Abstract
In recent years, there have been significant advances in our understanding of the functions of mitochondrial complex I other than the generation of energy. These include its role in generation of reactive oxygen species, involvement in the hypoxic tissue response and its possible regulation by nitric oxide (NO) metabolites. In this review, we will focus on the hypoxic conformational change of this mitochondrial enzyme, the so-called active/deactive transition. This conformational change is physiological and relevant to the understanding of certain pathological conditions including, in the cardiovascular system, ischaemia/reperfusion (I/R) damage. We will discuss how complex I can be affected by NO metabolites and will outline some potential mitochondria-targeted therapies in I/R damage.
Keywords: modulation of mitochondrial complex I, therapeutic intervention, ischaemia/reperfusion, nitric oxide, ROS
1. Mitochondrial complex I
Mitochondria have a well-recognized role in cellular energy production and in the generation of biosynthetic intermediates required for cell replication. Oxidative phosphorylation or the generation of energy by the aerobic catabolism of carbohydrates, fatty acids and proteins includes the transfer of electrons from metabolites to NAD+ to form NADH, the universal carrier of reducing equivalents. The only enzyme of the mitochondrial respiratory chain which catalyses the oxidation of matrix NADH by the respiratory chain is complex I or NADH:ubiquinone oxidoreductase. This is the major entry point for electrons into the respiratory chain. The enzyme has an L-shape and consists of two domains: hydrophilic, protruding into the matrix and hydrophobic, embedded in the membrane. All known redox centres of this enzyme (FMN and eight FeS clusters) are located within core subunits in the hydrophilic domain of the enzyme [1]. Electron transfer is linearly organized from the flavin as a first redox centre via FeS clusters towards a pocket for ubiquinone. Release of redox energy at the last step at terminal cluster N2 results in the formation of ubiquinol and most likely drives long-range conformational changes within the membrane part of the enzyme where proton translocation (4H+ per NADH) takes place [2–4]. This process is expected to be carried out by several Na+/H+ antiporter-like subunits localized in the membrane domain. Unlike the 14 core subunits of prokaryotic enzyme, mitochondrial complex I also contains around 30 additional accessory subunits, many with still unknown functions.
The catalytic properties of eukaryotic complex I are versatile (see [5] for a review). First of all, the NADH:ubiquinone reductase reaction catalysed by the enzyme is reversible. In vitro, during reverse electron transfer (RET), complex I can carry electrons upstream from ubiquinol for NAD+ reduction at the expense of proton-motive force [5]. In addition, under physiological conditions, complex I can catalyse the formation of reactive oxygen species (ROS) such as superoxide and hydrogen peroxide [6–9] and at the same time can be a target of ROS [10,11]. Several accessory subunits of complex I also carry out some additional activities such as rhodanese [12], acyl-carrier proteins [13], thioredoxin [14] and are also involved in cellular signalling in apoptotic and inflammation pathways [15].
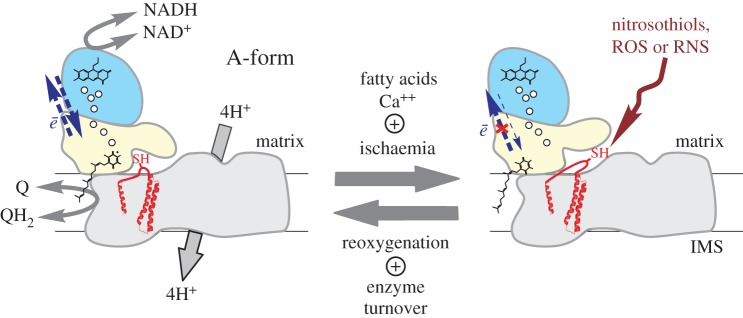
The intriguing property of this mitochondrial enzyme from mammals is the active/dormant (A/D) transition [16–19]. In mammals, the A-form operates at physiological temperatures if substrates (NADH and ubiquinone) are present catalysing the redox reaction at a high rate of 104 per minute. If the activity of the enzyme is hindered, i.e. when oxygen is lacking or in metabolic hypoxia [20], complex I spontaneously converts into the deactive, dormant D-form. This form can potentially undergo re-activation given both substrates' availability. As a result of slow (several times per minute) catalytic turnover the D-form is converted back to the A-form [16,21]. Based on the different sensitivities of the A- and D-forms to SH-reagents, Galkin et al. identified Cys-39 of the mitochondrially encoded ND3 subunit as the residue exposed only in the D-form of the enzyme (figure 1) [25,26]. The Cys-39-containing hydrophilic loop between transmembrane domains 1 and 2 of the ND3 was predicted to be facing the mitochondrial matrix [25]. This was later confirmed by the X-ray structure of the bacterial and mitochondrial enzyme [22,27]. However, the exact location of the loop was not very well defined in the initially published structures [22,28], probably indicating its high flexibility. At least four human pathogenic mutations in the vicinity of Cys-39 were identified, indicating the importance of this region for complex I function [29–31] (figure 1). Covalent modification of critical Cys-39 in the D-form is most likely the cause of arrest of activation even in the presence of substrates.
Figure 1.
The A/D transition of mitochondrial complex I. The active form of the enzyme catalyses both forward and RET. Ischaemia drives deactivation of the enzyme which can be greatly accelerated by free fatty acids and calcium. The D-form is unable to catalyse RET but reactivation (D → A) occurs after reperfusion, when the enzyme is in slow turnover. Blue, yellow and grey domains stand for NADH-dehydrogenase, quinone reduction and proton translocation modules, respectively. Relative location of the hydrophilic loop of ND3 subunit (red) based on X-ray structure of Yarrowia lipolytica [22] and mammalian enzyme [23,24] (PDB ID: 4WZ7, 5LNK and 5LDW, respectively). Note that critical Cys-39 of the ND3 subunit is exposed only in the D-form and can be specifically modified by nitrosothiols, ROS and RNS. Small spheres indicate the position of FeS clusters, the positions of FMN and ubiquinone are assigned by respective schematic structural formulae.
The exact molecular mechanism of the A/D transition is unknown. The current consensus view is that the change in position of the flexible loop ND3 is the main event occurring during the A/D transition. This hydrophilic ND3 loop is located between the Q and P modules of the enzyme (figure 1) close to the N-terminus of the core 49-kDa subunit [23,24]. The lack of catalytic activity of the D-form can be attributed to the disruption of the quinone binding site [23,24] brought on by the A to D conformational change. Most likely, a conformational change resulting in the exposure of critical Cys-39 of ND3 involves not only this loop, but also the components of other subunits in the vicinity [32]. Thus, intermembrane helices of ND1 subunits are confined between helixes of ND3, so that the cysteine-containing hydrophilic loop is positioned above ND1. In addition, the long N-terminal β-sheet of the core 49 kDa subunit is probably in close contact with parts of the ND3 loop [33]. Only the mitochondrial enzyme exhibits the A/D conformational change. Therefore, it seems likely that this transition involves at least one of the accessory subunits, e.g. 39 kDa (NDUFA9) [32], B14 (NDUFA6) and SDAP-α (NDUFAB1) [34] or B13 (NDUFA5) [35] which are located in close proximity to the hydrophilic loop of ND3 [23,24,28]. At least two different conformational states of complex I were observed in recent cryo-electron microscopy studies [23,24]. This information might help to further characterize the structural differences between the A- and D-forms and to better understand the link between A/D transition and the catalytic cycle of the enzyme.
