Abstract
Hypersecretion of mucus is an important component of airway remodeling and contributes to the mucus plugs and airflow obstruction associated with severe asthma phenotypes. Lyn has been shown to down-regulate allergen-induced airway inflammation. However, the role of Lyn in mucin gene expression remains unresolved. In this study, we first demonstrate that Lyn overexpression decreased the mucus hypersecretion and levels of the muc5ac transcript in mice exposed to ovalbumin (OVA). Lyn overexpression also decreased the infiltration of inflammatory cells and the levels of IL-13 and IL-4 in OVA-challenged airways. Whereas Lyn knockdown increased the IL-4 or IL-13-induced MUC5AC transcript and protein levels in the human bronchial epithelial cell line, 16HBE, Lyn overexpression decreased IL-4- or IL-13-induced MUC5AC transcript and protein levels. Overexpression of Lyn also decreased the expression and phosphorylation of STAT6 in OVA-exposed mice, whereas Lyn knockdown increased STAT6 and MUC5AC levels in 16HBE cells. Finally, chromatin immunoprecipitation analysis confirmed that Lyn overexpression decreased the binding of STAT6 to the promoter region of Muc5ac in mice exposed to OVA. Collectively, these findings demonstrated that Lyn overexpression ameliorated airway mucus hypersecretion by down-regulating STAT6 and its binding to the MUC5AC promoter.
Mucous metaplasia is an important component of airway remodeling associated with severe asthma phenotypes. However, mucus dysfunction is often underappreciated by clinicians whose attention is primarily focused on reversing the bronchoconstriction and inflammation in asthma. Mucins are the major macromolecular component of the mucus1, and MUC5AC and MUC5B are the primary mucins in human airways. MUC5B is the principal gel-forming mucin in small airways under baseline conditions in humans and mice. MUC5B, but not MUC5AC, is essential for airway homeostasis and antibacterial defense2. MUC5AC is the principal gel-forming mucin that is up-regulated in airway inflammation3. Up-regulated production of MUC5AC contributes to mucous plugs and airflow obstruction in asthmatic patients4,5,6. Airflow limitation caused by mucus hypersecretion hampers the reversal of inflammation and the clearance of the excess secretions7.
The levels of T helper type 2 cytokines such as interleukin-4 (IL-4) and interleukin-13 (IL-13) are significantly increased during the pathogenesis of allergic asthma, and these cytokines induce mucus production in the airways8,9. IL-13 plays a critical role in asthmatic mucus overproduction. Glucocorticoids are not sufficient to suppress IL-13-induced goblet cell hyperplasia10. It has been shown that the phosphoinositide 3-kinase (PI3K)-nuclear factor of activated T cells (NFAT) pathway and STAT6/SAM domain-containing prostate-derived Ets factor (SPDEF) are involved in IL-13-induced MUC5AC expression11,12. STAT6 is an important transcription factor and is activated by IL-4 and IL-13 via the IL-4Ra subunit in their cognate receptors13. The IL-4Ra receptor and STAT6 play key roles in the IL-13-induced mucus production in mouse airway epithelial cells14. STAT6-knockout mice were protected from airway inflammation in the murine model of OVA-induced allergic airway inflammation15. However, it remains unclear whether STAT6 directly regulates the levels of MUC5AC or of other mucus regulators.
Lyn kinase, a member of the Src kinase family, is a non-receptor cytoplasmic tyrosine kinase that regulates various cellular processes and plays a crucial role in the immune response and inflammatory reactions. Lyn is associated with asthma due to its participation in IL-5 receptor signaling and Janas M. et al. also have reported that Lyn is a negative regulator of IL-4 signaling16,17,18,19. Lyn has been regarded as an activating regulator, but it exhibits both activating and inhibitory roles in different diseases or body’s conditions20,21,22. Lyn was necessary for the antiapoptotic effect of IL-5 in eosinophils. Suppressing Lyn kinase activity blocked the ability of IL-5 to prevent eosinophil death18. However, Lyn-deficient mice are prone to severe and persistent asthma, indicating that Lyn negatively regulates the progression of asthma23. However, the effect of Lyn overexpression in asthma remains unclear, including the molecular mechanisms by which Lyn modulates asthmatic pathology, especially airway mucus hypersecretion. Herein, we examined the regulatory role of Lyn knockdown and over-expression in mucous secretion using our recently created Lyn-transgenic mice and human airway epithelial cells. These findings demonstrated that Lyn overexpression ameliorated airway mucus hypersecretion via down-regulation of STAT6 as well as binding to the MUC5AC promoter.
Methods
Reagents
A Lyn-specific siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The antibodies for histology were purchased from Santa Cruz Biotechnology: MUC5AC (mucin 5AC, Clone # K-20; Cat # sc-16903), STAT6 (Clone # S-20, Cat # sc-621), β-actin (Clone # N-21, Cat # sc-130656), and phospho-STAT6 (Clone # Tyr641, Cat # sc-11762). The ELISA kits for IL-4, and IL-13 were purchased from Beijing Yonghui biotechnology Co. Ltd (Beijing, China). The MUC5AC-pGL3 luciferase vector was constructed by our laboratory based on the pGL3 luciferase reporter vector (Promega) following the manufacturer’s instructions.
Immunization and challenge protocol
Sensitization of the Lyn-transgenic(Lyntg) and wild type (WT) mice with OVA was performed using previously described methods24. The Lyntg and WT mice were sensitized by an intraperitoneal injection of 20 μg of OVA and 1 mg of aluminum hydroxide on days 0 and 14. The mice were challenged with an intranasal instillation of 10 μg of OVA in 50 μl of phosphate buffered saline (PBS) 5 times/week from week 3 to week 8 (for duration of 5 weeks). The Lyntg mice and WT mice in the control group were given PBS alone. The mice were sacrificed with an intraperitoneal injection of 40 mg/kg ketamine 24 hours after the final intranasal challenge. The lung tissues were stored in liquid nitrogen or fixed in 10% neutral-buffered formalin and embedded in paraffin. The Lyn-transgenic mice on a C57BL/6 J genetic background were generated by Cyagen Biosciences, Inc. (Guangzhou, China). The mice were backcrossed to a C57BL/6 background for at least two generations and maintained under pathogen-free conditions, and experiments were initiated when mice were 6 to 8 weeks of age. Genotyping was performed by PCR and Western blotting. The Lyn-transgenic mice and C57BL/6 mice were maintained under specific pathogen–free conditions in the Animal Experimental Center of Southwest Medical University. All animal experiments were performed in accordance with the guidelines of the Animal Experiments Center of Southwest Medical University. All experimental protocols were approved by the Ethics Committee in Affiliated Hospital of Southwest Medical University, including any relevant details.
Histological assessment
The mouse tracheas were cannulated with a 20 gauge catheter, and the lungs were lavaged four times with 1.0 ml of PBS. The cells in the bronchoalveolar lavage (BAL) fluid were enumerated using a hemocytometer. The BAL fluid was centrifuged (500 × g for 5 minutes at 4 °C) to obtain the cells, which were fixed and stained as described previously25. Differential cell counts for 200 cells from each sample were performed in duplicate on coded slides. The lung tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin. Sections (5 μm) of the lung specimens were stained with standard hematoxylin-eosin staining (H&E) methods and periodic acid–Schiff (PAS) reagent. The inflammation index in the H&E-stained slides was scored for the severity of the inflammatory cell infiltrates around airways and vessels using previously described methods26. The index was calculated by multiplying severity by extent, with a maximum possible score of 9.
Lung tissue homogenization and cytokines assay
The lung tissues were crushed and homogenized in radioimmunoprecipitation assay (RIPA) buffer. The protein concentrations were quantified using an Epoch multi-volume spectrophotometer system (Biotech, USA). The cytokine levels (IL-4 and IL-13) were determined in triplicate in total lung lysates from each animal using ELISAs.
Cell culture
The human airway epithelial cells (16HBE) were cultured in DMEM/F12 culture medium with 10% fetal calf serum (FCS) at 37 °C in 5% CO2. After the 16HBE cells reached 85% confluence in 24-well and 6-well plastic plates, the medium was replaced with serum-free DMEM/F12 culture medium. The cells were then treated with IL-4 or IL-13 in serum-free culture medium. Recombinant human IL-4 and IL-13 were purchased from Peprotech, Inc. (Rocky Hill, New Jersey, USA), and were dissolved in PBS with 0.1% BSA for use at a final concentration of 1 ng/ml.
