ABSTRACT
Relapsing fever (RF) spirochetes colonize and are transmitted to mammals primarily by Ornithodoros ticks, and little is known regarding the pathogen's life cycle in the vector. To further understand vector colonization and transmission of RF spirochetes, Borrelia turicatae expressing a green fluorescent protein (GFP) marker (B. turicatae-gfp) was generated. The transformants were evaluated during the tick-mammal infectious cycle, from the third nymphal instar to adult stage. B. turicatae-gfp remained viable for at least 18 months in starved fourth-stage nymphal ticks, and the studies indicated that spirochete populations persistently colonized the tick midgut and salivary glands. Our generation of B. turicatae-gfp also revealed that within the salivary glands, spirochetes are localized in the ducts and lumen of acini, and after tick feeding, the tissues remained populated with spirochetes. The B. turicatae-gfp generated in this study is an important tool to further understand and define the mechanisms of vector colonization and transmission.
IMPORTANCE In order to interrupt the infectious cycle of tick-borne relapsing fever spirochetes, it is important to enhance our understanding of vector colonization and transmission. Toward this, we generated a strain of Borrelia turicatae that constitutively produced the green fluorescent protein, and we evaluated fluorescing spirochetes during the entire infectious cycle. We determined that the midgut and salivary glands of Ornithodoros turicata ticks maintain the pathogens throughout the vector's life cycle and remain colonized with the spirochetes for at least 18 months. We also determined that the tick's salivary glands were not depleted after a transmission blood feeding. These findings set the framework to further understand the mechanisms of midgut and salivary gland colonization.
KEYWORDS: relapsing fever spirochetes, Borrelia turicatae, Ornithodoros, soft tick, argasid, vector colonization
INTRODUCTION
Defining the intricacies of pathogen colonization and transmission from hematophagous arthropod vectors is essential for disease detection, control, and prevention. The primary vectors of relapsing fever (RF) spirochetes are argasid ticks of the genus Ornithodoros. The biology of these vectors is complex and unique compared to other pathogen-transmitting arthropods. Throughout their life history, Ornithodoros ticks may pass through six or more nymphal stages before molting to adults, and each stage requires a blood meal (1). The vectors also live up to 20 years and can also endure prolonged periods (five years) of starvation (1, 2).
Blood feeding is an important process for the survival of Ornithodoros species, and it influences the tick's colonization and subsequent transmission of RF spirochetes. Ornithodoros ticks quickly engorge, completing the blood meal within five to 60 min after host attachment (1, 3). Therefore, RF Borrelia spp. evolved mechanisms to facilitate colonization and maintenance through the complex life history of their long-lived rapid-feeding vectors. However, very little is known regarding the life cycle of RF Borrelia spp. in Ornithodoros ticks.
Two current systems used to study the tick-mammal transmission cycle of RF spirochetes include the Borrelia hermsii-Ornithodoros hermsi and Borrelia turicatae-Ornithodoros turicata models (4–6). These models demonstrated initial midgut colonization following an infectious blood meal, and in the following weeks, spirochetes migrated to colonize the salivary glands (6, 7). Given the tick's short blood meal duration, the salivary gland population is important to continue the pathogen's life cycle in the mammal (4, 8). Moreover, after tick feeding and through the molt, RF spirochetes are maintained transstadially (8, 9). While the generalities are known, the details of vector colonization throughout the tick's life cycle and localization in different tissues remain to be fully understood. This knowledge could be translated into disease control approaches that interrupt essential stages in the life cycle of RF spirochetes.
In this study, our goal was to begin to understand the intricacies of vector colonization by RF spirochetes. Toward that, recent advances in B. turicatae genetics were utilized (7), and a gene coding for the green fluorescent protein (gfp) was inserted into a plasmid. We demonstrated that after genetic manipulation, the transformants retained their ∼10 plasmids. The spirochetes constitutively expressed gfp, and we evaluated vector colonization and transmission. Visualization of B. turicatae-gfp during the infectious cycle, beginning with infected third-stage nymphal instars, indicated that the spirochetes were maintained in O. turicata ticks through the adult stage. Interestingly, evaluation of the tick revealed that the midgut and salivary glands remain persistently colonized with two spirochete populations. This study lays the foundation to further define and identify essential processes in the life cycle of RF spirochetes in the tick vector.
RESULTS
Insertion of gfp into B. turicatae lp150 and assessment of transformants in vitro.
Integration of gfp into B. turicatae was accomplished with the PCR2.1::gfp suicide vector. Four clones were assessed, and all spirochetes within a given population fluoresced (data not shown). Interestingly, PCR evaluation of clone 1 for integration of PflaB kan-gfp between fhbA and bta003, which are located on lp150, failed to generate a larger amplicon of the expected size (Fig. 1). Primer pair F1-R2 produced the parent-sized amplicon, and the primer pairs F1-R3 and F3-R2 did not produce an amplicon, which indicated that gfp was not inserted in the intended location on lp150 (Fig. 1A and B). However, primer pair F3-R3 amplified PflaB kan-gfp from genomic DNA of B. turicatae-gfp, showing that the construct remained intact within the transformants (Fig. 1A and B). Also, PCR of the three remaining clones was similar and further indicated that PflaB kan-gfp unexpectedly recombined elsewhere in the genome (data not shown).
FIG 1.
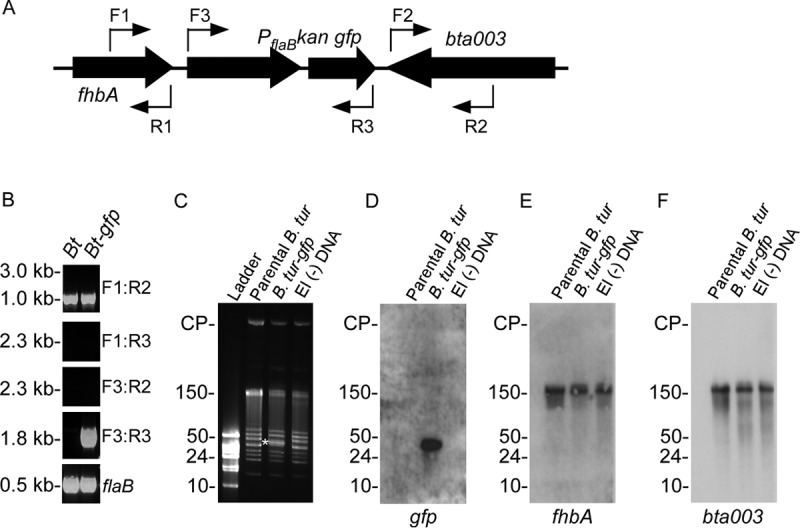
Integration of PflaB kan-gfp into B. turicatae. (A) PflaB kan-gfp was initially targeted for insertion between fhbA and bta003 with primer locations shown as thin arrows. (B) PCR was performed using wild-type B. turicatae (Bt) or B. turicatae-gfp (Bt-gfp) with primer combinations shown on the right of each gel image. (C to F) Pulsed-field electrophoresis (C) and Southern blotting (D to F) were performed to determine the recombination location of PflaB kan-gfp within the B. turicatae genome. (C) A shift in molecular weight was observed for a 40-kb linear plasmid of B. turicatae-gfp (white asterisk), which was not observed in the parental strain (parental B. tur) or B. turicatae that was electroporated without DNA (−DNA). (D to F) A probe designed for gfp localized the gene to a 40-kb linear plasmid (D), while fhbA and bta003 remained localized to lp150 (E and F). The probes for gfp, fhbA, and bta003 are shown beneath each Southern blot image. Molecular weights and circular plasmids (CP) are shown on the left of the gels and Southern blot.