The physiological role of the A/D transition is still under discussion. Most likely, the reversible A/D transition is an adaptive mechanism of metabolic response to variation of oxygen supply. As shown previously, no complex I was found in the D-from in cultured cells at ambient oxygen conditions [36]. However, in highly metabolizing tissues at physiological oxygen concentrations [17,19,37], a significant fraction of the enzyme (5–15%) was present in the D-form. It can be concluded that in situ, part of the energy released during steady-state NADH oxidation is used to maintain the catalytically competent A-form [4]. Energy-dependent maintenance of the fraction of the enzyme in the D-form would permit a fast response to changes in conditions such as oxygen availability and ATP demand resembling the so-called excess capacity of cytochrome c oxidase [38,39]. Therefore, the A/D transition could be one of the mechanisms for the fine-tuning of oxidative phosphorylation activity in different tissues.
At low oxygen pressure, when the respiration rate is decreased, all components of the respiratory chain are over-reduced due to the lack of terminal electron acceptors and low activity of cytochrome c oxidase. The ratio of membrane ubiquinone : ubiquinol is very low and complex I activity dramatically drops because of the lack of substrate. Therefore, the steady-state equilibrium in the A ↔ D reaction would be shifted to the right and the enzyme would be readily converted into the D-form within minutes in tissues such as the brain and heart [17,19,37]. The time course of complex I deactivation after cardiac arrest reveals a much faster deactivation in brain compared with heart tissue [37] and may explain the greater vulnerability of brain function to oxygen deprivation. The rate of reactivation is strongly decreased by calcium [21] and fatty acids [40]. Free fatty acid content of the mitochondrial membrane increases several fold in acute cerebral or cardiac ischaemia [41–43]. In ischaemic cardiac and cerebral tissue, deactivation of complex I [36,37] concurs with accumulation of free fatty acids, [41–43] as well as various acyl-carnitine and acyl-CoA esters [44] making these metabolites plausible endogenous effectors of A/D transition in situ. Tissue reoxygenation results in the return of the enzyme A/D equilibrium to its basal pre-hypoxic level, indicating that restoration of oxygen reactivates the D-form of the enzyme and converts it to the A-form [17,19,37].
Deactivation of complex I has physiological significance in a process of ischaemia/reperfusion (I/R) injury. The molecular details of this mechanism are explained below. Complex I has been recognized for a long time as one of the major sources of ROS production by the mitochondrial respiratory chain—mostly superoxide (O2−) and hydrogen peroxide (H2O2) [7–9,45–51]. It has been recognized that in vitro ROS production is significantly higher when complex I is in conditions when electron transfer is reversed by the proton-motive force from ubiquinol upstream towards enzyme FMN for NAD+ reduction in [8,9,47,48,52–58]. It should be stressed that there is no evidence of RET occurrence in mammalian tissue physiologically because succinate is generated stoichiometrically by NAD-dependent dehydrogenases in the Krebs cycle and the net electron flux through complex I is always forward. At the same time, high levels of succinate can accumulate under ischaemia/hypoxia in various tissues [59–62]. After reintroduction of oxygen, complex I can be contained in a ‘RET-like’ state since succinate is used to keep the quinone pool in the reduced state and potential across the membrane is present. In these conditions, complex I could potentially generate superoxide at a considerable rate. As shown in pioneering work of Vinogradov's lab, the D-form of the enzyme is unable to catalyse RET [16]. In the D-form, transfer of electrons from ubiquinol upstream is interrupted. Therefore, all redox centres of complex I including flavin and FeS clusters cannot be reduced. This fully blocks ROS production from the enzyme in conditions when RET is expected, i.e. at the early phase of reperfusion. It follows that, after ischaemia, mitochondria containing the D-form of complex I would have reduced capacity to generate ROS during reoxygenation.
This mechanistic model readily explains the experimental observations on I/R. Increased ROS production by mitochondria is associated with detrimental consequences of I/R injury [63–65] and decreased ROS production and oxidative stress protects tissues during reperfusion [11,64,66,67]. At the same time, transient inhibition of complex I during post-ischaemic reperfusion would protect mitochondria and decrease I/R damage in various highly metabolizing tissues [61,68–72]. Thus, the deactivation of complex I can be considered an intrinsic switch preventing the sharp burst of respiration associated with ROS generation during the initial phase of post-ischaemic reperfusion. This mechanism offers a unified and verifiable explanation to the complex changes in cell oxidative metabolism caused by I/R. The process of deactivation of complex I in ischaemia provides a novel potential target for pathology-activated pharmacological intervention.
2. Complex I and nitric oxide metabolites
Nitric oxide (NO) is a gaseous endogenous mediator playing an important role in signal transduction in the cardiovascular and immune systems, as well as in carcinogenesis [20,73–76]. NO structure resembles that of an oxygen molecule, therefore, the interaction of NO with cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, was described long before the biological significance of NO through activation of soluble guanylate cyclase was recognized [20,77]. NO is a competitive inhibitor of the enzyme with respect to oxygen, but unlike the latter, NO binds to both the reduced and the oxidized forms of the cytochrome c oxidase [78]. It was shown that signalling consequences in hypoxia may be profoundly modified by NO via inhibition of this enzyme [79]. Upon NO binding to cytochrome c oxidase, available oxygen is redistributed towards non-respiratory oxygen-dependent targets such as prolyl hydroxylases so that they degrade HIF-1α and therefore cells do not register hypoxia [79].
It should be stressed that since NO is a competitive inhibitor, lowering the apparent affinity of cytochrome c oxidase to oxygen [20,80], it would strongly synergize with hypoxia and induce deactivation of complex I as explained previously. It can be expected that in the presence of physiological concentrations of NO, complex I can undergo deactivation even in mild hypoxia. More generally, inhibition of energy transfer downstream of complex I in situ (endogenous inhibition of respiration by carbon monoxide [78] or fatty acids [81,82] as well as release of cytochrome c [83]) also would shift the A/D ratio of the enzyme towards accumulation of the D-form. In addition, NO is a precursor of several so-called NO metabolites such as peroxynitrite (ONOO−) and low molecular weight nitrosothiols, e.g. S-nitrosoglutathione [84,85]. The latter could be involved in transnitrosation/oxidation reactions of protein-free thiols providing a cGMP-independent NO signalling pathway for regulation of enzymes structure and activity.
