Abstract
Background
Intervertebral disc degeneration (IDD) has been widely recognized as a major contributor to low back pain. Accumulating evidence suggests that IDD is linked to various pro-inflammatory cytokines and metabolites. Recently, numerous studies have demonstrated that microRNAs (miRNAs) play a pivotal role in the development of most disorders, including degenerative disc diseases. Previous reports have revealed that miRNA-146a (miR-146a) could attenuate neuropathic pain in the spinal cord. The aim of this study was to investigate the role of miR-146a in the inflammatory response of IDD.
Material/Methods
Quantitative real-time (RT)-PCR was performed to investigate the levels of miR-146a in the PBMCs (peripheral blood mononuclear cells) of patients with IDD. Human nucleus pulposus (NP) cells were transiently transfected with miR-146a mimic; control NP cell transfections lacked miR-146a. Then all NP cells were treated with LPS (10 μM) to induce inflammation. The mRNA levels of miR-146a in NP cells were determined by RT-PCR. In addition, the mRNA and protein expression levels of tumor necrosis factor (TNF), receptor-associated factor 6 (TRAF6), and nuclear factor (NF)-κB in NP cells were evaluated by quantitative RT-PCR and Western blot analysis, respectively.
Results
We found that miR-146a was significantly downregulated in the PBMCs of patients. Moreover, overexpression of miR-146a significantly decreased the levels of pro-inflammatory cytokines in LPS-stimulated NP cells. The mRNA and protein levels of TRAF6 and NF-κB were downregulated by miR-146a overexpression.
Conclusions
These results suggest that overexpression of miR-146a could promote IDD through the TRAF/NF-κB pathway. Our findings also highlight miR-146a as a novel possible therapeutic target for IDD.
MeSH Keywords: Inflammation, Intervertebral Disc Degeneration, MicroRNAs, NF-kappa B, TNF Receptor-Associated Factor 6
Background
Low back pain (LBP) is the sixth leading cause of disability worldwide and one of the most costly musculoskeletal pain syndromes of modern society [1]; 84% of all people suffer from LBP at some time in their life [2]. The absolute number of people with low back pain is likely to increase substantially over the coming decades as the population ages [3]. Intervertebral disc degeneration (IDD) plays a central role in the pathogenesis of discogenic pain, disc herniation, and spinal instability and stenosis, and is widely recognized as a contributor to low back pain [4,5]. Numerous investigations have proven that IDD is linked to various pro-inflammatory cytokines and metabolites, including interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α).
MicroRNAs (miRNAs) are a type of small non-coding RNA molecule of 20–22 nucleotides in length [6]. Increasing evidence suggests these newly defined gene regulators, miRNAs, play a pivotal role in the development of most disorders [7,8], including degenerative disc diseases [9]. MicroRNA-146a (miR-146a), one of the earliest miRNAs identified in cartilage, has been implicated in some degenerative diseases, including osteoarthritis [10,11]. Moreover, it has been suggested that miR-146a functions as a negative feedback regulator of inflammation response by targeting TRAF6 [12], a member of the tumor necrosis factor (TNF) receptor-associated factors (TRAFs) family [13]. TRAF6 participates in signal transduction of both the TNF receptor (TNFR) and interleukin (IL)-1 receptor/Toll-like receptor (IL-1R/TLR) superfamily [14]; it triggers the IκB kinase, and in turn, the downstream nuclear factor (NF)-κB transcription factors, and results in overexpression of pro-inflammatory cytokine secretion. Recently, miR-146a was demonstrated to attenuate neuropathic pain in the spinal cord partially through the suppression of inflammation [15], which suggests a potential role for miR-146a in inhibiting inflammation in IDD.
Thus, the aim of the present study was to investigate the expression and role of miR-146a in IDD and explore the pathological links between miR-146a, IDD, and inflammatory pathways associated with IDD. In this study, we found miR-146a expression was significantly downregulated in patients with IDD versus healthy controls. Subsequently, we investigated the role of miR-146a in a series of experiments performed in LPS-stimulated human nucleus pulposus (NP) cells using miR-146a mimics.
Material and Methods
Materials and reagents
Lipopolysaccharides (LPS) were purchased from Sigma (St. Louis, MO, USA). ELISA kits for TNF-α, IL-1β, and IL-6 were obtained from Dakewe Biotech Co., Ltd. (Shenzhen, P. R. China). Antibodies against TRAF6, NF-κB, and β-actin and horseradish peroxidase (HRP)-linked anti-rabbit IgG antibody were obtained from Cell Signaling Technology (Danvers, MA, USA). All of the other chemicals and reagents were of analytical grade and purchased from Sinopharm Chemical Reagent (Shanghai, P. R. China).
