Abstract
Emerging evidence suggests that dietary fats may influence testicular function. However, most of the published literature on this field has used semen quality parameters as the only proxy for testicular function. We examined the association of fat intake with circulating reproductive hormone levels and testicular volume among healthy young Spanish men. This is a cross-sectional study among 209 healthy male volunteers conducted between October 2010 and November 2011 in Murcia Region of Spain. Participants completed questionnaires on lifestyle, diet, and smoking, and each underwent a physical examination, and provided a blood sample. Linear regression was used to examine the association between each fatty acid type and reproductive hormone levels and testicular volumes. Monounsaturated fatty acids intake was inversely associated with serum blood levels of calculated free testosterone, total testosterone, and inhibin B. A positive association was observed between the intake of polyunsaturated fatty acids, particularly of omega-6 polyunsaturated fatty acids, and luteinizing hormone concentrations. In addition, the intake of trans fatty acids was associated with lower total testosterone and calculated free testosterone concentrations (Ptrend = 0.01 and 0.02, respectively). The intake of omega-3 polyunsaturated fatty acids was positively related to testicular volume while the intake of omega-6 polyunsaturated fatty acids and trans fatty acids was inversely related to testicular volume. These data suggest that fat intake, and particularly intake of omega 3, omega 6, and trans fatty acids, may influence testicular function.
Keywords: fat intake, reproductive health, reproductive hormones, trans fatty acids, young healthy men
INTRODUCTION
Two meta-analyses1,2 and several single-center studies3,4,5,6,7 have documented a downward trend in semen quality in Western countries. Some have also reported a concomitant downward trend in circulating testosterone levels among men.8,9,10,11 These downward trends have coincided with numerous changes in environmental and lifestyle factors that may directly contribute to diminished testicular function. Most notably, increased exposure to endocrine-disrupting chemicals,12,13,14,15 increased worldwide prevalence of obesity,16,17 and worsening diet quality in Western countries18,19,20,21,22 have been previously related to semen quality, suggesting that these may explain some of the downward trends in semen quality and testosterone levels.
Most of the published work about fat intake and markers of male testicular function have been focused on semen quality parameters,23,24,25,26 and only a few on reproductive hormones. In addition, studies examining the relation between fat consumption and male sex hormones have focused on middle-aged and older men,27,28,29,30 and to our knowledge, there are no data on the association between dietary fats and reproductive hormones in young men. Moreover, the potential effect of dietary fat intake on testicular volume has not been explored so far. To address these gaps, we examined the relations between dietary fats with serum reproductive hormones and testicular volumes among young men in Spain.
MATERIALS AND METHODS
Study population
The Murcia Young Men's Study (MYMS) was a cross-sectional study conducted between October 2010 and November 2011 in the Murcia Region of Spain. Healthy young university students born in Spain, aged 18 to 23 years, were eligible to join the study. Recruitment flyers were posted at university campuses to invite students to participate in this study. A total of 240 students contacted the study staff, 17 of whom were ineligible. Of the remaining 223 men, 215 completed a study visit and agreed to participate in the study. We further excluded 6 men who reported implausible total caloric intake leaving 209 men (97.2%) for the current analysis. With this sample size, we had 80% statistical power to detect differences of 35% standard deviations of the outcome measures between men in extreme quartiles of dietary factors. Men received a €50 gift card for their participation. During the study visit, men completed questionnaires on lifestyle, diet, and smoking, underwent a physical examination, and provided blood and semen samples. Written informed consent was obtained from all cases. The Research Ethics Committee of the University of Murcia approved this study.
Physical examination and testicular volume assessment
Body weight and height were measured using a digital scale (Tanita SC 330-S, London, UK). Body mass index (BMI) was calculated as weight in kilograms divided by squared height in meters. The presence of varicocele or other scrotal abnormalities was also evaluated, and classified as grade I (only detected during Valsalva maneuver), grade II (palpable), or grade III (visible). Testicular volume was measured using a Prader orchidometer (Andrology Australia, Clayton, Victoria, Australia). Mean testicular volume was calculated as the mean of right and left testicular volumes. All physical examinations were performed by the same investigator (Jaime Mendiola) to minimize variability in study procedures.
Semen quality assessment
Men were asked to abstain from ejaculation for at least 48 h before sample collection but were not excluded if they failed to follow this instruction (n = 30). Abstinence time was recorded as the time between current and previous ejaculation as reported by the study subject. Men collected semen samples by masturbation at the clinic; no lubricants were used. Ejaculate volume was estimated by specimen weight, assuming a semen density of 1.0 g ml−1. Sperm concentration was evaluated by hemocytometer (Improved Neubauer; Hauser Scientific, Inc., Horsham, PA, USA). Spermatozoa were classified as either motile or immotile according to the World Health Organization (WHO) criteria,31 and the percentage of motile spermatozoa (progressive + nonprogressive) was calculated. Smears for morphology were prepared, air-dried, fixed, Papanicolaou stained, and assessed using strict criteria.32 Total sperm count (volume × sperm concentration) was also calculated. The same specialist biologist carried out all the semen analyses. An external quality control on semen samples throughout the study period was carried out in collaboration with the University of Copenhagen's Department of Growth and Reproduction. To assess interlaboratory variation in sperm concentration analysis, five sets of duplicate semen samples (600 ml each) were sent by mail during the study period from the University of Copenhagen's Department of Growth and Reproduction to the Murcia Andrology Laboratory. The specimens were coded, undiluted fresh sperm samples from regular semen donors that were preserved by adding 10 ml of a 3 mol l−1 sodium azide solution per 1 ml of the ejaculate after liquefaction. No systematic differences in the results were identified. The mean interexaminer coefficient of variation was 4.0%, ranging between 1.7% and 7.1%.
