Abstract
The mammalian gut microbiota influences various metabolic and physiological processes. Substantial metabolic changes occur during a healthy pregnancy that may be related to microbiota composition dynamics. However, the effect of diet on intestinal microbiota composition and diversity during pregnancy remains unclear. We examined the ileal contents of Huanjiang mini-pigs at two pregnancy stages to determine the effects of dietary nutrient levels on such microbial communities. Animals received either a higher-nutrient (HN) diet formulated to meet US National Research Council requirements or a lower-nutrient (LN) diet that met the Chinese National Feeding Standard recommendations. On day 45 or 75 of pregnancy, sows were euthanized and their ileal contents sampled. Experimental diet and pregnancy stage did not affect ileal bacterial richness or diversity, as determined by Chao1 and ACE species richness measures and Shannon and Simpson indices, respectively. The phyla Firmicutes and Proteobacteria, accounting for 69.99–85.44% and 5.82–15.17% of the total reads, respectively, predominated regardless of diet. At the genus level, diet significantly affected the abundance of Lactobacillus species, which was greater in pigs given HN feed (P < 0.05), but had little impact on that of Megasphaera species (P = 0.096). Pregnancy stage had a minimal effect on Proteobacteria numbers (P = 0.053). The number of bacteria of the phylum Firmicutes and genus Lactobacillus decreased, while that of the phylum Proteobacteria, family Enterobacteriaceae, and genus Bacteroides increased between days 45 and 75 of pregnancy. Of the short-chain fatty acids (SCFAs) measured, only propionate levels changed significantly, with higher concentrations observed on day 45 than on day 75. Our findings indicate that Firmicutes and Proteobacteria dominate pregnant sow ileal bacterial profiles. Excepting a tendency for the number of Proteobacteria to increase as pregnancy progressed, pregnancy stage and diet had little effect on ileal microbiotic composition and diversity and luminal SCFA concentrations.
Introduction
Bacterial communities play a very important role in the biological transformation of organic matter from dietary and endogenous origins [1], and influence host metabolism and physiology [2,3]. The composition of the intestinal microbiota is affected by various factors, including the intestinal environment, nutritional and non-nutritional dietary components, and antibiotic use, among other factors [4]. For example, in mammals, proteins and their amino acid-derived metabolites can affect the relationship between the gut microbiota and intestinal mucosal morphology, metabolism, and physiology, depending on diet quality and quantity [5,6]. Surprisingly, little is known regarding the effects of pregnancy on microbial composition, diversity, and metabolic activity in the small and large intestines. In swine, these organs’ microbiotas are thought to influence health and performance. Therefore, microbial populations in various segments of the intestinal tract should be monitored. A previous study reported that colonic bacterial richness decreased in pregnant Huanjiang mini-pigs as gestational age increased. In addition, elevated nutrient levels heightened the production of metabolites related to nitrogen metabolism (Kong et al., unpublished data). The ileum contains a larger and more complex microbiota than the proximal sections of the small intestine (i.e., the duodenum and jejunum) [7]. Therefore, it is also important to determine changes in microbial community composition and diversity in the ileal contents of pregnant sows fed various diets.
Reproduction is clearly critical to animal farming, being the principal process for producing offspring and preserving genetic resources [8]. During a healthy pregnancy, substantial hormonal, immunological, and metabolic changes occur in the body [9]. The mammalian gut is inhabited by a complex micro-ecosystem. Moreover, recent work suggests that changes in its microbiota can cause metabolic diseases involving inflammation and obesity, and reduce insulin sensitivity [10], and pig models can be used to gain insight into such human diseases [11–13]. Gut bacterial load has been reported to increase during gestation, and microbial diversity may be modified during pregnancy [14]. Although it has been demonstrated that the gut microbiota can induce symptoms of metabolic syndrome in non-pregnant hosts, the consequences of modified host-microbiota interactions in pregnancy remain only partially characterized [15].
During pregnancy, it is common practice to maintain sows at restricted feeding levels, as excessive feeding in early pregnancy can lead to an increase in embryonic mortality [16]. In addition, increased energy intake during gestation increases the body fat content of sows, which may lead to a subsequent reduction in feed consumption during the lactation period, and cause various reproductive problems [17]. Our previous study showed that a higher-nutrient diet improves nutrient metabolism, promotes the growth and development of sows and their fetuses, and is not deleterious for reproductive performance and body composition (including fat ratio and muscle ratio) of pregnant Huanjiang mini-pigs [18]. In addition, Koren et al. [15] demonstrated that pregnancy was associated with profound alterations to the gut microbiota. Therefore, we hypothesized that the composition and richness of the ileal microbiota and its metabolic activity in pregnant sows might change according to dietary conditions and pregnancy stage. The present investigation was conducted to compare bacterial community composition and diversity in samples of ileal contents from Huanjiang mini-pigs fed diets with higher or lower nutrient levels from the mid- to early-late stages of pregnancy. We also analyzed levels of short-chain fatty acids (SCFAs) and branched-chain fatty acids (BCFAs), i.e., metabolites typically produced by intestinal bacteria, in the ilea of pregnant sows.
Materials and methods
Animals, diets, and treatments
This present study was carried out in accordance with the Chinese guidelines for animal welfare and experimental protocols, and was approved by the Animal Care and Use Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences.
Thirty-two primiparous Huanjiang mini-pigs with a mean body weight (BW) of 46.38 ± 6.08 kg were obtained from a mini-pig farm located in Jixiang town, Huanjiang county, Guangxi province, China (108°27'40.8" E, 25°9'50" N). These sows were randomly assigned to one of the two dietary groups post-service (16 sows per dietary group and two sows per pen). One group of sows was fed a diet with a higher nutrient level (HN), while the other received feed of a lower nutrient level (LN). The HN diet was formulated to meet the nutrient recommendations of the US National Research Council [19], and contained 14.73 MJ/kg digestible energy, 13.11% crude protein, and 4.56% crude fiber. This diet is widely used in commercial crossbreed pig farms. The LN diet was formulated according to the recommendations of the Chinese National Feeding Standard for Swine, and contained 12.24 MJ/kg digestible energy, 9.77% crude protein, and 6.86% crude fiber. The LN diet is commonly used in commercial Huanjiang mini-pig farms (Table 1).
Table 1. Ingredients and nutritional composition of the two experimental diets (air-dried basis, %).
| Ingredients1 | HN diet | LN diet | Nutrient level3 | HN diet | LN diet |
|---|---|---|---|---|---|
| Corn | 58.20 | 57.20 | Digestible energy (MJ/kg) | 14.50 | 12.20 |
| Soybean meal | 11.00 | 0.00 | Crude protein | 13.10 | 11.00 |
| Wheat bran | 11.50 | 11.00 | Crude protein/digestible energy | 0.90 | 0.90 |
| Rice bran | 4.00 | 13.00 | Crude fiber | 4.56 | 6.86 |
| Alfalfa meal | 3.00 | 14.00 | Ether extract | 9.34 | 5.00 |
| Soybean oil | 7.50 | 0.00 | Ca | 0.62 | 0.58 |
| Dicalcium phosphate | 1.15 | 1.15 | Total P | 0.52 | 0.44 |
| Limestone | 0.79 | 0.79 | Available P | 0.28 | 0.26 |
| Salt | 0.30 | 0.30 | Lys | 1.11 | 0.83 |
| Premix2 | 1.00 | 1.00 | Met + Cys | 0.65 | 0.52 |
| Lys | 0.88 | 0.88 | |||
| Met | 0.27 | 0.27 | |||
| Thr | 0.33 | 0.33 | |||
| Tyr | 0.08 | 0.08 |
HN, higher-nutrient; LN, lower-nutrient.
