Abstract
Cordyceps sinensis (C. sinensis) is a natural product that has diverse nutritional and medicinal values. Since the availability of natural C. sinensis becomes limited its authentication and quality control is of high significance. Herein we report on profiling of metals in C. sinensis by using inductively coupled plasma mass spectrometry (ICP-MS). The analysis reveals that C. sinensis contains a wide array of essential elements, including P, Mg, Zn, Cu, Fe, etc. Toxic metals detected are Cd, Pb, and As. In all five samples analyzed Pb contents are below 2.0 ppm. Arsenic level in C. sinensis caterpillar is significantly higher than that in its mycelium and varies from 3.0 to 32 ppm likely due to soil contamination. It’s for the first time demonstrated in this work that clustering analysis on the proposed metal profiles consisting of 24 elements is very useful to identify “abnormal” C. sinensis samples, thus adding another dimension to the effective means for authentication and quality assessment of this highly demanded previous natural product.
Keywords: Toxic metals, Clustering analysis, ICP-MS, Metal Profile, Cordyceps sinensis, Natural products
Graphical Abstract
1. Introduction
Cordyceps sinensis (C. sinensis) is a parasitic complex of fungus and caterpillar known to have diversified nutritional and medicinal values [1–5]. It has been in great demand for hundreds of years as a tonic food and herbal medicine. Its health benefits demonstrated so far include anti-fatigue, anti-aging, anti-oxidant, immunological regulation, anti-inflammation, anti-cancer, antiplatelet and protection of kidney, liver and lung [1, 6–11]. Several classes of bioactive ingredients such as nucleosides, polysaccharides, sterols, fatty acids, etc. were detected in C. sinensis [1–2, 12–14]. Since its growth requires a very restricted habitat, the availability of natural C. sinensis is very limited. Therefore, its authentication and quality control is of high significance. Various chemical markers have been proposed for these purposes [14–17]. Although the chemical composition of C. sinensis was intensively studied over the past years, investigation on its metal profiles has been very limited so far [15, 18].
Metal contamination of foodstuffs and dietary supplements has been always a serious concern [19–20]. It can be caused by polluted environment, fertilizer and pesticide usage in agriculture, inappropriate storage techniques, etc. There were several reported cases of heavy metal poisoning caused by consuming wild Chinese herbs, including C. sinensis [21, 22]. Lead contamination to C. sinensis was very recently alerted by Chinese Food and Drug Administration on Feb 16, 2016. However, to our knowledge, no study has been done on profiling trace metals in C. sinensis. Analytical methods based on various techniques such as stripping voltammetry, flame atomic absorption spectrometry (FAAS), graphite furnace atomic absorption spectrometry (GF-AAS), inductively coupled plasma-atomic emission spectrometry (ICP-AES), and inductively coupled plasma mass spectrometry (ICP-MS) have been developed for determination of metals at trace levels[23]. Compared with other analytical techniques used for metal analysis, ICP-MS is the most versatile technique for metal analysis and provides part-per-trillion detection limits. ICP-MS methods offer simultaneous multiple element measurement capability, quantification specificity, and high determination sensitivity among other significant advantages [24–25]. These combined make the ICP-MS technique best suited for profiling of metals in natural products.
In this work an assay protocol based on the ICP-MS technique was developed and validated to simultaneously determine 24 essential, non-essential, and toxic metals for the first time in C. sinensis. Samples of authentic C. sinensis were collected from authority-certified herbal stores located in different regions of China. Each piece of C. sinensis was cut into its caterpillar host and mycelium, and then analyzed to determine the metal contents. The metal profiles obtained were comparatively studied by using HCL hierarchical clustering analysis, enhancing our capability of authentication and quality control for this precious natural product.
2. Materials and methods
2.1. Chemicals and reagents
Hydrogen peroxide (H2O2, >30% ultra-trace analysis grade) was from Sigma Aldrich (St. Louis, MO). Nitric acid (HNO3, trace metal grade) was purchased from BDH Chemicals (Atlanta, GA). High purity de-ionized water (18.2 MΩ/cm resistivity) was prepared by a Milli-Q water purification system (Millipore, Bedford, MA) and used throughout the work.
