Abstract
The short-wavelength sensitive (S-) opsin gene OPN1SW is pseudogenized in some nocturnal primates and retained in others, enabling dichromatic colour vision. Debate on the functional significance of this variation has focused on dark conditions, yet many nocturnal species initiate activity under dim (mesopic) light levels that can support colour vision. Tarsiers are nocturnal, twilight-active primates and exemplary visual predators; they also express different colour vision phenotypes, raising the possibility of discrete adaptations to mesopic conditions. To explore this premise, we conducted a field study in two stages. First, to estimate the level of functional constraint on colour vision, we sequenced OPN1SW in 12 wild-caught Philippine tarsiers (Tarsius syrichta). Second, to explore whether the dichromatic visual systems of Philippine and Bornean (Tarsius bancanus) tarsiers—which express alternate versions of the medium/long-wavelength sensitive (M/L-) opsin gene OPN1MW/OPN1LW—confer differential advantages specific to their respective habitats, we used twilight and moonlight conditions to model the visual contrasts of invertebrate prey. We detected a signature of purifying selection for OPN1SW, indicating that colour vision confers an adaptive advantage to tarsiers. However, this advantage extends to a relatively small proportion of prey–background contrasts, and mostly brown arthropod prey amid leaf litter. We also found that the colour vision of T. bancanus is advantageous for discriminating prey under twilight that is enriched in shorter (bluer) wavelengths, a plausible idiosyncrasy of understorey habitats in Borneo.
This article is part of the themed issue ‘Vision in dim light’.
Keywords: Tarsius, colour vision, opsins, nocturnality
1. Introduction
Light is a basic requirement for any image-forming visual system, yet most animals are routinely active under dark (scotopic) light conditions [1]. Given that vision is essential to survival and reproduction—underpinning central behaviours such as predator avoidance, foraging and mate recognition—many or most animals have traits that enable or enhance vision in darkness [1–4]. This truism extends to nocturnal mammals, but puzzling variation exists in their capacity for cone-mediated colour vision, a trait of negligible utility in darkness; i.e. light levels below the threshold of mammalian cone activation, approximately 0.02 cd m−2 [5–7]. In consequence, many scholars view the colour vision of nocturnal mammals as being immaterial to their visual ecology. A problem with this assumption is that it conflates the activity pattern of a species (nocturnality) with vision in darkness, ignoring behaviours under cone-active light conditions such as twilight. A greater appreciation for behaviours under dim (mesopic) light levels may inform the functional ecology of colour vision among nocturnal mammals, a topic we investigate here.
Colour vision is based on the expression of two or more spectrally distinct photoreceptors, and most mammals possess two classes of cone—those expressing short-wavelength (S-) and long-wavelength (L-) sensitive opsins [8]. A common exception to this pattern occurs when disabling mutations accumulate on the S-opsin gene (OPN1SW), resulting in M- or L-cone monochromatic vision, or colour blindness, a phenotype with several independent origins among scotopic-active mammals [9–13]. Some authors attribute degenerate opsins to the relaxation of natural selection under light-impoverished conditions, and view colour vision as a functionless anachronism for all nocturnal mammals [14]. This hypothesis is weakened, however, by systematic variation in closely related species with similar nocturnal behaviours. For example, S-opsin genes were lost in some lineages of bats, but retained for many millions of years in others [15–20]. The enduring preservation of dichromatic vision in some bats is strongly suggestive of one or more adaptive functions [17,18], a premise that informs recent research on nocturnal primates.
Primates are extremely visual mammals [21,22], with numerous derivations of the visual system, including convergent orbits, high concentrations of cones and ganglion cells, and (in haplorhine taxa) extreme cortical magnification of retinal foveal regions [23–26]. The collective function of these traits is to increase visual acuity, but they do so in tandem with colour vision, a trait of striking variation across primates. Some nocturnal primates are M- or L-cone monochromats, whereas others have retained the capacity for dichromatic vision [27–36], a pattern that has been interpreted as a state of evolutionary disequilibrium [14]. Under this view, dichromatic vision is a functionless anachronism for any nocturnal primate. Yet signatures of selection on OPN1SW speak to the adaptive function of dichromatic vision for tarsiers [32] and some nocturnal lemurs (Daubentonia [34], Lepilemur, Avahi, Microcebus [35]), demonstrating that colour vision is compatible with nocturnality even if it is incompatible with darkness.
This apparent paradox has fuelled interest in the visual ecology of nocturnal lemurs and shifted attention away from categorical classifications of darkness. Recent fieldwork has focused on dim-light conditions (twilight, moonlight [37]) and the integration of population genetics with field observations and spectral modelling [38–41]. Here we adopt a similar approach with tarsiers (Tarsius), a nocturnal haplorhine taxon. Tarsiers differ from lemurs in having a macula lutea and a fovea with much higher densities of cone photoreceptors [26], traits that enhance visual acuity and support visually mediated predation in the forest understorey [42]. Adaptations for greater visual sensitivity include hyper-enlarged eyes and orbits [24]. The latter trait has deep antiquity in the genus, occurring in Middle Eocene [43] and Middle Miocene [44] fossils. It follows that nocturnal visual predation is an enduring aspect of tarsier biology, yet some evidence points to a diurnal ancestor in the recent past. For example, the rod cell nuclei have a conventional architecture, a strong diurnal trait [45]; and different dichromatic phenotypes suggest an ancestral M/L-opsin polymorphism [29], a trait that appears to be incompatible with nocturnality.
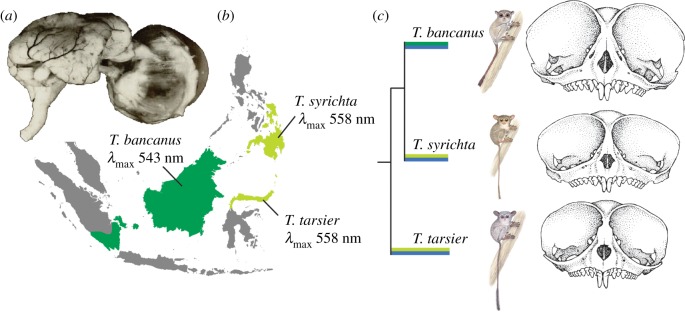
The dichromatic vision of Sulawesi (T. tarsier) and the Philippine tarsiers (T. syrichta) is based on an L-opsin gene [36], which corresponds with small and intermediate ocular morphologies in the genus, respectively [46,47]. In contrast, the dichromatic vision of Bornean tarsiers (T. bancanus) is based on an M-opsin gene [29] and coupled with extreme ocular hypertrophy [46,47]. The covariation of these visual traits is curious (figure 1), and it motivates two related questions: (i) is there an underlying signature of selection associated with the preservation of colour vision in tarsiers? and, if so, (ii) is phenotypic variation in colour vision better explained by genetic drift or natural selection? To explore these questions and to examine the evolution and visual ecology of tarsiers, we initiated an integrative study of opsin genes and prey colour.
Figure 1.
(a) Heinrich Sprankel's preparation of the eye and brain of T. bancanus [48] illustrates the similar volume of the two structures [46]. The eyes of T. bancanus are therefore enormous, both in absolute size and in proportion to the size of the 120–134 g animal. Polyak [49] concluded that the eye size relative to body size of tarsiers is unsurpassed by any mammal. (b) Geographical distribution of M- and L-opsins in Tarsius. (c) Covariation between M- and L-opsins and orbit size (tarsier illustrations © Stephen D. Nash/IUCN SSC Primate Specialist Group, reproduced with permission; skulls after Musser and Dagosto [47], drawn to scale).
