ABSTRACT
The DNA sensing pathway triggers innate immune responses against DNA virus infection, and NF-κB signaling plays a critical role in establishing innate immunity. We report here that the herpes simplex virus 1 (HSV-1) ubiquitin-specific protease (UL36USP), which is a deubiquitinase (DUB), antagonizes NF-κB activation, depending on its DUB activity. In this study, ectopically expressed UL36USP blocked promoter activation of beta interferon (IFN-β) and NF-κB induced by cyclic GMP-AMP synthase (cGAS) and stimulator of interferon genes (STING). UL36USP restricted NF-κB activation mediated by overexpression of STING, TANK-binding kinase 1, IκB kinase α (IKKα), and IKKβ, but not p65. UL36USP was also shown to inhibit IFN-stimulatory DNA-induced IFN-β and NF-κB activation under conditions of HSV-1 infection. Furthermore, UL36USP was demonstrated to deubiquitinate IκBα and restrict its degradation and, finally, abrogate NF-κB activation. More importantly, the recombinant HSV-1 lacking UL36USP DUB activity, denoted as C40A mutant HSV-1, failed to cleave polyubiquitin chains on IκBα. For the first time, UL36USP was shown to dampen NF-κB activation in the DNA sensing signal pathway to evade host antiviral innate immunity.
IMPORTANCE It has been reported that double-stranded-DNA-mediated NF-κB activation is critical for host antiviral responses. Viruses have established various strategies to evade the innate immune system. The N terminus of the HSV-1 UL36 gene-encoded protein contains the DUB domain and is conserved across all herpesviruses. This study demonstrates that UL36USP abrogates NF-κB activation by cleaving polyubiquitin chains from IκBα and therefore restricts proteasome-dependent degradation of IκBα and that DUB activity is indispensable for this process. This study expands our understanding of the mechanisms utilized by HSV-1 to evade the host antiviral innate immune defense induced by NF-κB signaling.
KEYWORDS: HSV-1, DNA sensor, UL36, NF-κB, IκBα
INTRODUCTION
Cells have developed plenty of ways to govern their homeostasis, and the recognition of double-stranded DNA (dsDNA) by DNA sensors is a central host cellular defense against DNA virus infection. Among these DNA sensors, cyclic GMP-AMP synthase (cGAS), which is a nucleotidyltransferase, has been demonstrated to be the predominant cytosolic DNA sensor. Upon binding to DNA fragments, cGAS utilizes GTP and ATP to produce cyclic-GMP-AMP (cGAMP) through its enzymatic activity, and cGAMP activates an endoplasmic reticulum (ER)-resident receptor, stimulator of interferon genes (STING) (1). Activated STING then recruits TANK-binding kinase 1 (TBK1) and traffics from the ER to a perinuclear endosomal compartment, leading to the activation of transcription factors NF-κB and interferon (IFN) regulatory factor 3 (IRF3), which induce IFN-β production (1–4).
Ubiquitination plays an important role in regulating the antiviral immune responses. It has been illustrated that ubiquitin has seven internal lysine residues (K6, K11, K27, K29, K33, K48, and K63), all of which can form long polyubiquitin chains (5, 6). Lys63-linked polyubiquitination is involved in signal transduction, and Lys48-linked polyubiquitination leads to the proteasomal degradation of target proteins (7, 8). As a posttranslational modulator, ubiquitination is critical for multiple cascades within the NF-κB signal pathway. In mammals, the NF-κB family contains five members: p65 (RelA), RelB, c-Rel, p50, and p52. NF-κB is retained in the cytoplasm in resting cells, binding with an inhibitor of NF-κB proteins, namely, IκBα. Stimulation with viral, microbial, cytokine, or other stimuli can lead to NF-κB activation (9). In the tumor necrosis factor alpha (TNF-α)-induced canonical NF-κB pathway, Lys63-linked polyubiquitin chains of receptor interacting protein 1 act as scaffolds and recruit the NF-κB essential modulator (NEMO) and transforming growth factor β (TGF-β)-activated kinase 1 (TAK1) complex (10–12). NEMO, a Lys63-selective ubiquitin receptor also known to bind linear ubiquitin chains, functions together with the TAK1 complex to promote IκB kinase (IKK) complex activation (13). The activated IKK complex phosphorylates IκBα, and the latter undergoes Lys48-linked polyubiquitination, resulting in its proteasomal degradation (14). Subsequently, NF-κB (such as p65/p50 heterodimer) is released from cytoplasmic sequestration and translocates into the nucleus, where it binds to κB sites in promoters and initiates NF-κB-dependent gene transcription (15). Moreover, it has been shown that STING is a novel target for ubiquitination and that K48-linked ubiquitination leads to the degradation of STING, whereas K11-linked and K63-linked ubiquitination stimulate STING activation (16–19). In addition, it has been demonstrated that K27-linked ubiquitination also acts in a positive regulatory role (20).
Herpes simplex virus 1 (HSV-1) belongs to the alphaherpesvirus subfamily, is a large dsDNA virus with a genome of about 152 kb, and encodes over 80 open reading frames (21, 22). As the largest tegument protein encoded by HSV-1, UL36 contains an approximately 420-amino-acid deubiquitinase (DUB) domain in its N terminus, also known as UL36USP, which is cleaved from full-length UL36 and can be detected at 12 h after viral infection (23). Our previous study demonstrated that UL36USP inhibits RIG-I-like receptor (RLR)-induced IFN-β production by removing the Lys63-linked ubiquitin chains from TNF receptor-associated factor 3 (TRAF3) and blocking IRF3 activation (24). It has been reported that UL36USP could particularly cleave Lys48-linked polyubiquitin chains but not the conjugates of ISGylation, Neddylation, SUMOylation, or Lys63-linked polyubiquitination (23). However, Kim et al. found that UL36USP cleaved both Lys48- and Lys63-linked polyubiquitin chains and showed higher DUB activities for Lys-63 linkages, which were comparable to those of our study (25). Recently, NF-κB signaling was shown to play an essential role in dsDNA-triggered IFN-β activation and to be critical for inhibiting HSV-1 replication (3). Given the importance of NF-κB in host antiviral responses, we intended to define the role of HSV-1 in the regulation of NF-κB signal transduction downstream of the DNA sensing signal pathway. In this study, we demonstrated that ectopically expressed UL36USP decreased cGAS-STING-induced IFN-β and NF-κB promoter activation. UL36USP was also shown to inhibit IFN-stimulatory DNA (ISD)-induced IFN-β and NF-κB activation under conditions of HSV-1 infection. Furthermore, UL36USP inhibited NF-κB activation by deubiquitinating IκBα and restricting its degradation and, consequently, dampened the cellular DNA sensing signal pathway and subsequent IFN production. In summary, these findings expand our understanding of the evasion of host antiviral innate immune responses by HSV-1.