Previous work reported segmental recombination of B. turicatae plasmids during in vitro cultivation (10). Therefore, we performed pulsed-field electrophoresis and Southern blotting, which indicated that PflaB kan-gfp had recombined to a smaller plasmid (Fig. 1C to F). Imaging of plasmid profiles of the parental spirochetes, B. turicatae-gfp, and B. turicatae that were transformed without DNA indicated a change in the molecular weight of an ∼40-kb plasmid only in genomic DNA isolated from B. turicatae-gfp (Fig. 1C). Southern blotting with a probe for gfp localized the gene to an ∼40-kb plasmid (Fig. 1D), while probes for fhbA (Fig. 1E) and bta003 (Fig. 1F) localized the genes on lp150. All four clones evaluated demonstrated the recombination of PflaB kan-gfp to this smaller plasmid (data not shown). Given that B. turicatae-gfp grew comparably in vitro to the parental strain (Fig. 2) and that the transformants fluoresced, we proceeded with infection of mice and evaluation of O. turicata colonization.
FIG 2.
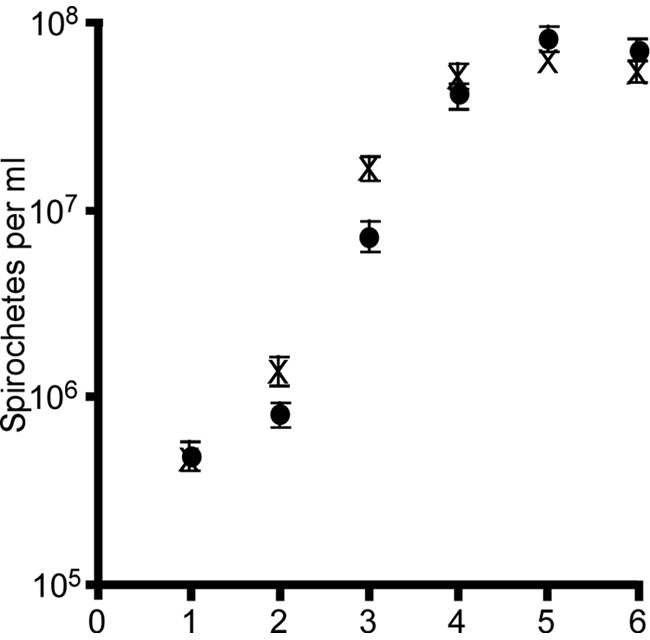
In vitro growth of B. turicatae-gfp (●) and the parental strain (X). Cultures were inoculated with 5 × 105 spirochetes and counted for six consecutive days. Error bars represent the standard deviations for triplicate counts.
B. turicatae-gfp expression after needle inoculation of mice.
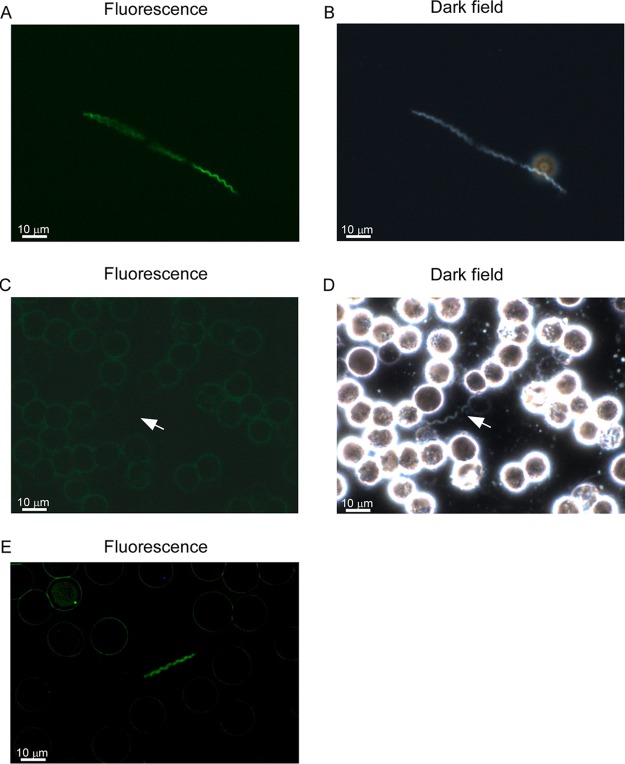
During in vitro cultivation, B. turicatae-gfp was visualized by microscopy (Fig. 3A and B). To evaluate gfp expression in vivo, mice were needle inoculated with 1 × 105 B. turicatae-gfp or wild-type spirochetes, and within 2 days, there were ∼1 × 107 spirochetes/ml of blood. The parental strain spirochetes were not detected by fluorescence microscopy (Fig. 3C) but were detected by dark-field microscopy (Fig. 3D). B. turicatae-gfp fluoresced in the blood (Fig. 3E). These results further indicated that gfp remained stable in B. turicatae during murine infection.
FIG 3.
Evaluation of B. turicatae-gfp in vitro and in murine blood after needle inoculation. B. turicatae-gfp grown in vitro is shown under fluorescent (A) and dark-field microscopy (B). In the blood, wild-type spirochetes failed to fluoresce (C) yet were detected by dark-field microscopy (D). (E) B. turicatae-gfp remained fluorescent in the blood after needle inoculation. Images were captured using a 63× oil immersion objective, and a scale is shown as a white bar toward the bottom left of each image.
Acquisition of B. turicatae-gfp by O. turicata and subsequent transmission.
