Abstract
Proteases perform a diverse array of biological functions. From simple peptide digestion for nutrient absorption to complex signaling cascades, proteases are found in organisms from prokaryotes to humans. In the human airway, proteases are associated with the regulation of the airway surface liquid layer, tissue remodeling, host defense and pathogenic infection and inflammation. A number of proteases are released in the airways under both physiological and pathophysiological states by both the host and invading pathogens. In airway diseases such as cystic fibrosis, proteases have been shown to be associated with increased morbidity and airway disease progression. In this review, we focus on the regulation of proteases and discuss specifically those proteases found in human airways. Attention then shifts to the epithelial sodium channel (ENaC), which is regulated by proteolytic cleavage and that is considered to be an important component of cystic fibrosis disease. Finally, we discuss bacterial proteases, in particular, those of the most prevalent bacterial pathogen found in cystic fibrosis, Pseudomonas aeruginosa.
Keywords: Protease, Epithelial sodium channel (ENaC), Cystic fibrosis (CF), Pseudomonas aeruginosa
Proteases
Proteases (peptidases, proteinases) are broadly classified as hydrolases that act on the peptide bond (Levene 1905). These enzymes are ubiquitous and control a range of biological functions across all kingdoms of life (Lopez-Otin and Bond 2008). This includes polypeptide cleavage in digestion and nutrient absorption to complex cell signaling, as in the caspase and blood-clotting cascades. Cleavage of the peptide bond results in the liberation of amino acid or peptide sequences from the original polypeptide polymer. These hydrolysis events can be used post-translationally to regulate substrate activity, localization and expression levels. A diversity of proteases are expressed from prokaryotes to humans and have been broadly classified into six family groups based on their active site chemistry, namely, serine proteases, threonine proteases, cysteine proteases, aspartate proteases and metalloproteases (MMPs) (Rawlings et al. 2010; Studholme et al. 2003). In addition, a small number of glutamic proteases have been isolated, although their identification has been limited to filamentous fungi, to date (Oda 2012).
Over 550 proteases have been identified in the human genome (Puente et al. 2003; Quesada et al. 2009). Of these, ~90 are thought to be homologs that are catalytically inactive, potentially contributing to physiological regulation via competitive interactions with their respective substrates. The location of cleavage is specific to each protease (or family) and can be broadly categorized as being either endoprotease (internal cleavage site) or exoprotease (cleavage of terminal residues) in nature (Overall et al. 2004). The substrate sequence and cleavage site is also specific to each protease or protease family. Specificities can be extremely permissive when cleavage sites are defined by a small number of amino acids, as with Arg-C, Asp-N, Lys-C, or trypsin (Quesada et al. 2009; Rawlings et al. 2012). Protease specificity can be greatly increased when the recognition sequences are more extended peptide sequences, as with thrombin and multiple caspases (Di Cera and Cantwell 2001; Talanian et al. 1997). Additional specificity is seen in proteases that recognize protein tertiary structures, as with the de-sumoylating and de-ubiquitylating proteases (Mossessova and Lima 2000).
The specific reaction mechanisms vary by active site composition and, in some cases, are still disputed. However, the general mechanism of hydrolysis proceeds via a nucleophilic attack on the backbone carbonyl of the protein/peptide substrate (Erez et al. 2009). Protease residues can accomplish this attack directly or through activated water and/or the coordinate metal ion in the active site. In addition to the catalytic domains, many proteases contain additional domains that regulate activity and localization. These domains serve to regulate protease function by changes in post-translational modification, ligand binding and cleavage. In addition, accessory domains serve to regulate membrane and cellular localization and facilitate protein-protein interactions in proteolytic cascades.
In this review, the regulation of proteases will be briefly discussed and then our focus will shift to proteases in the human airway and their role in the disease progression of cystic fibrosis (CF). Particular attention will be paid to proteases linked to pathogenesis and CF disease and to the impact of proteases on the regulation of the epithelial sodium channel (ENaC).
Protease regulation
Protease activity is tightly regulated at a minimum of four levels, which include transcriptional regulation, post-translational modification, physical compartmentalization and functional inhibition (Chow et al. 1995; Gorogh et al. 2006; Lopez-Otin and Matrisian 2007; Mirghomizadeh et al. 2009; Muzio et al. 1997). The multiple levels of protease regulation provide for tight tissue, temporal and environmentally responsive control of activity. Dysregulation of these regulatory events putatively contributes to multiple disease pathophysiologies. Transcriptional and epigenetic regulation of protease expression has been shown to be a major determinant across multiple families of proteases. A variety of cancer models suggest that the overexpression of proteases responsible for tissue remodeling is correlated with tumor proliferation and disease progression (Lopez-Otin and Matrisian 2007).
In addition to phosphorylation and glycosylation, a common means of protease regulation is through multiple post-translational modifications. Whereas a variety of mechanisms have been described for specific proteases, one of the most common is the proteolytic activation of pro-proteases (Salvesen and Riedl 2008). A variety of protease families are initially expressed, trafficked and/or secreted in an inactive state. Often, this inactive state is associated with a pro-peptide sequence that inhibits enzymatic activity. Removal of the pro-peptide, by protease cascades or by autoproteolytic cleavage, results in a disinhibition of protease activity and an active enzyme (Egnell and Flock 1992). This type of proteolytic regulation is often associated with secreted proteases and the pro-peptide cleavage is accomplished after secretion from the cell or with specific environmental triggers. Intracellular proteases can be regulated in a similar manner with the most well-studied cascade being that of the caspase apoptotic pathway (Salvesen and Riedl 2008). Such cascades often rely on an initial receptor or another physiological sensor that mediates the activation of an upstream protease. The cascade is triggered when cleavage of the downstream pro-peptides occurs as a result of upstream protease activation.
In addition to pro-peptide cleavage, ligand-induced activation is associated with a variety of proteases (Baumann 1994; Ravaud et al. 2003). Recent work on serralysin proteases has demonstrated that Ca2+ serves as a critical co-factor for protease folding and subsequent activation (Zhang et al. 2012). In the absence of Ca2+, the proteases remain in an unfolded conformation and are inactive. Calcium binding induces the folding of a chaperone domain, which subsequently serves to facilitate the folding and activation of the proteolytic domain.
Compartmentalization also serves as a major mechanism to regulate protease activities. Membrane and organelle targeting are key to regulating the functions of the extracellular MMPs and organelle-specific proteases (Fritz et al. 1987; Kametaka et al. 2003). In many cases, pro-peptide cleavage and/or additional post-translational modifications are associated with proper secretion or trafficking. This compartmentalization serves to regulate the activities of the activated protease spatially, with classic examples being lysosomal proteases and furin, a protease found in the trans-Golgi network. In addition, a unique mechanism to regulate protease activity intracellularly is the auto-compartmentalization seen with the proteasome (Song et al. 2003; Tomisugi et al. 2000; Unno et al. 2002). Structurally, the proteasome is a barrel formed of 28 core polypeptides. Within the core of the barrel, multiple protein subunits form active sites that provide for proteolysis with a range of substrate specificities. Regulatory complexes found at either or both ends of the proteasome regulate access to the central cavity and active sites. As a complex, access to the active sites within the barrel provide for the regulation of this important enzyme. Coupled with the ubiquitin modification system, the proteasome is recognized as one of the most critical components regulating the proteome in cells, degrading a majority of folded and misfolded substrates and regulating a wide range of physiological processes (Ciechanover 1998; Hershko and Ciechanover 1998).
Finally, inhibitors (or anti-proteases) provide an additional layer of protease regulation. Emerging evidence suggests that the protease-inhibitor balance is critical to a variety of normal and disease states in humans (Gorogh et al. 2006; Guyot et al. 2008; Myerburg et al. 2006; Quesada et al. 2009). On their initial discovery, protease inhibitors were thought to protect the host from unwanted hydrolytic activities, both spatially and temporally. However, recent work suggests that the protease-anti-protease balance is a mechanism to fine-tune protease activities. As an example, work on the airway surface fluid in patients with compromised pulmonary systems (to be discussed later in this review) suggests fluid volume is sensed via changes in this protease/anti-protease balance (Kleyman et al. 2009; Mall et al. 2004; Myerburg et al. 2006; Tan et al. 2011; Tarran et al. 2006). These changes in the protease/ anti-protease balance serve as a regulatory loop that alters water secretion across the epithelia. Thus, the changes in the protease/anti-protease balance both act as a signal and regulate water secretion across the airway epithelium.
Proteases, inhibitors and disease
Altered protease function and regulation have been associated with a large number of pathophysiological conditions (Quesada et al. 2009). Both sporadic and hereditary diseases are associated with endogenous protease dysregulation or dysfunction (Table 1). A large body of work has evolved looking specifically at the role of MMPs in cancer development and progression and in cardiac disease. The roles of these proteases were originally thought to be restricted to modification of the extracellular matrix. However, recent work suggests broader roles for the protease in other physiological and pathophysiological states.
Table 1.
Human proteases, inhibitors and disease (adapted from Quesada et al. 2009)
| Protease | Gene | Disease |
|---|---|---|
| Angiotensin-converting enzyme | ACE | Renal tubular dysgenesis |
| ADAM9 | ADAM9 | Cone-rod dystrophy |
| ADAMTS-13 | ADAMTS- 13 |
Thrombotic thrombocytopenic purpura |
| Afg3-like protein 2 | AFG3L2 | Ataxia(s) |
| Complement factor B | BF | Atypical hemolytic uremic syndrome |
| Calpain-3 | CAPN3 | Limb-girdle muscular dystrophy type 2A |
| Caspase-2 | CASP2 | Autosomal recessive intellectual disability |
| Caspase-8 | CASP8 | Autoimmune lymphoproliferative syndrome (I) |
| Carboxypeptidase E | CPE | Hyperproinsulinemia and diabetes |
| DJ-1 | DJ1 | Parkinson’s disease type VII |
| Neutrophil elastase | ELA2 | Cyclic neutropenia |
| Thrombin | F2 | Hyperprothrombinemia/hypoprothrombinemia |
| Mitochondrial inner membrane protease2 |
IMMP2L | Tourette syndrome |
| Neurotrypsin | PRSS12 | Nonsyndromic mental retardation |
| Presenilin 1 | PSEN1 | Alzheimer type III |
| Presenilin 2 | PSEN2 | Alzheimer type IV |
| Renin | REN | Renal tubular dysgenesis |
| Paraplegin | SPG7 | Spastic paraplegia |
| Transmembrane protease, serine 3 | TMPRSS3 | Deafness |
| Ubiquitin C-terminal hydrolase I | UCHL1 | Parkinson’s disease type V |
| Ubiquitin-specific protease 26 | USP26 | Sertoli-cell-only syndrome |
| Ubiquitin-specific protease 9Y | USP9Y | Azoospermia and hypospermatogenesis |
| FACE1/ZMPSTE24 | FACE1 | Progeria, mandibuloacral dysplasia |
| Chymotrypsin C | CTRC | Hereditary pancreatitis |
| Procollagen C-proteinase | BMP1 | Osteogenesis imperfect |
| Ataxin 3 | MJD1 | Machado-Joseph disease |
| Proprotein convertase 1 | PCSK1 | Obesity |
| Lysosomal carboxypeptidase A | PPGB | Galactosialidosis |
| Proteasome catalytic subunit 3i | PSMB8 | Nakajo-Nishimura sundrome |
In addition, a growing number of hereditary diseases are associated with alterations in protease inhibitor expression and function (Table 2) (Quesada et al. 2009). Whereas the exact mechanisms associated with the pathophysiological states vary by disease, evidence for both the direct and indirect involvement of proteases and protease inhibitors suggests that balanced protease activities are critical for regulating physiological processes.
Table 2.