RESULTS
UL36USP inhibited cGAS-STING-mediated IFN-β and NF-κB activation.
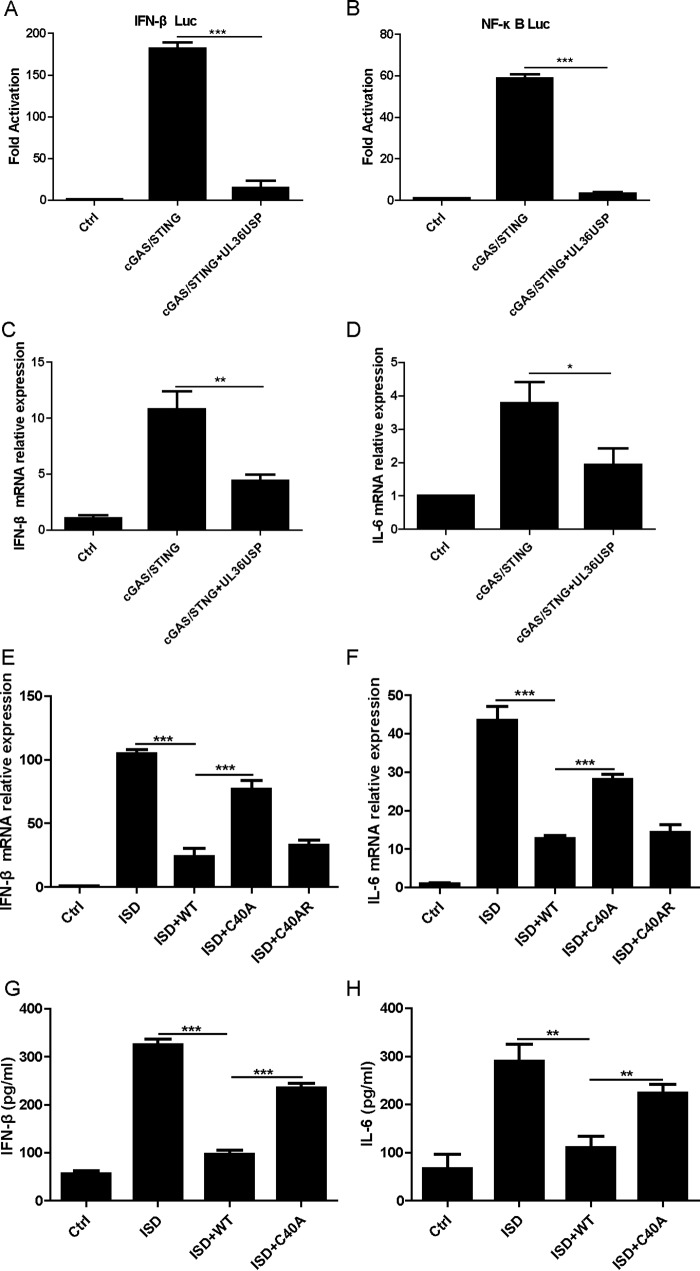
To investigate the role of UL36USP in DNA sensing signaling, we first explored whether UL36USP could modulate the cGAS-STING-mediated activation of the IFN-β and NF-κB promoters. Exogenous expression of a minimal amount of either cGAS or STING alone was unable to induce the activation of IFN-β in HEK293T cells, and the IFN-β promoter could be activated only when minimal amounts of cGAS and STING were cotransfected (1, 26). HEK293T cells were cotransfected with the IFN-β-Luc or NF-κB-Luc reporter plasmid, cGAS, and STING expression plasmids, along with an empty vector or a plasmid encoding UL36USP. After 24 h, the cells were harvested and subjected to a dual-luciferase reporter (DLR) assay. Ectopically expressed UL36USP blocked activation of the IFN-β promoter and the NF-κB promoter stimulated by cGAS and STING (Fig. 1A and B). Considering that IL-6 is expressed downstream of NF-κB activation, we verified the DLR results by examining the mRNA levels of IFN-β and IL-6 using quantitative PCR (qPCR), and similar results were obtained (Fig. 1C and D).
FIG 1.
HSV-1 UL36USP inhibited cGAS-STING-mediated IFN-β and NF-κB activation. (A and B) HEK293T cells were cotransfected with IFN-β-Luc (A) and NF-κB-Luc (B) reporter plasmid, along with an empty vector or plasmids encoding cGAS (15 ng) and STING (2.5 ng) with or without UL36USP, as indicated. Luciferase activity was measured at 24 h posttransfection, and the fold activation was determined compared to that for the empty vector. (C and D) HEK293T cells were cotransfected with a plasmid encoding cGAS, a plasmid encoding STING, and either an empty vector or a plasmid encoding UL36USP. At 24 h posttransfection, the cells were harvested and subjected to qPCR analysis to detect IFN-β (C) or IL-6 (D) mRNA. (E and F) HFF cells were infected with WT HSV-1, C40A mutant HSV-1, or C40AR HSV-1 at a multiplicity of infection (MOI) of 5 for 2 h and then transfected with ISD (4 μg/ml) for another 10 h. The cells were harvested and subjected to RT-PCR to detect IFN-β (E) or IL-6 (F) mRNA. (G and H) HFF cells were infected with WT HSV-1 or C40A mutant HSV-1 and then transfected with ISD for another 16 h. The supernatants were subjected to ELISA to detect IFN-β (G) or IL-6 (H). The data represent results from one of three independent experiments. Error bars represent standard deviations (SD) from three independent experiments. Statistical analysis was performed using Student's t test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant).