2.2. Cordyceps sinensis samples and sample preparation
Five samples of authentic C. sinensis were collected from authority-certified herbal stores located in different regions of China. Each piece of C. sinensis was thoroughly washed/rinsed with Milli-Q water and cut into its caterpillar host and mycelium. The samples were cryogenically ground and homogenized to obtain a uniform matrix. A powdered sample (~0.15g) was digested with 5.0 mL concentrated HNO3 and 2.5 mL H2O2 in 60-mL screw-capped PTFE tubes (Savillex) at 110 °C for 1 h. Additional 2.5 mL of H2O2 was added at the completion of the first digestion cycle and heated for another 1 h. The content was evaporated to near dryness. The residue was re-dissolved in 5.0 mL water and reheated to dryness to remove excess HNO3 and H2O2. The sample was finally re-constituted in 10.0 mL 1.0% HNO3 for ICP-MS analysis.
2.3. Instrumentation and ICP-MS analysis
Measurements were performed with a Varian 820MS inductively coupled plasma mass spectrometer (Varian, Australia). The instrument was equipped with a peltier–cooled double–pass glass spray chamber, a Teflon Ari–mist nebulizer (SCP Science, Champlain, NY), quartz torch, Ni sampler, skimmer cones, and all–digital detector. Samples were introduced manually. The instrument was optimized daily for sensitivity, doubly charged ions (<1%) and oxides (<3%) with 5.0 μg L−1 138Ba, 25Mg, 115In, 140Ce, 208Pb solution. Data collection was achieved by ICP–MS Expert software package (version 2.2 b126). The operating parameters of the instrument are summarized in Table 1. Germanium (72Ge) and rhodium (103Rh) were used as internal standard (IS) elements to correct for possible instrumental drift and sensitivity changes. 72Ge was used for As, Mg, Ca, Fe, Cr, Co, Cu, Mn, Mo, Ni, P, Se, V, Zn, while 103Rh was assigned to Ag, Ba, Cd, Hg, Pb, Sb, Sn, and Tl. The internal standards (Ge and Rh, each at 5.0 μg L−1 final concentration) were added to each sample solution. Two isotopes of the multi-isotopic elements (e.g., 63Cu and 65Cu for Cu, 66Zn and 68Zn for Zn, 60Ni and 62Ni for Ni, 95Mo and 98Mo for Mo, 111Cd and 114Cd for Cd, 200Hg and 202Hg for Hg, and 206Pb and 208Pb for Pb, 203Tl and 205Tl for Pb were utilized during the data collection. Before measurements calibration of the ICP-MS spectrometer was carried out using a multiple elemental mixture of metal standards (Agilent Technologies). Five calibration solutions at a concentration range from 0.050 to 5.0 ppm were prepared by dilution with1% nitric acid. The calibration curve for all the elements revealed a good linearity over the concentration range tested with r2 values > 0.998. To determine the limits of detection (LODs), five replicate analyses of the 1% nitric acid solution were performed. LODs of the studied isotopes (i.e. 27Al, 75As,135Ba, 114Cd, 60Ni, 208Pb, and 121Sb) were found between 0.5ppt for Sb and 12.3ppt for Al.
Table 1.
Instrumental parameters used for the measurement
| Operating parameter | Value |
|---|---|
| RF Power | 1.4 kW |
| Plasma Ar flow | 18 L min−1 |
| Auxiliary Ar flow | 1.8 L min−1 |
| Nebulizer Ar flow | 1.10 L min−1 |
| Sheath Ar flow | 0.12 L min−1 |
| Sampling depth | 5.5 mm |
| Pump rate | 6; 0.2 rpm; mL min−1 |
| Stabilization time | 20 s |
| Spray chamber temperature | 4°C |
| Scan mode | Peak hopping |
| Dwell time | 20 ms |
| Points/peak | 2 |
| Scans/peak | 5 |
| Scans/replicate | 5 |
2.4. Statistical analysis
To assess metal content differences between samples one-way analysis of variance (ANOVA) was used. The level of statistical significance was set at p-value <0.05. Hierarchical clustering analysis of the results was performed to determine if the samples can be grouped into classes.