2. Material and methods
(a). Study animals and sample acquisition for genetic analysis
Twelve adult or subadult tarsiers (T. syrichta; figure 2a) were hand-captured in the vicinity of Motorpool, Surigao del Norte, Mindanao, Philippines (9.633 N, 125.55 E) and anaesthetized for ca 1 h in association with another study (5–8 mg kg−1 Telazol, supplemented with 3 mg kg−1 Telazol or 15 µg kg−1 dexmedetomidine [52]). Small (2 mm) ear biopsies were collected with a punch tool (Miltex, York, USA) and immersed in a stabilization reagent (RNAlater, Qiagen) for storage and export. A topical antibiotic (chlorhexadine) was applied to each biopsy site. The animals were examined to evaluate general health and given subdermal fluids if needed. Each tarsier was released unharmed at its capture location.
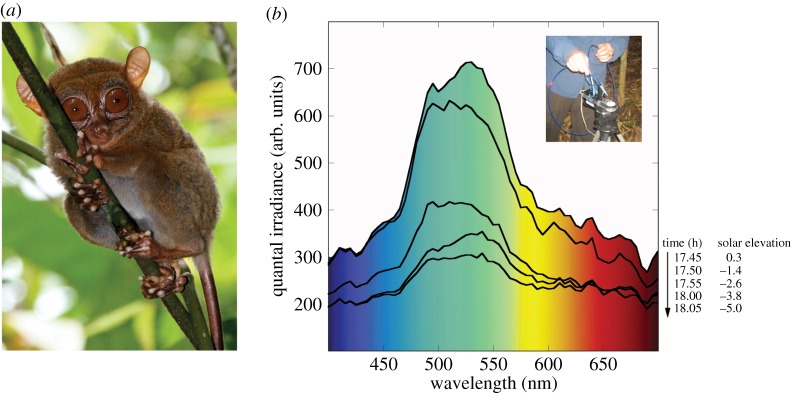
Figure 2.
(a) The Philippine tarsier (Tarsius syrichta) at our study site in Surigao del Norte, Mindanao, belongs to a distinct (Dinagat-Caraga) evolutionary lineage [50] (photograph by Andrew J. Cunningham, reproduced with permission). (b) Spectral composition of civil twilight in the forest understorey of Leyte, Philippines. Successive irradiance spectra correspond with the onset of tarsier activities. A broad peak at approximately 550 nm is typical of light transmission through canopy foliage [51]. Inset: USB2000 spectrometer modified for dim-light irradiance measures.
(b). Amplification and sequencing
We extracted genomic DNA (DNeasy blood and tissue kit, Qiagen) from each biopsy sample and amplified the S-opsin gene (OPN1SW) in two fragments, approximately 2 kb each. To identify the region of OPN1SW, we conducted a BLAST search of the reference genome tarSyr1 (T. syrichta) v. 66.1 in the Ensembl browser. Next, we used MEGA v. 5.0 [53] to align the sequence to the corresponding nucleotide sequence of T. bancanus (GenBank accession no. AB111463.1). Lastly, we used Primer3 [54] to design primers for polymerase chain reactions (PCRs; see electronic supplementary material, table S1).
To measure baseline nucleotide diversity, we also collected nucleotide sequence data from intergenic regions throughout the genome of T. syrichta. To avoid ascertainment bias, we used a random number generator to sample initial gene scaffolds from which we selected a sequence fragment of approximately 1 kb. Subsequent BLAST [55] and BLAT [56] analyses of the human genome sequence (GRCh37/hg19; assembly February 2009) allowed us to omit regions with putative gene identity and regions with highly repetitive elements. We then designed primers for the amplification and sequencing of six nuclear genome intergenic regions (see electronic supplementary material, table S1).
To amplify the OPN1SW fragments and intergenic regions, we carried out PCR using HotMaster Taq DNA Polymerase (5Prime), 10X HotMaster buffer (5Prime), 10 mM dNTPs (VWR) and 20 µM primers in 25 µl reactions. For the OPN1SW fragments, the PCR conditions were set at 95°C for 5 min followed by 35 cycles at 95°C for 30 s, 63°C for 30 s and 70°C for 120 s and a final hold at 70°C for 120 s. For our intergenic regions, the PCR conditions were set at 95°C for 5 min followed by 35 cycles at 95°C for 30 s, 60°C for 30 s and 70°C for 60 s and a final hold at 70°C for 120 s. All PCR products were purified using shrimp alkaline phosphatase and exonuclease I (Affymetrix). Nucleotide sequences were obtained using a 3730 DNA Analyser (Applied Biosystems) at the Molecular Biology Core Facility, Dartmouth College. All nucleotide sequences were deposited in GenBank (accession nos. KX132093-KX132101). Amplification was incomplete for three of 12 samples, and these were excluded from further analysis. For each of the nine remaining samples, the nucleotide sequences were aligned in Sequencher v. 4.2 (Gene Codes, Ann Arbor, MI) using the OPN1SW sequence of T. bancanus to identify exon–intron boundaries.
(c). OPN1SW and intergenic sequence analyses
To explore the functionality and potential variation of OPN1SW in our sample, we compiled and aligned all exon sequences and translated codons into amino acids. We also examined the critical sites that determine spectral absorbance [57,58]. Estimates of the nucleotide diversity population parameter θ for the OPN1SW gene and each of the six intergenic regions were calculated with two statistics: a sample-weighted estimate based on the number of single nucleotide polymorphisms (SNPs) per site (θW; [59]) and an estimate based on the average number of pairwise differences among sequences per site (θπ). The average estimates of θW and θπ for the six intergenic regions were weighted by fragment length. For OPN1SW, we examined each estimate of nucleotide diversity for non-synonymous and silent sites (synonymous sites + introns). We also computed Tajima's D [60] (which compares the values θW and θπ), to detect skews in the frequency distribution of SNPs.
Having no prior expectations for what neutral SNP frequency distribution would be, we performed coalescent simulations using the observed levels of variation at the intergenic regions, using both θW and θπ as expected neutral values. We simulated 5000 genealogies with no recombination to test how often the observed non-synonymous θπ fit simulated distributions under a standard model of neutrality. Finally, we compared the nucleotide diversity of non-synonymous (θπN) and silent sites (θπS) using the θπN/θπS ratio [34]. A ratio less than 1 would be consistent with purifying selection on non-synonymous SNP variation, whereas a ratio greater than or equal to 1 would suggest either relaxation of functional constraint or positive selection on non-synonymous SNP variation. Coalescent simulations, Tajima's D, θW and θπ estimates were calculated using DnaSP v. 4.1 [61].
(d). Irradiance and reflectance spectra
Tarsiers initiate travel and foraging under twilight [62–67]. We therefore measured the irradiance spectra of downwelling twilight in the understorey habitats of T. bancanus (Danum Valley Field Centre, Borneo, Malaysia [37]) and T. syrichta (Visayas State University, Leyte, Philippines [68,69]). Our instrumentation differed at each site. In Borneo, we used a spectrometer with a highly sensitive photomultiplier detector and an integrating sphere to ensure a cosine angular response (OL-770VIS; Gooch & Housego, Orlando, FL). For further details of the instrument and prevailing conditions, see Melin et al. [37]. In the Philippines, we used a portable spectrometer (USB2000; Ocean Optics, Dunedin, FL) modified for greater sensitivity by increasing the width of the entrance slit to 200 µm [70]. The USB2000 was fitted with a 1 mm diameter fibre-optic cable positioned at a 45° angle to a skyward-facing reflectance standard (WS1; Ocean Optics; figure 2b). The spectra were recorded at 1 nm intervals from 300 to 700 nm on 10 February 2010 (figure 2b).