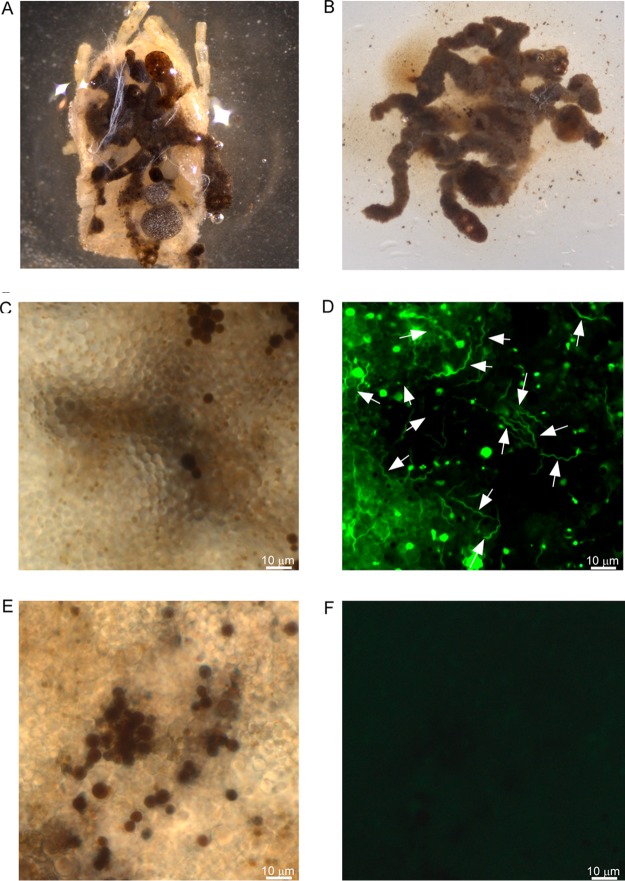
To evaluate GFP production in the tick, cohorts of third-stage nymphal O. turicata from Kansas (O. turicata-KS) and from southern Texas (O. turicata-TX) were fed to repletion when mice were spirochetemic with B. turicatae-gfp or wild-type spirochetes. Live bacteria were detected in the midgut of 10 ticks from the KS and TX colonies by fluorescence microscopy immediately following the blood meal, indicating that the ticks had ingested the pathogens (data not shown). Upon molting into fourth-stage nymphal instars (four weeks after the acquisition blood meal), 15 ticks were dissected, and B. turicatae-gfp was visualized in the midgut. The midgut from a single O. turicata (Fig. 4A to F) was representative of the remaining ticks that were evaluated. Similarly, salivary glands were excised (Fig. S1 and 5A), and evaluation of the tissues detected fluorescing spirochetes in regions of the lumen and duct of acini (Fig. 5B to I).
FIG 4.
Assessment of B. turicatae-gfp in the tick midgut. (A) Following the molt, the cuticle of O. turicata was removed exposing the midgut. The tissue was excised (B) and viewed by dark-field microscopy (C) and under fluorescence (D) using a 63× oil immersion objective. (D) White arrows point to fluorescing spirochetes. (E and F) Tick midguts infected with wild-type B. turicatae are shown as a negative control.
FIG 5.
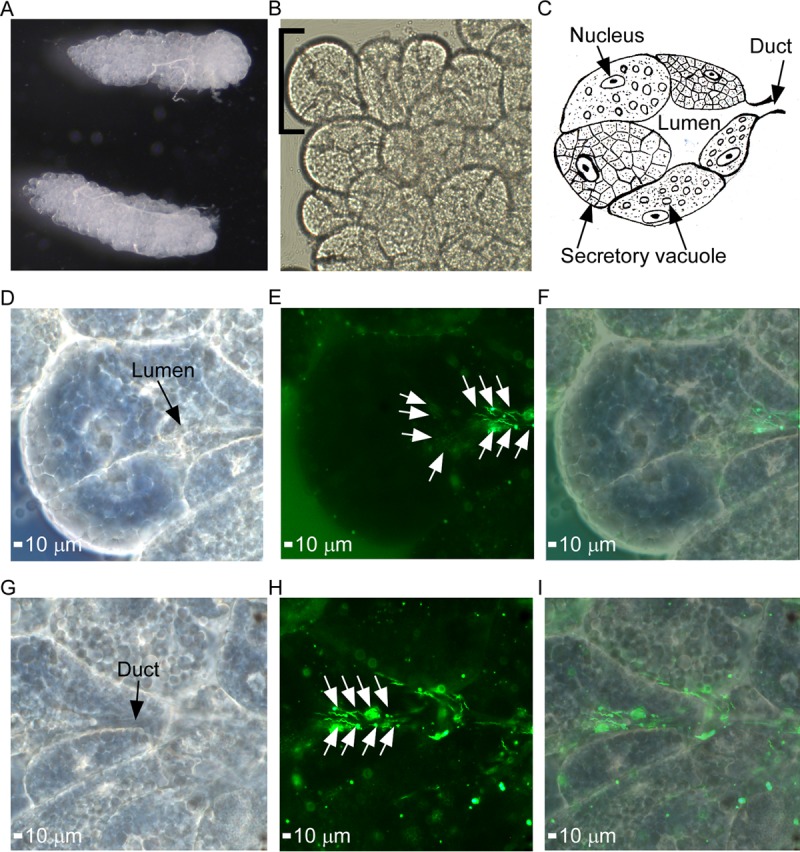
Demonstration of salivary gland colonization by B. turicatae-gfp. (A) Intact salivary glands were excised from infected O. turicata ticks. (B and C) Agranular acini along the peripheral margin of the posterior region of the salivary gland (B) were assessed for spirochete colonization based upon location of the lumen and efferent duct, as illustrated in the artistic rendition of salivary gland tissue (C). Each acinus is composed of secretory cells that produce saliva, which drains into the lumen and through the efferent duct. A single acinus is shown in panels D to I and was viewed with a 63× oil immersion objective. (D to F) Spirochete colonization in the acini lumen is demonstrated. A dark-field image shows the acinus structural outline and location of the lumen (D), followed by fluorescent-filtered image showing localization of fluorescing spirochetes (E), indicated by white arrows, and an overlay of dark-field and fluorescent images (F). (G to I) Spirochete colonization in the efferent duct region in the same acinus is also demonstrated. Shown is a dark-field image of the acinus structural outline and location of the efferent duct (G), followed by fluorescent-filter image showing the localization of fluorescing spirochetes (H), as indicated by the white arrows. (I) An overlay of dark-field and fluorescent images is also shown.