Protease inhibitors and human disease (adapted from Quesada et al. 2009)
| Inhibitor | Disease |
|---|---|
| α1 Antitrypsin | Thrombosis, emphysema |
| A1-Antichymotrypsin | Vascular disease |
| Kallistatin | Pancreatitis |
| Angiotensinogen | Hypertension, hypotension |
| Protein Z-dependent protease inhibitor | Venous thrombosis |
| Vaspin | Diabetes |
| Maspin | Cancer progression |
| Megsin | IgA nephropathy |
| Antithrombin II | Venous thrombosis |
| Heparin cofactor II | Venous thrombosis |
| Plasminogen activator inhibitor 1 | Bleeding disorders, myocardial infarction |
| Pigment epithelium-derived factor | Age-related macular disease |
Cystic fibrosis
CF is a disease of altered salt and water movement across epithelial tissues. Mutations within the CF transmembrane conductance regulator (CFTR) that result in a loss of CFTR function at the plasma membrane underlie the pathophysiologies of CF and CF-related diseases (Dean et al. 2001; Drumm et al. 1990; Mall et al. 1999; Riordan et al. 1989). The primary complications associated with CF are found in the digestive and pulmonary systems. Pancreatitis and associated nutritional deficiencies are associated with abnormal buffering of the pancreatic duct leading to premature zymogen activation (Choi et al. 2001; Ko et al. 2002; Marino et al. 1991; Stuhrmann et al. 1990). Similarly, lung function is chronically degraded as a result of decreased mucocilliary clearance and the persistence of airway pathogens (Rich et al. 1990). The ensuing inflammatory and immune responses result in chronic injury to the airway epithelium and a decrease in pulmonary function.
CFTR functions as a protein kinase A (PKA-) and ATP-regulated Cl− channel, facilitating the secretion of Cl− across the apical membranes of epithelial cells (Cheng et al. 1991; Gregory et al. 1990). In the airway, Cl− secretion is thought to impact the regulation of Na+ absorption through ENaC, either by an alteration in the electrogenic driving force for Na+ or by the direct regulation of ENaC by CFTR itself (Briel et al. 1998; Hopf et al. 1999; Ji et al. 2000; Kunzelmann et al. 1997; Reddy et al. 1999; Schreiber et al. 1999; Stutts et al. 1997). The luminal surfaces of the conducting airways are lined with a thin layer of fluid known as the airway surface liquid (ASL), which facilitates mucus clearance from the lung. The height of the ASL is determined by the net osmotic gradient established by Na+ absorption and Cl− secretion through these apically located ion channels. ENaC, in conjunction with the basolateral Na+ / K+ ATPase, is believed to be the predominant means for Na+ absorption across the airway epithelium. The loss of Cl− channel activity (as in CF) or an increase in Na+ absorption putatively results in increased water absorption and dehydration of the ASL. This dehydration, in turn, increases mucus viscosity, decreases the efficacy of the mucocilliary clearance and facilitates pathogen adherence and colonization in the lung (Tarran et al. 2006; Voynow et al. 2008).
The role of ENaC in contributing to human CF lung pathophysiology is still under investigation. Studies of the recently generated CF pig suggest that Na+ hyperabsorption is not associated with the CF phenotype (Abu-El-Haija et al. 2011; Chen et al. 2010; Itani et al. 2011). In ex vivo tissue and cell studies, significant changes in Cl− conductance but not in Na+ conductance have been observed. Similarly, ex vivo studies of human CF tracheal and bronchial epithelial cells indicate a primary role for altered Cl− conductance, associated with the loss of CFTR, in the CF tissues. However, they fail to show widespread changes in Na+ absorption (Itani et al. 2011; Reddy and Quinton 2003). These data stand in contrast to several in vivo and in vitro experiments that have implicated altered Na+ absorption in CF pathophysiology (Boucher 2004; Jiang et al. 2000; Myerburg et al. 2006). Thus, the exact nature of ENaC involvement has still not been fully elucidated from a functional and physiological perspective.
Although CFTR is thought to play the dominant role in CF pathophysiology, emerging evidence suggests a role for ENaC in the airway (Huber et al. 2010). Consistent with a role for ENaC in the CF phenotype, genetic studies of atypical CF patients have identified mutations in ENaC that putatively underlie their CF pathophysiology (Azad et al. 2009; Rauh et al. 2010). Further evidence for a role of ENaC in regulating lung function comes from transgenic mice overexpressing β-ENaC (Zhou et al. 2011). Disruption of the CFTR locus does not induce full CF-like lung pathophysiology in mice as a result of the divergent lung physiology (Kent et al. 1997). However, transgenic overexpression of ENaC recapitulates the CF-like lung physiology, consistent with a role for ENaC-associated Na+ absorption in the CF lung (Mall et al. 2004). Together, these data suggest that ENaC may partially regulate or contribute to the CF lung phenotype under specific spatial or physiological conditions.
Epithelial sodium channel
ENaC is expressed in the epithelial cells of several tissues, including the kidneys, airways, salivary ducts, sweat ducts, colon and taste cells (Butterworth 2010; Butterworth et al. 2009; Eaton et al. 2009; Hamm et al. 2010; Kleyman et al. 2009; Lang et al. 2010; Rossier and Stutts 2009). ENaC is a sodium-selective ion channel comprising three homologous subunits, namely, α-, β-, γ-, each composed of two membrane-spanning domains, a large folded extracellular domain and intracellular amino- and carboxy-termini (see Fig. 1). ENaC is the limiting step in the reabsorption of sodium across epithelia. Because of its role in the regulation of sodium homeostasis, ENaC has been associated with clinical defects of salt and water transport and implicated in a number of disease conditions including defects in airway surface hydration in CF (Capasso et al. 2005; Eaton et al. 2009; Ecelbarger and Tiwari 2006; Edelheit et al. 2005; Freundlich and Ludwig 2005; Hummler 1999; Li and Wang 2007; Matthay et al. 2005; Sun et al. 2011; Wagner et al. 2008).
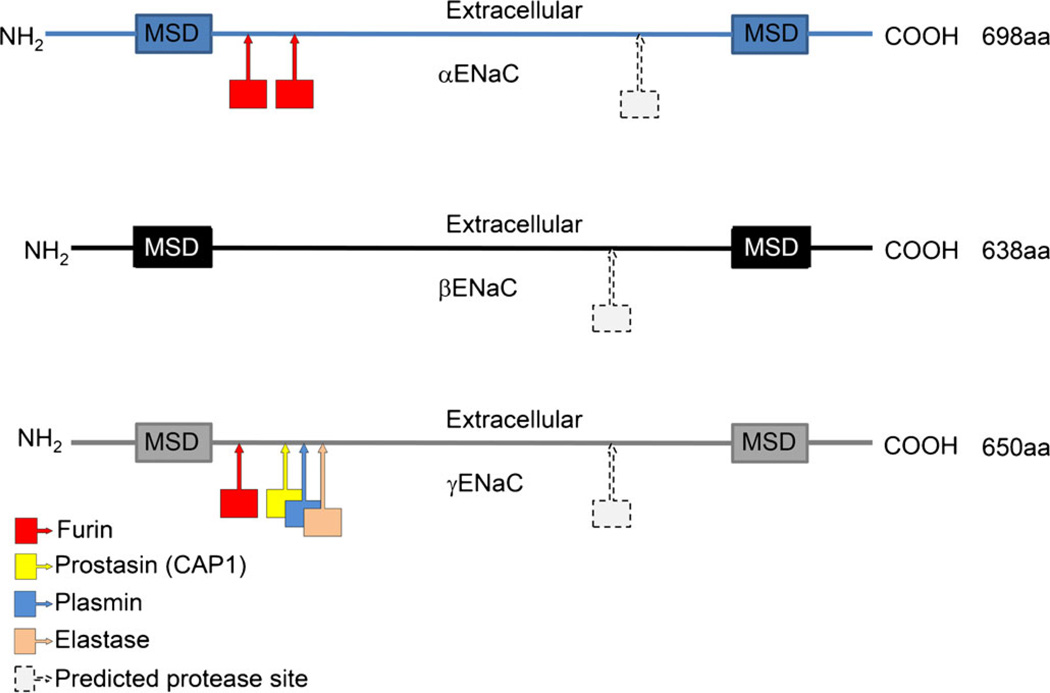
Fig. 1.
Representation of α-, β- and γ-ENaC structures with the approximate locations of confirmed and predicted protease cleavage sites (aa amino acids, MSD membrane spanning domain). Based on figures presented in Kleyman et al. (2009), Rossier and Stutts (2009) and Hamm et al. (2010)
ENaC is regulated by a number of intrinsic and external factors, the details of which have been reviewed previously (Bens et al. 2006; Bhalla and Hallows 2008; Butterworth 2010; Butterworth et al. 2009; Eaton et al. 2009; Ecelbarger and Tiwari 2006; Kleyman et al. 2009; Pochynyuk et al. 2006; Rossier and Stutts 2009). In every example of ENaC regulation, a limited number of options alter Na+ transport. ENaC activity can be changed either by altering the amount of time that the channel spends open (open probability or PO) or by modulating the surface membrane density (channel number or n) of the channel (Rossier 2002). Once fully active, the predominant mechanism to reduce sodium transport is to remove ENaC from the membrane surface by endocytosis. More recently, a new mode of regulation was appreciated that involved the activation of the channels by proteolytic cleavage (Adebamiro et al. 2007; Bruns et al. 2007; Ergonul et al. 2006; Hughey et al. 2003, 2004a, 2004b; Kleyman et al. 2009; Liu et al. 2002; Passero et al. 2008; Planes and Caughey, 2007; Planes et al. 2005; Tan et al. 2011; Vallet et al. 1997; Vuagniaux et al. 2002; Vuagniaux et al. 2000). In this instance, the PO is dramatically increased, in some cases altering ENaC from an electrically silent state to a fully active channel (Caldwell et al. 2004, 2005). Once ENaC is activated by proteases, however, the same mechanisms of retrieval and degradation need to be employed to reduce the net Na+ reabsorption.
ENaC activation by proteases
The potential for proteases and protease inhibitors to alter ENaC activity and Na+ transport was described in the early 1980s, before the molecular identity of the channel was known. The ability of protease inhibitors to block sodium flux was described in a number of model tissues without an underlying knowledge of the mechanisms behind these observations (Orce et al. 1980, 1981). The description of a serine protease that specifically activated ENaC, called a channel-activating protease (CAP1), opened the door to a new field of investigation in the regulation of ENaC (Liu et al. 2002; Vallet et al. 1997; Vuagniaux et al. 2000). CAP1 was eventually identified as prostasin or TMPRSS8 and these initial observations were quickly followed up with the descriptions of two additional channel-activating proteases, CAP2 (TMPRSS4) and CAP3 (matriptase; Planes and Caughey 2007; Vuagniaux et al. 2002). To date, the major class of proteases involved in ENaC cleavage belongs to the broad family of serine proteases, which all have a conserved serine in their active sites. The action of all these proteases is to cleave sites on the extracellular loops of the ENaC subunits at specific recognition sequences. Confirmed cleavage sites have been found clustered predominantly toward the n-terminal ends of α- and γ-ENaC, an area that is presumably exposed and accessible to protease action (Fig. 1; Adebamiro et al. 2007; Bruns et al. 2007; Caldwell et al. 2004; Gormley et al. 2003; Hamm et al. 2010; Hughey et al. 2004a, 2004b; Kleyman et al. 2009; Passero et al. 2008; Planes and Caughey 2007; Rossier 2004). However, a number of putative protease recognition sites have been mapped on all three subunits at both the N- and C-terminal sides of the extracellular loops of each subunit (Rossier and Stutts 2009). Protease action at these predicted sites has yet to be demonstrated experimentally. ENaC is acted on by proteases at intracellular locations or at the surface membrane, either with membrane-bound/anchored proteases or extracellular/free proteases.
