Abstract
Background
Objective and time-effective tools are needed to identify motor-cognitive impairment and facilitate early intervention.
Objective
We examined the feasibility, accuracy, and reliability of an instrumented trail-making task (iTMT), using a wearable sensor to identify motor-cognitive impairment, among older adults.
Methods
Thirty subjects (age = 82.2+6.1 years, body mass index = 25.7+4.8 kg/m2, female = 43.3%) in 3 age-matched groups, 11 healthy, 10 with amnestic mild cognitive impairment (aMCI), and 9 with Alzheimer’s disease (AD), were recruited. Subjects completed iTMT, using a wearable sensor attached to the leg, which translates the motion of the ankle into a human-machine interface. iTMT tests included reaching to 5 indexed circles on a computer screen by moving the ankle-joint while standing. iTMT was quantified by the time required to reach all circles in the correct sequence. Three iTMT tests were designed, including numbers (1-to-5) positioned in a fixed (iTMTfixed) or random (iTMTrandom) order, or numbers (1-to-3) and letters (A&B) positioned in random order (iTMTnumber-letter). Each test was repeated twice to examine test-retest reliability. In addition, the conventional trail-making task (TMT A&B), Montreal Cognitive Assessment (MoCA), and dual-task cost (DTC: gait-speed difference between walking alone and walking while counting backward) were used as references.
Results
Good-to-excellent reliability was achieved for all iTMT tests (ICC=0.742-0.836). Between-groups difference was more pronounced, when using iTMTnumber-letter, with average completion time of 26.3±12.4s, 37.8±14.1s, and 61.8±34.1s, respectively, for healthy, aMCI, and AD groups (p = 0.006). Pairwise comparison suggested strong effect sizes between AD and healthy (d=1.384, p=0.001) and between aMCI and AD (d=0.923, p=0.028). Significant correlation was observed when comparing iTMTnumber-letter with MoCA (r=−0.598, p=0.001), TMT A (r=0.519, p=0.006), TMT B (r=0.666, p<0.001), and DTC (r=0.713, p<0.001).
Conclusion
This study demonstrated proof of concept of a simple, safe, and practical iTMT system with promising results to identify cognitive and dual-task ability impairment among older adults, including those with aMCI and AD. Future studies need to confirm these observations in larger samples, as well as iTMT’s ability to track motor-cognitive decline over time.
Keywords: motor-cognitive impairment, gait, balance, technology, older adults, wearable technology, trail making, Alzheimer’s disease, amnestic mild cognitive impairment, dual task
Introduction
Motor-cognitive impairment impose serious challenges for the world’s medical care system as the older population grows, for which early detection may be beneficial [1,2]. Researchers have estimated that the number of adults with dementia will increase 2.5– 4-fold by 2050 because of population aging [3]. Mild cognitive impairment (MCI), which does not reach the threshold for dementia diagnosis, has a high prevalence at ~22% among US older adults [3,4]. Decline in cognitive functions leads to a loss of independent function that has a wide-ranging impact on individuals, families and healthcare systems [5]. Loss of cognitive performance is also known to be associated with increased risk of adverse events post intervention, complications of coexisting medical conditions, increased risk of falling, overall degradation in quality and satisfaction of life, decreased mobility, increased healthcare utilization, and substantial caregiver burden [2,6].
Precise and early diagnosis of motor-cognitive impairment in the older population is important because it may help provide intelligent and personalized interventions in early stages and, thus, delay further deteriorative progression and/or limit the consequences of motor-cognitive impairment, such as increasing risk of falling, decreasing mobility, and loss of independency. [2,7]. Early diagnosis of motor-cognitive impairment offers several direct benefits to persons at risk [8]. For example, detection can prompt evaluation of the patient for reversible causes of motor-cognitive decline [1,9]. When the course of the disease is expected to be chronic and progressive, pharmacologic intervention may slow motor-cognitive decline and/or limit the consequences, such as increasing risk of falling and decreasing mobility [9]. Early identification could also help us identify and understand remediable contributions to motor-cognitive impairment, such as substance use, medications, and sleep disorders [10]. Perhaps most importantly, early diagnosis provides time for patients and families to prepare for future care and maximizes patients’ opportunities to contribute to the care planning process [1]. Thus, a proactive approach to diagnosis and intervention may improve the well-being of both persons with risk of dementia and family members involved in their care [1].
Unfortunately, current diagnosis of motor-cognitive impairment is initiated mostly on a clinician’s suspicion, based on patient symptoms or caregivers’ concerns, usually in a primary care setting [1,11]. This is mainly due to impracticality of current modalities, which are often not suitable for routine use in busy clinics and/or outside clinics, including nursing homes and long-term settings. Thus, it is not surprising that a recent report suggests that 50% of persons with dementia are not diagnosed and that most persons are not diagnosed until late stages of the illness [1]. This is of increasing concern, given most investigators believe disease-modifying therapies will be most effective in the preclinical and early stages [12]. Therefore, designing a practical tool to identify motor-cognitive impairment irrespective of setting could be beneficial for early-stage diagnosis and implementing effective intervention.
In addition, the current modalities for identifying cognitive impairment (e.g. the Mini Mental State Examination [MMSE]) [13] often neglect or are unable to quantify the impact of cognitive impairment on motor performance such as the ability of dual-tasking (i.e. simultaneous performing a cognitive and a motor task), which is known to be an important risk factor for prospective falls, frailty, and restriction in daily physical activities [14,15]. Some recent tests such as Montreal Cognitive Assessment (MoCA) [16] includes a motor-cognitive test component such as ‘trail-making task (TMT)’, which requires both motor and cognitive performance to complete the task, but they are semi-objective and dependent on the examiner’s training and experience. And more importantly, the cognitive components are still dominating the motor components. Recently, dual-task gait tests [2,17] have been proposed to examine motor-cognitive performance with promising results to predict prospective falls, frailty, and restriction in physical activities as well as identifying mild cognitive impairment. However, these tests are often impractical for routine assessment in the home, clinic, or long-term care setting, where allocation of a walking pathway without any other distraction is usually unavailable or unaffordable.
Recent advances in designing wearable, virtual reality, and human-machine interface technologies have opened up new opportunities to design practical and cost-effective tools, which provide objective metrics to quantify functional performance, identify risk factors, and track health status irrespective of setting and across disciplines [17–24]. In this study we have designed an innovative instrumented trail-making task (iTMT) platform, based on wearable-sensor and human-machine interface technology. We examined the feasibility, reliability, and accuracy of iTMT in identifying motor-cognitive impairment among older adults, including those suffering from amnestic mild cognitive impairment (aMCI) and Alzheimer’s disease (AD). We also compared the results with other well established methods including MoCA, dual task walking, and TMT.