ISD is a dsDNA 60-bp oligonucleotide derived from the HSV-1 genome and has a large capacity to induce IFN-β secretion (27). To determine whether UL36 affected the activation of IFN-β and NF-κB induced by ISD under conditions of HSV-1 infection, human foreskin fibroblast (HFF) cells were infected with wild-type (WT) HSV-1, C40A mutant HSV-1, or C40A rescue (C40AR) HSV-1, followed by ISD stimulation. The cells were then harvested and subjected to qPCR to detect the expression of IL-6 and IFN-β. In response to the ISD stimulation, HFF cells exhibited a robust induction of IFN-β and IL-6 mRNA. Infection with both WT HSV-1 and C40AR HSV-1 exhibited impaired IFN-β and IL-6 mRNA expression, whereas infection with C40A mutant HSV-1 partly restored IFN-β and IL-6 mRNA expression (Fig. 1E and F), suggesting that UL36 inhibited ISD-induced IFN-β and NF-κB activation. To further confirm this assertion, the production of IFN-β and IL-6 in the supernatants was measured by enzyme-linked immunosorbent assay (ELISA) after transfection with ISD for 18 h. Both cytokines were significantly decreased in cells infected with WT HSV-1, whereas in C40A mutant HSV-1 infection, both cytokines were recovered to a certain extent (Fig. 1G and H). Collectively, these results indicated that UL36USP was capable of inhibiting the cGAS-STING-dependent DNA sensing signal pathway.
UL36USP inhibited the NF-κB signal pathway at a level between those of IKK and p65.
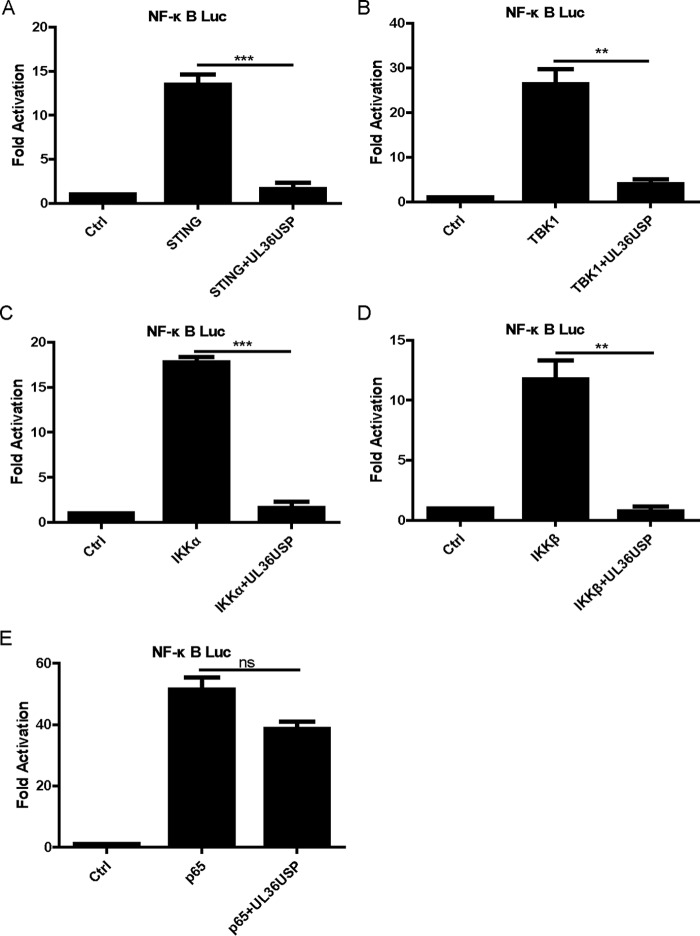
To clarify at which step the UL36USP protein restricted the NF-κB activation pathway, HEK293T cells were cotransfected with NF-κB-Luc reporter plasmid, UL36USP expression plasmid, and plasmids of adaptor proteins of NF-κB signal transduction pathways, including STING, TBK1, IKKα, IKKβ, and p65. Although ectopic expression of a minimal amount of STING alone failed to activate the IFN-β promoter, overexpression of STING plasmid could induce IFN-β promoter activation (26, 28). It was shown that the expression constructs resulted in an approximately 12- to 50-fold induction of NF-κB-Luc reporter activity (Fig. 2). NF-κB promoter activation driven by STING, TBK1, or IKK was reduced by UL36USP (Fig. 2A to D). However, UL36USP did not affect NF-κB promoter activation mediated by p65 overexpression (Fig. 2E). These findings suggested that UL36USP inhibited the NF-κB signal pathway at a level between those of IKK and p65.
FIG 2.
UL36USP inhibited the NF-κB signal pathway at a level between those of IKK and p65. (A to E) HEK293T cells were cotransfected with NF-κB-Luc, pRL-TK, and STING (A), TBK1 (B), IKKα (C), IKKβ (D), or p65 (E) expression plasmids, along with empty vector or plasmid encoding UL36USP. The cells were then harvested 24 h after transfection and subjected to DLR assay. The data represent results from one of the three independent experiments.
UL36USP restricted IκBα degradation via its DUB activity.