Lentiviral expression constructs and DNA plasmid constructs
To generate a Lyn expression construct, human Lyn cDNA was amplified and cloned into the pLV.ExBi.P/Puro-EF1α-IRES-eGFP/pLV.Des3d.P/Puro vector using the Gateway technology kit according to the manufacturer’s instructions (Life Technologies Corporation, Carlsbad, California, USA). The Lyn cDNA was amplified using the specific primers: Lyn sense: 5′-CAAGTTTGTACAAAAAAGCAGGCT-3′, Lyn antisense: 5′-CACTTTGTACAAGAAAGCTGG-3′. The vector constructs were confirmed by DNA sequencing. The pLV.ExBi.P/Puro-EF1α-IRES-eGFP/pLV.Des3d.P/Puro vectors were transfected into human embryo kidney cells (293 T cells) to create infectious lentiviral vector-containing particles.
Viral infection, transfection, and luciferase assay
The medium was replaced with serum-free DMEM/F12 culture medium after the 16HBE cells reached 85% confluence in 24-well and 6-well plastic plates. The lentiviral vector expressing Lyn was used at 109 particles per well in the 6-well plates and 108 particles per well in the 24-well plates. Lyn expression was analyzed by western blotting and by immunofluorescence using an SP5 Leica confocal microscope (Leica, Germany). The 16HBE cells were transfected with 20 mM Lyn siRNA (Santa Cruz Biotechnology) with LipofectAmine 2000 according to the manufacturer’s instructions. For the luciferase assay, a 1.5-kb segment of the 5′ flanking region of the human MUC5AC gene (from −1300 to +48) was cloned into the pGL3-Basic Luciferase Vector (Promega, Madison, WI). A PLR-TK vector was used as a control plasmid to measure transfection efficiency. The luciferase activity was measured according to our previous report24.
RNA isolation, RT-PCR and qRT-PCR
Total RNA was extracted with the TRIzol RNA Reagent (Invitrogen Life Technologies, Carlsbad, CA) as recommended by the manufacturer. The RNA was resuspended in 50 μl of nuclease-free water, and its concentration was determined using a Nanodrop instrument (Epoch, BioTek). The RNA samples were stored at −80 °C until use. cDNA was reverse transcribed from an equal amount of RNA (1.5 μg per reaction) using avian myeloblastosis virus reverse transcriptase with oligo (dT) as the primer. Routine reverse transcription-PCR (RT-PCR) and quantitative real-time PCR (qRT-PCR) were used in our study. The mRNA levels of muc1, muc2, muc3, muc4, muc5ac, muc5b and muc13 and ChIP assays were detected using RT-PCR. qRT-PCR analysis was performed using SYBR Advantage qPCR Premix (Clontech, USA). The muc5ac mRNA levels were analyzed using 2-ΔΔCq threshold method with the Light Cycler 480 Multiple Plate Analysis Software (Roche Diagnostics, USA). The primer sequences for RT-PCR were shown in Table 1.The primer sequence of Mus musculus Muc5ac for qRT-PCR were as follows: Forward primer: 5′-TCTACC ACTCCCTGCTTCT-3′; Reverse primer: 5′-TGACTAACCCTCTTGA CC A C-3′.
Table 1. The primer sequences for RT- PCR.
| Gene | Primer sequences | |
|---|---|---|
| Mus musculus mucin 1 (Muc1) | sense | 5′-TCCAACTACTACCAAGAACTGAA-3′ |
| antisense | 5′-CAAGGAAATAGACGATAGCCAA-3′ | |
| Mus musculus mucin 2 (Muc2) | sense | 5′-ACCTGAAGAAATGTGTCACTGGG-3′ |
| antisense | 5′-GTGGTAATGGTGGTAGAGATGGG-3′ | |
| Mus musculus mucin 3 (Muc3) | sense | 5′-CTGGTGGAGAGCGTAGAGATAGA-3′ |
| antisense | 5′-TTGGTGGCAGTGGAGTTGAAA-3′ | |
| Mus musculus mucin 4 (Muc4) | sense | 5′-AGTTTCACTCCCACCATCTCTAT-3′ |
| antisense | 5′-TTCTCCACTCCTCTTCTGCCT-3′ | |
| Muc5ac | sense | 5′-GCCAAGTGCCAAAAGCAGTAGAG-3 |
| antisense | 5′-GACCTGGGGTGTGGGTAGAAGA-3 | |
| Muc5b | sense | 5′-CCATCCTCTGGGCTGAGTTGCTT-3′ |
| antisense | 5′-TTGTGTTCTCGTCGGTCGCTTTC-3′ | |
| Muc6 | sense | 5′-GGCGTGTGTGTGGACTGGAGAA-3′ |
| antisense | 5′-GAGGATGGGGCTGAAGGTGGT-3′ | |
| Muc13 | sense | 5′-CATCCTCATCTTGCTGATTGCTTTT-3′ |
| antisense | 5′-TCTGCCCATTTCTCCTTGTCCT-3′ | |
| Muc19 | sense | 5′-CATTGGAGAGAAAGGAAAGTGTG-3′ |
| antisense | 5′-GCTTGCATGTACGAAGAGGAT-3′ | |
| GAPDH | sense | 5′-TCAACGGCACAGTCAAGG-3′ |
| antisense | 5′-ACCAGTGGATGCAGGGA-3′ | |
Immunofluorescent staining and Western blotting
Immunohistochemical staining was performed on glass slides using standard histological methods. The cells were fixed with cold methanol and blocked at room temperature. The cells were incubated with MUC5AC antibodies (Santa Cruz Biotechnology). A mouse isotype serum replaced the primary Ab as a negative control. FITC-conjugated goat anti-mouse or TRITC-conjugated anti-mouse secondary Abs were used to probe the primary Abs. The lung tissues removed from mice were cut by freezing microtome and applied for immunofluorescent staining as above. For western blotting, the lung tissues and cells were homogenized in RIPA buffer with an optional protease inhibitor mixture (Roche or Fisher Scientific), and the protein concentrations were measured using a Nanodrop instrument (Epoch, BioTek). Lysates from each sample were separated in a 10% SDS polyacrylamide gel electrophoresis at 100 V for 2 hours and transferred to a microporous polyvinylidene difluoride (PVDF) membrane at 100 mA for 2 hours. Lyn, β-actin, STAT6, phospho-Lyn and phospho-STAT6 antibodies (1:1000) were used for the Western blots. HRP-conjugated secondary antibodies were used to react with the primary Abs, and the bands were visualized using the Pierce ECL Western Blotting kit (Pierce Biotechnology, USA). AlphaEaseFC software was used to quantify protein expression.
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed using EZ-Magna ChIP™ and One-Day Chromatin Immunoprecipitation kits (Millipore) according to previously described methods27. Frozen tissues were cut into 1–3 mm pieces. A volume of 1 ml of PBS with protease inhibitors (Roche, Germany) was added per 100 mg of tissue. The DNA-protein complexes were cross-linked and then sheared to 200–1000 bp using a sonicator. The sonicated nuclear fractions were divided for input control and incubation with a negative control IgG or the STAT6 (D3H4) rabbit mAb (Cell Signaling Technology, USA). The antibody protein-DNA complex was then pulled down with magnetic beads coupled to anti-mouse IgG. The pellets were washed with a series of wash buffers, and the protein-DNA complexes were eluted with 100 μl of ChIP Elution Buffer and 1 μl of Proteinase K. The DNA was purified using spin columns. Finally, the Muc5ac promoter region was amplified using RT-PCR and quantitative real-time PCR with primers specific for the STAT6-binding elements of the MUC5AC promoter region (from −879 to +1 bp): forward primer: 5′-CCATCCCA GCAGACATGAAA-3′, reverse primer: 5′-CTATTAACCTCCTGAGC AACCC-3′. The specificity of each primer was confirmed by analyzing the melt curve and the amplification plot.
Statistical analysis
All data are presented as the mean ± s.d. The statistical analyses were performed using ANOVA. All the statistical analyses were conducted using SPSS 17.0 software. The level of significance was defined as a P value less than or equal to 0.05.
