Abstract
A relationship between hyperammonemia and Ureaplasma infection has been shown in lung transplant recipients. We have demonstrated that Ureaplasma urealyticum cases hyperammonemia in a novel immunocompromised murine model. Herein, we determined whether Ureaplasma parvum can do the same. Male C3H mice were given mycophenolate mofetil, tacrolimus and prednisone for seven days, and then challenged with U. parvum intratracheally (IT) and/or intraperitoneally (IP), while continuing immunosuppression over six days. Plasma ammonia concentrations were determined and compared using Wilcoxon ranks sum tests. Plasma ammonia concentrations of immunosuppressed mice challenged IT/IP with spent broth [median, 188 μmol/L; range, 102–340 μmol/L] were similar to those of normal [median, 226 μmol/L; range, 154–284 μmol/L, p>0.05], uninfected immunosuppressed [median, 231 μmol/L; range, 122–340 μmol/L, p>0.05], and U. parvum IT/IP challenged immunocompetent [median, 226 μmol/L; range, 130–330 μmol/L, p>0.05] mice. Immunosuppressed mice challenged with U. parvum IT/IP (median 343 μmol/L; range 136–1,000 μmol/L) or IP (median 307 μmol/L; range 132–692 μmol/L) had higher plasma ammonia concentrations than those challenged IT/IP with spent broth (p<0.001). U. parvum can cause hyperammonemia in pharmacologically immunocompromised mice.
Keywords: Ureaplasma parvum, transplant, hyperammonemia syndrome, immunocompromised murine model
Introduction
Hyperammonemia has been reported in a number of types of transplant recipients, most commonly lung transplant recipients [1–6]. In a recent retrospective cohort study involving 807 lung transplant recipients, 1% developed hyperammonemia, 75% of whom died [1]. Until recently, the cause of hyperammonemia in transplant recipients was unclear. Using specialized culture and a Ureaplasma parvum/urealyticum nucleic acid amplification test [7], we recently described a relationship between Ureaplasma infection and hyperammonemia in lung transplant recipients [4]. Based on this finding, we administered an immunosuppression regimen mimicking that administered to lung transplant recipients to laboratory mice, and showed that they developed hyperammonemia when challenged with a type strain of U. urealyticum [8]. In this study, we showed that the related organism, U. parvum, can also cause hyperammonemia in pharmacologically immunosuppressed mice.
Materials and Methods
1. Microorganism
A clinical U. parvum isolate (IDRL-10,774, multilocus sequence type 22 [9]) recovered from the bronchial washings of a lung transplant patient was studied. The patient from whom the isolate had been recovered was a 65 year old man with idiopathic pulmonary fibrosis who had undergone bilateral lung transplantation. Two days post-transplant, he had been noted to be lethargic; five days post-transplant he had developed acute mental status changes and hypoxia triggering intubation and mechanical ventilation. Seven days post-transplant, he had developed seizures; his ammonia level had been 1,100 μmol/L on the eighth post-transplant day. The following day, computed tomography of the head had shown cerebral edema and herniation, and he had died on the tenth post-transplant day. A bronchial washing from three days post-transplant, tested posthumously, was culture-positive the U. parvum strain studied here.
Inoculum was prepared by growing the organism in U9 broth (Hardy Diagnostics, Santa Maria, CA) at 37°C in room air until an orange color change was observed (~5 hours). The culture broth was then centrifuged at 4,000 rpm for 30 minutes to concentrate the cells. The final inoculum concentration ranged from 5×1010 to 2×1011 CFU/mL. For morphological identification and quantitation, U. parvum was plated onto A8 agar (Hardy Diagnostics) and incubated anaerobically at 37°C for five days. Colonies were enumerated by 100X microscopy. Freshly-prepared inoculum was used to challenge the mice.