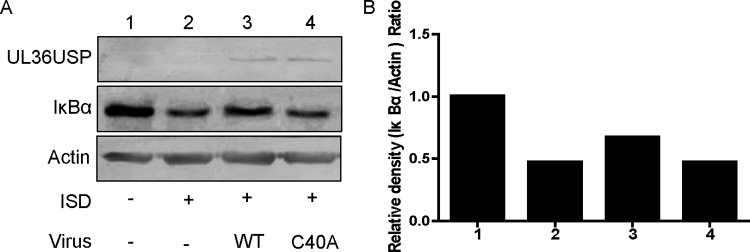
In the NF-κB signal pathway, activated IKK transduces the signal to IκBα and subjects it to phosphorylation, Lys-48 linked ubiquitination, and proteasomal degradation (14, 29, 30). The aforementioned results raised the hypothesis that UL36USP might deubiquitinate IκBα to block NF-κB transcription. It has been reported that the residue Cys65 in UL36USP of the HSV-1 KOS strain is indispensable for DUB activity (23), whereas for the HSV-1 F strain in this study, mutation of Cys40 to Ala abrogates the deubiquitinase activity of UL36USP (24). To substantiate our speculation, we first examined whether UL36USP could inhibit IκBα degradation during HSV-1 infection. HFF cells were infected with WT HSV-1 or C40A mutant HSV-1 for 2 h before ISD transfection. At 10 h after transfection, the cells were harvested and subjected to a Western blot (WB) assay to detect endogenous IκBα. In response to ISD treatment, the level of IκBα exhibited a remarkable degradation. WT HSV-1 infection inhibited the degradation of IκBα, but infection with the C40A mutant HSV-1 did not (Fig. 3A). Results for the relative density analysis of IκBα are shown in Fig. 3B. Consistent with our hypothesis, WT HSV-1 infection, but not C40A mutant HSV-1 infection, decreased ISD-induced IκBα degradation and DUB activity was critical for attenuating the degradation of IκBα.
FIG 3.
UL36USP restricted IκBα degradation via its DUB activity. (A) HFF cells were infected with WT HSV-1 or C40A mutant HSV-1 at an MOI of 1 for 2 h and transfected with ISD (10 μg/ml) for another 10 h. The cells were harvested and subjected to WB analysis. (B) Relative density analysis of IκBα. The data represent results from one of three independent experiments.
UL36USP deubiquitinated IκBα.
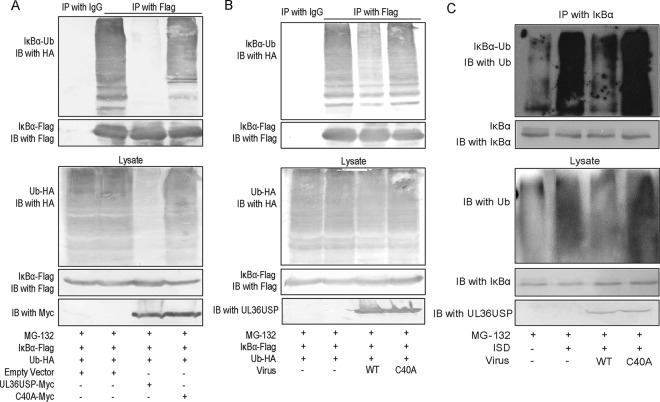
To delineate the mechanism by which UL36USP stabilized IκBα, we examined the ubiquitination of IκBα by coimmunoprecipitation (Co-IP) assays. Ub-HA and IκBα-Flag, along with empty vector, UL36USP, or C40A expression plasmids, were cotransfected into HEK293T cells. Twenty-four hours after transfection, IκBα was immunoprecipitated by anti-Flag antibody, and a WB assay was performed with anti-HA antibody to detect the ubiquitination of IκBα. As shown in Fig. 4A, the presence of UL36USP, but not the C40A mutant, reduced the ubiquitination of IκBα. To investigate the ubiquitination of IκBα during HSV-1 infection, HEK293T cells were transfected with IκBα-Flag and HA-Ub expression plasmids and subsequently infected with WT HSV-1 or C40A mutant HSV-1, and the cell lysates were subjected to a Co-IP assay to detect the ubiquitination of IκBα. As expected, the polyubiquitin chains of IκBα were significantly attenuated by WT HSV-1 but not by C40A mutant HSV-1 (Fig. 4B).
FIG 4.
UL36USP deubiquitinated IκBα. (A) HEK293T cells were transfected with Ub-HA and IκBα-Flag, along with an empty vector, UL36USP-Myc, or C40A-Myc. At 24 h posttransfection, the cells were treated with MG132 (10 μM) for 6 h. Co-IP assays were performed with anti-Flag antibody, and WB was performed with anti-HA antibody to detect the ubiquitination of IκBα. (B) HEK293T cells were transfected with Ub-HA and IκBα-Flag. At 24 h posttransfection, the cells were infected with WT HSV-1 or C40A mutant HSV-1 at an MOI of 3 for 16 h. Before harvest, the cells were treated with MG132 (10 μM) for 6 h. Co-IP experiments were performed using anti-Flag antibody, and WB was performed with anti-HA antibody to detect the ubiquitination of IκBα. (C) HFF cells were infected with WT HSV-1 or C40A mutant HSV-1 at an MOI of 1 for 2 h and transfected with ISD (10 μg/ml) for another 10 h. Before harvest, the cells were treated with MG132 (10 μM) for 6 h. Co-IP experiments were performed using anti-IκBα antibody, and WB was performed with anti-Ub antibody to detect the ubiquitination of endogenous IκBα. The data represent results from one of three independent experiments.
To further determine the role of UL36USP in deconjugating the ubiquitination of IκBα, HFF cells were infected with WT HSV-1 or C40A mutant HSV-1, followed by transfection with ISD. The cells were harvested and subjected to a Co-IP assay, and the ubiquitination of endogenous IκBα was measured. The polyubiquitination of IκBα was greatly increased after ISD stimulation compared to that of the control panel. Consistent with the observations above, WT HSV-1 infection substantially deubiquitinated IκBα. In contrast, enzyme-inactive C40A mutant HSV-1 lost its ability to remove ubiquitin modification from IκBα (Fig. 4C). These data together suggested that UL36USP deubiquitinated IκBα and restricted IκBα degradation by its DUB activity and blocked the activation of NF-κB.