Results
Overexpression of Lyn attenuated OVA-induced mucus hypersecretion and muc5ac expression
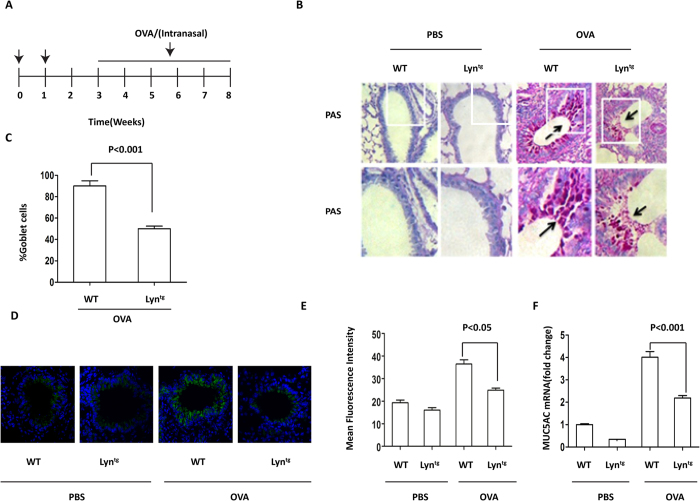
Based on our previous findings showing mucus hypersecretion during exposure of Lyn−/− (Lyn knockout) mice to HDM24, we investigated mucus secretion and muc5ac expression using a transgenic approach. To more thoroughly elucidate the critical role of Lyn in asthmatic pathology, we generated Lyn transgenic mice (Lyntg mice) and successfully verified the overexpression of Lyn in these mice by western-blot. We then backcrossed these mice with C57BL/6 J for 2 generations and challenged them as well as control mice with OVA. We employed an initial sensitization using an intraperitoneal (i.p.) injection of OVA followed by repeated intranasal instillations to induce chronic airway inflammation in the Lyntg and WT mice (Fig. 1A). We further confirmed that Lyn kinase regulates mucus secretion and muc5ac expression in a murine model of allergic airway inflammation. The lungs of the WT and Lyntg mice exposed to PBS remained normal, with few mucin-positive goblet cells and trace amounts of muc5ac (Fig. 1B,D,F). The lungs of the WT and Lyntg mice exposed to OVA (at 3, 6 and 8 weeks) showed an increased number of mucin-positive goblet cells (Fig. 1B,C; Supplementary Figure S1A). At 8 weeks, we observed a robust decrease in the number of mucin-positive goblet cells in the Lyntg mice exposed to OVA compared to the WT mice (2.0-fold decrease; Fig. 1C; P < 0.001). Immunofluorescent staining of lung tissue showed that expression of muc5ac in Lyntg mice exposed to OVA was less than WT mice exposed to OVA (Fig. 1D,E; Supplementary Figure S1B; P < 0.05. In Supplementary Figure S1B, “I&II” both refer to the negative control of immunohistochemistry staining in Fig. 1D. “I” indicates the sample’s autofluorescence. “II” shows non-specific fluorescence.). To examine the transcript levels of mucous secretion genes, RT-PCR was utilized to detect the mRNA levels of muc1, muc2, muc3, muc4, muc5ac, muc5b and muc13 in lung tissue. The transcript levels for muc1, muc2, muc3, muc4, muc5b and muc13 in lung tissue were unaltered, whereas the Muc5ac transcript was significantly increased in the WT mice exposed to OVA (Fig. 1F; Supplementary Figure S1C). We also observed a robust decrease in muc5ac transcript levels in the Lyntg mice exposed to OVA (at 8 weeks) compared with WT mice (Fig. 1F; P < 0.001). Taken together, these findings indicate that Lyn overexpression decreased mucus secretion and muc5ac transcript levels in mice exposed to OVA.
Figure 1. Mucus hypersecretion and muc5ac transcripts in OVA-induced Lyntg mice.
(A) Protocol for sensitization and challenge of Lyntg mice and WT mice with OVA (n = 8 mice for each group). (B) PAS staining of epithelial goblet cells in the lungs of WT and Lyntg mice exposed to OVA or PBS (original magnification, x200). (C) The PAS-positive cell percentage was quantified in 10 random fields (original magnification, x200). (D) Muc5ac in 16HBE cells was determined by immunofluorescence (original magnification, x200, the blue color is for DAPI). (E) The intensity of muc5ac was quantified. (F) The mRNA expression of muc5ac was determined in the lungs of Lyntg mice and WT mice using qRT-PCR (n = 3 mice for each group).
Overexpression of Lyn attenuated OVA-induced airway inflammation
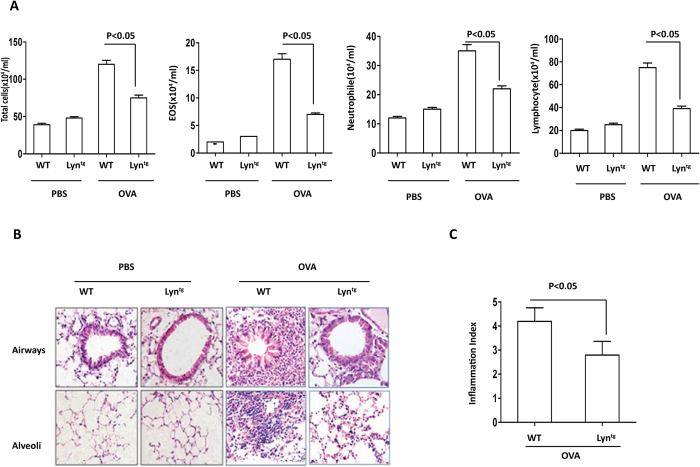
Having shown that the airway responds to inflammation with increased mucus secretion and that Lyn-deficiency enhanced the airway inflammation in mice exposed to HDM7, we analyzed the role of Lyn in regulating airway inflammation. The mice were challenged by intranasal instillations of 10 μg of OVA 5 times per week for 5 weeks. The total number of inflammatory cells as well as eosinophils, neutrophils and lymphocytes in the BAL had dramatic reduction in the OVA-treated Lyntg mice compared to the OVA-treated WT mice (Fig. 2A, P < 0.05). Inflammatory cell infiltration into the airways and alveoli was determined to be decreased in the OVA-treated Lyntg mice compared to the OVA-treated WT mice by H&E staining (Fig. 2B). The degree of cellular infiltration into the lungs was significantly decreased in the OVA-treated Lyntg mice compared to the OVA-treated WT mice (Fig. 2C, P < 0.05). These findings indicate that Lyn overexpression decreased the airway inflammation in mice exposed to OVA.
Figure 2. Airway inflammation in OVA-challenged Lyntg mice.
(A) The total number of inflammatory cells as well as eosinophils, neutrophils and lymphocytes in the BAL of the WT and Lyntg mice were determined by differential cell analysis. (B) The lung tissues were stained using H&E (original magnification, x200). (C)The inflammatory cell infiltration index was determined in the lungs in (B) (10 random areas).
Overexpression of Lyn decreased IL-13 and IL-4 during OVA-induced airway inflammation in mice
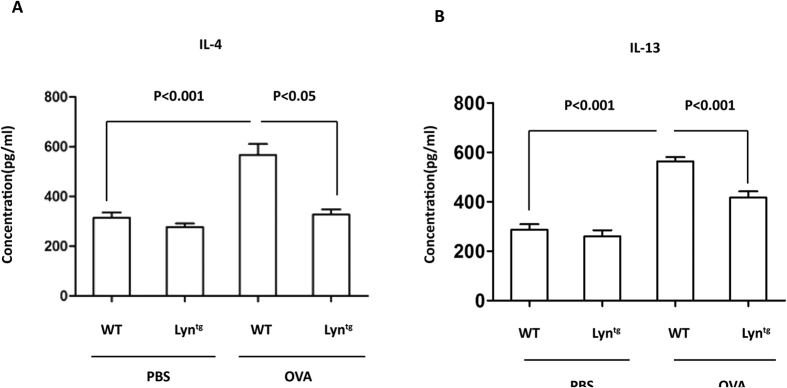
Cytokines such as IL-13 and IL-4 induce goblet cell metaplasia and muc5ac gene expression28,29 Lyn transgenic mice were used to investigate the effect of Lyn on IL-13 and IL-4 in OVA-induced airway inflammation. There is a significant up-regulation of IL-4 and IL-13 when PBS and OVA treatment are compared in WT mice (Fig. 3A,B; P < 0.001). The levels of IL-4 and IL-13 in the lungs were significantly lower in the OVA-treated Lyn-transgenic mice compared to the OVA-treated WT mice (Fig. 3A,B; P < 0.05 or P < 0.001). These findings show that Lyn overexpression decreased IL-13 and IL-4 expression during OVA-induced airway inflammation.