Methods
Study Population
Thirty ambulatory older adults (age 65 years or older) were recruited from the Memory Disorders Clinic at the Banner Sun Health Research Institute from March 2015 to August 2015. Subjects were assigned to AD, aMCI and healthy control groups based on the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) [25] and Peterson criteria [26] by a Board Certified Neurologist (MS) with expertise in Alzheimer’s and Memory Disorders. There were 10 subjects with clinically confirmed aMCI, 9 subjects with clinically confirmed AD, and 11 healthy controls without MCI or AD. Healthy controls included those healthy ambulatory subjects with age range of +/− 5 years compared to other groups. Subjects were excluded from the study if they were non-ambulatory or had a severe gait impairment (e.g., unable to walk independently with or without an assistive device); had other neurological conditions associated with cognitive impairment (stroke, Parkinson’s, Huntington’s, etc.); had a clinically significant psychiatric condition or substance abuse; had severe visual impairment; or were unwilling to participate. For subjects meeting the inclusion criteria, written informed consent was obtained by a board-certified neurologist (MS). A capacity to consent was administered to ensure the subject was able to understand the consent form and procedures of the study. This study was approved by the Banner and Western Institutional Review Boards.
Clinical and Motor-Cognition Performance Measures
Subjects’ demographics including age, gender, body mass, height, and body mass index (BMI), were collected. All subjects underwent clinical assessments, including MoCA [16], Center for Epidemiologic Studies Depression (CES-D) scale [27,28], Fried Frailty Criteria [28], and TMT assessment [29,30]. As secondary analysis, all subjects were re-assessed using MoCA test and based on a cut-of score of 25 or less, they were classified into cognitive intact or cognitive impaired groups [16]. The CES-D short-version scale was used for measuring self-reported depression symptoms. A cut-off of CES-D score of 16 or greater was used to identify subjects with depression [31]. The Fried Frailty Criteria, including unintentional weight loss, self-reported exhaustion, weakness (grip strength), slow gait speed (15-foot gait test), and self-reported low physical activity, were used for assessing prefrailty and frailty [28]. Subjects with 1 or 2 positive Fried criteria were considered prefrail, and those with 3 or more positive Fried criteria were considered frail. Subjects with all negative Fried criteria were considered nonfrail. TMT assessment requires a subject to connect a sequence of 25 consecutive targets on a sheet of paper. There are 2 parts to the test: in the first part (TMT A), the targets are all numbers (1, 2, 3, etc.), and the test taker needs to connect them in sequential order; in the second part (TMT B), the subject alternates between numbers and letters (1, A, 2, B, etc.). If the subject makes an error, the test administrator corrects it before the subject moves on to the next dot [30]. The direct score of each part is represented by the time of completion of the tasks. With the questionnaire survey, all subjects self-reported their experience of falls in the past year, and, if some occurred, how many times they fell in the past year.
Gait performance was measured using wearable sensors attached to both the left and right upper and lower legs (LegSys™, BioSensics, MA, USA). Subjects were asked to walk with their habitual gait speed for a distance of 20 meters with no cognitive task (single-task walking). Then they were asked to repeat the test while counting backward from a random number with loud voice (dual-task walking) [2, 17]. Gait speed was calculated using validated algorithms [14, 32]. The dual task cost (DTC) was estimated by changes in gait speed from single-task to dual-task walking [2, 15].
iTMT Design and Platform
We designed the iTMT platform, which is inspired by the conventional paper-and-pencil TMT described in the earlier section. The platform (Figure 1) has 1 wearable sensor (part of the product, LEGSys™, BioSensics LLC, MA, USA), which includes a triaxial accelerometer, gyroscope, and magnetometer for estimation of angles and position [32]. Sensor data are acquired and transmitted at 100-Hz frequency for real-time feedback in a virtual environment. The sensor is attached to the subject’s shin (Figure 1); using an elastic strap allows tracking of ankle motion in 3D and translates it to a human-machine interface installed on a computer (Figure 1) to examine cognitive (trail-making performance), as well as motor ability (ankle-reaching task performance) of the subject. By moving the ankle, the subject can navigate a cursor on the screen from a start circle to targets appearing on the same screen. The proposed system allows performance of the same iTMT tests while an individual is sitting or standing. However, for the purpose of this study, we focused on iTMT while standing.
Figure 1.

Instrumented trail-Making Task (iTMT) platform illustration. One inertial sensor, including a triaxial accelerometer, a triaxial gyroscope, and a triaxial magnetometer, was attached on the subject’s shin using a comfortable elastic band. The sensor allows measurement of 3D motion of the ankle joint in real time. The instantaneous measured joint angle with a sample frequency of 100Hz was wirelessly transferred to a computer laptop, using low-power Bluetooth to create a human-machine interface for the purpose of interactive iTMT tests. For safety purposes, a study administrator was in the room supervising the iTMT test at all times. After starting the iTMT test, the administrator did not provide any guidance; and only the interactive human-machine interface provided the necessary guidance and instruction to complete the test.
For the purpose of this design, we used a commercially available wearable technology named LEGSys™, which is based on 5 wearable sensors. However, iTMT uses only 1 of the sensors attached to the right shin; we used other sensors for measuring spatiotemporal parameters of gait, as described earlier. The human-machine interface was developed based on Matlab® 2013b and Psychophysics Toolbox Version 2.54.
Similar to conventional TMT test, to execute iTMT, subjects are required to simultaneously execute multiple tasks including visual search, scanning, speed of processing, mental flexibility, and executive functions [29]. However, the purpose of this study is to demonstrate feasibility, accuracy, and reliability of iTMT compared to well established methods rather than assessing its subcomponent tasks.
iTMT Procedure
The entire iTMT test was set up in a quiet, private room in the clinic. During the whole process, the subject stood in front of the computer screen, wearing the sensor set. The screen was adjusted to the subject’s eye level. For safety purposes, a study administrator was in the room supervising the iTMT test all the time (Figure 1). If the subject could not complete the test or felt uncomfortable, the study administrator would terminate the test to guarantee safety. Before starting the iTMTs, the administrator described the protocol to the subjects and then asked them to do a single ankle-reaching task without a cognitive component, using the Exergaming platform described in our previous publication [18]. In this ankle-reaching task, the subjects simply moved the ankle joint to navigate the cursor to targets shown up, down, and lateral on the computer screen, without any cognitive challenge. After starting the iTMT test, the administrator did not provide any further guidance; only the interactive human-machine interface provided the necessary guidance and instructions, as described in the following.