DISCUSSION
In the battle between host and viruses, viruses have evolved multiple strategies to antagonize the host innate immune defense. It has been documented that HSV-1 infection activates NF-κB signaling, hijacks NF-κB to block cell apoptosis, promotes protein transcription through the κB binding sites in the viral genome, and promotes virus replication (31–33). When NF-κB is activated, it induces cytokine and chemokine transcription, which leads to host immune responses. Therefore, HSV-1 also evolved strategies to evade NF-κB signaling during infection and to balance the promoting and suppressing effects for its propagation. Studies have reported that HSV-1 uses a variety of proteins, mostly tegument proteins, to maintain effective infection (34). ICP0, an E3 ubiquitin ligase, disrupts NF-κB activation by abrogating p65 nuclear translocation and promoting p50 to proteasomal degradation (35). US3 hyperphosphorylates p65 to inhibit NF-κB activation and inflammatory chemokine expression (36). ICP27 inhibits NF-κB signaling by stabilizing IκBα (37). UL42 abolishes NF-κB activation by interacting with p65/p50 and retaining them in the cytoplasm (38). VP16 suppresses NF-κB by binding to p65 and blocks IRF3-mediated transactivation by interfering with the formation of the CREB binding protein-IRF3 complex (39). The virion host shutoff protein abrogates the antiviral activity of some IFN-stimulated genes, such as those for tetherin, viperin, and zinc finger antiviral protein, by targeting their mRNA for degradation (40–42). γ134.5 targets TBK1 to prevent its interaction with IRF3 and therefore inhibits IFN production (43). Moreover, US3 also hyperphosphorylates IRF3 and blocks its nuclear translocation, thus inhibiting IFN-β production (44).
cGAS is essential for IFN production and the establishment of a host antiviral state; thus, viruses must evolve certain strategies to antagonize the cGAS-STING pathway for their effective infection (45). However, the mechanisms by which HSV-1 subverts cGAS-STING-dependent signaling have not been fully described. Recently, it was shown that Kaposi's sarcoma-associated herpesvirus (KSHV)-encoded viral IRF1, which is required for efficient viral reactivation and replication, prevents cGAS-STING-dependent IFN-β activation by competitively binding to STING and abrogates the interaction between STING and TBK1 (26). Moreover, Wu et al. reported that KSHV ORF52, which is highly conserved in gammaherpesviruses, abolishes the cGAS-dependent DNA sensing pathway by directly binding to DNA and cGAS and inhibiting cGAS enzymatic activity (46). A recent study from our lab revealed that VP24, a serine protease of HSV-1, blocks the DNA sensing signal pathway by abrogating the interaction between TBK1 and IRF3 (47).
A previous study reported that silencing of p65 led to compromised dsDNA-induced IFN-β activation and facilitated HSV-1 replication (3). The results suggested that NF-κB signaling was involved in dsDNA-triggered IFN-β production. We have shown that ISD-induced NF-κB activation could be reduced by infection with WT HSV-1 but not with C40A mutant HSV-1 through the deubiquitination of IκBα. Therefore, the inhibition of IFN-β production exhibited in Fig. 1 may be due to suppressed NF-κB signaling. As UL36USP is a ubiquitin-specific cysteine protease, its catalytic residues show high conservation across the herpesvirus family (48). Research has shown that the DUBs encoded by other members of the herpesvirus family could counteract the host innate immune responses to maintain the survival of viruses. The human cytomegalovirus UL48 contains DUB activity and is required for efficient viral replication, virion stabilization, and virus entry (25, 49). Epstein-Barr virus BPLF1 deubiquitinates TRAF6 to inhibit cellular NF-κB signal responses, resulting in extensive viral lytic DNA replication (50). KSHV ORF64 is capable of restricting RIG-I-mediated IFN signaling by decreasing the ubiquitination of RIG-I (51). Our previous study shows that UL36USP inhibits RLR signaling by deubiquitinating TRAF3 and blocking IRF3 activation (24). In the present study, we found that UL36USP abrogated NF-κB activation in the DNA sensing signal pathway. It will be interesting to investigate whether DUBs from other herpesviruses affect DNA sensor-induced signaling through a similar mechanism.
In summary, we demonstrated that ectopic expression of UL36USP or infection of WT HSV-1 was sufficient to inhibit DNA sensor-mediated IFN-β and NF-κB activation. Our data showed that UL36USP, but not the C40A mutant, inhibits the NF-κB signal pathway by deubiquitinating IκBα to restrict its degradation, indicating that the DUB activity of UL36USP is indispensable. These findings expand our knowledge of the mechanisms utilized by HSV-1 to evade the host antiviral defense induced by the NF-κB signal pathway, and this may provide a new target for the development of anti-HSV-1 therapy.
MATERIALS AND METHODS
Cells, viruses, and antibodies.
HEK293T cells, Vero cells, and HFF cells were grown in Dulbecco modified Eagle medium (Gibco-BRL) supplemented with 10% fetal bovine serum and 100 U of penicillin and streptomycin/ml. The WT HSV-1 F strain and its derivative UL36USP mutant HSV-1 strain (C40A mutant HSV-1) were propagated in Vero cells and titrated as described previously (52). Two-step Red-mediated recombination was performed to construct C40A rescue HSV-1 (C40AR) as previously described (24). The Ala40 of UL36 in the C40A mutant HSV-1 genome was changed back to Cys. The protease inhibitor cocktail mixtures, mouse anti-IκBα monoclonal antibody (MAb), and rabbit anti-ubiquitin (Ub) polyclonal antibody (pAb) were purchased from CST (Boston, MA). Mouse anti-Myc, anti-Flag, and anti-HA MAbs were purchased from ABmart (Shanghai, China). Rabbit anti-UL36USP pAb was made by GL Biochem, Ltd. (Shanghai, China). Mouse anti-β-actin MAb was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA).