Figure 3. The levels of IL-13 and IL-4 in OVA-challenged Lyntg mice.
The cytokine levels were measured by ELISA in triplicate samples of lung lysates from each animal. (A) IL-4 levels in lung tissue. (B) IL-13 levels in lung tissue (n = 3 mice for each group). The results are shown as the mean ± s.d.
Lyn deficiency increased MUC5AC in IL-4/IL-13-exposed HBE cells
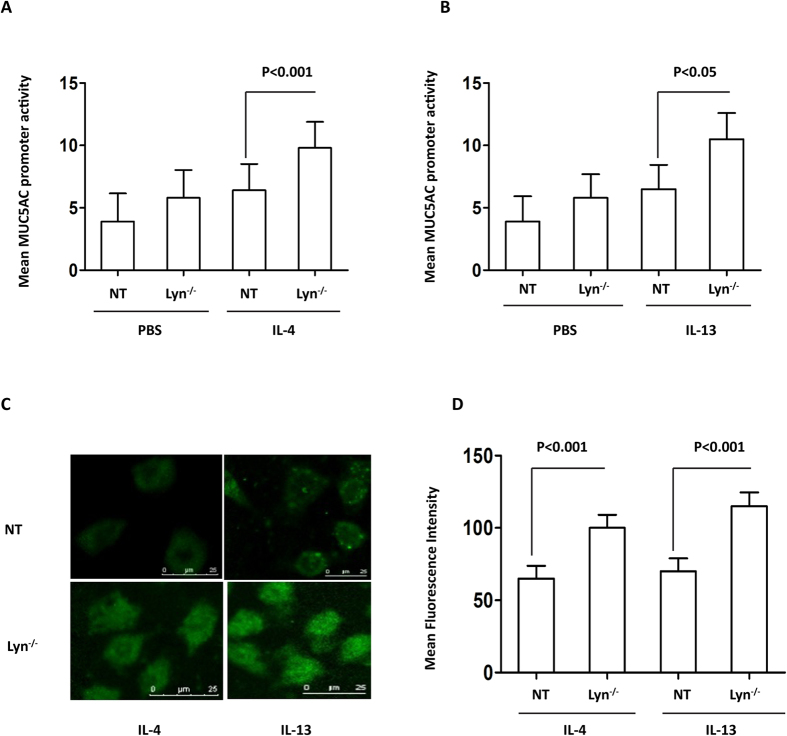
To further evaluate the involvement of Lyn in IL-4- and IL-13-associated mucus secretion, MUC5AC and muc5ac expression was examined in IL-4- or IL-13-treated Lyn knockdown 16HBE cells using immunostaining and the MUC5AC PGL3 luciferase reporter assay. In cells transfected with the MUC5AC promoter construct, luciferase reporter gene activity increased in IL-4-treated Lyn knockdown 16HBE cells (Lyn−/−) compared to cells untransfected with LynsiRNA (NT cells) (Fig. 4A, P < 0.001). A similar phenomenon was also observed for IL-13-stimulated MUC5AC promoter luciferase activity in IL-13-treated Lyn knockdown 16HBE cells compared to NT 16HBE cells (Fig. 4B, P < 0.05). Control (scrambled) siRNA had been used in our previous study24. Thus, we have not used control siRNA this time (based on similar conditions). These results indicated that the region of the MUC5AC promoter spanning −1300/+48 contained the cis-acting element that was required for both IL-4- and IL-13-stimulated gene expression. The protein levels of MUC5AC were measured with MUC5AC antibody immunostaining. The level of MUC5AC in NT 16HBE cells increased after treatment with IL-4 or IL-13 for 24 hours compared to PBS treatment (Supplementary Figure S2A, S2B and S2C). The level of MUC5AC also increased ∼1.57-fold in IL-13-treated Lyn−/−cells and in IL-4 treated Lyn−/− cells compared to NT cells (Fig. 4C,D, P < 0.001). Taken together, these studies suggest that Lyn regulates the transcription and translation of MUC5AC in airway epithelial cells exposed to IL-4 or IL-13. Lyn knockdown increased the IL-4 or IL-13-induced MUC5AC transcript and protein levels.
Figure 4. The levels of the MUC5AC transcript and protein in IL-4/IL-13-treated Lyn−/− 16HBE cells in vitro (Lyn−/− for siRNA treated cells, NT for untransfected cells).
MUC5AC activation was determined using a luciferase promoter assay. The human airway epithelial 16HBE cells were cotransfected with the MUC5AC promoter-luciferase plasmid and Lyn siRNA. (A) The cells were then stimulated with 1 ng/ml IL-4 for 24 hours, and the luciferase activity was measured. (B) The cells were stimulated with 1 ng/ml IL-13 for 24 hours, and the luciferase activity was measured. (C) Immunohistochemical analysis of MUC5AC in Lyn−/− cells and untransfected (NT) cells exposed to IL-4 or IL-13 for 24 hours. (D) Quantitation of the fluorescence intensity of MUC5AC per micrometer in 10 random fields as shown in (C). The results are shown as the mean ± s.d. All data are representative of three experiments, and a statistical analysis was performed.
Overexpression of Lyn decreased MUC5AC in IL-4/IL-13-treated HBE cells
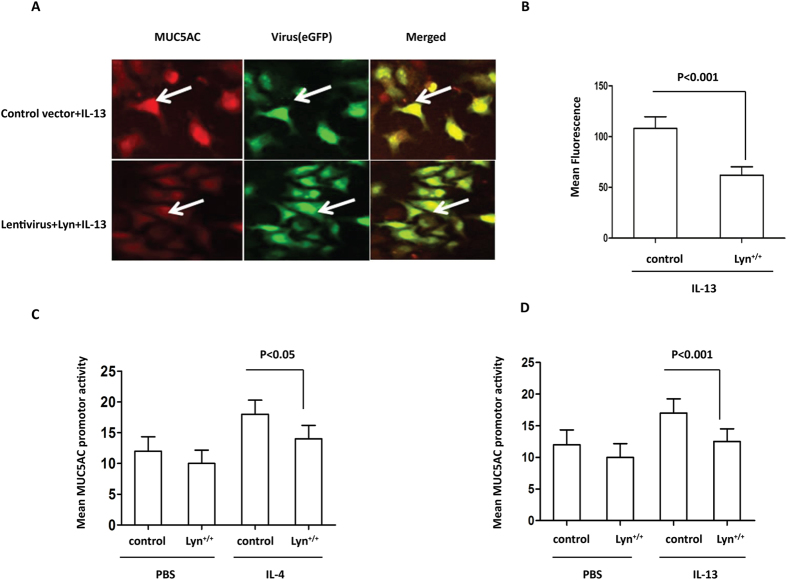
To further understand the role of Lyn, we assessed the MUC5AC levels in 16HBE cells using lentiviral Lyn-overexpression vectors. As shown in our previous studies, we generated Lyn-expression lentiviruses and infected 16HBE cells using 109 viral particles per well of a 24-well plate. We next examined the effect of IL-13 on MUC5AC in cells infected with the Lyn-expressing lentivirus (Lyn+/+) by immunostaining. Lyn-infected or control vector-infected 16HBE cells were stimulated with IL-13 for 24 hours. We observed robust increases in the levels of MUC5AC in the IL-13-treated 16HBE cells transfected with control vector (control). However, Lyn-expression via lentiviral transduction suppressed the expression of MUC5AC in IL-13-treated 16HBE cells (Fig. 5A). MUC5AC showed a ∼1.7-fold decrease in mean fluorescence intensity in cells infected with the Lyn-expressing lentivirus and exposed to IL-13 compared to the corresponding control cells (Fig. 5B).
Figure 5. The levels of the MUC5AC transcript and protein in IL-4/IL-13-treated Lyn+/+.16HBE cells in vitro.