After finishing clinic assessments and the questionnaire survey, subjects from all 3 groups attended 1 session of iTMT testing. This session lasted approximately 5 minutes and included: 1) fixed-order trail-making (iTMTfixed); 2) random-order trail-making (iTMTrandom); and 3) number-letter order trail-making (iTMTnumber-letter), as will be described in detail in later sections.
For trail-making, the subject needs to stand upright (always in double stance) and move the hip in the anterior-posterior (AP) direction in order to generate dorsiflexion/plantarflexion at the ankle without lifting the heel or toes. The subject navigate the cursor to correct targets in certain orders according to different tasks described in the following, by moving the ankle joint (i.e., ankle-reaching task [18]). The subject is expected to navigate the cursor to the right target within 0.5 to 2 seconds. If this takes more than 2 seconds (too slow), the target circle will turn green as a visual cue. If it is between 0.5 to 2 seconds (perfect), the border of the target circle will turn red; and the target circle will explode with a rewarding sound. If the subject makes a mistake in reaching the right order, he/she receives a visual and audio error signal. If the subject makes 3 consecutive mistakes, the right target blinks as a visual cue to guide him/her to reach the right target. No other graphical or audio effect was included in the interface, as it might distract older adults [33]. This simplistic design of the graphical user interface allows the subject to focus on cognitive tasks, better focus on the iTMT test and perceive errors (the difference between the actual motor output and the desired motor output).
Each iTMT test (i.e. iTMTfixed, iTMTrandom, and iTMTnumber-letter) was repeated three times. The first attempt was considered as the subject’s understanding and getting familiar with the test. The second and third attempts were considered valid experiments and also used for assessing test-retest reliability for each iTMT test. We selected the shortest completion time of the second and third attempts as our result.
Fixed-Order Trail-Making Task (iTMTfixed)
The iTMTfixed (Figure 2A) has the lowest level of cognitive complexity. In this iTMT, 6 circles appeared on the screen, 1 start circle in white and 5 target circles in yellow. The target circles were located in a fanwise position in front of the start circle (Figure 2). Each target circle had a number located in the center. From left to right, 5 target circles had fixed numbers, 1, 2, 3, 4, and 5 in sequence. At the beginning of the iTMT, position of the cursor was automatically calibrated to the center of the start circle. By rotating the ankle joint, the subject navigated the cursor to the center of the first target circle (with number “1” inside). Then the subject navigated the cursor back to the center of the start circle and went to the second target circle (with number “2” inside), and went on. If the subject navigated the cursor to a wrong target circle, a visual and audio feedback indicating a mistake was played. Then the subject needed to go back to the start circle and continue the trail-making task from where he/she made the previous mistake. If the subject made 3 consecutive mistakes, a computer-generated visual cue (flashing of correct target circle) appeared to guide him/her to correct the sequence.
Figure 2.

An illustration of different Instrumented Trail-Making Task (iTMT) tests. A) iTMTfixed with fixed numbers of 1 to 5 from left to right; B) iTMTrandom with randomized location for numbers of 1 to 5; and C) iTMTnumber-letter with both numbers and letters mixed, as well as randomized location.
Random-Order Trail-Making task (iTMTrandom)
The iTMTrandom (Figure 2B) was similar to the fixed order, but the order of numbers located at the center of target circles was no longer fixed as 1 to 5 from left to right. At the beginning of each trial (i.e. point to point ankle reaching task), numbers 1, 2, 3, 4, and 5 were randomly placed in the 5 target circles. During reaching trial, the subject needed to observe and find the correct target (i.e. correct sequential order) based on the previously reached target, which is not necessarily placed on the same location as previous trial. The iTMTrandom added more cognitive challenges than with the fixed-order test.
Number-Letter-Order Trail-Making Task (iTMTnumber-letter)
The iTMTnumber-letter (Figure 2C) has the highest level of cognitive complexity. In this task, not only the order of numbers in target circles was randomized, but also numbers were mixed with letters together. Instead of 1, 2, 3, 4, and 5, this task had 1, A, 2, B, and 3 located at the center of each target circle. The subject navigated the cursor to targets with numbers and letters alternately. Therefore, after completing target 1, instead of going to target 2, the subject should move the cursor to target A. In this task, besides observing and figuring out the correct location of the next target, the subject also needed to remember to switch between number and letter sequences.
Statistical Analysis
All continuous data were presented as mean ± standard deviation (SD). All categorical data were expressed as count (percentage). Analysis of variance (ANOVA), Mann-Whitney U-tests, and Chi-square-tests were used for between-group comparison according to the scale of the investigated variable and the distribution of the data. Analysis of covariance (ANCOVA) was employed to compare difference between groups for iTMT tests with and without adjustment for age, BMI, and education level. Post hoc analyses for significant (p<0.050) main effects or interactions were done by using a Sidak adjustment for pairwise comparison. Test-retest reliability was assessed using intra-class correlation and absolute agreement model (ICC (2,1)). Values ranging less than 0.40 are considered poor, between 0.40 and 0.75 are considered fair-to-good and greater than 0.75 are considered as excellent reliability [34]. The effect size to discriminate between groups was estimated using Cohen’s d effect size and represented as d in the results section. Values ranging from 0.20 to 0.49 indicate small; from 0.50 to 0.79 indicate medium, from 0.80 to 1.29 indicate large, and above 1.30 indicate very large effects [35]. Values less than 0.20 are considered as having no noticeable effect [35]. Spearman’s correlation coefficients were used to evaluate the agreement between iTMT with MoCA, conventional TMT, and DTC. For all comparisons, significance was accepted at p<0.05. All statistical analyses were performed using IBM Statistical Package for Social Sciences (SPSS Version 23, IBM, Chicago, IL, USA).
Results
Table 1 summarizes demographic and clinical data. The subjects’ ages ranged from 71 to 93 years. No between-group difference was observed for demographic information, including age, gender, height, body mass, BMI, and education level (p>0.05). However, all clinical examinations used for cognitive assessment were significantly different between groups (p≤0.001). When using MoCA to identify cognitive impairment, we found that 9.1%, 70%, and 100% of subjects were having cognitive impairment, respectively, in the healthy, aMCI, and AD groups. Among tested clinical examinations, the highest effect size to discrimination between healthy and aMCI groups was observed for MoCA (d=1.823, p=0.004). The lowest effect size was observed for TMT B (d=1.580, p=0.051). On the same note, the highest and lowest effect sizes to discriminate between healthy and AD, as well as between aMCI and AD, were MoCA (d=3.239, p<0.001) and TMT A (d=1.744, p<0.001), respectively, as well as MoCA (d=1.770, p<0.001) and TMT A (d=0.693, p=0.083), respectively. No between-group difference was observed for depression. However, with progression in cognitive impairment, a trend in the increasing history of falls and frailty symptoms was observed. But the difference achieved statistical significance only for frailty symptoms in our sample (p=0.038).