Dietary assessment
We used a validated33,34 101-food item semi-quantitative food frequency questionnaire (FFQ) to assess the usual intake of foods and nutrients (available at: http://bibliodieta.umh.es/files/2011/07/CFA101.pdf). Men were asked to report how often, on average, they had consumed each food item over the past year. Serving sizes were specified for each food item in the FFQ. The questionnaire offered nine options for frequency of consumption for each food, ranging from never or less than once a month to 6 or more times per day. Nutrient values for each food in the questionnaire were obtained from food composition tables of the US Department of Agriculture and supplemented with Spanish sources.35,36 Trans fatty acid intake was derived using published sources for Spanish foods.37,38 We calculated nutrient intakes (saturated fatty acids [SFA], monounsaturated fatty acids [MFA], polyunsaturated fatty acids [PUFA], omega-3 [n3], and omega-6 [n6]) by multiplying the frequency of use for each food by the nutrient composition of the portion size specified on the FFQ and by addition across all foods to obtain a total nutrient intake for each individual. Intake of energy-bearing nutrients was adjusted for total energy intake using the multivariate nutrient density method while non-energy-bearing nutrients were adjusted using the nutrient residual method.39
The reproducibility and validity of this FFQ is comparable to other widely used FFQs.40,41,42,43 The average of correlation coefficients between nutrient intakes estimated using four one-week prospectively collected diet records and those estimated with the FFQ were 0.44 for validity and 0.44 for reproducibility.44 This FFQ also showed satisfactory biochemical validity when compared to plasma levels of carotenoids and vitamin C.34
Reproductive hormones measurement
Blood samples were drawn from participants’ cubital veins in the afternoon and centrifuged; the serum was separated, stored, and frozen at −80°C. Serum samples were then shipped to Copenhagen, Denmark, on dry ice and stored at −20°C until hormone analysis was performed at Rigshospitalet. The methods have been described previously.45 Briefly, hormone assessments were performed simultaneously to reduce intralaboratory variations. Serum levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and sex hormone-binding globulin (SHBG) were determined using time-resolved immunofluorometric assays (DELFIA; PerkinElmer, Skovlund, Denmark). Intra- and inter-assay variations were <5% in each of the three assays. Serum total testosterone (TT) levels were determined using a time-resolved fluoroimmunoassay (DELFIA; PerkinElmer) with intra- and inter-assay variation of <8%. Estradiol (E2) was measured by radioimmunoassay (Pantex, Santa Monica, CA, USA) with an intraassay variation of <8% and an interassay variation of <13%. Inhibin B levels were determined by a specific two-sided enzyme immunometric assay (Oxford Bio-Innovation Ltd., Bicester, UK) with intra- and inter-assay variation of 13% and 18%, respectively. When inhibin B was above approximately 100 pg ml−1, the intraassay variation was <7% and the interassay variation was <6%. The majority of the men had levels above 100 pg ml−1. Calculated free testosterone (cFT) was determined using the equation of Vermeulen and colleagues assuming a fixed albumin of 43.8 g l−1. 46
Statistical analyses
Because the reproductive hormones, fat intakes, and the men's characteristics had a skewed distribution, the median and 5th and 95th percentiles were used to describe those variables. FSH and E2 concentrations were transformed using the natural log (ln) before analysis because they showed nonnormal distributions. Left and right testicular volumes were averaged, and the mean volume was used for analysis. Men were divided into quartiles of intake for total and major subtypes of dietary fat. Men with the lowest intake of each fat category were considered as the reference group. To test for associations across quartiles of intake, Kruskal-Wallis tests were used for continuous variables, and χ2 -tests for categorical variables. Linear regression was used to examine the association of each fatty acid category with testicular volume and reproductive hormone levels. Tests for linear trend were performed using the median values of fat categories in each quartile as a continuous variable and reproductive hormones as the response variable. We used analysis of covariance (ANCOVA) to calculate adjusted testicular volume and reproductive hormone levels for each quartile by relevant covariates. Regression coefficients for outcomes that were log-transformed for analysis (FSH and E2 serum levels) were exponentiated (“back-transformed”) to allow the presentation of adjusted means in the scale variables that were originally measured. In addition, the presence of nonlinear associations was tested modeling fat intake as a continuous variable and modeling them as a linear and quadratic term. We considered that an association was present when we found a linear trend across quartiles <0.05.