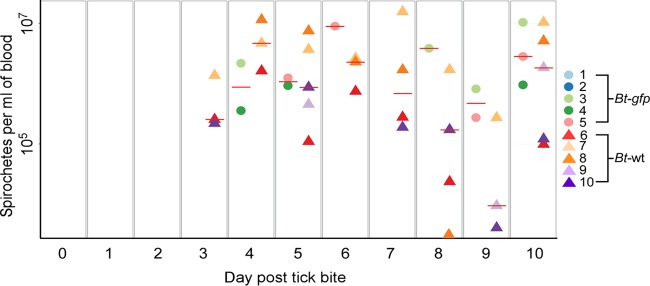
Tick transmission and relapse of B. turicatae-gfp or the parental strain in mice were visually confirmed under fluorescence (Movie S1) and by quantitative PCR (qPCR) (Fig. 6). All five animals that were fed upon by ticks colonized by the parental strain of B. turicatae became infected and relapsed within 10 days (Fig. 6). Three of five animals were infected with B. turicatae-gfp, and a relapse of fluorescing spirochetes in the blood was detected within 10 days after tick feeding (Fig. 6). Within a population of B. turicatae-gfp, all spirochetes visualized by dark-field microscopy fluoresced when assessed by fluorescent microcopy. The two mice that were negative by qPCR failed to seroconvert (Fig. 7A and B), indicating that a low-level infection that was under the limit of detection by qPCR did not occur. Interestingly, the antibody response generated against B. turicatae-gfp appeared less robust than that in mice infected with wild-type spirochetes.
FIG 6.
Transmission of B. turicatae-gfp by tick bite. Five mice were fed upon by ticks infected with B. turicatae expressing gfp (Bt-gfp, colored circles) or wild-type spirochetes with the same genetic background (Bt-wt, colored triangles). Mice were sampled daily and spirochete densities in the blood determined by qPCR. A horizontal red line within a given day represents the average spirochete density for the group of positive animals.
FIG 7.
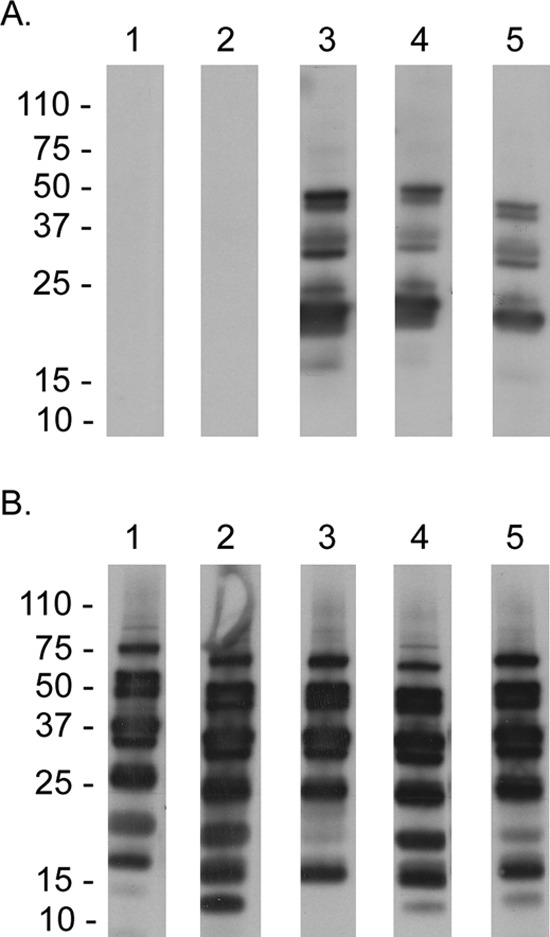
Immunoblotting using serum samples from mice infected with B. turicatae-gfp (A) or wild-type spirochetes (B). Animal number is indicated above each immunoblot, and molecular weight standards are shown on the left.
Similar successful transmission frequencies were detected regardless of whether O. turicata-KS or -TX colonies were used in the study. In total, we observed 100% (15/15) and 60% (9/15) murine infection rates for ticks colonized with the parental strain of B. turicatae or B. turicatae-gfp, respectively. Similar transmission frequencies were observed at the fifth nymphal stage and as adult ticks, which confirmed the maintenance of B. turicatae-gfp through three molts and as adults.
Since successful transmission frequencies were not identical between positive-control ticks and those infected with B. turicatae-gfp, the cohort of O. turicata that failed to deliver an infectious dose was further evaluated. The remaining 12 live ticks were mixed in one vial, separated into two six-tick cohorts, and salivary glands were assessed by fluorescence microscopy and PCR. Live spirochetes were visualized in four of six salivary gland sets by fluorescence microscopy, while PCR using primers for gfp detected spirochete DNA in three of six salivary glands (Fig. 8A). Uninfected ticks were used as a negative control in the assays (Fig. 8B). In total, evidence of salivary gland colonization by B. turicatae-gfp was detected in seven of 12 ticks. From ticks that successfully transmitted B. turicatae-gfp, spirochetes were visualized in 10 of 12 salivary gland sets. These results suggested that a lower infectious dose of B. turicatae-gfp may have been delivered during the blood meal.
FIG 8.
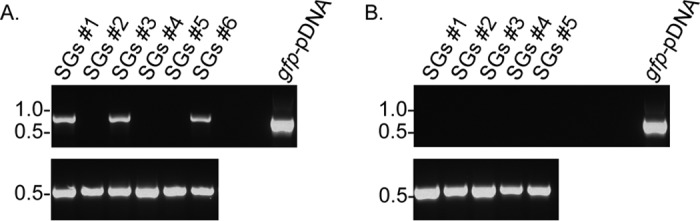
PCR to detect B. turicatae-gfp DNA in tick salivary glands (SGs). Primers for gfp were used with DNA from infected (A, top) and uninfected (B, top) ticks. Plasmid DNA containing gfp (gfp-pDNA) was used as a positive control in the assays in addition to primers for O. turicata 28s (A and B, bottom). Molecular weights are shown to the left of each image.
Evaluation of the stability of gfp during the tick-mammal transmission cycle.
To further assess stability of the gfp cassette after transmission to mice by tick bite, B. turicatae-gfp was reisolated from murine blood after feeding of infected O. turicata. Southern blotting using genomic DNA of wild-type spirochetes, B. turicatae-gfp initially used to needle inoculate mice, and B. turicatae-gfp reisolated from murine blood after tick transmission indicated that the gfp cassette remained stable and localized on the ∼40-kb plasmid (Fig. 9). Moreover, PCR analysis failed to amply portions of the suicide vector and indicated that only PflaB kan-gfp integrated into the ∼40-kb plasmid (data not shown). While the mechanism of gfp-recombination remains unclear, the gene was not mobile during the tick-mammal transmission cycle.
FIG 9.
Assessment of gfp localization from spirochetes isolated from murine blood. Southern blotting using a probe for gfp and genomic DNA from wild-type B. turicatae (Bt-wt), B. turicatae-gfp that was originally used to needle inoculate mice (Bt-gfp #1) prior to tick feeding, and B. turicatae-gfp that was recovered from mouse blood after infected ticks fed (Bt-gfp #2). Molecular weights are indicated on the left of the Southern blot.
Long-term maintenance of B. turicatae-gfp in the tick vector.
