Abstract
Background
HIV-associated neurocognitive disorders (HAND) persist in the post-HAART era, characterized by asymptomatic neurocognitive impairment (ANI) and mild neurocognitive disorders (MND). High mobility group box 1 (HMGB1) is a non-histone chromosomal protein widely expressed in the nucleus of all eukaryotic cells, including brain cells, which acts as a potent proinflammatory cytokine when actively secreted from immune cells. Recent reports suggested that HMGB1 acts on microglial cells to promote neuroinflammation. In this study, our aim was to determine whether HMGB1 is involved in HAND, but also to identify early new markers of neurological impairment in HIV-infected patients.
Methods
CSF and serum were collected from 103 HIV-1-infected patients enrolled in Neuradapt, a prospective study of the prevalence of HAND in HIV-1 infected patients at Nice University Hospital. Stored fluids were assessed for immunological, virological, and brain metabolite parameters. In addition to HIV RNA and DNA measurements, expression of T-cell surface markers of activation (CD38 and HLA-DR) was analyzed on whole blood. Concentration of 27 cytokines and chemokines was measured using multiplex bead assays on serum and CSF. Concentration of HMGB1 and anti-HMGB1 IgG autoantibodies were also measured on the same samples. Changes in cerebral metabolites N-acetyl aspartate (NAA), Choline (Cho) and creatinine (Cr) were assessed by magnetic resonance microscopy (MRS).
Results
Clinical, virological and immunological characteristics were comparable between HAND (n = 30) and no HAND (n = 73) patients, except the absolute numbers of CD8+ T cells, which were higher in patients with HAND. Among the 29 molecules tested, only 4 of them were significantly upregulated in the CSF from HAND patients as compared to healthy donors i.e. HMGB1, anti-HMGB1 IgG antibodies, IP-10 and MCP1. CSF HMGB1 levels were positively correlated with HIV-1 DNA in aviremic HAND patients, suggesting a positive impact of HMGB1 on HIV reservoirs. Moreover, in contrast to NAA/Cr and Cho/NAA ratios, circulating anti-HMGB1 IgG antibody levels could discriminate patients with no HAND from patients with no HAND and a single deficit (average ROC-AUC = 0.744, p = 0.03 for viremic patients), thus enabling the identification of a very early stage of neurocognitive impairment,
Conclusion
We report that brain injury in chronically HIV-infected patients on stable HAART is strongly associated with persistent CNS inflammation, which is correlated with increased levels of HMGB1 and anti-HMGB1 IgG in the CSF. Moreover, we identified circulating anti-HMGB1 IgG as a very early biomarker of neurological impairment in patients without HAND. These results might have important implication for the identification of patients who are at high risk of developing neurological disorders.
Keywords: Neuroscience
1. Introduction
Neurocognitive deficits are common in HIV-infected patients and they are independent of either antiretroviral therapy or disease state [1, 2]. Before the introduction of combined antiretroviral therapies, HIV dementia after AIDS onset developed at an annual rate of 7% [3]. In the post-HAART era, the frequency of HIV-associated neurocognitive disorder (HAND) in long-standing aviremic patients is high [4], with prevalence estimates of 33% for asymptomatic neurocognitive impairment (ANI), 12% for mild neurocognitive disorder (MND), and only 2% for HIV-associated dementia [5]. Persistence of neurocognitive disorders in successfully treated HIV-infected patients is not well understood. Penetration of the brain by HIV-1 is possible during the acute phase of the infection through migrating infected monocytes and CD4 T cells that cross the blood brain barrier and facilitate infection of microglial cells [6]. Effective antiretroviral treatment significantly decreases intrathecal immunoactivation, but a significant proportion of patients continue to show signs of macrophage/microglia activation and immunoglobulin production in the CNS [7].
The risk factors associated with HAND in patients despite virological successful therapy included severe co-morbidities (such as hepatitis C virus co-infection or drug abuse), evidence of failure of HIV therapy, or host factors (genetic predisposition, metabolic disorders or aging) [5, 8, 9]. Attempts to identify predictive biomarkers for HAND have been made. Chemokines in CSF, including IP-10, and MCP1, appeared to be associated with MRS cerebral metabolites, which were validated to be an assessment tool for HAND [10, 11]. Plasma soluble CD14 has potential as a biomarker to monitor HAND progression and therapeutic responses [12], and the burden of HIV DNA in circulating monocytes may identify increased risk for HAND [13, 14]. Thus, increased trafficking of activated monocytes to the brain may lead to neurocognitive impairment.
The high mobility group box 1 (HMGB1) protein is a chromatin-associated protein that acts as a DNA chaperone involved in replication, transcription, DNA repair and genome stability. It is expressed in the nucleus of all eukaryotic cells and, when actively secreted from innate immune cells, including activated macrophages, dendritic cells (DCs) or NK cells, it acts as a danger associated molecular pattern molecule (DAMP) that amplifies proinflammatory response. Moreover, HMGB1 promotes DC functional maturation and migration to lymphoid organs in response to chemokines, involving its specific receptor for advanced glycation end products (RAGE). Thus, HMGB1 acts in an autocrine/paracrine fashion and sustains long-term repair and defense programs [15, 16]. HMGB1 was shown to trigger HIV-replication in HIV-infected DCs, thus contributing to the constitution of viral reservoirs in DCs [17]. Moreover, HMGB1 was able to induce the resistance of HIV-infected DCs to NK killing through the upregulation in DCs of potent anti-apoptotic molecules [18], a process that was reversible by HMGB1 inhibitors. These findings provide new insights into the pivotal role of HMGB1 in promoting viral dissemination and maintaining viability of long-term reservoirs [19].
Stimulated astrocytes specifically release large amounts of HMGB1, and in neuroblastoma cells stimulated with retinoic acid HMGB1 is accumulated in a membrane-bound form at the level of neurite outgrowths, suggesting a role for HMGB1 in promoting cell-cell interaction and development of nerve tissues [20]. Moreover, HMGB1 was identified as a biomarker in multiple sclerosis [21], sepsis [22], involved in post-operative neuroinflammation and cognitive dysfunction [23], and found to be expressed in atherosclerotic lesions of human cerebral arteries [24]. HMGB1 is highly expressed in mouse brain neurons and astrocytes, it is released during brain ischemia, and it contributes to neuroinflammation and postischemic brain damage [25]. Because these findings suggest that HMGB1 contributes to the chronicity of neuroinflammation through a mechanism involving infiltrating macrophages and microglia, we examined a possible involvement of HMGB1 in HAND. We herein demonstrate that increased levels of HMGB1 and anti-HMGB1 antibodies were detected in cerebrospinal fluid (CSF) from a cohort of HIV-infected patients enrolled in the Neuradapt study [26], which were correlated with clinical parameters, immune activation, CSF pattern of cytokines, and cerebral metabolites measured by proton magnetic resonance spectroscopy. Overall, our data suggest that HMGB1 is involved in the pathogenesis of HAND and provide a very early marker of neurological impairment that could identify a population at risk of neurological progression.
2. Methods
2.1. Study design and participants
Neuradapt is a prospective study investigating the prevalence of HAND among HIV-infected patients. Study participants were randomly selected in the Nadis cohort, a large prospective French cohort of HIV-infected patients followed in Nice University Hospital [27], to undergo neuropsychological (NP) evaluation from 2007 to 2013 [28] [26]. No limit on CD4 cell count or viral load were set for inclusion. Exclusion criteria included active opportunistic infection (OI), change or introduction of psychoactive drugs within the last three weeks or any history of neurological disorder. The Ethics Committee in Montpellier (France) approved this study and all subjects gave informed consent prior to screening and enrollment. A control group of healthy donors (HD) was studied. HD were included by the Department of Neurology of Nice University Hospital (France), according to the following criteria: no clinical sign of neurological disorder, normal CSF analysis, and no comorbidities. Ten age-matched HD were studied, M/F sex ratio (3/7), median age 39 years (Interquartile range (IQR) 26–56).
According to the American Academy of Neurology (AAN) 2007 revised criteria [29], the two most frequent categories of impairment are ANI (asymptomatic neurocognitive impairment involving at least two cognitive domains without interference in daily functioning), in which initial symptoms are absent, and MND (mild neurocognitive disorder involving at least two cognitive domains with mild interference in daily functioning), in which symptoms are subtle. Patients with HAD (HIV-associated dementia) exhibit more severe neurocognitive impairment, characterized by at least two cognitive domains with marked interference in daily functioning. A diagnosis of HAND was made for 29% of the Neuradapt cohort, which is comparable to that in other similar cohorts [30].
