y, de Stanchina E, Massague J. Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell. 2016;165:45–60. doi: 10.1016/j.cell.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nature reviews Cancer. 2012;12:323–334. doi: 10.1038/nrc3261. [DOI] [PubMed] [Google Scholar]
- McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nature cell biology. 2014;16:717–727. doi: 10.1038/ncb3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto DT, Zheng Y, Wittner BS, Lee RJ, Zhu H, Broderick KT, Desai R, Fox DB, Brannigan BW, Trautwein J, Arora KS, Desai N, Dahl DM, Sequist LV, Smith MR, Kapur R, Wu CL, Shioda T, Ramaswamy S, Ting DT, Toner M, Maheswaran S, Haber DA. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science. 2015;349:1351–1356. doi: 10.1126/science.aab0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux G, Geyer FC, Magnay FA, McCarthy A, Kendrick H, Natrajan R, Mackay A, Grigoriadis A, Tutt A, Ashworth A, Reis-Filho JS, Smalley MJ. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell stem cell. 2010;7:403–417. doi: 10.1016/j.stem.2010.07.010. [DOI] [PubMed] [Google Scholar]
- Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PloS one. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouw JK, Yui Y, Damiano L, Bainer RO, Lakins JN, Acerbi I, Ou G, Wijekoon AC, Levental KR, Gilbert PM, Hwang ES, Chen YY, Weaver VM. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nature medicine. 2014;20:360–367. doi: 10.1038/nm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Hermelink N, Braumüller H, Pichler B, Wieder T, Mailhammer R, Schaak K, Ghoreschi K, Yazdi A, Haubner R, Sander CA, Mocikat R, Schwaiger M, Förster I, Huss R, Weber WA, Kneilling M, Röcken M. TNFR1 Signaling and IFN-γ Signaling Determine whether T Cells Induce Tumor Dormancy or Promote Multistage Carcinogenesis. Cancer cell. 2008;13:507–518. doi: 10.1016/j.ccr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nature reviews Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- Naxerova K, Jain RK. Using tumour phylogenetics to identify the roots of metastasis in humans. Nature reviews Clinical oncology. 2015;12:258–272. doi: 10.1038/nrclinonc.2014.238. [DOI] [PubMed] [Google Scholar]
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nature reviews Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer research. 1999;59:1295–1300. [PubMed] [Google Scholar]
- Nieto MA, Huang Ruby Y-J, Jackson Rebecca A, Thiery Jean P. EMT: 2016. Cell. 2016;166:21–45. doi: 10.1016/j.cell.2016.06.028. [DOI] [PubMed] [Google Scholar]
- Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Obenauf AC, Massague J. Surviving at a distance: organ specific metastasis. Trends in cancer. 2015;1:76–91. doi: 10.1016/j.trecan.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenauf AC, Zou Y, Ji AL, Vanharanta S, Shu W, Shi H, Kong X, Bosenberg MC, Wiesner T, Rosen N, Lo RS, Massague J. Therapy-induced tumour secretomes promote resistance and tumour progression. Nature. 2015;520:368–372. doi: 10.1038/nature14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocana OH, Corcoles R, Fabra A, Moreno-Bueno G, Acloque H, Vega S, Barrallo-Gimeno A, Cano A, Nieto MA. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer cell. 2012;22:709–724. doi: 10.1016/j.ccr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, Massague J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nature medicine. 2011;17:867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson T, Batlle E, Massague J. Metastatic stem cells: sources, niches, and vital pathways. Cell stem cell. 2014;14:306–321. doi: 10.1016/j.stem.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk S, Papageorgis P, Wong CK, Lambert AW, Abdolmaleky HM, Thiagalingam A, Cohen HT, Thiagalingam S. SDPR functions as a metastasis suppressor in breast cancer by promoting apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:638–643. doi: 10.1073/pnas.1514663113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, Massague J. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Hu Z, Barney KA, Degen JL. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood. 2007;110:133–141. doi: 10.1182/blood-2007-01-065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirouskova M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–185. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- Pang R, Law WL, Chu AC, Poon JT, Lam CS, Chow AK, Ng L, Cheung LW, Lan XR, Lan HY, Tan VP, Yau TC, Poon RT, Wong BC. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell stem cell. 2010;6:603–615. doi: 10.1016/j.stem.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nature medicine. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ, Morrison SJ. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–191. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsypanina K, Du YC, Jechlinger M, Beverly LJ, Hambardzumyan D, Varmus H. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. 2008;321:1841–1844. doi: 10.1126/science.1161621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proia TA, Keller PJ, Gupta PB, Klebba I, Jones AD, Sedic M, Gilmore H, Tung N, Naber SP, Schnitt S, Lander ES, Kuperwasser C. Genetic predisposition directs breast cancer phenotype by dictating progenitor cell fate. Cell stem cell. 2011;8:149–163. doi: 10.1016/j.stem.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nature reviews Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PloS one. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nature medicine. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352:175–180. doi: 10.1126/science.aaf4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed ZA, Yang J, Wang Q, Kowalski J, Freed I, Murter C, Hong SM, Koorstra JB, Rajeshkumar NV, He X, Goggins M, Iacobuzio-Donahue C, Berman DM, Laheru D, Jimeno A, Hidalgo M, Maitra A, Matsui W. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. Journal of the National Cancer Institute. 2010;102:340–351. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revenu C, Gilmour D. EMT 2.0: shaping epithelia through collective migration. Current opinion in genetics & development. 2009;19:338–342. doi: 10.1016/j.gde.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Reymond N, d’Agua BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nature reviews Cancer. 2013;13:858–870. doi: 10.1038/nrc3628. [DOI] [PubMed] [Google Scholar]
- Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JB, Huh D, Noble LB, Tavazoie SF. Identification of molecular determinants of primary and metastatic tumour re-initiation in breast cancer. Nature cell biology. 2015;17:651–664. doi: 10.1038/ncb3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, Damti P, Lumbroso D, Polyansky L, Sionov RV, Ariel A, Hovav AH, Henke E, Fridlender ZG, Granot Z. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10:562–573. doi: 10.1016/j.celrep.2014.12.039. [DOI] [PubMed] [Google Scholar]
- Sampson VB, David JM, Puig I, Patil PU, de Herreros AG, Thomas GV, Rajasekaran AK. Wilms’ tumor protein induces an epithelial-mesenchymal hybrid differentiation state in clear cell renal cell carcinoma. PloS one. 2014;9:e102041. doi: 10.1371/journal.pone.0102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliekelman MJ, Taguchi A, Zhu J, Dai X, Rodriguez J, Celiktas M, Zhang Q, Chin A, Wong CH, Wang H, McFerrin L, Selamat SA, Yang C, Kroh EM, Garg KS, Behrens C, Gazdar AF, Laird-Offringa IA, Tewari M, Wistuba II, Thiery JP, Hanash SM. Molecular portraits of epithelial, mesenchymal, and hybrid States in lung adenocarcinoma and their relevance to survival. Cancer research. 2015;75:1789–1800. doi: 10.1158/0008-5472.CAN-14-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer cell. 2013;24:130–137. doi: 10.1016/j.ccr.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Sethi N, Kang Y. Unravelling the complexity of metastasis - molecular understanding and targeted therapies. Nature reviews Cancer. 2011;11:735–748. doi: 10.1038/nrc3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenich L, Bowman RL, Mason SD, Quail DF, Rapaport F, Elie BT, Brogi E, Brastianos PK, Hahn WC, Holsinger LJ, Massague J, Leslie CS, Joyce JA. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nature cell biology. 