Table 1.
General characteristics of the study population.
| Variable | Healthy (n = 11) |
aMCI (n = 10) |
AD (n = 9) |
p Value |
|---|---|---|---|---|
| Age, years (mean±SD) | 80.5±6.3 | 85.2±4.6 | 80.8±6.6 | 0.152 |
| Female, n (%) | 6.0 (54.5) | 5.0 (50.0) | 2.0 (22.2) | 0.305 |
| Height, cm (mean±SD) | 168.9±9.5 | 161.0±10.6 | 172.5±10.7 | 0.058 |
| Body mass, kg (mean±SD) | 67.6±14.0 | 69.4±10.7 | 83.9±25.7 | 0.112 |
| BMI, kg/m2 (mean±SD) | 23.5±3.4 | 26.3±3.7 | 27.7±6.4 | 0.130 |
| Education level, years (mean±SD) | 15.2±3.0 | 13.8±2.3 | 14.6±1.8 | 0.451 |
| MoCA score, 0–30 (mean±SD) | 27.7±1.8 | 23.3±2.9 | 16.6±4.5 | <0.001* |
| TMT A, s (mean±SD) | 34.8±10.5 | 54.8±12.9 | 68.8±25.5 | 0.001* |
| TMT B, s (mean±SD) | 77.7±32.9 | 164.3±70.2 | 278.0±157.5 | <0.001* |
| Cognitive impairment, n (%)** | 1.0 (9.1) | 7.0 (70.0) | 9.0 (100.0) | <0.001* |
| Depression, n (%)*** | 1.0 (9.1) | 1.0 (10.0) | 1.0 (11.1) | 0.989 |
| History of fall, n (%) | 1.0 (9.1) | 5.0 (50.0) | 3.0 (33.3) | 0.120 |
| Prefrail/frail, n (%) | 6.0 (54.5) | 8.0 (80.0) | 8.0 (88.9) | 0.038* |
BMI: Body Mass Index
MoCA: Montreal Cognitive Assessment
TMT: Trail-Making Task
aMCI: amnestic mild cognitive impairment
AD: Alzheimer disease
p ≤ 0.05
According to MoCA score with cut-off of 25 or smaller
According to CES-D score with cut-off of 16 or greater
Table 2 summarizes iTMT values for different groups, including those with and without cognitive impairment, as confirmed by the MoCA test. Table 3 summarizes between-group comparisons with and without adjustment by age, BMI, and education level. The highest effect size for separation between groups with and without cognitive impairment was obtained using iTMTnumber-letter (d=1.024, p=0.015). The duration to complete iTMTnumber-letter was 27.8±11.9s in the group without impairment and was significantly increased on average by 44.5% in the group with cognitive impairment (p=0.015, Figure 3). Similarly, the highest effect size to discriminate between the healthy, aMCI, and AD group was observed for iTMTnumber-letter, with large-to-very-large effect size ranging from 0.866 for comparison between healthy and aMCI, to 1.384 for comparing between healthy and AD. The duration for completing iTMTnumber-letter in the healthy group was 26.3±12.4s and was increased on average by 30.7% and 57.4%, respectively, in the aMCI and AD groups (Table 2 and Figure 3). However, the between-group difference achieved statistical significance when comparing between healthy and AD groups (d=1.384, p=0.001), as well as between aMCI and AD groups (d=0.920, p=0.029). When results were adjusted for demographics covariates including age, BMI, and education level, the results were essentially unchanged (Table 3). Similarly, gender or frailty level had no significant effect on the iTMT results.
Table 2.
iTMT values for different groups.
| iTMTfixed | iTMTrandom | iTMTnumber-letter | ||
|---|---|---|---|---|
| Cognitive disease status | Healthy | 25.3±8.7 sec | 24.5±6.5 sec sec | 26.3±12.4 sec |
| aMCI | 30.2±11.9 sec | 26.7±6.9 sec | 37.8±14.1 sec | |
| AD | 28.5±12.2 sec | 31.4±9 sec | 61.8±34.1 sec | |
| p Value | 0.634 | 0.198 | 0.006 | |
| Cognitive impairment status | No cognitive impairment | 24.9±8.2 sec | 25.1±6.3 sec | 27.8±11.9 sec |
| Cognitive impairment | 30.3±12 sec | 28.9±8.4 sec | 50.1±28.4 sec | |
| p Value | 0.213 | 0.229 | 0.015 |
iTMT: Instrumented Trail-Making Task
aMCI: amnestic mild cognitive impairment
AD: Alzheimer disease
Significant difference between groups were indicated in bold
Table 3.
Between-group iTMT comparisons with and without adjustment for age, BMI, and education level.
| iTMTfixed | iTMTrandom | iTMTnumber-letter | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Difference Mean (%) | p Value | d | Difference Mean (%) | p Value | d | Difference Mean (%) | p Value | d | |
| Without adjustment | |||||||||
| With vs. without cognitive impairment | 5.4 (17.8) | 0.213 | 0.525 | 3.8 (13.1) | 0.229 | 0.512 | 23.0 (45.3) | 0.015 | 1.024 |
| Healthy vs. aMCI | 4.8 (15.9) | 0.349 | 0.470 | 2.1 (7.9) | 0.544 | 0.321 | 11.6 (30.7) | 0.221 | 0.866 |
| Healthy vs. AD | 3.1 (10.9) | 0.576 | 0.302 | 6.9 (22.0) | 0.078 | 0.876 | 35.5 (57.4) | 0.001 | 1.384 |
| aMCI vs. AD | -1.7 (5.6) | 0.759 | 0.141 | 4.7 (15.0) | 0.217 | 0.591 | 24.0 (38.8) | 0.029 | 0.920 |
| With adjustment for age, BMI, and education level | |||||||||
| With vs. without cognitive impairment | 4.6 (15.5) | 0.305 | 0.448 | 4.0 (13.7) | 0.205 | 0.532 | 22.8 (44.8) | 0.038 | 1.005 |
| Healthy vs. aMCI | 2.5 (8.8) | 0.669 | 0.238 | 2.2 (8.3) | 0.547 | 0.318 | 11.2 (31.1) | 0.327 | 0.782 |
| Healthy vs. AD | 4.2 (14.0) | 0.483 | 0.393 | 8.4 (25.8) | 0.033 | 1.068 | 40.7 (62.1) | 0.002 | 1.568 |
| aMCI vs. AD | 1.7 (5.7) | 0.780 | 0.142 | 6.2 (19.0) | 0.116 | 0.760 | 29.5 (44.9) | 0.022 | 1.119 |
iTMT: Instrumented Trail-Making Test
aMCI: amnestic mild cognitive impairment
AD: Alzheimer disease
Significant difference between groups were indicated in bold
Effect sizes were calculated as Cohen’s d
Figure 3.