Confounding was assessed using a hybrid method that combines previous knowledge using directed acyclic graphs (DAGs)47 and a statistical method on change in point estimate. The variables considered as potential confounders included factors previously related to semen quality or reproductive hormones in this or other studies, and factors associated with fat intake and reproductive outcomes in this study, regardless of whether they had been previously described as predictors of male reproductive health. Using this method, final models included terms for BMI (kg m−2), smoking (current smoker versus not current smoker), time to blood sampling (min), mean daily alcohol intake (g day−1), and mean daily caffeine intake (mg day−1). Mean daily intakes of micronutrients previously related to semen quality in this population (vitamin C, ®- cryptoxantin, lycopene, and ®-carotene)20 were forced into the model. In order to adjust for total energy intake, terms for total calorie intake, intake of protein, and the remaining types of fat were included in the model,39 regardless of statistical significance, to allow the interpretation of the regression parameter of interest as the isocaloric substitution of a specific type of fat for the same amount of energy from carbohydrates. Statistical analyses were performed with the IBM Statistical Package for the Social Sciences 19.0 (IBM Corporation, Armonk, NY, USA).
RESULTS
Almost all the participants were Caucasian (98%) with a median (interquartile range [IQR]) age of 20.4 (19.6, 21.4) years. Sixty-eight (33%) of the men were smokers and the median (IQR) BMI was 23.7 (21.8, 25.5). Four (1.9%) men had a history of cryptorchidism and 32 (15.3%) men had a left varicocele diagnosed during the examination. Men in the highest quartile of total fat intake had lower intake of carbohydrates, vitamin C, cryptoxanthin, and alcohol, compared with men in the lowest quartile (Table 1).