MUC5AC activation was determined using a luciferase promoter assay. We infected 16HBE cells with the Lyn-eGFP-expression lentiviral vector and control vector respectively (108 viral particles per well of a 24-well plate). (A) The cells were then stimulated with 1 ng/ml IL-13 for 24 hours. Immunohistochemical analysis of MUC5AC in Lyn+/+cells and control cells. The arrows indicate relevant markers in the positively stained cells: MUC5AC (red); eGFP or Lyn-eGFP (green). One representative experiment out of three is shown (original magnification x400). (B) Quantitation of the fluorescence intensity of MUC5AC per micrometer in 10 random fields of the images as shown in (A). (C) A MUC5AC promoter construct was transfected into control cells and Lyn+/+ cells. The cells were then stimulated with 1 ng/ml IL-4 for 24 hours. (D) The MUC5AC promoter construct was transfected into control cells and Lyn+/+ cells. The cells were then stimulated with 1 ng/ml IL-13 for 24 hours. The results are shown as the mean ± s.d. All data are representative of three experiments, and a statistical analysis was performed.
To further characterize the role of Lyn overexpression in regulating muc5ac transcription, we measured the luciferase activity in cultured 16HBE cells. In 16HBE cells transfected with the muc5ac promoter construct, the luciferase reporter gene activity decreased in IL-4-treated cells infected with the Lyn-expressing lentivirus compared to the corresponding control cells (Fig. 5C, P < 0.05). A similar result was also observed for the muc5ac promoter luciferase activity in IL-13-treated 16HBE cells infected with the Lyn-expressing lentivirus compared to those infected with the control vector (Fig. 5D, P < 0.001). These studies indicate that the levels of the muc5ac transcript and protein were clearly reduced in the Lyn-overexpressing human airway epithelial cells in response to IL-4/IL-13 treatment that simulated airway inflammation.
STAT6 was critical for both IL-4- and IL-13-induced MUC5AC expression in Lyn- deficient 16HBE cells
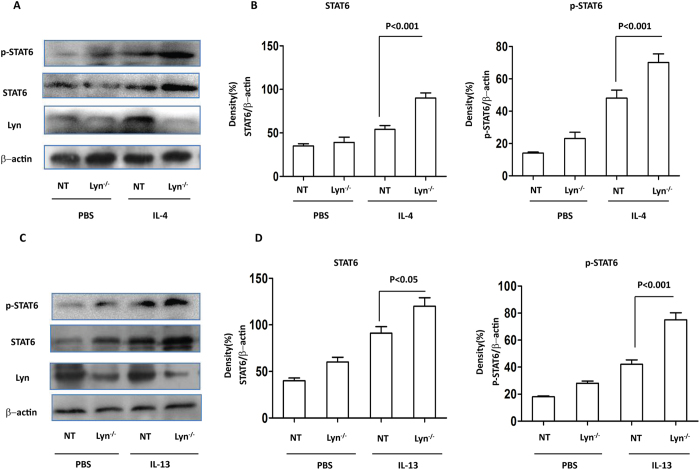
Because Lyn regulated MUC5AC in Lyn-transgenic mice and in IL-4/IL-13-treated cells, we next analyzed the transcriptional mechanism governing MUC5AC expression under these conditions. Previous studies have shown that STAT6 is critical to the development of allergen-induced AHR and mucus production30. IL-4 and/or IL-13 can initiate a cascade of STAT6-dependent signaling events in epithelial cells that lead to the formation of goblet cells31. The repression of STAT6 inhibits the IL-13-induced expression of MUC5AC11. However, the effect of STAT6 on MUC5AC remains undetermined. To further evaluate the involvement of STAT6 in IL-4/IL-13-induced MUC5AC expression, the expression and phosphorylation of STAT6 were studied in siRNA-mediated Lyn knockdown 16HBE cells (Lyn−/−) in vitro using Western blotting. IL-4 increased the protein expression and phosphorylation of STAT6 in Lyn knockdown 16HBE cells compared with untransfected (NT) 16HBE cells (Fig. 6A). The expression and phosphorylation of STAT6 increased ∼1.72-fold and ∼1.53-fold, respectively, in IL-4-treated cells compared to NT cells (Fig. 6B, P < 0.001). The expression and phosphorylation of STAT6 also increased by ∼1.22-fold and ∼1.75-fold in the IL-13-treated cells compared to the NT cells (Fig. 6C,D; P < 0.05 and P < 0.001, respectively). These results indicated that Lyn may play a key role in regulating the expression and phosphorylation of STAT6 to regulate MUC5AC.
Figure 6. The expression and phosphorylation of STAT6 in IL-4/IL-13- challenged Lyn/− 16HBE cells.
The 16HBE cells were transfected with Lyn siRNA. (A) Western blot analysis of Lyn, STAT6 and the phosphorylation of STAT6 in Lyn−/− and untransfected (NT) cells exposed to IL-4. (B) Relative density of STAT6 and phosphorylation of STAT6 in IL-4-treated Lyn knockdown and NT cells. (C) Western blot analysis of Lyn, STAT6 and phosphorylated STAT6 in Lyn−/− and NT cells exposed to IL-13. (D) Relative density of STAT6 and phosphorylated STAT6 in IL-13-treated Lyn−/− and NT cells. β-actin was used as the loading control. All data are representative of three experiments, and a statistical analysis was performed.
To further determine whether the IL-4/IL-13-induced MUC5AC promoter activity was due to STAT6, the muc5ac transcript was measured in IL-4- or IL-13-treated STAT6 deficient 16HBE cells (STAT6−/−) using the MUC5AC PGL3 luciferase reporter vector in vitro. The knockdown of STAT6 by siRNA was shown in Sup Figure S3A. The luciferase reporter gene activity was significantly decreased ∼2.2-fold in IL-4-treated STAT6 knockdown 16HBE cells (STAT6−/−) compared to NT 16HBE cells (Supplementary Figure S3B). A similar observation was made for the IL-13-stimulated MUC5AC promoter luciferase activity in IL-13-treated STAT6 knockdown 16HBE cells compared to NT 16HBE cells. The luciferase reporter gene activity was significantly decreased (∼3.1-fold) in the IL-13-induced STAT6 knockdown 16HBE cells compared to the NT 16HBE cells (Supplementary Figure S3C). Taken together, these results indicated that STAT6 was critical for the IL-4/IL-13-induced MUC5AC expression. IL-4 and/or IL-13 can initiate a cascade of STAT6-dependent signaling events that culminate in MUC5AC expression.
Overexpression of Lyn decreased STAT6 in IL-4/IL-13-treated 16HBE cells
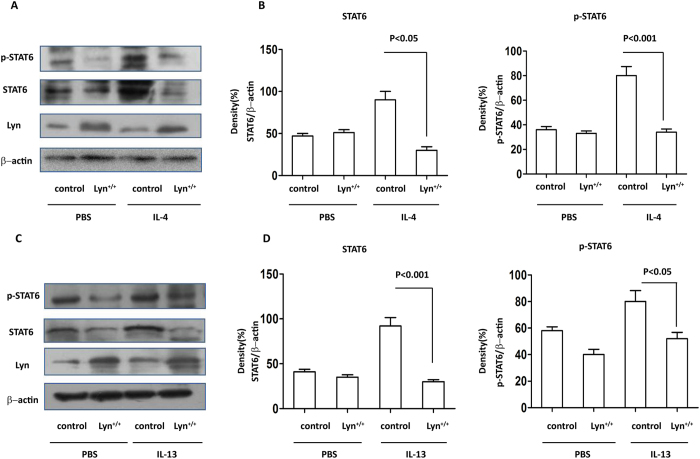
The above studies implied that STAT6 is critical for both IL-4- and IL-13-induced MUC5AC expression in Lyn-deficient 16HBE cells in vitro. We next extended these studies to Lyn-overexpressing 16HBE cells (Lyn+/+) challenged with IL-4/IL-13. We infected 16HBE cells with the Lyn-expressing lentiviral vector and examined the effect of Lyn overexpression on STAT6 following IL-4/IL-13 challenge in vitro. The 16HBE cells infected with Lyn-expressing lentivirus were stimulated with IL-4/IL-13 for 24 hours. Figure 7A,B shows an approximately 2.96-fold decrease in STAT6 expression and an approximately 2.5-fold decrease in STAT6 phosphorylation in the IL-4-challenged Lyn+/+cells compared to the cells transfected with control vector (control). Furthermore, the expression of STAT6 also decreased approximately 3.4-fold in IL-13-challenged Lyn+/+cells compared to control cells. The phosphorylation of STAT6 decreased approximately 1.6-fold in the IL-13-treated Lyn+/+cells compared to the control cells (Fig. 7C,D). Collectively, these studies suggest that Lyn overexpression attenuated MUC5AC expression via negative regulation of the expression and phosphorylation of STAT6 in IL-4/IL-13-treated cells.