2.2. Neuropsychological evaluation
For each patient, a battery of neuropsychological tests was administered by a single neuropsychologist. Tests explored a wide spectrum of cognitive domains according to AAN revised criteria [29]: learning and recall, episodic memory, attention/concentration, working memory, executive functions, language, visual agnosia and motor/psychomotor speed, i.e.: – Mini Mental State Evaluation (MMSE) for evaluation of global cognitive function; – Grober & Buschke test for episodic memory; – “Four seconds” Paced Auditory Serial Addition Task (PASAT) for attention/concentration and working memory; – Stroop test for attention/concentration and speed of information processing; – Modified Card Sorting Test for executive functions; – Motor and Psychomotor Test (timed finger tapping and timed alternating hand sequence test) for motor and psychomotor speed abilities; – Verbal Fluency for executive functions and language; – Protocole Montreal-Toulouse d’Evaluation des gnosies visuelles for visual agnosia. The NP scores from each test were converted to z-scores as described elsewhere [29] and were adjusted for age, gender and years of education, using standardized norms. Patients also performed the Montgomery and Asberg Depression Rating Scale (MADRS) and the Neuropsychiatric Inventory (NPI) in order to diagnose a behavioral disorder.
According to the AAN revised criteria, a definition of HAND included the 3 following categories: 1) ANI, defined as HIV-associated asymptomatic neurocognitive impairment involving at least two cognitive domains and documented by a performance of at least 1 SD below the mean on NP tests, without interference in everyday functioning. The asymptomatic characteristics of impairment were defined by the Instrumental Activity of Daily Living short version battery and by interviewing the patient. 2) MND, defined as HIV-associated mild neurocognitive disorder involving at least two cognitive domains and documented by a performance of at least 1 SD below the mean on NP tests, with mild interference in daily functioning. 3) HAD, involving at least two cognitive domains and documented by performing below 2 SD from the normative mean on NP tests, with marked interference on daily functioning. According to the NP test results, HIV-infected patients were classified into two main groups: HAND (i.e. ANI, MND or HAD) and No HAND.
2.3. Magnetic resonance spectroscopy analysis
A single Magnetic Resonance Spectroscopy (MRS) assessment was done on all subjects with HAND. MRS can measure relative changes in cerebral metabolites, including N-acetyl aspartate (NAA), which reflects neuronal injury or loss, Choline (Cho), which reflects membrane remodeling, and creatinine (Cr), which reflects cellular metabolism. According to studies reported in the literature, the NAA/Cr ratio is considered as a marker of neuronal integrity, while the ratios Cho/NAA and Cho/Cr are markers of inflammation [31, 32]. Metabolic changes in basal ganglia (BG) were calculated from a strict middle sagittal T1-weighted slice used to map a set of 24 axial-oblique Fast Spin Echo T2-weighted slices (TR = 4000 ms, TE = 103 ms, ETL = 23, FOV = 240 mm, imaging matrix 320 × 512, 6 mm thickness, no gap) parallel to the line running beneath the rostrum and splenium of the corpus callosum (bi-callosal line). This set of T2-weighted slices was then used to map the spectroscopic studies. Two single voxel (70 × 50 × 24 mm) using a PRESS sequence were acquired. A spectrum with a long TE (TR = 2000 ms, TE = 288 ms) was acquired at the level of left and right BG, the inferior border of the voxel centered on the bi-callosal line.
2.4. Assessment of T-cell surface markers of activation
The expression of surface activation markers was studied on fresh blood kept at room temperature until it is used. CD38 and HLA-DR expression was measured on CD4 and CD8 T cells by six-color flow cytometry using a whole blood cell procedure. Briefly, 50 μL of whole blood were incubated with a mixture of antibodies (all from BD Biosciences, San Jose, CA, USA) namely CD3 − fluorescein isothiocyanate (FITC), CD8 −peridinin-chrorophyll-protein-cyanin 5.5 (PerCP-Cy5.5), CD4 − phycoerythrin cyanin 7 (PC7), CD45 − allophycocyanin 7 (APC-Cy7) and either CD38 − phycoerythrin (PE) and HLA-DR − allophycocyanin (APC) or their irrelevant counterparts, for 15 min in the dark. The red blood cells were lysed using 2 ml of FACS lysing solution (BD Biosciences), washed before being suspended in 500 μL of Cell Wash (BD Biosciences). A minimum of 10,000 lymphocytes was acquired for each condition. Flow cytometric acquisition and analysis were performed on a FACSCanto flow cytometer and analysis was performed using FACSDiva software (v6.1, BD Biosciences).
2.5. Plasma HIV-1 RNA and DNA measurements
Quantification of plasma HIV-1 RNA viral load (VL) was performed by RT-PCR (Ampliprep/CobasTaqman Roche Molecular system), with a lower detection limit of 40 copies/ml. HIV-1 DNA was determined by a modified version of the Amplicor HIV-1 Monitor test (version1.5 Roche Diagnostics) with an internal HIV-1 DNA standard provided by Roche Laboratories (limit detection of 10 copies/106 PBMC). CD4 and CD8 counts were assessed by standard flow-cytometry.
2.6. Multiplex-bead assay of cytokines in CSF and serum
We used Luminex MAP technology for multiplexed quantification of cytokines in the CSF and serum. At patient’s inclusion, blood was drawn and lumbar puncture was performed. Serum and aliquots of the CSF sample were centrifuged and the supernatant immediately frozen at −80 °C. Samples were stored until use. Assessment of cytokine concentration on CSF and serum samples was performed using a 27-plex kit in accordance with the instructions provided (Biorad Bio-Plex Pro Human cytokine assay). Analyzed cytokines included (lower detection limit given within brackets) IL-1β (3.2 pg/ml), IL-2 (2.1 pg/ml), IL-4 (2.2 pg/ml), IL-5 (3.1 pg/ml), IL-6 (2.3 pg/ml), IL-7 (3.1 pg/ml), IL-8 (1.9 pg/mL), IL-9 (2.1 pg/ml), IL-10 (2.2 pg/ml), IL-12 p70 (3.3 pg/ml), IL-13 (3.7 pg/ml), IL-15 (2.1 pg/ml), IL-17 (4.9 pg/ml), Eotaxin (40.9 pg/ml), basic FGF (27.2 pg/ml), G-CSF (2.4 pg/ml), GM-CSF (63.3 pg/ml), IFN-γ (92.6 pg/mL), IP-10 (18.8 pg/ml), MCP1 (2.1 pg/ml), MIP-1α (1.4 pg/ml), MIP-1ß (2 pg/ml), PDGF-BB (7 pg/ml), RANTES (2.2 pg/ml), TNF-α (5.8 pg/ml) and GM-CSF (0.75 pg/ml), VEGF (5.5 pg/ml). Briefly, 50 μL of CSF or serum were incubated with antibody-linked magnetic beads for 2 h, washed twice and incubated for 1 h with biotinylated secondary antibodies. A final incubation step of 30 min with streptavidin-phycoerythrin secondary antibodies preceded acquisition on the Luminex 100IS and the data were acquired using the StarStation 2.0 software (Applied cytometry systems, Sheffield, UK).
