Abstract
Rationale and Objectives
Severe progressive multi-focal heterotopic ossification (HO) is a rare occurrence seen predominantly in patients who have fibrodysplasia ossificans progressiva (FOP) and is difficult to quantitate due to patient-, disease-, logistical-, and radiation-related issues. The purpose of this study was to develop and validate a scoring system based on plain radiographs for quantitative assessment of HO lesions in FOP patients.
Materials and Methods
IRB approval was obtained from the University of Pennsylvania and all data comply with HIPAA regulations. The University of Pennsylvania Institutional Animal Care and Use Committee (IACUC) approved the use of mice in this study. First, we used a mouse model of FOP-like HO to validate a semi-quantitative analogue scale for estimating relative heterotopic bone volume. Second, we used this validated scale to estimate the relative amount of HO from a retrospective analysis of plain radiographs from 63 patients with classic FOP. Finally, the scale was applied to a retrospective analysis of CT images from three FOP patients.
Results
In the FOP-mouse model, the observed rating on the analogue scale is highly correlated to heterotopic bone volumes measured by micro-computed tomography (R2 = 0.89). The scoring system that was applied to radiographs of FOP patients captured the clinical range of HO typically present at all axial and appendicular sites. Analysis of CT scans of FOP patients found that observed radiograph ratings were highly correlated with HO volume (R2 = 0.80).
Conclusion
The scoring system described here could enable practical, quantitative assessment of HO in clinical trials to evaluate new treatment modalities, especially for FOP.
Clinical Relevance
The development of the six-point analogue scale described here provides and validates a much-needed, reproducible and quantifiable method for describing and assessing heterotopic ossification in FOP patients. This scale has the potential to be a key descriptor that can inform FOP patients and clinicians about disease progression and response of heterotopic ossification lesions to interventions and treatments.
Introduction
Fibrodysplasia ossificans progressiva (FOP) (Mendelian Inheritance in Man [MIM] #135100) is a severely disabling heritable disorder characterized by congenital malformations of the great toes and progressive heterotopic ossification (HO) that forms qualitatively normal bone at extra-skeletal sites.[1] During the first decade of life, affected children experience episodic exacerbations of painful soft tissue swellings (flare-ups), often precipitated by soft tissue injury, intramuscular injections, viral infection, muscular stretching, falls or muscular fatigue. [1, 2] These flare-ups transform skeletal muscles, tendons, ligaments, fascia, and aponeuroses into heterotopic bone, rendering movement restricted or impossible. Classic FOP is caused by a recurrent activating mutation (c.617G>A; R206H) in the gene encoding Activin A receptor type I/Activin-like kinase 2 (ACVR1/ALK2), a bone morphogenetic protein (BMP) type I receptor [3]. At present, there is no definitive treatment, but emerging drug development offers the possibility of beneficial interventions [4–8].
Although multiple classification systems grade the severity of focal HO, [9–13] none is applicable to progressive multi-focal HO, nor does any adequately measure volumetric bone by plain radiographs. A simple method for quantifying extra-skeletal bone formation would be useful to assess the effectiveness of evolving treatments, especially for FOP, where rigid immobility due to progressive multi-focal HO often precludes serial quantitative computed tomographic (CT) scans at major urban medical centers.
We developed an analogue scoring system of HO based on radiographic appearance and relative size (Table 1). For early lesions, the score is based on the former, while for more advanced lesions the score is based on the later. The purpose of this study is to develop and validate a scoring system based on radiographs for semi-quantitative assessment of HO lesions in FOP patients.
Table 1.
Analogue scale to measure HO on plain radiographs in FOP patients*
| 0 | No HO |
| 1 | Single or multiple spicules (punctate) or islands (non-contiguous) of HO |
| 2 | Coalescing islands or reticular complexes of heterotopic bone |
| 3 | Single contiguous HO having longest dimension ≤ ½ the diameter of the reference normotopic bone** in any projection |
| 4 | Single contiguous HO with longest dimension > ½ but ≤1 diameter of the reference normotopic bone** in any projection |
| 5 | Single contiguous HO with longest dimension >1 but ≤2 diameter of reference normotopic bone* in any projection |
| 6 | Single contiguous HO with longest dimension >2 diameters of reference normotopic bone** in any projection |
Score is assigned based on highest grade feature.