Patients and control participants
Between May 2013 and March 2014, a total of 21 patients with degenerative disc disease (IDD) and 21 healthy human volunteers were enrolled in the study. Routine MRI scans of the lumbar spine were taken of these patients, the degree of disc degeneration was graded from T2-weighted images using a modified Pfirrmann classification [16]. According to the modified classification system of the International Society for the Study of the Lumbar Spine [17], 12 samples were protrusions, five were sequestration, and four were subligamentous extrusion. PBMCs (peripheral blood mononuclear cells) were isolated from the peripheral blood of IDD patients and from healthy human volunteers as described previously and collected in heparinized tubes. Separation of human PBMCs was performed using density centrifugation with Lymphoprep (Fresenius Kabi Norge AS, Oslo, Norway). The study protocol were approved by the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong University, and informed written consents were obtained from all participants.
Culture of human NP cells
Human NP cells were purchased from ScienCell Research Laboratories (Carlsbad, CA, USA) and cultured in NP cell medium containing 10% fetal bovine serum (FBS) (GIBCO, NY, USA), 100 mg/mL streptomycin, 100 U/mL penicillin, and 1% NP cell growth supplement, and then incubated at 37°C in a humidified atmosphere with 95% (v/v) air and 5% (v/v) CO2. The medium was changed every two days.
Treatment of human NP cells
Human NP cells were transiently transfected with miR-146a mimic (pre-microRNA mimics, Applied Biosystems) using Lipofectamine plus (Invitrogen, Carlsbad, CA, USA), with control transfections lacking miR-146a. After 24 hours, cells were stimulated with LPS (10 ng/mL) in serum-free medium for 24 hours at 37°C under 5% CO2. Supernatants were collected 24 hours after the initiation of each treatment and prepared for either cell lysates or total RNA extraction followed by qRT-PCR analyses.
miR-146a quantitative RT-PCR
Total RNA was isolated from human PBMCs and human NP cells using the total RNA isolation system according to manufacturer recommendations. RT reactions were performed using the TaqMan microRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. All miRNA quantification data were normalized to 5S rRNA expression and mRNA quantification data were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Relative amounts of transcript were calculated using the comparative Ct method.
ELISA kits
Cellular supernatant IL-1β, IL-6, and TNF-α levels were determined by ELISA kits following the manufacturer’s instructions.
Real-time PCR
RT-PCR was performed using standard procedures with the QuantiTect SYBR Green PCR kit (Qiagen) in ABI PRISM 7900HT detection systems (Applied Biosystems). Sequences of primers used for qPCR are presented in Table 1. Expression levels of each gene were normalized to GAPDH and expressed as a fold of control.
Table 1.
Sequences of primers.
| Gene name | Forward primer | Reverse primer |
|---|---|---|
| miRNA-146a | 5′-GGGTGAGAACTGAATTCCA-3′ | 5′-CAGTGCGTGTCGTGGAGT-3′ |
| TRAF6 | 5′-GAGTTTGACCCACCTCTGGA-3′ | 5′-TTTCATTGTCAACTGGGCACT-3′ |
| NF-κB | 5′-GAGGTGTATTTCACGGGACC-3′ | 5′-GAAGTCCATGTCCGCAATGG-3′ |
| GAPDH | 5′-GCACCGTCAAGGCTGAGAAC-3′ | 5′-TGGTGAAGACGCCAGTGGA-3′ |
Western blot analysis
Western bolt analysis was performed as previously described [18]. In general, the cell lysates were centrifuged at 12,000 rpm for 15 minutes at 4°C, and the supernatants were collected to obtain the total proteins. A BCA protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA) was used to determine the protein concentration. Then, the proteins were resolved by 10% SDS-PAGE (10% (v/v) gel and transferred to PVDF membrane (Millipore, Billerica, MA, USA). The membrane was incubated with primary antibodies against TRAF6 (1:1,000), NF-κB (1:1,000), and β-actin (1:10,000) overnight at 4°C, and Western blot bands were visualized using Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific, Rockford, IL, USA).
Statistical analysis
The data were expressed as the means ±SEM. The number of independent experiments was represented by “n.” Multiple comparisons were performed using one-way ANOVA followed by Tukey’s multiple-comparison test, where p<0.05 was considered significant.