Table 1.
Demographic characteristics of men in the Murcia Young Men's Study among total fat
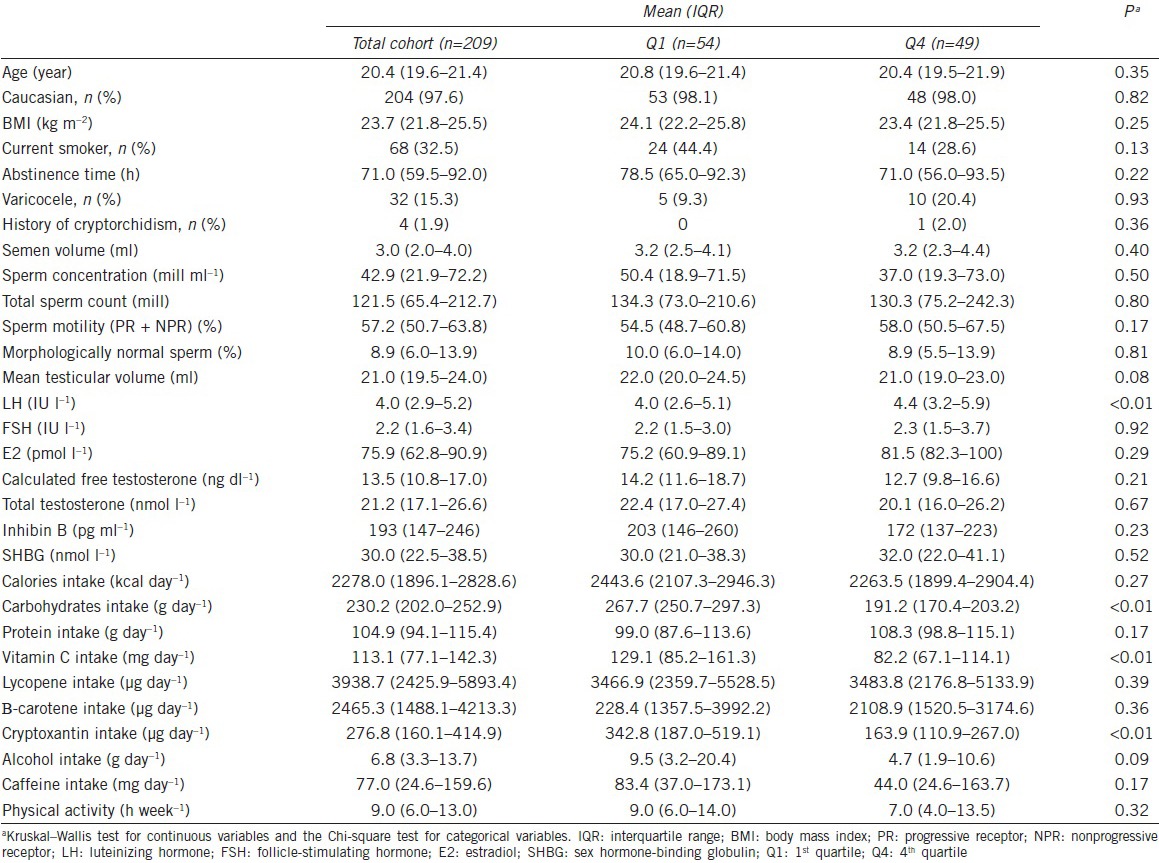
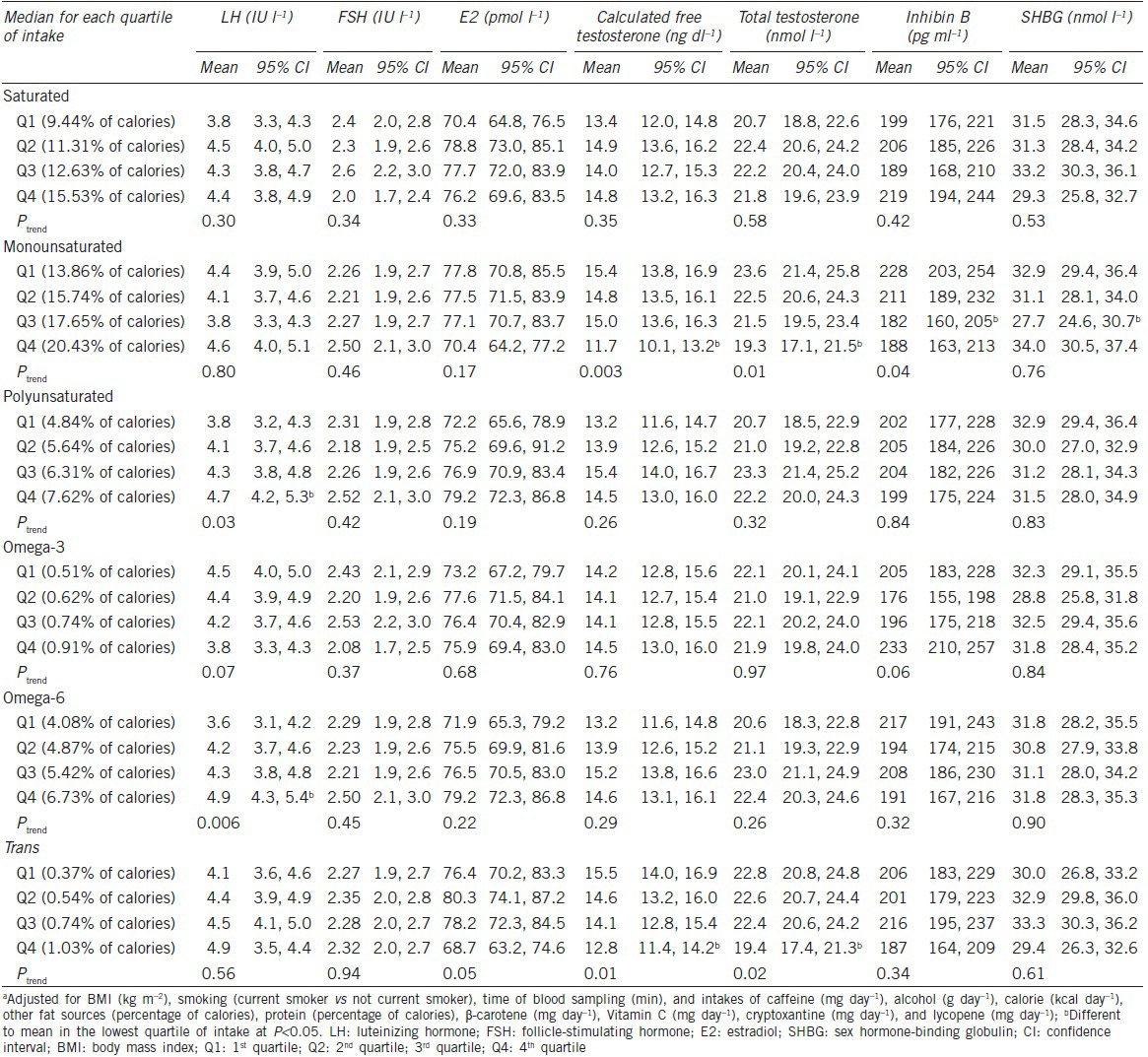
Table 2 shows the multivariate-adjusted associations between fatty acid intake and reproductive hormones. In these models, MFA intake was inversely related with blood levels of cFT, TT, and inhibin B (Ptrend < 0.05). There was a positive relation between intakes of PUFAs, particularly of omega-6, and LH concentrations. In addition, intake of trans fatty acids was associated with lower cFT and TT concentrations (Ptrends = 0.01 and 0.02, respectively). Relative to men in the lowest quartile of trans fatty acid intake, the adjusted difference (95% Confidence Interval [CI]) of TT blood levels for men in the 2nd, 3rd, and 4th quartiles were −0.3 (−3.0–2.5), −0.4 (−3.2–2.4), and −3.4 (−6.4–−0.4) nmol l−1, respectively.
Table 2.
Multivariate adjusteda associations of fat intake with reproductive hormones (n=209)
We then evaluated the relation between fatty acid intake and testicular volume. Intake of omega-3 PUFAs was positively related to testicular volume while intakes of omega-6 PUFAs and trans fatty acids were inversely related to testicular volume (Figure 1). The adjusted median (95% CI) total testicular volumes (ml) for men in increasing quartiles of fatty acid intake were 20.7 (19.7, 21.7), 21.0 (20.1, 22.0), 20.9 (20.0, 21.9), and 22.9 (21.9, 23.9) for omega-3 fatty acids; 22.2 (21.1, 23.3), 21.9 (21.0, 22.8), 21.5 (20.6, 22.4), and 20.0 (18.9, 21.0) for omega-6 fatty acids; and 21.3 (20.3, 22.3), 22.0 (21.1, 22.9), 21.9 (20.9, 22.8), and 20.4 (19.5, 21.4) for trans fatty acids. Intake of other fatty acids was not related to testicular volume (data not shown). Further adjustment by physical activity did not modify the associations (data not shown).