1 All dietary components except for the alfalfa meal were provided by a feed manufacturer (Guilin city, China), and all components were mashed and pelletized. Alfalfa meal was purchased from Gansu Tianmu Co., Ltd. (Lanzhou city, China).
2 Premix provided the following per kg of feed: vitamin A, 12,040 IU; vitamin D3, 2,112 IU; vitamin E, 29.7 IU; vitamin K3, 2.8 mg; vitamin B1, 1.2 mg; vitamin B2, 7.1 mg; vitamin B6, 1.3 mg; vitamin B12, 0.03 mg; nicotinic acid, 42.9 mg; pantothenic acid, 21.6 mg; folic acid, 0.44 mg; biotin, 0.12 mg; choline, 320 mg; Fe, 80 mg; Cu, 40 mg; Zn, 140 mg; Mn, 52 mg; I, 0.56 mg; Co, 1.4 mg; and Se, 0.33 mg.
3 Digestible energy, crude protein, Ca, total phosphorus, and available phosphorus are calculated values, while other factors are shown as measured values.
All animals were housed in 2 × 3 m pens with cement-sclerified flooring. Each pen was equipped with a feeder and a nippled drink dispenser. The room temperature was maintained at 22–28°C. All pigs had ad libitum access to drinking water and were fed twice daily (at 08:30 and 16:30, with approximately 2.5% of their BW) after service. All sows were checked twice daily throughout the experimental period to monitor food intake, amount of excreta, and any evidence of pain, distress, or unusual behavior.
Sample collection
Five and eight pregnant gilts in the HN and LN diet groups, respectively, were examined 45 days after service, and six pregnant gilts in both groups were tested 75 days after service. Initially, eight sows were included in each group, but owing to unsuccessful matings, the final group numbers differed. This was addressed in our statistical analysis.
In a report by Johnston and Trottier (1999), the early, middle, and late stages of pregnancy in pigs are defined as days 1 to 30, 30 to 75, and 75 to delivery, respectively [20]. Considering the size of Huanjiang mini-pigs and the difficulty of collecting conceptus samples to determine fetal development, we chose 45 and 75 days post-service to represent the middle and early-late stages of pregnancy, respectively.
Sows were euthanized for sample collection 12 h after the last feeding on day 45 or 75 post-service [12]. Briefly, general anesthesia was induced by intravenous injection of 4% sodium pentobarbital solution (40 mg/kg BW) and euthanasia carried out by exsanguination following severing of the carotid artery [21]. The ileum was then recovered and its luminal contents collected from a region 10 cm anterior to the ileocecal valve. These were stored at −80°C for subsequent analysis of gut microbial composition and SCFA concentrations.
Microbial DNA isolation and PCR amplification
Total bacterial DNA was extracted from ileal contents using a QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions. The DNA concentration of each extract was measured with a NanoDrop ND-1000 instrument (NanoDrop Technologies Inc., Wilmington, DE, USA). The 260/280 nm absorption ratio of all samples was between 1.8 and 2.0.
Bacterial community diversity and composition in each ileal sample was determined by high-throughput sequencing of microbial 16S rDNA genes. Using a previously published protocol [22], DNA was amplified by PCR with primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′), which target the V4 region of the 16S rRNA gene. The reverse primer contained a 6-bp error-correcting barcode unique to each sample. DNA samples were sent to a commercial service provider (Novogene, Beijing, China) for pyrosequencing on an Illumina MiSeq platform according to the manufacturer’s instructions. Raw data were obtained, before being screened and assembled using the QIIME [23] and FLASH [24] software packages. Sequencing reads were assigned to samples based on the barcodes. Reads flagged as chimeric were removed to form an “effective sequences” collection for each sample. The QIIME software package and UPARSE pipeline were used to analyze these effective sequences and determine operational taxonomic units (OTUs) [25]. Subsequently, the UCLUST algorithm [23] was employed to cluster sequences into OTUs with an identity threshold of 97%. Each OTU was assigned to a taxonomic level with RDP Classifier [26]. The sequences obtained in the present study were deposited in the National Center for Biotechnology Information Sequence Read Archive under accession numbers SRR4156412 to SRR4156415.
SCFA and BCFA analyses
Straight-chain fatty acids, namely acetate, propionate, butyrate, and valerate, and BCFAs, namely isobutyrate and isovalerate, were analyzed as described previously [27]. To ensure their homogeneity, intestinal samples were freeze-dried using a vacuum freeze-dryer (ALPHA 2-4/LSC; Martin Christ, Osterode am Harz, Germany) at −80°C. Our preliminary data indicate that freeze-drying has little effect on the concentration of organic acids in biological samples (S1 Table). Briefly, the freeze-dried samples (0.5–0.6 g) were placed in 10-mL centrifuge tubes, mixed with 8 mL double-distilled H2O, homogenized, and centrifuged in sealed tubes at 7,000 × g at 4°C for 10 min. The resulting supernatant (0.9 mL) was mixed with 0.1 mL 25% metaphosphoric acid solution in a sealed 2-mL tube, and left to stand at 4°C for over 2 h, before being centrifuged at 20,000 × g at 4°C for 10 min. The supernatant was then passed through a 0.45-μm polysulfone filter and analyzed on an Agilent 6890 gas chromatograph (Agilent Technologies, Inc., Palo Alto, CA, USA) connected to a flame ionization detector and a 1.82 m × 0.2 mm (length × internal diameter) glass column packed with 10% SP-1200/1% H3PO4 on 80/100 Chromosorb W/AW (HP, Inc., Boise, ID, USA).
Statistical analyses
Clustering and determination of alpha and beta diversity were performed in QIIME [23]. Apparent relative abundance at the phylum and genus levels, alpha diversity indices of bacterial communities, and ileal luminal SCFA concentrations were analyzed using a completely randomized design with a general linear model implemented in SAS (SAS Institute, Inc., Cary, NC, USA). Principal coordinate analysis (PCoA) of overall microbial community diversity based on an unweighted UniFrac metric was performed by the Bray-Curtis distance method to compare all samples. Rarefaction curves were created using Excel 2010 (Microsoft, Redmond, WA, USA). Phyla and genera with relative abundances below 0.5% in sows of both diet groups were excluded from further analysis. Differences were deemed statistically significant when associated with a P-value < 0.05. P < 0.10 was considered to indicate a trend toward significance.
Results
DNA sequence coverage and alpha diversity of bacteria from ileal contents
To assess the impact of diet and pregnancy stage on bacterial communities, sequences of the 16S rRNA gene V4 region were amplified. A total of 1,050,719 sequences (42,028.76 ± 2,520.12 per sample) were obtained, including 43,195, 42,229, 41,653, and 41,356 raw reads acquired from samples in the HN diet group on days 45 and 75 of pregnancy and those in the LN diet group at the same time points, respectively. After trimming, assembly, and quality filtering, 41,167, 40,116, 39,601, and 39,007 sequences from samples in these groups, respectively, were selected for further analysis. Considering all samples, sequence read number ranged from 32,169 to 43,472 per sample, with an average of 39,895. The average sequence read length after primer removal was 253 bp. A total of 31,460 effective sequences were extracted from each sample for comparisons at the same sequencing depth. Overall, 3,381 OTUs were detected according to a nucleotide sequence identity of 97% between reads (S2 Table).