More than 30 years ago, inhibition of complex I-mediated respiration was demonstrated in cultured cells after incubation with activated macrophages [86] which later was identified to be due to NO [87]. Subsequent studies identified ONOO− as a compound responsible for the NO-dependent inactivation of several components of the respiratory chain [88–90]. Moncada's group first found that prolonged exposure of cells to high concentrations of NO led to persistent inhibition of complex I which was attributed to nitrosation of protein thiol residue(s) [91–93], a finding that has been confirmed by other groups [94–98]. Although the degree of inhibition of complex I caused by various NO donors has been extensively investigated, the precise mechanism and nature of the targeted subunit(s) as well as the possibility of nitrosation of complex I in vivo have not been established.
It was shown long ago [99] that deactivation increases susceptibility of complex I to covalent modifications by SH-reagents. This was later found to be due to the conformational change resulting in exposure of Cys-39 of ND3 subunit upon enzyme deactivation (A to D transition) [25]. We found that sensitivity of complex I to S-nitrosothiols and peroxynitrite is governed by the A/D status of the enzyme in vitro [36,100]. NO-metabolites can react with the critical Cys-39 subunit in the D-form and block enzyme activation. The activity of the S-nitrosated enzyme could be restored by thiol-reducing agents [36,100], while treatment by peroxynitrite or ROS was found to be irreversible [19,36,100]. Cys-39 of the ND3 subunit of complex I in the D-form was found to be a target for ROS or RNS, so inhibition of the enzyme in mouse heart mitochondria was a result of this thiol oxidation [19]. In a further elegant study from Murphy's laboratory, Cys-39 was identified as a critical residue nitrosated by mitochondrially targeted nitrosothiol MitoSNO [71]. This novel compound is composed of two structural units: one provides hydrophobicity and positive charge ensuring membrane permeability and accumulation in mitochondria in which potential across the membrane is present (negative inside). Another unit is S-nitroso-N-acetylpenicillamine, a low molecular weight nitrosothiol able to donate an NO-moiety to protein thiols. Administration of MitoSNO before reperfusion in a mouse model of cardiac infarction inhibited complex I and significantly decreased the volume of cardiac infarction most probably via lessening oxidative damage [71,101]. The ischaemic state of the tissue is characterized by accumulation of significant amounts of succinate [59–62,102] so that upon reperfusion complex II can potentially oxidize the accumulated succinate, which drives RET through mitochondrial complex I [62]. These RET-like conditions upon reperfusion could lead to increased ROS generation from the enzyme flavin. Nitrosation of critical Cys-39 of ND3 delays activation of the enzyme during early reperfusion prevents electron transfer to flavin and therefore decreases production of ROS. This thiol group can be subsequently recovered via denitrosating by the thiol-reducing system in the mitochondrial matrix, therefore providing reversibility of complex I inhibition [71,103–105]. The exact time frame of such protective nitrosation in vivo is not currently known, but it is likely to occur within minutes [71].
Several points should be considered when discussing the mechanism above:
1. After an ischaemic episode all of the redox components of the respiratory chain including quinone and pyridine nucleotides (NAD and NADP) are in the reduced state. The process of RET, as defined originally [106], cannot proceed in the absence of oxygen since there is no NAD+ to accept electrons from complex I, therefore there is no actual ‘transfer’ of electrons from quinol upstream. However, over-reduction of complex I flavin with partial leakage of electrons to oxygen rather than true reduction of NAD+ сan take place when the potential across the membrane is present and the quinone : quinol ratio is very low.
2. Ischaemic accumulation of succinate in tissues found in several studies on rodents [59–62,71,102] may not be present in human tissues [107].
3. Superoxide production of complex I is strongly inhibited by NADH or NADPH in the micromolar range [6,48], whereas the concentration of these nucleotides in the mitochondrial matrix is millimolar [108]. In these conditions, the capacity of complex I to generate ROS is low. Thus, the possibility of other enzymatic systems upstream of complex I involved in ROS generation in the RET-like condition is very likely. For example, a role of ketoglutarate dehydrogenase should be further reinforced, since this enzyme is linked to the NADH/NAD+ pool together with complex I [109–112].
4. Reversible nitrosation of Cys-39 in the D-form may not only block ROS generation from complex I, but also protect this critical thiol from irreversible oxidation by ROS or peroxynitrite known to form during the early phase of I/R [113,114].
3. Modulation of A/D as an example of pathologically activated therapeutics
Identified conformational differences between the A- and D-forms offer an opportunity for the development of the so-called pathologically activated therapeutics for ischaemia-associated pathologies. For example, the Cys-39 residue of the ND3 subunit is only exposed in the D-form, which is accumulated in the ischaemic area of the tissue. Thus, any treatment aimed towards this critical thiol would influence the enzyme only in the affected area. The enzyme in normoxic tissue would not be affected by pharmacological agents targeting only the D-form. Taking into account that there are other subunits, such as 39 kDa, involved in deactivation [32], several pharmacological approaches can be used to affect the D-form only.
On the other hand, compounds that modulate the A/D transition may be promising for widening the window for pharmacological intervention in ischaemic conditions, such as stroke, heart infarction and organ transplantation. Compounds with the ability to shift the A/D ratio towards the A- or the D-form may be very useful tools.
It has been shown that metformin, the common biguanide-type drug for the treatment of type II diabetes, directly inhibits complex I [115–117]. Deactivation greatly enhances the sensitivity of the enzyme to metformin [118]. The rate of inhibition of the enzyme was significantly higher when metformin was pre-incubated with the D-form (resting enzyme), than with the A-form [117]. It was suggested that deactivation of the enzyme may facilitate the binding of metformin to complex I in the region of the critical ND3 loop [117]. Apparent suppression of complex I activity observed in several studies [117,118] can be explained by specific effects of biguanides on the rate of activation rather than classical inhibition of the D-form. When activation is decelerated, the rate of NADH oxidation corresponds to the (small) fraction of the A-form that is present during the initial phase of the assay. Similar to free fatty acids or divalent cations, biguanides may decrease the rate of activation. In the conventionally used kinetic assays, this could be manifested as apparent inhibition of the activity [109]. Thus, metformin and similar compounds act on stabilization of the D-form and this effect may contribute to their therapeutic effects.
Observations made in our laboratory and other laboratories suggest that development of pharmaceutical agents targeted towards Cys-39 of mitochondrial complex I may provide a pathologically activated therapy for various cardiovascular disorders associated with ischaemia aiming to prevent I/R injury.
4. Conclusion and perspectives
Impressive progress has been achieved in recent years in our understanding of the structure of mitochondrial complex I [22–24,28]. The surprising success of potential complex I inhibitors in protection against I/R injury [61,68–72] and Alzheimer's disease [119] requires additional studies for understanding the molecular details underlying their therapeutic effects. Studies of the regulation of complex I remain an important area for current translational medicine [71,119–124].