Intracellular proteases
To be fully active, with a high PO, ENaC needs to be cleaved more than once on the large extracellular domains of the α–and γ–subunits. One of the first steps in ENaC cleavage appears to occur by the action of pro-protein convertases, most likely located intracellularly (Fig. 2a; Schafer et al. 1995). The convertase furin is the most well-studied and best candidate for this intracellular protease function (Bosshart et al. 1994; Bruns et al. 2007; Hughey et al. 2004a, 2004b; Schafer et al. 1995). Furin is responsible for cleaving both α– and γ–subunits through a consensus sequence R/S-XX-R (where R is an arginine, S a serine and X any amino acid; Bruns et al. 2007; Hughey et al. 2004a, 2004b; Sheng et al. 2006). In the case of the α-subunit, furin is able to cleave at two distinct sites in the extracellular domain of the channel (Fig. 1). Cleavage at both of these locations releases a small inhibitory peptide. This cleavage putatively relieves structural constraints and allows the channel to become partially activated. Likewise, a furin site has been found on the extracellular domain of the γ-subunit (Bruns et al. 2007). A second cleavage event by an additional protease is required to achieve full activation of ENaC. The location of the distal γ-ENaC cleavage site varies with the specific protease investigated; however, it is within approximately 60 amino acids from the first furin cleavage site (Fig. 1). This second cleavage event also releases an inhibitory fragment, similar to α-ENaC. To date, a number of other serine proteases have been implicated in the cleavage of ENaC subunits and include members of the elastase family, plasmin, prostasin, chymotrypsin and trypsin (Adebamiro et al. 2007; Caldwell et al. 2005; Carattino et al. 2008; Kleyman et al. 2009; Passero et al. 2008; Rossier and Stutts 2009; Vuagniaux et al. 2002). In most cases, a double-cleavage event appears to release a small portion of the extracellular domain of ENaC, thereby activating the channel.
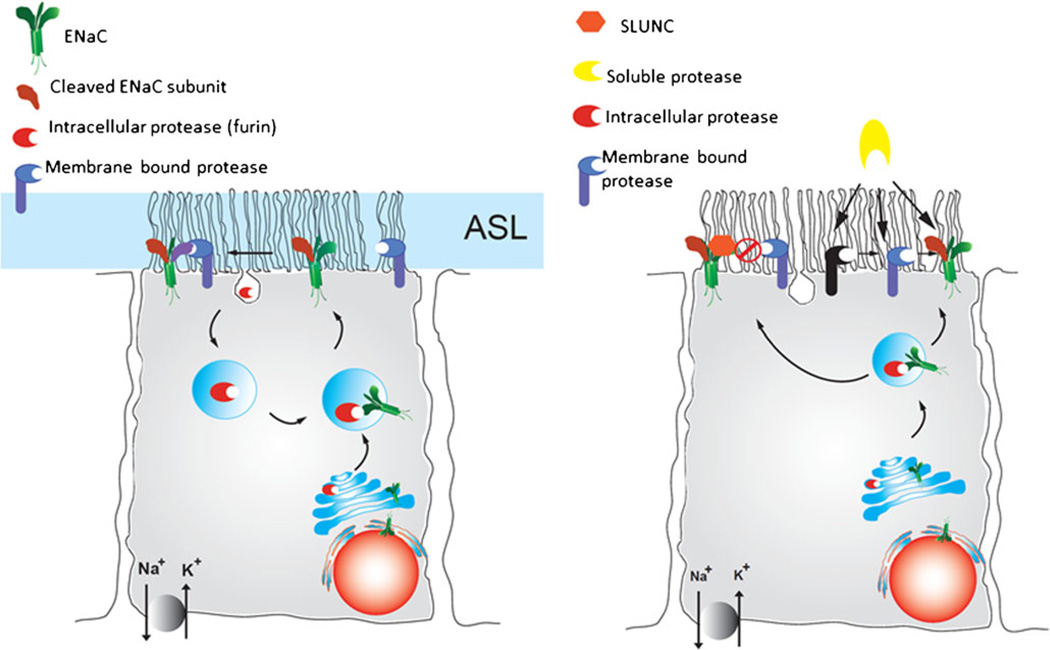
Fig. 2.
Representation of protease activation of ENaC in airway epithelial cells. a A double-cleavage event is required for full ENaC activation (ASL airway surface liquid). The first step probably occurs by intracellular proteases (furin), followed by a second cleavage event at the surface (membrane protease). b Multiple cleavage events or a cascade of proteolysis might be involved in achieving full ENaC activation. In this scenario, ENaC is cleaved by intracellular proteases and reaches the membrane surface in either an uncleaved or partially cleaved state. It can then be further activated by membrane-bound proteases or by soluble proteases. In addition, soluble protease might initiate a cascade of proteolytic events eventually resulting in full ENaC activation. Soluble protease inhibitors and proteins that prevent ENaC cleavage (SPLUNC) might modulate the extent of ENaC activation. Images based on previous reviews (Rossier and Stutts 2009; Gaillard et al. 2010)
Functional and biochemical studies investigating ENaC cleavage do not always make it clear precisely where in the cell and when in the life of the channel the cleavage events occur. However, the need for the double-cleavage seems to be a requirement for full activation of ENaC. Therefore, ENaC might be found in a range of processed states depending on the longevity of the channel and the opportunity for interaction with both intracellular and extracellular proteases. A range of channel-open probabilities are therefore likely until full activation has been achieved; this observation has been made in the biophysical description of ENaC (Caldwell et al. 2004, 2005).
Membrane-bound extracellular proteases
Following biosynthesis and trafficking to the Golgi, ENaC is likely to encounter the intracellular protease furin, as this convertase has been shown to recycle between the Golgi and plasma membrane (Fig. 2a). Once ENaC is inserted into the plasma membrane, the opportunity exists for full proteolytic activation of the channel by extracellular proteases (Fig. 2b). The three CAPs described above are all reported to be membrane bound. CAP1 is anchored by glycosylphosphatidylinositol (GPI) and can be shed from the membrane surface. The CAP2 and CAP3 proteases are membrane-bound serine proteases with portions of these proteins being located intracellularly. Therefore, a direct association between ENaC and these proteases would probably be required to achieve proteolytic cleavage (Gormley et al. 2003; Rossier 2004; Vuagniaux et al. 2002).
Some uncertainty exists about the absolute requirement for the catalytic activity of CAP1 to achieve ENaC activation. Purported CAP1 catalytic mutants were still capable of activating ENaC; however, mutation of the proposed prostasin recognition sequence on γENaC (RKRK) eliminates the ability of prostasin to activate ENaC (Harris et al. 2008; Kleyman et al. 2009; Rossier 2004; Rossier and Stutts 2009). Several authors have suggested the possibility of an indirect mechanism for CAP1 activation of ENaC or the role of another protease in a cascade of proteolytic activity (Gaillard et al. 2010; Rossier and Stutts 2009; Tarran et al. 2006). Both of these possibilities remain plausible. In line with these suggestions and observations, we consider that a multi-step process might be required for full ENaC activation (see below; unpublished observations; Fig. 2b). Unlike the discrepancies for CAP1, greater consensus has been achieved with regard to the requirement for catalytic activities of CAP2 and CAP3 to activate ENaC fully and these cleavage sites have been mapped by using model expression systems (Planes and Caughey 2007; Planes et al. 2005; Vuagniaux et al. 2002).
One of the factors that might contribute to the seemingly contradictory studies for CAP1 activation of ENaC is that protease activity can itself be modulated by protease inhibitors, as described above. The interplay between protease activity and the action of protease inhibitors has been proposed as an underlying factor in excessive airway surface liquid reabsorption in the CF airway (Donaldson et al. 2002; Gaillard et al. 2010; Hughey et al. 2007; Myerburg et al. 2010; Rossier and Stutts 2009; Tan et al. 2011). Several protease inhibitors have been identified that result in the direct inhibition of the target proteases. These include the Kunitz-like inhibitors (aprotinin) and the serpins (protease nexin 1; Adebamiro et al. 2002, 2005, 2007; Myerburg et al. 2010; Orce et al. 1980, 1981; Planes et al. 2005; Tarran et al. 2006; Tong et al. 2004; Vallet et al. 1997). Alternatively, inhibitors might bind to ENaC directly and prevent protease access and subsequent activation of the channel (Fig. 2b). The protein SPLUNC1 appears to bind to ENaC and prevent access of proteases to cleavage sequences, thereby inhibiting proteolytic activation of ENaC (Gaillard et al. 2010; Rollins et al. 2010).
Added to this interplay between proteases and their inhibitors is the possibility that a series of proteolytic steps is required for full ENaC activation. In this scenario, the activity of a protease might activate a protease pathway or a cascade of proteases that cleave ENaC. This could involve the inactivation or degradation of a protease inhibitor or conversion of a protease from an inactive to active form (Fig. 2b). These possibilities have been recently suggested in the literature but evidence for this activation and the nature of these proposed cascades have yet to be demonstrated experimentally for ENaC (Chambers et al. 2007; Gaillard et al. 2010; Tarran et al. 2006).
ENaC and proteases in the airway
Previous work from our group and others indicates that a balance between the protease activity of membrane-tethered channel-activating proteases (CAPs) and soluble protease inhibitors in the ASL modulates ENaC activity and therefore Na+ absorption across human bronchial epithelial (HBE) cells (Gaillard et al. 2010; Kleyman et al. 2006; Mall 2008; Myerburg et al. 2006, 2010; Rossier and Stutts 2009). In this scheme, when the ASL volume is low, soluble protease inhibitors would achieve a sufficiently high local concentration in the ASL to minimize constitutive activation of ENaC by CAPs. This would lead to reduced Na+ and water reabsorption. Conversely, when the ASL volume is expanded, the soluble protease inhibitors are diluted, relieving the inhibition of CAPs. ENaC activation would presumably occur from membrane-bound proteases that cannot be removed from the airway surface.
A number of proteases have been identified in the airway, not all of which are associated with ENaC activation (Cottrell et al. 2004; Elizur et al. 2008). The most common and abundant proteases found in the extracellular milieu in human airway are the serine and metallo-proteases (Conese et al. 2003; Elizur et al. 2008; Voynow et al. 2008). In the airways of CF patients, extracellular proteases are derived predominantly from two sources: external and intrinsic. Proteases are released by invading bacterial pathogens as virulence factors that facilitate infection and colonization. As a consequence of immune, inflammatory and cell damage/repair responses, the host also secretes proteases (Bainbridge and Fick 1989; Conese et al. 2003; Elizur et al. 2008; Terheggen-Lagro et al. 2005; Voynow et al. 2008). These proteases, combined with a number of endogenous and epithelial-cell-derived proteases, make up the large number of active proteases in the surface fluid overlaying the airway epithelium and have a range of impacts in the airway. Serine proteases have been shown to increase mucus expression, which decreases ciliary beating and results in ciliary injury (Griese et al. 2008). In addition, serine proteases induce goblet cell hyperplasia and degrade extracellular matrix (Coraux et al. 2008; Elizur et al. 2008; Voynow et al. 2008; Wynn 2008). The proteases also induce increased interleukin expression (IL-8), causing downstream pro-inflammatory signaling. Finally, the serine proteases degrade innate and adaptive immune molecules, altering immune signaling in the airway (Downey et al. 2007a, 2007b, 2009).
On balance these proteases are detrimental to the host, while aiding the pathogenesis of bacterial infection. In response to invasion, the host innate immune response might exacerbate the inflammation and protease damage, particularly in patients with CF and progressive lung diseases. One of the downstream consequences of infection is the accumulation of neutrophils at the site of infection (Downey et al. 2009). A large number of proteases are released by neutrophils themselves. These include neutrophil serine proteases, neutrophil elastase (which is known to activate ENaC), proteinase 3 and cathepsin G. Metalloproteases, collagenase and gelatinase have also been detected in the ASL under inflammatory conditions (Downey et al. 2009; Elizur et al. 2008; Voynow et al. 2008). In addition, bacterial pathogens are known to secrete a variety of proteases that are capable of host remodeling (see below). These external bacterial proteases might also serve to activate ENaC (Fig. 2b).