Some tarsiers increase ranging and foraging activities under moonlight (lunar philia [71,72]), and some authors have suggested that moonlight could be sufficient for primate cone activation and colour discrimination [34,37]. We therefore measured the irradiance spectrum of moonlight (91% full) in the understorey habitat of T. bancanus. For further details, see Melin et al. [37].
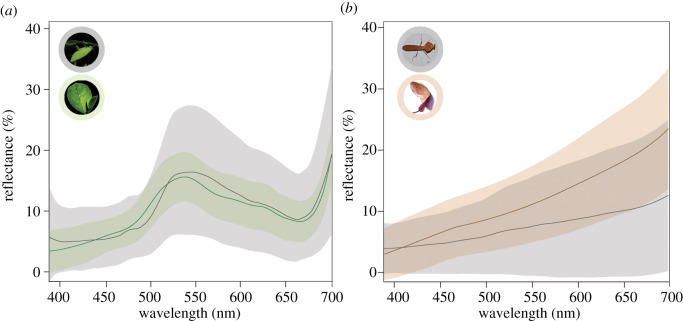
We recorded the reflectance spectra of invertebrate prey at four sites: (i) Motorpool, Surigao del Norte, Mindanao, Philippines (9.633 N, 125.550 E); (ii) Visayas State University, Baybay City, Leyte, Philippines (10.747 N, 124.803 E); (iii) Tangkoko Dua Saudara Nature Reserve, Bitung, Sulawesi, Indonesia (1.566 N, 125.233 E); and (iv) Cabang Panti Research Site, West Kalimantan, Borneo, Indonesia (1.216 S, 100.117 E). The inhabiting tarsiers are T. syrichta (sites i and ii), T. tarsier (site iii) and T. bancanus (site iv). At each site, we collected arthropod prey (mostly orthopteran insects) with sweep nets and by hand in areas where tarsiers were observed foraging. The reflectance spectra of prey and their corresponding backgrounds (mature foliage or leaf litter; figure 3) were measured with a USB2000 spectrometer calibrated against the WS1 reflectance standard.
Figure 3.
The reflectance spectra of (a) invertebrate green prey (n = 65) and mature foliage (n = 495) and (b) invertebrate brown prey (n = 110) and leaf litter (n = 200). Lines depict the mean reflectance spectrum, whereas shading represents the standard deviation.
(e). Luminance and chromaticity contrasts
The irradiance spectra of twilight and moonlight were used to estimate the radiance spectrum of prey items and to calculate the relative quantum catch (Qi = M/L, S) of cone photoreceptors. The quantum catches of the S and M/L cone classes were calculated by multiplying the reflectance spectrum of the stimulus, R(λ), the illumination spectra, I(λ), the spectral sensitivity function of the ith photoreceptor and integrating the resulting spectrum over wavelength (equation (2.1)) [73]. The quantum catches were modelled for both dichromatic phenotypes such that QL defines the luminance and QS/(QS + QL) defines the yellow-blueness.
| 2.1 |
To determine the conspicuousness of cryptic prey, we calculated the chromatic and luminance contrasts, or differences, of green prey and brown prey against a background of mature green foliage or leaf litter, respectively. These contrasts were calculated for 60 prey items against a sample of 60 corresponding background leaves, yielding a total of 3600 contrasts for each dichromatic phenotype for green and brown prey. Comparisons of significance were investigated using Welch's two sample t-tests. The significance for all tests was set at α = 0.05.
Finally, we calculated colour contrast comparisons in units of just notable differences (JNDs) using approximate cone densities [30] and considering quantum noise (calculated as the sum of neural and receptor noise, and proportional to the Weber fraction and inversely proportional to the intensity of the quantum catches [74]). When the colour contrast of two objects (target and background) produces a value that exceeds the threshold of one JND, the target is considered to be detectable against the background. Cone sensitivity curves were approximations based on an S-opsin λmax of 430 nm [29] and an M/L-opsin λmax of 543 or 558 nm for T. bancanus and T. syrichta, respectively [14]. Visual modelling, including estimates of cone sensitivity curves, was conducted with the package ‘pavo’ [75] in R v. 3.3.1 [76]. The effects of cone optical density and filtering by the macular pigment and lens were not considered.
(f). Measures of canopy structure (openness)
Variation in canopy openness is expected to affect the amount of light in the forest understorey. To estimate canopy openness, we collected hemispherical photographs with a tripod-mounted camera (CoolPix 8700 outfitted with an FC-E9 fish-eye lens; Nikon) at each study site, with additional data collected at the Danum Valley Field Centre, Sabah, Borneo, Malaysia (4.966 N, 117.800 E). Photographs were acquired at 10 m increments along 50 m transects in areas where tarsiers were observed foraging. To calculate canopy openness, we used Gap Light Analyser v. 2.0 [77]. We performed all statistical tests using R v. 3.3.1 [76] and JMP v. 11.0.0 for Macintosh.
3. Results and discussion
(a). Spectral absorbance of the S-opsin
We sequenced OPN1SW in nine individuals of T. syrichta. The coding sequences were free of indels (insertions/deletions), nonsense mutations and premature stop codons, indicating strict conservation and functional preservation of the gene. Among vertebrates, the spectral absorbance (λmax) of the S-opsin is determined by residues present at seven sites (46, 49, 52, 86, 93, 114 and 118) [57,58]. Here we detected Val46, Ser49, Ile52, Leu86, Pro93, Gly114 and Ser118, residues that indicate a λmax of 430 nm. This finding replicates an earlier study of T. syrichta [14] and agrees well with studies of T. bancanus [14,32].
(b). Evidence for purifying selection
Our analysis of nucleotide sequence data from six intergenic regions (3416 kb in total) revealed wide variance in estimates of SNP diversity, with two regions containing no polymorphism and one region containing four SNPs. We observed similar patterns for SNPs in magnitude and frequency, both for the overall dataset of 3416 bp (θW = 0.100% and θπ = 0.085%, respectively) and for the six regions independently (table 1). We found that θW and θπ were significantly different in one intergenic region (chr18q12.1); however, this difference did not differ statistically from our coalescent simulation (p = 0.98). Thus, it appears that variation within our intergenic regions can be considered an appropriate proxy for neutral variation in the tarsier genome.
Table 1.
Tarsier nucleotide diversity statistics for six autosomal intergenic regions.
| regiona | bpb | Sc | θw(%)d | θπ(%)e | Tajima's Df |
|---|---|---|---|---|---|
| chr2q11.2 | 681 | 0 | 0 | 0 | n.a. |
| chr6q24.2 | 700 | 0 | 0 | 0 | n.a. |
| chr4q24 | 724 | 1 | 0.039 | 0.026 | −0.592 |
| chr1q31.2 | 639 | 2 | 0.188 | 0.195 | 0.095 |
| chr2q24.1 | 672 | 3 | 0.122 | 0.160 | 0.801 |
| chr18q12.1 | 700 | 4 | 0.172 | 0.289 | 2.127f |
| total | 3416 | 10 | 0.100 | 0.085 | 0.530 |
aNames based on the corresponding chromosome position in humans (see Methods).
bIntergenic fragments were sequenced in nine individuals.
cNumber of SNPs.
dNucleotide diversity based on the number of SNPs [60].
eNucleotide diversity based on the average pairwise sequence divergence.
fθw and θπ were significantly different in only one comparison (p < 0.05). This difference did not reach significance using a coalescent simulation.