Reference normotopic bone for the indicated location of HO is defined as follows: TMJ – height of cervical vertebral body nearest to HO midpoint; Jaw/chin – width of hyoid; Neck – height of cervical vertebral body nearest to HO midpoint (lateral projection); Back or chest – height of cervical vertebral body nearest to HO midpoint; Proximal upper extremity – humeral shaft; Distal upper extremity – radial shaft; Hip/proximal lower extremity – width of femoral neck; Knee – femoral shaft; Distal lower extremity – tibial shaft; Ankle – tibial shaft; Foot – metatarsal
Materials and Methods
Animals and Injury-Induced HO Model
The University of Pennsylvania Institutional Animal Care and Use Committee (IACUC) approved the use of mice in this study. A transgenic mouse model of FOP-like HO containing a constitutively active (ca) ACVR1/ALK2 allele flanked by loxP sites (caALK2 mice) was used in all experiments [4, 7, 14]. To induce expression of caALK2, 50 μl of 0.9% NaCl solution containing 5×1010 genome copies (GC) of recombinant adenovirus-expressing Cre recombinase (University of Pennsylvania Vector Core) and cardiotoxin (10μM solution; Sigma-Aldrich, St. Louis, MO) was injected into the left hind limb musculature of mice at 3 weeks of age. Tissues were recovered 7, 10, and 14 days post-injection (n = 10, 10, and 14, respectively).
Micro Computed Tomography
Micro computed tomography was performed on the adenovirus-cre/cardiotoxin injected legs of caALK2 mice post mortem. A Scanco VivaCT 40 (Bruettisellen, Switzerland) was used to determine the volume of heterotopic bone and obtain a two-dimensional image of the medial view of the sagittal plane of the limb analogous to radiographs. Scanning was performed using a source voltage of 55 kV, a source current of 142 μA, and an isotropic voxel size of 10.5 μm. Skeletal and HO bone was differentiated from “non-bone” by an upper threshold of 1000 Hounsfield units and a lower threshold of 150 Hounsfield units. A recent study demonstrated the usefulness of using micro-CT as an imaging tool for the evaluation of HO in animal models [15].
Patients
Classic FOP was diagnosed by clinical criteria and confirmed by mutation analysis (c.617G>A; R206H in the gene ACVR1/ALK2). Plain radiographs from 63 FOP patients and CT scans from a further 3 FOP patients (2 males, 1 female; ranging from 8 to 13 years of age) were retrospectively reviewed in accordance with institutional review board approval at the University of Pennsylvania.
Assessment of Radiographs
To determine if the HO scale could be used to estimate the amount of HO seen on plain radiographs, films available in the FOP film library of The Center for Research in FOP and Related Disorders from FOP patients were retrospectively reviewed. Each HO lesion seen on radiographs was classified according to the six-point analogue scale described in Table 1 by two raters (FSK and RJP) independently blinded to prior classification to determine inter-rater reliability of the scoring system.
Clinical CT Imaging and HO Volume Analysis
Retrospective examination of CT scans (obtained as part of a comprehensive spinal deformity analysis) from three FOP patients was performed as a gold standard for comparative radiographic analysis on concomitant 2D images. Two scans had been performed on Siemens-manufactured CT machines at the Children’s Hospital of Philadelphia and one on a GE medical systems-manufactured CT machine at the Cardinal Glennon Children’s Medical Center. Scanning was performed using a source voltage of 120kV. Slices were acquired in the axial orientation covering the skull, neck, torso, and pelvis. However, slice thickness varied between the three patients (0.625mm, 3mm, and 5mm thick, respectively). 3D modeling and analysis of CT images were performed using the DICOM viewer OsiriX® (Pixmeo, Geneva, Switzerland). Three-dimensional modeling and calculation of HO lesion volumes were performed following visual lesion identification. An intensity threshold was set to automatically segment HO lesions from soft tissue. Each individual HO lesion was identified manually from normotopic skeletal structures in the three-dimensional viewing window and HO volumes were calculated.