2. Immunosuppressive Agents
Mice were pharmacologically immunosuppressed using intraperitoneal (IP) mycophenolate mofetil (90 mg/kg) (Cellcept Intravenous, Roche Laboratories, Inc., Nutley, NJ), IP tacrolimus (1.2 mg/kg) (Prograf, Astellas Pharma US, Inc., Northbrook, IL), and oral prednisone (6 mg/kg) (Prednisone Intensol, Roxane Laboratories, Inc., Columbus, OH) administered daily for seven days prior to challenge with U. parvum and continued over six days of microbial challenge (i.e., until the day before sacrifice).
3. Experimental Mouse Model
Immunocompetent C3H male mice (24–29 g, Charles River Laboratories, Wilmington, MA) were studied. As controls, normal mice were studied (n= 12), with additional controls consisting of mice receiving the immunosuppression regimen for 13 days (n=12), and immunocompetent mice (n=12) challenged with U. parvum IT/IP. Pharmacologically immunosuppressed mice were challenged with U. parvum in U9 broth over six days by IP challenge every day (n=21), or by IP challenge every day combined with IT challenge every other day (n=27). For IT challenge, mice were anesthetized with ketamine/xylazine (90/10 mg/kg) and placed in a vertical position; 50 μl of U. parvum suspension was placed into the trachea using a 22G curved gavage needle. Mice remained vertical for two minutes and were monitored until awake. For IP challenge, 500 μl of U. parvum suspension was injected into the peritoneum. A fourth group of control animals (n = 18), consisted of immunosuppressed mice challenged IT/IP challenged with spent U9 broth (filtered through a 0.1 μm filter). U. parvum challenge was administered five hours after administration of immunosuppressive agents. Animals were monitored for decreased activity, decreased body temperature (as assessed by touch), hunched stature, distress, and inability to eat and drink; if these findings were severe, animals were euthanized. Eleven mice died before the experimental endpoint. Because of rapid coagulation of blood, blood of most deceased mice was unobtainable for ammonia determination.
4. Measurement of Plasma Ammonia Concentrations
Mice were euthanized with CO2 asphyxiation 24 hours after the last IP and 48 hours after the last IT challenge. Blood was collected via cardiac puncture and placed into 1.5 ml EDTA tubes. EDTA blood was immediately centrifuged at 8,000 rpm for 3 minutes and plasma frozen at −80°C. Plasma ammonia concentrations were determined using a Vitros 350 (Ortho Clinical Diagnostics, Inc., Raritan, NJ).
5. U. parvum Culture
Cardiac blood, lung tissue and a peritoneal cavity swab were cultured for U. parvum in U9 broth at 37°C for five days. Due to color changes in the U9 media, a maximum of 50 μl of blood could be cultured. Whole lung was homogenized with 3 ml MicroTest M5 transport media (Remel, Lenexa, KS); 200 μl of tissue homogenate was cultured. Positive cultures were confirmed by plating to A8 agar and by a previously-described real-time PCR assay [7], performed here using a LightCycler 1.5 real-time PCR instrument (Roche Diagnostic Gmbh, Mannheim, Germany) and with probe dye Red-640.
6. Statistical Analysis
Plasma ammonia concentrations between study groups were compared using Wilcoxon ranks sum tests. All tests were two sided; p-values less than 0.05 were considered statistically significant. No adjustment for multiple comparisons was made due to the small sample sizes. Analysis was performed using SAS version 9.4 (SAS Inc. Cary, NC).
Results
1. Plasma Ammonia Concentrations
The plasma ammonia concentrations of normal (non-immunosuppressed, uninfected) C3H mice were 154–284 μmol/L (median, 226 μmol/L), while those of uninfected immunosuppressed mice were 122–340 μmol/L (median, 231 μmol/L) and those of immunocompetent mice challenged IT/IP with U. parvum were 130–330 μmol/L (median, 226 μmol/L). The plasma ammonia concentrations of pharmacologically immunosuppressed mice challenged with spent U9 vehicle IT/IP were 102–340 μmol/L (median, 188 μmol/L). There was no difference in the plasma ammonia concentrations in this fourth group compared with the first three control groups (P values are shown in the table).