Previous work reported the ability of B. turicatae to remain viable in ticks that were starved for 5 years (2). To begin understanding long-term vector colonization, we assessed a cohort of B. turicatae-gfp-infected ticks that had not fed in 18 months. Persistent colonization of the spirochetes in infected O. turicata-KS ticks was demonstrated by visualizing fluorescing spirochetes in the midgut and salivary glands from 10 starved ticks. Notably, the midgut remained populated with spirochetes after 18 months, and the pathogens were motile and fluorescing (Movie S2).
Characterization of salivary gland colonization after a blood meal.
With the short blood meal duration of Ornithodoros ticks (9), the salivary gland population of RF spirochetes is essential for transmission and establishing mammalian infection. However, it is unknown whether these tissues become entirely depleted of spirochetes after feeding. Evaluation of salivary glands from O. turicata-KS and -TX cohorts that were excised and rinsed immediately after a blood meal revealed that a population of spirochetes remained localized in the lumen region (Table 1 and Fig. S2). In one tick, B. turicatae-gfp was undetectable by microscopy in the midgut and salivary glands after feeding (Table 1), which suggested that the tick may not have imbibed the infectious dose required for vector colonization. These results indicated that the salivary glands remain populated through the transmission cycle of RF spirochetes.
TABLE 1.
Detection of B. turicatae-gfp in the midgut and salivary glands of unfed O. turicata and immediately after ticks fed
| Tick no. | Unfed ticks |
Fed ticks |
||
|---|---|---|---|---|
| Midgut | Salivary glands | Midgut | Salivary glands | |
| 1 | + | + | + | + |
| 2 | + | + | + | + |
| 3 | − | + | − | + |
| 4 | + | + | + | + |
| 5 | − | − | − | + |
| 6 | + | + | + | + |
| 7 | + | + | + | + |
| 8 | + | + | + | + |
| 9 | + | + | + | − |
| 10 | + | + | − | − |
| Total (no. infected/total no.) | 8/10 | 9/10 | 7/10 | 8/10 |
DISCUSSION
In this study, we demonstrated the stable integration of gfp into B. turicatae and the ability of O. turicata ticks to maintain the genetically modified spirochetes and transmit them during blood feeding on mice. The spirochetes that successfully colonized immature O. turicata ticks were transmitted transstadially to the adult-stage tick. B. turicatae-gfp also remained fluorescing in ticks starved for 18 months. Collectively, these findings indicate that B. turicatae-gfp can be utilized to understand the mechanisms of Ornithodoros colonization and transmission.
The site for PflaB kan-gfp insertion between fhbA and bta003, at the 5′ end of the B. turicatae megaplasmid, was selected because previous work with B. hermsii demonstrated integration of gfp into that region (11). However, in our study, the gfp insert recombined into an ∼40-kb linear plasmid (Fig. 1). Given that PflaB kan-gfp was detected on this smaller plasmid in clones passaged once after transformation, recombination likely occurred early after genetic manipulation. Additionally, plasmid profiles from spirochetes that were transformed without DNA were identical to the parental B. turicatae strain. This observation suggests that transformation alone did not have a role in plasmid recombination. Our attempts to determine the precise location of the PflaB kan-gfp were hindered because a complete assembly of B. turicatae plasmids was not available at the time of this study.
Genetic relocalization was previously observed for RF spirochetes. In B. hermsii and B. turicatae, relocalization of the plasmid genes resT, chbC, and fhbA was reported after continuous spirochete passage in vitro, which did not alter infectivity by needle inoculation (10). In our study, gfp remained localized on an ∼40-kb plasmid during the tick-mammal infectious cycle, and B. turicatae transformants were infectious by tick feeding. While the association of gfp recombination and the observed transmissibility of the spirochetes from the tick will be addressed in future studies, B. turicatae-gfp was utilized to further understand vector colonization.
Visualization of GFP-producing spirochetes in the salivary gland lumen and ducts indicated that the pathogens are localized in regions that would promote rapid transmission upon tick bite. Surprisingly, a population of B. turicatae-gfp was also consistently detected in the salivary glands immediately after a blood meal, revealing that the tissues were not depleted of RF spirochetes after tick feeding. The results, along with the observed transmission of B. turicatae within 15 s of tick attachment (4), further indicate that a relatively low inoculum is delivered to the host at the bite site during blood feeding.
An additional population of RF spirochetes is present in the midgut of Ornithodoros species after they imbibe an infectious blood meal (6, 7); yet, their role in pathogenesis remains vague. By indirect immunofluorescence assay, Schwan and Hinnebusch detected B. hermsii in the midguts of 80% of O. hermsi ticks during the nearly 5 months after the ticks ingested spirochetes (6). In our current report, the midgut population was still detected in ticks 18 months after they initially imbibed an infectious blood meal. It is unlikely that the midgut population is involved with transmission to the host during tick blood feeding, as observed with Lyme disease-causing spirochetes. For example, Borrelia burgdorferi persistently colonizes the midgut of Ixodes species (12–14), and during a blood meal, a 36- to 48-h lapse is required for the pathogens to migrate from the midgut to the salivary glands before transmission to vertebrate hosts can occur (12–14).
Depending on the life stage, Ornithodoros species complete the blood meal within five to 60 min (9), and a full blood meal is not required for RF spirochete transmission (4). Transmission kinetics indicate that there is insufficient time for the midgut population to migrate to the salivary glands and enter the vertebrate host. One putative role of the midgut population may be to replenish the salivary glands after a blood meal. Ornithodoros ticks infected with RF spirochetes maintain and transmit the pathogens throughout their life history (2, 9, 15), which can include over five nymphal instars. Adult ticks also feed and reproduce multiple times (1, 16). Therefore, as RF spirochete densities in the tick diminish over time, the nutrient-rich blood meal may facilitate replication in the midgut and subsequent migration to the salivary glands. This is an aspect of pathogenesis that can now be evaluated using the B. turicatae-gfp reported here.
The utilization of B. turicatae-gfp could also help define the mechanisms of vector competence observed for RF spirochetes, because of the specific vector-pathogen interactions that have evolved between species of RF Borrelia and Ornithodoros ticks. An early example of vector specificity was demonstrated in 1953 when Luiz Mazzotti recovered an RF spirochete, later named Borrelia mazzotti, from Ornithodoros talaje collected in Mexico (17). Several Ornithodoros species from Latin America were infected with B. mazzotti; however, subsequent tick feedings on mice indicated that only O. talaje successfully transmitted the pathogens. More recent work with O. hermsi, Ornithodoros parkeri, and O. turicata infected with B. hermsii indicated that the tick's salivary glands may be a restrictive environment for vector competence (5). O. hermsi, O. parkeri, and O. turicata were allowed to engorge on a mouse infected with B. hermsii. Spirochetes disseminated out of the midgut from the three tick species, but only O. hermsi transmitted B. hermsii. The ability to infect ticks with fluorescing B. turicatae will further enable vector competency studies to assess the kinetics of salivary gland colonization and subsequent pathogen transmission between Ornithodoros species.