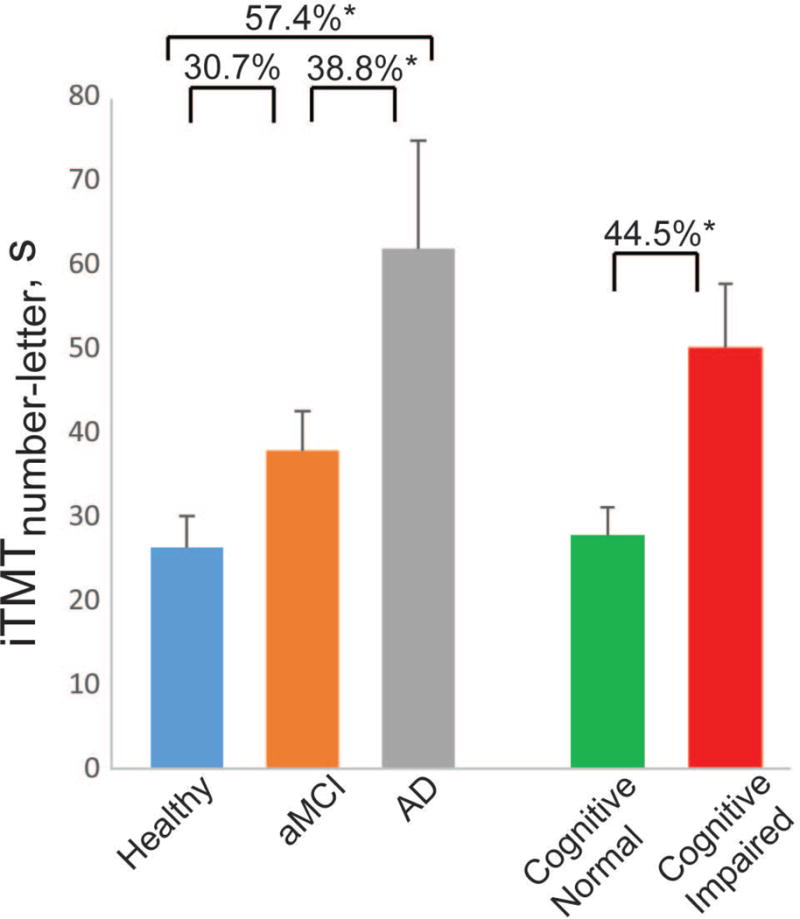
The duration for completing Instrumented Trail-Making Task (iTMTnumber-letter) for all groups, including older adults suffering from amnestic mild cognitive impairment, Alzheimer disease, and healthy age-matched controls. Error bar represents the standard error. The results were adjusted by subjects’ age, body mass index, and education level. ‘*’ denotes when the pairwise group comparison achieved a statistically significant level (p<0.050).
The test-retest reliability was fair to good for iTMTfixed (ICC=0.742) and reached excellent reliability for both iTMTrandom (ICC=0.836) and iTMTnumber-letter (ICC=0.826) tests.
The agreement between iTMT and traditional cognitive assessments was also highest for iTMTnumber-letter. In summary, a relatively fair-to-good agreement was observed between iTMTnumber-letter and MoCA (r=−0.598, p=0.001, Fig. 4A), as well as between iTMTnumber-letter and both TMT A (r=0.519, p=0.006, Fig. 4B) and TMT B (r=0.666, p<0.001, Fig. 4C). Similarly, no significant difference was observed between the number of errors recorded using TMT B and iTMTnumber-letter test (2.3±4.5 errors in iTMTnumber-letter versus 1.9±4.1 errors in TMT B, p=0.748). Despite fair-to-good agreement between TMT B and iTMTnumber-letter, results suggest that on average the total time to complete TMT B is 4.2 times longer than the time required to complete iTMTnumber-letter (165.2±24.6 sec for TMT B versus 39.3±4.7 sec for iTMTnumber-letter, p<0.001, 95% CI = [77.2,174.6] sec).
Figure 4.
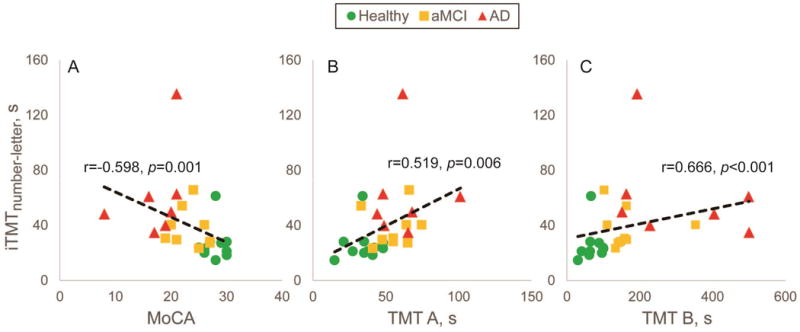
Agreement between duration required to complete Instrumented Trail-Making Task (iTMTnumber-letter) and traditional cognitive impairment assessments, including A) Montreal Cognitive Assessment, B) Trail-Making Task A, C) and Trail-Making Tak B.
When we compared DTC, and indicator of impact of cognitive impairment on motor performance, we observed a relatively good agreement between iTMTnumber-letter and DTC (r=0.713, p<0.001, Fig 5A). Interestingly, no noticeable correlation was observed between DTC with MoCA and conventional TMT A&B (p>0.050, Figure 5), indicating lack of ability of MoCA and conventional TMT to evaluate motor task deterioration due to cognitive decline.
Figure 5.