The A/D transition of complex I can be considered an attractive target for modulation of complex I activity; however, it is still not clear what the driving force is for A/D transition and what subunits are involved in the conformational change. We think that the identification of pharmaceutical compounds targeting the rate of activation or deactivation, and modulating the A/D state of the enzyme in situ is an attractive area of research. Further characterization of the A/D transition may provide a better understanding of the regulation of the mitochondrial response to oxygen deprivation and oxidative stress. It may help to develop novel therapeutic compounds for cardiovascular conditions, such as cardiac infarction, stroke and other ischaemia-associated pathologies.
Acknowledgements
The authors also thank Anna Bunin and Suzanne Burstein for help in preparation of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
Work performed in the authors' laboratories was supported by MRC UK grant nos. G1100051 and MR/L007339/1 (to A.G.).
References
- 1.Sazanov LA, Hinchliffe P. 2006. Structure of the hydrophilic domain of respiratory complex I from Thermus thermophilus. Science 311, 1430–1436. ( 10.1126/science.1123809) [DOI] [PubMed] [Google Scholar]
- 2.Galkin AS, Grivennikova VG, Vinogradov AD. 1999. H+/2e− stoichiometry in NADH-quinone reductase reactions catalyzed by bovine heart submitochondrial particles. FEBS Lett. 451, 157–161. ( 10.1016/S0014-5793(99)00575-X) [DOI] [PubMed] [Google Scholar]
- 3.Galkin A, Dröse S, Brandt U. 2006. The proton pumping stoichiometry of purified mitochondrial complex I reconstituted into proteoliposomes. Biochim. Biophys. Acta 1757, 1575–1581. ( 10.1016/j.bbabio.2006.10.001) [DOI] [PubMed] [Google Scholar]
- 4.Ripple MO, Kim N, Springett R. 2013. Mammalian complex I pumps 4 protons per 2 electrons at high and physiological proton motive force in living cells. J. Biol. Chem. 288, 5374–5380. ( 10.1074/jbc.M112.438945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinogradov AD. 1998. Catalytic properties of the mitochondrial NADH-ubiquinone oxidoreductase (complex I) and the pseudo-reversible active/inactive enzyme transition. Biochim. Biophys. Acta 1364, 169–185. ( 10.1016/S0005-2728(98)00026-7) [DOI] [PubMed] [Google Scholar]
- 6.Grivennikova VG, Vinogradov AD. 2013. Partitioning of superoxide and hydrogen peroxide production by mitochondrial respiratory complex I. Biochim. Biophys. Acta 1827, 446–454. ( 10.1016/j.bbabio.2013.01.002) [DOI] [PubMed] [Google Scholar]
- 7.Hinkle PC, Butow RA, Racker E, Chance B. 1967. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XV. Reverse electron transfer in the flavin-cytochrome beta region of the respiratory chain of beef heart submitochondrial particles. J. Biol. Chem. 242, 5169–5173. [PubMed] [Google Scholar]
- 8.Kussmaul L, Hirst J. 2006. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc. Natl Acad. Sci. USA 103, 7607–7612. ( 10.1073/pnas.0510977103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galkin A, Brandt U. 2005. Superoxide radical formation by pure complex I (NADH:ubiquinone oxidoreductase) from Yarrowia lipolytica. J. Biol. Chem. 280, 30 129–30 135. ( 10.1074/jbc.M504709200) [DOI] [PubMed] [Google Scholar]
- 10.Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS. 2004. Characterization of superoxide-producing sites in isolated brain mitochondria. J. Biol. Chem. 279, 4127–4135. ( 10.1074/jbc.M310341200) [DOI] [PubMed] [Google Scholar]
- 11.Dröse S, Brandt U, Wittig I. 2014. Mitochondrial respiratory chain complexes as sources and targets of thiol-based redox-regulation. Biochim. Biophys. Acta 1844, 1344–1354. ( 10.1016/j.bbapap.2014.02.006) [DOI] [PubMed] [Google Scholar]
- 12.Abdrakhmanova A, Dobrynin K, Zwicker K, Kerscher S, Brandt U. 2005. Functional sulfurtransferase is associated with mitochondrial complex I from Yarrowia lipolytica, but is not required for assembly of its iron-sulfur clusters. FEBS Lett. 579, 6781–6785. ( 10.1016/j.febslet.2005.11.008) [DOI] [PubMed] [Google Scholar]
- 13.Dobrynin K, Abdrakhmanova A, Richers S, Hunte C, Kerscher S, Brandt U. 2010. Characterization of two different acyl carrier proteins in complex I from Yarrowia lipolytica. Biochim. Biophys. Acta 1797, 152–159. ( 10.1016/j.bbabio.2009.09.007) [DOI] [PubMed] [Google Scholar]
- 14.Brockmann C, Diehl A, Rehbein K, Strauss H, Korn B, Kuhne R, Oschkinat H. 2004. The oxidized subunit B8 from human complex I adopts a thioredoxin fold. Structure 12, 1645–1654. ( 10.1016/j.str.2004.06.021) [DOI] [PubMed] [Google Scholar]
- 15.Fearnley IM, Carroll J, Shannon RJ, Runswick MJ, Walker JE, Hirst J. 2001. Grim-19, a cell death regulatory gene product, is a subunit of bovine mitochondrial NADH:ubiquinone oxidoreductase (complex I). J. Biol. Chem. 276, 38 345–38 348. ( 10.1074/jbc.C100444200) [DOI] [PubMed] [Google Scholar]
- 16.Kotlyar AB, Vinogradov AD. 1990. Slow active/inactive transition of the mitochondrial NADH-ubiquinone reductase. Biochim. Biophys. Acta 1019, 151–158. ( 10.1016/0005-2728(90)90137-S) [DOI] [PubMed] [Google Scholar]
- 17.Maklashina E, Kotlyar AB, Karliner JS, Cecchini G. 2004. Effect of oxygen on activation state of complex I and lack of oxaloacetate inhibition of complex II in Langendorff perfused rat heart. FEBS Lett. 556, 64–68. ( 10.1016/S0014-5793(03)01369-3) [DOI] [PubMed] [Google Scholar]
- 18.Babot M, Birch A, Labarbuta P, Galkin A. 2014. Characterisation of the active/de-active transition of mitochondrial complex I. Biochim. Biophys. Acta 1837, 1083–1092. ( 10.1016/j.bbabio.2014.02.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorenkova N, Robinson E, Grieve D, Galkin A. 2013. Conformational change of mitochondrial complex I increases ROS sensitivity during ischaemia. Antioxid. Redox. Signal. 19, 1459–1468. ( 10.1089/ars.2012.4698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moncada S, Erusalimsky JD. 2002. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat. Rev. Mol. Cell Biol. 3, 214–220. ( 10.1038/nrm762) [DOI] [PubMed] [Google Scholar]