Figure 7. The expression and phosphorylation of STAT6 in IL-4/IL-13-treated Lyn+/+ 16HBE cells (Lyn+/+ for Lyn-expression lentiviral transfectd cells, control for control vector transfected cells).
The 16HBE cells were infected with the Lyn-expression lentiviral vector and control vector respectively (109 viral particles per well of a 6-well plate). (A) Western blot analysis of Lyn, STAT6 and phosphorylated STAT6 in Lyn+/+ and control cells after exposure to IL-4. (B) Relative density of STAT6 and phosphorylated STAT6 in IL-4-treated Lyn+/+ and control cells. (C) Western blot analysis of Lyn, STAT6 and phosphorylated STAT6 in Lyn+/+ and control cells after exposure to IL-13. (D) Relative density of STAT6 and phosphorylated STAT6 in IL-13-treated Lyn+/+ and control cells. β-actin was used as the loading control. All data are representative of three experiments, and a statistical analysis was performed.
Overexpression of Lyn attenuated STAT6 expression and STAT6 binding to the MUC5AC promoter in OVA-induced airway inflammation in mice
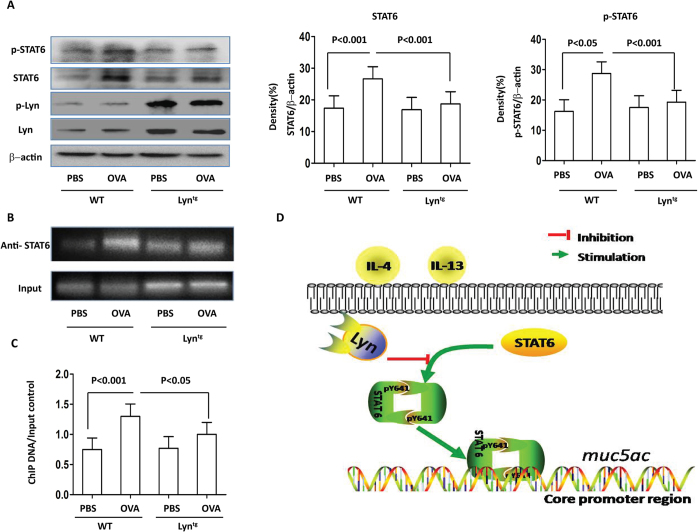
Mice lacking STAT6 are protected from all of the pulmonary effects of IL-13. Reconstitution of STAT6 in epithelial cells only was sufficient for IL-13-induced mucus production9. Our previous studies established that the expression and phosphorylation of STAT6 increases in HDM-exposed Lyn−/− mice24. We next investigated the expression and phosphorylation of STAT6 in OVA-exposed Lyntg mice. In our present study, the expression and phosphorylation of STAT6 in the lungs were decreased in OVA-exposed Lyntg mice compared to WT mice. Furthermore, the expression and phosphorylation of STAT6 decreased by approximately 1.44-fold and approximately 1.52-fold, respectively, in OVA-exposed Lyntg mice compared to OVA-exposed WT mice (Fig. 8A). Our studies provide the first evidence that overexpression of Lyn decreased the expression and phosphorylation of STAT6 during OVA-induced allergic airway inflammation in mice.
Figure 8. STAT6 and STAT6 binding to MUC5AC promoter during OVA-induced airway inflammation in mice.
(A) Western blot analysis of Lyn, STAT6, phosphorylated STAT6 and phosphorylated Lyn in OVA-exposed Lyntg and WT mice. Relative density of STAT6 and phosphorylated STAT6 in lung tissue. (B) ChIP assays for STAT6 binding to the muc5ac promoter region in the lungs of OVA-challenged mice. Cross-linked chromatin was isolated and fragmented to 200–1000 bp. Anti-STAT6 Ab or control IgG was used to precipitate the DNA fragments bound to STAT6 or nonspecific proteins. The precipitation of the protein and DNA was measured by RT-PCR analysis with primers targeting the MUC5AC promoter regions. (C) Relative density of ChIP DNA/input control (in (B)) in lung tissue. (D) Diagram showing the cell signaling pathway associated with Lyn kinase.
It is unknown whether STAT6 regulates MUC5AC via direct interaction between STAT6 and the muc5ac promoter. To determine whether muc5ac promoter activity is due to a direct interaction between STAT6 and the muc5ac promoter, ChIP assays were conducted on the lung tissue from OVA-induced allergic mice. The STAT6-binding elements of the muc5ac promoter region were amplified by RT-PCR. The nuclear fractions were incubated with a negative control IgG (Supplementary Figure S4) We found that precipitation using STAT6 Abs in the lung tissue from the OVA-challenged WT mice yielded a high PCR DNA band/input DNA ratio compared to that from the PBS control mice (Fig. 8B). In contrast, the PCR DNA band/input DNA ratio was approximately 1.34-fold lower in the OVA-exposed Lyntg mice compared to the OVA-exposed WT mice (Fig. 8C).
To further confirm these results, the relative gene expression data were analyzed using quantitative real-time PCR and the 2-△△CT method. Our studies found that the fold enrichment of muc5ac expression increased approximately 5.9-fold in the OVA-exposed WT mice compared to the PBS-treated control mice (Supplementary Figure S5). Furthermore, applied bioinformatics for the identification of regulatory elements was performed using Jaspar (http://jaspar.genereg.net/) software. We found that the MUC5AC promoter activity may be due to a direct interaction between STAT6 and the MUC5AC promoter in the region flanking the STAT6 binding site (−879 ~ +1 bp). Taken together, these studies identified a novel pathway by which Lyn affected mucus hypersecretion by regulating STAT6. This is the first demonstration of STAT6 binding to the MUC5AC promoter in allergen-induced MUC5AC, muc5ac expression and the key role of Lyn in regulating STAT6 (Fig. 8D).
Discussion
Mucus is an important component of both the physiological and pathological processes in airways32. A total of 20 human mucin (MUC) genes have been identified. However, their relative contributions to the development of asthma remain unclear33. In the present study, we demonstrated that the transcript levels for muc1, muc2, muc3, muc4, muc5b and muc13 in lung tissue were unaltered, whereas muc5ac transcript expression was significantly increased during allergen-induced airway inflammation in mice (Supplementary Figure S1C). These results indicated that muc5ac expression plays a key role in airway inflammation and goblet cell metaplasia after antigen challenge. Mucus hypersecretion is induced in a time-dependent manner in mouse models of chronic airway inflammation. The number of mucin-positive goblet cells at 8 weeks was greater than that at 3 weeks (Fig. 1B and Supplementary Figure S1A). Although Lyn kinase has been implicated in the pathogenesis of asthma, only a few studies have addressed the role of Lyn in mucus secretion. In our previous studies18,34,35, Lyn-deficient mice exposed to house dust mite allergen exhibited mucus hypersecretion and increased muc5ac mRNA and MUC5AC protein expression24. The present study has carefully elaborated a novel and key role for Lyn in the regulation of airway mucin production. Lyn overexpression decreased mucus secretion and muc5ac transcription in mice exposed to allergens.
The pathology of asthma is characterized by structural changes, including goblet cell metaplasia and an increase in epithelial mucin stores18. The formation of pathological intraluminal mucus results in airway narrowing during asthma exacerbations. Goblet cell metaplasia results from chronic airway inflammation36. Previous studies have demonstrated that Lyn deficiency enhances the airway inflammatory response24. In the present study, the overexpression of Lyn attenuated airway inflammation and decreased total inflammatory cells including eosinophils, neutrophils and lymphocytes in the BAL as well as the degree of cellular infiltration in the lungs. The decreased airway inflammation was associated with lower levels of IL-13 and IL-4 in the Lyn transgenic mice exposed to allergen (Fig. 3). Our findings further confirmed that the Lyn-mediated decrease of IL-13 and IL-4 may contribute to lowered airway inflammatory responses.