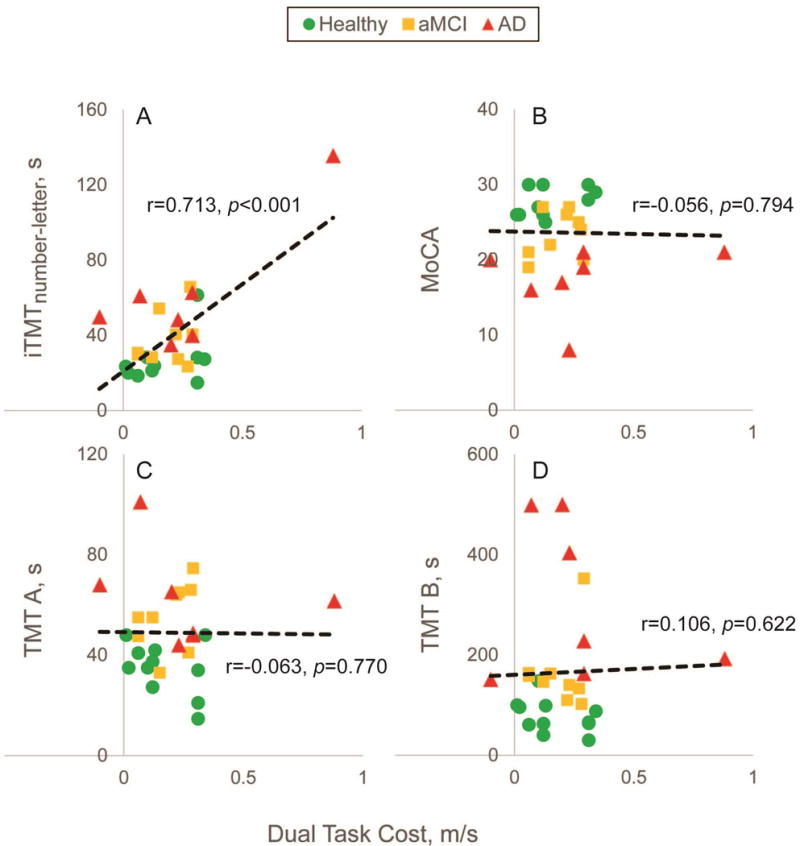
Correlation between dual-ask cost (i.e., the difference between gait speed during single-task walking and dual-task walking) and A) Instrumented Trail-Making Task (iTMTnumber-letter), B) Montreal Cognitive Assessment, C) Trail-Making Task A, and D) Trail-Making Task B. Only iTMTnumber-letter had a significant correlation with dual-task cost, indicating its ability to evaluate motor-cognitive performance.
Discussion
This study demonstrated feasibility and proof of concept of an innovative and instrumented TMT to identify motor-cognitive impairment among older adults, including those suffering from aMCI and AD. This instrument benefits from a low-cost wearable sensor combined with a human-machine interface installable in any standard computer. The test is simple and its execution, on average, takes less than 1 minute (excluding time for explanation and sensor attachment), making it suitable for busy clinics. All subjects, including patients with aMCI and AD, were able to complete all iTMT tests without any support from the study administrator, while often subjects in the aMCI and AD groups required involvement from the study administrator to perform conventional TMT tests. Furthermore, none of the participants stopped or were overtaxed during the test indicating its feasibility for older adults including those with MCI and dementia. No adverse events, including loss of balance, were observed during any iTMT tests, despite more than 50% of subjects in the healthy group and more than 80% in groups with cognitive impairment being frail or prefrail. While the test is simple, short, safe, and easy to administer, it has a large-to-very-large effect size to separate between groups with and without cognitive impairment, as well as to differentiate between healthy, aMCI, and AD groups. In addition, fair-to-good agreement were observed with traditional cognitive assessments, such as MoCA and TMT A and B. Furthermore, iTMT required on average 40 sec (excluding time for explanation and sensor attachment) to be completed, which is 4.2-fold less than the conventional TMT B test (excluding time for explanation). Minimal supervision from the research coordinator was required to administer the test given its interactive human-machine interface, which facilitates standard and accurate execution. However, the test still requires supervision for safety, particularly in older adults with poor balance or severe cognitive impairment.
We examined 3 scenarios of iTMT, by a virtual ankle-reaching task to 5 circles indexed with fixed-position numbers (iTMTfixed), random-positioned numbers (iTMTrandom) and alteration of numbers and letters (iTMTnumber-letter). Our comparative results with traditional cognitive test instruments suggest that iTMTnumber-letter is the most sensitive test to identify motor-cognitive impairment among older adults. In addition, excellent test-retest reliability was achieved, when iTMT with alteration between numbers and letters was used (iTMTnumber-letter).
The key innovation of the proposed platform is that it uses a computerized routine, which makes it easy for use by nonexpert users. Since it uses a wearable sensor and a standard computer laptop, it has application irrespective of setting, including acute and long-term settings, as well as at a subject’s home. It provides objective metrics to identify motor-cognitive impairment, which can reduce bias from the examiner, with the potential to track changes in motor-cognitive impairment over time.
Over the past decade, several researchers have focused on investigating dual-task ability to study involvement of attention in gait control [2]. Dual-task gait allows isolating the cognitive control component of locomotion and exposes cognitive deficits through the evaluation of activities which simultaneously demand attention resources. Dual-task gait performance, as quantified by difference in gait speed during walking while doing a cognitively demanding task (reciting words or calculation) and walking along (single task), has been shown to be sensitive to distinguish amnestic MCI from non-amnestic MCI [36], as well as between healthy and MCI groups [2]. It has also been demonstrated to be more sensitive to detect cognitive-related gait changes, predict prospective falls in comparison with single-task assessment [2]. However, gait assessment is often impractical in busy clinics and requires dedication of appropriate space to administer gait tests, which is often unaffordable in small clinics and impractical for the home setting. In addition, dual-task gait test-retest reliability is highly dependent on walking distance [14] and could be too demanding for individuals suffering from dementia [2]. Our results suggest that iTMT has good agreement with DTC and could be used as a more practical and reliable alternative to dual-task gait. In addition, our results suggest that traditional cognitive assessment tools, including TMT A, TMT B, and MoCA, are not sensitive to identify DTC and are, thus, unable to evaluate the cognitive control component of locomotion, which is essential to evaluate the risk (e.g., risk of falling) and ability to live independently among older adults suffering from motor-cognitive impairment.