Results
Downregulation of miR-146a in the PBMCs
To investigate the potential role of miRNA-146 in IDD, we first measured the relative mRNA expression of miR-146a in PBMCs separated from IDD patients and control participants. As shown in Figure 1, obviously decreased mRNA levels of miR-146a were observed in PBMCs of patients with IDD in comparison to those in control participants.
Figure 1.
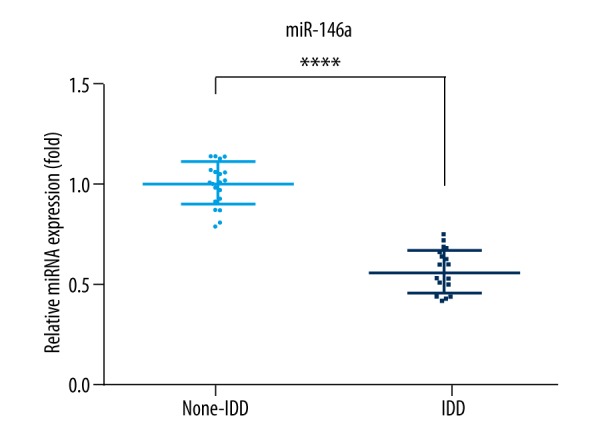
Quantitative reverse transcription polymerase chain reaction analysis of expression of miR-146a in PBMCs separated from patients with IDD and control participants. Values are the mean ±SD, n=21 for each group. * p<0.05, patients with IDD compared to control participants (non-IDD).
Overexpression of miR-146a decreased pro-inflammatory cytokine levels in LPS-stimulated NP cells
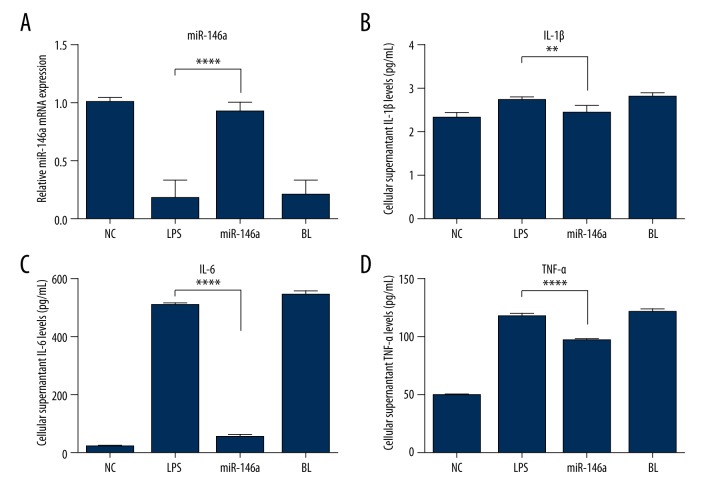
In order to evaluate the effects of miR-146a in IDD, we induced miR-146a expression using miR-146a mimic, while NC oligonucleotides were used as the control. NP cells were transfected with miR-146a mimic and NC oligonucleotides. Then miR-146a expression was analyzed using RT-PCR. As shown in Figure 2A, miR-146a expression was upregulated efficiently. Compared with the LPS group, the overexpression of miR-146a significantly decreased the levels of pro-inflammatory cytokines, including IL-1β, TNF-α, and IL-6 (Figure 2B–2D).
Figure 2.
Effects of miR-146a on pro-inflammatory cytokine levels in NP cells. (A) miR-146a mRNA expression was increased after mimic treatment. (B, C) Overexpression of miR-146a decreased IL-1β, IL-6, and TNF-α in LPS-stimulated NP cells. Values are the mean ±SD, n=4 for each group. * p<0.05, compared to LPS treated group.