Plasmid construction.
All enzymes for cloning procedures were purchased from Thermo Fisher Scientific (Waltham, MA). UL36USP and C40A were cloned into pCMV-Myc vectors (Beyotime, Shanghai, China) as previously described (24). Commercial reporter plasmids include NF-κB-Luc (Stratagene, La Jolla, CA) and pRL-TK plasmid (Promega). Gift plasmids include the following: cGAS-flag (1), STING-HA, pcDNA3.1-Flag TBK1 (53), pHA-IKKα (54), pFlag-IKKβ (55), pCMV-p65-Flag (56), and IFN-β promoter reporter plasmid p125-luc (57).
RNA isolation and qPCR.
Total RNA was extracted with TRIzol (Invitrogen) according to the manufacturer's manual. Samples were digested with DNase I and subjected to reverse transcription (RT) as described in our previous study (58). The cDNA was used as a template for qPCR to investigate the expression patterns of human IFN-β and interleukin-6 (IL-6). The reaction mix contained 5 μl of 2× SYBR green master mix (with ROX), 1 μl of the diluted cDNA templates, and 2 μl of each primer (1 μmol/liter). 18S rRNA was used as an internal control for transcription analysis.
Transfection and dual-luciferase reporter (DLR) assay.
HEK293T cells were cotransfected with IFN-β-Luc, NF-κB-Luc reporter plasmids, and an internal control plasmid, pRL-TK, with or without expression plasmids by standard calcium phosphate coprecipitation (59). At 24 h posttransfection, luciferase assays were performed with a luciferase assay kit (Promega, Madison, WI) as previously described (58).
ELISA.
Concentrations of IFN-β and IL-6 in cell culture supernatants were determined by using a VeriKine human interferon beta ELISA kit from PBL Assay Science (Piscataway, NJ) and by using a RayBio human IL-6 ELISA kit from RayBiotech (Norcross, GA), respectively, according to the manufacturers' manuals.
Co-IP and Western blot analysis.
Coimmunoprecipitation (Co-IP) assays and Western blot (WB) analysis were performed as previously described (52). Briefly, cells were transfected or infected with WT HSV-1 or C40A mutant HSV-1 as indicated in the figure legends. Harvested cells were lysed on ice with 500 μl of lysis buffer. The lysates were incubated with the antibodies referred to in the figure legends and 30 μl of a 1:1 slurry of protein A/G Plus-Agarose (Santa Cruz Biotechnology) overnight at 4°C. The beads were washed four times with lysis buffer containing 150 mM NaCl, and WB analysis was performed to detect the interaction of proteins. The Co-IP assays were repeated three times, and similar data were obtained.
Statistical analysis.
Data are presented as means ± standard deviations (SD). A two-tailed unpaired Student's t test was used to determine differences. A P value of <0.05 was considered statistically significant and marked with asterisks (*, P < 0.05; **, P < 0.01; ***, P < 0.001) on the figures, whereas “ns” indicates the comparison was not significant.
ACKNOWLEDGMENTS
Work in the Zheng laboratory relevant to this article was supported by grants from the National Natural Science Foundation of China (grants 81371795 and 81571974), the Innovative Research Team in Soochow University (PCSIRT IRT1075), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (YX13400214).
We thank Takashi Fujita for the IFN-β-Luc, Zhijian Chen for the cGAS-flagN1 plasmid, and Rongtuan Lin for the STING plasmid.
REFERENCES
- 1.Sun L, Wu J, Du F, Chen X, Chen ZJ. 2013. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu JX, Sun LJ, Chen X, Du FH, Shi HP, Chen C, Chen ZJJ. 2013. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abe T, Barber GN. 2014. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-κB activation through TBK1. J Virol 88:5328–5341. doi: 10.1128/JVI.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishikawa H, Barber GN. 2011. The STING pathway and regulation of innate immune signaling in response to DNA pathogens. Cell Mol Life Sci 68:1157–1165. doi: 10.1007/s00018-010-0605-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeda F, Crosetto N, Dikic I. 2010. What determines the specificity and outcomes of ubiquitin signaling? Cell 143:677–681. doi: 10.1016/j.cell.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Peng J, Schwartz D, Elias JE, Thoreen CC, Cheng D, Marsischky G, Roelofs J, Finley D, Gygi SP. 2003. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol 21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 7.Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. 2000. Recognition of the polyubiquitin proteolytic signal. EMBO J 19:94–102. doi: 10.1093/emboj/19.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen ZJ, Sun LJ. 2009. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell 33:275–286. doi: 10.1016/j.molcel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Dev A, Iyer S, Razani B, Cheng G. 2011. NF-κB and innate immunity. Curr Top Microbiol Immunol 349:115–143. [DOI] [PubMed] [Google Scholar]
- 10.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. 2006. Activation of IKK by TNF-α requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell 22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 11.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. 2006. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-κB activation (corrected). Nat Cell Biol 8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 12.Kanayama A, Seth RB, Sun L, Ea CK, Hong M, Shaito A, Chiu YH, Deng L, Chen ZJ. 2004. TAB2 and TAB3 activate the NF-κB pathway through binding to polyubiquitin chains. Mol Cell 15:535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Rahighi S, Ikeda F, Kawasaki M, Akutsu M, Suzuki N, Kato R, Kensche T, Uejima T, Bloor S, Komander D, Randow F, Wakatsuki S, Dikic I. 2009. Specific recognition of linear ubiquitin chains by NEMO is important for NF-κB activation. Cell 136:1098–1109. doi: 10.1016/j.cell.