2.7. Assessment of HMGB1 and anti-HMGB1 autoantibody concentrations in CSF and serum
Quantification of CSF and circulating HMGB1 was performed with an ELISA kit (IBL, Hamburg). Considering that HMGB1 forms highly inflammatory complexes with ssDNA, LPS, IL-1beta, nucleosomes, and also antibodies [33], and taking into account our own data aimed at identifying what form of HMGB1 (bound or unbound) is detected by this ELISA kit, we considered that this kit only detected the unbound HMGB1 molecule. We called it “residual HMGB1”. The concentrations of anti-HMGB1 autoantibodies in CSF and serum from patients’ samples were determined with an in house quantitative ELISA assay. 96-well plates were coated overnight at 4∘C with recombinant HMGB1 (HMGBiotech, HM-115) in PBS. Simultaneously, coating of serial dilutions of the calibrator human serum IgG (Sigma) was performed. After washing the plates with PBS/0.05% (v/v) Tween® 20, unbound sites were blocked with PBS/2% (w/v) BSA. Since anti-HMGB1 antibodies were reported to bind HMGB1 in serum [34], we dissociated these complexes before titration of the antibodies. Thus, CSF and serum samples were treated with 1.5 M Glycine and further diluted with 1.5 M Tris, v/v, pH 9,0. Treated samples were then immediately diluted (from 1/10 to 1/1000) and distributed on coated plates. Goat anti-human IgG alkaline phosphatase-conjugated antibodies were added, and detection of HMGB1-specific antibodies was performed after 30 min of incubation at 37 °C with 100 μl pNPP substrate. Concentration of HMGB1-specific antibodies was calculated according to the standard curve obtained from standard immunoglobulin solution absorbance by Ascent software, Thermo Electrocorp, as we previously reported [35]. The data are expressed in ng/ml of total anti-HMGB1 antibodies detected.
2.8. Quantification of plasma LPS
LPS was determined in heat-inactivated plasma using a commercially available limulus amebocyte lysate assay (LAL) test (Lonza, Basel, Switzerland) according to the manufacturer's instructions. Briefly, samples diluted 1/5 with endotoxin-free water were heated at 70 °C for 10 min to inactivate interfering plasma components. After centrifugation and incubation with the LAL reagent and the chromogen, samples were measured in duplicate at 405 nm in a photometric plate reader, and background, if present, was subtracted.
2.9. Statistical analyses
Data are expressed as Median and IQR range. Statistical comparisons were performed using a two-tailed unpaired non-parametric Mann–Whitney U test for comparison between groups, and the Wilcoxon’s signed rank- tests was used for comparisons within study group. The relationship between the panel of immunological and neurological biomarkers and early neurological impairment was assessed by receiver operating characteristics area under curve (ROC-AUC) analysis. A Spearman rank test was used for all correlations. Statistical analyses were performed with Prism version 6.0a (GraphPad Software, Inc).
3. Results
3.1. Demographic parameters and clinical characteristics
103 patients from the Neuradapt cohort participated to the immunological ancillary study. Table 1 summarizes the clinical characteristics of the patients upon inclusion. The median age was 43 years, 65% had undetectable viral load, and median plasma HIV-1 RNA was 1.6 log10 cp/ml. Median CD4 count was 495 cells/μL, median nadir CD4 count was 227 cells/μL, and median CD8 count was 807 cells/μL. 29% of the patients were HCV co-infected, and the median time since infection by HIV-1 was 14.5 years. 77% of the patients were on combined antiretroviral therapy (c-ART) and the median time on current treatment was 2 years. Among the 103 patients studied, 73 had no HAND (71%) and they included 37 patients classified as no HAND with a single deficit. The 30 patients diagnosed with HAND (29%) included 16 patients with ANI, 12 patients with MND, and 2 patients with HAD. No major forms of depression were identified. The main alterations detected were impairments in the speed of information processing, recall memory, attention/concentration, working memory and motor skills. As far as viraemia is concerned, 13 among the 36 no HAND patients were viremic, as well as 12 among 37 no HAND patients with a single deficit, 5 among 16 ANI patients, and 7 among 14 MND + HAD patients. Some of the patients were treated with HAART, including 29 among the 36 no HAND patients (23 were aviremic), 30 among 37 no HAND patients with a single deficit (25 were aviremic), 14 among 16 ANI patients (11 were aviremic), and 11 among 14 MND + HAD patients (7 were aviremic).
Table 1.
Patient demographics and clinical characteristics.
| HIV+ Group | no HAND | HAND | p* | |
|---|---|---|---|---|
| Number of patients (%) | 103 | 73 (71%) | 30 (29%) | |
| Age at Baseline (years) Median (IQR)# |
43 (38–50) | 43 (36–49) | 44 (40–55) | 0.12 |
| HCV positive (%) | 29% | 27% | 30% | 0.66 |
| HIV RNA plasma (log10 cp/ml) Median (IQR) |
1.6 (1.6–2.4) | 1.6 (1.6–2.6) | 1.6 (1.6–2) | 0.87 |
| Undetectable HIV RNA (%) | 65% | 65% | 64% | 0.85 |
| Nadir CD4 count (cells/μL) Median (IQR) |
227 (76–350) | 216 (78–372) | 238 (58–350) | 0.74 |
| CD4+ cell count (cells/μL) Median (IQR) | 495 (358–747) | 480 (372–729) | 504 (351-765) | 0.83 |
| CD8+ cell count (cells/μL) Median (IQR) |
807 (549–1089) | 765 (543–1023) | 1045 (732–1399) | 0.02 |
| Duration of HIV infection (years) Median (IQR) | 14.5 (5–19) | 13.5 (5–18) | 15.5 (7.5–20) | 0.49 |
| On antiretroviral therapy (%) | 77% | 75% | 77% | 0.47 |
| Years of current treatment Median (IQR) | 2 (1–2.8) | 1.45 (1–2.35) | 2 (1.5–3) | 0.1 |
*Wilcoxon Mann Whitney test comparison between HAND and no HAND patients. Statistically significant p value ≤0.05 is written in bold.
#IQR: Interquartile range.
Patient baseline characteristics were not different between the HAND and no HAND groups, except the absolute number of CD8 T cells that was higher in the HAND group (Table 1). The median number of CD4 T cells was quite high in both groups (504 and 480 cells/μL, respectively), and plasma viral load was undetectable in two third of the patients (64 and 65%, respectively). Same proportions of patients were under cART in both groups (77% and 75% respectively) and they had a stable treatment for a median of 18 to 24 months. Duration of HIV infection was also similar (median duration 15.5 and 13.5 years for HAND and no HAND groups, respectively).
3.2. Specific cytokine pattern in CSF from HAND patients
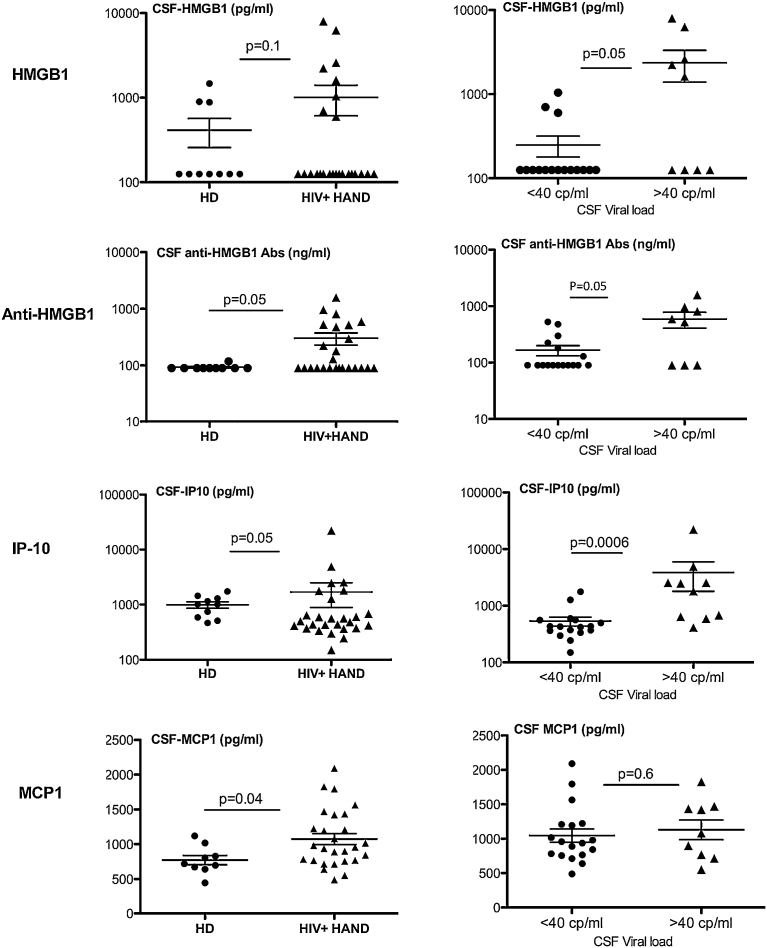
CSF from all HAND patients and healthy donors (HD) were tested for their content in cytokines with the MAP technology. Moreover, the levels of HMGB1 and anti-HMGB1 autoantibodies were measured, as described in the Methods section. Among the 29 molecules tested (including the 27 cytokines listed in the Methods section), only 4 of them were significantly increased in CSF from HAND patients as compared to healthy donors. Those are HMGB1, anti-HMGB1 antibodies (p = 0.05), IP-10 (p = 0.05) and MCP1 (p = 0.04) (Fig. 1). Except for MCP1, CSF levels of these molecules were significantly higher in viremic HAND patients (p = 0.05 for HMGB1 and anti-HMGB1, and p = 0.0006 for IP-10) (Fig. 1), and they were positively correlated with immune activation, as measured by the percentages of peripheral CD8+ T cells expressing the surface activation markers CD38 and/or HLA-DR (Table 2, see below). Moreover, CSF levels of anti-HMGB1 antibodies (r = −0.343 and p = 0.05), IP-10 (r = −0.644 and p = 0.0005), and MCP1 (r = −0.477, p = 0.016) were correlated with disease evolution, as measured by the decreased number of blood CD4+ T cells (Table 2).