Overexpression of miR-146a suppressed the activation of TRAF6/NF-κB in LPS-stimulated NP cells
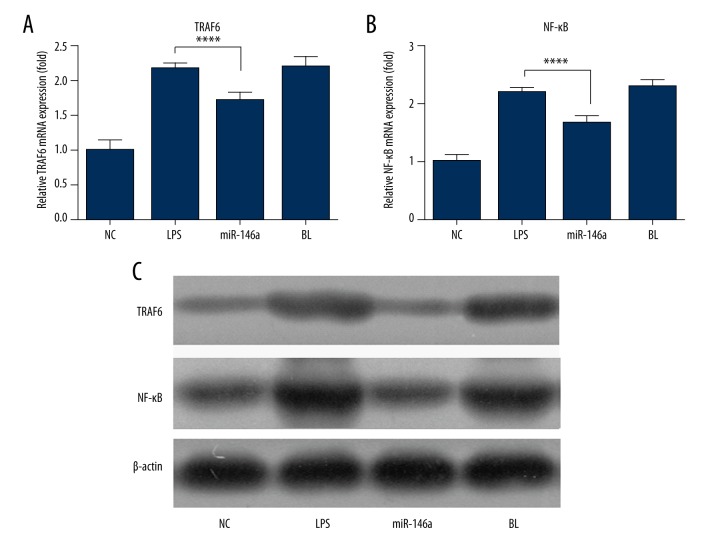
We next investigated whether miR-146a could regulate inflammation via the TRAF6/NF-κB pathway. As shown in Figure 3, overexpression of miR-146a significantly reduced the mRNA levels of TRAF6 and its downstream effector, NF-κB in LPS-stimulated NP cells, while NC oligonucleotides showed no significant effect on the expression of TRAF6/NF-κB in LPS-stimulated NP cells.
Figure 3.
Effects of miR-146a on mRNA (A, B) and protein (C) expression of TRAF6 and NF-κB in NP cells. Values are the mean ±SD, n=4 for each group. * p<0.05, compared to LPS treated group.
Discussion
Increasing evidence shows that miRNAs play an important role in many normal physiological processes and in the development of most diseases. In the present study, we found that miR-146a was significantly downregulated in PBMCs separated from patients and control subjects. Furthermore, overexpression of miR-146a greatly increased the levels of pro-inflammatory cytokines, including IL-1β, TNF-α and IL-6, in LPS-stimulated NP cells. In addition, miR-146a could regulate LPS-induced inflammation via the TRAF6/NF-κB pathway in NP cells.
MiR-146a has been reported to be implicated in multiple diseases, including age-related macular degeneration [19], sepsis [20], atherosclerosis [21], Alzheimer’s disease [22], and osteoarthritis [10]. However, expression of miR-146a and its role in IDD are still not clear. In this study, miR-146a levels were significantly decreased in PBMCs in patients with IDD. Decreased levels of miR-146a may be associated with the inflammation in intervertebral discs. Previous studies have shown that inflammatory processes exacerbated by cytokines TNF-α, IL-1β, and IL-6 are believed to be key mediators of disc degeneration and low back pain [23–25]. In the present study, we found that the overexpression of miR-146a markedly decreased pro-inflammatory cytokines levels in NP cells. These data suggest that increased pro-inflammatory cytokines levels induced by downregulation of miR-146a might participate in the development of IDD.
TRAF6, an essential factor that mediates receptor signaling in response to ligands of the TNFα superfamily, has been identified as the target of miR-146a. In the present study, overexpression of miR-146a led to the decrease of TRAF6 and its downstream NF-κB expression both in mRNA and in protein levels. It has been demonstrated that overexpression of miR-146 controls cytokine signaling through a negative feedback regulation loop by inhibiting TRAF6 expression and impairing NF-κB activity [12,26]. These findings are in agreement with previous work that showed that miR-146a negatively regulates TRAF6 activity and the inflammatory pathway in the spinal cord [15]. Therefore, these data indicate that overexpression of miR-146a might play an important role in IDD development, in part by suppressing TRAF6/NF-κB expression.
Conclusions
The results in the present study showed that miR-146a was decreased in the PBMCs of IDD patients compared to healthy controls. Moreover, overexpression of miR-146a significantly decreased the levels of pro-inflammatory cytokines by targeting the TARF6/NF-κB pathway, indicating a role for miR-146a in IDD. Furthermore, our findings suggest that overexpression of miR-146a might potentially be a therapeutic target in IDD.