We compared variation in the intergenic regions with variation at non-synonymous, or amino acid-changing sites, in the OPN1SW gene. The levels of nucleotide diversity at non-synonymous sites were lower than those at intergenic regions (θπ = 0.041% versus θπ = 0.085%, respectively; table 2). We also used coalescent modelling to simulate neutral distributions of nucleotide diversity based on the observed levels of variation at our intergenic regions, both as θW and θπ, and then tested whether the observed nucleotide diversity at non-synonymous sites is lower than expected under neutral evolution. Under purifying selection, the observed nucleotide diversity at non-synonymous sites is expected to be lower than the simulated distributions, reflecting a history of natural selection removing or reducing non-synonymous diversity. Indeed, we found that the pattern of variation at non-synonymous sites in the S-opsin gene (θπ = 0.041%) was significantly lower than expected under neutrality (p = 0.012), suggesting purifying selection for retaining OPN1SW functionality.
Table 2.
Comparison of tarsier S-opsin and intergenic region nucleotide diversity.
| region | functional classa | total bp | Sb | θw (%)c | θπ (%)d |
|---|---|---|---|---|---|
| S-opsin (n = 9) | silent | 3005 | 7 | 0.074 | 0.064 |
| non-synonymous | 785 | 2 | 0.068 | 0.041 | |
| six autosomal intergenic | silent | 3416 | 10 | 0.100 | 0.085 |
aOpsin gene silent class includes intronic and synonymous coding region sites.
bNumber of SNPs.
cNucleotide diversity based on the number of SNPs [60].
dNucleotide diversity based on the average pairwise sequence divergence.
*θπN/θπs = 0.64 and is consistent with purifying selection.
For OPN1SW itself, we combined the introns and synonymous sites within exons into a ‘silent’ class of variation. Although selection may act on such sites, they better represent our expectation for neutral evolution compared with changes that alter amino acids and protein sequences. We compared the level of genetic diversity between the non-synonymous and silent sites [34]. Under neutrality, the frequency distributions of these two classes of diversity are expected to be similar, yielding a value ≈1 for the statistic θπN/θπs. Values substantially less than or greater than 1 may signify negative (purifying) or positive selection, respectively. Our estimate of θπN/θπS is 0.64, consistent with a history of purifying selection on non-synonymous mutations (table 2).
Taken together, our analysis indicates the functional preservation of OPN1SW, and therefore, dichromatic vision, in a population of Philippine tarsiers. This finding reinforces an earlier result based on T. bancanus [32] and complements recent studies of nocturnal lemurs (Daubentonia [34], Lepilemur, Avahi and Microcebus [35]). The preservation of colour vision in these nocturnal primates, and not others, is an enduring puzzle, and it motivated our exploration of dim-light conditions and the potential advantages of chromatic discrimination.
(c). Activities during sunset and civil twilight
For tarsiers, sunset and civil twilight (when the solar elevation angle, θs, is between 0° and −6°) appear to be important cues for initiating activity. Observations of tarsiers exiting their sleeping sites and initiating travel are strikingly consistent across species, occurring 15 min before sunset [63] or between 18.00 and 18.15 hours (range: 17.45–19.10 hours) [62–67]. In Borneo, Crompton & Andau [64] recorded light levels at the onset of activity (mean: 6.8 lux; range: 1.4–15 lux), demonstrating behavioural synchronization with light conditions that support colour vision. It is perhaps significant that nocturnal lemurs are similarly attuned to prevailing light conditions, but mono- and dichromatic taxa (Phaner and Lepilemur, respectively [35]) initiate activities under much darker conditions [78].
Here we report the spectral composition of civil twilight in the understorey of our study site on Leyte, Philippines (figure 2b). We found that T. syrichta initiated travel in the light with a peak of approximately 550 nm, a green colour that is typical of light transmitted through canopy foliage [51]. This spectrum differs from that of Melin et al. [37], who recorded understorey irradiance in Sabah, Borneo at 18.50 (θs of −6°). Their spectrum contained a broad peak around 450 nm, a decidedly blue colour that is typical of civil twilight in open habitats [51,70,79].
Differences in the spectral composition of understorey light can be attributed to differences in canopy openness. Denser canopy foliage will attenuate and filter downwelling light to a greater extent (resulting in darker, greener understoreys) [51]; and it follows that systematic variation in canopy openness will exert large effects on the colour and amount of light available to tarsiers. We therefore examined geographical variation in canopy openness to better contextualize differences in their visual systems.
(d). Geographical variation in canopy openness
We examined 150 hemispherical canopy photos from Borneo (Danum Valley and Cabang Panti; n = 99) and the Philippines (our field sites on Leyte and Mindanao; n = 51) and compared per cent site openness. Our results suggest that the forest canopies of Borneo are more open (F23.51, d.f. = 140, p < 0.0001) and more variable (Levene's test, p < 0.0006) than those of Leyte and Mindanao (figure 4). Although it is premature to characterize large heterogeneous islands on the basis of such limited data, our results support the enduring perception of Bornean canopies as being relatively porous, particularly those habitats dominated by dipterocarp trees [80].
Figure 4.
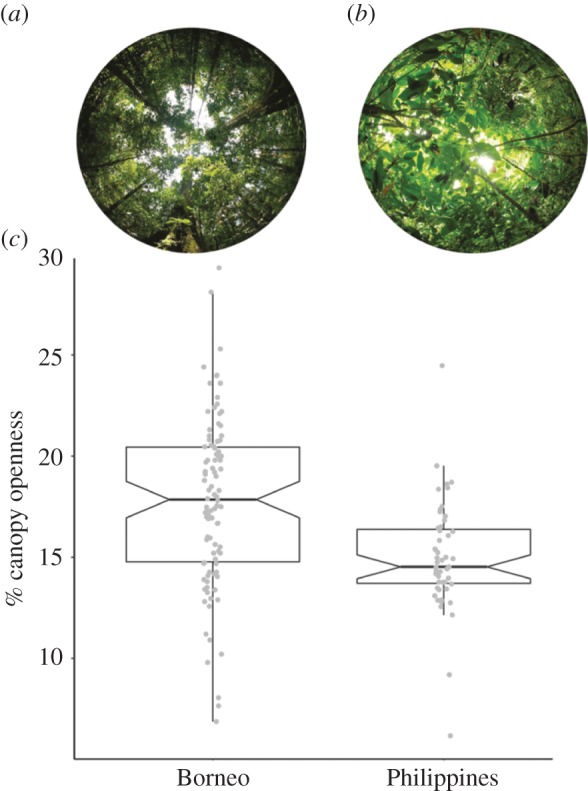
Representative hemispherical photographs from (a) Gunung Palung National Park, Borneo and (b) Mindanao, Philippines. (c) Notched box plots illustrate varying levels of canopy openness in the forests of Borneo (n = 3 sites; 99 total photographs) and the Philippines (n = 2 sites; 51 total photographs), as measured by hemispherical photography. Taken together, the two tarsier-inhabited forests of Borneo are more open (p < 0.0001) and more variable (p < 0.0006) than those in the Philippines. (Online version in colour.)