HO Scoring System
Appearance of bone was scored by comparing the diameters of heterotopic bone to a nearby ‘reference’ normotopic bone as described in Table 1. The reference normotopic bone was defined based on anatomical location of HO.
Statistics
Coefficient of determination (R2) was used to describe the relationship between the analogue HO score and the bone volume. A logarithmic transformation was employed to account for the wide distribution of bone volumes. Inter-observer agreement was calculated as the percentage of identical scores by two independent observers (FSK and RJP). Statistical analysis was performed using Microsoft Excel version 2007 (Malvern, PA, USA).
Results
In the mouse model, we found a very strong correlation (R2 =0.89) between the radiograph-based HO score and the bone volume derived from micro-CT (Figure 1), indicating the potential to quantify the amount of HO from radiographs in FOP. One-hundred percent of caALK2 mice are induced to form HO by day 14 after adenovirus-cre/cardiotoxin injection (data not shown). Figure 2 shows representative three-dimensional renditions of HO corresponding to the analogue scores 0 through 6.
Figure 1.
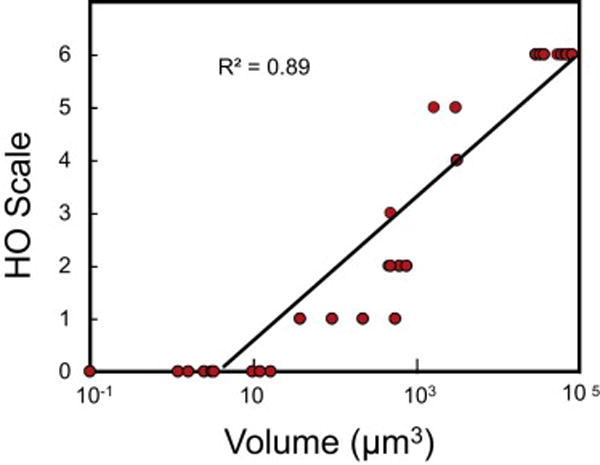
High correlation between microCT-quantified bone volume and HO scores on the analogue scale (n=34) in a mouse model of HO. R2, coefficient of determination.
Figure 2.
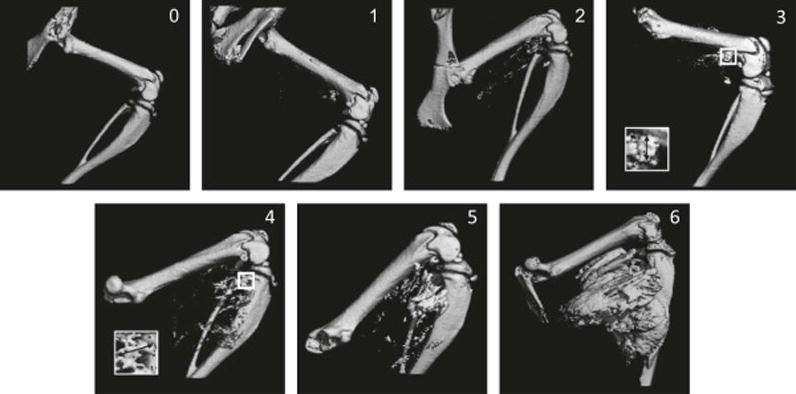
Two-dimensional renderings of reconstructed micro-CT images in an inducible HO mouse model of FOP. Shown are representative images of HO corresponding to the indicated analogue scores 0–6, as defined in Table 1. Insets for scores 3 and 4 show magnified views of the longest contiguous ectopic bone (↔) corresponding to the indicated grade.
Review of radiographs in FOP patients showed that the clinical range of HO typically present at all axial and appendicular locations in FOP patients was represented by the analogue scale (Figure 3 and Table 2). The vast majority of radiographic lesions identified in FOP patients (82%; 171 out of 208) were scored a 6 (Table 2 and Figure 4). Use of adjacent reference normotopic bone in the same projection allowed a simple estimation of the amount of HO. Inter-observer agreement was excellent in our study (96.7% over all anatomic sites) and differences in scores never varied by more than one analogue point. Even excluding the HO scores of 6, there is no significant difference in the inter-observer agreement.