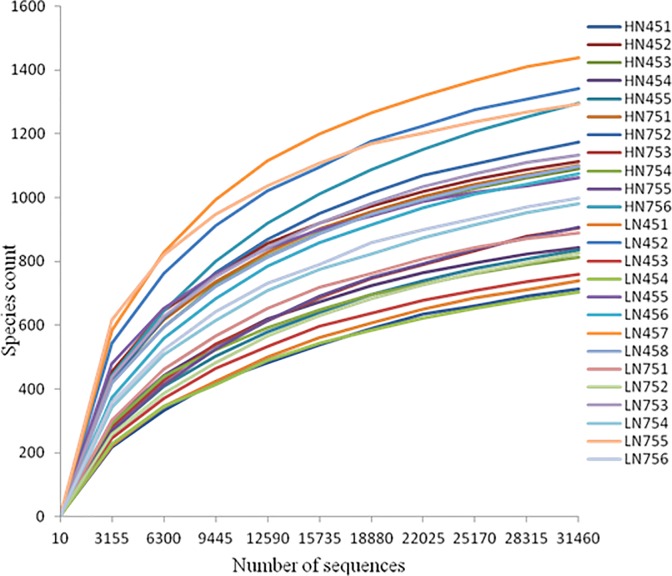
Based on normalized subsamples of 31,460 reads per sample, rarefaction curves showed that the selected sequences were sufficient to determine the majority of bacterial diversity parameters (Fig 1). Indices of community richness (Chao1 and ACE) and diversity (Shannon and Simpson indices), with cut-off values of 0.03, are shown in Table 2. None of these measures were significantly affected by diet or pregnancy stage, although a trend toward fewer OTUs in the later stage of pregnancy (P = 0.083) was observed (Table 2).
Fig 1. Rarefaction curves of bacterial species abundance in the ileal luminal contents of pregnant Huanjiang mini-pigs.
HN45 and HN75 represent samples obtained from Huanjiang mini-pigs fed a higher-nutrient diet for 45 and 75 days, respectively. LN45 and LN75 represent samples obtained from those fed a lower-nutrient diet for 45 and 75 days, respectively.
Table 2. Alpha diversity indices of ileal bacterial communities in Huanjiang mini-pigs at different stages of pregnancya.
| Items | HN45 | HN75 | LN45 | LN75 | SEM | P values | ||
|---|---|---|---|---|---|---|---|---|
| Diet | Stage | Diet × Stage | ||||||
| OTU | 1,107.80 | 1,247.00 | 1,223.38 | 1,208.00 | 44.84 | 0.690 | 0.521 | 0.424 |
| Chao1 | 1,073.19 | 1,232.02 | 1,198.90 | 1,159.29 | 44.22 | 0.778 | 0.527 | 0.297 |
| ACE | 1,110.08 | 1,279.08 | 1,224.47 | 1,265.04 | 42.8 | 0.578 | 0.251 | 0.478 |
| Shannon | 4.81 | 4.94 | 5.31 | 5.47 | 0.01 | 0.126 | 0.284 | 0.802 |
| Simpson | 0.85 | 0.87 | 0.88 | 0.91 | 0.21 | 0.256 | 0.739 | 0.971 |
| Coverage | 99.25 | 99.08 | 99.19 | 99.17 | 0.03 | 0.716 | 0.083 | 0.163 |
a Based on 31,460 reads. Here and in the following tables, HN45 and HN75 represent samples obtained from Huanjiang mini-pigs fed a higher-nutrient diet for 45 and 75 days, respectively, and LN45 and LN75 represent samples obtained from those fed a lower-nutrient diet for 45 and 75 days, respectively.
Bacterial community composition in ileal contents
In total, genetic material from 37 bacterial phyla was identified across all ileal samples. There were six phyla with a relative abundance greater than 0.5% in at least one experimental group: Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, Spirochaetes, and Tenericutes. Of these six phyla, Firmicutes predominated in all samples, with a relative abundance of 69.99–82.22%, followed by Proteobacteria, at 5.82–15.17%. From day 45 to 75 of pregnancy, Firmicutes abundance exhibited a decreasing trend (P = 0.069), whereas levels of Proteobacteria tended to increase (P = 0.053). Diet marginally affected the presence of Tenericutes (P = 0.079). However, as for the effect of pregnancy stage on Firmicutes and Proteobacteria, this change was not statistically significant (Table 3).
Table 3. Composition of ileal bacterial communities at the phylum level in Huanjiang mini-pigs at different pregnancy stages (%).
| Items | HN45 | HN75 | LN45 | LN75 | SEM | P values | ||
|---|---|---|---|---|---|---|---|---|
| Diet | Stage | Diet × Stage | ||||||
| Actinobacteria | 5.58 | 2.56 | 2.91 | 5.45 | 1.20 | 0.966 | 0.926 | 0.286 |
| Bacteroidetes | 3.08 | 3.05 | 4.97 | 8.06 | 1.28 | 0.205 | 0.568 | 0.562 |
| Firmicutes | 82.22 | 77.16 | 81.98 | 69.99 | 2.28 | 0.414 | 0.069 | 0.607 |
| Proteobacteria | 7.20 | 15.17 | 5.82 | 13.04 | 1.89 | 0.641 | 0.053 | 0.922 |
| Spirochaetes | 0.77 | 0.65 | 2.08 | 0.89 | 0.35 | 0.293 | 0.374 | 0.466 |
| Tenericutes | 0.49 | 0.55 | 0.82 | 1.48 | 0.18 | 0.079 | 0.306 | 0.388 |
| Unclassified bacteria | 0.15 | 0.18 | 0.26 | 0.24 | 0.03 | 0.242 | 0.920 | 0.711 |
| Other bacteria | 0.51 | 0.68 | 1.17 | 0.85 | 0.23 | 0.396 | 0.875 | 0.611 |
Of the 17 genera with a relative abundance greater than 0.5% in at least one of the experimental groups, only Lactobacillus was significantly affected by diet (P < 0.05). Sows fed the HN diet displayed higher numbers of sequences assigned to this genus than those given LN feed. A similar, though not statistically significant (P = 0.096), effect was observed in relation to Megasphaera. Both of these genera belong to the phylum Firmicutes. No statistically significant effect of pregnancy stage on the relative abundance of these bacteria was evident. The effect of the interaction between diet and pregnancy stage trended towards statistical significance for Pseudomonas (P = 0.080) and Sutterella (P = 0.084), members of the phylum Proteobacteria (Table 4).
Table 4. Composition of ileal bacterial communities at the genus level in Huanjiang mini-pigs at different stages of pregnancy (%).