This result also speaks to the differential history of cyclone disturbance across insular Southeast Asia; cyclones seldom reach Borneo, but they visit the islands of Leyte and Mindanao regularly (see electronic supplementary material, figure S1). The effects of cyclones on understorey light levels are twofold: initially, there is an immediate increase in the light owing to the removal of canopy vegetation; however, light is soon extinguished by the rapid growth of understorey vegetation as it fills vacant space in the mid- and upper canopy [81,82]. Counterintuitively, then, a history of recurrent disturbance will produce darker understoreys, and it stands to reason that Philippine tarsiers (T. syrichta) are light-limited relative to Bornean tarsiers (T. bancanus). This conjecture requires testing, but it has the advantage of framing curious morphological patterns, such as the apparent trade-off between relative eye size and ear size in the genus [83] (figure 1). It is tempting to suggest that darker understorey conditions favour degenerate eyes and a greater reliance on auditory localization.
(e). Visual modelling results
Our working hypothesis—that geographical variation in understorey light conditions has shaped the evolution of tarsier visual systems—has the advantage of generating testable predictions. For example, given that the performance of photoreceptors diminishes as they become photon-noise-limited, the λmax of opsins could be important for maximizing signal-to-noise ratios in dim light. To test this prediction, we estimated the chromatic contrasts of arthropod prey and calculated JNDs under site-specific twilight conditions and standard moonlight conditions.
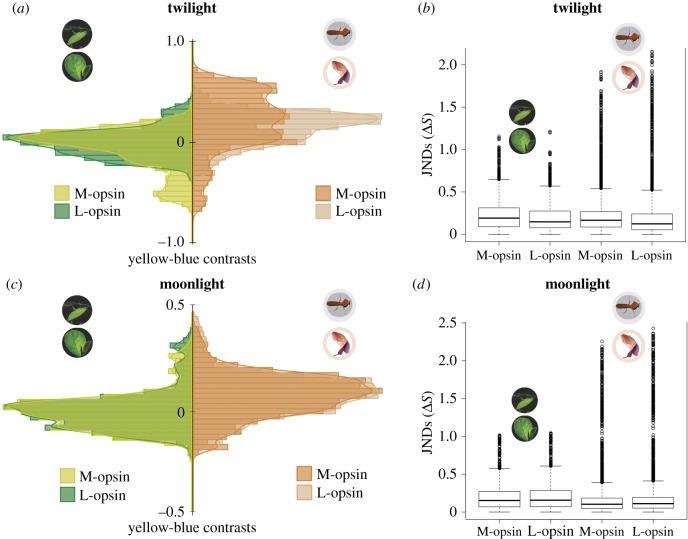
We found that most prey–background combinations were well matched chromatically, and that each colour vision phenotype produced distinct distributions of chromatic contrasts in twilight (green prey: t14.86, d.f. = 6283.9, p < 0.001; brown prey: t−11.84, d.f. = 5611.5, p < 0.001; figure 5a). We also detected phenotypic differences in the discrimination of achromatic (luminance) contrasts (green prey: t10.00, d.f. = 5428.1, p < 0.001; brown prey: t−14.54, d.f. = 5711.6, p < 0.001). However, only a small proportion of the prey–background contrasts exceeded 1 JND, a theoretical threshold for chromatic discrimination (figure 5b). Under moonlight, we detected no phenotypic differences in chromatic discrimination (green prey: t1.13, d.f. = 7185.2, p = 0.26; brown prey: t0.01, d.f. = 7196.2, p = 0.96; figure 5c). Similarly, we found no phenotypic difference in the luminance contrasts of green prey (t1.40, d.f. = 7161.6, p = 0.16); however, we did detect a difference among brown prey (t−2.66, d.f. = 7185.2, p = 0.008). Again, only a small proportion of prey–background contrasts exceeded 1 JND, a theoretical threshold for chromatic discrimination (figure 5d).
Figure 5.
(a) Distributions of chromatic contrasts for arthropod prey (green prey: n = 60; brown prey: n = 60) against representative mature leaves (n = 60) or leaf litter (n = 60) backgrounds under Bornean and Philippine twilight conditions for the M- and L-opsin phenotypes, respectively. (b) Prey–background chromatic contrasts under twilight conditions in units of JNDs; values >1 exceed the theoretical detection threshold. (c) Distributions of chromatic contrasts for arthropod prey (green prey: n = 60; brown prey: n = 60) against representative mature leaves (n = 60) or leaf litter (n = 60) backgrounds under moonlight conditions for the M- and L-opsin phenotypes. (d) Prey–background chromatic contrasts under moonlight conditions in units of JNDs; values >1 exceed the theoretical detection threshold.
The significance of these findings is threefold. First, they suggest that mesopic-active tarsiers are unable to discriminate a majority of prey on the basis of colour vision. Second, they suggest that the M-opsin of T. bancanus is advantageous for discriminating prey in twilight that is enriched in shorter (bluer) wavelengths, a plausible idiosyncrasy of understorey habitats across much of Borneo. Third, the greatest potential for chromatic discrimination was always associated with brown arthropod prey, suggesting that visual predation amid leaf litter is perhaps the primary activity that drives the evolution of tarsier opsins.
(f). Summary conclusions
Our findings reveal a signature of purifying selection for OPN1SW, indicating that dichromatic colour vision confers an adaptive advantage to tarsiers. However, the nature of this advantage is uncertain. We measured the spectral composition of twilight and moonlight in the understorey habitats of tarsiers and modelled 7200 prey–background combinations, finding few chromatic contrasts. At the same time, our models suggest that the colour vision of tarsiers can discriminate some prey under dim light, particularly brown prey amid leaf litter. Our models also suggest that the M-opsin of T. bancanus is advantageous for visual predation in the relatively light-enriched understorey habitats of Borneo. Taken together, our findings suggest that the M-opsin gene and hyper-enlarged eyes of T. bancanus are coupled, adaptive derivations within Tarsius. However, if Borneo was colonized by a small founding population with an M/L-opsin gene polymorphism [36], then fixation of the M-opsin allele by drift [84] is also plausible.
Supplementary Material
Acknowledgements
The authors thank the organizers, E. Warrant and D. O'Carroll, for the invitation to contribute to this themed issue and for constructive comments. We thank the Mamanwa, S. F. Matugas (Governor of Surigao del Norte), L. H. Dagsaan and M. G. T. Pascua (NCIP National and Regional Offices), E. S. Buiser (DENR Regional Executive Director, Region 10), T. M. S. Lim (Director, DENR-PAWB), M. R. Duya, M. Pedregosa-Hospodarsky and A. U. Luczon (Institute of Biology, UP Diliman), B. E. B. Magsaganay (Mamanwa Tribal Leader), and the following individuals: E. M. Bade Jr., M. L. Eludo, L. M. Gales, J. D. Quiñonez and G. A. Romarate Jr. For logistical support at Visayas State University, we thank V. B. Asio and M. J. Ceniza. We also acknowledge the following individuals for assistance: B. Calsbeek, R. G. Calsbeek, R. M. Cox, A. J. Cunningham, M. C. Duryea, L. R. Heaney, P. W. Lucas, A. J. Marshall, D. O. Schmitt, H. M. ter Hofstede, E. R. Vogel and C. V. Williams.