Figure 1.
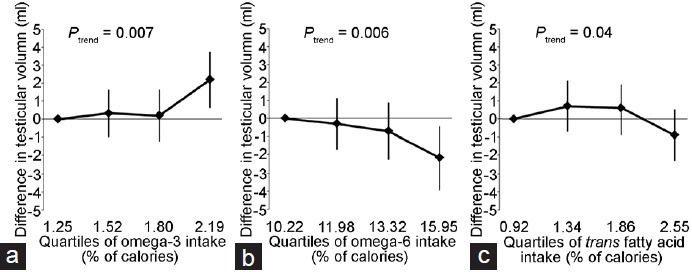
Difference in testicular volume by increasing quartiles of some nutrient intakes. (a) Omega-3. (b) Omega-6. (c) Trans fatty acids. Values represent the difference and 95% confidence interval in the mean (right and left) of testicular volume for men in the 2nd, 3rd, and 4th quartile of intakes when compared to men in the first quartile of intake. Models are adjusted for BMI (kg m−2), smoking (current smoker vs not current smoker), time of blood sampling (min), and intakes of caffeine (mg day−1), alcohol (g day−1), calorie (kcal day−1), other fat sources (% of calories), protein (% of calories), β-carotene (mg day−1), vitamin C (mg day−1), cryptoxanthin (mg day−1), and lycopene (mg day−1). BMI: body mass index.
DISCUSSION
We examined the cross-sectional associations of fat intake with reproductive hormone levels and testicular volume in a population of young healthy Spanish men. We found inverse associations of monounsaturated fat intake with cFT and TT and inhibin B concentrations. Intake of omega-6 PUFAs was positively associated with LH concentrations and inversely related to testicular volume. Similarly, intake of trans fatty acids was inversely related to cFT and TT levels and testicular volume. In addition, we found a positive association between intake of omega-3 PUFAs and testicular volume. The findings for trans fatty acids are of particular interest given our previous report of an association between trans fat intake and lower sperm count in these men26 and previous experimental work in rodents showing a deleterious effect of trans fats on testicular function48,49,50,51 supporting a causal interpretation of this association.
We found inverse associations of MFA intake with three reproductive hormones (inhibin B, cFT, and TT concentrations). In this population, monounsaturated fats are primarily consumed from animal products (27%: from red and processed meats and dairy products) and from olive oil (20%). Jensen and colleagues found a borderline and inverse association between monounsaturated fatty acid intake and morphologically normal sperm among Danish men from general population.25 Jensen's finding could be interpreted as consistent with our results, suggesting that monounsaturated fat intake may impair male reproductive health. However, we have previously reported no association between monounsaturated fat intake and semen quality in this population26 and others have found no relation between monounsaturated fats and reproductive hormones in older men.27,28 Nevertheless, intake of red and processed meats and of dairy foods has also been previously related to poor semen quality,18,52,53 suggesting that the observed relation in this study could reflect the relation with these foods rather than with monounsaturated fats per se. Given the paucity of data on the relation between fat intake with reproductive hormone concentrations and testicular volume in men, additional work is necessary to evaluate these relations further.
We observed a positive association between intake of omega-6 PUFAs and LH levels coupled with an inverse relation between intake of these fats and testicular volume. Elevated LH levels are characteristic of primary Leydig cell failure. While none of the men in the study had abnormally high LH levels and all had a testicular volume within the normal range, the co-occurrence of higher LH levels and lower testicular volume among men with the highest intake of omega-6 PUFAs may suggest that these fats could negatively influence testicular function acting directly on the testis. As we did not observe a relation to testosterone, it might indicate a situation with a compensated state (i.e., unchanged testosterone on a background of increased stimulation with LH). We did not observe a relation between intake of omega-6 PUFAs with FSH (expected in the case of spermatogenic dysfunction) raising the possibility that the observed relation to testis volume could be due to chance. The literature on the relation between these fats and markers of testicular function is sparse. PUFA intake was unrelated to sex hormones among 696 British men aged 70 years and older54 and also in middle-aged US men.27 We also found that intake of omega-3 PUFAs was not associated with reproductive hormones, but it was positively related with testicular volume. Although there is little consistency in the relation between omega-3 PUFA intake and reproductive hormones among men,27,28,29,30 it seems that omega-3 consumption may have a positive effect on semen quality parameters, in agreement with our finding regarding testicular volume. Afeiche and colleagues have recently found that fish intake, an important source of omega-3 fatty acids, was positively associated with sperm counts and morphologically normal sperm among men attending a fertility center, and that relation appeared to be mediated through intake of long-chain omega-3 fats.21 In agreement with these results, omega-3 fatty acid supplementation increased the total percentage of sperm with normal morphology among oligoasthenoteratospermic Iranian men,23 and Jensen et al.25 found a positive and borderline association between omega-3 fat intake and semen volume among the general population (Ptrend = 0.05). Moreover, experimental models also showed a positive effect of omega-3 PUFAs on male reproductive health.55,56
We are not aware of previous reports between the relation of trans fat intake and testosterone levels and testicular volume. Nevertheless, our findings are in agreement with our previous report on the relation between intake of these fats and total sperm count in this study population.26 Specifically, we had previously reported that men in the highest quartile of trans fat intake had 37% lower total sperm count;26 these same men were also found to have 15% lower T levels and 4% lower testicular volume than men in the lowest quartile of intake. These findings are also in agreement with a study among fertility patients where sperm trans fat levels, a biomarker of intake, were inversely related to sperm concentration.19 Moreover, rodent models of trans fat supplementation provide strong evidence supporting a causal interpretation of these relations. Supplementing the diet of male mice with trans fatty acids results in reproductive impairment including the accumulation of trans fats in the testis,48,49 decreased serum testosterone concentrations and sperm counts, and at the highest levels of supplementation, arrest of spermatogenesis and testicular degeneration.48,50,51 Findings from these rodent models closely parallel our findings in humans. However, given the scarcity of data in humans, it is important that these relations are further evaluated in different populations.