3. Results and discussion
3.1. Validation of the analytical method
To validate the analytical method, a standard reference material for trace metal analysis (SRM 1570a - Trace Elements in Spinach Leaves, NIST) was added to a pooled sample of powdered C. sinensis at four different concentrations: 0, 10.0% (w/w), 50.0%, and 100%. The spiked samples were then digested and measured by ICP-MS for three times. Metal contents in these mixed sample were determined to assess the accuracy and precision (RSD) of this method. The results for selected metals, i.e. Al, As, and P are summarized in Table 2. Results for other elements certified in SRM 1570a concurred with the results for Al, As, and P listed in the table. These results indicate that the present ICP-MS based protocol is accurate and repeatable for quantifying metals in C. sinensis.
Table 2.
Evaluation of the ICP-MS Method by a NIST standard reference material (SRM 1570a—Trace Elements in Spinach Leaves)*
| Sample | Element | Claimed value (ppm) | Found value (ppm) | RSD (n=3),% | Error % |
|---|---|---|---|---|---|
|
| |||||
| SRM 1570a | Al | 310 | 320 | 2.1 | 3.2 |
| As | 0.068 | 0.065 | 4.2 | −4.4 | |
| P | 5187 | 5102 | 3.7 | −1.6 | |
|
| |||||
| Pooled C. sinensis | Al | n/a | 403.3 | 2.9 | n/a |
| As | n/a | 6.50 | 3.6 | n/a | |
| P | n/a | 4284 | 4.2 | n/a | |
|
| |||||
| 10.0% SRM 1570a in C. sinenesis | Al | 325 | 318 | 1.8 | −2.2 |
| As | 5.86 | 6.02 | 3.3 | 2.7 | |
| P | 4374 | 4335 | 2.5 | −0.9 | |
|
| |||||
| 50.0% SRM 1570a in C. sinenesis | Al | 356.7 | 341.0 | 4.1 | −4.4 |
| As | 3.28 | 3.32 | 2.4 | 1.2 | |
| P | 4736 | 4845 | 1.7 | 2.3 | |
Results for other elements certified in SRM 1570a are not shown that concurred with the results for Al, As, and P listed in the table.
3.2. Metals detected in C. sinensis
By using the ICP-MS based protocol, metal profiles consisting of 24 elements were determined for C. sinensis in this work. Five natural C. sinensis samples were collected, and each was separated into its caterpillar host and mycelium for the investigation. Metal contents found are listed in Table 3. As shown, a wide array of essential metals, including Mg, P, Ca, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Sr, Mo, and Sn were detected in C. sinensis at different levels. Mg, Fe, Zn and P levels are very high (>100 ppm) while other essential metals are present at ppb levels. It’s obvious that C. sinensis is a good source of essential elements, and therefore, promotes human health in various aspects associated with the benefits from these elements. It’s worth noting, however, that Se, an important essential element, was not detected in C. sinensis. Four non-essential metals (i.e. Al, Ag, Ba, and Tl) were investigated. Tl was not detected. Al and Ba were found at ppm levels. Interestingly, their levels varied drastically from sample to sample, likely reflecting the soil chemical composition at the land where the C. sinensis sample was harvested. Five toxic metals (i.e. As, Cd, Sb, Hg, and Pb) were included in the metal profiling. Highly toxic Hg was not detected in C. sinensis. Levels of Cd and Sb were found extremely low (<50 ppb). Pb content ranged from 220 to 1493 ppb. All are below the limit of 5 ppm set for herbal medicines in both European [26] and Chinese [27] Pharmacopeia. These results show that lead in natural