Ethics
Animal research was approved by the Institutional Animal Care and Use Committee, Dartmouth College (protocol nos. 10-11-02 and 11-06-06AT). Research in the Philippines was approved by the Office of the Punong Barangay, the Office of the Municipal Mayor, Congressman G. A. Romarate, the National Commission on Indigenous Peoples, and the Protected Areas and Wildlife Bureau of the Department of Environment and Natural Resources (DENR; Gratuitous Permit no. R13-2010-00 and Local Transport Permit no. R13-2010-004). Tissue export and import was approved, respectively, by the DENR (CITES permit no. 14281 A-2010) and the US Centers for Disease Control and Prevention (permit no. 2010-07-146). Research in Sabah, Borneo, Malaysia was approved by the Danum Valley Management Committee and the Sabah Biodiversity Council (permit nos. DVMC 2011/05 and JKM/MBS.1000-2/2(26)). Research in West Kalimantan, Borneo, Indonesia was approved by the Indonesian State Ministry for Research and Technology (RISTEK; permit no. 0185/FRP/SM/VIII/2009).
Data accessibility
Sequence data has been deposited on GenBank under accession numbers: KX132093-KX132101. Spectral data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.q29c0.
Authors' contributions
G.L.M. and N.J.D. conceived the study. G.L.M. and P.S.O. conducted field research. G.H.P. designed molecular methods. G.L.M. performed the lab work and ensuing analyses. G.L.M. and N.J.D. drafted the manuscript and all authors were involved in revising drafts. All authors contributed to the overall design and interpretation.
Competing interests
We have no competing interests.
Funding
Funding was received from the David and Lucile Packard Foundation (Fellowship in Science and Engineering no. 2007-31754 N.J.D.) and a Sigma Xi Grant-in-Aid of Research (no. G2009102163 to G.L.M.).
References
- 1.Warrant EJ, Johnsen S. 2013. Vision and the light environment. Curr. Biol. 23, R990–R994. ( 10.1016/j.cub.2013.10.019) [DOI] [PubMed] [Google Scholar]
- 2.Davies WL, Collin SP, Hunt DM. 2012. Molecular ecology and adaptation of visual photopigments in craniates. Mol. Ecol. 21, 3121–3158. ( 10.1111/j.1365-294X.2012.05617.x) [DOI] [PubMed] [Google Scholar]
- 3.Johnsen S. 2012. The optics of life. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Cronin TW, Johnsen S, Marshall NJ, Warrant EJ. 2014. Visual ecology. Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.Roth LSV, Balkenius A, Kelber A. 2008. The absolute threshold of colour vision in the horse. PLoS ONE 3, e3711 ( 10.1371/journal.pone.0003711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umino Y, Solessio E, Barlow RB. 2008. Speed, spatial, and temporal tuning of rod and cone vision in mouse. J. Neurosci. 28, 189–198. ( 10.1523/jneurosci.3551-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naarendorp F, Esdaille TM, Banden SM, Andrews-Labenski J, Gross OP, Pugh EN. 2010. Dark light, rod saturation, and the absolute and incremental sensitivity of mouse cone vision. J. Neurosci. 30, 12 495–12 507. ( 10.1523/jneurosci.2186-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peichl L. 2005. Diversity of mammalian photoreceptor properties: adaptations to habitat and lifestyle? Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 287, 1001–1012. ( 10.1002/ar.a.20262) [DOI] [PubMed] [Google Scholar]
- 9.Jacobs GH. 2013. Losses of functional opsin genes, short-wavelength cone photopigments, and color vision—a significant trend in the evolution of mammalian vision. Visual Neurosci. 30, 39–53. ( 10.1017/S0952523812000429) [DOI] [PubMed] [Google Scholar]
- 10.Meredith RW, Gatesy J, Emerling CA, York VM, Springer MS. 2013. Rod monochromacy and the coevolution of cetacean retinal opsins. PLoS Genet. 9, e1003432 ( 10.1371/journal.pgen.1003432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emerling CA, Springer MS. 2014. Eyes underground: regression of visual protein networks in subterranean mammals. Mol. Phylogenet. Evol. 78, 260–270. ( 10.1016/j.ympev.2014.05.016) [DOI] [PubMed] [Google Scholar]
- 12.Emerling CA, Springer MS. 2014. Genomic evidence for rod monochromacy in sloths and armadillos suggests early subterranean history for Xenarthra. Proc. R. Soc. B 282, 20142192 ( 10.1098/rspb.2014.2192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melin AD, et al. 2016. Euarchontan opsin variation brings new focus to primate origins. Mol. Biol. Evol. 33, 1029–1041. ( 10.1093/molbev/msv346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan Y, Yoder AD, Yamashita N, Li W-H. 2005. Evidence from opsin genes rejects nocturnality in ancestral primates. Proc. Natl Acad. Sci. USA 102, 14 712–14 716. ( 10.1073/pnas.0507042102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D, Oakley T, Mower J, Shimmin LC, Yim S, Honeycutt RL, Tsao H, Li WH. 2004. Molecular evolution of bat color vision genes. Mol. Biol. Evol. 21, 295–302. ( 10.1093/molbev/msh015) [DOI] [PubMed] [Google Scholar]
- 16.Müller B, Goodman SM, Peichl L. 2007. Cone photoreceptor diversity in the retinas of fruit bats (Megachiroptera). Brain Behav. Evol. 70, 90–104. ( 10.1159/000102971) [DOI] [PubMed] [Google Scholar]
- 17.Müller B, Glösmann M, Peichl L, Knop GC, Hagemann C, Ammermüller J. 2009. Bat eyes have ultraviolet-sensitive cone photoreceptors. PLoS ONE 4, e6390 ( 10.1371/journal.pone.0006390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H, Rossiter SJ, Teeling EC, Li C, Cotton JA, Zhang S. 2009. The evolution of color vision in nocturnal mammals. Proc. Natl Acad. Sci. USA 106, 8980–8985. ( 10.1073/pnas.0813201106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao H, Xu D, Zhou Y, Flanders J, Zhang S. 2009. Evolution of opsin genes reveals a functional role of vision in the echolocating little brown bat (Myotis lucifugus). Biochem. Syst. Ecol. 37, 154–161. ( 10.1016/j.bse.2009.03.001) [DOI] [Google Scholar]
- 20.Melin AD, Danosi CF, McCracken GF, Dominy NJ. 2014. Dichromatic vision in a fruit bat with diurnal proclivities: the Samoan flying fox (Pteropus samoensis). J. Comp. Physiol. A 200, 1015–1022. ( 10.1007/s00359-014-0951-x) [DOI] [PubMed] [Google Scholar]
- 21.Le Gros Clark WE. 1971. The antecedents of man. Edinburgh, UK: Edinburgh University Press. [Google Scholar]
- 22.Martin RD. 1990. Primate origins and evolution: a phylogenetic reconstruction. Princeton, NJ: Princeton University Press. [Google Scholar]