Bacterial infection in CF
The major opportunistic pathogen associated with CF lung disease is Pseudomonas aeruginosa, a gram-negative rod-shaped bacterium (Burke et al. 1991; Jagger et al. 1982; Parmely et al. 1987). Pseudomonas infection and colonization presents early in the patient’s life and persists in the CF lung as a result of the ability of the bacterium to evade and neutralize the host’s defenses (Lazdunski et al. 1990; Lyczak et al. 2000; Suter 1994). Pseudomonas colonization is aided by the compromised mucociliary clearance associated with CF. The persistence of Pseudomonas induces lung injury by both direct and indirect mechanisms (Hobden 2002; Kharazmi et al. 1984a, 1984b; Lyczak et al. 2002; Parmely et al. 1990; Sarkisova et al. 2005a; Suter 1994). The secretion of multiple virulence factors results in direct injury to the epithelial tissue (Kudoh et al. 1994; Wiener-Kronish et al. 1993). Additionally, the chronic involvement of immune and inflammatory responses, resulting from Pseudomonas colonization, leads to indirect injury (Granstrom et al. 1984; Jagger et al. 1982; Kharazmi et al. 1984a; Parmely and Horvat 1986; Parmely et al. 1984; Reeves and McElvaney 2012; Saadane et al. 2006; Sagel et al. 2002). Specifically, the long-term recruitment and activation of neutrophils is associated with epithelial damage as a result of sustained secretion of bacterial killing proteins (Conese et al. 2003; Kercsmar and Davis 1993).
Pseudomonas has evolved multiple mechanisms to facilitate adherence and colonization. Among these, the secretion of virulence factors and the formation of biofilms are thought to contribute to Pseudomonas resistance to host and antibiotic insults (Kudoh et al. 1994; Lazdunski et al. 1990). Biofilm formation provides a physical barrier between the bacterial cells and the extracellular environment, decreases the efficacy of host and pharmacological agents and facilitates adherence (Byrd et al. 2011; Kobayashi 2005; Lee et al. 2011; Sarkisova et al. 2005b). Composed of DNA, polysaccharides and proteins, the biofilms form a matrix that encapsulates the bacterial cells (Byrd et al. 2011; Lee et al. 2011). The development of this matrix is a compounding factor in the treatment of Pseudomonas infection. Virulence factors also play key roles in modulating the virulence of many pathogens and include proteins that modulate innate and adaptive immune signaling and inflammatory signaling and that break down epithelial barriers (Kobayashi 2005; Sarkisova et al. 2005b; Tomlin et al. 2001).
Multiple proteases have been implicated in the virulence of Pseudomonas, including elastase A and B (LasA and LasB), protease IV (PIV) and alkaline protease (AP) (Jagger et al. 1982, 1983). Bronchiolar lavage and sputum from CF patients have demonstrated the presence of multiple bacterial proteases in the Pseudomonas-infected lung. Similarly, the expression and secretion of these proteases have been reported in other modes of Pseudomonas infection, including burn and ocular injury (Hobden 2002; Kharazmi et al. 1984a). Multiple proteolytic targets are associated with the expression of these proteases and might contribute to changes in the physiology of infected tissue.
The two elastase proteases are metalloproteases and are both capable of degrading human elastin; LasB protease is also capable of degrading collagen (Goldberg and Ohman 1987; Johnson et al. 1967; Kessler and Safrin 1988; Kessler et al. 1993; Rust et al. 1996; Saulnier et al. 1989; Toder et al. 1994; Uss et al. 1969; Voynow et al. 2008). Both elastin and collagen are significant biopolymers and contribute to tissue plasticity. Changes in elastin and collagen content in the lung are associated with pulmonary fibrosis (Voynow et al. 2008). In addition, both proteases have also been shown to be involved in the invasive phenotype of multiple Pseudomonas strains (Azghani et al. 2002; Fleiszig et al. 1997). LasB has also been shown to degrade IgA and IgG and surfactant proteins A and D (SP-A and D), both being associated with the identification and elimination of pathogens (Bainbridge and Fick 1989; Heck et al. 1990; Mariencheck et al. 2003).
PIV is a serine protease that has been implicated in corneal virulence of Pseudomonas (Engel et al. 1997; O’Callaghan et al. 1996). As with the elastase proteases, PIV can degrade surfactant and iron-binding proteins isolated from bronchoalveolar lavage (Britigan et al. 1993; Malloy et al. 2005). In addition, PIV can bind plasminogen and cleave fibrinogen, which are both involved in regulating blood clotting (Caballero et al. 2001; Engel et al. 1998). Specifically, the degradation of fibrinogen is associated with hemorrhage, which is often associated with Pseudomonas infection.
AP is a Ca2+-regulated Zn2+ metalloprotease (Baumann 1994; Baumann et al. 1993; Duong et al. 1992; Guzzo et al. 1990; Zhang et al. 2012). The active protease is capable of degrading γ-interferon and putatively inactivates multiple protease inhibitors (Guyot et al. 2010; Horvat et al. 1989; Leidal et al. 2003; Parmely et al. 1990). In addition, AP is thought to alter the function of neutrophils and leukocytes, facilitating evasion of host defenses (Kharazmi et al. 1984a, 1984b; Parmely et al. 1984). Whereas its substrate specificity is currently unknown, the protease is thought to be capable of cleaving a broad range of physiological targets (Louis et al. 1998). Previous studies suggest that AP expression is regulatory, both spatially and temporally (Lazdunski et al. 1990). AP expression is modulated temporally during the infection and colonization of the host tissue and is highly enriched in bio-films (Manos et al. 2008, 2009; Salunkhe et al. 2005).
Bacterial proteases, ENaC and CF
Although long-term and widespread changes in ENaC activation might not directly contribute to the CF phenotype per se, they might contribute significantly to local and acute changes within the lung. Recent work suggests that, in addition to the host proteases that are present in the lung, secreted pathogen proteases might contribute to colonization and infection, as detailed above. In such models, the secretion of bacterial proteases would lead to the activation of ENaC, either directly through its cleavage or indirectly through the activation of a host protease. Evidence for the possibility of an indirect cascade activation is suggested by the ability of a number of bacterial proteases to cleave host proteases and protease inhibitors in vitro (Guyot et al. 2008, 2010; Johnson et al. 1967; Kessler and Safrin 1994; Kessler et al. 1998; Leidal et al. 2003). The nature and molecular identities of these putative cascades have not been characterized at this point in vivo.
The roles for the bacterial proteases in colonization, infection and exacerbation are also not well established. Secreted bacterial proteins and peptides are often immunogenic and, in many cases, become neutralized after recognition by the adaptive immune system (Granstrom et al. 1984; Jagger et al. 1982). However, both the initial response and generation of neutralizing antibodies occurs on timescales that would not be predicted to alter acute expression and secretion during early steps in infection and colonization. Evidence for the acute expression of Pseudomonas proteases is found in analysis of bronchoalveolar lavage, wherein protease secretion and activation occurs extremely early in infection and colonization (Burke et al. 1991; Lyczak et al. 2000; Suter 1994). The increase in local protease activity might directly influence host defenses. In addition, the increase in protease activity might result in local tissue remodeling that facilitates adhesion and colonization. Moreover, the secreted proteases, including elastase and alkaline protease, might contribute to local remodeling by ENaC cleavage and alterations in its activity. The resulting changes in ASL would putatively facilitate adhesion by decreasing ASL and mucociliary clearance. This, coupled with changes in epithelial permeability, might lead to an increase in pathogen invasion and infection.
Concluding remarks
The role of proteases in activating Na+ conductance through ENaC is now a well-established mechanism in ENaC regulation. The appreciation of this mode of channel modulation has prompted studies into underlying misregulation of proteases in pathophysiological disease states such as CF. From the brief summary provided above, we can clearly see that the airway is awash with a large number of proteases and protease-interacting proteins that are derived from a variety of sources, both host and invading pathogen. Comprehension of the pathways that link the potential protease interactions and cascades remains a challenging undertaking for a full understanding of the physiology of the airway.
Acknowledgments
This work was supported by NIH grants to M.B. (K99DK078917, P30DK079307) and P.T. (R01DK083284).
Contributor Information
P. H. Thibodeau, Department of Cell Biology, University of Pittsburgh School of Medicine, 3500 Terrace Street, S327 Biomedical Science Tower, Pittsburgh PA 15261, USA
M. B. Butterworth, Department of Cell Biology, University of Pittsburgh School of Medicine, 3500 Terrace Street, S314 Biomedical Science Tower, Pittsburgh PA 15261, USA, michael7@pitt.edu
References
- Abu-El-Haija M, Sinkora M, Meyerholz DK, Welsh MJ, McCray PB, Jr, Butler J, Uc A. An activated immune and inflammatory response targets the pancreas of newborn pigs with cystic fibrosis. Pancreatology. 2011;11:506–515. doi: 10.1159/000332582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebamiro A, Johnson JP, Bridges RJ. Aprotinin decreases the number of active Na+ channels in the apical membrane of A6 epithelia. Pediatr Pulmonol Suppl. 2002;24:211–212. [Google Scholar]
- Adebamiro A, Cheng Y, Johnson JP, Bridges RJ. Endogenous protease activation of ENaC: effect of serine protease inhibition on ENaC single channel properties. J Gen Physiol. 2005;126:339–352. doi: 10.1085/jgp.200509285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebamiro A, Cheng Y, Rao US, Danahay H, Bridges RJ. A segment of gamma ENaC mediates elastase activation of Na+ transport. J Gen Physiol. 2007;130:611–629. doi: 10.1085/jgp.200709781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad AK, Rauh R, Vermeulen F, Jaspers M, Korbmacher J, Boissier B, Bassinet L, Fichou Y, Georges Mdes Stanke F, De Boeck K, Dupont L, Balascakova M, Hjelte L, Lebecque P, Radojkovic D, Castellani C, Schwartz M, Stuhrmann M, Schwarz M, Skalicka V, Monestrol Ide, Girodon E, Ferec C, Claustres M, Tummler B, Cassiman JJ, Korbmacher C, Cuppens H. Mutations in the amiloride-sensitive epithelial sodium channel in patients with cystic fibrosis-like disease. Hum Mutat. 2009;30:1093–1103. doi: 10.1002/humu.21011. [DOI] [PubMed] [Google Scholar]
