Abstract
Mangroves in China are severely affected by the rapid invasion of the non-native species Spartina alterniflora. Although many studies have addressed the possible impacts of S. alterniflora on the performance of mangrove seedlings, how excessive nitrogen (N) input due to eutrophication affects the interactions between mangrove species and S. alterniflora remains unknown. Here, we report the results from a mesocosm experiment using seedlings of the native mangrove species Kandelia obovata and the exotic S. alterniflora grown in monoculture and mixed culture under no nitrogen addition and nitrogen (N) addition treatments for 18 months. Without N addition, the presence of S. alterniflora inhibited the growth of K. obovata seedlings. Excessive N addition significantly increased the growth rate of K. obovata in both cultures. However, the positive and significantly increasing relative interaction intensity index under excessive N input suggested that the invasion of S. alterniflora could favour the growth of K. obovata under eutrophication conditions. Our results imply that excessive N input in southeastern China can increase the competitive ability of mangrove seedlings against invasive S. alterniflora.
Keywords: mangrove ecosystem, coastal wetland, biological invasion, smooth cordgrass, nitrogen loading
1. Introduction
Located along coastlines throughout the tropical and subtropical regions of the world, mangrove wetlands are recognized as one of the most biologically important ecosystems [1]. However, many mangrove ecosystems are severely disturbed and threatened by human activities, causing many biological and environmental changes, particularly in China and other Southeast Asian countries [2]. Worldwide, mangrove forests are lost at an annual rate of 1–2% [3,4]; however, this rate is even higher in Southeast Asia [2].
Spartina alterniflora, commonly known as smooth cordgrass in the USA, was introduced to China in 1979. Since then, it has aggressively spread and invaded native mangrove habitats in China [5]. Although extensive studies have evaluated the possible impacts of S. alterniflora on native mangroves, the interactions between S. alterniflora and native mangrove seedlings have long been a subject of great debate. For example, some studies have shown that by stabilizing the sediment and ameliorating soil properties, S. alterniflora could play a ‘nursing’ role for mangrove seedlings [6]. By contrast, other studies reported that S. alterniflora inhibited the establishment and growth of mangrove seedlings by occupying their niche and shading their leaves owing to its fast growth [7–9].
Mangrove wetlands in China have seriously suffered from increasing eutrophication due to sewage discharge and aquaculture activities [10]. The results from a previous study suggest that eutrophication caused by nitrogen (N) enrichment can increase the survival and growth of mangroves, particularly young seedlings, because they are highly sensitive to changes in soil physico-chemical conditions [11,12]. However, whether excessive N can change the impact of S. alterniflora invasion on mangrove seedlings is still unclear. On the northeastern coast of Mexico, where mangroves have severely invaded S. alterniflora marshes, excessive N input had no effect on the interactions between mangroves and S. alterniflora [7]. In mangrove wetlands of China invaded by S. alterniflora, the effect of excessive N input on the interactions between S. alterniflora and mangrove seedlings is in urgent need of better understanding.
In this study, we examined the responses of Kandelia obovata (a common native mangrove species in China) seedlings and S. alterniflora grown in monoculture and mixed culture to excessive N loading typical of coastal regions in southeast China. The objectives of this study were to evaluate (i) how excessive N affects the growth of K. obovata seedlings with or without neighbouring S. alterniflora and (ii) the ability of excessive N to alleviate the negative impacts of S. alterniflora on mangrove seedlings.
2. Material and methods
(a). Experimental design
A mesocosm experiment was conducted in a greenhouse at the Graduate School at Shenzhen, Tsinghua University, People's Republic of China (22°59′ N, 113°97′ E). The mesocosm system consisted of 18 cement tanks (1.2 m width × 2.0 m length and 0.5 m depth) as experimental mesocosms and two cement tanks (0.5 m width × 3.0 m length and 1.2 m depth) as seawater reservoirs. One seawater reservoir tank was connected to nine control mesocosms with no N addition (hereafter ‘CK’). The other was connected to nine N addition treatment mesocosms (hereafter ‘N addition’). For the N addition treatment, 450 g NH4Cl (NH4+-N was the dominant N form in eutrophication-seawater column in coastal regions of southeast China) was added to the 3000 l seawater reservoir to create a high concentration of approximately 150 mg l−1 NH4+-N, which was similar to the concentration occurring in the coastal seawater of southeast China. Healthy seedlings of K. obovata and young ramets of S. alterniflora approximately 15 cm in height were transplanted into each mesocosm in August 2012. Each K. obovata seedling and S. alterniflora ramet covered an area of 0.15 × 0.15 m and 0.25 × 0.25 m, respectively. This mimics typical seedling densities of these two species according to our observations in the field. We simulated three types of vegetation compositions: (i) K. obovata monoculture, (ii) S. alterniflora monoculture, and (iii) mixed culture of the two species. Each vegetation type was tested with and without N addition (n = 3). For the monoculture mesocosms, there were 140 seedlings or ramets per tank, while for the mixed culture mesocosms there were 70 seedlings and 70 ramets cross-planted per tank. A schematic representation of the experimental mesocosms is presented in the electronic supplementary material, figure S1, and more detailed information is available in [13].