T-helper type 2 (Th2) inflammation, mediated by IL-4, IL-5 and IL-13, is considered the central molecular mechanism underlying asthma37. IL-13 induces goblet cell metaplasia via forkhead box protein A2 (FoxA2), FoxA3 and SAM-pointed domain-containing ETS transcription factor (SPDEF). IL-17A induces mucus production and MUC5AC in a mechanism involving NFκB-mediated transcription, and NFκB specifically binds to position-3594/−3582 in the promoter of MUC5AC38,39. No previous study had linked Lyn kinase to the regulation of airway mucin production by IL-4/IL-13, but available databases led us to consider this possibility. We evaluated the relevance of Lyn-regulated muc5ac transcription in Lyn-knockdown cells and Lyn-overexpressing cells. Using a reporter-based promoter study, we demonstrated that Lyn indeed regulates the muc5ac transcript in vitro. Lyn deficiency increased the levels of the muc5ac transcript. In contrast, the overexpression of Lyn suppressed the IL-4/IL-13-induced transcription of the muc5ac gene. This is the first report describing a crucial role of Lyn in the transcriptional regulation of airway MUC5AC and muc5ac expression in response to IL-4 and IL-13.
IL-13 is a mediator of mucus hyperplasia. Therefore, IL-13 is an attractive drug target for treating asthma40. STAT6 plays a key role in affecting the transcription of many IL-4/IL-13-dependent genes41. Lyn influences the phosphorylation of various signaling molecules and transcription factors, including the STAT proteins42,43. Previous studies showed that Lyn interacted with STAT5 and directly activated STAT5. Maverick Lau et al. reported that genetic deletion of STAT6 in lupus-prone Lyn-deficient mice promotes autoimmune disease44. However, whether Lyn interacts with STAT6 or regulates STAT6 remains unclear. In the present study, using Lyn siRNA, we found that knocking down Lyn substantially increased the expression and phosphorylation of STAT6 in IL-4- and IL-13-treated 16HBE cells. However, using a Lyn-overexpressing lentiviral vector, we demonstrated that Lyn overexpression significantly decreased the expression and phosphorylation of STAT6 in the IL-4- and IL-13-treated 16HBE cells. These results indicated that Lyn exhibited a dual regulatory role regarding IL-4/IL-13-induced STAT6 activity.
Muc5ac is elevated by IL-13 via a STAT6-dependent pathway in mouse lungs45, and previous studies strongly support a pivotal role for IL-4 or IL-13 and STAT6 activation in mucus hypersecretion and plugging in the airways30. Using siRNA targeting STAT6 in our present studies, STAT6 knockdown strongly decreased the IL-4/IL-13-induced Muc5ac promoter activity (Supplementary Figure S3). Moreover, considering the similar findings in the Lyn knockout mice exposed to allergen, our data suggest that the downregulation of mucin production resulting from a decrease in STAT6 phosphorylation is dependent on Lyn overexpression in Lyn transgenic mice exposed to allergen (Fig. 8A). Thus, our data suggest that STAT6 is strongly implicated in IL-4/IL-13-mediated mucin production in vitro.
However, the sequence analysis of the Muc5ac 5′ flanking region showed no consensus motif for STAT6 (5′-TTCN4GAA-3′)33. The metaplasia airway mucous cells are incompletely dependent on the JAK/STAT6 pathway45. Previous results have found that SPDEF plays a critical role in mediating IL-13-induced MUC5AC synthesis in a manner dependent on STAT6. Phosphorylation of p38 MAPK is involved in IL-13-induced mucus cell metaplasia46. The IL-13-induced mucin expression may be associated with TGF-β2 up-regulation47. These results implicate a different gene regulatory mechanism. Sequence analysis of the Muc5ac promoter region was performed using applied bioinformatics software (http://jaspar.genereg.net/). The Muc5ac promoter in the region contains two STAT6 binding site motifs (−879 ∼ +1 bp). Using ChIP analysis of tissue from WT and Lyn transgenic mice exposed to allergen, we report for the first time that Lyn plays a crucial role in the transcriptional regulation of MUC5AC expression through STAT6 and the identified STAT6 response element locates in the promoter of the MUC5AC gene in vivo.
In summary, we have found that overexpression of Lyn attenuates OVA-induced mucus hypersecretion, muc5ac expression and airway inflammation. We further showed that Lyn kinase regulates the expression and phosphorylation of STAT6, which is associated with MUC5AC and muc5ac expression in allergen-induced mice and IL-4/IL-13-treated cells. We have further identified a critical STAT6 binding site in the muc5ac promoter essential for gene induction in OVA-induced mucus hypersecretion in mice. This study indicates that Lyn kinase plays a central role in mucus hypersecretion in antigen-challenged mice. Lyn regulated STAT6, which was associated with the development and progression of mucus hypersecretion and MUC5AC expression in vivo and in vitro.
Additional Information
How to cite this article: Wang, X. et al. Lyn regulates mucus secretion and MUC5AC via the STAT6 signaling pathway during allergic airway inflammation. Sci. Rep. 7, 42675; doi: 10.1038/srep42675 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This project was supported by the Chinese National Science Foundation (81170032).
Footnotes
The authors declare no competing financial interests.
Author Contributions Conceived and designed the study: Guoping Li, Min Wu and Nanshan Zhong. Performed the experiments: Xiaoyun Wang, Yin Li, Deyu Luo, Xing Wang, and Yun Zhang. Analyzed the data: Xiaoyun Wang, Yin Li, Deyu Luo, Zhigang Liu, and Xing Wang. Wrote the paper: Guoping Li, Yin Li, and Min Wu. All authors read and approved the final manuscript.
References
- Johnson D. C. Airway mucus function and dysfunction. The New England journal of medicine 364, 978; author reply 978, doi: 10.1056/NEJMc1014719#SA1 (2011). [DOI] [PubMed] [Google Scholar]
- Rose M. C. & Voynow J. A. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiological reviews 86, 245–278, doi: 10.1152/physrev.00010.2005 (2006). [DOI] [PubMed] [Google Scholar]
- Evans C. M., Kim K., Tuvim M. J. & Dickey B. F. Mucus hypersecretion in asthma: causes and effects. Current opinion in pulmonary medicine 15, 4–11, doi: 10.1097/MCP.0b013e32831da8d3 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M. R. & Boucher R. C. Mucus clearance as a primary innate defense mechanism for mammalian airways. The Journal of clinical investigation 109, 571–577, doi: 10.1172/JCI15217 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft M. et al. Mycoplasma pneumoniae induces airway epithelial cell expression of MUC5AC in asthma. The European respiratory journal 31, 43–46, doi: 10.1183/09031936.00103307 (2008). [DOI] [PubMed] [Google Scholar]
- Evans C. M. et al. The polymeric mucin Muc5ac is required for allergic airway hyperreactivity. Nature communications 6, 6281, doi: 10.1038/ncomms7281 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin B. K., Priftis K. N., Schmidt H. J. & Henke M. O. Secretory hyperresponsiveness and pulmonary mucus hypersecretion. Chest 146, 496–507, doi: 10.1378/chest.13-2609 (2014). [DOI] [PubMed] [Google Scholar]
- Oh C. K., Geba G. P. & Molfino N. Investigational therapeutics targeting the IL-4/IL-13/STAT-6 pathway for the treatment of asthma. European respiratory review : an official journal of the European Respiratory Society 19, 46–54, doi: 10.1183/09059180.00007609 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman D. A. et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nature medicine 8, 885–889, doi: 10.1038/nm734 (2002). [DOI] [PubMed] [Google Scholar]
- Nakano T. et al. Niflumic acid suppresses interleukin-13-induced asthma phenotypes. American journal of respiratory and critical care medicine 173, 1216–1221, doi: 10.1164/rccm.200410-1420OC (2006). [DOI] [PubMed] [Google Scholar]
- Yu H., Li Q., Kolosov V. P., Perelman J. M. & Zhou X. Interleukin-13 induces mucin 5AC production involving STAT6/SPDEF in human airway epithelial cells. Cell communication & adhesion 17, 83–92, doi: 10.3109/15419061.2010.551682 (2010). [DOI] [PubMed] [Google Scholar]
- Yan F. et al. Interleukin-13-induced MUC5AC expression is regulated by a PI3K-NFAT3 pathway in mouse tracheal epithelial cells. Biochemical and biophysical research communications 446, 49–53, doi: 10.1016/j.bbrc.2014.02.051 (2014). [DOI] [PubMed] [Google Scholar]