Although a human-machine interface could be designed using other interactive motion-tracking systems, such as the Microsoft Kinect or Nintendo Wii Fit, we believe wearable technology has particular advantages for evaluating motor-cognitive performance in older adults. For example, although Kinect uses a camera to capture the subject’s motion and, thus, may be a low-cost alternative for a similar purpose, it needs a distance of at least 1.83 meters between the camera and subject. For older adults, this could be too far to see the computer screen and execute the tasks. More importantly, unlike the Microsoft Kinect, a wearable sensor system does not require a continuous unobstructed sightline; and we are able to place a chair in front of the subject as a mechanism to prevent falls during the test. The examiner could also be next to the subject, unlike with the camera-based system. This safety feature is especially important during the iTMT in older adults, in particular, those with MCI and dementia, who have increased fall risk. On the same note, a force platform such as the Nintendo Wii Fit restrict the base of support during testing, which may cause falls during dynamic tests [33]. In addition, it does not provide any information about joint angles, which are key inputs for the iTMT. Thus, using a wearable sensor for the purpose of iTMT is superior to other motion-tracking and virtual-reality alternatives, allowing easier and safer administration of the test in any preferred position with any auxiliary support (e.g., cane, walker, chair, etc.).
Limitations and Future Directions
The small sample size was the major limitation in this study. However, we included 3 geriatric-age matched groups with different levels of cognitive performance, ranging from intact cognitive performance (MoCA = 30.0) to severe cognitive impairment (MoCA = 8.0), and age ranged from 71 years to 93 years, which allowed us to evaluate feasibility, practicality, and proof of concept of iTMT to evaluate motor-cognitive performance among the geriatric population. Additionally, given the large-to-very-large effect sizes, a small sample size was adequate to clinically validate the benefit of iTMT to identify cognitive impairment, as well as differentiate between aMCI, AD, and healthy groups. Since, this an exploratory study and the proposed platform is novel, we didn’t estimate prior sample size and power. However, based on observed effect size from this study, we performed a post hoc analysis. Based on post hoc analysis, with 10 subjects per group and reported effect sizes for each pairwise comparison, the estimated power was ranged from 45% to 83% assuming significant level of 0.050 and two tails independent means comparison. Considering the observed effect size of 1.4 for iTMT difference between AD and healthy, to achieve a minimum power of 80%, a minimum sample size of 10 subjects per group are required to observe a statistical significant of 5% or lower using two tails independent sample comparisons. Using the above assumptions, the sample size to identify aMCI from healthy is 21 subjects based on estimated effect size of 0.9 and the sample size to identify aMCI from AD is 17 subjects based on estimated effect size of 1.0.
Our test-retest reliability protocol is not optimal because of the very brief time between tests, which limits the conclusion about reliability of the proposed test platform. This limitation should be addressed in a follow-up study in which the introduction and retest is done on another day and administered by a different examiner.
Another major limitation of the proposed study is inability of the iTMT using the proposed assessment to separate cognitive performance from motor performance. In other words, the iTMT involves both motor (ankle-reaching task while standing, which requires dynamic postural control) and cognitive (trail-making) tasks, thus assessing dual-task performance. While all subjects completed all tasks while standing, another study might examine feasibility and accuracy of the iTMT while sitting, which could be easier and safer to administer. In addition, by comparing the results of the iTMT test performed while sitting (with focus on cognitive performance) to the results of the test performed while standing (with focus on motor-cognitive performance), cognitive and motor components of the iTMT test can be isolated. Alternatively, indicators of motor performance could be extracted from iTMT such as ankle reaching velocity. In addition, future studies are warranted to examine the ability of iTMT to track changes in motor-cognitive performance over time, its ability to predict prospective falls, its ability to evaluate the ability to live independently, and its ability to identify the earliest stages of dementia and the change from normal cognition to aMCI and prodromal AD, to identify early symptoms of dementia.
Conclusion
This study proposed a computerized dual-task paradigm based on wearable technology and a human-machine interface named the iTMT, with promising results to identify motor-cognitive impairment among older adults. The key advantages of the proposed platform are ease of use (with minimum involvement from test administrator due to its fully computerized test), being objective, time efficiency (on average less than 1 minute to complete the test), and practicality to evaluate dual-task performance (no need to use a walking test). This pilot study provides feasibility, reliability, and proof-of-concept accuracy of the iTMT platform to identify motor-cognitive impairment in older adults and discriminate between groups with aMCI, AD, and healthy age-matched controls. Results suggest that, while iTMT has relatively fair-to-good agreement with traditional cognitive assessments, including conventional TMT A&B and MoCA, it is less time consuming, is objective, and is able to capture dual-task performance. Further study is required to validate the findings of this study in a larger sample, as well as the ability of iTMT to track motor-cognitive decline over time.
Acknowledgments
Partial support was provided by the National Institutes of Health/National Institute on Aging (award number 2R42AG032748), the Flinn Foundation, Arizona Aging and Cognitive Collaborative grant (award number 1907), and Baylor College of Medicine, Michael E. DeBakey Department of Surgery. The content is solely the responsibility of the authors and does not necessarily represent the official views of National Institute of Health, Flinn Foundation, or Baylor College of Medicine. We thank Ms. Sonora Hudson for reviewing and editing this manuscript. Authors also thank Gurtej Grewal, Ana Enriquez, and Ivan Marin for assisting with data analysis and coordination of this research study between involved key investigators.