Fig. 1.
Specific cytokine/chemokine pattern in CSF from HAND patients compared with healthy donors. CSF from HAND patients (n = 30) and healthy donors (HD) (n = 10) were tested for their content in cytokines and chemokines with the MAP technology (27-plex). In addition, circulating residual HMGB1 and total (residual + HMGB1 bound) HMGB1-specific IgG antibodies were measured with an in-house ELISA assay, as described in M&M. Only 4 molecules, HMGB1, anti-HMGB1, IP-10 and MCP1, were significantly increased in CSF from HAND patients vs HD. Left panel: two-sided Mann-Whitney statistical comparison of CSF levels of the indicated molecules in HAND patients vs HD, p values are indicated. Right panel: CSF levels of the indicated molecules as a function of viral load, with a cut-off at 40 cp HIV-RNA/ml). Two-sided Mann-Whitney p values are indicated.
Table 2.
CSF cytokine/chemokine levels as a function of clinical variables and immune activation markers.
| All HAND(a) |
Aviremic HAND(a) |
|||
|---|---|---|---|---|
| r(b) | p(b) | r(b) | p(b) | |
| CSF HMGB1 | ||||
| CSF-VL | 0.590 | 0.003 | 0.157 | NS |
| HIV-DNA | 0.697 | 0.0002 | 0.646 | 0.003 |
| CD8+HLA-DR+ T | 0.697 | 0.0006 | 0.511 | 0.050 |
| CSF Anti − HMGB1 | ||||
| nCD4 | −0.343 | 0.050 | −0.114 | NS |
| CD8+HLA-DR+ T | 0.455 | 0.033 | 0.239 | NS |
| CD4+HLA-DR+ T | 0.455 | 0.033 | 0.354 | NS |
| CSF IP-10 | 0.477 | 0.015 | 0.159 | NS |
| CSF IP10 | ||||
| nCD4 | −0.644 | 0.0005 | −0.566 | 0.014 |
| CSF VL | 0.567 | 0.003 | 0.159 | NS |
| CD8+CD38+ T cells | 0.455 | 0.022 | 0.295 | NS |
| CD8+CD38+HLA-DR+ T | 0.533 | 0.007 | 0.359 | NS |
| CSF anti-HMGB1 | 0.477 | 0.016 | 0.236 | NS |
| CSF MCP1 | 0.422 | 0.036 | 0.456 | 0.050 |
| CSF MCP1 | ||||
| Nadir CD4 | −0.590 | 0.009 | −0.539 | 0.021 |
| nCD4 | −0.477 | 0.016 | −0.475 | 0.045 |
| CD8+CD38+ | 0.347 | 0.08 | 0.541 | 0.02 |
| CSF IP-10 | 0.422 | 0.036 | 0.456 | 0.050 |
| CSF VL | ||||
| Plasma VL | 0.638 | 0.0003 | ND | ND |
| CSF HMGB1 | 0.590 | 0.003 | 0.157 | NS |
| CSF IP10 | 0.567 | 0.003 | 0.159 | NS |
(a): all HAND (n = 25), aviremic HAND (n = 18). Aviremic refers to plasma viral load.
(b): Spearman’s correlation coefficient (r) and p values for differences between groups are indicated.
Values in italics highlight significant correlations persisting in aviremic HAND patients.
ND: not done.
3.3. Correlation of CSF immune mediators with immune activation and HIV-DNA
Table 2 shows the correlations between CSF levels of HMGB1, anti-HMGB1 antibodies, IP-10 and MCP1, and clinical variables in either all HAND or aviremic HAND patients. In all HAND patients, concentrations of CSF HMGB1 and IP-10 correlated with CSF viral load, while concentrations of all four mediators correlated with clinical evolution, as measured by CD4 cell numbers. Strikingly, CSF HMGB1 levels were positively correlated with HIV-DNA (r = 0.697, p = 0.0002) in all HAND patients, and this correlation persisted in aviremic patients (r = 0.511, p = 0.003). suggesting a positive impact of HMGB1 on HIV reservoirs. Persistent immune activation had a positive impact on CSF levels of the four mediators in all HAND patients since they were positively correlated with the percentages of CD8+ T cells expressing HLA-DR and/or CD38, and these correlations were still detected in aviremic patients for HMGB1 and MCP1. A positive correlation between CSF levels of IP-10 and MCP1 was found in all HAND patients (r = 0.422, p = 0.036) and it persisted in aviremic patients (r = 0.456, p = 0.05). IP-10 and anti-HMGB1 concentrations in CSF were also positively correlated (r = 0.477, p = 0.016) in all HAND patients (Table 2). Lastly, we analyzed the relationship between plasma and CSF viral load in all HAND patients. A strong positive correlation was found in these two compartments (r = 0.638) (Table 2). While almost 2/3 (60%) of HAND patients had undetectable viral load in both plasma and CSF, 23% were viremic in both compartments (range 58–240.000 cp/ml in plasma, 50 − 90.000 cp/ml in CSF), and 15% were viremic in plasma (range 120 − 1500 cp/ml) but aviremic in CSF. None of the patients with undetectable viral load in plasma had detectable viral load in CSF. Of note, comparison of HIV-1 genotypes in LCR and plasma in patients viremic in both compartments showed no difference, excluding the hypothesis of a compartmentalization of the CNS.
3.4. Circulating levels of immune mediators in HAND vs no HAND patients
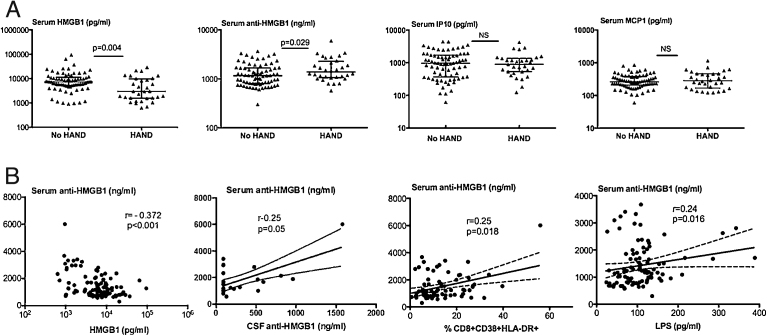
The circulating levels of HMGB1, anti-HMGB1 antibodies, IP-10 and MCP1 were assessed and compared between the HAND and no HAND groups (Fig. 2A). While the levels of IP-10 and MCP1 were not different between the two groups, anti-HMGB1 antibody levels were significantly increased in patients with HAND (p = 0.029). In parallel, circulating residual HMGB1 levels were significantly decreased in HAND patients, and accordingly an inverse correlation was found between serum HMGB1 and anti-HMGB1 levels (Fig. 2B). Interestingly, the concentrations of serum and CSF anti-HMGB1 antibodies were positively correlated (Fig. 2B). As found for CSF anti-HMGB1 antibodies (Table 2), serum levels of anti-HMGB1 antibodies were correlated with immune activation, as measured by the percentages of CD8+CD38+HLA-DR+ T cells (Fig. 2B), and LPS concentrations (Fig. 2B). Of note, the association of serum anti-HMGB1 antibodies and activated CD8 T cells persisted under HAART (r = 0.25, p = 0.018).
Fig. 2.