Our findings with B. turicatae-gfp revealed key aspects of RF spirochete pathogenesis that warrant future investigation. For example, details of the kinetics and molecular mechanisms of RF spirochete escape from the midgut, migration through the hemocoel, and salivary gland colonization remain to be fully understood. An understanding of whether B. turicatae migration to the salivary glands is a stochastic process is needed, and identification of the molecular signals is essential. Additionally, understanding whether the midgut population of B. turicatae serves as a depot to replenish the salivary glands upon blood feeding is important toward developing strategies to interrupt the pathogen's life cycle in the tick. As progress with the genetic manipulation of RF spirochetes has been made (7, 11, 18, 19), a refined mechanistic understanding of vector colonization and transmission is foreseeable. These studies will help advance new control and prevention methods to mitigate the health burden of tick-borne RF Borrelia species.
MATERIALS AND METHODS
Ethical statement.
Mouse transmission studies were approved by the Institutional Animal Care and Use Committee (IACUC) at Baylor College of Medicine, protocol numbers AN-6563 and AN-6580, whose laboratory animal program complies with standards and guidance established by the Association for Assessment and Accreditation of Laboratory Animal Care and the National Institutes of Health Office of Laboratory Animal Welfare. Animal husbandry was provided by the veterinary staff and animal care technicians.
Constructing a suicide vector for kan-gfp insertion.
The 91E135 isolate of B. turicatae (20) was used in this study, and gfp was targeted for insertion between fhbA and bta003 on the 150-kb linear megaplasmid (lp150), in an approach similar to that described by Fine and colleagues for B. hermsii (11). The GoTaq Flexi DNA polymerase kit (Promega, Madison, WI, USA) was used to amplify ∼500 bp of fhbA and bta003 with primer combinations F1-R1 and F2-R2, respectively (Table 2 and Fig. 10A). Amplicons were digested with SphI, ligated, and cloned into PCR2.1 (Life Technologies, Carlsbad, CA, USA) (Fig. 10B to D), and Escherichia coli was transformed by electroporation. Colonies were screened by PCR for insertion, PCR2.1::fhbA-bta003 was purified using QIAprep (Qiagen, Valencia, CA) from positive colonies, and DNA sequencing was performed to confirm that errors were not introduced during PCR (Fig. 10B to D). The gfp gene was amplified from the pABG5 cloning vector (21) using gfp F SpeI and gfp R SgrAI primers (Table 2) and cloned into a B. turicatae vector, which contained the flagellin (flaB) promoter driving the kanamycin acetyltransferase (Fig. 10D). The construct (PflaB kan-gfp) was amplified with F3 and R3 primers, which contained AvrII and NheI restriction sites, respectively. The amplicon was cloned into PCR2.1::fhbA-bta003, generating the PCR2.1::gfp suicide vector (Fig. 10E). E. coli was transformed, colonies were selected for plasmid isolation, and sequencing was performed.
TABLE 2.
Primers used in the study
| Primer | Sequence (5′ to 3′) |
|---|---|
| F1 | GGTAAGTTCTACTTATGATGCTTATGC |
| R1 | GGGGCATGCGGGCCTAGGATGAACTTAACTTTCTAAAAGTGACATTATTCTC |
| F2 | GGGGCATGCGGGGCTAGCTTTTAACACTTAAGATTTATCTCTTACACGC |
| R2 | TTCTCAATTTGATTTAACTGATTACC |
| F3 | GGGCCTAGGGGCAATTCCTAATCAGAAAAATGTGG |
| R3 | GGGGCTAGCTTATTTGTATAGTTCATCCATGCCATGTG |
| gfp F SpeI | GCGACTAGTAAAGATTAACTTTATAAGGAGGAAAAACATATGAGTAAAGGAGAAGAACTTTTCACTGG |
| gfp R SgrAI | GCGCGCCGGTGTTATTTGTATAGTTCATCCATGCCATGTGTAATCC |
FIG 10.
Pictorial depiction of the approach to construct the gfp insertion vector. fhbA and bta003 were amplified using primers (A), adding the appropriate restriction enzyme sites (B), and digested and ligated (C). The ligation reaction was cloned into the PCR2.1 TOPO vector, generating PCR2.1::fhbA-bta003 (D, left plasmid). The kanamycin acetyltransferase gene and gfp driven by the B. turicatae flaB promoter (PflaB kan-gfp) were amplified by PCR adding AvrII and NheI restriction sites to the 5′ and 3′ ends of the amplicon, respectively, and cloned into PCR2.1::fhbA-bta003 (D, left plasmid, and E).
Generation of B. turicatae-gfp.
Electrocompetent B. turicatae 91E135, passaged 17 times in modified Barbour-Stoenner-Kelly (mBSK) medium (18, 22), was produced as previously described and transformed with 20 μg of PCR2.1::gfp (7, 18). Spirochetes were transferred into 5 ml of mBSK medium for 24 h, after which the cultures were added to 40 ml of mBSK medium with 200 μg/ml kanamycin. Once live spirochetes were observed by dark-field microscopy, 1 ml was transferred to a vial containing 4 ml of fresh mBSK medium and 200 μg/ml kanamycin. After spirochetes attained stationary growth, the transformants were evaluated for fluorescence with an EVOS FL microscope (Thermo Fisher Scientific, Waltham, MA, USA) and cloned by limiting dilution, as previously described (7, 18).
To evaluate the in vitro growth of transformants compared to that of the parental strain of B. turicatae, 5-ml culture tubes containing mBSK medium were inoculated in triplicate with a given clone at 5 × 105 spirochetes. The bacteria were quantified for six consecutive days using Petroff-Hausser counting chambers (Hausser Scientific, Horsham, PA, USA) and a Zeiss Axio Imager A2 dark-field microscope (Zeiss, Munich, Germany). Daily counts were performed in triplicate, and spirochete averages and standard deviations calculated. Fifty-milliliter cultures were also generated from a given clone, and genomic DNA was isolated by phenol-chloroform extraction, as detailed below. Insertion of gfp into B. turicatae was assessed by PCR and Southern blotting, as previously described (20, 23).
Genomic DNA isolation, pulsed-field gel electrophoresis, and Southern blotting.