(b). Measurements of plant height and biomass
We randomly sampled one plant of the two species per mesocosm one month after planting and every three months thereafter. We measured the stem height and then harvested the aboveground biomass. The roots were sampled by the soil core method. Four soil cores (10 cm diameter and 30 cm depth) around the sampled plants were systematically taken and sieved to extract the total root biomass of each plant. All samples were oven-dried and weighed. The dry weights of the aboveground plant and roots were summed for the total biomass. There were in total seven plants per species per tank sampled during the experiment periods. Though this sampling would have changed the density and interaction of the two species, we believe that the data were credible considering the large number of plants in each tank.
(c). Calculation of the relative interaction intensity index
To examine whether K. obovata growth was significantly affected by S. alterniflora, we calculated the relative interaction intensity index (RII) [14] for each mesocosm as follows:
| 2.1 |
where Bmixed is the K. obovata biomass in the mixed culture, and Bmono is that in the monoculture.
(d). Statistical analyses
A three-way ANOVA was performed to examine the effects of N treatment, S. alterniflora invasion, sampling month and their possible interactions on K. obovata seedling growth. One-way ANOVA was used to examine significant differences in height and biomass for each sampling time and in the RII for K. obovata under the two N addition treatments. All data analyses were performed using SPSS v. 19.0.
3. Results
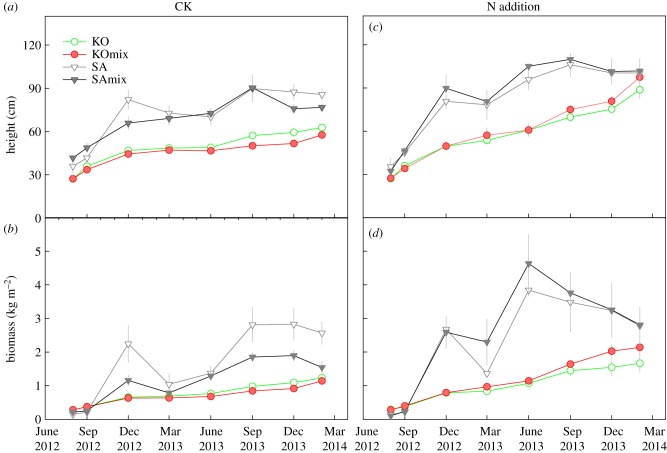
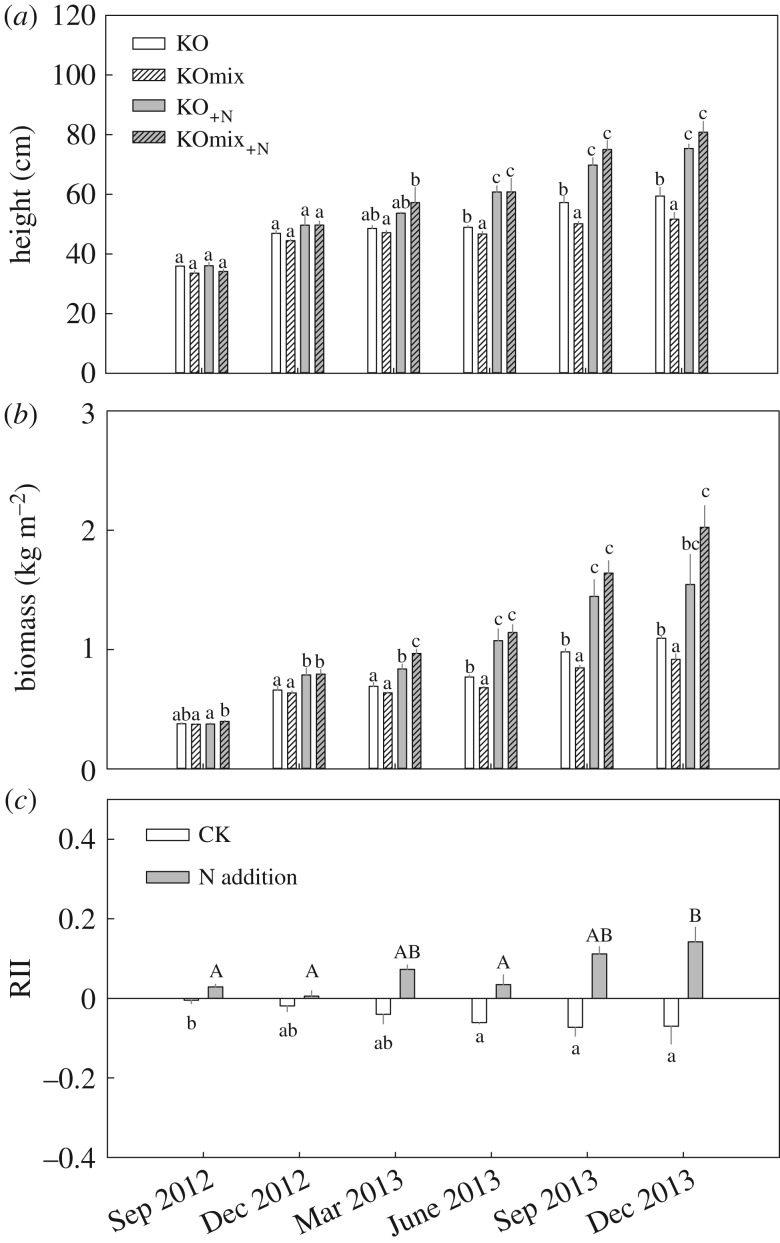
The height and biomass of K. obovata seedlings gradually increased in both mono- and mixed cultures under the CK treatment (figure 1a,b). There was no significant difference in the height or biomass of K. obovata between the mono- and mixed cultures during the period from September 2012 to March 2013, but the K. obovata in monoculture showed a greater height and biomass than those in mixed culture from June 2013 until the experiment ended (figure 2a,b). During the experimental period, the RII was significantly negative (p < 0.01, figure 2c), indicating that competition by S. alterniflora invasion suppressed the growth of K. obovata seedlings.