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Karin M, Ben-Neriah Y. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu Rev Immunol 18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 15.Hayden MS, Ghosh S. 2004. Signaling to NF-κB. Genes Dev 18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 16.Zhong B, Zhang L, Lei C, Li Y, Mao AP, Yang Y, Wang YY, Zhang XL, Shu HB. 2009. The ubiquitin ligase RNF5 regulates antiviral responses by mediating degradation of the adaptor protein MITA. Immunity 30:397–407. doi: 10.1016/j.immuni.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Qin Y, Zhou MT, Hu MM, Hu YH, Zhang J, Guo L, Zhong B, Shu HB. 2014. RNF26 temporally regulates virus-triggered type I interferon induction by two distinct mechanisms. PLoS Pathog 10:e1004358. doi: 10.1371/journal.ppat.1004358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuchida T, Zou J, Saitoh T, Kumar H, Abe T, Matsuura Y, Kawai T, Akira S. 2010. The ubiquitin ligase TRIM56 regulates innate immune responses to intracellular double-stranded DNA. Immunity 33:765–776. doi: 10.1016/j.immuni.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Hu MM, Wang YY, Shu HB. 2012. TRIM32 protein modulates type I interferon induction and cellular antiviral response by targeting MITA/STING protein for K63-linked ubiquitination. J Biol Chem 287:28646–28655. doi: 10.1074/jbc.M112.362608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q, Liu X, Cui Y, Tang Y, Chen W, Li S, Yu H, Pan Y, Wang C. 2014. The E3 ubiquitin ligase AMFR and INSIG1 bridge the activation of TBK1 kinase by modifying the adaptor STING. Immunity 41:919–933. doi: 10.1016/j.immuni.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 21.McGeoch DJ, Dalrymple MA, Davison AJ, Dolan A, Frame MC, McNab D, Perry LJ, Scott JE, Taylor P. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol 69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 22.McGeoch DJ, Cook S. 1994. Molecular phylogeny of the alphaherpesvirinae subfamily and a proposed evolutionary timescale. J Mol Biol 238:9–22. doi: 10.1006/jmbi.1994.1264. [DOI] [PubMed] [Google Scholar]
- 23.Kattenhorn LM, Korbel GA, Kessler BM, Spooner E, Ploegh HL. 2005. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol Cell 19:547–557. doi: 10.1016/j.molcel.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Wang K, Li J, Zheng C. 2013. Herpes simplex virus 1 ubiquitin-specific protease UL36 inhibits beta interferon production by deubiquitinating TRAF3. J Virol 87:11851–11860. doi: 10.1128/JVI.01211-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim ET, Oh SE, Lee YO, Gibson W, Ahn JH. 2009. Cleavage specificity of the UL48 deubiquitinating protease activity of human cytomegalovirus and the growth of an active-site mutant virus in cultured cells. J Virol 83:12046–12056. doi: 10.1128/JVI.00411-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma Z, Jacobs SR, West JA, Stopford C, Zhang Z, Davis Z, Barber GN, Glaunsinger BA, Dittmer DP, Damania B. 2015. Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses. Proc Natl Acad Sci U S A 112:E4306–E4315. doi: 10.1073/pnas.1503831112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, Fitzgerald KA, Paludan SR, Bowie AG. 2010. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol 11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishikawa H, Barber GN. 2008. STING is an endoplasmic reticulum adaptor that facilitates innate immune signaling. Nature 455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, Maniatis T. 1995. Signal-induced site-specific phosphorylation targets IκBα to the ubiquitin-proteasome pathway. Genes Dev 9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 30.DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. 1996. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol 16:1295–1304. doi: 10.1128/MCB.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santoro MG, Rossi A, Amici C. 2003. NF-κB and virus infection: who controls whom. EMBO J 22:2552–2560. doi: 10.1093/emboj/cdg267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodkin ML, Ting AT, Blaho JA. 2003. NF-κB is required for apoptosis prevention during herpes simplex virus type 1 infection. J Virol 77:7261–7280. doi: 10.1128/JVI.77.13.7261-7280.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amici C, Rossi A, Costanzo A, Ciafre S, Marinari B, Balsamo M, Levrero M, Santoro MG. 2006. Herpes simplex virus disrupts NF-κB regulation by blocking its recruitment on the IκBα promoter and directing the factor on viral genes. J Biol Chem 281:7110–7117. doi: 10.1074/jbc.M512366200. [DOI] [PubMed] [Google Scholar]
- 34.Su C, Zhan G, Zheng C. 2016. Evasion of host antiviral innate immunity by HSV-1, an update. Virol J 13:38. doi: 10.1186/s12985-016-0495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Wang K, Wang S, Zheng C. 2013. Herpes simplex virus 1 E3 ubiquitin ligase ICP0 protein inhibits tumor necrosis factor alpha-induced NF-κB activation by interacting with p65/RelA and p50/NF-κB1. J Virol 87:12935–12948. doi: 10.1128/JVI.01952-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang K, Ni L, Wang S, Zheng C. 2014. Herpes simplex virus 1 protein kinase US3 hyperphosphorylates p65/RelA and dampens NF-κB activation. J Virol 88:7941–7951. doi: 10.1128/JVI.03394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JC, Lee SY, Kim SY, Kim JK, Kim HJ, Lee HM, Choi MS, Min JS, Kim MJ, Choi HS, Ahn JK. 2008. HSV-1 ICP27 suppresses NF-κB activity by stabilizing IκBα. FEBS Lett 582:2371–2376. doi: 10.1016/j.febslet.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Wang S, Wang K, Zheng C. 2013. Herpes simplex virus 1 DNA polymerase processivity factor UL42 inhibits TNF-α-induced NF-κB activation by interacting with p65/RelA and p50/NF-κB1. Med Microbiol Immunol 202:313–325. doi: 10.1007/s00430-013-0295-0. [DOI] [PubMed] [Google Scholar]