- Foster P. S., Webb D. C., Yang M., Herbert C. & Kumar R. K. Dissociation of T helper type 2 cytokine-dependent airway lesions from signal transducer and activator of transcription 6 signalling in experimental chronic asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 33, 688–695 (2003). [DOI] [PubMed] [Google Scholar]
- Kuperman D. A. et al. IL-4 receptor signaling in Clara cells is required for allergen-induced mucus production. Journal of immunology 175, 3746–3752 (2005). [DOI] [PubMed] [Google Scholar]
- Miyata S. et al. STAT6 deficiency in a mouse model of allergen-induced airways inflammation abolishes eosinophilia but induces infiltration of CD8+ T cells. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 29, 114–123 (1999). [DOI] [PubMed] [Google Scholar]
- Ingley E. Functions of the Lyn tyrosine kinase in health and disease. Cell communication and signaling : CCS 10, 21, doi: 10.1186/1478-811X-10-21 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi S. et al. Src kinases mediate STAT growth pathways in squamous cell carcinoma of the head and neck. The Journal of biological chemistry 278, 31574–31583, doi: 10.1074/jbc.M303499200 (2003). [DOI] [PubMed] [Google Scholar]
- Pazdrak K., Olszewska-Pazdrak B., Stafford S., Garofalo R. P. & Alam R. Lyn, Jak2, and Raf-1 kinases are critical for the antiapoptotic effect of interleukin 5, whereas only Raf-1 kinase is essential for eosinophil activation and degranulation. The Journal of experimental medicine 188, 421–429 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janas M. L., Hodgkin P., Hibbs M. & Tarlinton D. Genetic evidence for Lyn as a negative regulator of IL-4 signaling. Journal of immunology 163, 4192–4198 (1999). [PubMed] [Google Scholar]
- Umemori H. et al. Impairment of N-methyl-D-aspartate receptor-controlled motor activity in LYN-deficient mice. Neuroscience 118, 709–713 (2003). [DOI] [PubMed] [Google Scholar]
- Goldenberg-Furmanov M. et al. Lyn is a target gene for prostate cancer: sequence-based inhibition induces regression of human tumor xenografts. Cancer research 64, 1058–1066 (2004). [DOI] [PubMed] [Google Scholar]
- Bates R. C., Edwards N. S., Burns G. F. & Fisher D. E. A CD44 survival pathway triggers chemoresistance via lyn kinase and phosphoinositide 3-kinase/Akt in colon carcinoma cells. Cancer research 61, 5275–5283 (2001). [PubMed] [Google Scholar]
- Beavitt S. J. et al. Lyn-deficient mice develop severe, persistent asthma: Lyn is a critical negative regulator of Th2 immunity. Journal of immunology 175, 1867–1875 (2005). [DOI] [PubMed] [Google Scholar]
- Li G. et al. Lyn mitigates mouse airway remodeling by downregulating the TGF-beta3 isoform in house dust mite models. Journal of immunology 191, 5359–5370, doi: 10.4049/jimmunol.1301596 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Liu Z., Zhong N., Liao B. & Xiong Y. Therapeutic effects of DNA vaccine on allergen-induced allergic airway inflammation in mouse model. Cellular & molecular immunology 3, 379–384 (2006). [PubMed] [Google Scholar]
- Suzaki Y. et al. A small-molecule compound targeting CCR5 and CXCR3 prevents airway hyperresponsiveness and inflammation. The European respiratory journal 31, 783–789, doi: 10.1183/09031936.00111507 (2008). [DOI] [PubMed] [Google Scholar]
- Pahl M. C. et al. Transcriptional (ChIP-Chip) Analysis of ELF1, ETS2, RUNX1 and STAT5 in Human Abdominal Aortic Aneurysm. International journal of molecular sciences 16, 11229–11258, doi: 10.3390/ijms160511229 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistemaker L. E. et al. Tiotropium attenuates IL-13-induced goblet cell metaplasia of human airway epithelial cells. Thorax 70, 668–676, doi: 10.1136/thoraxjnl-2014-205731 (2015). [DOI] [PubMed] [Google Scholar]
- Fujisawa T. et al. Regulation of airway MUC5AC expression by IL-1beta and IL-17A; the NF-kappaB paradigm. Journal of immunology 183, 6236–6243, doi: 10.4049/jimmunol.0900614 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman D., Schofield B., Wills-Karp M. & Grusby M. J. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. The Journal of experimental medicine 187, 939–948 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman D. A. & Schleimer R. P. Interleukin-4, interleukin-13, signal transducer and activator of transcription factor 6, and allergic asthma. Current molecular medicine 8, 384–392 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber H. P., Foley S. C. & Hamid Q. Mucin overproduction in chronic inflammatory lung disease. Canadian respiratory journal 13, 327–335 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H. W. et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. American journal of respiratory cell and molecular biology 37, 273–290, doi: 10.1165/rcmb.2005-0460OC (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. & Bertics P. J. Chemoattractant-induced signaling via the Ras-ERK and PI3K-Akt networks, along with leukotriene C4 release, is dependent on the tyrosine kinase Lyn in IL-5- and IL-3-primed human blood eosinophils. Journal of immunology 186, 516–526, doi: 10.4049/jimmunol.1000955 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H. et al. Streptochlorin suppresses allergic dermatitis and mast cell activation via regulation of Lyn/Fyn and Syk signaling pathways in cellular and mouse models. PloS one 8, e74194, doi: 10.1371/journal.pone.0074194 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurzem J. R. et al. Chronic inflammation is associated with an increased proportion of goblet cells recovered by bronchial lavage. Chest 100, 389–393 (1991). [DOI] [PubMed] [Google Scholar]
- Woodruff P. G. et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. American journal of respiratory and critical care medicine 180, 388–395, doi: 10.1164/rccm.200903-0392OC (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H. et al. Sensitivity of MUC5AC to topical corticosteroid is negatively associated with interleukin-17A in patients with allergic rhinitis. American journal of rhinology & allergy 26, 359–364, doi: 10.2500/ajra.2012.26.3795 (2012). [DOI] [PubMed] [Google Scholar]
- Hashimoto K. et al. Respiratory syncytial virus in allergic lung inflammation increases Muc5ac and gob-5. American journal of respiratory and critical care medicine 170, 306–312, doi: 10.1164/rccm.200301-030OC (2004). [DOI] [PubMed] [Google Scholar]
- Newcomb D. C. et al. Human TH17 cells express a functional IL-13 receptor and IL-13 attenuates IL-17A production. The Journal of allergy and clinical immunology 127, 1006–1013 e1001–1004, doi: 10.1016/j.jaci.2010.11.043 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rael E. L. & Lockey R. F. Interleukin-13 signaling and its role in asthma. The World Allergy Organization journal 4, 54–64, doi: 10.1097/WOX.0b013e31821188e0 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuh M., Morse B. A., Heidecker G. & Derse D. Association of SRC-related kinase Lyn with the interleukin-2 receptor and its role in maintaining constitutive phosphorylation of JAK/STAT in human T-cell leukemia virus type 1-transformed T cells. Journal of virology 85, 4623–4627, doi: 10.1128/JVI.00839-10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infantino S. et al. The tyrosine kinase Lyn limits the cytokine responsiveness of plasma cells to restrict their accumulation in mice. Science signaling 7, ra77, doi: 10.1126/scisignal.2005105 (2014). [DOI] [PubMed] [Google Scholar]
- Lau M. et al. Loss of STAT6 promotes autoimmune disease and atopy on a susceptible genetic background. Journal of autoimmunity 39, 388–397, doi: 10.1016/j.jaut.2012.06.003 (2012). [DOI] [PubMed] [Google Scholar]
- Thai P., Chen Y., Dolganov G. & Wu R. Differential regulation of MUC5AC/Muc5ac and hCLCA-1/mGob-5 expression in airway epithelium. American journal of respiratory cell and molecular biology 33, 523–530, doi: 10.1165/rcmb.2004-0220RC (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T. et al. Involvement of the p38 MAPK pathway in IL-13-induced mucous cell metaplasia in mouse tracheal epithelial cells. Respirology 13, 191–202, doi: 10.1111/j.1440-1843.2008.01237.x (2008). [DOI] [PubMed] [Google Scholar]
- Chu H. W. et al. Transforming growth factor-beta2 induces bronchial epithelial mucin expression in asthma. The American journal of pathology 165, 1097–1106 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.