- 21.Kotlyar AB, Sled VD, Vinogradov AD. 1992. Effect of Ca2+ ions on the slow active/inactive transition of the mitochondrial NADH-ubiquinone reductase. Biochim. Biophys. Acta 1098, 144–150. ( 10.1016/S0005-2728(05)80329-9) [DOI] [PubMed] [Google Scholar]
- 22.Zickermann V, Wirth C, Nasiri H, Siegmund K, Schwalbe H, Hunte C, Brandt U. 2015. Mechanistic insight from the crystal structure of mitochondrial complex I. Science 347, 44–49. ( 10.1126/science.1259859) [DOI] [PubMed] [Google Scholar]
- 23.Fiedorczuk K, Letts JA, Degliesposti G, Kaszuba K, Skehel M, Sazanov LA. 2016. Atomic structure of the entire mammalian mitochondrial complex I. Nature 537, 644–648. ( 10.1038/nature19774) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu J, Vinothkumar KR, Hirst J. 2016. Structure of mammalian respiratory complex I. Nature 536, 354–358. ( 10.1038/nature19095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galkin A, Meyer B, Wittig I, Karas M, Schägger H, Vinogradov A, Brandt U. 2008. Identification of the mitochondrial ND3 subunit as a structural component involved in the active/deactive enzyme transition of respiratory complex I. J. Biol. Chem. 283, 20 907–20 913. ( 10.1074/jbc.M803190200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gavrikova EV, Vinogradov AD. 1999. Active/de-active state transition of the mitochondrial complex I as revealed by specific sulfhydryl group labeling. FEBS Lett. 455, 36–40. ( 10.1016/S0014-5793(99)00850-9) [DOI] [PubMed] [Google Scholar]
- 27.Efremov RG, Sazanov LA. 2011. Structure of the membrane domain of respiratory complex I. Nature 476, 414–420. ( 10.1038/nature10330) [DOI] [PubMed] [Google Scholar]
- 28.Vinothkumar KR, Zhu J, Hirst J. 2014. Architecture of mammalian respiratory complex I. Nature 515, 80–84. ( 10.1038/nature13686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Werner KG, et al. 2009. Rolandic mitochondrial encephalomyelopathy and MT-ND3 mutations. Pediatr. Neurol. 41, 27–33. ( 10.1016/j.pediatrneurol.2009.02.010) [DOI] [PubMed] [Google Scholar]
- 30.Sarzi E, Brown MD, Lebon S, Chretien D, Munnich A, Rotig A, Procaccio V. 2007. A novel recurrent mitochondrial DNA mutation in ND3 gene is associated with isolated complex I deficiency causing Leigh syndrome and dystonia. Am. J. Med. Genet. A 143, 33–41. ( 10.1002/ajmg.a.31565) [DOI] [PubMed] [Google Scholar]
- 31.Nesbitt V, Morrison PJ, Crushell E, Donnelly DE, Alston CL, He L, McFarland R, Taylor RW. 2012. The clinical spectrum of the m.10191T>C mutation in complex I-deficient Leigh syndrome. Dev. Med. Child. Neurol. 54, 500–506. ( 10.1111/j.1469-8749.2012.04224.x) [DOI] [PubMed] [Google Scholar]
- 32.Babot M, Labarbuta P, Birch A, Kee S, Fuszard M, Botting CH, Wittig I, Heide H, Galkin A. 2014. ND3, ND1 and 39 kDa subunits are more exposed in the de-active form of bovine mitochondrial complex I. Biochim. Biophys. Acta 1837, 929–939. ( 10.1016/j.bbabio.2014.02.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wirth C, Brandt U, Hunte C, Zickermann V. 2016. Structure and function of mitochondrial complex I. Biochim. Biophys. Acta 1857, 902–914. ( 10.1016/j.bbabio.2016.02.013) [DOI] [PubMed] [Google Scholar]
- 34.Angerer H, Radermacher M, Mankowska M, Steger M, Zwicker K, Heide H, Wittig I, Brandt U, Zickermann V. 2014. The LYR protein subunit NB4M/NDUFA6 of mitochondrial complex I anchors an acyl carrier protein and is essential for catalytic activity. Proc. Natl Acad. Sci. USA 111, 5207–5212. ( 10.1073/pnas.1322438111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ushakova AV, Duarte M, Vinogradov AD, Videira A. 2005. The 29.9 kDa subunit of mitochondrial complex I is involved in the enzyme active/de-active transitions. J. Mol. Biol. 351, 327–333. ( 10.1016/j.jmb.2005.06.005) [DOI] [PubMed] [Google Scholar]
- 36.Galkin A, Abramov AY, Frakich N, Duchen MR, Moncada S. 2009. Lack of oxygen deactivates mitochondrial complex I: implications for ischemic injury? J. Biol. Chem. 284, 36 055–36 061. ( 10.1074/jbc.M109.054346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babot M, Galkin A. 2013. Molecular mechanism and physiological role of active–deactive transition of mitochondrial complex I. Biochem. Soc. Trans. 41, 1325–1330. ( 10.1042/BST20130088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kudin A, Vielhaber S, Elger CE, Kunz WS. 2002. Differences in flux control and reserve capacity of cytochrome c oxidase (COX) in human skeletal muscle and brain suggest different metabolic effects of mild COX deficiencies. Mol. Biol. Rep. 29, 89–92. ( 10.1023/A:1020374924550) [DOI] [PubMed] [Google Scholar]
- 39.Palacios-Callender M, Hollis V, Frakich N, Mateo J, Moncada S. 2007. Cytochrome c oxidase maintains mitochondrial respiration during partial inhibition by nitric oxide. J. Cell Sci. 120, 160–165. ( 10.1242/jcs.03308) [DOI] [PubMed] [Google Scholar]
- 40.Loskovich MV, Grivennikova VG, Cecchini G, Vinogradov AD. 2005. Inhibitory effect of palmitate on the mitochondrial NADH:ubiquinone oxidoreductase (complex I) as related to the active–de-active enzyme transition. Biochem. J. 387, 677–683. ( 10.1042/BJ20041703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun D, Gilboe DD. 1994. Ischemia-induced changes in cerebral mitochondrial free fatty acids, phospholipids, and respiration in the rat. J. Neurochem. 62, 1921–1928. ( 10.1046/j.1471-4159.1994.62051921.x) [DOI] [PubMed] [Google Scholar]
- 42.Jones RM, Bagchi M, Das DK. 1992. Preconditioning of heart by repeated stunning: adaptive modification of myocardial lipid membrane. Clin. Physiol. Biochem. 9, 41–46. ( 10.1007/bf00788663) [DOI] [PubMed] [Google Scholar]
- 43.Goto Y, Okamoto S, Yonekawa Y, Taki W, Kikuchi H, Handa H, Kito M. 1988. Degradation of phospholipid molecular species during experimental cerebral ischemia in rats. Stroke 19, 728–735. ( 10.1161/01.STR.19.6.728) [DOI] [PubMed] [Google Scholar]
- 44.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. 2010. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 90, 207–258. ( 10.1152/physrev.00015.2009) [DOI] [PubMed] [Google Scholar]
- 45.Boveris A, Cadenas E. 1975. Mitochondrial production of superoxide anions and its relationship to the antimycin insensitive respiration. FEBS Lett. 54, 311–314. ( 10.1016/0014-5793(75)80928-8) [DOI] [PubMed] [Google Scholar]