Figure 1.
Changes in height and biomass of K. obovata (KO: monoculture; KOmix: mixed culture) and S. alterniflora (SA: monoculture; SAmix: mixed culture) under no nitrogen addition (CK, a and b) and nitrogen addition (N addition, c and d). (Online version in colour.)
Figure 2.
Seasonal dynamics in stem height (a) and biomass (b) of K. obovata seedlings under the four treatments, and the mean relative interaction intensity (RII, c) of neighbouring S. alterniflora on K. obovata seedlings under the two nitrogen addition treatments.
Nitrogen addition significantly increased the growth rates of K. obovata and S. alterniflora in both mono- and mixed cultures (p < 0.001, table 1; figure 1c,d). The interactive effects of N addition and S. alterniflora invasion on the height and biomass of K. obovata seedlings were significant (p < 0.001; table 1). There was no difference in the height or biomass of K. obovata between the mono- and mixed cultures under the N addition treatment (p > 0.05; figure 2a,b). The RII was positive and increased significantly during the experiment (p < 0.01; figure 2b), indicating that the presence of S. alterniflora favoured the growth of K. obovata seedlings.
Table 1.
p-Values of three-way ANOVA for the effects of S. alterniflora invasion (I), nitrogen addition treatment (N), sampling time (T), and their interactions on height and biomass of K. obovata seedlings.
| d.f. | height | biomass | |
|---|---|---|---|
| I | 1 | 0.828 | 0.132 |
| N | 1 | <0.001 | <0.001 |
| T | 4 | <0.001 | <0.001 |
| I × N | 1 | <0.001 | 0.006 |
| I × T | 4 | 0.551 | 0.723 |
| N × T | 4 | <0.001 | <0.001 |
| I × N × T | 4 | <0.001 | 0.057 |
4. Discussion
Without N addition, S. alterniflora inhibited the growth of K. obovata seedlings, which could be due to the following reasons. First, the high root uptake capacity of fast-growing S. alterniflora could deplete nutrient pools (electronic supplementary material, figure S2), resulting in a low growth rate of K. obovata. Second, the fast increase in the height of S. alterniflora could have inhibited photosynthesis in K. obovata by shading [15].