Figure 3.
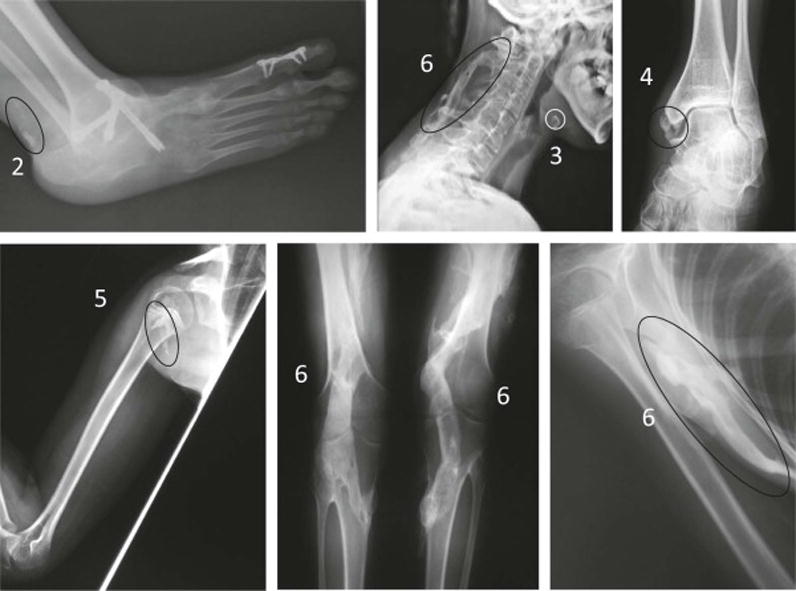
Grading of radiographic lesions of HO in patients with FOP by the analogue scoring system. Shown are representative images from plain X-rays corresponding to the indicated analogue scores 2–6, as defined in Table 1.
Table 2.
Localization and grading of radiographic HO lesions.
| HO Grade | Totals by location | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| Neck | 2 | 21 | 23 | ||||
| Back/Chest | 1 | 1 | 47 | 49 | |||
| Shoulders | 3 | 5 | 4 | 49 | 61 | ||
| Elbows | 2 | 8 | 10 | ||||
| Wrists | 0 | ||||||
| Hips | 1 | 3 | 8 | 27 | 39 | ||
| Knees | 1 | 12 | 13 | ||||
| Distal lower extremities (incl. Ankles/Feet) | 2 | 3 | 1 | 7 | 13 | ||
| Totals by grade | 0 | 4 | 5 | 9 | 19 | 171 | 208 |
Figure 4.
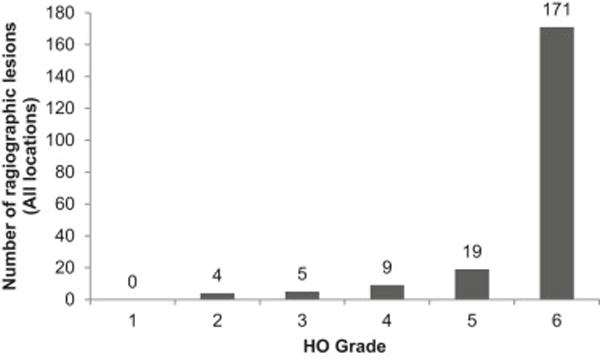
Distribution of radiographic HO lesions by score on the analogue scale in FOP patients.
There was a strong correlation (R2 =0.80) between the HO score and the bone volume derived from CT images in FOP patients (Figure 5), indicating the feasibility of quantifying HO volume on radiographs when three-dimensional imaging is not practical. Interestingly, among the few FOP patients who had undergone CT examinations, the entire range of HO scores was observable (Figure 6). However, 45% (9 out of 20) of lesions were scored a 6, indicating advanced disease stage. Three-dimensional rendition of CT images allowed clear differentiation of HO lesions from normotopic bone in the entire body region (Figure 7).