- 23.Walls GL. 1942. The vertebrate eye and its adaptive radiation. Bloomfield Hills, MI: Cranbrook Institute of Science. [Google Scholar]
- 24.Allman J. 1977. Evolution of the visual system in the early primates. Prog. Psychobiol. Physiol. Psychol 7, 1–53. [Google Scholar]
- 25.Ross CF. 2000. Into the light: the origin of Anthropoidea. Annu. Rev. Anthropol. 29, 147–194. ( 10.1146/annurev.anthro.29.1.147) [DOI] [Google Scholar]
- 26.Ross CF. 2004. The tarsier fovea: functionless vestige or nocturnal adaptation? In Anthropoid origins: new visions (eds Ross CF, Kay RF), pp. 477–537. New York, NY: Kluwer Academic/Plenum Publishers. [Google Scholar]
- 27.Wikler K, Rakic P. 1990. Distribution of photoreceptor subtypes in the retina of diurnal and nocturnal primates. J. Neurosci. 10, 3390–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs GH, Neitz M, Neitz J. 1996. Mutations in S-cone pigment genes and the absence of colour vision in two species of nocturnal primate. Proc. R. Soc. Lond. B 263, 705–710. ( 10.1098/rspb.1996.0105) [DOI] [PubMed] [Google Scholar]
- 29.Tan Y, Li W-H. 1999. Trichromatic vision in prosimians. Nature 402, 36 ( 10.1038/46947) [DOI] [PubMed] [Google Scholar]
- 30.Hendrickson A, Djajadi HR, Nakamura L, Possin DE, Sajuthi D. 2000. Nocturnal tarsier retina has both short and long/medium-wavelength cones in an unusual topography. J. Comp. Neurol. 424, 718–730. ( 10.1002/1096-9861(20000904)424:4%3C718::aid-cne12%3E3.0.co;2-z) [DOI] [PubMed] [Google Scholar]
- 31.Dkhissi-Benyahya O, Szel A, Degrip WJ, Cooper HM. 2001. Short and mid-wavelength cone distribution in a nocturnal strepsirrhine primate (Microcebus murinus). J. Comp. Neurol. 438, 490–504. ( 10.1002/cne.1330) [DOI] [PubMed] [Google Scholar]
- 32.Kawamura JH, Kubotera N. 2004. Ancestral loss of short wave-sensitive cone visual pigment in lorisiform prosimians, contrasting with its strict conservation in other prosimians. J. Mol. Evol. 58, 314–321. ( 10.1007/s00239-003-2553-z) [DOI] [PubMed] [Google Scholar]
- 33.Levenson DH, Fernandez-Duque E, Evans S, Jacobs GH. 2007. Mutational changes in S-cone opsin genes common to both nocturnal and cathemeral Aotus monkeys. Am. J. Primatol. 69, 757–765. ( 10.1002/ajp.20402) [DOI] [PubMed] [Google Scholar]
- 34.Perry GH, Martin RD, Verrelli BC. 2007. Signatures of functional constraint at aye-aye opsin genes: the potential of adaptive color vision in a nocturnal primate. Mol. Biol. Evol. 24, 1963–1970. ( 10.1093/molbev/msm124) [DOI] [PubMed] [Google Scholar]
- 35.Veilleux CC, Louis EE, Bolnick DA. 2013. Nocturnal light environments influence color vision and signatures of selection on the OPN1SW opsin gene in nocturnal lemurs. Mol. Biol. Evol. 30, 1420–1437. ( 10.1093/molbev/mst058) [DOI] [PubMed] [Google Scholar]
- 36.Melin AD, Matsushita Y, Moritz GL, Dominy NJ, Kawamura S. 2013. Inferred L/M cone opsin polymorphism of ancestral tarsiers sheds dim light on the origin of anthropoid primates. Proc. R. Soc. B 280, 20130189 ( 10.1098/rspb.2013.0189) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melin AD, Moritz GL, Fosbury RAE, Kawamura S, Dominy NJ. 2012. Why aye-ayes see blue. Am. J. Primatol. 74, 185–192. ( 10.1002/ajp.21996) [DOI] [PubMed] [Google Scholar]
- 38.Veilleux CC, Cummings ME. 2012. Nocturnal light environments and species ecology: implications for nocturnal color vision in forests. J. Exp. Biol. 215, 4085–4096. ( 10.1242/jeb.071415) [DOI] [PubMed] [Google Scholar]
- 39.Valenta K, Burke RJ, Styler SA, Jackson DA, Melin AD, Lehman SM. 2013. Colour and odour drive fruit selection and seed dispersal by mouse lemurs. Sci. Rep. 3, 2424 ( 10.1038/srep02424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veilleux CC, Jacobs RL, Cummings ME, Louis EE, Bolnick DA. 2014. Opsin genes and visual ecology in a nocturnal folivorous lemur. Int. J. Primatol. 35, 88–107. ( 10.1007/s10764-013-9708-6) [DOI] [Google Scholar]
- 41.Valenta K, et al. 2015. Visual ecology of true lemurs suggests a cathemeral origin for the primate cone opsin polymorphism. Funct. Ecol. 30, 932–942. ( 10.1111/1365-2435.12575) [DOI] [Google Scholar]
- 42.Moritz GL, Melin AD, Tuh Yit Yu F, Bernard H, Ong PS, Dominy NJ. 2014. Niche convergence suggests functionality of the nocturnal fovea. Front. Integr. Neurosci. 8, 61 ( 10.3389/fnint.2014.00061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossie JB, Ni X, Beard C. 2006. Cranial remains of an Eocene tarsier. Proc. Natl Acad. Sci. USA 103, 4381–4385. ( 10.1073/pnas.0509424103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chaimanee Y, Lebrun R, Yamee C, Jaeger J-J. 2011. A new Middle Miocene tarsier from Thailand and the reconstruction of its orbital morphology using a geometric-morphometric method. Proc. R. Soc. B 278, 1956–1963. ( 10.1098/rspb.2010.2062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joffe B, Peichl L, Hendrickson A, Leonhardt H, Solovei I. 2014. Diurnality and nocturnality in primates: an analysis from the rod photoreceptor nuclei perspective. Evol. Biol. 41, 1–11. ( 10.1007/s11692-013-9240-9) [DOI] [Google Scholar]
- 46.Castenholz A. 1984. The eye of Tarsius. In Biology of tarsiers (ed. Niemitz C.), pp. 303–318. Stuttgart, Germany: Gustav Fischer Verlag. [Google Scholar]
- 47.Musser GG, Dagosto M. 1987. The identity of Tarsius pumilus, a pygmy species endemic to the montane mossy forests of Central Sulawesi. Am. Mus. Novit. 2867, 1–53. [Google Scholar]
- 48.Sprankel H. 1965. Untersuchungen an Tarsius I. Morphologie des schwanzes nebst ethologischen bemerkungen. Folia Primatol. 3, 153–188. ( 10.1159/000155027) [DOI] [PubMed] [Google Scholar]
- 49.Polyak S. 1957. The vertebrate visual system. Chicago, IL: University of Chicago Press. [Google Scholar]
- 50.Brown RM, et al. 2014. Conservation genetics of the Philippine tarsier: cryptic genetic variation restructures conservation priorities for an island archipelago primate. PLoS ONE 9, e104340 ( 10.1371/journal.pone.0104340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Endler JA. 1993. The color of light in forests and its implications. Ecol. Monogr. 63, 1–27. ( 10.2307/2937121) [DOI] [Google Scholar]
- 52.Ramsier MA, Cunningham AJ, Moritz GL, Finneran JJ, Williams CV, Ong PS, Gursky-Doyen SL, Dominy NJ. 2012. Primate communication in the pure ultrasound. Biol. Lett. 8, 508–511. ( 10.1098/rsbl.2011.1149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739. ( 10.1093/molbev/msr121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. 2012. Primer3 - new capabilities and interfaces. Nucleic Acids Res. 40, e115 ( 10.1093/nar/gks596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215, 403–410. ( 10.1016/S0022-2836(05)80360-2) [DOI] [PubMed] [Google Scholar]