Genomic DNA was isolated from four clones of B. turicatae-gfp, untransformed parental strain, and the parental strain that had been electroporated without DNA. Spirochetes were grown in 50 ml of 1× mBSK medium, and genomic DNA was prepared when they attained a density of 1 × 107 bacteria/ml. B. turicatae was centrifuged and pellets washed with 1× phosphate-buffered saline (PBS) and 5 mM MgCl2. Spirochete pellets were resuspended in 2.4 ml of N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid (TES) buffer (50 mM Tris [pH 8.0], 50 mM EDTA, 15% sucrose) and cells lysed with 4 mg/ml lysozyme for 10 min on ice. Cell homogenate was treated with 3 ml of TES and 1% deoxycholic acid on a rocker for 10 min at room temperature. Genomic DNA was organically isolated by phenol-chloroform extraction using phase-lock gel conical tubes (VWR, Houston, TX), precipitated, and resuspended in Tris-EDTA (TE) buffer, as previously described (24). DNA concentration was determined by NanoDrop (Thermo Fisher Scientific).
Reverse-field electrophoresis was performed as previously described (20). Total genomic DNA samples were electrophoresed into a 1% agarose gel at 100 V for 15 min and then on Program 3 of a PPI-200 programmable power inverter (MJ Research, Watertown, MA) for ∼20 h. Gels were stained with GelRed (Phenix Research, Candler, NC) at a 1:10,000 dilution and visualized with UV transillumination. Transfer of DNA onto polyvinylidene difluoride (PVDF) membranes and Southern blotting were performed as previously described (20). Probes were generated for fhbA, gfp, and bta003 using the PCR DIG Probe synthesis kit (Roche Applied Science, Indianapolis, IN, USA).
Murine infection by needle inoculation.
B. turicatae-gfp and the parental strain were cultured at 35°C in mBSK medium, and 1 × 108 spirochetes were inoculated by intraperitoneal injection into Institute of Cancer Research (ICR) mice (a Swiss derivative in colony at Baylor College of Medicine). Blood was collected by tail nick for two consecutive days, and 2.5 μl was viewed by dark-field microscopy and under fluorescence with a Zeiss Axio Imager A2 (Zeiss) to evaluate gfp expression. For fluorescence microscopy, images were captured with an exposure time of 300 to 500 ms. Another 2.5 μl of blood was added to 47.5 μl of lysis stabilization buffer (Agilent Technologies, Santa Clara, CA, USA) for quantitative PCR (qPCR). Within 2 days after needle inoculation, the animals had ∼1 × 107 spirochetes/ml of blood, and O. turicata ticks were allowed to feed on the mice.
O. turicata colonies and B. turicatae-gfp acquisition and transmission by tick bite.
Nymphal ticks used in this study were progeny of adult O. turicata from laboratory-reared colonies, and an uninfected cohort was reared through the second-instar nymphs. The two colonies originated from Kansas (O. turicata-KS) (7) and southern Texas (O. turicata-TX) (25). Ticks were housed at 25°C and 85% relative humidity (26). Acquisition of B. turicatae-gfp or the parental strain was performed by feeding third-instar nymphal ticks on infected mice. Salivary glands and midguts from specimens in a given tick colony were assessed at the following time points: (i) immediately after the spirochete acquisition blood meal (O. turicata-KS and -TX colonies), (ii) immediately following the molt (O. turicata-KS and -TX), (iii) 18 months after the B. turicatae-gfp acquisition blood meal (O. turicata-KS), and (iv) immediately following the subsequent transmission blood meal by fourth-instar nymphs (O. turicata-KS and -TX). Fifteen to 20 ticks were evaluated at each experimental procedure. For fluorescence microscopy, images and movies were captured with an exposure time of 300 to 500 ms. Transmission was assessed by feeding 10 ticks infected with B. turicatae-gfp or the parental strain per mouse, and five animals were used per study. Mice were sampled for 10 consecutive days for qPCR, as previously described (7, 27). Following transmission, ticks were kept in separate vials based on the animal upon which they fed, and transmission was reevaluated on naive mice at the fifth nymphal instar and adult stage.
Reisolation of B. turicatae-gfp from mice.
gfp recombination into other B. turicatae plasmids was evaluated by reisolating B. turicatae-gfp from murine blood after tick transmission. O. turicata-KS ticks, which were infected for over 18 months, were used. Upon visualization of spirochetes in the blood, animals were exsanguinated, and 50 to 100 μl of blood was used to inoculate mBSK medium. Once spirochetes attained stationary growth, they were inoculated into a 50-ml culture of mBSK medium. Genomic DNA from B. turicatae-gfp was isolated from the cultures and Southern blotting performed, as stated above.
Assessment of B. turicatae-gfp in ticks.
Tick dissections involved excising midguts using an Axio Stemi (Zeiss) dissecting microscope and placing the tissues in 5 to 10 μl of mBSK medium on a clean microscope slide. A coverslip was gently placed on the midguts, and 50 microscopic fields were scanned for B. turicatae-gfp. Similarly, salivary glands were excised from the same cohort of ticks, rinsed with 1× PBS-MgCl2, and transferred to a clean slide containing mBSK medium, and a coverslip was placed over the tissues. Midguts and salivary glands were evaluated by microscopy for B. turicatae-gfp colonization within 5 min after dissection. Imaging B. turicatae-gfp was accomplished with an Axio Imager A2 (Zeiss) fluorescence microscope, and images were analyzed with the ZEN 2012 digital imaging software (Zeiss).
qPCR of murine blood and detection of B. turicatae-gfp DNA in ticks.
Quantification of B. turicatae-gfp and the parental strain in murine blood was performed as previously described (7). Blood samples in lysis buffer were thawed and diluted 1:10 in nuclease-free water. qPCR was performed using a primer and probe set for the flagellin gene (7) and the Brilliant II QPCR master mix (Agilent Technologies). Assays were run on the Applied Biosystems ViiA7 real-time PCR system, as previously described (7).
To detect B. turicatae-gfp in O. turicata salivary glands, infected ticks were dissected. The cuticle was cut longitudinally, peeled back, and the midgut exposed. Intact salivary glands were excised, rinsed with 1× PBS, and frozen at −80°C in 50 μl of 1× PBS. DNA was extracted using the DNeasy blood and tissue kit (Qiagen), according to the manufacturer's instructions. PCR for gfp was performed as previously described (23), using primers 5′-GCGACTAGTAAAGATTAACTTTATAAGGAGGAAAAACATATGAGTAAAGGAGAAGAACTTTTCACTGG-3′ and 5′-GCGCGCCGGTGTTATTTGTATAGTTCATCCATGCCATGTGTAATCC-3′.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sandy Stewart, Julie Lopez, and Brittany Armstrong for critical review of the manuscript.
This research was supported by 1P20GM103646 (to J.A.T.), the U.S. Defense Threat Reduction Agency [Cooperative Biological Engagement Program Agreement IAA# U.S.C. 3318(b)-15217 with the USDA-ARS] (to J.E.L. and A.A.P.D.L.), and AI103724 and AI091652 (to J.E.L.).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.02503-16.