We cannot discount the limitations of our study. Reverse causation is possible due to the cross-sectional study. However, we selected young men with untested fertility and we did not communicate the results of the semen analyses or reproductive hormones to them, reducing the possibility of this bias. Second, as is the case in all observational epidemiologic studies, we cannot exclude the possibility of unmeasured confounding. Nevertheless, we adjusted for a large number of known and suspected confounders. Adjustment for these factors had a minimal impact on the results, suggesting that any unmeasured confounding is unlikely to have a major impact on the results. Based on the number of tested associations, we cannot completely rule out the possibility that observed associations may result from chance. Third, misclassification of dietary intakes is possible as well. However, we used a previously validated FFQ.33,34,57 Fourth, associations with testicular volume are more difficult to evaluate since little is known about how much this outcome could be influenced by environmental factors, such as diet, among adult men. Whether and to what extent testicular volume might change in response to modifiable environmental factors remains an open question. Interpretation of these findings since we used an orchidometer to assess testicular volume which limited the precision of the measurements. Fifth, only one serum sample was obtained for each man opening the possibility of error due to within-person variability in reproductive hormones. Nevertheless, previous work has shown that a single sample can be used to classify men's reproductive hormone function.58 Sixth, the intra- and inter-assay variation for assessing inhibin B levels could be considered as high (13%–18%); however, the majority of the men had inhibin B levels above 100 pg ml−1, which is translated to a lower intra- and inter-assay variation (<7%).
In summary, we found that omega-3 fatty acids may be positively associated with testicular function as indicated by testicular volume whereas intake of omega-6 fatty acids and trans fatty acids appear to be negatively related to it. Some of these findings, most notably those for omega-3 and trans fats, are in agreement with animal experimental data as well as preclinical and clinical data. However, because data in humans on this topic are scarce, further work is necessary to clarify the nature of these relations as well as their clinical relevance.
AUTHOR CONTRIBUTIONS
LMA and JEC carried out the nutrition studies and drafted the manuscript. NJ and JM carried out the hormone analysis. JEC, LMA, CT, JV, and NJ participated in the design of the study and performed the statistical analysis. AMTC, NJ, JM, and MR conceived of the study, and participated in its design and coordination and helped draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declared no competing interests.
ACKNOWLEDGMENTS
The authors gratefully acknowledge C. Ruiz, E. Belmonte, F. Mas, and all the Quirón Dexeus Murcia clinic staff for their assistance in data collection; and the young men of the study for their participation. We would also like to thank L. Sarabia and G. Vivero for semen analyses, K. Ruiz and E. Estrella for database management, and E.M. Navarrete-Muñoz for dietary assessment. This work was supported by The Seneca Foundation, Regional Agency of Science and Technology, grant n° 08808/PI/08, Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III (FIS), grant n° PI10/00985, and grant P30 DK46200 from the National Institutes of Health.