| Phylum | Genus | HN45 | HN75 | LN45 | LN75 | SEM | P values | ||
|---|---|---|---|---|---|---|---|---|---|
| Diet | Stage | Diet × Stage | |||||||
| Actinobacteria | Bifidobacterium | 5.37 | 2.03 | 2.55 | 4.94 | 1.17 | 0.985 | 0.849 | 0.257 |
| Bacteroidetes | Prevotella | 0.65 | 0.53 | 0.78 | 1.49 | 0.24 | 0.292 | 0.567 | 0.413 |
| Firmicutes | Acidaminococcus | 0.86 | 0.03 | 0.02 | 0.01 | 0.16 | 0.194 | 0.204 | 0.211 |
| Allobaculum | 0.73 | 0.37 | 0.09 | 0.03 | 0.14 | 0.106 | 0.488 | 0.621 | |
| Clostridium | 11.00 | 13.40 | 21.75 | 18.27 | 2.74 | 0.181 | 0.925 | 0.607 | |
| Dialister | 0.54 | 0.04 | 0.02 | 0.02 | 0.09 | 0.127 | 0.167 | 0.169 | |
| Lactobacillus | 29.83 | 20.18 | 12.96 | 4.82 | 3.15 | 0.010 | 0.131 | 0.895 | |
| Megasphaera | 0.55 | 0.21 | 0.10 | 0.10 | 0.08 | 0.096 | 0.320 | 0.295 | |
| Oscillospira | 0.90 | 0.82 | 1.28 | 1.30 | 0.18 | 0.278 | 0.940 | 0.904 | |
| Roseburia | 0.46 | 0.40 | 0.68 | 0.30 | 0.11 | 0.788 | 0.357 | 0.507 | |
| Ruminococcus | 1.62 | 1.54 | 2.19 | 2.35 | 0.33 | 0.325 | 0.960 | 0.864 | |
| Turicibacter | 12.47 | 15.35 | 16.89 | 19.16 | 2.47 | 0.442 | 0.630 | 0.955 | |
| Proteobacteria | Acinetobacter | 0.18 | 0.45 | 0.20 | 2.14 | 0.39 | 0.281 | 0.170 | 0.290 |
| Escherichia | 0.24 | 0.82 | 0.13 | 0.40 | 0.13 | 0.335 | 0.123 | 0.574 | |
| Pseudomonas | 0.24 | 0.36 | 0.53 | 0.21 | 0.06 | 0.567 | 0.426 | 0.080 | |
| Sutterella | 1.48 | 0.20 | 0.76 | 1.40 | 0.26 | 0.659 | 0.553 | 0.084 | |
| Spirochaetes | Treponema | 0.76 | 0.64 | 2.06 | 0.88 | 0.35 | 0.297 | 0.375 | 0.471 |
| Unclassified bacteria | 27.90 | 37.54 | 31.94 | 36.90 | 2.64 | 0.759 | 0.197 | 0.673 | |
| Other bacteria | 4.23 | 5.10 | 5.07 | 5.28 | 0.49 | 0.630 | 0.612 | 0.757 | |
OTU diversity
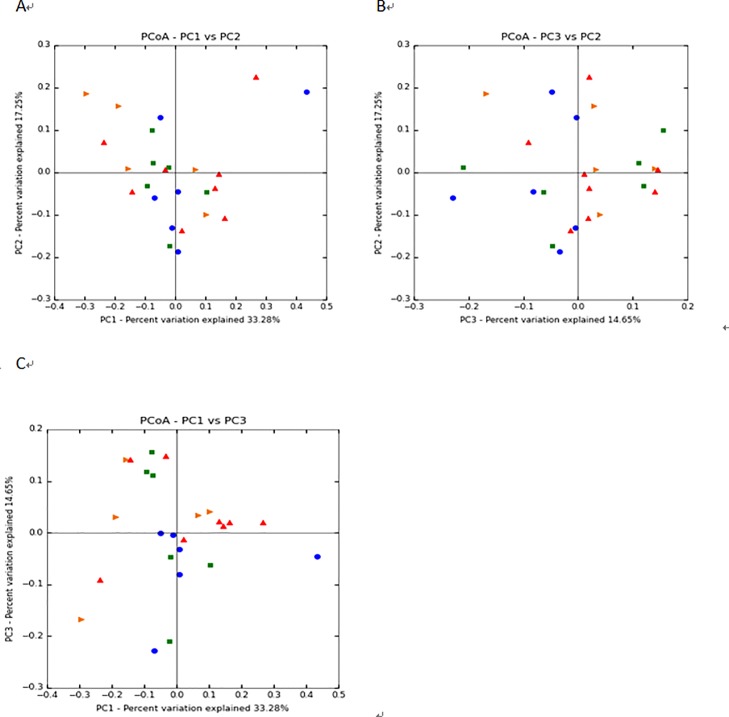
Fig 2 shows that the bacterial communities of sows in each diet group did not substantially differ. However, those sampled on day 75 of pregnancy demonstrated a greater degree of scatter compared with those obtained on day 45.
Fig 2. PCoA based on the UniFrac distance metric.
To evaluate similarities between bacterial communities, graphs A, B, and C were generated using OTUs, based on the UniFrac distance metrics PC1 and PC2, PC3 and PC2, and PC1 and PC3 (on a two-dimensional array), respectively. Samples from each treatment group are represented as follows: ►, HN45; ■, HN75; ▲, LN45; and ●, LN75. HN45 and HN75 represent samples obtained from Huanjiang mini-pigs fed the higher-nutrient diet for 45 and 75 days, respectively. LN45 and LN75 indicate samples obtained from those given the lower-nutrient diet for 45 and 75 days, respectively.
OTUs present at <0.5% in any samples were excluded from the analysis. Of those in the phylum Firmicutes, levels of OTU-2 and OTU-906 (genus: Lactobacillus), OTU-45 (species: Lactobacillus delbrueckii), and OTU-460 (genus Mitsuokella) were significantly altered by diet (P < 0.05). All of these organisms as well as OTU-29 (species Megasphaera elsdenii, P = 0.077) were more abundant in sows fed the HN diet than in those fed the LN diet. In contrast, the presence of OTU-4 (genus Clostridium, P = 0.078), OTU-38 (order Clostridiales, P = 0.053), and OTU-2219 (family Clostridiaceae, P = 0.091) in sows given the HN diet was lower than in those fed the LN diet. Concerning pregnancy stage, levels of OTU-45 (species Lactobacillus delbrueckii, P < 0.05), OTU-906 (genus Lactobacillus, P < 0.05), and OTU-28 (family Streptococcaceae, P = 0.051) tended to be higher on day 45 than on day 75. In addition, OTU-10 (family Enterobacteriaceae, P = 0.080) and OTU-184 (genus Bacteroides, P = 0.067) were less abundant on day 45 than on day 75 of pregnancy (Table 5). A significant effect of the interaction between diet and pregnancy stage was noted for OTU-45 (species Lactobacillus delbrueckii, P < 0.05) and OTU-906 (genus Lactobacillus, P < 0.05). This effect was also evident to a certain degree, close to reaching statistical significance, for OTU-460 (genus Mitsuokella, P = 0.089).
Table 5. Effect of diet and pregnancy stage on OTU levels (%) in the ileal bacterial communities of Huanjiang mini-pigs*.
| Items | HN45 | HN75 | LN45 | LN75 | SEM | P values | Annotation | Phylum | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Diet | Stage | Diet × Stage | ||||||||
| OTU-2 | 26.65 | 16.23 | 8.33 | 3.63 | 0.03 | 0.005 | 0.140 | 0.568 | g: Turicibacter | Firmicutes |
| OTU-4 | 6.36 | 5.37 | 11.94 | 10.63 | 0.02 | 0.078 | 0.697 | 0.957 | g: Clostridium | Firmicutes |
| OTU-10 | 3.43 | 10.67 | 2.32 | 7.57 | 0.02 | 0.541 | 0.080 | 0.773 | f: Enterobacteriaceae | Proteobacteria |
| OTU-28 | 0.29 | 0.05 | 0.27 | 0.06 | <0.01 | 0.972 | 0.051 | 0.903 | f: Streptococcaceae | Firmicutes |
| OTU-29 | 0.54 | 0.21 | 0.09 | 0.07 | <0.01 | 0.077 | 0.271 | 0.343 | s: Megasphaera elsdenii | Firmicutes |
| OTU-38 | 0.12 | 0.09 | 0.21 | 0.26 | <0.01 | 0.053 | 0.833 | 0.580 | o: Clostridiales | Firmicutes |
| OTU-45 | 0.65 | 0.17 | 0.08 | 0.05 | <0.01 | <0.01 | <0.01 | <0.01 | s: Lactobacillus delbrueckii | Firmicutes |
| OTU-184 | 0.04 | 0.23 | 0.09 | 0.24 | <0.01 | 0.744 | 0.067 | 0.869 | g: Bacteroides | Bacteroidetes |
| OTU-460 | 0.26 | 0.05 | 0.02 | 0.03 | <0.01 | 0.038 | 0.119 | 0.089 | g: Mitsuokella | Firmicutes |
| OTU-906 | 1.10 | 0.03 | 0.06 | 0.03 | <0.01 | 0.013 | 0.010 | 0.014 | g: Lactobacillus | Firmicutes |
| OTU-2219 | 0.78 | 0.99 | 1.45 | 1.56 | <0.01 | 0.091 | 0.649 | 0.897 | f: Clostridiaceae | Firmicutes |
*Only OTUs significantly affected by diet or pregnancy stage are shown. OTUs were present at ≥ 0.5% in all cases.
f: family; g: genus; o: order; s: species.