Circulating levels of immune mediators in HAND and no HAND patients. Comparative levels of circulating levels of residual HMGB1, total anti-HMGB1 IgG antibodies, IP-10 and MCP1 in serum from HAND (n = 30) and no HAND patients (n = 73). Two-sided Mann-Whitney p values are indicated (A). Spearman correlations between total anti-HMGB1 antibodies and serum residual HMGB1, CSF total anti-HMGB1 antibodies, % HLA-DR+CD38+CD8 T cells, and circulating LPS levels (B).
3.5. Circulating anti-HMGB1 IgG autoantibodies identify a very early stage of neurological impairment
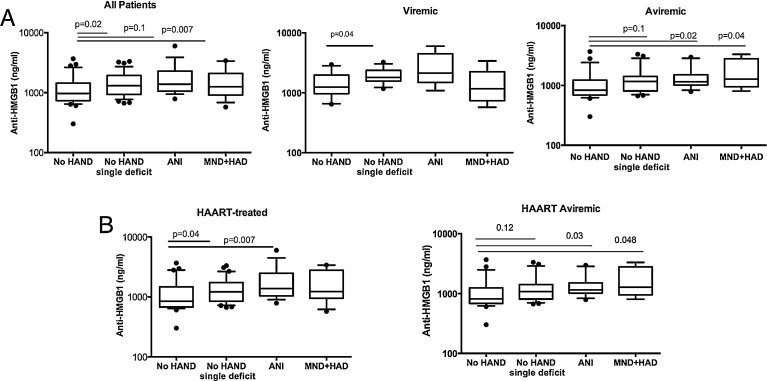
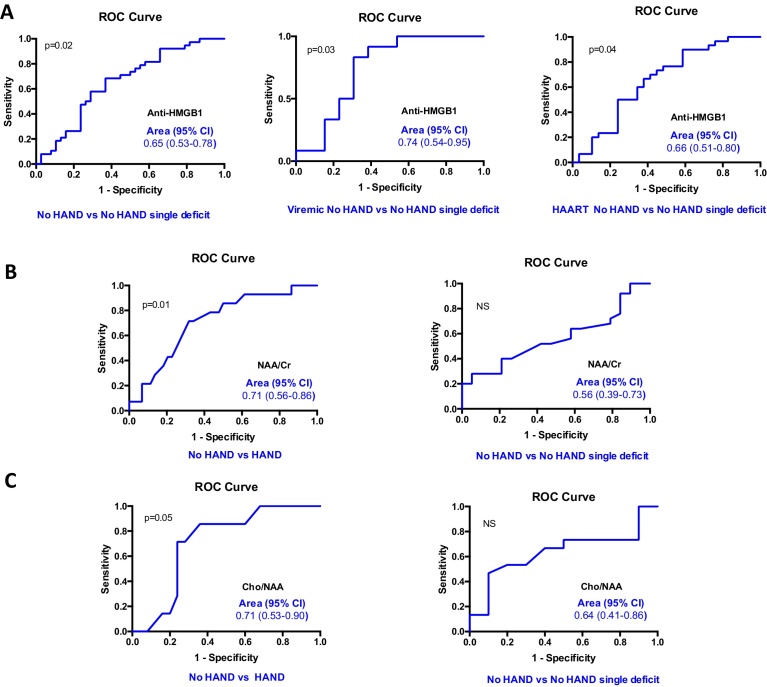
With the aim to identify biomarkers associated with early stages of neurocognitive disorders, the 103 studied patients were stratified into four groups i.e. no HAND, no HAND with a single deficit, ANI and MND + HAD according to NP test results (as detailed in the Methods section) and AAN revised criteria [29]. Among the four biomarkers identified in CSF to be significantly increased in HAND patients as compared to healthy donors (Fig. 1) serum level of total anti-HMGB1 IgG antibodies was the only one that allowed the identification of a very early stage of neurocognitive impairment, i.e. patients with no HAND and a single deficit (Fig. 3A). This finding persisted when patients were stratified according to plasma viral load and, in aviremic HAND patients, the levels of total anti-HMGB1 IgG antibodies were significantly increased in ANI and MND + HAD patients while a trend was still detected in no HAND patients with a single deficit as compared to no HAND patients (Fig. 3A). The identification of serum level of total anti-HMGB1 IgG antibodies as a biomarker of a very early stage of neurological impairment was also true for patients under HAART, and it persisted as a trend in aviremic HAART patients (Fig. 3B). The discriminatory power of anti-HMGB1 IgG antibodies as a very early marker of neurological impairment was characterized using a ROC-AUC analysis of patients with no HAND vs patients with no HAND with a single deficit (Fig. 4A). The average of ROC-AUC was 0.744 for the viremic patients (p = 0.03), 0.654 for all patients (p = 0.02) and 0.655 for patients under HAART (p = 0.04).
Fig. 3.
Circulating anti-HMGB1 antibodies identify a very early stage of neurological impairment. The patients (n = 103) were stratified into four groups i.e. no HAND (n = 37), no HAND with a single deficit (n = 37), ANI (n = 16) and MND + HAD (n = 13) according to NP test results and AAN revised criteria, as detailed in M&M. Comparative levels of circulating total anti-HMGB1 IgG antibodies were compared between the four groups of patients. Same analysis was performed for the viremic patients no HAND (n = 13), no HAND with a single deficit (n = 12), ANI (n = 5) and MND + HAD (n = 6), and aviremic patients: no HAND (n = 24), no HAND with a single deficit (n = 25), ANI (n = 11) and MND + HAD (n = 7). Two-sided Mann-Whitney p values are indicated (A). Same analysis was performed for HAART patients [no HAND (n = 29), no HAND with a single deficit (n = 30), ANI (n = 14) and MND + HAD (n = 11)], and aviremic HAART patients [no HAND (n = 23), no HAND with a single deficit (n = 24), ANI (n = 11) and MND + HAD (n = 7). Two-sided Mann-Whitney p values are indicated (B).
Fig. 4.
Receiver operating characteristic (ROC) analysis of anti-HMGB1 antibodies, NAA/Cr and Cho/NAA in relation with HAND status. ROC curve analysis comparing the performance of anti-HMGB1 antibodies (panel A), NAA/Cr (panel B) and Cho/NAA (panel C) for discrimination of HAND vs no HAND patients, and no HAND vs no HAND with a single deficit patients. Area under the curve and 95% CI are indicated.
Altogether, these observations suggest that serum levels of total anti-HMGB1 IgG autoantibodies are an early marker of the onset of HIV-associated neurological impairment.
3.6. Association between total anti-HMGB1 IgG autoantibodies, chemokines and cerebral metabolites
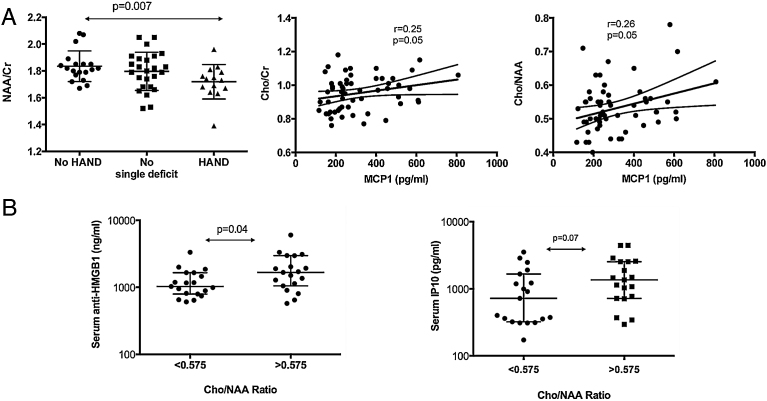
Changes in cerebral metabolites were analyzed and their relationships with the studied biomarkers were assessed. The ratio of NAA to total creatinine [NAA/Creatinine (Cr)] is a marker of neuronal metabolism and neuronal injury. NAA/Cr levels in the frontal white matter were significantly lower in the HAND group compared to the no HAND group (p = 0.007), while they did not differ in the no HAND group with a single deficit (Fig. 5A). The discriminatory power of NAA/Cr ratio as a marker of neurological impairment was characterized using a ROC-AUC analysis (Fig. 4B). The average of ROC-AUC was 0.711, confirming that NAA/Cr ratio provides good discrimination between patients with no HAND vs patients with HAND. However, NAA/Cr ratio was not able to discriminate between patients with no HAND and patients with no HAND with a single deficit, the average AUC being 0.563 and p > 0.05 (Fig. 4B).