C. sinensis presents at low levels and should cause no toxicological concerns. Unfortunately, high levels of arsenic were detected in C. sinensis. In the five samples tested arsenic level ranged from 0.379 to 32 ppm. Some are well above the limit of 2.0 ppm set for herbal medicines in Chinese Pharmacopeia. It should be pointed out that these results of toxic metals are in good agreement with those found in a previous study where 6 heavy metals in C. sinensis were determined by AAS. In the study Pb was found in a range from 0.22 to 5.12 ppm, and As from 1.92 to 17.68 ppm [15]. It’s worth noting that As level in caterpillar is a lot higher than that in mycelium for all the five C. sinensis samples tested, suggesting that soil arsenic contributes to arsenic level in the caterpillar samples. As shown in Table 3, the range of As content in the five caterpillar samples is from 2.95 ppm to 32.2 ppm while As contents in all the mycelium samples are below 2 ppm except for that in sample #4. Considering the extraordinarily high level of arsenic in the caterpillar of sample #1 and #4, it safe to say these samples were harvested at a location where soil arsenic level was high. The U.S. Food and Drug Administration (FDA) has established tolerance levels for arsenic in byproducts of animals. These permissible levels range from 0.5 ppm in eggs and uncooked edible tissues of chickens and turkeys to 2 ppm in certain uncooked edible byproducts of swine. American Herbal Products Association (AHPA) guidance on maximum quantitative limits for heavy metals in herbal supplements are 10 μg per day for As and Pb [28]. Based on this information, arsenic intake from consuming C. sinensis at a normal dose should be safe. To our knowledge, the metal profile of C. sinensis is studied for the first time in this work. There are currently more than 400 documented species of Cordyceps [2]. Their metal profiles may be different due to their different habitat environments, life cycles, etc. For example, the metal profile of C. kyushuensis Kawam reported previously [18] was significantly different from that we found for C. sinensis. A major difference is that in C. kyushuensis Kawam metal contents in stroma (mycelium) are higher than the respective ones in worm (caterpillar) for all 20 metals included in the profile. Statistical analysis on the metal profiles of different samples determines the sample similarity, and thus helps with their authentication and quality assessment.
Table 3.
Metal Contents in Cordyceps sinensis*
| Sample # | Mg25 ppm | Al27 ppm | P31 ppm | Ca43 ppm | V51 ppb | Cr53 ppb | Mn55 ppm | Fe57 ppm | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Caterpillar host | 1233.6 | 87.8 | 4648.3 | 302.4 | 409.8 | 172.5 | 43.6 | 194.3 |
| Mycelium | 1121.1 | 352.3 | 3644.2 | 318.2 | 988.7 | 236.8 | 43.3 | 505.5 | |
| 2 | Caterpillar host | 1428.3 | 182.9 | 5894.9 | 278.4 | 696.1 | 274.8 | 48.8 | 360.6 |
| Mycelium | 1720.8 | 705.8 | 7185.0 | 995.5 | 1751.2 | 658.4 | 40.2 | 1008.3 | |
| 3 | Caterpillar host | 1794.9 | 83.1 | 6042.7 | 222.7 | 387.6 | 87.7 | 42.0 | 174.1 |
| Mycelium | 806.1 | 241.1 | 4691.9 | 270.4 | 700.2 | 221.3 | 18.4 | 393.2 | |
| 4 | Caterpillar host | 1440.9 | 546.9 | 4705.5 | 166.4 | 983.2 | 852.4 | 56.7 | 810.8 |
| Mycelium | 1777.7 | 1849.9 | 969.3 | 808.4 | 3759.9 | 2315.1 | 80.7 | 1747.3 | |