- Azghani AO, Baker JW, Shetty S, Miller EJ, Bhat GJ. Pseudomonas aeruginosa elastase stimulates ERK signaling pathway and enhances IL-8 production by alveolar epithelial cells in culture. Inflamm Res. 2002;51:506–510. doi: 10.1007/pl00012420. [DOI] [PubMed] [Google Scholar]
- Bainbridge T, Fick RB., Jr Functional importance of cystic fibrosis immunoglobulin G fragments generated by Pseudomonas aeruginosa elastase. J Lab Clin Med. 1989;114:728–733. [PubMed] [Google Scholar]
- Baumann U. Crystal structure of the 50 kDa metallo protease from Serratia marcescens. J Mol Biol. 1994;242:244–251. doi: 10.1006/jmbi.1994.1576. [DOI] [PubMed] [Google Scholar]
- Baumann U, Wu S, Flaherty KM, McKay DB. Three-dimensional structure of the alkaline protease of Pseudomonas aeruginosa: a two-domain protein with a calcium binding parallel beta roll motif. EMBO J. 1993;12:3357–3364. doi: 10.1002/j.1460-2075.1993.tb06009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bens M, Chassin C, Vandewalle A. Regulation of NaCl transport in the renal collecting duct: lessons from cultured cells. Pflugers Arch. 2006;453:133–146. doi: 10.1007/s00424-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Bhalla V, Hallows KR. Mechanisms of ENaC regulation and clinical implications. J Am Soc Nephrol. 2008;19:1845–1854. doi: 10.1681/ASN.2008020225. [DOI] [PubMed] [Google Scholar]
- Bosshart H, Humphrey J, Deignan E, Davidson J, Drazba J, Yuan L, Oorschot V, Peters PJ, Bonifacino JS. The cytoplasmic domain mediates localization of furin to the trans-Golgi network en route to the endosomal/lysosomal system. J Cell Biol. 1994;126:1157–1172. doi: 10.1083/jcb.126.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher RC. New concepts of the pathogenesis of cystic fibro-sis lung disease. Eur Respir J. 2004;23:146–158. doi: 10.1183/09031936.03.00057003. [DOI] [PubMed] [Google Scholar]
- Briel M, Greger R, Kunzelmann K. Cl transport by cystic fibrosis transmembrane conductance regulator (CFTR) contributes to the inhibition of epithelial Na+ channels (ENaCs) in Xenopus oocytes co-expressing CFTR and ENaC. J Physiol. 1998;508:825–836. doi: 10.1111/j.1469-7793.1998.825bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan BE, Hayek MB, Doebbeling BN, Fick RB., Jr Transferrin and lactoferrin undergo proteolytic cleavage in the Pseudomonas aeruginosa-infected lungs of patients with cystic fibrosis. Infect Immun. 1993;61:5049–5055. doi: 10.1128/iai.61.12.5049-5055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the {gamma}-subunit. J Biol Chem. 2007;282:6153–6160. doi: 10.1074/jbc.M610636200. [DOI] [PubMed] [Google Scholar]
- Burke V, Robinson JO, Richardson CJ, Bundell CS. Longitudinal studies of virulence factors of Pseudomonas aeruginosa in cystic fibrosis. Pathology. 1991;23:145–148. doi: 10.3109/00313029109060814. [DOI] [PubMed] [Google Scholar]
- Butterworth MB. Regulation of the epithelial sodium channel (ENaC) by membrane trafficking. Biochim Biophys Acta. 2010;1802:1166–1177. doi: 10.1016/j.bbadis.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth MB, Edinger RS, Frizzell RA, Johnson JP. Regulation of the epithelial sodium channel by membrane trafficking. Am J Physiol Renal Physiol. 2009;296:F10–F24. doi: 10.1152/ajprenal.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd MS, Pang B, Hong W, Waligora EA, Juneau RA, Armbruster CE, Weimer KE, Murrah K, Mann EE, Lu H, Sprinkle A, Parsek MR, Kock ND, Wozniak DJ, Swords WE. Direct evaluation of Pseudomonas aeruginosa biofilm mediators in a chronic infection model. Infect Immun. 2011;79:3087–3095. doi: 10.1128/IAI.00057-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero AR, Moreau JM, Engel LS, Marquart ME, Hill JM, O’Cal-laghan RJ. Pseudomonas aeruginosa protease IV enzyme assays and comparison to other Pseudomonas proteases. Anal Biochem. 2001;290:330–337. doi: 10.1006/abio.2001.4999. [DOI] [PubMed] [Google Scholar]
- Caldwell RA, Boucher RC, Stutts MJ. Serine protease activation of near-silent epithelial Na+ channels. Am J Physiol Cell Physiol. 2004;286:C190–C194. doi: 10.1152/ajpcell.00342.2003. [DOI] [PubMed] [Google Scholar]
- Caldwell RA, Boucher RC, Stutts MJ. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am J Physiol Lung Cell Mol Physiol. 2005;288:L813–L819. doi: 10.1152/ajplung.00435.2004. [DOI] [PubMed] [Google Scholar]
- Capasso G, Cantone A, Evangelista C, Zacchia M, Trepiccione F, Acone D, Rizzo M. Channels, carriers, and pumps in the pathogenesis of sodium-sensitive hypertension. Semin Nephrol. 2005;25:419–424. doi: 10.1016/j.semnephrol.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Carattino MD, Passero CJ, Steren CA, Maarouf AB, Pilewski JM, Myerburg MM, Hughey RP, Kleyman TR. Defining an inhibitory domain in the alpha-subunit of the epithelial sodium channel. Am J Physiol Renal Physiol. 2008;294:F47–F52. doi: 10.1152/ajprenal.00399.2007. [DOI] [PubMed] [Google Scholar]
- Chambers LA, Rollins BM, Tarran R. Liquid movement across the surface epithelium of large airways. Respir Physiol Neurobiol. 2007;159:256–270. doi: 10.1016/j.resp.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, Rector MV, Reznikov LR, Launspach JL, Chaloner K, Zabner J, Welsh MJ. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell. 2010;143:911–923. doi: 10.1016/j.cell.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SH, Rich DP, Marshall J, Gregory RJ, Welsh MJ, Smith AE. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell. 1991;66:1027–1036. doi: 10.1016/0092-8674(91)90446-6. [DOI] [PubMed] [Google Scholar]
- Choi JY, Muallem D, Kiselyov K, Lee MG, Thomas PJ, Muallem S. Aberrant CFTR-dependent HCO3 transport in mutations associated with cystic fibrosis. Nature. 2001;410:94–97. doi: 10.1038/35065099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow SC, Weis M, Kass GE, Holmstrom TH, Eriksson JE, Orrenius S. Involvement of multiple proteases during Fas-mediated apoptosis in T lymphocytes. FEBS Lett. 1995;364:134–138. doi: 10.1016/0014-5793(95)00370-o. [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 1998;17:7151–7160. doi: 10.1093/emboj/17.24.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conese M, Copreni E, Di Gioia S, De Rinaldis P, Fumarulo R. Neutrophil recruitment and airway epithelial cell involvement in chronic cystic fibrosis lung disease. J Cyst Fibros. 2003;2:129–135. doi: 10.1016/S1569-1993(03)00063-8. [DOI] [PubMed] [Google Scholar]
- Coraux C, Roux J, Jolly T, Birembaut P. Epithelial cell-extracellular matrix interactions and stem cells in airway epithelial regeneration. Proc Am Thorac Soc. 2008;5:689–694. doi: 10.1513/pats.200801-010AW. [DOI] [PubMed] [Google Scholar]
- Cottrell GS, Amadesi S, Grady EF, Bunnett NW. Trypsin IV, a novel agonist of protease-activated receptors 2 and 4. J Biol Chem. 2004;279:13532–13539. doi: 10.1074/jbc.M312090200. [DOI] [PubMed] [Google Scholar]
- Dean M, Hamon Y, Chimini G. The human ATP-binding cassette (ABC) transporter superfamily. J Lipid Res. 2001;42:1007–1017. [PubMed] [Google Scholar]
- Di Cera E, Cantwell AM. Determinants of thrombin specificity. Ann N Y Acad Sci. 2001;936:133–146. doi: 10.1111/j.1749-6632.2001.tb03502.x. [DOI] [PubMed] [Google Scholar]
- Donaldson SH, Hirsh A, Li DC, Holloway G, Chao J, Boucher RC, Gabriel SE. Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem. 2002;277:8338–8345. doi: 10.1074/jbc.M105044200. [DOI] [PubMed] [Google Scholar]
- Downey DG, Brockbank S, Martin SL, Ennis M, Elborn JS. The effect of treatment of cystic fibrosis pulmonary exacerbations on airways and systemic inflammation. Pediatr Pulmonol. 2007a;42:729–735. doi: 10.1002/ppul.20646. [DOI] [PubMed] [Google Scholar]
- Downey DG, Martin SL, Dempster M, Moore JE, Keogan MT, Starcher B, Edgar J, Bilton D, Elborn JS. The relationship of clinical and inflammatory markers to outcome in stable patients with cystic fibrosis. Pediatr Pulmonol. 2007b;42:216–220. doi: 10.1002/ppul.20553. [DOI] [PubMed] [Google Scholar]
- Downey DG, Bell SC, Elborn JS. Neutrophils in cystic fibrosis. Thorax. 2009;64:81–88. doi: 10.1136/thx.2007.082388. [DOI] [PubMed] [Google Scholar]
- Drumm ML, Pope HA, Cliff WH, Rommens JM, Marvin SA, Tsui LC, Collins FS, Frizzell RA, Wilson JM. Correction of the cystic fibrosis defect in vitro by retrovirus-mediated gene transfer. Cell. 1990;62:1227–1233. doi: 10.1016/0092-8674(90)90398-x. [DOI] [PubMed] [Google Scholar]
- Duong F, Lazdunski A, Cami B, Murgier M. Sequence of a cluster of genes controlling synthesis and secretion of alkaline protease in Pseudomonas aeruginosa: relationships to other secretory pathways. Gene. 1992;121:47–54. doi: 10.1016/0378-1119(92)90160-q. [DOI] [PubMed] [Google Scholar]
- Eaton DC, Helms MN, Koval M, Bao HF, Jain L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annu Rev Physiol. 2009;71:403–423. doi: 10.1146/annurev.physiol.010908.163250. [DOI] [PubMed] [Google Scholar]
- Ecelbarger CA, Tiwari S. Sodium transporters in the distal nephron and disease implications. Curr Hypertens Rep. 2006;8:158–165. doi: 10.1007/s11906-006-0013-z. [DOI] [PubMed] [Google Scholar]
- Edelheit O, Hanukoglu I, Gizewska M, Kandemir N, Tenenbaum-Rakover Y, Yurdakok M, Zajaczek S, Hanukoglu A. Novel mutations in epithelial sodium channel (ENaC) subunit genes and phenotypic expression of multisystem pseudohypoaldosteronism. Clin Endocrinol. 2005;62:547–553. doi: 10.1111/j.1365-2265.2005.02255.x. [DOI] [PubMed] [Google Scholar]
- Egnell P, Flock JI. The autocatalytic processing of the subtilisin Carlsberg pro-region is independent of the primary structure of the cleavage site. Mol Microbiol. 1992;6:1115–1119. doi: 10.1111/j.1365-2958.1992.tb01549.x. [DOI] [PubMed] [Google Scholar]
- Elizur A, Cannon CL, Ferkol TW. Airway inflammation in cystic fibrosis. Chest. 2008;133:489–495. doi: 10.1378/chest.07-1631. [DOI] [PubMed] [Google Scholar]
- Engel LS, Hobden JA, Moreau JM, Callegan MC, Hill JM, O’Callaghan RJ. Pseudomonas deficient in protease IV has significantly reduced corneal virulence. Invest Ophthalmol Vis Sci. 1997;38:1535–1542. [PubMed] [Google Scholar]
- Engel LS, Hill JM, Caballero AR, Green LC, O’Callaghan RJ. Protease IV, a unique extracellular protease and virulence factor from Pseudomonas aeruginosa . J Biol Chem. 1998;273:16792–16797. doi: 10.1074/jbc.273.27.16792. [DOI] [PubMed] [Google Scholar]
- Erez E, Fass D, Bibi E. How intramembrane proteases bury hydrolytic reactions in the membrane. Nature. 2009;459:371–378. doi: 10.1038/nature08146. [DOI] [PubMed] [Google Scholar]
- Ergonul Z, Frindt G, Palmer LG. Regulation of maturation and processing of ENaC subunits in the rat kidney. Am J Physiol Renal Physiol. 2006;291:F683–F693. doi: 10.1152/ajprenal.00422.2005. [DOI] [PubMed] [Google Scholar]