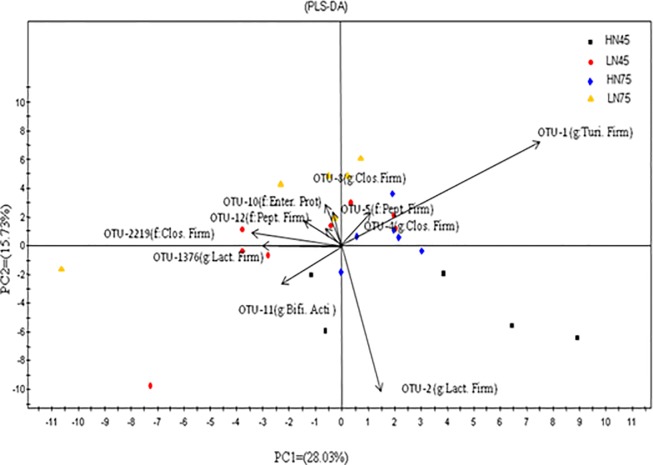
Principal component analysis at the genus level (Fig 3) revealed a tendency for each experimental group to form a distinct cluster, although some overlap was apparent. Ten dominant OTUs contributing to the variation between these groups were determined. OTU-1 and OTU-4, identified as Turicibacter spp. and Clostridium spp., respectively, were partly responsible for separating the HN diet group on day 75 of pregnancy from the other treatment groups. OTU-5 (family Peptostreptococcaceae), OTU-8 (genus Clostridium), OTU-10 (family Enterobacteriaceae), OTU-12 (family Peptostreptococcaceae), and OTU-2219 (family Clostridiaceae) partially distinguished the LN diet group on day 75 of pregnancy and the HN diet group on day 45 of pregnancy from the three other experimental groups. OTU-1376, OTU-11, and OTU-2 were identified as Lactobacillus spp., Bifidobacterium spp., and Lactobacillus spp., respectively. In addition, samples from the LN diet group on day 45 of pregnancy were more widely dispersed compared with those of other groups.
Fig 3. Principal component analysis of bacterial OTUs in ileal contents and the 10 predominant OTUs.
Partial least squares-discriminant analysis of bacterial OTUs in ileal contents, using diet and pregnancy stage as factors. The 10 OTUs principally responsible for the separation of samples are shown as follows: OTU-1, genus: Turicibacter, phylum: Firmicutes; OTU-2, genus: Lactobacillus, phylum: Firmicutes; OTU-4, genus: Clostridium, phylum: Firmicutes; OTU-5, family: Peptostreptococcaceae, phylum: Firmicutes; OTU-8, genus: Clostridium, phylum: Firmicutes; OTU-10, family Enterobacteriaceae, phylum: Proteobacteria; OTU-11, genus: Bifidobacterium, phylum: Actinobacteria; OTU-12, family: Peptostreptococcaceae, phylum: Firmicutes; OTU-1376, genus Lactobacillus, phylum: Firmicutes; OTU-2219, family: Clostridiaceae, phylum: Firmicutes.
Concentrations of SCFA and BCFA in ileal contents
The total SCFA concentration was not significantly affected by diet. Of the individual straight-chain fatty acids and BCFAs tested, only propionate was influenced by any of the experimental factors, its concentration being significantly higher (P < 0.05) on day 45 of pregnancy than on day 75 (Table 6).
Table 6. Effect of diet and pregnancy stage on ileal SCFA and BCFA concentrations (mg/g) in Huanjiang mini-pigs.
| Items | HN45 | HN75 | LN45 | LN75 | SEM | P values | ||
|---|---|---|---|---|---|---|---|---|
| Diet | Stage | Diet × Stage | ||||||
| Acetate | 2.97 | 2.61 | 2.59 | 2.05 | 0.441 | 0.295 | 0.311 | 0.838 |
| Propionate | 0.31 | 0.16 | 0.24 | 0.17 | 0.134 | 0.429 | 0.016 | 0.339 |
| Isobutyrate | 0.06 | 0.06 | 0.06 | 0.05 | 0.076 | 0.757 | 0.991 | 0.740 |
| Butyrate | 0.39 | 0.33 | 0.23 | 0.17 | 0.184 | 0.055 | 0.482 | 0.993 |
| Isovalerate | 0.11 | 0.09 | 0.10 | 0.09 | 0.094 | 0.864 | 0.537 | 0.989 |
| Valerate | 0.05 | 0.03 | 0.02 | 0.02 | 0.064 | 0.062 | 0.453 | 0.241 |
| A/P | 11.67 | 15.44 | 11.79 | 12.47 | 1.091 | 0.600 | 0.416 | 0.569 |
| BCFA | 0.16 | 0.15 | 0.16 | 0.14 | 0.119 | 0.810 | 0.698 | 0.887 |
| Straight-chain fatty acids | 3.72 | 3.13 | 3.08 | 2.41 | 0.487 | 0.212 | 0.248 | 0.940 |
| BCFA/Straight-chain fatty acids | 0.04 | 0.06 | 0.05 | 0.06 | 0.068 | 0.961 | 0.218 | 0.675 |
| Total SCFAs | 3.88 | 3.29 | 3.24 | 2.56 | 0.493 | 0.225 | 0.257 | 0.936 |
A/P: acetate/propionate. BCFAs comprised isobutyrate and isovalerate; straight-chain fatty acids comprised acetate, propionate, butyrate, and valerate.
Discussion
Owing to the potentially important roles of the intestinal microbiota in swine health and growth performance, its composition and metabolic activities in various physiological and nutritional contexts deserve close attention [28]. Changes in the composition of the intestinal (including bacterial profiles of the digesta and mucosa) and fecal microbiota have been demonstrated in pigs from birth to finishing phases [29–30] and in pregnant animals [31]. To the best of our knowledge, the present study represents the first analysis of changes in the ileal luminal bacterial profiles of Huanjiang mini-pigs.
In this study, on average, 39,895 effective reads were obtained for each sample, with high coverage (>99.08%). In general, alpha diversity indices were not influenced by diet or pregnancy stage. Coverage was marginally lower on day 45 of pregnancy compared to day 75. However, this difference was not statistically significant. The PCoA of overall diversity indicated that differences among individual pigs became greater as gestational age increased, as did indices of alpha diversity. The ileal microbial communities of pregnant Huanjiang mini-pigs were dominated by Firmicutes (69.99–82.22% of the total microbial content) and Proteobacteria (5.82–15.17%). This observation was consistent with the findings of Isaacson and Kim [32]. In the present study, Clostridium (11.00–21.75%), Lactobacillus (4.82–29.83%), and Turicibacter (12.47–19.16%) were the dominant bacterial genera in the ileal contents of Huanjiang mini-pigs, in accordance with previous surveys of the porcine ileal digesta- and mucosa-associated microbiota [33]. Collectively, these data support the assertion that Firmicutes constitutes the dominant phylum in the gut microbiota of mammals, including mice and humans [34].