Footnotes
Conflict of Interest: None
References
- 1.Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care: Prevalence and contributing factors. Alzheimer disease and associated disorders. 2009;23:306–314. doi: 10.1097/WAD.0b013e3181a6bebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahureksa L, Najafi B, Saleh A, Sabbagh M, Coon D, Mohler MJ, Schwenk M. The impact of mild cognitive impairment on gait and balance: A systematic review and meta-analysis of studies using instrumented assessment. Gerontology. 2016 doi: 10.1159/000445831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzheimer’s A. 2015 alzheimer’s disease facts and figures. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB. Prevalence of dementia in the united states: The aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, McArdle JJ, Willis RJ, Wallace RB. Prevalence of cognitive impairment without dementia in the united states. Annals of internal medicine. 2008;148:427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Gelder BM, Tijhuis MA, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Physical activity in relation to cognitive decline in elderly men: The fine study. Neurology. 2004;63:2316–2321. doi: 10.1212/01.wnl.0000147474.29994.35. [DOI] [PubMed] [Google Scholar]
- 7.Amariglio RE, Donohue MC, Marshall GA, Rentz DM, Salmon DP, Ferris SH, Karantzoulis S, Aisen PS, Sperling RA, Alzheimer’s Disease Cooperative S Tracking early decline in cognitive function in older individuals at risk for alzheimer disease dementia: The alzheimer’s disease cooperative study cognitive function instrument. JAMA Neurol. 2015;72:446–454. doi: 10.1001/jamaneurol.2014.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moise P, Schwarzinger M, Um M-Y, Dementia experts’ group . In: Dementia care in 9 oecd countries: A comparative analysis. Development OfECa, editor. Paris: 2004. p. 13. [Google Scholar]
- 9.Standridge JB. Pharmacotherapeutic approaches to the prevention of alzheimer’s disease. Am J Geriatr Pharmacother. 2004;2:119–132. doi: 10.1016/s1543-5946(04)90017-7. [DOI] [PubMed] [Google Scholar]
- 10.Tannenbaum C, Paquette A, Hilmer S, Holroyd-Leduc J, Carnahan R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, gabaergic and opioid drugs. Drugs Aging. 2012;29:639–658. doi: 10.1007/BF03262280. [DOI] [PubMed] [Google Scholar]
- 11.Iliffe S, Robinson L, Brayne C, Goodman C, Rait G, Manthorpe J, Ashley P, De NPCCSG Primary care and dementia: 1. Diagnosis, screening and disclosure. Int J Geriatr Psychiatry. 2009;24:895–901. doi: 10.1002/gps.2204. [DOI] [PubMed] [Google Scholar]
- 12.Association As. 2015 alzheimer’s disease facts and figures: Alzheimer’s & Dementia. 2015;11:332. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Cockrell JR, Folstein MF. Mini-mental state examination. Principles and practice of geriatric psychiatry. 2002:140–141. [Google Scholar]
- 14.Najafi B, Helbostad JL, Moe-Nilssen R, Zijlstra W, Aminian K. Does walking strategy in older people change as a function of walking distance? Gait Posture. 2009;29:261–266. doi: 10.1016/j.gaitpost.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Plummer P, Grewal G, Najafi B, Ballard A. Instructions and skill level influence reliability of dual-task performance in young adults. Gait Posture. 2015;41:964–967. doi: 10.1016/j.gaitpost.2015.03.348. [DOI] [PubMed] [Google Scholar]
- 16.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The montreal cognitive assessment, moca: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 17.Schwenk M, Howe C, Saleh A, Mohler J, Grewal G, Armstrong D, Najafi B. Frailty and technology: A systematic review of gait analysis in those with frailty. Gerontology. 2014;60:79–89. doi: 10.1159/000354211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grewal GS, Schwenk M, Lee-Eng J, Parvaneh S, Bharara M, Menzies RA, Talal TK, Armstrong DG, Najafi B. Sensor-based interactive balance training with visual joint movement feedback for improving postural stability in diabetics with peripheral neuropathy: A randomized controlled trial. Gerontology. 2015;61:567–574. doi: 10.1159/000371846. [DOI] [PubMed] [Google Scholar]
- 19.Schwenk M, Grewal GS, Holloway D, Muchna A, Garland L, Najafi B. Interactive sensor-based balance training in older cancer patients with chemotherapy-induced peripheral neuropathy: A randomized controlled trial. Gerontology. 2015 doi: 10.1159/000442253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Najafi B, Armstrong DG, Mohler J. Novel wearable technology for assessing spontaneous daily physical activity and risk of falling in older adults with diabetes. J Diabetes Sci Technol. 2013;7:1147–1160. doi: 10.1177/193229681300700507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toosizadeh N, Joseph B, Heusser MR, Orouji Jokar T, Mohler J, Phelan HA, Najafi B. Assessing upper-extremity motion: An innovative, objective method to identify frailty in older bed-bound trauma patients. J Am Coll Surg. 2016 doi: 10.1016/j.jamcollsurg.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor-Piliae RE, Mohler MJ, Najafi B, Coull BM. Objective fall risk detection in stroke survivors using wearable sensor technology: A feasibility study. Top Stroke Rehabil. 2016:1–7. doi: 10.1179/1074935715Z.00000000059. [DOI] [PubMed] [Google Scholar]
- 23.Mohler MJ, Wendel CS, Taylor-Piliae RE, Toosizadeh N, Najafi B. Motor performance and physical activity as predictors of prospective falls in community-dwelling older adults by frailty level: Application of wearable technology. Gerontology. 2016 doi: 10.1159/000445889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grewal G, Sayeed R, Yeschek S, Menzies RA, Talal TK, Lavery LA, Armstrong DG, Najafi B. Virtualizing the assessment: A novel pragmatic paradigm to evaluate lower extremity joint perception in diabetes. Gerontology. 2012;58:463–471. doi: 10.1159/000338095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R. The diagnosis of dementia due to alzheimer’s disease: Recommendations from the national institute on aging-alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimer’s & dementia. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of internal medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 27.Orme JG, Reis J, Herz EJ. Factorial and discriminant validity of the center for epidemiological studies depression (ces-d) scale. Journal of clinical psychology. 1986;42:28–33. doi: 10.1002/1097-4679(198601)42:1<28::aid-jclp2270420104>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 28.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research G Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 29.Tombaugh TN. Trail making test a and b: Normative data stratified by age and education. Archives of clinical neuropsychology: the official journal of the National Academy of Neuropsychologists. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 30.Bowie CR, Harvey PD. Administration and interpretation of the trail making test. Nature protocols. 2006;1:2277–2281. doi: 10.1038/nprot.2006.390. [DOI] [PubMed] [Google Scholar]
- 31.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: A validation study. American journal of epidemiology. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 32.Grewal G, Sayeed R, Yeschek S, Menzies RA, Talal TK, Lavery LA, Armstrong DG, Najafi B. Virtualizing the assessment: A novel pragmatic paradigm to evaluate lower extremity joint perception in diabetes. Gerontology. 2012;58:463–471. doi: 10.1159/000338095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerling KM, Schild J, Masuch M. Exergaming for elderly: Analyzing player experience and performance. Mensch & Computer 2011: 11 fachübergreifende Konferenz für interaktive und kooperative Medien Oldenbourg Verlag. 2011:401. [Google Scholar]
- 34.Menz HB, Latt MD, Tiedemann A, Mun San Kwan M, Lord SR. Reliability of the gaitrite walkway system for the quantification of temporo-spatial parameters of gait in young and older people. Gait Posture. 2004;20:20–25. doi: 10.1016/S0966-6362(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 35.Ellis P. Thresholds for interpreting effect sizes. Retrieved January. 2009;13:2014. [Google Scholar]
- 36.Montero-Odasso M, Oteng-Amoako A, Speechley M, Gopaul K, Beauchet O, Annweiler C, Muir-Hunter SW. The motor signature of mild cognitive impairment: Results from the gait and brain study. J Gerontol A Biol Sci Med Sci. 2014;69:1415–1421. doi: 10.1093/gerona/glu155. [DOI] [PMC free article] [PubMed] [Google Scholar]