- Fleiszig SM, Evans DJ, Do N, Vallas V, Shin S, Mostov KE. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect Immun. 1997;65:2861–2867. doi: 10.1128/iai.65.7.2861-2867.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundlich M, Ludwig M. A novel epithelial sodium channel beta-subunit mutation associated with hypertensive Liddle syndrome. Pediatr Nephrol. 2005;20:512–515. doi: 10.1007/s00467-004-1751-2. [DOI] [PubMed] [Google Scholar]
- Fritz LC, Haidar MA, Arfsten AE, Schilling JW, Carilli C, Shine J, Baxter JD, Reudelhuber TL. Human renin is correctly processed and targeted to the regulated secretory pathway in mouse pituitary AtT-20 cells. J Biol Chem. 1987;262:12409–12412. [PubMed] [Google Scholar]
- Gaillard EA, Kota P, Gentzsch M, Dokholyan NV, Stutts MJ, Tarran R. Regulation of the epithelial Na+ channel and airway surface liquid volume by serine proteases. Pflugers Arch. 2010;460:1–17. doi: 10.1007/s00424-010-0827-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JB, Ohman DE. Cloning and transcriptional regulation of the elastase lasA gene in mucoid and nonmucoid Pseudomonas aeruginosa . J Bacteriol. 1987;169:1349–1351. doi: 10.1128/jb.169.3.1349-1351.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gormley K, Dong Y, Sagnella GA. Regulation of the epithelial sodium channel by accessory proteins. Biochem J. 2003;371:1–14. doi: 10.1042/BJ20021375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorogh T, Beier UH, Baumken J, Meyer JE, Hoffmann M, Gottschlich S, Maune S. Metalloproteinases and their inhibitors: influence on tumor invasiveness and metastasis formation in head and neck squamous cell carcinomas. Head Neck. 2006;28:31–39. doi: 10.1002/hed.20298. [DOI] [PubMed] [Google Scholar]
- Granstrom M, Ericsson A, Strandvik B, Wretlind B, Pavlovskis OR, Berka R, Vasil ML. Relation between antibody response to Pseudomonas aeruginosa exoproteins and colonization/infection in patients with cystic fibrosis. Acta Paediatr Scand. 1984;73:772–777. doi: 10.1111/j.1651-2227.1984.tb17774.x. [DOI] [PubMed] [Google Scholar]
- Gregory RJ, Cheng SH, Rich DP, Marshall J, Paul S, Hehir K, Ostedgaard L, Klinger KW, Welsh MJ, Smith AE. Expression and characterization of the cystic fibrosis transmembrane conductance regulator. Nature. 1990;347:382–386. doi: 10.1038/347382a0. [DOI] [PubMed] [Google Scholar]
- Griese M, Kappler M, Gaggar A, Hartl D. Inhibition of airway proteases in cystic fibrosis lung disease. Eur Respir J. 2008;32:783–795. doi: 10.1183/09031936.00146807. [DOI] [PubMed] [Google Scholar]
- Guyot N, Butler MW, McNally P, Weldon S, Greene CM, Levine RL, O’Neill SJ, Taggart CC, McElvaney NG. Elafin, an elastase-specific inhibitor, is cleaved by its cognate enzyme neutrophil elastase in sputum from individuals with cystic fibrosis. J Biol Chem. 2008;283:32377–32385. doi: 10.1074/jbc.M803707200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot N, Bergsson G, Butler MW, Greene CM, Weldon S, Kessler E, Levine RL, O’Neill SJ, Taggart CC, McElvaney NG. Functional study of elafin cleaved by Pseudomonas aeruginosa metalloproteinases. Biol Chem. 2010;391:705–716. doi: 10.1515/BC.2010.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzzo J, Murgier M, Filloux A, Lazdunski A. Cloning of the Pseudomonas aeruginosa alkaline protease gene and secretion of the protease into the medium by Escherichia coli . J Bacteriol. 1990;172:942–948. doi: 10.1128/jb.172.2.942-948.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm LL, Feng Z, Hering-Smith KS. Regulation of sodium transport by ENaC in the kidney. Curr Opin Nephrol Hypertens. 2010;19:98–105. doi: 10.1097/MNH.0b013e328332bda4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M, Garcia-Caballero A, Stutts MJ, Firsov D, Rossier BC. Preferential assembly of ENaC subunits in Xenopus oocyte: role of furin-mediated endogenous proteolysis. J Biol Chem. 2008;283:7455–7463. doi: 10.1074/jbc.M707399200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck LW, Alarcon PG, Kulhavy RM, Morihara K, Russell MW, Mestecky JF. Degradation of IgA proteins by Pseudomonas aeruginosa elastase. J Immunol. 1990;144:2253–2257. [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- Hobden JA. Pseudomonas aeruginosa proteases and corneal virulence. DNA Cell Biol. 2002;21:391–396. doi: 10.1089/10445490260099674. [DOI] [PubMed] [Google Scholar]
- Hopf A, Schreiber R, Mall M, Greger R, Kunzelmann K. Cystic fibrosis transmembrane conductance regulator inhibits epithelial Na+ channels carrying Liddle’s syndrome mutations. J Biol Chem. 1999;274:13894–13899. doi: 10.1074/jbc.274.20.13894. [DOI] [PubMed] [Google Scholar]
- Horvat RT, Clabaugh M, Duval-Jobe C, Parmely MJ. Inactivation of human gamma interferon by Pseudomonas aeruginosa proteases: elastase augments the effects of alkaline protease despite the presence of alpha 2-macroglobulin. Infect Immun. 1989;57:1668–1674. doi: 10.1128/iai.57.6.1668-1674.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Krueger B, Diakov A, Korbmacher J, Haerteis S, Einsiedel J, Gmeiner P, Azad AK, Cuppens H, Cassiman JJ, Korbmacher C, Rauh R. Functional characterization of a partial loss-of-function mutation of the epithelial sodium channel (ENaC) associated with atypical cystic fibrosis. Cell Physiol Biochem. 2010;25:145–158. doi: 10.1159/000272059. [DOI] [PubMed] [Google Scholar]
- Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, Harkleroad KL, Carattino MD, Kleyman TR. Maturation of the epithelial Na+ channel involves proteolytic processing of the alpha- and gamma-subunits. J Biol Chem. 2003;278:37073–37082. doi: 10.1074/jbc.M307003200. [DOI] [PubMed] [Google Scholar]
- Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem. 2004a;279:18111–18114. doi: 10.1074/jbc.C400080200. [DOI] [PubMed] [Google Scholar]
- Hughey RP, Bruns JB, Kinlough CL, Kleyman TR. Distinct pools of epithelial sodium channels are expressed at the plasma membrane. J Biol Chem. 2004b;279:48491–48494. doi: 10.1074/jbc.C400460200. [DOI] [PubMed] [Google Scholar]
- Hughey RP, Carattino MD, Kleyman TR. Role of proteolysis in the activation of epithelial sodium channels. Curr Opin Nephrol Hypertens. 2007;16:444–450. doi: 10.1097/MNH.0b013e32821f6072. [DOI] [PubMed] [Google Scholar]
- Hummler E. Implication of ENaC in salt-sensitive hypertension. J Steroid Biochem Mol Biol. 1999;69:385–390. doi: 10.1016/s0960-0760(99)00073-4. [DOI] [PubMed] [Google Scholar]
- Itani OA, Chen JH, Karp PH, Ernst S, Keshavjee S, Parekh K, Klesney-Tait J, Zabner J, Welsh MJ. Human cystic fibrosis airway epithelia have reduced Cl conductance but not increased Na+ conductance. Proc Natl Acad Sci USA. 2011;108:10260–10265. doi: 10.1073/pnas.1106695108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger KS, Robinson DL, Franz MN, Warren RL. Detection by enzyme-linked immunosorbent assays of antibody specific for Pseudomonas proteases and exotoxin A in sera from cystic fibro-sis patients. J Clin Microbiol. 1982;15:1054–1058. doi: 10.1128/jcm.15.6.1054-1058.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger KS, Bahner DR, Warren RL. Protease phenotypes of Pseudomonas aeruginosa isolated from patients with cystic fibro-sis. J Clin Microbiol. 1983;17:55–59. doi: 10.1128/jcm.17.1.55-59.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji HL, Chalfant ML, Jovov B, Lockhart JP, Parker SB, Fuller CM, Stanton BA, Benos DJ. The cytosolic termini of the beta-and gamma-ENaC subunits are involved in the functional interactions between cystic fibrosis transmembrane conductance regulator and epithelial sodium channel. J Biol Chem. 2000;275:27947–27956. doi: 10.1074/jbc.M002848200. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Li J, Dubroff R, Ahn YJ, Foskett JK, Engelhardt J, Kleyman TR. Epithelial sodium channels regulate cystic fibrosis transmembrane conductance regulator chloride channels in Xenopus oocytes. J Biol Chem. 2000;275:13266–13274. doi: 10.1074/jbc.275.18.13266. [DOI] [PubMed] [Google Scholar]
- Johnson GG, Morris JM, Berk RS. The extracellular protease from Pseudomonas aeruginosa exhibiting elastase activity. Can J Microbiol. 1967;13:711–719. doi: 10.1139/m67-093. [DOI] [PubMed] [Google Scholar]
- Kametaka S, Shibata M, Moroe K, Kanamori S, Ohsawa Y, Waguri S, Sims PJ, Emoto K, Umeda M, Uchiyama Y. Identification of phospholipid scramblase 1 as a novel interacting molecule with beta-secretase (beta-site amyloid precursor protein (APP) cleaving enzyme (BACE)) J Biol Chem. 2003;278:15239–15245. doi: 10.1074/jbc.M208611200. [DOI] [PubMed] [Google Scholar]
- Kent G, Iles R, Bear CE, Huan LJ, Griesenbach U, McKerlie C, Frndova H, Ackerley C, Gosselin D, Radzioch D, O’Brodovich H, Tsui LC, Buchwald M, Tanswell AK. Lung disease in mice with cystic fibrosis. J Clin Invest. 1997;100:3060–3069. doi: 10.1172/JCI119861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kercsmar CM, Davis PB. Resistance of human tracheal epithelial cells to killing by neutrophils, neutrophil elastase, and Pseudomonas elastase. Am J Respir Cell Mol Biol. 1993;8:56–62. doi: 10.1165/ajrcmb/8.1.56. [DOI] [PubMed] [Google Scholar]
- Kessler E, Safrin M. Partial purification and characterization of an inactive precursor of Pseudomonas aeruginosa elastase. J Bacteriol. 1988;170:1215–1219. doi: 10.1128/jb.170.3.1215-1219.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler E, Safrin M. The propeptide of Pseudomonas aeruginosa elastase acts an elastase inhibitor. J Biol Chem. 1994;269:22726–22731. [PubMed] [Google Scholar]
- Kessler E, Safrin M, Olson JC, Ohman DE. Secreted LasA of Pseudomonas aeruginosa is a staphylolytic protease. J Biol Chem. 1993;268:7503–7508. [PubMed] [Google Scholar]
- Kessler E, Safrin M, Gustin JK, Ohman DE. Elastase and the LasA protease of Pseudomonas aeruginosa are secreted with their propeptides. J Biol Chem. 1998;273:30225–30231. doi: 10.1074/jbc.273.46.30225. [DOI] [PubMed] [Google Scholar]
- Kharazmi A, Doring G, Hoiby N, Valerius NH. Interaction of Pseudomonas aeruginosa alkaline protease and elastase with human polymorphonuclear leukocytes in vitro. Infect Immun. 1984a;43:161–165. doi: 10.1128/iai.43.1.161-165.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazmi A, Hoiby N, Doring G, Valerius NH. Pseudomonas aeruginosa exoproteases inhibit human neutrophil chemiluminescence. Infect Immun. 1984b;44:587–591. doi: 10.1128/iai.44.3.587-591.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyman TR, Myerburg MM, Hughey RP. Regulation of ENaCs by proteases: an increasingly complex story. Kidney Int. 2006;70:1391–1392. doi: 10.1038/sj.ki.5001860. [DOI] [PubMed] [Google Scholar]
- Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem. 2009;284:20447–20451. doi: 10.1074/jbc.R800083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO3 transport in cystic fibrosis. EMBO J. 2002;21:5662–5672. doi: 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H. Airway biofilms: implications for pathogenesis and therapy of respiratory tract infections. Treat Respir Med. 2005;4:241–253. doi: 10.2165/00151829-200504040-00003. [DOI] [PubMed] [Google Scholar]