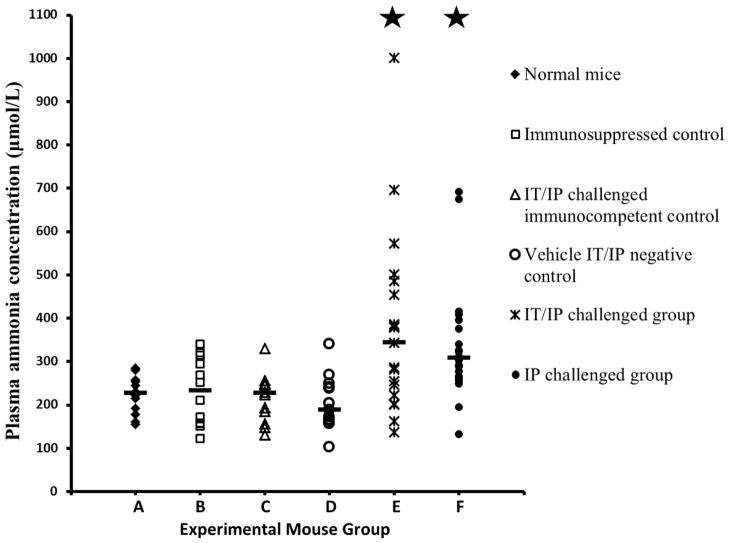
Mice in the IT/IP challenge group had elevated plasma ammonia concentrations (median 343 μmol/L, range 136–1,000 μmol/L) compared to vehicle IT/IP negative control mice (p<0.001). Mice in the IP challenge group also had elevated plasma ammonia concentrations (median 307 μmol/L, range 132–692 μmol/L) compared to vehicle IT/IP negative control mice (p<0.001). A comparison of plasma ammonia concentrations for each group is shown in figure 1.
Figure 1. Plasma ammonia concentrations in the six groups of experimental mice.
The median of each immunosuppressed and infected group is shown by a dash. Starred groups represent those with significantly elevated ammonia concentrations compared with group D, immunosuppressed mice inoculated with spent U. parvum-free U9 broth intratracheally (IT) every other day and intraperitoneally (IP) every day (P<0.001). Groups from left to right: A). Immunocompetent and uninfected mice (n=12). B). Immunosuppressed but uninfected mice (n=12). C). Immunocompetent mice challenged with U. parvum IT every other day and IP every day (n=12). D). Immunosuppressed mice challenged with spent U9 broth without bacteria IT every other day and IP every day (n=16). E). Immunosuppressed mice challenged with U. parvum IT every other day and IP every day (n=19). F). Immunosuppressed mice challenged with U. parvum IP every day (n=20).
2. U. parvum Culture
Culture results are shown in the table. Uninfected immunosuppressed mice, IT/IP challenged immunocompetent mice, and spent U9 IT/IP vehicle control mice had negative cultures. Among immunosuppressed mice challenged with U. parvum IP or IT/IP, all cardiac blood cultures results were negative. In immunosuppressed mice challenged with IT/IP U. parvum, 68% (13/19) of lung tissues was culture-positive, and 47% (9/19) of mice were both lung and peritoneal swab culture-positive. All mice in the IP challenge group had culture-negative lung tissues, but 40% (8/20) had positive peritoneal swab cultures. Among immunocompetent mice challenged with U. parvum IT/IP, 50% (6/12) of lung tissues and 42% (5/12) of peritoneal swabs were culture-positive. Only 25% (3/12) of these immunocompetent mice challenged IT/IP were both lung and peritoneal swab culture-positive.