REFERENCES
- 1.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–13. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934-1996. Environ Health Perspect. 2000;108:961–6. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995;332:281–5. doi: 10.1056/NEJM199502023320501. [DOI] [PubMed] [Google Scholar]
- 4.Swan SH, Brazil C, Drobnis EZ, Liu F, Kruse RL, et al. Geographic differences in semen quality of fertile U.S. males. Environ Health Perspect. 2003;111:414–20. doi: 10.1289/ehp.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendiola J, Jorgensen N, Minguez-Alarcon L, Sarabia-Cos L, Lopez-Espin JJ, et al. Sperm counts may have declined in young university students in Southern Spain. Andrology. 2013;1:408–13. doi: 10.1111/j.2047-2927.2012.00058.x. [DOI] [PubMed] [Google Scholar]
- 6.Rolland M, Le Moal J, Wagner V, Royere D, De Mouzon J. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum Reprod. 2013;28:462–70. doi: 10.1093/humrep/des415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jorgensen N, Vierula M, Jacobsen R, Pukkala E, Perheentupa A, et al. Recent adverse trends in semen quality and testis cancer incidence among Finnish men. Int J Androl. 2011;34:e37–48. doi: 10.1111/j.1365-2605.2010.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson AM, Jensen TK, Juul A, Petersen JH, Jorgensen T, et al. Secular decline in male testosterone and sex hormone binding globulin serum levels in Danish population surveys. J Clin Endocrinol Metab. 2007;92:4696–705. doi: 10.1210/jc.2006-2633. [DOI] [PubMed] [Google Scholar]
- 9.Travison TG, Araujo AB, Hall SA, McKinlay JB. Temporal trends in testosterone levels and treatment in older men. Curr Opin Endocrinol Diabetes Obes. 2009;16:211–7. doi: 10.1097/med.0b013e32832b6348. [DOI] [PubMed] [Google Scholar]
- 10.Nyante SJ, Graubard BI, Li Y, McQuillan GM, Platz EA, et al. Trends in sex hormone concentrations in US males: 1988-1991 to 1999-2004. Int J Androl. 2012;35:456–66. doi: 10.1111/j.1365-2605.2011.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perheentupa A, Makinen J, Laatikainen T, Vierula M, Skakkebaek NE, et al. A cohort effect on serum testosterone levels in Finnish men. Eur J Endocrinol. 2013;168:227–33. doi: 10.1530/EJE-12-0288. [DOI] [PubMed] [Google Scholar]
- 12.Boisen KA, Kaleva M, Main KM, Virtanen HE, Haavisto AM, et al. Difference in prevalence of congenital cryptorchidism in infants between two Nordic countries. Lancet. 2004;363:1264–9. doi: 10.1016/S0140-6736(04)15998-9. [DOI] [PubMed] [Google Scholar]
- 13.Bray F, Richiardi L, Ekbom A, Pukkala E, Cuninkova M, et al. Trends in testicular cancer incidence and mortality in 22 European countries: continuing increases in incidence and declines in mortality. Int J Cancer. 2006;118:3099–111. doi: 10.1002/ijc.21747. [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen R, Moller H, Thoresen SO, Pukkala E, Kjaer SK, et al. Trends in testicular cancer incidence in the Nordic countries, focusing on the recent decrease in Denmark. Int J Androl. 2006;29:199–204. doi: 10.1111/j.1365-2605.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- 15.Nassar N, Bower C, Barker A. Increasing prevalence of hypospadias in Western Australia, 1980-2000. Arch Dis Child. 2007;92:580–4. doi: 10.1136/adc.2006.112862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32:1431–7. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 17.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2014;384:766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendiola J, Torres-Cantero AM, Moreno-Grau JM, Ten J, Roca M, et al. Food intake and its relationship with semen quality: a case-control study. Fertil Steril. 2009;91:812–8. doi: 10.1016/j.fertnstert.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Chavarro JE, Furtado J, Toth TL, Ford J, Keller M, et al. Trans-fatty acid levels in sperm are associated with sperm concentration among men from an infertility clinic. Fertil Steril. 2011;95:1794–7. doi: 10.1016/j.fertnstert.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minguez-Alarcon L, Mendiola J, Lopez-Espin JJ, Sarabia-Cos L, Vivero-Salmeron G, et al. Dietary intake of antioxidant nutrients is associated with semen quality in young university students. Hum Reprod. 2012;27:2807–14. doi: 10.1093/humrep/des247. [DOI] [PubMed] [Google Scholar]
- 21.Afeiche MC, Gaskins AJ, Williams PL, Toth TL, Wright DL, et al. Processed meat intake is unfavorably and fish intake favorably associated with semen quality indicators among men attending a fertility clinic. J Nutr. 2014;144:1091–8. doi: 10.3945/jn.113.190173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod. 2012;27:2899–907. doi: 10.1093/humrep/des298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Safarinejad MR. Effect of omega-3 polyunsaturated fatty acid supplementation on semen profile and enzymatic anti-oxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: a double-blind, placebo-controlled, randomised study. Andrologia. 2011;43:38–47. doi: 10.1111/j.1439-0272.2009.01013.x. [DOI] [PubMed] [Google Scholar]
- 24.Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, et al. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod. 2012;27:1466–74. doi: 10.1093/humrep/des065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen TK, Heitmann BL, Jensen MB, Halldorsson TI, Andersson AM, et al. High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. Am J Clin Nutr. 2013;97:411–8. doi: 10.3945/ajcn.112.042432. [DOI] [PubMed] [Google Scholar]