The addition of N significantly increased the growth rate of K. obovata in both mono- and mixed cultures, implying its growth is N-limited (electronic supplementary material, figure S3). To our surprise, N addition led to a positive and increasing RII value, suggesting that the invasion of S. alterniflora could facilitate rather than inhibit the growth of K. obovata under excessive N input.
It has been theorized that biological interactions between plant species are affected by changes in nutrient availability and the environment [16] and that competition between plant species is more intense under high fertility conditions [17]. Thus, under high soil N conditions, competition for N resources between K. obovata seedlings and S. alterniflora could increase significantly, favouring the growth of S. alterniflora because of its higher growth rate and nutrient uptake capacity. However, in this study, excessive N input reversed the suppressive effects of S. alterniflora on K. obovata, suggesting that other factors may be involved in the changes in K. obovata seedling growth under excessive N inputs. Previous studies have indicated that, under some extreme conditions, the survival and growth of mangrove seedlings are facilitated by the presence of herbaceous species because they can ameliorate soil properties [18]. In this study, the regular loadings of excessive N in the form of NH4+-N might have exceeded the carrying capacity of the soils in the mesocosms, resulting in detrimental effects to the growth of both species due to physical pressure and/or ammonia toxicity [19,20]. Therefore, we hypothesize that the effects of S. alterniflora on K. obovata are likely to change from competition to facilitation under excessive N input, leading to a higher growth rate of K. obovata in the mixed culture than in the monoculture.
Our study provides direct experimental evidence that excessive N input due to eutrophication provides some hope for the survival and spread of K. obovata seedlings in southeastern China, where S. alterniflora invasions have become progressively more serious. More studies are needed to validate whether this phenomenon also occurs in other native mangrove species in China. Furthermore, the Chinese government has made great effects in mangrove afforestation during the last few decades, and more than 2000 ha of mangrove forests have been successful restored [21]. Our results might shed some light on how we can govern and protect the restored mangrove forests under the influences of Spartina invasion. In addition, this study lasted only 18 months and examined only one scenario of possible interactions between S. alterniflora and K. obovata, so more experimental studies with longer experimental period and a greater number of scenarios of mangrove–Spartina interactions are needed to better understand the eutrophication effects on the interactions between mangrove seedlings and S. alterniflora.
Supplementary Material
Acknowledgements
We thank Cunxin Ning, Ronghao Peng and Weizhi Lu for sampling assistance, three anonymous reviewers for valuable comments and Lynne Hyman for language editing.
Data accessibility
Raw data are available from Dryad: http://dx.doi.org/10.5061/dryad.j5148 [22].
Authors' contributions
G.L. conceived and designed the experiment. J.F., D.J., J.G., Z.W., H.W. and F.Q. conducted the experiment and collected data. X.C., W.S., J.F., J.L. and G.L. analysed the data and wrote the initial draft of the manuscript. All authors contributed to the editing and revising of the final version of the manuscript. The authors approved the final version for publication and agree to be held accountable for the content therein.
Competing interests
We have no competing interests.
Funding
This research was supported by the National Key Basic Research Program of China (973 program, 2013CB956601) and State Oceanic Administration of China (201305021).
References
- 1.Donato DC, Kauffman JB, Murdiyarso D, Kurnianto S, Stidham M, Kanninen M. 2011. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 4, 293–297. ( 10.1038/ngeo1123) [DOI] [Google Scholar]
- 2.Richards DR, Friess DA. 2016. Rates and drivers of mangrove deforestation in Southeast Asia, 2000–2012. Proc. Natl Acad. Sci. USA 113, 344–349. ( 10.1073/pnas.1510272113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alongi DM. 2002. Present state and future of the world's mangrove forests. Environ. Conserv. 29, 331–349. ( 10.1017/s0376892902000231) [DOI] [Google Scholar]
- 4.Duke NC, et al. 2007. A world without mangroves? Science 317, 41–42. ( 10.1126/science.317.5834.41b) [DOI] [PubMed] [Google Scholar]
- 5.An SQ, et al. 2007. Spartina invasion in China: implications for invasive species management and future research. Weed Res. 47, 183–191. ( 10.1111/j.1365-3180.2007.00559.x) [DOI] [Google Scholar]
- 6.Lewis RR. 2005. Ecological engineering for successful management and restoration of mangrove forests. Ecol. Eng. 24, 403–418. ( 10.1016/j.ecoleng.2004.10.003) [DOI] [Google Scholar]
- 7.McKee K, Rooth JE. 2008. Where temperate meets tropical: multi-factorial effects of elevated CO2, nitrogen enrichment, and competition on a mangrove-salt marsh community. Glob. Change Biol. 14, 971–984. ( 10.1111/j.1365-2486.2008.01547.x) [DOI] [Google Scholar]
- 8.Zhang Y, Huang G, Wang W, Chen L, Lin G. 2012. Interactions between mangroves and exotic Spartina in an anthropogenically disturbed estuary in southern China. Ecology 93, 588–597. ( 10.1890/11-1302.1) [DOI] [PubMed] [Google Scholar]
- 9.Pickens CN. 2012. Influence of climatic change on the ecophysiology and restoration ecology of black mangrove (Avicennia germinans (L.) L.). PhD dissertation, University of Louisiana, USA.