Fig. 5.
Association between serum total anti-HMGB1 IgG antibodies, chemokines and cerebral metabolites. Changes in cerebral metabolites were analyzed and their relationships with the studied biomarkers were assessed. The ratio of NAA to total creatinine [NAA/Creatinine (Cr)] is a marker of neuronal metabolism and neuronal injury, while the ratio of Choline (Cho)/Cr and Cho/NAA are markers of inflammation. NAA/Cr levels in the frontal white matter were compared between the HAND group (n = 19), the no HAND group with a single deficit (n = 19) and the HAND group (n = 9). Two-sided Mann-Whitney p values are indicated (left-hand side). Spearman correlations between serum MCP1 levels and Cho/Cr ratio (n = 57) and Cho/NAA ratio (n = 57) are shown (right-hand side)(A). The Cho/NAA ratio with a cut-off identified at 0.575 was found to be the best diagnostic performance using Roc curves. Serum total anti-HMGB1 IgG antibody and IP-10 levels were compared based on this ratio (n = 19 in each group). Two-sided Mann-Whitney p values are indicated (B).
Regarding the correlations of chemokine concentrations with cerebral metabolites, higher levels of MCP1 correlated with higher levels of Cho/Cr (Spearman r = 0.25, p = 0.05) and higher levels of Cho/NAA (Spearman r = 0.26, p = 0.05), both ratios being markers of inflammation (Fig. 5A). Using ROC curves, the Cho/NAA ratio displayed the best diagnostic performance for identifying patients with HAND, with a cut-off identified at 0.575 (data not shown). Considering this cut-off, significantly increased levels of anti-HMGB1 were associated with higher levels of Cho/NAA (p = 0.04). In a similar way, IP-10 levels were increased in patients with Cho/NAA ratio >0.575, although the statistical significance was not reached (Fig. 5B).
ROC-AUC analysis confirmed that Cho/NAA ratio provides good discrimination between patients with no HAND and patients with HAND (ROC-AUC = 0.711, p = 0.05). However, it revealed that Cho/NAA ratio is not a marker of early neurological impairment (ROC-AUC = 0.636 for comparison of patients with no HAND and patients with no HAND with a single deficit, p > 0.05) (Fig. 4C).
Altogether, these data argue for the use of circulating anti-HMGB1 IgG autoantibodies as a very early biomarker of neurological impairment in patients infected with HIV.
4. Discussion
Extracellular HMGB1 is a danger signal that originates from damaged cells and cells of the innate immune system, and it is an important trigger of inflammation and a stimulus for tissue reconstruction. It exhibits several molecular features including the ability to switch among mutually exclusive redox states, reduced cysteines making HMGB1 a chemoattractant, whereas a disulfide bonds makes it a proinflammatory cytokine [36]. HMGB1 forms highly inflammatory complexes with LPS, ssDNA and cytokines, including IL-1ß and CXCL12, which interact with TLR4, TLR9, IL-1-R and CXCR4, respectively [33]. In the rodent brain, HMGB1 is expressed in the nucleus of both neurons and astrocytes, it translocates to the cytoplasm of neurons in ischemic brain tissue and it is then released in the CSF and serum [25]. Brain microinjection of HMGB1 increases ischemic volumes in the mouse, it concomitantly increases transcript levels of inducible nitric oxide synthase (iNOS) and interleukin-1ß, suggesting that HMGB1 plays an active role in progression of ischemic brain injury [25]. In an in vitro rat blood brain barrier (BBB) system, HMGB1 increased the permeability of the BBB with morphological changes in endothelial cells. In vivo administration of anti-HMGB1 monoclonal antibodies ameliorated ischemic brain damage [37]. In humans, the participation of HMGB1 to neuropathology is suggested by reports indicating that plasma levels of HMGB1 are elevated in stroke patients [38], brains from patients affected by Alzheimer disease show increased levels of HMGB1 in microglia, and the highest HMGB1 serum levels observed in relapsing-remitting patients with multiple sclerosis compared to primary progressive and secondary progressive patients [39].
In the present study, we report for the first-time increased levels of HMGB1 in the CNS from HIV-infected individuals. Moreover, we show that HMGB1 is part of a CSF molecular pattern characterizing patients with HAND. This pattern also includes anti-HMGB1 IgG autoantibodies. We developed a homemade assay aimed at quantifying “total” circulating anti-HMGB1 antibodies in CSF and serum from healthy subjects or HIV-infected patients. Since anti-HMGB1 antibodies were reported to form a complex with HMGB1 in serum [34], we dissociated these complexes with an acidic treatment before titration of the antibodies. Circulating anti-HMGB1 antibodies were reported in patients with autoimmune diseases including SLE, which positively correlated with the SLEDAI score [40]. However, we herein report that anti-HMGB1 autoantibodies were also detected in most healthy individuals, both in CSF and serum. Accordingly, Urbonaviciute et al. reported the detection in plasma and serum of IgG antibodies binding to HMGB1 and interfering with its detection by ELISA [34]. The physiological role of anti-HMGB1 autoantibodies in healthy subjects is unclear. However, it has been reported that anti-cytokine autoantibodies are ubiquitous in healthy individuals [41], and it was suggested that they may regulate the biological activities of cytokines. Anti-HMGB1 autoantibodies may neutralize HMGB1 to regulate its proinflammatory activity, HMGB1-autoantibody complexes may act as reservoir for HMGB1 and also exhibit bioactivity via Fcγ receptor, as suggested for antibodies to other cytokines [41]. In HIV-infected patients, we found an inverse correlation between total circulating anti-HMGB1 IgG antibodies and HMGB1, suggesting a neutralizing activity of the antibodies that modulates the proinflammatory activity of HMGB1. However, reliable quantification of HMGB1 remains an issue since it may be impeded by its propensity to form complexes with unidentified serum proteins, and ELISA may detect only predominant free bioavailable HMGB1, as suggested by Urbonaviciute et al. [34] who noticed that lower concentrations of HMGB1 were detected by ELISA in comparison with Western blot analysis.
Comparison of CSF cytokines levels in patients with HAND vs healthy individuals revealed that only two molecules were significantly increased in HAND patients, e.g. IP-10 and MCP1. Higher levels of these two chemokines in CSF were associated with persistent immune activation (as measured by the frequency of CD8+ T cells expressing HLA-DR and/or CD38), CSF viral load and disease evolution. The analyses of cerebral metabolites revealed a strong positive association between MCP1 and Cho/Cr and Cho/NAA, which was not found for IP-10. These observations are in agreement with a recent study showing a positive correlation between MCP1 and Cho in BG [42], and they extend prior evidence of the association between CSF MCP1 levels and a composite score of glial markers that included Cho in BG [43]. Thus, monocyte chemotaxis, as indicated by MCP1, may have a significant role in brain inflammation. Besides MCP1, already identified as a biomarker of HAND, our study provides evidence for a new biomarker, namely anti-HMGB1 IgG. This is the first report of the detection of anti-HMGB1 IgG antibodies in CSF, and their levels were associated with the percentage of peripheral activated CD8+ HLA-DR+ CD38+ T cells, IP-10 concentration in CSF and disease evolution.
We also report a positive correlation between serum anti-HMGB1 IgG levels and LPS concentration. Taking into account the association between LPS levels and HAND that we previously reported [28], we suggest that increased trafficking of activated monocytes to the brain, triggered by MCP1, associated with hyper-expression of CCR5 related to LPS plasma levels contributes to the appearance of HIV-associated neurocognitive disorders [44]. Using Roc curves, the Cho/NAA ratio appeared to display the best diagnostic performance for identifying HAND, with a cut-off at 0.575. Considering this cut-off, significantly increased levels of anti-HMGB1 antibodies were associated with higher Cho/NAA ratios. Interestingly, among the four biomarkers that we found associated with HAND, anti-HMGB1 IgG level was the only one that enabled to identify patients with no HAND but a single neurocognitive deficit. Moreover, it is important to stress that changes in anti-HMGB1 IgG levels preceded changes in cerebral metabolite patterns, and ROC analyses demonstrated that serum anti-HMGB1 IgG levels provide good discrimination between patients with no HAND and patients with no HAND and a single deficit, in contrast to NAA/Cr and Cho/NAA ratios.