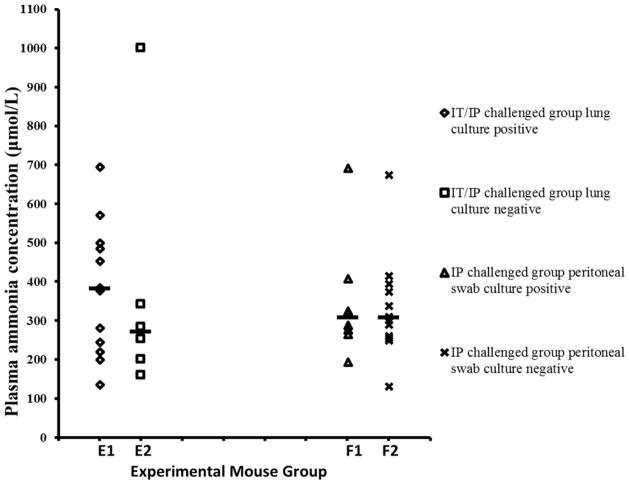
Immunosuppressed mice challenged with U. parvum IT/IP were divided into lung culture-positive and -negative subgroups (figure 2); there was no difference in ammonia concentrations between the two subgroups (p=0.43). Similarly, immunosuppressed mice challenged with U. parvum IP were divided into peritoneal swab culture-positive and -negative subgroups (figure 2); ammonia levels were similar in the subgroups (p=0.94).
Figure 2. Relationship between culture and plasma ammonia concentrations.
Plasma ammonia concentrations were not statistically different between culture-positive mice and -negative mice. Groups from left to right: E1). Immunosuppressed mice challenged with U. parvum IT every other day and IP every day, lung culture-positive (n=13). E2). Immunosuppressed mice challenged with U. parvum IT every other day and IP every day, lung culture-negative (n=6). F1). Immunosuppressed mice challenged with U. parvum IP every day, peritoneal swab culture-positive (n=8). F2). Immunosuppressed mice challenged with U. parvum IP every day, peritoneal swab culture-negative (n=12). The median of each subgroup is shown by a short dash.
3. Mortality Prior to the Experimental Endpoint
Eleven animals died before the planned experimental endpoint and therefore are not included in the table (except in the footnote) or figures 1 or 2; details are shown in the footnote to the table.
Discussion
Recently, we established an immunocompromised experimental mouse model in which we showed that U. urealyticum ATCC 27618 can cause hyperammonemia [8]. Here, we show that a clinical U. parvum isolate can cause hyperammonemia using the same model. U. urealyticum and U. parvum produce large amounts of urease which hydrolyses urea to produce ATP, generating ammonia in the process [10]. These organisms may cause urogenital infections in immunocompetent adults and invasive diseases, such as pneumonia and bacteremia, in neonates [11]. Since they cannot grow in or on routine laboratory culture media, clinicians and microbiologists may overlook them as causes of infection or, as reported herein, a metabolic syndrome.
Hyperammonemia syndrome is a rare, acute and fatal complication of lung transplantation, reported to occur in 1–4.1% of lung transplant recipients; onset is typically in the first two weeks post-transplant [1, 12]. We have described a relationship between Ureaplasma infection and hyperammonemia in lung transplant recipients [4]. Consistent with findings in humans infected with Ureaplasma species, some of our experimental mice showed acute-onset hyperammonemia during a 13-day immunosuppression period, with a few mice dying with hyperammonemia and positive blood cultures.
Similar with results of our previous study with U. urealyticum [8], none of the mice not receiving pharmacologic immunosuppression challenged IT/IP with U. parvum developed elevated plasma ammonia concentrations, while immunosuppressed mice challenged with U. parvum IT/IP or IP had higher plasma ammonia levels (up to 1,000 μmol/L) than vehicle-challenged controls (p<0.001). Additionally, in this study, we were able to measure plasma ammonia concentrations of a few mice that died just before the experimental endpoint and found ammonia concentrations up to 1,000 μmol/L or greater. Thus, the ability of two Ureaplasma species, U. ureaplasma and U. parvum, to cause hyperammonemia has now been established in our immunocompromised murine model.