- 46.Takeshige K, Minakami S. 1979. NADH- and NADPH-dependent formation of superoxide anions by bovine heart submitochondrial particles and NADH-ubiquinone reductase preparation. Biochem. J. 180, 129–135. ( 10.1042/bj1800129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lambert AJ, Brand MD. 2004. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I). J. Biol. Chem. 279, 39 414–39 420. ( 10.1074/jbc.M406576200) [DOI] [PubMed] [Google Scholar]
- 48.Grivennikova VG, Vinogradov AD. 2006. Generation of superoxide by the mitochondrial complex I. Biochim. Biophys. Acta 1757, 553–561. ( 10.1016/j.bbabio.2006.03.013) [DOI] [PubMed] [Google Scholar]
- 49.Ohnishi ST, Shinzawa-Itoh K, Ohta K, Yoshikawa S, Ohnishi T. 2010. New insights into the superoxide generation sites in bovine heart NADH-ubiquinone oxidoreductase (complex I): the significance of protein-associated ubiquinone and the dynamic shifting of generation sites between semiflavin and semiquinone radicals. Biochim. Biophys. Acta 1797, 1901–1909. ( 10.1016/j.bbabio.2010.05.012) [DOI] [PubMed] [Google Scholar]
- 50.Dröse S, Brandt U. 2012. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv. Exp. Med. Biol. 748, 145–169. ( 10.1007/978-1-4614-3573-0_6) [DOI] [PubMed] [Google Scholar]
- 51.Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD. 2013. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox. Biol. 1, 304–312. ( 10.1016/j.redox.2013.04.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Votyakova TV, Reynolds IJ. 2001. ΔΨm-dependent and -independent production of reactive oxygen species by rat brain mitochondria. J. Neurochem. 79, 266–277. ( 10.1046/j.1471-4159.2001.00548.x) [DOI] [PubMed] [Google Scholar]
- 53.Lambert AJ, Brand MD. 2004. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem. J. 382, 511–517. ( 10.1042/BJ20040485) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vinogradov AD, Grivennikova VG. 2005. Generation of superoxide-radical by the NADH:ubiquinone oxidoreductase of heart mitochondria. Biochemistry (Mosc.) 70, 120–127. ( 10.1007/s10541-005-0090-7) [DOI] [PubMed] [Google Scholar]
- 55.Treberg JR, Quinlan CL, Brand MD. 2011. Evidence for two sites of superoxide production by mitochondrial NADH-ubiquinone oxidoreductase (complex I). J. Biol. Chem. 286, 27 103–27 110. ( 10.1074/jbc.M111.252502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quinlan CL, Orr AL, Perevoshchikova IV, Treberg JR, Ackrell BA, Brand MD. 2012. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J. Biol. Chem. 287, 27 255–27 264. ( 10.1074/jbc.M112.374629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muller FL, Liu YH, Abdul-Ghani MA, Lustgarten MS, Bhattacharya A, Jang YC, Van Remmen H. 2008. High rates of superoxide production in skeletal-muscle mitochondria respiring on both complex I- and complex II-linked substrates. Biochem. J. 409, 491–499. ( 10.1042/BJ20071162) [DOI] [PubMed] [Google Scholar]
- 58.Starkov AA. 2008. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N Y Acad. Sci. 1147, 37–52. ( 10.1196/annals.1427.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pisarenko OI, Solomatina ES, Studneva IM. 1986. The role of amino acid catabolism in the formation of the tricarboxylic acid cycle intermediates and ammonia in anoxic rat heart. Biochim. Biophys. Acta 885, 154–161. ( 10.1016/0167-4889(86)90083-2) [DOI] [PubMed] [Google Scholar]
- 60.Pisarenko O, Studneva I, Khlopkov V, Solomatina E, Ruuge E. 1988. An assessment of anaerobic metabolism during ischemia and reperfusion in isolated guinea pig heart. Biochim. Biophys. Acta 934, 55–63. ( 10.1016/0005-2728(88)90119-3) [DOI] [PubMed] [Google Scholar]
- 61.Niatsetskaya ZV, Sosunov SA, Matsiukevich D, Utkina-Sosunova IV, Ratner VI, Starkov AA, Ten VS. 2012. The oxygen free radicals originating from mitochondrial complex I contribute to oxidative brain injury following hypoxia–ischemia in neonatal mice. J. Neurosci. 32, 3235–3244. ( 10.1523/JNEUROSCI.6303-11.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chouchani ET, et al. 2014. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435. ( 10.1038/nature13909) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. 2009. Mitochondrial calcium and the permeability transition in cell death. Biochim. Biophys. Acta 1787, 1395–1401. ( 10.1016/j.bbabio.2009.06.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Hüttemann M. 2013. Molecular mechanisms of ischemia–reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 47, 9–23. ( 10.1007/s12035-012-8344-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Napankangas JP, Liimatta EV, Joensuu P, Bergmann U, Ylitalo K, Hassinen IE. 2012. Superoxide production during ischemia–reperfusion in the perfused rat heart: a comparison of two methods of measurement. J. Mol. Cell Cardiol. 53, 906–915. ( 10.1016/j.yjmcc.2012.09.011) [DOI] [PubMed] [Google Scholar]
- 66.Di Lisa F, Canton M, Menabo R, Kaludercic N, Bernardi P. 2007. Mitochondria and cardioprotection. Heart Fail Rev. 12, 249–260. ( 10.1007/s10741-007-9028-z) [DOI] [PubMed] [Google Scholar]
- 67.Murphy E, Steenbergen C. 2008. Mechanisms underlying acute protection from cardiac ischemia–reperfusion injury. Physiol. Rev. 88, 581–609. ( 10.1152/physrev.00024.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. 2004. Blockade of electron transport during ischemia protects cardiac mitochondria. J. Biol. Chem. 279, 47 961–47 967. ( 10.1074/jbc.M409720200) [DOI] [PubMed] [Google Scholar]
- 69.Chen Q, Hoppel CL, Lesnefsky EJ. 2006. Blockade of electron transport before cardiac ischemia with the reversible inhibitor amobarbital protects rat heart mitochondria. J. Pharmacol. Exp. Ther. 316, 200–207. ( 10.1124/jpet.105.091702) [DOI] [PubMed] [Google Scholar]
- 70.Riepe MW, Ludolph AC. 1997. Chemical preconditioning: a cytoprotective strategy. Mol. Cell Biochem. 174, 249–254. ( 10.1023/A:1006820927262) [DOI] [PubMed] [Google Scholar]
- 71.Chouchani ET, et al. 2013. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat. Med. 19, 753–759. ( 10.1038/nm.3212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ambrosio G, et al. 1993. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J. Biol. Chem. 268, 18 532–18 541. [PubMed] [Google Scholar]