Thus, circulating total anti-HMGB1 IgG antibodies levels appear to be a very early marker of HIV-associated neurocognitive deficit, preceding changes in chemokine levels and MRS metabolites, and valid for the whole cohort of patients, viremic patients and HAART-treated patients as well. In addition, the clinical usefulness of anti-HMGB1 IgG is demonstrated by the easy detection in serum since a clear positive correlation was found between serum and LCR levels of anti-HMGB1 IgG, thus avoiding an invasive procedure to collect CSF. Indeed, it is essential to identify as early as possible patients at risk for HAND because it was demonstrated that asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline [45], and in any case these cognitive deficits are associated with poor compliance to antiretroviral treatment, lowest quality of life and higher mortality [1, 46].
Another interesting and unexpected finding in this study is the positive association between CSF HMGB1 levels and CSF HIV DNA in all HAND patients, but also in aviremic HAND patients. This finding suggests that HMGB1 may contribute to the establishment and maintenance of HIV reservoirs [19]. Indeed, we previously reported several mechanisms whereby HMGB1 may contribute to HIV persistence and dissemination. For instance, HMGB1 was found to contribute to the persistence of infected cells by triggering the upregulation of two potent apoptosis inhibitors, c-FLIP and c-IAP2, protecting infected DCs from NK-mediated TRAIL-dependent apoptosis [18]. Moreover, HMGB1 may be involved in viral dissemination, as it could reactivate ex-vivo quiescent HIV-1 from latently infected PBMC from aviremic HIV-infected patients [47], and it could also promote viral replication in latently infected DCs [17]. Circulating extracellular HMGB1 can combine with microbial products, including LPS with which it forms highly inflammatory complexes that signal through the TLR4 [33], and HMGB1-LPS complexes may be important in perpetuating inflammatory amplification loops in HIV infection. Therefore, in the context of a chronic viral infection, increased circulating levels of HMGB1 may contribute to viral persistence and immune activation, and subsequent CNS inflammation.
4.1. Concluding remarks
Taken together, our observations suggest that brain injury in chronically HIV-infected patients on stable combination antiretroviral therapy is strongly associated with persistent CNS inflammation, combined with increased levels of CNS HMGB1 and anti-HMGB1 autoantibodies, HMGB1 being identified as a marker of HIV reservoir. Elevated CSF levels of MCP1 and IP-10 chemokines were also detected in patients with HAND that may promote the infiltration of pathogenic inflammatory cells or directly induce neuronal cell death [48]. Moreover, our data strongly suggest that circulating anti-HMGB1 IgG autoantibodies may be considered as an early clinical biomarker of the onset of HIV-associated neurocognitive impairment, which precedes changes in the levels of chemokines and MRS metabolites, and which would help to identify patients who are at high risk of developing neurological impairment.
Declarations
Author contribution statement
Marie-Lise Gougeon: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Béatrice Poirier-Beaudouin, Héla Saïdi, Valérie Seffer, Michel Ticchioni, Stephane Chanalet, Helene Carsenti: Performed the experiments; Analyzed and interpreted the data.
Jacques Durant, Christine Lebrun-Frenay, Christian Pradier, Pierre Dellamonica: Conceived and designed the experiments.
Alexandra Harvey-Langton, Muriel Laffon, Jacqueline Cottalorda: Performed the experiments.
Matteo Vassallo: Conceived and designed the experiments.
Competing interest statement
The authors declare no conflict of interest.
Funding statement
The Neuradapt study received funding from Abbott, Glaxo-Smith-Kline, Boehringer, Gilead, Tibotec and Merck. These pharmaceutical companies took no part in the design or implementation of the study, the collection, management, analysis or interpretation of the data or the preparation, review or approval of the manuscript.
Additional information
No additional information is available for this paper.
Acknowledgements
We wish to thank the patients who agreed to take part in the Neuradapt study. We are grateful for the help of Monique Massard, Valerie Martinez, Francoise Alexis, Ghislaine Valentini, Suzanne Raynaud and Marie-Ange Serini, who performed blood sample collection.
References
- 1.Heaton R.K., Franklin D.R., Ellis R.J., McCutchan J.A., Letendre S.L., Leblanc S. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mateen F.J., Shinohara R.T., Carone M., Miller E.N., McArthur J.C., Jacobson L.P. Neurologic disorders incidence in HIV+ vs HIV- men: Multicenter AIDS Cohort Study, 1996–2011. Neurology. 2012;79(18):1873–1880. doi: 10.1212/WNL.0b013e318271f7b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McArthur J.C., Hoover D.R., Bacellar H., Miller E.N., Cohen B.A., Becker J.T. Dementia in AIDS patients: incidence and risk factors: Multicenter AIDS Cohort Study. Neurology. 1993;43(11):2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- 4.Simioni S., Cavassini M., Annoni J.M., Rimbault Abraham A., Bourquin I., Schiffer V. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 5.Heaton R.K., Clifford D.B., Franklin D.R., Jr., Woods S.P., Ake C., Vaida F. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivey N.S., MacLean A.G., Lackner A.A. Acquired immunodeficiency syndrome and the blood-brain barrier. J. Neurovirol. 2009;15(2):111–122. doi: 10.1080/13550280902769764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eden A., Price R.W., Spudich S., Fuchs D., Hagberg L., Gisslen M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J. Infect. Dis. 2007;196(12):1779–1783. doi: 10.1086/523648. [DOI] [PubMed] [Google Scholar]
- 8.Kallianpur A.R., Levine A.J. Host genetic factors predisposing to HIV-associated neurocognitive disorder. Curr. HIV/AIDS Rep. 2014;11(3):336–352. doi: 10.1007/s11904-014-0222-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill A.J., Kolson D.L. Chronic inflammation and the role for cofactors (hepatitis C, drug abuse, antiretroviral drug toxicity, aging) in HAND persistence. Curr. HIV/AIDS Rep. 2014;11(3):325–335. doi: 10.1007/s11904-014-0210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letendre S.L., Zheng J.C., Kaul M., Yiannoutsos C.T., Ellis R.J., Taylor M.J. Chemokines in cerebrospinal fluid correlate with cerebral metabolite patterns in HIV-infected individuals. J. Neurovirol. 2011;17(1):63–69. doi: 10.1007/s13365-010-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson A.M., Harezlak J., Bharti A., Mi D., Taylor M.J., Daar E.S. Plasma and cerebrospinal fluid biomarkers predict cerebral injury in HIV-infected individuals on stable combination antiretroviral therapy. J. Acquir Immune Defic Syndr. 2015;69(1):29–35. doi: 10.1097/QAI.0000000000000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyons J.L., Uno H., Ancuta P., Kamat A., Moore D.J., Singer E.J. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J. Acquir. Immune Defic. Syndr. 2011;57(5):371–379. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valcour V.G., Shiramizu B.T., Shikuma C.M. HIV DNA in circulating monocytes as a mechanism to dementia and other HIV complications. J. Leukoc. Biol. 2010;87(4):621–626. doi: 10.1189/jlb.0809571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiramizu B., Ananworanich J., Chalermchai T., Siangphoe U., Troelstrup D., Shikuma C. Failure to clear intra-monocyte HIV infection linked to persistent neuropsychological testing impairment after first-line combined antiretroviral therapy. J. Neurovirol. 2012;18(1):69–73. doi: 10.1007/s13365-011-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bianchi M.E., Manfredi A.A. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 16.Lotze M.T., Tracey K.J. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5(4):331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 17.Saidi H., Melki M.T., Gougeon M.L. HMGB1-dependent triggering of HIV-1 replication and persistence in dendritic cells as a consequence of NK-DC cross-talk. PLoS One. 2008;3(10):e3601. doi: 10.1371/journal.pone.0003601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melki M.T., Saidi H., Dufour A., Olivo-Marin J.C., Gougeon M.L. Escape of HIV-1-infected dendritic cells from TRAIL-mediated NK cell cytotoxicity during NK-DC cross-talk-a pivotal role of HMGB1. PLoS Pathog. 2010;6(4):e000862. doi: 10.1371/journal.ppat.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gougeon M.L., Melki M.T., Saidi H. HMGB1, an alarmin promoting HIV dissemination and latency in dendritic cells. Cell Death Differ. 2012;19(1):96–106. doi: 10.1038/cdd.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Passalacqua M., Patrone M., Picotti G.B., Del Rio M., Sparatore B., Melloni E. Stimulated astrocytes release high-mobility group 1 protein, an inducer of LAN-5 neuroblastoma cell differentiation. Neuroscience. 1998;82(4):1021–1028. doi: 10.1016/s0306-4522(97)00352-7. [DOI] [PubMed] [Google Scholar]