Footnotes
Source of support: Departmental sources
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Morlion B. Chronic low back pain: pharmacological, interventional and surgical strategies. Nat Rev Neurol. 2013;9:462–73. doi: 10.1038/nrneurol.2013.130. [DOI] [PubMed] [Google Scholar]
- 2.Walker BF. The prevalence of low back pain: A systematic review of the literature from 1966 to 1998. J Spinal Disord Tech. 2000;13:205–17. doi: 10.1097/00002517-200006000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64:2028–37. doi: 10.1002/art.34347. [DOI] [PubMed] [Google Scholar]
- 4.Colombier P, Clouet J, Hamel O, et al. The lumbar intervertebral disc: From embryonic development to degeneration. Joint Bone Spine. 2014;81:125–29. doi: 10.1016/j.jbspin.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Ren S, Liu Y, Ma J, et al. Treatment of rabbit intervertebral disc degeneration with co-transfection by adeno-associated virus-mediated SOX9 and osteogenic protein-1 double genes in vivo. Int J Mol Med. 2013;32:1063–68. doi: 10.3892/ijmm.2013.1497. [DOI] [PubMed] [Google Scholar]
- 6.Van Wynsberghe PM, Chan SP, Slack FJ, Pasquinelli AE. Analysis of microRNA expression and function. Methods Cell Biol. 2011;106:219–52. doi: 10.1016/B978-0-12-544172-8.00008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Min W, Wang B, Li J, et al. The expression and significance of five types of miRNAs in breast cancer. Med Sci Monit Basic Res. 2014;20:97–104. doi: 10.12659/MSMBR.891246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu M, Zhang Q, Deng M, et al. An analysis of human microRNA and disease associations. PloS One. 2008;3:e3420. doi: 10.1371/journal.pone.0003420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang C, Wang W-J, Yan Y-G, et al. MicroRNAs: New players in intervertebral disc degeneration. Clin Chim Acta. 2015;450:333–41. doi: 10.1016/j.cca.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Gibson G, Kim J-S, et al. MicroRNA-146a is linked to pain-related pathophysiology of osteoarthritis. Gene. 2011;480:34–41. doi: 10.1016/j.gene.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Kroin JS, Kc R, et al. Altered apinal microRNA-146a and the microRNA-183 cluster contribute to osteoarthritic pain in knee joints. J Bone Miner Res. 2013;28:2512–22. doi: 10.1002/jbmr.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taganov KD, Boldin MP, Chang K-J, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–86. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao Z, Xiong J, Takeuchi M, et al. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–46. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 14.Ye H, Arron JR, Lamothe B, et al. Distinct molecular mechanism for initiating TRAF6 signalling. Nature. 2002;418:443–47. doi: 10.1038/nature00888. [DOI] [PubMed] [Google Scholar]
- 15.Lu Y, Cao D-L, Jiang B-C, et al. MicroRNA-146a-5p attenuates neuropathic pain via suppressing TRAF6 signaling in the spinal cord. Brain Behav Immun. 2015;49:119–29. doi: 10.1016/j.bbi.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–78. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 17.Williams AL, Haughton VM, Meyer GA, Ho KC. Computed tomographic appearance of the bulging annulus. Radiology. 1982;142:403–8. doi: 10.1148/radiology.142.2.7054829. [DOI] [PubMed] [Google Scholar]
- 18.Wang X-H, Hong X, Zhu L, et al. Tumor necrosis factor alpha promotes the proliferation of human nucleus pulposus cells via nuclear factor-κB, c-Jun N-terminal kinase, and p38 mitogen-activated protein kinase. Exp Biol Med. 2015;240:411–17. doi: 10.1177/1535370214554533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szemraj M, Bielecka-Kowalska A, Oszajca K, et al. Serum microRNAs as potential biomarkers of AMD. Med Sci Monit. 2015;21:2734–42. doi: 10.12659/MSM.893697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng HS, Sivachandran N, Lau A, et al. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. Embo Mol Med. 2013;5:1017–34. doi: 10.1002/emmm.201202318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo M, Mao X, Ji Q, et al. miR-146a in PBMCs modulates Th1 function in patients with acute coronary syndrome. Immunol Cell Biol. 2010;88:555–64. doi: 10.1038/icb.2010.16. [DOI] [PubMed] [Google Scholar]
- 22.Wang L-L, Huang Y, Wang G, Chen S-D. The potential role of microRNA-146 in Alzheimer’s disease: Biomarker or therapeutic target? Med Hypotheses. 2012;78:398–401. doi: 10.1016/j.mehy.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Johnson Z, Schoepflin Z, Choi H, et al. Disc in flames: Roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur Cell Mater. 2015;30:104–17. doi: 10.22203/ecm.v030a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang W, Yu X-H, Wang C, et al. Interleukin-1β in intervertebral disk degeneration. Clin Chim Acta. 2015;450:262–72. doi: 10.1016/j.cca.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat Rev Rheumatol. 2014;10:44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhaumik D, Scott G, Schokrpur S, et al. Expression of microRNA-146 suppresses NF-κB activity with reduction of metastatic potential in breast cancer cells. Oncogene. 2008;27:5643–47. doi: 10.1038/onc.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]