| 5 | Caterpillar host | 1011.0 | 73.0 | 4317.9 | 122.7 | 380.4 | 244.5 | 23.2 | 138.9 |
| Mycelium | 805.1 | 399.7 | 5327.1 | 159.9 | 909.3 | 472.5 | 23.3 | 660.3 | |
| Sample # | Co59 ppb | Ni60 ppb | Cu63 ppb | Zn66 ppm | As75 ppb | Se82 ppb | Sr88 ppb | Mo95 ppb | |
| 1 | Caterpillar host | 201.2 | 339.6 | 8348.7 | 60.6 | 17177.8 | ND | 794.0 | 2326.1 |
| Mycelium | 482.9 | 1916.5 | 8229.8 | 55.5 | 896.8 | ND | 1259.1 | 144.2 | |
| 2 | Caterpillar host | 146.4 | 427.0 | 7537.4 | 155.3 | 8192.4 | ND | 906.0 | 473.2 |
| Mycelium | 634.0 | 2565.4 | 15288.8 | 96.8 | 1118.4 | ND | 3918.6 | 158.2 | |
| 3 | Caterpillar host | 126.7 | 325.7 | 5763.1 | 174.1 | 7627.8 | ND | 1285.5 | 281.7 |
| Mycelium | 131.8 | 1348.7 | 11648.0 | 78.1 | 379.8 | ND | 942.2 | 32.7 | |
| 4 | Caterpillar host | 457.7 | 822.7 | 8753.6 | 111.7 | 32169.3 | ND | 1001.0 | 1804.7 |
| Mycelium | 1279.2 | 3760.8 | 11979.0 | 73.0 | 2300.3 | ND | 4299.2 | 177.6 | |
| 5 | Caterpillar host | 99.8 | 276.6 | 7253.1 | 77.3 | 2951.1 | 34.5 | 824.6 | 496.9 |
| Mycelium | 315.5 | 882.1 | 13236.3 | 115.7 | 501.0 | ND | 825.0 | 82.8 | |
| Sample # | Ag109 ppb | Cd111 ppb | Sn120 ppb | Sb121 ppb | Ba135 ppb | Hg200 ppb | Tl205 ppb | Pb208 ppb | |
| 1 | Caterpillar host | 17.8 | 27.9 | 13.9 | 20.5 | 5101.9 | ND | ND | 676.1 |
| Mycelium | 103.2 | 87.0 | 2.4 | 34.2 | 3635.5 | ND | ND | 747.0 | |
| 2 | Caterpillar host | 125.5 | 37.5 | 11.2 | 6.5 | 7039.0 | ND | ND | 1493.2 |
| Mycelium | 116.8 | 99.8 | 4.3 | ND | 7581.7 | ND | ND | 1117.2 | |
| 3 | Caterpillar host | 71.5 | 54.8 | 2.4 | 13.7 | 13100.1 | ND | ND | 1632.0 |
| Mycelium | 759.6 | 163.6 | 2.1 | 4.3 | 3920.3 | ND | ND | 220.5 | |
| 4 | Caterpillar host | 40.4 | 130.8 | 48.8 | 34.5 | 9071.0 | ND | ND | 240.1 |
| Mycelium | 5.5 | 125.8 | 3.9 | ND | 11347.3 | ND | 6.5 | 320.0 | |
| 5 | Caterpillar host | 15.9 | 32.5 | 35.5 | 15.6 | 5297.1 | 4.6 | 3.6 | 441.1 |
| Mycelium | 8.6 | 14.3 | 1.1 | ND | 3451.5 | 14.3 | ND | 391.1 |
Averages calculated from 3 measurements, RSD <2.0% for all averages. “ND”= not detected.
3.3. Hierarchical clustering analysis on metal profiles
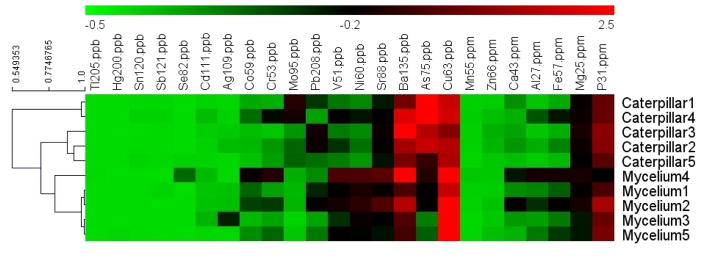
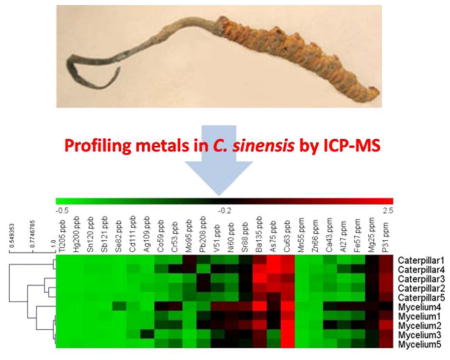
Metal profiles of 10 samples classified as 5 caterpillars and 5 myceliums were subjected to hierarchical clustering analysis. According to similarity of the contents of 24 metals 10 samples were classified into 3 main cluster groups as shown in the dendrogram on the left side of Figure 1. The heatmap roughly shows a difference between caterpillar and mycelium in terms of metal contents. We can see the caterpillar (especially caterpillar #1 & #4) has higher contents of Mo and As than mycelium. Moreover, the content of Al is lower in most caterpillar samples (4 out of 5) than in mycelium. Mycelium #1–5 are grouped together and showed the similarity in most of the metal contents. However, among 5 caterpillar samples, caterpillar #1 and #4 are separated from other caterpillar samples due to the very high contents of As & Mo which is likely caused by soil contamination. These results prove that comparing the proposed metal profiles of unknown samples with those of authentic C. sinensis by statistical clustering analysis adds another dimension of the effective means for authentication and quality assessment of C. sinensis samples.