- 21.Andersson A., Covacu R., Sunnemark D., Danilov A.I., Dal Bianco A., Khademi M. Pivotal advance: HMGB1 expression in active lesions of human and experimental multiple sclerosis. J. Leukoc. Biol. 2008;84(5):1248–1255. doi: 10.1189/jlb.1207844. [DOI] [PubMed] [Google Scholar]
- 22.Sunden-Cullberg J., Norrby-Teglund A., Rouhiainen A., Rauvala H., Herman G., Tracey K.J. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit. Care Med. 2005;33(3):564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 23.Vacas S., Degos V., Tracey K.J., Maze M. High-mobility group box 1 protein initiates postoperative cognitive decline by engaging bone marrow-derived macrophages. Anesthesiology. 2014;120(5):1160–1167. doi: 10.1097/ALN.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umahara T., Uchihara T., Koyama S., Hashimoto T., Akimoto J., Haraoka J. Local extension of HMGB1 in atherosclerotic lesions of human main cerebral and carotid arteries. Histol. Histopathol. 2014;29(2):235–242. doi: 10.14670/HH-29.235. [DOI] [PubMed] [Google Scholar]
- 25.Faraco G., Fossati S., Bianchi M.E., Patrone M., Pedrazzi M., Sparatore B. High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo. J Neurochem. 2007;103(2):590–603. doi: 10.1111/j.1471-4159.2007.04788.x. [DOI] [PubMed] [Google Scholar]
- 26.Vassallo M., Durant J., Lebrun-Frenay C., Fabre R., Ticchioni M., Andersen S. Virologically suppressed patients with asymptomatic and symptomatic HIV-associated neurocognitive disorders do not display the same pattern of immune activation. HIV Med. 2015;16(7):431–440. doi: 10.1111/hiv.12246. [DOI] [PubMed] [Google Scholar]
- 27.Pugliese P., Cuzin L., Cabie A., Poizot-Martin I., Allavena C., Duvivier C. A large French prospective cohort of HIV-infected patients: the Nadis Cohort. HIV Med. 2009;10(8):504–511. doi: 10.1111/j.1468-1293.2009.00719.x. [DOI] [PubMed] [Google Scholar]
- 28.Vassallo M., Dunais B., Durant J., Carsenti-Dellamonica H., Harvey-Langton A., Cottalorda J. Relevance of lipopolysaccharide levels in HIV-associated neurocognitive impairment: the Neuradapt study. J. Neurovirol. 2013;19(4):376–382. doi: 10.1007/s13365-013-0181-y. [DOI] [PubMed] [Google Scholar]
- 29.Antinori A., Arendt G., Becker J.T., Brew B.J., Byrd D.A., Cherner M. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robertson K.R., Smurzynski M., Parsons T.D., Wu K., Bosch R.J., Wu J. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21(14):1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 31.Yiannoutsos C.T., Ernst T., Chang L., Lee P.L., Richards T., Marra C.M. Regional patterns of brain metabolites in AIDS dementia complex. Neuroimage. 2004;23(3):928–935. doi: 10.1016/j.neuroimage.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 32.Lee P.L., Yiannoutsos C.T., Ernst T., Chang L., Marra C.M., Jarvik J.G. A multi-center 1H MRS study of the AIDS dementia complex: validation and preliminary analysis. J. Magn. Reson. Imag. 2003;17(6):625–633. doi: 10.1002/jmri.10295. [DOI] [PubMed] [Google Scholar]
- 33.Bianchi M.E. HMGB1 loves company. J. Leukoc. Biol. 2009;86(3):573–576. doi: 10.1189/jlb.1008585. [DOI] [PubMed] [Google Scholar]
- 34.Urbonaviciute V., Furnrohr B.G., Weber C., Haslbeck M., Wilhelm S., Herrmann M. Factors masking HMGB1 in human serum and plasma. J. Leukoc. Biol. 2007;81(1):67–74. doi: 10.1189/jlb.0306196. [DOI] [PubMed] [Google Scholar]
- 35.Launay O., Sadorge C., Jolly N., Poirier B., Bechet S., van der Vliet D. Safety and immunogenicity of SC599, an oral live attenuated Shigella dysenteriae type-1 vaccine in healthy volunteers: results of a Phase 2, randomized, double-blind placebo-controlled trial. Vaccine. 2009;27(8):1184–1191. doi: 10.1016/j.vaccine.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 36.Venereau E., Casalgrandi M., Schiraldi M., Antoine D.J., Cattaneo A., De Marchis F. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J. Exp. Med. 2012;209(9):1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J., Takahashi H.K., Liu K., Wake H., Liu R., Maruo T. Anti-high mobility group box-1 monoclonal antibody protects the blood-brain barrier from ischemia-induced disruption in rats. Stroke. 2011;42(5):1420–1428. doi: 10.1161/STROKEAHA.110.598334. [DOI] [PubMed] [Google Scholar]
- 38.Muhammad S., Barakat W., Stoyanov S., Murikinati S., Yang H., Tracey K.J. The HMGB1 receptor RAGE mediates ischemic brain damage. J. Neurosci. 2008;28(46):12023–12031. doi: 10.1523/JNEUROSCI.2435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang P., Schachner M., Shen Y.Q. HMGB1 in development and diseases of the central nervous system. Mol. Neurobiol. 2012;45(3):499–506. doi: 10.1007/s12035-012-8264-y. [DOI] [PubMed] [Google Scholar]
- 40.Abdulahad D.A., Westra J., Bijzet J., Limburg P.C., Kallenberg C.G., Bijl M. High mobility group box 1 (HMGB1) and anti-HMGB1 antibodies and their relation to disease characteristics in systemic lupus erythematosus. Arthritis Res Ther. 2011;13(3) doi: 10.1186/ar3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe M., Uchida K., Nakagaki K., Kanazawa H., Trapnell B.C., Hoshino Y. Anti-cytokine autoantibodies are ubiquitous in healthy individuals. FEBS Lett. 2007;581(10):2017–2021. doi: 10.1016/j.febslet.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 42.Anderson A.M., Fennema-Notestine C., Umlauf A., Taylor M.J., Clifford D.B., Marra C.M. CSF biomarkers of monocyte activation and chemotaxis correlate with magnetic resonance spectroscopy metabolites during chronic HIV disease. J. Neurovirol. 2015;21(5):559–567. doi: 10.1007/s13365-015-0359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang L., Ernst T., St Hillaire C., Conant K. Antiretroviral treatment alters relationship between MCP-1 and neurometabolites in HIV patients. Antivir. Ther. 2004;9(3):431–440. doi: 10.1177/135965350400900302. [DOI] [PubMed] [Google Scholar]
- 44.Ancuta P., Kamat A., Kunstman K.J., Kim E.Y., Autissier P., Wurcel A. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3(6):e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grant I., Franklin D.R., Jr., Deutsch R., Woods S.P., Vaida F., Ellis R.J. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014;82(23):2055–2062. doi: 10.1212/WNL.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan P., Brew B.J. HIV associated neurocognitive disorders in the modern antiviral treatment era: prevalence, characteristics, biomarkers, and effects of treatment. Curr. HIV/AIDS Rep. 2014;11(3):317–324. doi: 10.1007/s11904-014-0221-0. [DOI] [PubMed] [Google Scholar]
- 47.Thierry S., Gozlan J., Jaulmes A., Boniface R., Nasreddine N., Strauss F. High-mobility group box 1 protein induces HIV-1 expression from persistently infected cells. AIDS. 2007;21(3):283–292. doi: 10.1097/QAD.0b013e3280115b50. [DOI] [PubMed] [Google Scholar]
- 48.Hosking M.P., Lane T.E. The role of chemokines during viral infection of the CNS. PLoS Pathog. 2010;6(7):e1000937. doi: 10.1371/journal.ppat.1000937. [DOI] [PMC free article] [PubMed] [Google Scholar]