- 56.Kent WJ. 2002. BLAT – the BLAST-like alignment tool. Genome Res. 4, 656–664. ( 10.1101/gr.229202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi Y, Yokoyama S. 2003. Molecular analysis of the evolutionary significance of ultraviolet vision in vertebrates. Proc. Natl Acad. Sci. USA 100, 8308–8313. ( 10.1073/pnas.1532535100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carvalho LS, Davies WL, Robinson PR, Hunt DM. 2011. Spectral tuning and evolution of primate short-wavelength-sensitive visual pigments. Proc. R. Soc. B 279, 387–393. ( 10.1098/rspb.2011.0782) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watterson GA. 1975. On the number of segregating sites in genetical models without recombination. Theor. Popul. Biol. 7, 256–276. ( 10.1016/0040-5809(75)90020-9) [DOI] [PubMed] [Google Scholar]
- 60.Tajima F. 1989. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123, 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rozas J, Sánchez-DelBarrio JC, Messeguer X, Rozas R. 2003. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19, 2496–2497. ( 10.1093/bioinformatics/btg359) [DOI] [PubMed] [Google Scholar]
- 62.MacKinnon J, MacKinnon K. 1980. The behavior of wild spectral tarsiers. Int. J. Primatol. 1, 361–379. ( 10.1007/BF02692280) [DOI] [Google Scholar]
- 63.Niemitz C. 1984. Activity rhythms and use of space in semi-wild Bornean tarsiers, with remarks on wild spectral tarsiers. In Biology of tarsiers (ed. Niemitz C.), pp. 85–115. Stuttgart, Germany: Gustav Fischer. [Google Scholar]
- 64.Crompton RH, Andau PM. 1987. Ranging, activity rhythms, and sociality in free-ranging Tarsius bancanus: a preliminary report. Int. J. Primatol. 8, 43–71. ( 10.1007/bf02737113) [DOI] [Google Scholar]
- 65.Neri-Arboleda I, Stott P, Arboleda NP. 2002. Home ranges, spatial movements and habitat associations of the Philippine tarsier (Tarsius syrichta) in Corella, Bohol. J. Zool. 257, 387–402. ( 10.1017/S0952836902000997) [DOI] [Google Scholar]
- 66.Merker S. 2006. Habitat-specific ranging patterns of Dian's tarsiers (Tarsius dianae) as revealed by radiotracking. Am. J. Primatol. 68, 111–125. ( 10.1002/ajp.20210) [DOI] [PubMed] [Google Scholar]
- 67.Grow N, Gursky-Doyen S. 2010. Preliminary data on the behavior, ecology, and morphology of pygmy tarsiers (Tarsius pumilus). Int. J. Primatol. 31, 1174–1191. ( 10.1007/s10764-010-9456-9) [DOI] [Google Scholar]
- 68.Dagosto M, Gebo DL, Dolino C. 2001. Positional behavior and social organization of the Philippine tarsier (Tarsius syrichta). Primates 42, 233–243. ( 10.1007/bf02629639) [DOI] [Google Scholar]
- 69.Dagosto M, Gebo DL, Dolino CN. 2003. The natural history of the Philippine tarsier (Tarsius syrichta). In Tarsiers: past, present, and future (eds Wright PC, Simons EL, Gursky S), pp. 237–259. New Brunswick, NJ: Rutgers University Press. [Google Scholar]
- 70.Johnsen S, Kelber A, Warrant E, Sweeney AM, Widder EA, Lee RL, Hernandez-Andres J. 2006. Crepuscular and nocturnal illumination and its effects on color perception by the nocturnal hawkmoth Deilephila elpenor. J. Exp. Biol. 209, 789–800. ( 10.1242/jeb.02053) [DOI] [PubMed] [Google Scholar]
- 71.Gursky S. 2003. Lunar philia in a nocturnal primate. Int. J. Primatol. 24, 351–367. ( 10.1023/A:1023053301059) [DOI] [Google Scholar]
- 72.Gursky SL. 2007. The spectral tarsier. Upper Saddle River, NJ: Pearson. [Google Scholar]
- 73.Osorio D, Smith AC, Vorobyev M, Buchanan-Smith HM. 2004. Detection of fruit and the selection of primate visual pigments for color vision. Am. Nat. 164, 696–708. ( 10.1086/425332) [DOI] [PubMed] [Google Scholar]
- 74.Vorobyev M, Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358. ( 10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maia R, Eliason CM, Bitton PP, Doucet SM, Shawkey MD. 2013. pavo: an R package for the analysis, visualization and organization of spectral data. Methods Ecol. Evol. 4, 906–913. ( 10.1111/2041-210X.12069) [DOI] [Google Scholar]
- 76.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; https://www.R-project.org/. [Google Scholar]
- 77.Frazer GW, Canham CD, Lertzman KP. 1999. Gap Light Analyzer (GLA), Version 2.0: imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, users manual and program documentation. Burnaby, BC: Simon Fraser University; Millbrook, NY: Institute of Ecosystem Studies. [Google Scholar]
- 78.Pariente G. 1974. Influence of light on the activity rhythms of two Malagasy lemurs: Phaner furcifer and Lepilemur mustelinus leucopus. In Prosimian biology (eds Doyle GA, Walker AC), pp. 183–198. Pittsburgh, PA: University of Pittsburgh Press. [Google Scholar]
- 79.Pariente GF. 1980. Quantitative and qualitative study of the light available in the natural biotope of Malagasy prosimians. In Nocturnal Malagasy primates: ecology, physiology and behaviour (eds Charles-Dominique P, Cooper HM, Hladik A, Hladik CM, Pages E, Pariente GF, Petter-Rousseaux A, Petter JJ, Schilling A), pp. 117–134. New York, NY: Academic Press. [Google Scholar]
- 80.Corlett RT, Primack RB. 2011. Tropical rain forests: an ecological and biogeographical comparison, 2nd edn Chichester, UK: Wiley-Blackwell. [Google Scholar]
- 81.Bellingham PJ, Tanner EV, Rich PM, Goodland TC. 1996. Changes in light below the canopy of a Jamaican montane rainforest after a hurricane. J. Trop. Ecol. 12, 699–722. ( 10.1017/S0266467400009883) [DOI] [Google Scholar]
- 82.Fernandez DS, Fetcher N. 1991. Changes in light availability following Hurricane Hugo in a subtropical montane forest in Puerto Rico. Biotropica 23, 393–399. ( 10.2307/2388257) [DOI] [Google Scholar]
- 83.Niemitz C. 1984. Taxonomy and distribution of the genus Tarsius Storr, 1780. In Biology of tarsiers (ed. Niemitz C.), pp. 1–16. Stuttgart, Germany: Gustav Fischer. [Google Scholar]
- 84.Jacobs RL, Bradley BJ. 2016. Considering the influence of nonadaptive evolution on primate color vision. PLoS ONE 11, e0149664 ( 10.1371/journal.pone.0149664) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data has been deposited on GenBank under accession numbers: KX132093-KX132101. Spectral data are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.q29c0.