Figure 5.
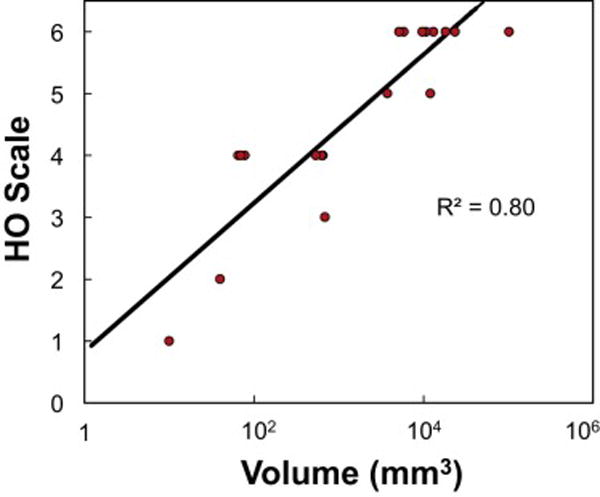
Correlation between bone volumes measured by CT image analysis with HO scores on the analogue scale (n=20). R2, coefficient of determination.
Figure 6.
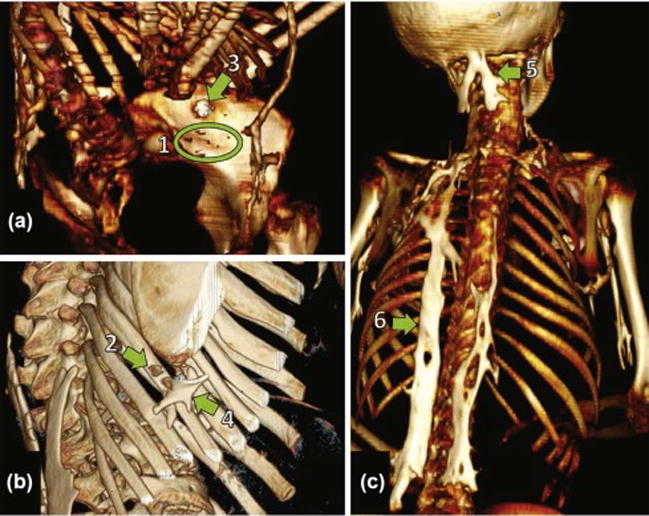
Examples of HO grading in 3D reconstructions of CT images by the analogue scoring system in patients with FOP. HO lesions are displayed with the indicated analogue scores 1–6. Each image is from a different FOP patient (A, B, C).
Figure 7.
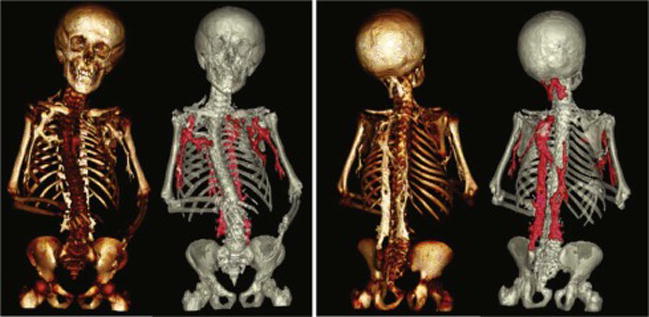
Anterior and posterior 3D reconstructions of CT images obtained from an FOP patient with HO lesions highlighted in red. 3D reconstructions of CT images can aid in identifying lower grade lesions and in determining how lesions connect to and impact skeletal structure on a systemic level.
Discussion
Clinically useful radiographic classification systems for HO must include a scale for the estimation of volumetric bone. Our study reports a scale, validated in an animal model of FOP and applied to FOP patients, describing the clinical spectrum of radiographic lesions seen in this population. While the locations of radiographic lesions cannot be predicted by this or any other scoring system, the HO scale categorizes the relative amount of extra-skeletal bone at diverse anatomical sites. This analogue scale is meant to be used not as an isolated assessment, but in combination with either objective measurement of range of motion or patient self-report of associated joint function. Such a scale will be immensely useful to assess potential therapeutic interventions for the prevention of HO in FOP, a condition in which serial CT scans would be logistically impossible.