- 73.Moncada S, Higgs A. 1993. The l-arginine–nitric oxide pathway. N. Engl. J. Med. 329, 2002–2012. ( 10.1056/NEJM199312303292706) [DOI] [PubMed] [Google Scholar]
- 74.Fukumura D, Kashiwagi S, Jain RK. 2006. The role of nitric oxide in tumour progression. Nat. Rev. Cancer 6, 521–534. ( 10.1038/nrc1910) [DOI] [PubMed] [Google Scholar]
- 75.Bogdan C. 2001. Nitric oxide and the immune response. Nat. Immunol. 2, 907–916. ( 10.1038/ni1001-907) [DOI] [PubMed] [Google Scholar]
- 76.Moncada S, Bolanos JP. 2006. Nitric oxide, cell bioenergetics and neurodegeneration. J. Neurochem. 97, 1676–1689. ( 10.1111/j.1471-4159.2006.03988.x) [DOI] [PubMed] [Google Scholar]
- 77.Galkin A, Higgs A, Moncada S. 2007. Nitric oxide and hypoxia. Essays Biochem. 43, 29–42. ( 10.1042/bse0430029) [DOI] [PubMed] [Google Scholar]
- 78.Cooper CE, Brown GC. 2008. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J. Bioenerg. Biomembr. 40, 533–539. ( 10.1007/s10863-008-9166-6) [DOI] [PubMed] [Google Scholar]
- 79.Hagen T, Taylor CT, Lam F, Moncada S. 2003. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1α. Science 302, 1975–1978. ( 10.1126/science.1088805) [DOI] [PubMed] [Google Scholar]
- 80.Cleeter MWJ, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AHV. 1994. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 345, 50–54. ( 10.1016/0014-5793(94)00424-2) [DOI] [PubMed] [Google Scholar]
- 81.Schewe T, Albracht SP, Ludwig P, Rapoport SM. 1985. Two modes of irreversible inactivation of the mitochondrial electron-transfer system by tetradecanoic acid. Biochim. Biophys. Acta 807, 210–215. ( 10.1016/0005-2728(85)90124-0) [DOI] [PubMed] [Google Scholar]
- 82.Batayneh N, Kopacz SJ, Lee CP. 1986. The modes of action of long chain alkyl compounds on the respiratory chain-linked energy transducing system in submitochondrial particles. Arch. Biochem. Biophys. 250, 476–487. ( 10.1016/0003-9861(86)90752-6) [DOI] [PubMed] [Google Scholar]
- 83.Brown GC, Borutaite V. 1999. Nitric oxide, cytochrome c and mitochondria. Biochem. Soc. Symp. 66, 17–25. ( 10.1042/bss0660017) [DOI] [PubMed] [Google Scholar]
- 84.Beltran B, Orsi A, Clementi E, Moncada S. 2000. Oxidative stress and S-nitrosylation of proteins in cells. Br. J. Pharmacol. 129, 953–960. ( 10.1038/sj.bjp.0703147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith BC, Marletta MA. 2012. Mechanisms of S-nitrosothiol formation and selectivity in nitric oxide signaling. Curr. Opin. Chem. Biol. 16, 498–506. ( 10.1016/j.cbpa.2012.10.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Granger DL, Lehninger AL. 1982. Sites of inhibition of mitochondrial electron transport in macrophage-injured neoplastic cells. J. Cell Biol. 95, 527–535. ( 10.1083/jcb.95.2.527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stuehr DJ, Gross SS, Sakuma I, Levi R, Nathan CF. 1989. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J. Exp. Med. 169, 1011–1020. ( 10.1084/jem.169.3.1011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lizasoain I, Moro MA, Knowles RG, Darley-Usmar V, Moncada S. 1996. Nitric oxide and peroxynitrite exert distinct effects on mitochondrial respiration which are differentially blocked by glutathione or glucose. Biochem. J. 314, 877–880. ( 10.1042/bj3140877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Radi R, Rodriguez M, Castro L, Telleri R. 1994. Inhibition of mitochondrial electron transport by peroxynitrite. Arch. Biochem. Biophys. 308, 89–95. ( 10.1006/abbi.1994.1013) [DOI] [PubMed] [Google Scholar]
- 90.Cassina A, Radi R. 1996. Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch. Biochem. Biophys. 328, 309–316. ( 10.1006/abbi.1996.0178) [DOI] [PubMed] [Google Scholar]
- 91.Clementi E, Brown GC, Feelisch M, Moncada S. 1998. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc. Natl Acad. Sci. USA 95, 7631–7636. ( 10.1073/pnas.95.13.7631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Beltran B, Mathur A, Duchen MR, Erusalimsky JD, Moncada S. 2000. The effect of nitric oxide on cell respiration: a key to understanding its role in cell survival or death. Proc. Natl Acad. Sci. USA 97, 14 602–14 607. ( 10.1073/pnas.97.26.14602) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Orsi A, Beltran B, Clementi E, Hallen K, Feelisch M, Moncada S. 2000. Continuous exposure to high concentrations of nitric oxide leads to persistent inhibition of oxygen consumption by J774 cells as well as extraction of oxygen by the extracellular medium. Biochem. J. 346, 407–412. ( 10.1042/bj3460407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Borutaite V, Budriunaite A, Brown GC. 2000. Reversal of nitric oxide-, peroxynitrite- and S-nitrosothiol-induced inhibition of mitochondrial respiration or complex I activity by light and thiols. Biochim. Biophys. Acta 1459, 405–412. ( 10.1016/S0005-2728(00)00178-X) [DOI] [PubMed] [Google Scholar]
- 95.Riobo NA, Clementi E, Melani M, Boveris A, Cadenas E, Moncada S, Poderoso JJ. 2001. Nitric oxide inhibits mitochondrial NADH:ubiquinone reductase activity through peroxynitrite formation. Biochem. J. 359, 139–145. ( 10.1042/bj3590139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pearce LL, Kanai AJ, Epperly MW, Peterson J. 2005. Nitrosative stress results in irreversible inhibition of purified mitochondrial complexes I and III without modification of cofactors. Nitric Oxide 13, 254–263. ( 10.1016/j.niox.2005.07.010) [DOI] [PubMed] [Google Scholar]
- 97.Dahm CC, Moore K, Murphy MP. 2006. Persistent S-nitrosation of complex I and other mitochondrial membrane proteins by S-nitrosothiols but not nitric oxide or peroxynitrite: implications for the interaction of nitric oxide with mitochondria. J. Biol. Chem. 281, 10 056–10 065. ( 10.1074/jbc.M512203200) [DOI] [PubMed] [Google Scholar]
- 98.Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. 2006. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem. J. 394, 627–634. ( 10.1042/BJ20051435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Minakami S, Schindler FJ, Estabrook RW. 1964. Hydrogen transfer between reduced diphosphopyridine nucleotide dehydrogenase and the respiratory chain. I. Effect of sulfhydryl inhibitors and phospholipase. J. Biol. Chem. 239, 2042–2048. [PubMed] [Google Scholar]