Lactobacillus species are known for their potentially beneficial effects on gut function and health [35]. In our experiments, species of this genus were relatively more common in sows fed the HN diet (containing 11% soybean meal, a highly digestible plant protein source), which is consistent with a previous report that pigs fed normal levels of protein exhibit a greater abundance of Lactobacillus in the cecum compared with those given lower-protein feed [36]. Our previous in vitro studies indicated that soybean oligosaccharides (SBOS), major bioactive components of soybean meal, can be selectively fermented by commensal bacteria present in the colon, thus improving gut microbiota balance and modulating metabolism [37]. Dietary SBOS supplementation increases SCFAs, but decreases protein-derived catabolites in the intestinal luminal contents of weaned Huanjiang mini-piglets, which may have beneficial effects on the gut [27]. In addition, our results agree with a previously published report describing elevated numbers of lactobacilli in the ilea of pigs given a barley-based diet compared to those fed primarily on corn [28]. At the OTU level, the presence of OTU-45 (species: Lactobacillus delbrueckii) and OTU-906 (genus: Lactobacillus) decreased from day 45 to day 75 of pregnancy. Lactobacillus species have been associated with weight change in humans and animals [38]. However, the mechanism by which these microbes induce body weight loss or gain remains unclear.
Regarding other Firmicutes taxa, Megasphaera abundance was slightly lower in pigs fed the HN diet than in those given LN feed. Overall, our findings are similar to those of Pedersen et al. [39], who found bacteria of this phylum to be more abundant in the terminal ilea of obese pigs. Moreover, excess energy intake, obesity, and glucose intolerance are associated with increased presence of Firmicutes in humans [40,41].
In the present study, OTU-1, OTU-2, OTU-4, OTU-5, OTU-8, OTU-12, and OTU-1376 were among the 10 OTUs whose relative abundances distinguished, to a certain extent, species composition under different experimental conditions, i.e., diet group and pregnancy stage. All of these belonged to the phylum Firmicutes. In addition, considering it as a single indicator, this phylum was more affected by pregnancy stage than by diet.
Representatives of the phylum Proteobacteria were significantly less abundant than those of Firmicutes. Proteobacteria presence tended to be increased in the later stage of pregnancy (day 75). Koren et al. [15] showed that the relative abundance of Proteobacteria in fecal samples from pregnant women is higher in the third trimester of pregnancy than in the first. Moreover, a significant increase in the abundance of Proteobacteria has been associated with gastrointestinal inflammation in response to environmental and genetic factors [42], as observed in inflammation-associated dysbioses [43]. Shin et al. reported that members of Proteobacteria constitute a microbial signature of gut microbiota dysbiosis [44]. In the present study, this phylum was prominent and primarily represented by the genus Sutterella (0.20–1.48%). The abundance of OTU-10, a family (Enterobacteriaceae) within Proteobacteria, was also higher on day 75 than on day 45 of pregnancy. Several studies reported that active inflammatory bowel disease is associated with significantly elevated levels of Proteobacteria (members of Enterobacteriaceae in particular) [45]. Therefore, our results are compatible with suggestions that the structure and composition of bacterial communities in pregnant hosts are reminiscent of disease-associated dysbiosis.
Previous investigations indicated an association between raised Firmicutes/Bacteroidetes ratios and obesity [46]. Clostridium bacteria are also suspected to play a role in energy harvesting because they are found at higher levels in obese individuals than in people with low body weights [35]. Our prior study showed that the average back-fat thickness of pregnant Huanjiang mini-pigs in both HN and LN diet groups increases from day 45 (27.20 and 26.90 mm, respectively) to day 75 (36.60 and 28.10 mm, respectively) post-service [18]. The live body weights of sows in these treatment groups also increase over this period, from 73.82 and 67.52 kg to 86.14 and 75.28 kg, respectively (Kong et al., unpublished data). These data suggest that the sows became obese during pregnancy, especially those in the HN group. In the present work, the level of Firmicutes tended to be lower on day 75 than on day 45 of pregnancy. Bacteroidetes levels were stable between the two measured time points in the HN diet group, but were higher during the later stage of pregnancy in sows fed the LN diet. Therefore, the Firmicutes/Bacteroidetes ratio was lower on day 75 than on day 45 of pregnancy in both diet groups.
The presence of Prevotella species positively correlates with the proportion of carbohydrates in the diet. In a previously published study in which samples were clustered according to the prevalence of dietary components, representatives of this genus were found to be more abundant in a “carbohydrate” than in a “fat-protein” cluster [47]. In the current investigation, ileal levels of OTU-184 (genus: Bacteroides) tended to be higher on day 75 than on day 45 of pregnancy in Huanjiang mini-pigs. Bacteria of the genus Prevotella were more abundant in pigs fed the LN diet, whereas pregnancy stage did not significantly affect their numbers. This is consistent with observations of elevated Prevotella levels in goats fed a diet with reduced grain content [48]. In summary, the ratio of Bacteroides to Prevotella was less affected by the LN diet than by the HN diet. The abundance of Proteobacteria and Bacteroidetes in the ileal contents samples was higher on day 75 than on day 45 of pregnancy, suggesting an increase in these bacteria between the first and second trimesters.
Together, the results of the present study showed that bacteria of the phylum Firmicutes and genus Lactobacillus decreased in number, while those of the phylum Proteobacteria, family Enterobacteriaceae, and genus Bacteroides increased from day 45 to 75 of pregnancy. These changes in the gut microbiota are similar to those observed in inflammatory bowel disease, during which, the numbers of several species within Firmicutes are reduced, including those of Lactobacillus. Moreover, Enterobacteriaceae is among the Proteobacteria families whose levels appear to be consistently increased in this condition [49,50].
SCFAs are produced by the microbiota of the large intestine from both indigestible carbohydrates [51] and certain amino acids originating from partially digested dietary and endogenous proteins [52]. SCFAs regulate colonic physiology, metabolism, and gene expression [53]. They are also produced in the small intestine, but at concentrations lower than those in the colon, with the exception of acetate [5]. These molecules are produced via fermentation of indigestible polysaccharides by saccharolytic bacteria [54]. For example, species of Ruminococcus, Oscillospira, Clostridium, and Pseudobutyrivibrio metabolize fiber, while those of Prevotella metabolize hemicellulose, producing acetate and propionate [55]. In our study, the concentration of propionate in the ilea of sows was higher on day 45 than day 75 of pregnancy, which may be related to significant changes in Bacteroides abundance during this period. Notably, propionate is known to inhibit the synthesis of lipids from acetate [56].
In the large intestine, concentrations of BCFAs, bacterial metabolites produced exclusively from amino acids, are generally lower than those of SCFAs [57]. BCFAs such as isobutyrate and isovalerate are breakdown products of fermentation by proteolytic bacteria, including members of Bacteroides and Clostridium [58]. Various Clostridium, Bacillus, Lactobacillus, and Streptococcus species as well as many Proteobacteria species play major roles in the utilization of amino acids by their hosts [59].