Compared with our previous U. ureaplasma-related hyperammonemia study using the same model, more lung cultures were positive in U. parvum IT/IP challenged mice. Two factors may explain this finding. First, the U. parvum inoculum used in this study was higher than the U. urealyticum inoculum used in our previous study. Second, a clinical U. parvum strain, that had caused hyperammonemia in a lung transplant recipient, was used in this study. This clinical strain may by more virulent (e.g., have stronger adherence to respiratory epithelium) or possibly be a stronger urease producer than the type strain of U. urealyticum studied previously.
Interestingly, culture positivity did not correlate with high ammonia concentrations. Immunocompetent mice challenged with U. parvum IT/IP had positive cultures with normal ammonia levels. Among immunosuppressed mice challenged with U. parvum IP or IT/IP, mice with positive lung or peritoneal swab cultures did not have higher ammonia levels than mice with negative cultures. In our unpublished experiments using NOD SCID mice (severe combined immunodeficiency mice with non-obese diabetic background, 23–29 g, Charles River Laboratories, Wilmington, MA) challenged with U. urealyticum and U. parvum, we did not observe hyperammonemia (data not shown). The role of specific immunosuppressive agents in Ureaplasma-associated hyperammonemia, and correlates of protection against both infection and hyperammonemia deserve to be further investigated. Our animal model makes possible further research on pathogenesis, treatment, and prophylaxis of Ureaplasma-related hyperammonemia.
In conclusion, using a clinical strain isolated from the respiratory tract of a lung transplant recipient who died of hyperammonemia syndrome, we have verified that U. parvum can cause hyperammonemia in pharmacologically immunosuppressed mice.
Table 1.
Experimental mouse groups, interventions and culture results
| Experimental Mouse Group | Number | Immunosuppressed | Ureaplasma parvum challenge | Positive culture | P value* | ||
|---|---|---|---|---|---|---|---|
| Blood | Lung | Peritoneal cavity swab | |||||
| Normal control (immunocompetent, uninfected) | 12 | No | No | NA | NA | NA | 0.16 |
| Immunosuppressed control | 12 | Yes | No | 0 | 0 | 0 | 0.42 |
| IT/IP challenged immunocompetent control | 12 | No | Yes | 0 | 6 | 5 | 0.47 |
| Vehicle IT/IP negative control1 | 16 | Yes | Spent broth | 0 | 0 | 0 | Reference |
| IT/IP challenge2 | 19 | Yes | Yes | 0 | 13 | 9 | 0.0008 |
| IP challenge3 | 20 | Yes | Yes | 0 | 0 | 8 | <0.0001 |
IT, intratracheal; IP, intraperitoneal; NA, not applicable
P value for ammonia concentrations compared to spent vehicle broth
Two deaths prior to experimental endpoint (days 10 and 12), attributed to anesthesia and IT challenge.
Eight deaths prior to experimental endpoint. One mouse died on day 11 after having been noted to feel cool to touch. One mouse developed bladder enlargement and died after immunosuppressive drug administration on day 11; it was not possible to assess its ammonia level but blood and bladder aspirate cultures were positive. One mouse was sacrificed on day 11 because of tachypnea, hunched stature and lethargy; cultures of blood, lung and a peritoneal swab were positive and ammonia was 955 μmol/L. Four mice died after IT/IP inoculation on day 12, of which two developed an enlarged bladder and died about 15 minutes after IT/IP inoculation with ammonia levels >1000 μmol/L and positive blood and bladder aspirate cultures. One mouse was sacrificed on day 12 because of tachypnea, hunched stature and lethargy; cultures of lung and a peritoneal swab were positive and ammonia was 326 μmol/L.
One death just before sacrifice on day 14; the animal had hunched stature and lethargy after inoculation on day 13.