- 10.Vaiphasa C, De Boer WF, Panitchart S, Vaiphasa T, Bamrongrugsa N, Santitamnont P. 2007. Impact of solid shrimp pond waste materials on mangrove growth and mortality: a case study from Pak Phanang, Thailand. Hydrobiologia 591, 47–57. ( 10.1007/s10750-007-0783-6) [DOI] [Google Scholar]
- 11.Feller IC. 1995. Effects of nutrient enrichment on growth and herbivory of dwarf red mangrove (Rhizophora mangle). Ecol. Monogr. 65, 477–505. ( 10.2307/2963499) [DOI] [Google Scholar]
- 12.Feller IC, Lovelock C, McKee K. 2007. Nutrient addition differentially affects ecological processes of Avicennia germinans in nitrogen versus phosphorus limited mangrove ecosystems. Ecosystems 10, 347–359. ( 10.1007/s10021-007-9025-z) [DOI] [Google Scholar]
- 13.Jia D, et al. 2016. Co-regulations of Spartina alterniflora invasion and exogenous nitrogen loading on soil N2O efflux in subtropical mangrove mesocosms. PLoS ONE 11, e0146199 ( 10.1371/journal.pone.0146199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armas C, Ordiales R, Pugnaire FI. 2004. Measuring plant interactions: a new comparative index. Ecology 85, 2682–2686. ( 10.1890/03-0650) [DOI] [Google Scholar]
- 15.Rogers K, Saintilan N, Heijnis H. 2005. Mangrove encroachment of salt marsh in Western Port Bay, Victoria: the role of sedimentation, subsidence, and sea level rise. Estuaries 28, 551–559. ( 10.1007/BF02696066) [DOI] [Google Scholar]
- 16.Callaway RM. 2007. Interaction between competition and facilitation. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 17.Keddy P, Fraser LH. 2000. Four general principles for the management and conservation of wetlands in large lakes: the role of water levels, nutrients, competitive hierarchies and centrifugal organization. Lakes Reserv. Res. Manage. 5, 177–185. ( 10.1046/j.1440-1770.2000.00111.x) [DOI] [Google Scholar]
- 18.McKee KL, Rooth JE, Feller IC. 2007. Mangrove recruitment after forest disturbance is facilitated by herbaceous species in the Caribbean. Ecol. Appl. 17, 1678–1693. ( 10.1890/06-1614.1) [DOI] [PubMed] [Google Scholar]
- 19.Lovelock CE, Ball MC, Martin KC, Feller IC. 2009. Nutrient enrichment increases mortality of mangroves. PLoS ONE 4, e5600 ( 10.1371/journal.pone.0005600) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alongi DM. 2011. Early growth responses of mangroves to different rates of nitrogen and phosphorus supply. J. Exp. Mar. Biol. Ecol. 397, 85–93. ( 10.1016/j.jembe.2010.11.021) [DOI] [Google Scholar]
- 21.Chen LZ, Wang YH, Lin GH. 2009. Recent progresses in mangrove conservation, restoration and research in China. J. Plant Ecol. 2, 45–54. ( 10.1093/jpe/rtp009) [DOI] [Google Scholar]
- 22.Cui X, et al. 2017. Data from: Increased nitrogen input enhances Kandelia obovata seedling growth in the presence of invasive Spartina alterniflora in subtropical regions of China. Dryad Digital Repository. ( 10.5061/dryad.j5148) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data are available from Dryad: http://dx.doi.org/10.5061/dryad.j5148 [22].