The majority of HO lesions in FOP patients identified in this study corresponded to advanced stages of disease. Since the timing of plain radiographs does not often clinically coincide with the initiation of HO after flare-ups in FOP, it is not surprising that we did not identify many instances of radiographic evidence of lesions with a score of 1; however, these early lesions are likely to occur with suboptimal blockade of HO in FOP clinical trials.
Current HO classification systems, including the Brooker system [9], are not easily applied to HO lesions in FOP due to the wide variety of anatomic sites involved in FOP lesions and the erratic nature and extent of soft tissue involved with FOP flare-ups. Although the modified Brooker system [13] attempts to correct for the same (high) grade lesion having disparate functional consequences, this too is not sufficient to classify FOP lesions since the grading system still does not adequately account for the variable amount of HO at different extra-skeletal locations. The HO scale described here overcomes this limitation and may be broadly applied to genetic and non-hereditary forms of multifocal HO.
In this study, we retrospectively reviewed plain radiographs from 63 patients, representing 208 radiographic lesions of HO. This is an underestimation of the number of established lesions since skeletal surveys are not routinely performed in FOP; rather, these were isolated radiographs from a wide-range of anatomic locations obtained at the time of clinical decision-making.
The ultimate size of a radiographic HO lesion reflects the size of the initial soft connective tissue involvement after injury or other insult. Therefore, a drug that inhibits ossification, but not earlier stages of a lesion, could result in the underestimation of the lesion size by a scale that only assesses radiographic HO. Thus, the analogue scale does not provide information about lesional maturation, only information about relative size of mature (ossified) lesions. Radiographic studies that might distinguish between ossified and non-ossified lesions (e.g., magnetic resonance imaging, radionuclide bone scanning) would be difficult to perform on many patients with FOP due to severe mobility issues and other physical constraints.
HO can occur within soft tissue, unconnected to bone, or originate from periosteal outgrowth. Determining the relative amount and location of HO compared to normotopic bone, particularly with relation to critical anatomical sites such as joints, is critical for assessment of patient symptoms and understanding the use of future interventional options. Towards this end, the HO-scale presented here could be used in conjunction with two orthogonal radiographic projections (e.g., lateral and AP) to improve the determination of relative amount and spatial location of HO.
Although other radiographic techniques are available to assess clinically significant extra-skeletal bone formation, including computed tomography, magnetic resonance imaging, and radionuclide bone scanning, none offer a superior benefit relative to availability, convenience, cost, and exposure to radiation (which is particularly relevant in the pediatric FOP population). Clinically, our global experience with FOP patients worldwide for the past 25 years [16–19] has never dictated the choice of these modalities over plain radiographs in the diagnosis and management of the condition.
Clearly, CT provides volumetric information not provided by plain radiographs to evaluate HO. However, for reasons stated above, routine use in the clinical setting, and now a method for HO classification, plain radiographs offer an overall advantage. Low dose CT has a role in clinical studies, including interventional trials and low dose (low radiation) CT can be used for longitudinal studies in children if cumulative radiation dosage is carefully monitored and the head is excluded; however, as a routine imaging modality it is not so practical in FOP. Neither plain radiographs, nor low dose CT, is required for diagnostic purposes in FOP. Both in clinical practice and for potential design of clinical trials involving patients with disabilities that preclude them from obtaining a CT (e.g., inability to lie flat), the ability to assess HO involvement on plain films is a distinct advantage.