Conclusions
The ileal bacterial profiles of Huanjiang mini-pigs were dominated primarily by Firmicutes and Proteobacteria, and, in particular, representatives of the genera Lactobacillus, Clostridium, and Turicibacter. The effects of varying animal feed on the composition and diversity of the large intestinal microbiota also need to be elucidated, considering their potential importance. The HN diet was associated with a higher Lactobacillus abundance in pregnant Huanjiang mini-pigs. Since this diet differs from the LN diet in various characteristics (more digestible energy, higher relative protein content, and lower relative crude fiber content), it was not feasible in the current study to identify one particular dietary component associated with modifications in bacterial communities. In addition, the rice bran fiber and alfalfa used in both diets, but in inverse proportions, are characterized by different fiber type compositions [60,61]. Members of the phylum Firmicutes and genus Lactobacillus decreased, while those of the phylum Proteobacteria, family Enterobacteriaceae, and genus Bacteroides increased in number from day 45 to 75 of pregnancy. Notably, changes in bacterial community structure (e.g., increased number of Proteobacteria) as pregnancy progressed were similar to those observed in disease-associated dysbiosis (e.g., morbid obesity), indicating the need for further studies on a possible causal link between these parameters.
Supporting information
(DOC)
(DOC)
Acknowledgments
The present work was jointly supported by grants from the “Western Light” Key Program for Talent Cultivation from the Chinese Academy of Sciences, National Nature Science Foundation of China (31270044, 31572421), National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2012BAC17B0102), and Chinese Academy of Sciences Visiting Professorship for Senior International Scientists (F. Blachier, 2013T2S0014). The authors thank Professor Francois Blachier from UMR PNCA, AgroParisTech, INRA, Université Paris-Saclay, Paris, France for his helpful reviewing on the manuscript. All authors have read and approved the final version of the manuscript and have declared that no competing interests exist.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The present work was jointly supported by grants from the “Western Light” key Program for Talent Cultivation from Chinese Academy of Sciences, National Nature Science Foundation of China (31270044, 31572421), National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2012BAC17B0102, 2014BAD08B06), and Chinese Academy of Sciences Visiting Professorship for Senior International Scientists (F. Blachier, 2013T2S0014).
References
- 1.Drury B, Rosi-Marshall E, Kelly JJ (2013) Wastewater treatment effluent reduces the abundance and diversity of benthic bacterial communities in urban and suburban rivers. Appl Environ Microb 79(6): 1897–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richards JD, Gong J, de Lange CFM (2005) The gastrointestinal microbiota and its role in monogastric nutrition and health with an emphasis on pigs: Current understanding, possible modulations, and new technologies for ecological studies. Can J Anim Sci 85(4): 421–435. [Google Scholar]
- 3.Nielsen S, Nielsen DS, Lauritzen L, Jakobsen M, Michaelsen KF (2007) Impact of diet on the intestinal microbiota in 10-month-old infants. J Pediatr Gastr Nutr 44(5): 613–618. [DOI] [PubMed] [Google Scholar]
- 4.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, et al. (2006) Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118(2): 511–521. 10.1542/peds.2005-2824 [DOI] [PubMed] [Google Scholar]
- 5.Boudry G, Jamin A, Chatelais L, Gras-Le Guen C, Michel C, et al. (2013) Dietary protein excess during neonatal life alters colonic microbiota and mucosal response to inflammatory mediators later in life in femal pigs. J Nutr 143(8): 1225–1232. 10.3945/jn.113.175828 [DOI] [PubMed] [Google Scholar]
- 6.Lan A, Andriamihaja M, Blouin JM, Liu X, Descatoire V, et al. (2015) High-protein diet differently modifies intestinal goblet cell characteristics and mucosal cytokine expression in ileum and colon. J Nutr Biochem 26(1): 91–98. 10.1016/j.jnutbio.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 7.Knarreborg A, Simon MA, Engberg RM, Jensen BB, Tannock GW (2002) Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages. Appl Environ Microb 68(12): 5918–5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai Z, Wu Z, Hang S, Zhu W, Wu G (2015) Amino acid metabolism in intestinalbacteria and its potential implications for mammalian reproduction. Mol Hum Reprod 21(5): 389–409. 10.1093/molehr/gav003 [DOI] [PubMed] [Google Scholar]
- 9.Newbern D, Freemark M (2011) Placental hormones and the control of maternal metabolism and fetal growth. Curr Opin Endocrinol Diabetes Obes 18(6): 409–416. 10.1097/MED.0b013e32834c800d [DOI] [PubMed] [Google Scholar]
- 10.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, et al. (2010) Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328(5975): 228–231. 10.1126/science.1179721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawaguchi H, Miyoshi N, Miura N, Fujiki M, Horiuchi M, et al. (2011) Microminipig, a nonrodent experimental animal optimized for life science research: novel atherosclerosis model induced by high fat and cholesterol diet. J Pharmacol Sci 115(2): 115–121. [DOI] [PubMed] [Google Scholar]
- 12.Liu YY, Li FN, He LY, Tan BE, Deng JP, et al. (2015) Dietary protein intake affects expression of genes for lipid metabolism in porcine skeletal muscle in a genotype-dependent manner. Brit J Nutr 113: 1069–1077. 10.1017/S0007114514004310 [DOI] [PubMed] [Google Scholar]
- 13.Liu YY, Li FN, Kong XF, Tan BE, Li YH, et al. (2015) Signaling pathways related to protein synthesis and amino acid concentration in pig skeletal muscles depend on the dietary protein level, strain and developmental stages. PLoS ONE 10(9): e0138277 10.1371/journal.pone.0138277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collado MC, Isolauri E, Laitinen K, Salminen S (2008) Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: a prospective follow-up study initiated in early pregnancy. Am J Clin Nutr 92: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 15.Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, et al. (2012) Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150(3): 470–480. 10.1016/j.cell.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jindal R, Cosgrove JR, Aherne FX, Foxcroft GR (1996) Effect of nutrition on embryonal mortality in gilts: Association with progesterone. J Anim Sci 74(3): 620–624. [DOI] [PubMed] [Google Scholar]
- 17.Weldon WC, Lewis AJ, Louis GF, Kovar JL, Giesemann MA, et al. (1994) Postpartum hypophagia in primiparous sows: I. Effects of gestation feeding level on feed intake, feeding behavior, and plasma metabolite concentrations during lactation. J Anim Sci 72(2): 387–394. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Q, Ji YJ, Li HW, Guo QP, Kong XF (2016) Effects of diets with high- or low-nutrient level on reproductive performance, body composition, and plasma biochemical parameters in pregnant huanjiang mini-Pigs. Chin J Anim Nutr 28(5): 1534–1540. [Google Scholar]
- 19.NRC (1998) Nutrient Requirements of Swine. 10th ed. Washington, DC: National Academy Press; 1–212. [Google Scholar]
- 20.Johnston L, Trottier N (1999) Nutritional methods to improve sow productivity examined. Feedstuffs 10: 12–17. [Google Scholar]
- 21.Kong XF, Yin YL, Wu GY, Liu HJ, Yin FG, et al. (2007) Dietary supplementation with Acanthopanax senticosus extract modulates cellular and humoral immunity in weaned piglets. Asian-Aust J Anim Sci 20(9): 1453–1461. [Google Scholar]
- 22.Qin JJ, Li RQ, Raes J, Arumugam M, Burgdorf KS, et al. (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464(7285): 59–65. 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, et al. (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5): 335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magoc T, Salzberg SL (2011) FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27(21): 2957–2963. 10.1093/bioinformatics/btr507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10(10): 996–998. 10.1038/nmeth.2604 [DOI] [PubMed] [Google Scholar]