Acknowledgments
We are grateful to Suzannah M. Schmidt Malan and Javier Fernandez Dominguez for their technical input.
Funding
This work was supported by the Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN. R.P. is supported by the National Institutes of Health [grant numbers R01 AR056647 and R01 AI91594]. X.W. was supported by the State Scholarship Fund from the China Scholarship Council as a Visiting Scientist at Mayo Clinic. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of Interest
No conflicts of interest.
Ethical approval
This study was carried out in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and was approved by Mayo Clinic Institutional Animal Care and Use Committee (protocol number: A8115).
References
- 1.Chen C, Bain KB, Iuppa JA, Yusen RD, Byers DE, Patterson GA, et al. Hyperammonemia syndrome after lung transplantation: a single center experience. Transplantation. 2016;100(3):678–684. doi: 10.1097/TP.0000000000000868. [DOI] [PubMed] [Google Scholar]
- 2.Uygun V, Karasu G, Daloglu H, Hazar V, Yesilipek A. Idiopathic hyperammonemia after hematopoietic stem cell transplantation: A case report. Pediatr Transplant. 2015;19(4):E104–105. doi: 10.1111/petr.12467. [DOI] [PubMed] [Google Scholar]
- 3.Kiberenge RK, Lam H. Fatal hyperammonemia after repeat renal transplantation. J Clin Anesth. 2015;27(2):164–167. doi: 10.1016/j.jclinane.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Bharat A, Cunningham SA, Scott Budinger GR, Kreisel D, DeWet CJ, Gelman AE, et al. Disseminated Ureaplasma infection as a cause of fatal hyperammonemia in humans. Sci Transl Med. 2015;7(284):284re283. doi: 10.1126/scitranslmed.aaa8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anwar S, Gupta D, Ashraf MA, Khalid SA, Rizvi SM, Miller BW, et al. Symptomatic hyperammonemia after lung transplantation: Lessons learnt. Hemodial Int. 2014;18(1):185–191. doi: 10.1111/hdi.12088. [DOI] [PubMed] [Google Scholar]
- 6.Wylam ME, Kennedy CC, Hernandez NM, Peters SG, Maleszewski JJ, Cassivi SD, et al. Fatal hyperammonaemia caused by Mycoplasma hominis. Lancet. 2013;382(9908):1956. doi: 10.1016/S0140-6736(13)62115-7. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham SA, Mandrekar JN, Rosenblatt JE, Patel R. Rapid PCR Detection of Mycoplasma hominis, Ureaplasma urealyticum, and Ureaplasma parvum. International journal of bacteriology. 2013 doi: 10.1155/2013/168742. Article ID 168742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Karau MJ, Greenwood-Quaintance KE, Block DR, Mandrekar JN, Cunningham SA, et al. Ureaplasma urealyticum causes hyperammonemia in an experimental immunocompromised murine model. PLoS ONE. 2016;11(8):e0161214. doi: 10.1371/journal.pone.0161214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez J, Karau MJ, Cunningham SA, Greenwood-Quaintance KE, Patel R. Antimicrobial susceptibility and clonality of clinical Ureaplasma isolates in the United States. Antimicrob Agents Chemother. 2016;60(8):4793–4798. doi: 10.1128/AAC.00671-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waites KB, Katz B, Schelonka RL. Mycoplasmas and Ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005;18(4):757–789. doi: 10.1128/CMR.18.4.757-789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gwee A, Curtis N. Ureaplasma--Are you sitting comfortably? J Infect. 2014;68(Suppl 1):S19–23. doi: 10.1016/j.jinf.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenstein GR, Yang YX, Nunes FA, Lewis JD, Tuchman M, Tino G, et al. Fatal hyperammonemia after orthotopic lung transplantation. Ann Intern Med. 2000;132(4):283–287. doi: 10.7326/0003-4819-132-4-200002150-00006. [DOI] [PubMed] [Google Scholar]