- Kudoh I, Wiener-Kronish JP, Hashimoto S, Pittet JF, Frank D. Exoproduct secretions of Pseudomonas aeruginosa strains influence severity of alveolar epithelial injury. Am J Physiol. 1994;267:L551–L556. doi: 10.1152/ajplung.1994.267.5.L551. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Kiser GL, Schreiber R, Riordan JR. Inhibition of epithelial Na+ currents by intracellular domains of the cystic fibrosis transmembrane conductance regulator. FEBS Lett. 1997;400:341–344. doi: 10.1016/s0014-5793(96)01414-7. [DOI] [PubMed] [Google Scholar]
- Lang F, Huang DY, Vallon V. SGK, renal function and hypertension. J Nephrol. 2010;23(Suppl 16):S124–S129. [PMC free article] [PubMed] [Google Scholar]
- Lazdunski A, Guzzo J, Filloux A, Bally M, Murgier M. Secretion of extracellular proteins by Pseudomonas aeruginosa . Biochimie. 1990;72:147–156. doi: 10.1016/0300-9084(90)90140-c. [DOI] [PubMed] [Google Scholar]
- Lee B, Schjerling CK, Kirkby N, Hoffmann N, Borup R, Molin S, Hoiby N, Ciofu O. Mucoid Pseudomonas aeruginosa isolates maintain the biofilm formation capacity and the gene expression profiles during the chronic lung infection of CF patients. APMIS. 2011;119:263–274. doi: 10.1111/j.1600-0463.2011.02726.x. [DOI] [PubMed] [Google Scholar]
- Leidal KG, Munson KL, Johnson MC, Denning GM. Metalloproteases from Pseudomonas aeruginosa degrade human RANTES, MCP-1, and ENA-78. J Interferon Cytokine Res. 2003;23:307–318. doi: 10.1089/107999003766628151. [DOI] [PubMed] [Google Scholar]
- Levene PA. The cleavage of products of proteoses. J Biol Chem. 1905;1:45–58. [Google Scholar]
- Li J, Wang DH. Function and regulation of epithelial sodium transporters in the kidney of a salt-sensitive hypertensive rat model. J Hypertens. 2007;25:1065–1072. doi: 10.1097/HJH.0b013e3280a8b87d. [DOI] [PubMed] [Google Scholar]
- Liu L, Hering-Smith KS, Schiro FR, Hamm LL. Serine protease activity in m-1 cortical collecting duct cells. Hypertension. 2002;39:860–864. doi: 10.1161/01.hyp.0000013055.48885.8d. [DOI] [PubMed] [Google Scholar]
- Lopez-Otin C, Bond JS. Proteases: multifunctional enzymes in life and disease. J Biol Chem. 2008;283:30433–30437. doi: 10.1074/jbc.R800035200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–808. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- Louis D, Bernillon J, Wallach JM. Specificity of Pseudomonas aeruginosa serralysin revisited, using biologically active peptides as substrates. Biochim Biophys Acta. 1998;1387:378–386. doi: 10.1016/s0167-4838(98)00144-7. [DOI] [PubMed] [Google Scholar]
- Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2:1051–1060. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall MA. Role of cilia, mucus, and airway surface liquid in mucociliary dysfunction: lessons from mouse models. J Aerosol Med Pulm Drug Deliv. 2008;21:13–24. doi: 10.1089/jamp.2007.0659. [DOI] [PubMed] [Google Scholar]
- Mall M, Bleich M, Kuehr J, Brandis M, Greger R, Kunzelmann K. CFTR-mediated inhibition of epithelial Na+ conductance in human colon is defective in cystic fibrosis. Am J Physiol. 1999;277:G709–G716. doi: 10.1152/ajpgi.1999.277.3.G709. [DOI] [PubMed] [Google Scholar]
- Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- Malloy JL, Veldhuizen RA, Thibodeaux BA, O’Callaghan RJ, Wright JR. Pseudomonas aeruginosa protease IV degrades surfactant proteins and inhibits surfactant host defense and biophysical functions. Am J Physiol Lung Cell Mol Physiol. 2005;288:L409–L418. doi: 10.1152/ajplung.00322.2004. [DOI] [PubMed] [Google Scholar]
- Manos J, Arthur J, Rose B, Tingpej P, Fung C, Curtis M, Webb JS, Hu H, Kjelleberg S, Gorrell MD, Bye P, Harbour C. Transcriptome analyses and biofilm-forming characteristics of a clonal Pseudomonas aeruginosa from the cystic fibrosis lung. J Med Microbiol. 2008;57:1454–1465. doi: 10.1099/jmm.0.2008/005009-0. [DOI] [PubMed] [Google Scholar]
- Manos J, Arthur J, Rose B, Bell S, Tingpej P, Hu H, Webb J, Kjelleberg S, Gorrell MD, Bye P, Harbour C. Gene expression characteristics of a cystic fibrosis epidemic strain of Pseudomonas aeruginosa during biofilm and planktonic growth. FEMS Microbiol Lett. 2009;292:107–114. doi: 10.1111/j.1574-6968.2008.01472.x. [DOI] [PubMed] [Google Scholar]
- Mariencheck WI, Alcorn JF, Palmer SM, Wright JR. Pseudo-monas aeruginosa elastase degrades surfactant proteins A and D. Am J Respir Cell Mol Biol. 2003;28:528–537. doi: 10.1165/rcmb.2002-0141OC. [DOI] [PubMed] [Google Scholar]
- Marino CR, Matovcik LM, Gorelick FS, Cohn JA. Localization of the cystic fibrosis transmembrane conductance regulator in pancreas. J Clin Invest. 1991;88:712–716. doi: 10.1172/JCI115358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay MA, Robriquet L, Fang X. Alveolar epithelium: role in lung fluid balance and acute lung injury. Proc Am Thorac Soc. 2005;2:206–213. doi: 10.1513/pats.200501-009AC. [DOI] [PubMed] [Google Scholar]
- Mirghomizadeh F, Bullwinkel J, Orinska Z, Janssen O, Petersen A, Singh PB, Bulfone-Paus S. Transcriptional regulation of mouse mast cell protease-2 by interleukin-15. J Biol Chem. 2009;284:32635–32641. doi: 10.1074/jbc.M109.015446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossessova E, Lima CD. Ulp1-SUMO crystal structure and genetic analysis reveal conserved interactions and a regulatory element essential for cell growth in yeast. Mol Cell. 2000;5:865–876. doi: 10.1016/s1097-2765(00)80326-3. [DOI] [PubMed] [Google Scholar]
- Muzio M, Salvesen GS, Dixit VM. FLICE induced apoptosis in a cell-free system. Cleavage of caspase zymogens. J Biol Chem. 1997;272:2952–2956. doi: 10.1074/jbc.272.5.2952. [DOI] [PubMed] [Google Scholar]
- Myerburg MM, Butterworth MB, McKenna EE, Peters KW, Frizzell RA, Kleyman TR, Pilewski JM. Airway surface liquid volume regulates ENaC by altering the serine protease-protease inhibitor balance: a mechanism for sodium hyperabsorption in cystic fibrosis. J Biol Chem. 2006;281:27942–27949. doi: 10.1074/jbc.M606449200. [DOI] [PubMed] [Google Scholar]
- Myerburg MM, Harvey PR, Heidrich EM, Pilewski JM, Butterworth MB. Acute regulation of the epithelial sodium channel in airway epithelia by proteases and trafficking. Am J Respir Cell Mol Biol. 2010;43:712–719. doi: 10.1165/rcmb.2009-0348OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan RJ, Engel LS, Hobden JA, Callegan MC, Green LC, Hill JM. Pseudomonas keratitis. The role of an uncharacterized exoprotein, protease IV, in corneal virulence. Invest Ophthalmol Vis Sci. 1996;37:534–543. [PubMed] [Google Scholar]
- Oda K. New families of carboxyl peptidases: serine-carboxyl peptidases and glutamic peptidases. J Biochem. 2012;151:13–25. doi: 10.1093/jb/mvr129. [DOI] [PubMed] [Google Scholar]
- Orce GG, Castillo GA, Margolius HS. Inhibition of short-circuit current in toad urinary bladder by inhibitors of glandular kallikrein. Am J Physiol. 1980;239:F459–F465. doi: 10.1152/ajprenal.1980.239.5.F459. [DOI] [PubMed] [Google Scholar]
- Orce GG, Castillo GA, Margolius HS. Kallikrein inhibitors decrease short-circuit current by inhibiting sodium uptake. Hypertension. 1981;3:92–95. doi: 10.1161/01.hyp.3.6_pt_2.ii-92. [DOI] [PubMed] [Google Scholar]
- Overall CM, Tam EM, Kappelhoff R, Connor A, Ewart T, Morrison CJ, Puente X, Lopez-Otin C, Seth A. Protease degradomics: mass spectrometry discovery of protease substrates and the CLIP-CHIP, a dedicated DNA microarray of all human proteases and inhibitors. Biol Chem. 2004;385:493–504. doi: 10.1515/BC.2004.058. [DOI] [PubMed] [Google Scholar]
- Parmely MJ, Horvat RT. Antigenic specificities of Pseudomonas aeruginosa alkaline protease and elastase defined by human T cell clones. J Immunol. 1986;137:988–994. [PubMed] [Google Scholar]
- Parmely MJ, Iglewski BH, Horvat RT. Identification of the principal T lymphocyte-stimulating antigens of Pseudomonas aeruginosa . J Exp Med. 1984;160:1338–1349. doi: 10.1084/jem.160.5.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmely MJ, Horvat RT, Iglewski BH, Kanarek J, Furtado D, Van Enk R. The antigenicity of a pulmonary pathogen defined by monoclonal T cells. Adv Exp Med Biol. 1987;216B:1043–1051. [PubMed] [Google Scholar]
- Parmely M, Gale A, Clabaugh M, Horvat R, Zhou WW. Proteolytic inactivation of cytokines by Pseudomonas aeruginosa . Infect Immun. 1990;58:3009–3014. doi: 10.1128/iai.58.9.3009-3014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passero CJ, Mueller GM, Rondon-Berrios H, Tofovic SP, Hughey RP, Kleyman TR. Plasmin activates epithelial Na+ channels by cleaving the gamma subunit. J Biol Chem. 2008;283:36586–36591. doi: 10.1074/jbc.M805676200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planes C, Caughey GH. Regulation of the epithelial Na+ channel by peptidases. Curr Top Dev Biol. 2007;78:23–46. doi: 10.1016/S0070-2153(06)78002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planes C, Leyvraz C, Uchida T, Angelova MA, Vuagniaux G, Hummler E, Matthay M, Clerici C, Rossier B. In vitro and in vivo regulation of transepithelial lung alveolar sodium transport by serine proteases. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1099–L1109. doi: 10.1152/ajplung.00332.2004. [DOI] [PubMed] [Google Scholar]
- Pochynyuk O, Tong Q, Staruschenko A, Ma HP, Stockand JD. Regulation of the epithelial Na+ channel (ENaC) by phosphatidylinositides. Am J Physiol Renal Physiol. 2006;290:F949–F957. doi: 10.1152/ajprenal.00386.2005. [DOI] [PubMed] [Google Scholar]
- Puente XS, Sanchez LM, Overall CM, Lopez-Otin C. Human and mouse proteases: a comparative genomic approach. Nat Rev Genet. 2003;4:544–558. doi: 10.1038/nrg1111. [DOI] [PubMed] [Google Scholar]
- Quesada V, Ordonez GR, Sanchez LM, Puente XS, Lopez-Otin C. The Degradome database: mammalian proteases and diseases of proteolysis. Nucleic Acids Res. 2009;37:D239–D243. doi: 10.1093/nar/gkn570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh R, Diakov A, Tzschoppe A, Korbmacher J, Azad AK, Cuppens H, Cassiman JJ, Dotsch J, Sticht H, Korbmacher C. A mutation of the epithelial sodium channel associated with atypical cystic fibrosis increases channel open probability and reduces Na+ self inhibition. J Physiol. 2010;588:1211–1225. doi: 10.1113/jphysiol.2009.180224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravaud S, Gouet P, Haser R, Aghajari N. Probing the role of divalent metal ions in a bacterial psychrophilic metalloprotease: binding studies of an enzyme in the crystalline state by X-ray crystallography. J Bacteriol. 2003;185:4195–4203. doi: 10.1128/JB.185.14.4195-4203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ, Bateman A. MEROPS: the peptidase database. Nucleic Acids Res. 2010;38:D227–D233. doi: 10.1093/nar/gkp971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ, Bateman A. MEROPS: the database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res. 2012;40:D343–D350. doi: 10.1093/nar/gkr987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MM, Quinton PM. Functional interaction of CFTR and ENaC in sweat glands. Pflugers Arch. 2003;445:499–503. doi: 10.1007/s00424-002-0959-x. [DOI] [PubMed] [Google Scholar]