- 26.Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microb 73(16): 5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou XL, Kong XF, Lian GQ, Blachier F, Geng MM, et al. (2014) Dietary supplementation with soybean oligosaccharidesincreases short-chain fatty acids but decreases protein-derived catabolites in the intestinal luminal contents of weaned Huanjing mini-piglets. Nutr Res 34(9): 780–788. 10.1016/j.nutres.2014.08.008 [DOI] [PubMed] [Google Scholar]
- 28.Hill JE, Hemmingsen SM, Goldade BG, Dumonceaux TJ, Klassen J, et al. (2005) Comparison of ileum microflora of pigs fed corn-, wheat-, or barley-based diets by chaperonin-60 sequencing and quantitative PCR. Appl Environ Microb 71(2): 867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konstantinov SR, Awati AA, Williams BA, Miller BG, Jones P, et al. (2006) Post-natal development of the porcine microbiota composition and activities. Environ Microbiol 8(7): 1191–1199. 10.1111/j.1462-2920.2006.01009.x [DOI] [PubMed] [Google Scholar]
- 30.Kim HB, Borewicz K, White BA, Singer RS, Sreevatsan S, et al. (2011) Longitudinal investigation of the age-related bacterial diversity in the feces of commercial pigs. Vet Microbiol 153: 124–133. 10.1016/j.vetmic.2011.05.021 [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Nguyen SG, Guevarra RB, Lee I, Unno T (2015) Analysis of swine fecal microbiota at various growth stages. Arch Microbiol 197(6): 753–759. 10.1007/s00203-015-1108-1 [DOI] [PubMed] [Google Scholar]
- 32.Isaacson R, Kim HB (2012) The intestinal microbiome of the pig. Anim Health Res Rev 13(1): 100–109. 10.1017/S1466252312000084 [DOI] [PubMed] [Google Scholar]
- 33.Rettedal E, Vilain S, Lindblom S, Lehnert K, Scofield C, et al. (2009) Alteration of the ileal microbiota of weanling piglets by the growth-promoting antibiotic chlortetracycline. Appl Environ Microb 75(17): 5489–5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, et al. (2008) Evolution of mammals and their gut microbes. Science 320(5883): 1647–1651. 10.1126/science.1155725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konstantinov SR, Favier CF, Zhu WY, Williams BA, Klüss J, et al. (2004) Microbial diversity studies of the porcine gastrointestinal ecosystem during the weaning transition. Anim Res 54(4): 317–324. [Google Scholar]
- 36.Zhou LZ, Fang LD, Sun Y, Su Y, Zhu WY (2016) Efects of the dietary protein level on the microbial compositon and metabolomic profile in the hingut of the pig. Anaerobe 38: 61–69. 10.1016/j.anaerobe.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 37.Zhou XL, Kong XF, Yang XJ, Yin YL (2012) Soybean oligosaccharides alter colon short-chain fatty acid production and microbial population in vitro. J Anim Sci 90: 37–39. [DOI] [PubMed] [Google Scholar]
- 38.Million M, Angelakis E, Paul M, Armougom F, Leibovici L, et al. (2012) Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Micro Pathog 53(2): 100–108. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen R, Andersen AD, Hermann-Bank ML, Stagsted J, Boye M (2013) The effect of high-fat diet on the compositon of the gut microbiota in cloned and non-cloned pigs of lean and obese phenotype. Gut Microbes 4(5): 371–381. 10.4161/gmic.26108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, et al. (2011) Energy-balance studied reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr 94(1): 58–65. 10.3945/ajcn.110.010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, et al. (2013) Richness of human gut microbiome correlates with metabolic markers. Nature 500(7464): 541–546. 10.1038/nature12506 [DOI] [PubMed] [Google Scholar]
- 42.Carvalho FA, Koren O, Goodrich JK, Johansson ME, Nalbantoglu I, et al. (2012) Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe 122: 139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukhopadhya I, Hansen R, El-Omar EM, Hold GL (2012) IBD-what role do Proteobacteria play? Nat Rev Gastro Hepat 9(4): 219–230. [DOI] [PubMed] [Google Scholar]
- 44.Shin NR, Whon TW, Bae JW (2015) Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 33(9): 496–503. 10.1016/j.tibtech.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 45.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, et al. (2007) Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. P Natl Acad Sci USA 104(34): 13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsai F, Coyle WJ (2009) The microbiome and obesity: is obesity linked to our gut flora? Curr Gastroent Rep 11(4): 307–313. [DOI] [PubMed] [Google Scholar]
- 47.Egshatyan LV, Kashtanova DA, Popenko AS, Tkacheva ON, Tyakht A, et al. (2016) Gut microbiota and diet in patients with different glucose tolerance. Endocr Connect 5(1): 1–9. 10.1530/EC-15-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huo WJ, Zhu WY, Mao SY (2014) Impact of subacute ruminal acidosis on the diversity of liquid and solid associated bacteria in the rumen of goats. World J Microbiol Biot 30(2): 669–680. [DOI] [PubMed] [Google Scholar]
- 49.Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, et al. (2012) Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 13: R79 10.1186/gb-2012-13-9-r79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Basson A, Trotter A, Rodriguez-Palacios A, Cominelli F (2016) Mucosal interactions between genetics, diet, and microbiome in inflammatory bowel disease. Front Immunol 7: 290 10.3389/fimmu.2016.00290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ (2008) Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 27(2): 104–119. 10.1111/j.1365-2036.2007.03562.x [DOI] [PubMed] [Google Scholar]
- 52.Davila AM, Blachier F, Gotteland M, Andriamihaja M, Benetti PH, et al. (2013) Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host. Pharmacol Res 68(1): 95–107. 10.1016/j.phrs.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 53.Thibault R, Blachier F, Darcy-Vrillon B, de Coppet P, Bourreille A et al. (2010) Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm Bowel Dis 16(4): 684–695. 10.1002/ibd.21108 [DOI] [PubMed] [Google Scholar]
- 54.Macfarlane S, Macfarlane GT (2003) Regulation of short-chain fatty acid production. P Nutr Soc 62(1): 67–72. [DOI] [PubMed] [Google Scholar]
- 55.Salyers AA (1984) Bacteroides of the human lower intestinal tract. Annu Rev Microbiol 38: 293–313. 10.1146/annurev.mi.38.100184.001453 [DOI] [PubMed] [Google Scholar]
- 56.Nadal I, Santacruz A, Marcos A, Warnberg J, Garagorri JM, et al. (2009) Shifts in Clostridia, Bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. Int J Obes (Lond) 33(7): 758–767. [DOI] [PubMed] [Google Scholar]
- 57.Andriamihaja M, Davila AM, Eklou-Lawson M, Petit N, Delpal S, et al. (2010) Colon luminal content and epithelial cell morphology are markedly modified in rats fed with a high-protein diet. Am J Physiol Gastrointest Liver Physiol 299(5): G1030–G1037. 10.1152/ajpgi.00149.2010 [DOI] [PubMed] [Google Scholar]
- 58.Blachier F, Mariotti F, Huneau JF, Tomé D (2007) Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 33(4): 547–562. 10.1007/s00726-006-0477-9 [DOI] [PubMed] [Google Scholar]
- 59.Booijink CCGM (2009) Analysis of diversity and function of the human small intestinal microbiota. Wageningen University, Wageningen, the Netherlands. [Google Scholar]
- 60.Sera N, Morita K, Nagasoe M, Tokieda H, Kitaura T, Tokiwa H (2005) Binding effect of polychlorinated compounds and environmental carcinogens on rice bran fiber. J Nutr Biochem 16: 50–58. 10.1016/j.jnutbio.2004.09.005 [DOI] [PubMed] [Google Scholar]
- 61.Ai J, Tschirner U (2010) Fiber length and pulping characteristics of switchgrass, alfalfa stems, hybrid poplar and willow biomasses. Bioresour Technol 101: 215–221. 10.1016/j.biortech.2009.07.090 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.