REFERENCES
- 1.Balashov YS. 1972. Bloodsucking ticks (Ixodoidea)-vectors of diseases of man and animals. Entomol Soc Am Misc Publ 8:161–376. [Google Scholar]
- 2.Francis E. 1938. Longevity of the tick Ornithodoros turicata and of Spirochaeta recurrentis with this tick. Public Health Rep 53:2220–2241. doi: 10.2307/4582740. [DOI] [Google Scholar]
- 3.Sonenshine DE, Roe RM. 2014. Biology of ticks, 2nd ed, vol 1 Oxford University Press, New York, NY. [Google Scholar]
- 4.Boyle WK, Wilder HK, Lawrence AM, Lopez JE. 2014. Transmission dynamics of Borrelia turicatae from the arthropod vector. PLoS Negl Trop Dis 8:e2767. doi: 10.1371/journal.pntd.0002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwan TG. 1996. Ticks and Borrelia: model systems for investigating pathogen-arthropod interactions. Infect Agents Dis 5:167–181. [PubMed] [Google Scholar]
- 6.Schwan TG, Hinnebusch BJ. 1998. Bloodstream- versus tick-associated variants of a relapsing fever bacterium. Science 280:1938–1940. doi: 10.1126/science.280.5371.1938. [DOI] [PubMed] [Google Scholar]
- 7.Lopez JE, Wilder HK, Hargrove R, Brooks CP, Peterson KE, Beare PA, Sturdevant DE, Nagarajan V, Raffel SJ, Schwan TG. 2013. Development of genetic system to inactivate a Borrelia turicatae surface protein selectively produced within the salivary glands of the arthropod vector. PLoS Negl Trop Dis 7:e2514. doi: 10.1371/journal.pntd.0002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis GE. 1941. Ornithodoros turicata: the male; feeding and copulation habits, fertility, span of life, and the transmission of relapsing fever spirochetes. Public Health Rep 56:1799–1802. doi: 10.2307/4583854. [DOI] [Google Scholar]
- 9.Varma MGR. 1962. Transmission of relapsing fever spirochetes by ticks. The Zoological Society of London, Regent's Park, London, United Kingdom. [Google Scholar]
- 10.Lopez JE, Schrumpf ME, Raffel SJ, Policastro PF, Porcella SF, Schwan TG. 2008. Relapsing fever spirochetes retain infectivity after prolonged in vitro cultivation. Vector Borne Zoonotic Dis 8:813–820. doi: 10.1089/vbz.2008.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fine LM, Earnhart CG, Marconi RT. 2011. Genetic transformation of the relapsing fever spirochete Borrelia hermsii: stable integration and expression of green fluorescent protein from linear plasmid 200. J Bacteriol 193:3241–3245. doi: 10.1128/JB.05037-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piesman J. 1993. Dynamics of Borrelia burgdorferi transmission by nymphal Ixodes dammini ticks. J Infect Dis 167:1082–1085. doi: 10.1093/infdis/167.5.1082. [DOI] [PubMed] [Google Scholar]
- 13.Piesman J. 1995. Dispersal of the Lyme disease spirochete Borrelia burgdorferi to salivary glands of feeding nymphal Ixodes scapularis (Acari: Ixodidae). J Med Entomol 32:519–521. doi: 10.1093/jmedent/32.4.519. [DOI] [PubMed] [Google Scholar]
- 14.Piesman J, Mather TN, Sinsky RJ, Spielman A. 1987. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol 25:557–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis GE, Walker ME. 1940. Ornithodoros hermsi: feeding and molting habits in relation to the acquisition and transmission of relapsing fever spirochetes. Public Health Rep 55:492–504. doi: 10.2307/4583218. [DOI] [Google Scholar]
- 16.Davis GE. 1942. Tick vectors and life cycles of ticks. Am Assoc Adv Sci 18:67–76. [Google Scholar]
- 17.Davis GE, Mazzotti L. 1953. The non-transmission of the relapsing fever spirochete, Borrelia dugesii (Mazzotti, 1949) by the argasid tick Ornithodoros turicata (Dugès, 1876). J Parasitol 39:663–666. doi: 10.2307/3274086. [DOI] [PubMed] [Google Scholar]
- 18.Battisti JM, Raffel SJ, Schwan TG. 2008. A system for site-specific genetic manipulation of the relapsing fever spirochete Borrelia hermsii. Methods Mol Biol 431:69–84. [DOI] [PubMed] [Google Scholar]
- 19.Raffel SJ, Battisti JM, Fischer RJ, Schwan TG. 2014. Inactivation of genes for antigenic variation in the relapsing fever spirochete Borrelia hermsii reduces infectivity in mice and transmission by ticks. PLoS Pathog 10:e1004056. doi: 10.1371/journal.ppat.1004056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwan TG, Raffel SJ, Schrumpf ME, Policastro PF, Rawlings JA, Lane RS, Breitschwerdt EB, Porcella SF. 2005. Phylogenetic analysis of the spirochetes Borrelia parkeri and Borrelia turicatae and the potential for tick-borne relapsing fever in Florida. J Clin Microbiol 43:3851–3859. doi: 10.1128/JCM.43.8.3851-3859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Granok AB, Parsonage D, Ross RP, Caparon MG. 2000. The RofA binding site in Streptococcus pyogenes is utilized in multiple transcriptional pathways. J Bacteriol 182:1529–1540. doi: 10.1128/JB.182.6.1529-1540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbour AG. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med 57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez JE, Schrumpf ME, Nagarajan V, Raffel SJ, McCoy BN, Schwan TG. 2010. A novel surface antigen of relapsing fever spirochetes can discriminate between relapsing fever and Lyme borreliosis. Clin Vaccine Immunol 17:564–571. doi: 10.1128/CVI.00518-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simpson WJ, Garon CF, Schwan TG. 1990. Analysis of supercoiled circular plasmids in infectious and non-infectious Borrelia burgdorferi. Microb Pathog 8:109–118. doi: 10.1016/0882-4010(90)90075-2. [DOI] [PubMed] [Google Scholar]
- 25.Donaldson TG, Perez de Leon AA, Li AI, Castro-Arellano I, Wozniak E, Boyle WK, Hargrove R, Wilder HK, Kim HJ, Teel PD, Lopez JE. 2016. Assessment of the geographic distribution of Ornithodoros turicata (Argasidae): climate variation and host diversity. PLoS Negl Trop Dis 10:e0004383. doi: 10.1371/journal.pntd.0004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winston PW, Bates DH. 1960. Saturated solutions for the control of humidity in biological research. Ecology 41:232–237. doi: 10.2307/1931961. [DOI] [Google Scholar]
- 27.McCoy BN, Raffel SJ, Lopez JE, Schwan TG. 2010. Bloodmeal size and spirochete acquisition of Ornithodoros hermsi (Acari: Argasidae) during feeding. J Med Entomol 47:1164–1172. doi: 10.1603/ME10175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.