Figure 1.
Dendrogram and heatmap obtained by HCL hierarchical clustering analysis of 24 metal concentrations in 10 samples. The data are separated into two groups (the ones with ppb unit on the left and the ones with ppm unit on the right) with the order of the columns based on the averaged contents of each metal. The heatmap is based on global Z-scoring transformation of the metal contents.
4. Conclusions
Profiling of metals in C. sinensis, a precious natural product by using an ICP-MS method has been performed for the first time. The profiling reveals that C. sinensis contains a wide array of essential elements. Among them P, Mg, Fe, and Zn are present at high levels. It also contains toxic metals, including Pb and As. While Pb level is below the limit of 5 ppm set for Pb in herbal medicines according to Pharmacopeia it is found that As level in caterpillar samples can be as high as 32 ppm likely due to soil contamination. Clustering analysis on the proposed metal profiles consisting of 24 elements proves effective for identifying “abnormal” C. sinensis samples.
Acknowledgments
Financial support from US National Institutes of Health (GM089557 and partially G12MD007581) is gratefully acknowledged.
References
- 1.Zhu JS, Halpern GM, Jones K. Scientific discovery of a previous ancient Chinese herbal regimen: Cordyceps sinensis (Part I and Part II) J Altern Complement Med. 1998;4:289–303. 429–457. doi: 10.1089/acm.1998.4.3-289. [DOI] [PubMed] [Google Scholar]
- 2.Zhou X, Gong Z, Su Y. Cordyceps fungi: natural products, pharmacological functions and developmental products. J Pharm Pharmacol. 2009;61:279–291. doi: 10.1211/jpp/61.03.0002. [DOI] [PubMed] [Google Scholar]
- 3.Das SK, Masuda M, Sakurai A, Sakakibara M. Medicinal uses of the mushroom Cordyceps militaris: current state and prospects. Fitoterapia. 2010;81:961–968. doi: 10.1016/j.fitote.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Chen PX, Wang SA, Nie SP, Marcone M. Properties of Cordyceps Sinensis: A review. J Funct Foods. 2013;5:550–569. doi: 10.1016/j.jff.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou XW, Li LJ, Tian EW. Advances in research of the artificial cultivation of Ophiocordyceps sinensis in China. Crit Rev Biotechnol. 2014;34:233–43. doi: 10.3109/07388551.2013.791245. [DOI] [PubMed] [Google Scholar]
- 6.Chang Y, Hsu WH, Lu WJ, Jayakumar T, Liao JC, Lin MJ, Wang SH, Geraldine P, Lin KH, Sheu JR. Inhibitory mechanisms of CME-1, a novel polysaccharide from the mycelia of Cordyceps sinensis, in platelet activation. Curr Pharm Biotechnol. 2015;16:451–61. [PubMed] [Google Scholar]
- 7.Nakamura K, Shinozuka K, Yoshikawa N. Anticancer and antimetastatic effects of cordycepin, an active component of Cordyceps sinensis. J Pharmacol Sci. 2015;127:53–56. doi: 10.1016/j.jphs.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Zhang HW, Lin ZX, Tung YS, Kwan TH, Mok CK, Leung C, Chan LS. Cordyceps sinensis (a traditional Chinese medicine) for treating chronic kidney disease. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD008353.pub2.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng L, Hao L, Ma H, Tian C, Li T, Sun X, Jia M, Jia L. Production and in vivo antioxidant activity of Zn, Ge, Se-enriched mycelia by Cordyceps sinensis SU-01. Curr Microbiol. 2014;69:270–276. doi: 10.1007/s00284-014-0582-z. [DOI] [PubMed] [Google Scholar]