- Reddy MM, Light MJ, Quinton PM. Activation of the epithelial Na+ channel (ENaC) requires CFTR Cl− channel function. Nature. 1999;402:301–304. doi: 10.1038/46297. [DOI] [PubMed] [Google Scholar]
- Reeves EP, McElvaney NG. The facilitating effect of hypertonic saline on resolution of airway inflammation in cystic fibrosis. Am J Respir Crit Care Med. 2012;185:226–227. doi: 10.1164/ajrccm.185.2.226a. [DOI] [PubMed] [Google Scholar]
- Rich DP, Anderson MP, Gregory RJ, Cheng SH, Paul S, Jefferson DM, McCann JD, Klinger KW, Smith AE, Welsh MJ. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990;347:358–363. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rollins BM, Garcia-Caballero A, Stutts MJ, Tarran R. SPLUNC1 expression reduces surface levels of the epithelial sodium channel (ENaC) in Xenopus laevis oocytes. Channels (Austin) 2010;4:255–259. doi: 10.4161/chan.4.4.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier BC. Hormonal regulation of the epithelial sodium channel ENaC: N or Po? J Gen Physiol. 2002;120:67–70. doi: 10.1085/jgp.20028638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier BC. The epithelial sodium channel: activation by membrane-bound serine proteases. Proc Am Thorac Soc. 2004;1:4–9. doi: 10.1513/pats.2306007. [DOI] [PubMed] [Google Scholar]
- Rossier BC, Stutts MJ. Activation of the epithelial sodium channel (ENaC) by serine proteases. Annu Rev Physiol. 2009;71:361–379. doi: 10.1146/annurev.physiol.010908.163108. [DOI] [PubMed] [Google Scholar]
- Rust L, Pesci EC, Iglewski BH. Analysis of the Pseudomonas aeruginosa elastase (lasB) regulatory region. J Bacteriol. 1996;178:1134–1140. doi: 10.1128/jb.178.4.1134-1140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadane A, Soltys J, Berger M. Acute Pseudomonas challenge in cystic fibrosis mice causes prolonged nuclear factor-kappa B activation, cytokine secretion, and persistent lung inflammation. J Allergy Clin Immunol. 2006;117:1163–1169. doi: 10.1016/j.jaci.2006.01.052. [DOI] [PubMed] [Google Scholar]
- Sagel SD, Sontag MK, Wagener JS, Kapsner RK, Osberg I, Accurso FJ. Induced sputum inflammatory measures correlate with lung function in children with cystic fibrosis. J Pediatr. 2002;141:811–817. doi: 10.1067/mpd.2002.129847. [DOI] [PubMed] [Google Scholar]
- Salunkhe P, Smart CH, Morgan JA, Panagea S, Walshaw MJ, Hart CA, Geffers R, Tummler B, Winstanley C. A cystic fibrosis epidemic strain of Pseudomonas aeruginosa displays enhanced virulence and antimicrobial resistance. J Bacteriol. 2005;187:4908–4920. doi: 10.1128/JB.187.14.4908-4920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen GS, Riedl SJ. Caspase mechanisms. Adv Exp Med Biol. 2008;615:13–23. doi: 10.1007/978-1-4020-6554-5_2. [DOI] [PubMed] [Google Scholar]
- Sarkisova S, Patrauchan MA, Berglund D, Nivens DE, Franklin MJ. Calcium-induced virulence factors associated with the extracellular matrix of mucoid Pseudomonas aeruginosa bio-films. J Bacteriol. 2005a;187:4327–4337. doi: 10.1128/JB.187.13.4327-4337.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisova S, Patrauchan MA, Berglund D, Nivens DE, Franklin MJ. Calcium-induced virulence factors associated with the extracellular matrix of mucoid Pseudomonas aeruginosa bio-films. J Bacteriol. 2005b;187:4327–4337. doi: 10.1128/JB.187.13.4327-4337.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulnier JM, Curtil FM, Duclos MC, Wallach JM. Elastolytic activity of Pseudomonas aeruginosa elastase. Biochim Biophys Acta. 1989;995:285–290. doi: 10.1016/0167-4838(89)90048-4. [DOI] [PubMed] [Google Scholar]
- Schafer W, Stroh A, Berghofer S, Seiler J, Vey M, Kruse ML, Kern HF, Klenk HD, Garten W. Two independent targeting signals in the cytoplasmic domain determine trans-Golgi network localization and endosomal trafficking of the proprotein convertase furin. EMBO J. 1995;14:2424–2435. doi: 10.1002/j.1460-2075.1995.tb07240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Hopf A, Mall M, Greger R, Kunzelmann K. The first-nucleotide binding domain of the cystic-fibrosis transmembrane conductance regulator is important for inhibition of the epithelial Na+ channel. Proc Natl Acad Sci USA. 1999;96:5310–5315. doi: 10.1073/pnas.96.9.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng S, Carattino MD, Bruns JB, Hughey RP, Kleyman TR. Furin cleavage activates the epithelial Na+ channels by relieving Na+ self-inhibition. Am J Physiol Renal Physiol. 2006;290:F1488–F1496. doi: 10.1152/ajprenal.00439.2005. [DOI] [PubMed] [Google Scholar]
- Song HK, Bochtler M, Azim MK, Hartmann C, Huber R, Ramachandran R. Isolation and characterization of the prokaryotic proteasome homolog HslVU (ClpQY) from Thermotoga maritima and the crystal structure of HslV. Biophys Chem. 2003;100:437–452. doi: 10.1016/s0301-4622(02)00297-1. [DOI] [PubMed] [Google Scholar]
- Studholme DJ, Rawlings ND, Barrett AJ, Bateman A. A comparison of Pfam and MEROPS: two databases, one comprehensive, and one specialised. BMC Bioinformatics. 2003;4:17. doi: 10.1186/1471-2105-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhrmann M, Macek M, Jr, Reis A, Schmidtke J, Tummler B, Dork T, Vavrova V, Macek M, Krawczak M. Genotype analysis of cystic fibrosis patients in relation to pancreatic sufficiency. Lancet. 1990;335:738–739. doi: 10.1016/0140-6736(90)90862-y. [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Rossier BC, Boucher RC. Cystic fibrosis transmembrane conductance regulator inverts protein kinase A-mediated regulation of epithelial sodium channel single channel kinetics. J Biol Chem. 1997;272:14037–14040. doi: 10.1074/jbc.272.22.14037. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhang JN, Zhao D, Wang QS, Gu YC, Ma HP, Zhang ZR. Role of the epithelial sodium channel in salt-sensitive hypertension. Acta Pharmacol Sin. 2011;32:789–797. doi: 10.1038/aps.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter S. The role of bacterial proteases in the pathogenesis of cystic fibrosis. Am J Respir Crit Care Med. 1994;150:S118–S122. doi: 10.1164/ajrccm/150.6_Pt_2.S118. [DOI] [PubMed] [Google Scholar]
- Talanian RV, Quinlan C, Trautz S, Hackett MC, Mankovich JA, Banach D, Ghayur T, Brady KD, Wong WW. Substrate specificities of caspase family proteases. J Biol Chem. 1997;272:9677–9682. doi: 10.1074/jbc.272.15.9677. [DOI] [PubMed] [Google Scholar]
- Tan CD, Selvanathar IA, Baines DL. Cleavage of endogenous gammaENaC and elevated abundance of alphaENaC are associated with increased Na transport in response to apical fluid volume expansion in human H441 airway epithelial cells. Pflugers Arch. 2011;462:431–441. doi: 10.1007/s00424-011-0982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol. 2006;127:591–604. doi: 10.1085/jgp.200509468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terheggen-Lagro SW, Rijkers GT, van der Ent CK. The role of airway epithelium and blood neutrophils in the inflammatory response in cystic fibrosis. J Cyst Fibros. 2005;4(Suppl 2):15–23. doi: 10.1016/j.jcf.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Toder DS, Ferrell SJ, Nezezon JL, Rust L, Iglewski BH. lasA and lasB genes of Pseudomonas aeruginosa: analysis of transcription and gene product activity. Infect Immun. 1994;62:1320–1327. doi: 10.1128/iai.62.4.1320-1327.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomisugi Y, Unno M, Mizushima T, Morimoto Y, Tanahashi N, Tanaka K, Tsukihara T, Yasuoka N. New crystal forms and low resolution structure analysis of 20 S proteasomes from bovine liver. J Biochem. 2000;127:941–943. doi: 10.1093/oxfordjournals.jbchem.a022709. [DOI] [PubMed] [Google Scholar]
- Tomlin KL, Coll OP, Ceri H. Interspecies biofilms of Pseudomonas aeruginosa and Burkholderia cepacia . Can J Microbiol. 2001;47:949–954. [PubMed] [Google Scholar]
- Tong Z, Illek B, Bhagwandin VJ, Verghese GM, Caughey GH. Prostasin, a membrane-anchored serine peptidase, regulates sodium currents in JME/CF15 cells, a cystic fibrosis airway epithelial cell line. Am J Physiol Lung Cell Mol Physiol. 2004;287:L928–L935. doi: 10.1152/ajplung.00160.2004. [DOI] [PubMed] [Google Scholar]
- Unno M, Mizushima T, Morimoto Y, Tomisugi Y, Tanaka K, Yasuoka N, Tsukihara T. The structure of the mammalian 20 S proteasome at 2.75 Å resolution. Structure. 2002;10:609–618. doi: 10.1016/s0969-2126(02)00748-7. [DOI] [PubMed] [Google Scholar]
- Uss RH, Fenton JW2nd, Muraschi TF, Miller KD. Two distinct elastases from different strains of Pseudomonas aeruginosa . Biochim Biophys Acta. 1969;191:179–181. doi: 10.1016/0005-2744(69)90332-5. [DOI] [PubMed] [Google Scholar]
- Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 1997;389:607–610. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- Voynow JA, Fischer BM, Zheng S. Proteases and cystic fibrosis. Int J Biochem Cell Biol. 2008;40:1238–1245. doi: 10.1016/j.biocel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuagniaux G, Vallet V, Jaeger NF, Pfister C, Bens M, Farman N, Courtois-Coutry N, Vandewalle A, Rossier BC, Hummler E. Activation of the amiloride-sensitive epithelial sodium channel by the serine protease mCAP1 expressed in a mouse cortical collecting duct cell line. J Am Soc Nephrol. 2000;11:828–834. doi: 10.1681/ASN.V115828. [DOI] [PubMed] [Google Scholar]
- Vuagniaux G, Vallet V, Jaeger NF, Hummler E, Rossier BC. Synergistic activation of ENaC by three membrane-bound channel-activating serine proteases (mCAP1, mCAP2, and mCAP3) and serum- and glucocorticoid-regulated kinase (Sgk1) in Xenopus oocytes. J Gen Physiol. 2002;120:191–201. doi: 10.1085/jgp.20028598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CA, Loffing-Cueni D, Yan Q, Schulz N, Fakitsas P, Carrel M, Wang T, Verrey F, Geibel JP, Giebisch G, Hebert SC, Loffing J. Mouse model of type II Bartter’s syndrome. II. Altered expression of renal sodium- and water-transporting proteins. Am J Physiol Ren Physiol. 2008;294:F1373–F1380. doi: 10.1152/ajprenal.00613.2007. [DOI] [PubMed] [Google Scholar]
- Wiener-Kronish JP, Sakuma T, Kudoh I, Pittet JF, Frank D, Dobbs L, Vasil ML, Matthay MA. Alveolar epithelial injury and pleural empyema in acute P. aeruginosa pneumonia in anesthetized rabbits. J Appl Physiol. 1993;75:1661–1669. doi: 10.1152/jappl.1993.75.4.1661. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Conway JF, Thibodeau PH. Calcium-induced folding and stabilization of the Pseudomonas aeruginosa alkaline protease. J Biol Chem. 2012;287:4311–4322. doi: 10.1074/jbc.M111.310300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Duerr J, Johannesson B, Schubert SC, Treis D, Harm M, Graeber SY, Dalpke A, Schultz C, Mall MA. The ENaC-overexpressing mouse as a model of cystic fibrosis lung disease. J Cyst Fibros. 2011;10(Suppl 2):S172–S182. doi: 10.1016/S1569-1993(11)60021-0. [DOI] [PubMed] [Google Scholar]