Limitations of this scale are salient and include the presence of extensive, existing HO (which may preclude the ability to resolve spatial landmarks that distinguish heterotopic from normotopic bone), the lack of a direct measurement of heterotopic bone volume in FOP patients, and the current lack of longitudinal data on radiographic progression of HO. In addition, radiographic appearance of HO lesions could not be assessed by this scale in regions of the skull and jaw and were necessarily excluded from analysis. We also note that validation of this scale was performed in a mouse model of FOP with HO induced to form about the knee joint only, but applied to radiographic lesions of clinical FOP at various anatomic locations. Further, lesions in the animal model were induced, whereas in FOP many lesions occur spontaneously. Comparison of HO score to bone volume was demonstrated only on a small cohort of FOP patients due to the extreme difficulty in obtaining CT scans in this cohort. Nevertheless, lesions corresponding to the entire spectrum of HO score were observed in the limited number of patients studied. It should be mentioned that this HO scale is not meant to stratify radiographic lesions of orthotopic ossification such as osteochrondromas, which are common in FOP, nor should it be used to assess radiographic lesions of intra-articular synovial osteochondromatosis (Figure 8).
Figure 8.
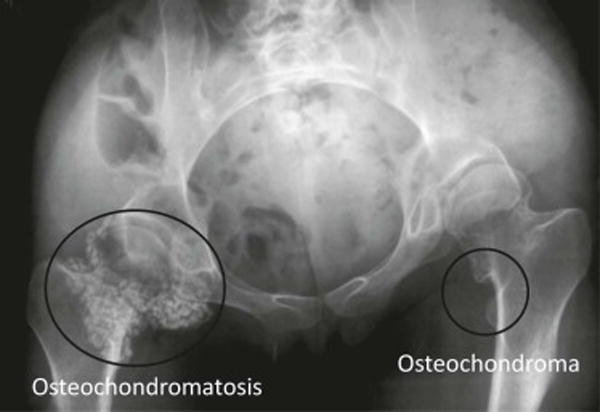
Example of intra-articular synovial osteochondromatosis and osteochrondromas of the hip. These radiographic lesions should not be graded on the HO scale.
We are entering an era of rapidly accelerating drug development for FOP [4–8]. It is critically important to have at hand simple algorithms for assessing the efficacy of potential therapeutics in FOP. A fundamental measurement in the assessment of HO is the estimation of volumetric HO from plain radiographs in the context of changes in joint function. We believe that our estimation tool is a first attempt to satisfy this criterion.
Acknowledgments
We acknowledge Meiqi Xu for ACVR1 genotyping to confirm patients with classic FOP. This work was supported in part by the International Fibrodysplasia Ossificans Progressiva Association (IFOPA), the Center for Research in FOP and Related Disorders, the Ian Cali Endowment for FOP Research, the Whitney Weldon Endowment for FOP Research, the Isaac and Rose Nassau Professorship of Orthopaedic Molecular Medicine (to F.S.K.), the Cali-Weldon Research Professorship in FOP (to E.M.S.), the Ian Cali Distinguished Clinician-Scientist (to RJP), the McGuire FOP Fund, the Ashley Martucci FOP Research Fund, the Penn Center for Musculoskeletal Disorders, and the National Institutes of Health (NIH R01 AR41916, R01 AR068382, and K25 AR060283).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pignolo RJ, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva: clinical and genetic aspects. Orphanet J Rare Dis. 2011;6:80. doi: 10.1186/1750-1172-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan FS, Le Merrer M, Glaser DL, Pignolo RJ, Goldsby RE, Kitterman JA, Groppe J, Shore EM. Fibrodysplasia ossificans progressiva. Best Pract Res Clin Rheumatol. 2008;22(1):191–205. doi: 10.1016/j.berh.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38(5):525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 4.Kan L, Lounev VY, Pignolo RJ, Duan L, Liu Y, Stock SR, McGuire TL, Lu B, Gerard NP, Shore EM, Kaplan FS, Kessler JA. Substance P signaling mediates BMP-dependent heterotopic ossification. J Cell Biochem. 2011;112(10):2759–2772. doi: 10.1002/jcb.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan FS, Glaser DL, Pignolo RJ, Shore EM. A new era for fibrodysplasia ossificans progressiva: a druggable target for the second skeleton. Expert Opin Biol Ther. 2007;7(5):705–712. doi: 10.1517/14712598.7.5.705. [DOI] [PubMed] [Google Scholar]