- 10.Peng Y, Tao Y, Wang Q, Shen L, Yang T, Liu Z, Liu C. Ergosterol Is the Active Compound of Cultured Mycelium Cordyceps sinensis on Antiliver Fibrosis. Evid Based Complement Alternat Med. 2014;2014 doi: 10.1155/2014/537234. Article ID 537234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Wang M, Ling Y, Fan W, Wang Y, Yin H. Structural determination and antioxidant activity of a polysaccharide from the fruiting bodies of cultured Cordyceps sinensis. Am J Chin Med. 2009;37:977–989. doi: 10.1142/S0192415X09007387. [DOI] [PubMed] [Google Scholar]
- 12.Yue K, Ye M, Zhou Z, Sun W, Lin X. The genus Cordyceps: a chemical and pharmacological review. J Pharm Pharmacol. 2013;65:474–93. doi: 10.1111/j.2042-7158.2012.01601.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Xie J, Wang LY, Li SP. Advanced development in chemical analysis of Cordyceps. J Pharma Biomed Anal. 2014;87:271–289. doi: 10.1016/j.jpba.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Li SP, Yang FQ, Tsim KW. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J Pharm Biomed Anal. 2006;41:1571–1584. doi: 10.1016/j.jpba.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 15.Zuo HL, Chen SJ, Zhang DL, Zhao J, Yang FQ, Xia ZN. Quality evaluation of natural Cordyceps sinensis from different collecting places in China by the contents of nucleosides and heavy metals. Anal Meth. 2013;5:5450–5456. [Google Scholar]
- 16.Wong YL, Wong KL, Shaw PC. Rapid authentication of Cordyceps by lateral flow dipstick. J Pharm Biomed Anal. 2015;111:306–310. doi: 10.1016/j.jpba.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Hu H, Xiao L, Zheng B, Wei X, Ellis A, Liu YM. Identification of chemical markers in Cordyceps sinensis by HPLC-MS/MS. Anal Bioanal Chem. 2015;407:8059–8066. doi: 10.1007/s00216-015-8978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang G, Zhao FY, Liu, Ling J, Lin J, Zhang C. Determination of essential and toxic elements in Cordyceps kyushuensis Kawam by inductively coupled plasma mass spectrometry. J Pharm Biomed Anal. 2013;72:172–176. doi: 10.1016/j.jpba.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Commission Regulation (CE) Setting maximum levels for certain contaminants in foodstuffs as regards heavy metals. Official J European Union. 2006;49(L364):5–24. [Google Scholar]
- 20.Stilinović N, Škrbić B, Živančev J, Mrmoš N, Pavlović N, Vukmirović S. The level of elements and antioxidant activity of commercial dietary supplement formulations based on edible mushrooms. Food Funct. 2014;5:3170–3178. doi: 10.1039/c4fo00703d. [DOI] [PubMed] [Google Scholar]
- 21.Wu TN, Yang KC, Wang CM, Lai JS, Ko KN, Chang PY, Liou SH. Lead poisoning caused by contaminated Cordyceps, a Chinese herbal medicine: two case reports. Sci Total Environ. 1996;182:193–195. doi: 10.1016/0048-9697(96)05054-1. [DOI] [PubMed] [Google Scholar]
- 22.Byard RW. A review of the potential forensic significance of traditional herbal medicines. J Forensic Sci. 2010;55:89–92. doi: 10.1111/j.1556-4029.2009.01252.x. [DOI] [PubMed] [Google Scholar]
- 23.Carter S, Fisher A, Garcia R, Gibson B, Lancaster S, Marshall J, Whiteside I. Review of advances in the analysis of metals, chemicals, and functional materials. J Anal Atomic Spectrom. 2015;30:2249–2294. [Google Scholar]
- 24.Ammann AA. Inductively coupled plasma mass spectrometry (ICP MS): a versatile tool. J Mass Spectrom. 2007;42:419–427. doi: 10.1002/jms.1206. [DOI] [PubMed] [Google Scholar]
- 25.Sanz-Mede A. “Heteroatom-tagged” quantification of proteins via ICP-MS. Anal Bioanal Chem. 2016;408:5393–5395. doi: 10.1007/s00216-016-9687-5. [DOI] [PubMed] [Google Scholar]
- 26.Herbal Drugs. European Pharmacopeia; 2005. [Google Scholar]
- 27.Chinese Pharmacopeia. (1) 2010;I [Google Scholar]
- 28.American Herbal Products Association. Heavy Metals: Analysis and Limits in Herbal Dietary Supplements. AHPA; Silver Spring, MD: Dec, 2009. [Google Scholar]