- 39.Xing J, Ni L, Wang S, Wang K, Lin R, Zheng C. 2013. Herpes simplex virus 1-encoded tegument protein VP16 abrogates the production of beta interferon (IFN) by inhibiting NF-κB activation and blocking IFN regulatory factor 3 to recruit its coactivator CBP. J Virol 87:9788–9801. doi: 10.1128/JVI.01440-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zenner HL, Mauricio R, Banting G, Crump CM. 2013. Herpes simplex virus 1 counteracts tetherin restriction via its virion host shutoff activity. J Virol 87:13115–13123. doi: 10.1128/JVI.02167-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen G, Wang K, Wang S, Cai M, Li ML, Zheng C. 2014. Herpes simplex virus 1 counteracts viperin via its virion host shutoff protein UL41. J Virol 88:12163–12166. doi: 10.1128/JVI.01380-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su C, Zhang J, Zheng C. 2015. Herpes simplex virus 1 UL41 protein abrogates the antiviral activity of hZAP by degrading its mRNA. Virol J 12:203. doi: 10.1186/s12985-015-0433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verpooten D, Ma Y, Hou S, Yan Z, He B. 2009. Control of TANK-binding kinase 1-mediated signaling by the γ134.5 protein of herpes simplex virus 1. J Biol Chem 284:1097–1105. doi: 10.1074/jbc.M805905200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang S, Wang K, Lin R, Zheng C. 2013. Herpes simplex virus 1 serine/threonine kinase US3 hyperphosphorylates IRF3 and inhibits beta interferon production. J Virol 87:12814–12827. doi: 10.1128/JVI.02355-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li XD, Wu J, Gao D, Wang H, Sun L, Chen ZJ. 2013. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341:1390–1394. doi: 10.1126/science.1244040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu JJ, Li W, Shao Y, Avey D, Fu B, Gillen J, Hand T, Ma S, Liu X, Miley W, Konrad A, Neipel F, Sturzl M, Whitby D, Li H, Zhu F. 2015. Inhibition of cGAS DNA sensing by a herpesvirus virion protein. Cell Host Microbe 18:333–344. doi: 10.1016/j.chom.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang D, Su C, Zheng C. 2016. Herpes simplex virus 1 serine protease VP24 blocks the DNA-sensing signal pathway by abrogating activation of interferon regulatory factor 3. J Virol 90:5824–5829. doi: 10.1128/JVI.00186-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlieker C, Korbel GA, Kattenhorn LM, Ploegh HL. 2005. A deubiquitinating activity is conserved in the large tegument protein of the herpesviridae. J Virol 79:15582–15585. doi: 10.1128/JVI.79.24.15582-15585.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim YE, Oh SE, Kwon KM, Lee CH, Ahn JH. 2016. Involvement of the N-terminal DUB domain of human cytomegalovirus UL48 tegument protein in auto-ubiquitination, virion stability, virus entry. J Virol 90:3229–3242. doi: 10.1128/JVI.02766-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saito S, Murata T, Kanda T, Isomura H, Narita Y, Sugimoto A, Kawashima D, Tsurumi T. 2013. Epstein-Barr virus deubiquitinase downregulates TRAF6-mediated NF-κB signaling during productive replication. J Virol 87:4060–4070. doi: 10.1128/JVI.02020-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inn KS, Lee SH, Rathbun JY, Wong LY, Toth Z, Machida K, Ou JH, Jung JU. 2011. Inhibition of RIG-I-mediated signaling by Kaposi's sarcoma-associated herpesvirus-encoded deubiquitinase ORF64. J Virol 85:10899–10904. doi: 10.1128/JVI.00690-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xing J, Wang S, Lin F, Pan W, Hu CD, Zheng C. 2011. Comprehensive characterization of interaction complexes of herpes simplex virus type 1 ICP22, UL3, UL4, and UL205. J Virol 85:1881–1886. doi: 10.1128/JVI.01730-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paz S, Vilasco M, Arguello M, Sun Q, Lacoste J, Nguyen TL, Zhao T, Shestakova EA, Zaari S, Bibeau-Poirier A, Servant MJ, Lin R, Meurs EF, Hiscott J. 2009. Ubiquitin-regulated recruitment of IκB kinase epsilon to the MAVS interferon signaling adapter. Mol Cell Biol 29:3401–3412. doi: 10.1128/MCB.00880-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park KA, Byun HS, Won M, Yang KJ, Shin S, Piao L, Kim JM, Yoon WH, Junn E, Park J, Seok JH, Hur GM. 2007. Sustained activation of protein kinase C downregulates nuclear factor-κB signaling by dissociation of IKK-γ and Hsp90 complex in human colonic epithelial cells. Carcinogenesis 28:71–80. doi: 10.1093/carcin/bgl094. [DOI] [PubMed] [Google Scholar]
- 55.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. 1997. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 56.Severa M, Coccia EM, Fitzgerald KA. 2006. Toll-like receptor-dependent and -independent viperin gene expression and counter-regulation by PRDI-binding factor-1/BLIMP1. J Biol Chem 281:26188–26195. doi: 10.1074/jbc.M604516200. [DOI] [PubMed] [Google Scholar]
- 57.Lin R, Lacoste J, Nakhaei P, Sun Q, Yang L, Paz S, Wilkinson P, Julkunen I, Vitour D, Meurs E, Hiscott J. 2006. Dissociation of a MAVS/IPS-1/VISA/Cardif-IKKε molecular complex from the mitochondrial outer membrane by hepatitis C virus NS3-4A proteolytic cleavage. J Virol 80:6072–6083. doi: 10.1128/JVI.02495-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu H, Zheng C, Xing J, Wang S, Li S, Lin R, Mossman KL. 2011. Varicella-zoster virus immediate-early protein ORF61 abrogates the IRF3-mediated innate immune response through degradation of activated IRF3. J Virol 85:11079–11089. doi: 10.1128/JVI.05098-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jordan M, Schallhorn A, Wurm FM. 1996. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res 24:596–601. doi: 10.1093/nar/24.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]