- 100.Galkin A, Moncada S. 2007. S-nitrosation of mitochondrial complex I depends on its structural conformation. J. Biol. Chem. 282, 37 448–37 453. ( 10.1074/jbc.M707543200) [DOI] [PubMed] [Google Scholar]
- 101.Methner C, Chouchani ET, Buonincontri G, Pell VR, Sawiak SJ, Murphy MP, Krieg T. 2014. Mitochondria selective S-nitrosation by mitochondria-targeted S-nitrosothiol protects against post-infarct heart failure in mouse hearts. Eur. J. Heart Fail. 16, 712–717. ( 10.1002/ejhf.100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. 2000. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc. Natl Acad. Sci. USA 97, 2826–2831. ( 10.1073/pnas.97.6.2826) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Benhar M, Forrester MT, Stamler JS. 2009. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat. Rev. Mol. Cell Biol. 10, 721–732. ( 10.1038/nrm2764) [DOI] [PubMed] [Google Scholar]
- 104.Chang AH, Sancheti H, Garcia J, Kaplowitz N, Cadenas E, Han D. 2014. Respiratory substrates regulate S-nitrosylation of mitochondrial proteins through a thiol-dependent pathway. Chem. Res. Toxicol. 27, 794–804. ( 10.1021/tx400462r) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Anand P, Stamler JS. 2012. Enzymatic mechanisms regulating protein S-nitrosylation: implications in health and disease. J. Mol. Med. 90, 233–244. ( 10.1007/s00109-012-0878-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chance B, Hollunger G. 1960. Energy-linked reduction of mitochondrial pyridine nucleotide. Nature 185, 666–672. ( 10.1038/185666a0) [DOI] [PubMed] [Google Scholar]
- 107.Wijermars LG, Schaapherder AF, Kostidis S, Wust RC, Lindeman JH. 2016. Succinate accumulation and ischemia–reperfusion injury: of mice but not men, a study in renal ischemia–reperfusion. Am. J. Transplant 16, 2741–2746. ( 10.1111/ajt.13793) [DOI] [PubMed] [Google Scholar]
- 108.Estabrook RW, Williamson JR, Frenkel R, Maitra PK. 1967. The fluorometric determination of mitochondrial adenine and pyridine nucleotides. Methods Enzymol. 10, 474–482. ( 10.1016/0076-6879(67)10079-7) [DOI] [Google Scholar]
- 109.Drose S, Stepanova A, Galkin A. 2016. Ischemic A/D transition of mitochondrial complex I and its role in ROS generation. Biochim. Biophys. Acta 1857, 946–957. ( 10.1016/j.bbabio.2015.12.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. 2004. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J. Neurosci. 24, 7779–7788. ( 10.1523/JNEUROSCI.1899-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Quinlan CL, Goncalves RL, Hey-Mogensen M, Yadava N, Bunik VI, Brand MD. 2014. The 2-oxoacid dehydrogenase complexes in mitochondria can produce superoxide/hydrogen peroxide at much higher rates than complex I. J. Biol. Chem. 289, 8312–8325. ( 10.1074/jbc.M113.545301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kareyeva AV, Grivennikova VG, Cecchini G, Vinogradov AD. 2011. Molecular identification of the enzyme responsible for the mitochondrial NADH-supported ammonium-dependent hydrogen peroxide production. FEBS Lett. 585, 385–389. ( 10.1016/j.febslet.2010.12.019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hayashi Y, Sawa Y, Ohtake S, Fukuyama N, Nakazawa H, Matsuda H. 2001. Peroxynitrite formation from human myocardium after ischemia–reperfusion during open heart operation. Ann. Thorac. Surg. 72, 571–576. ( 10.1016/S0003-4975(01)02668-6) [DOI] [PubMed] [Google Scholar]
- 114.Fukuyama N, Takizawa S, Ishida H, Hoshiai K, Shinohara Y, Nakazawa H. 1998. Peroxynitrite formation in focal cerebral ischemia–reperfusion in rats occurs predominantly in the peri-infarct region. J. Cereb. Blood Flow Metab. 18, 123–129. ( 10.1097/00004647-199802000-00001) [DOI] [PubMed] [Google Scholar]
- 115.Owen MR, Doran E, Halestrap AP. 2000. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 348, 607–614. ( 10.1042/bj3480607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Andrzejewski S, Gravel SP, Pollak M, St-Pierre J. 2014. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2, 12–26. ( 10.1186/2049-3002-2-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bridges HR, Jones AJ, Pollak MN, Hirst J. 2014. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem. J. 462, 475–487. ( 10.1042/BJ20140620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Matsuzaki S, Humphries KM. 2015. Selective inhibition of deactivated mitochondrial complex I by biguanides. Biochemistry 54, 2011–2021. ( 10.1021/bi501473h) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang L, et al. 2015. Modulation of mitochondrial complex I activity averts cognitive decline in multiple animal models of familial Alzheimer's disease. EBioMedicine 2, 294–305. ( 10.1016/j.ebiom.2015.03.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barber-Singh J, Seo BB, Matsuno-Yagi A, Yagi T. 2010. Protective role of rAAV-NDI1, serotype 5, in an acute MPTP mouse Parkinson's model. Parkinsons Dis. 2011, 438 370–438 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Koopman WJ, Nijtmans LG, Dieteren CE, Roestenberg P, Valsecchi F, Smeitink JA, Willems PH. 2010. Mammalian mitochondrial complex I: biogenesis, regulation, and reactive oxygen species generation. Antioxid. Redox. Signal. 12, 1431–1470. ( 10.1089/ars.2009.2743) [DOI] [PubMed] [Google Scholar]
- 122.Lim SC, Carey KT, McKenzie M. 2015. Anti-cancer analogues ME-143 and ME-344 exert toxicity by directly inhibiting mitochondrial NADH: ubiquinone oxidoreductase (Complex I). Am. J. Cancer Res. 5, 689–701. [PMC free article] [PubMed] [Google Scholar]
- 123.Koopman WJ, Verkaart S, van Emst-de Vries SE, Grefte S, Smeitink JA, Nijtmans LG, Willems PH. 2008. Mitigation of NADH: ubiquinone oxidoreductase deficiency by chronic Trolox treatment. Biochim. Biophys. Acta 1777, 853–859. ( 10.1016/j.bbabio.2008.03.028) [DOI] [PubMed] [Google Scholar]
- 124.Low Wang CC, Galinkin JL, Hiatt WR. 2015. Toxicity of a novel therapeutic agent targeting mitochondrial complex I. Clin. Pharmacol. Ther. 98, 551–559. ( 10.1002/cpt.178) [DOI] [PubMed] [Google Scholar]