2014;16:876–888. doi: 10.1038/ncb3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T, Brooks MW, Inan MF, Reinhardt F, Weinberg RA. The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discov. 2012;2:706–721. doi: 10.1158/2159-8290.CD-11-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T, Brooks MW, Weinberg RA. An integrin-linked machinery of cytoskeletal regulation that enables experimental tumor initiation and metastatic colonization. Cancer cell. 2013;24:481–498. doi: 10.1016/j.ccr.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T, Weinberg RA. Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10290–10295. doi: 10.1073/pnas.0904227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, Pienta MJ, Song J, Wang J, Loberg RD, Krebsbach PH, Pienta KJ, Taichman RS. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. The Journal of clinical investigation. 2011;121:1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nature medicine. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- Sosa MS, Avivar-Valderas A, Bragado P, Wen HC, Aguirre-Ghiso JA. ERK1/2 and p38alpha/beta signaling in tumor cell quiescence: opportunities to control dormant residual disease. Clin Cancer Res. 2011;17:5850–5857. doi: 10.1158/1078-0432.CCR-10-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nature reviews Cancer. 2014;14:611–622. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer JD, McDonald B, Cools-Lartigue JJ, Chow SC, Giannias B, Kubes P, Ferri LE. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer research. 2012;72:3919–3927. doi: 10.1158/0008-5472.CAN-11-2393. [DOI] [PubMed] [Google Scholar]
- Spiegel A, Brooks MW, Houshyar S, Reinhardt F, Ardolino M, Fessler E, Chen MB, Krall JA, DeCock J, Zervantonakis IK, Iannello A, Iwamoto Y, Cortez-Retamozo V, Kamm RD, Pittet MJ, Raulet DH, Weinberg RA. Neutrophils Suppress Intraluminal NK Cell-Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov. 2016;6:630–649. doi: 10.1158/2159-8290.CD-15-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg PS. Metastasis suppressors alter the signal transduction of cancer cells. Nature reviews Cancer. 2003;3:55–63. doi: 10.1038/nrc967. [DOI] [PubMed] [Google Scholar]
- Steeg PS. Targeting metastasis. Nature reviews Cancer. 2016;16:201–218. doi: 10.1038/nrc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J, Frederick DT, Cooper ZA, Chapman PB, Solit DB, Ribas A, Lo RS, Flaherty KT, Ogino S, Wargo JA, Golub TR. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strilic B, Yang L, Albarran-Juarez J, Wachsmuth L, Han K, Muller UC, Pasparakis M, Offermanns S. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature. 2016;536:215–218. doi: 10.1038/nature19076. [DOI] [PubMed] [Google Scholar]
- Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, True L, Nelson PS. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nature medicine. 2012;18:1359–1368. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer research. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nature reviews Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Trimboli AJ, Fukino K, de Bruin A, Wei G, Shen L, Tanner SM, Creasap N, Rosol TJ, Robinson ML, Eng C, Ostrowski MC, Leone G. Direct evidence for epithelial-mesenchymal transitions in breast cancer. Cancer research. 2008;68:937–945. doi: 10.1158/0008-5472.CAN-07-2148. [DOI] [PubMed] [Google Scholar]
- Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turajlic S, Swanton C. Metastasis as an evolutionary process. Science. 2016;352:169–175. doi: 10.1126/science.aaf2784. [DOI] [PubMed] [Google Scholar]
- Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E, Massague J. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;156:1002–1016. doi: 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanharanta S, Massague J. Origins of metastatic traits. Cancer cell. 2013;24:410–421. doi: 10.1016/j.ccr.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veracini L, Grall D, Schaub S, Beghelli-de la Forest Divonne S, Etienne-Grimaldi MC, Milano G, Bozec A, Babin E, Sudaka A, Thariat J, Van Obberghen-Schilling E. Elevated Src family kinase activity stabilizes E-cadherin-based junctions and collective movement of head and neck squamous cell carcinomas. Oncotarget. 2015;6:7570–7583. doi: 10.18632/oncotarget.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nature medicine. 2013;19:1450–1464. doi: 10.1038/nm.3391. [DOI] [PubMed] [Google Scholar]
- Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528:413–417. doi: 10.1038/nature16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nature reviews Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng D, Penzner JH, Song B, Koido S, Calderwood SK, Gong J. Metastasis is an early event in mouse mammary carcinomas and is associated with cells bearing stem cell markers. Breast cancer research : BCR. 2012;14:R18. doi: 10.1186/bcr3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott JM, Prechtl AM, Maine EA, Dang TT, Esparza MA, Sun H, Zhou Y, Xie Y, Pearson GW. An epigenetically distinct breast cancer cell subpopulation promotes collective invasion. The Journal of clinical investigation. 2015;125:1927–1943. doi: 10.1172/JCI77767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow MM, Dayton TL, Verhaak RG, Kim-Kiselak C, Snyder EL, Feldser DM, Hubbard DD, DuPage MJ, Whittaker CA, Hoersch S, Yoon S, Crowley D, Bronson RT, Chiang DY, Meyerson M, Jacks T. Suppression of lung adenocarcinoma progression by Nkx2-1. Nature. 2011;473:101–104. doi: 10.1038/nature09881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf MJ, Hoos A, Bauer J, Boettcher S, Knust M, Weber A, Simonavicius N, Schneider C, Lang M, Sturzl M, Croner RS, Konrad A, Manz MG, Moch H, Aguzzi A, van Loo G, Pasparakis M, Prinz M, Borsig L, Heikenwalder M. Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer cell. 2012;22:91–105. doi: 10.1016/j.ccr.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, Velculescu VE, Kinzler KW, Vogelstein B, Iacobuzio-Donahue CA. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates LR, Gerstung M, Knappskog S, Desmedt C, Gundem G, Van Loo P, Aas T, Alexandrov LB, Larsimont D, Davies H, Li Y, Ju YS, Ramakrishna M, Haugland HK, Lilleng PK, Nik-Zainal S, McLaren S, Butler A, Martin S, Glodzik D, Menzies A, Raine K, Hinton J, Jones D, Mudie LJ, Jiang B, Vincent D, Greene-Colozzi A, Adnet PY, Fatima A, Maetens M, Ignatiadis M, Stratton MR, Sotiriou C, Richardson AL, Lonning PE, Wedge DC, Campbell PJ. Subclonal diversification of primary breast cancer revealed by multiregion sequencing. Nature medicine. 2015;21:751–759. doi: 10.1038/nm.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, Weinberg RA. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015;525:256–260. doi: 10.1038/nature14897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Bardia A, Aceto N, Bersani F, Madden MW, Donaldson MC, Desai R, Zhu H, Comaills V, Zheng Z, Wittner BS, Stojanov P, Brachtel E, Sgroi D, Kapur R, Shioda T, Ting DT, Ramaswamy S, Getz G, Iafrate AJ, Benes C, Toner M, Maheswaran S, Haber DA. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 2014;345:216–220. doi: 10.1126/science.1253533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, Concannon KF, Donaldson MC, Sequist LV, Brachtel E, Sgroi D, Baselga J, Ramaswamy S, Toner M, Haber DA, Maheswaran S. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–584. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Ting DT, Stott SL, Wittner BS, Ozsolak F, Paul S, Ciciliano JC, Smas ME, Winokur D, Gilman AJ, Ulman MJ, Xega K, Contino G, Alagesan B, Brannigan BW, Milos PM, Ryan DP, Sequist LV, Bardeesy N, Ramaswamy S, Toner M, Maheswaran S, Haber DA. RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature. 2012;487:510–513. doi: 10.1038/nature11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XH, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, Foekens JA, Massague J. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Kannappan V, Chen X, Li J, Leng X, Zhang J, Xuan S. RBP2 induces stem-like cancer cells by promoting EMT and is a prognostic marker for renal cell carcinoma. Experimental & molecular medicine. 2016;48:e238. doi: 10.1038/emm.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]