- 6.Shimono K, Tung WE, Macolino C, Chi AH, Didizian JH, Mundy C, Chandraratna RA, Mishina Y, Enomoto-Iwamoto M, Pacifici M, Iwamoto M. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-gamma agonists. Nat Med. 2011;17(4):454–460. doi: 10.1038/nm.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, Kamiya N, Fukuda T, Mishina Y, Peterson RT, Bloch KD. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med. 2008;14(12):1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatsell SJ, Idone V, Wolken DM, Huang L, Kim HJ, Wang L, Wen X, Nannuru KC, Jimenez J, Xie L, Das N, Makhoul G, Chernomorsky R, D’Ambrosio D, Corpina RA, Schoenherr CJ, Feeley K, Yu PB, Yancopoulos GD, Murphy AJ, Economides AN. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci Transl Med. 2015;7(303):303ra137. doi: 10.1126/scitranslmed.aac4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooker AF, Bowerman JW, Robinson RA, Riley LH., Jr Ectopic ossification following total hip replacement. Incidence and a method of classification. J Bone Joint Surg Am. 1973;55(8):1629–1632. [PubMed] [Google Scholar]
- 10.Kawaguchi Y, Urushisaki A, Seki S, Hori T, Asanuma Y, Kimura T. Evaluation of ossification of the posterior longitudinal ligament by three-dimensional computed tomography and magnetic resonance imaging. Spine J. 2011;11(10):927–932. doi: 10.1016/j.spinee.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Kocic M, Lazovic M, Mitkovic M, Djokic B. Clinical significance of the heterotopic ossification after total hip arthroplasty. Orthopedics. 2010;33(1):16. doi: 10.3928/01477447-20091124-13. [DOI] [PubMed] [Google Scholar]
- 12.Mavrogenis AF, Guerra G, Staals EL, Bianchi G, Ruggieri P. A classification method for neurogenic heterotopic ossification of the hip. J Orthop Traumatol. 2012;13(2):69–78. doi: 10.1007/s10195-012-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuh A, Zeiler G. The modified Brooker classification for evaluation of heterotopic ossifications in total hip replacement. Zentralbl Chir. 2005;130(4):293–296. doi: 10.1055/s-2005-836783. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda T, Scott G, Komatsu Y, Araya R, Kawano M, Ray MK, Yamada M, Mishina Y. Generation of a mouse with conditionally activated signaling through the BMP receptor, ALK2. Genesis. 2006;44(4):159–167. doi: 10.1002/dvg.20201. [DOI] [PubMed] [Google Scholar]
- 15.Brownley RC, Agarwal S, Loder S, Eboda O, Li J, Peterson J, Hwang C, Breuler C, Kaartinen V, Zhou B, Mishina Y, Levi B. Characterization of Heterotopic Ossification Using Radiographic Imaging: Evidence for a Paradigm Shift. PLoS One. 2015;10(11):e0141432. doi: 10.1371/journal.pone.0141432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan FS, Shore EM, Pignolo RJ, FOP aTICCo The medical management of fibrodysplasia ossificans progressiva: current treatment considerations. Clin Proc Intl Clin Consort FOP. 2011;4:1–100. [Google Scholar]
- 17.Kaplan FS, Strear CM, Zasloff MA. Radiographic and scintigraphic features of modeling and remodeling in the heterotopic skeleton of patients who have fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1994;(304):238–247. [PubMed] [Google Scholar]
- 18.Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L, Delai P, Fastnacht-Urban E, Forman SJ, Gillessen-Kaesbach G, Hoover-Fong J, Koster B, Pauli RM, Reardon W, Zaidi SA, Zasloff M, Morhart R, Mundlos S, Groppe J, Shore EM. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat. 2009;30(3):379–390. doi: 10.1002/humu.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahboubi S, Glaser DL, Shore EM, Kaplan FS. Fibrodysplasia ossificans progressiva. Pediatr Radiol. 2001;31(5):307–314. doi: 10.1007/s002470100447. [DOI] [PubMed] [Google Scholar]