- 26.Chavarro JE, Minguez-Alarcon L, Mendiola J, Cutillas-Tolin A, Lopez-Espin JJ, et al. Trans fatty acid intake is inversely related to total sperm count in young healthy men. Hum Reprod. 2014;29:429–40. doi: 10.1093/humrep/det464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Field AE, Colditz GA, Willett WC, Longcope C, McKinlay JB. The relation of smoking, age, relative weight, and dietary intake to serum adrenal steroids, sex hormones, and sex hormone-binding globulin in middle-aged men. J Clin Endocrinol Metab. 1994;79:1310–6. doi: 10.1210/jcem.79.5.7962322. [DOI] [PubMed] [Google Scholar]
- 28.Nagata C, Takatsuka N, Kawakami N, Shimizu H. Relationships between types of fat consumed and serum estrogen and androgen concentrations in Japanese men. Nutr Cancer. 2000;38:163–7. doi: 10.1207/S15327914NC382_4. [DOI] [PubMed] [Google Scholar]
- 29.Demark-Wahnefried W, Price DT, Polascik TJ, Robertson CN, Anderson EE, et al. Pilot study of dietary fat restriction and flaxseed supplementation in men with prostate cancer before surgery: exploring the effects on hormonal levels, prostate-specific antigen, and histopathologic features. Urology. 2001;58:47–52. doi: 10.1016/s0090-4295(01)01014-7. [DOI] [PubMed] [Google Scholar]
- 30.Giltay EJ, Geleijnse JM, Heijboer AC, de Goede J, Oude Griep LM, et al. No effects of n-3 fatty acid supplementation on serum total testosterone levels in older men: the Alpha Omega Trial. Int J Androl. 2012;35:680–7. doi: 10.1111/j.1365-2605.2012.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: WHO Press; 2010. [Google Scholar]
- 32.Menkveld R. In: The basic semen analysis. Male Infertility. Diagnosis and Treatment. Oerhninger SC, Kruger TF, editors. Abingdon: Informa Healthcare; 2007. [Google Scholar]
- 33.Vioque J, Navarrete-Munoz EM, Gimenez-Monzo D, Garcia-de-la-Hera M, Granado F, et al. Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr J. 2013;12:26. doi: 10.1186/1475-2891-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vioque J, Weinbrenner T, Asensio L, Castello A, Young I, et al. Plasma concentrations of carotenoids and vitamin C are better correlated with dietary intake in normal weight than overweight and obese elderly subjects. Br J Nutr. 2007;97:977–86. doi: 10.1017/S0007114507659017. [DOI] [PubMed] [Google Scholar]
- 35.Palma I, Farran P, Cervera P. [Tables of Food Composition for household measures of habitual consumption in Spain: CESNID] 4th ed. Madrid: McGraw Hill; 2008. Article in Spanish. [Google Scholar]
- 36.US Department of Agriculture. Agricultural Research Service 2010 National Nutrient Database for Standard Reference, Release 23. Nutrient Data Laboratory. 2010. [Last accessed on 2016 Sep 27]. Available from: http://www.ars.usda.gov/ba/bhnrc/ndl .
- 37.Vicario IM, Griguol V, Leon-Camacho M. Multivariate characterization of the fatty acid profile of spanish cookies and bakery products. J Agric Food Chem. 2003;51:134–9. doi: 10.1021/jf0258297. [DOI] [PubMed] [Google Scholar]
- 38.Larqué E, Garaulet M, Pérez-Llamas F, Zamora S, Tebar J. [Fatty acid composition and nutritional relevance of most widely consumed margarines in Spain] Int J Fats Oils. 2003;54:65–70. Article in Spanish. [Google Scholar]
- 39.Willett W. Nutritional Epidemiology. 3rd ed. Oxford: Oxford University Press; 2013. [Google Scholar]
- 40.Willett W, Sampson L, Stampfer M, Rosner B, Bain C, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 41.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154:1089–99. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 42.Ocke MC, Bueno-de-Mesquita HB, Pols MA, Smit HA, van Staveren WA, et al. The Dutch EPIC food frequency questionnaire. II. Relative validity and reproducibility for nutrients. Int J Epidemiol. 1997;26(Suppl 1):S49–58. doi: 10.1093/ije/26.suppl_1.s49. [DOI] [PubMed] [Google Scholar]
- 43.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–35. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 44.Vioque J. In: [Validity of the evaluation of dietary intake]. [Nutrition and Public Health. Methods, Scientific Basis and Applications] 2nd ed. Serra-Majem L, Aranceta Bartrina J, editors. Barcelona: Masson-Elsevier; 2006. Article in Spanish. [Google Scholar]
- 45.Asklund C, Jorgensen N, Skakkebaek NE, Jensen TK. Increased frequency of reproductive health problems among fathers of boys with hypospadias. Hum Reprod. 2007;22:2639–46. doi: 10.1093/humrep/dem217. [DOI] [PubMed] [Google Scholar]
- 46.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 47.Weng HY, Hsueh YH, Messam LL, Hertz-Picciotto I. Methods of covariate selection: Directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol. 2009;169:1182–90. doi: 10.1093/aje/kwp035. [DOI] [PubMed] [Google Scholar]
- 48.Jensen B. Rat testicular lipids and dietary isomeric fatty acids in essential fatty acid deficiency. Lipids. 1976;11:179–88. doi: 10.1007/BF02532855. [DOI] [PubMed] [Google Scholar]
- 49.Privett OS, Phillips F, Shimasaki H, Nozawa T, Nickell EC. Studies of effects of trans fatty acids in the diet on lipid metabolism in essential fatty acid deficient rats. Am J Clin Nutr. 1977;30:1009–17. doi: 10.1093/ajcn/30.7.1009. [DOI] [PubMed] [Google Scholar]
- 50.Veaute C, Andreoli MF, Racca A, Bailat A, Scalerandi MV, et al. Effects of isomeric fatty acids on reproductive parameters in mice. Am J Reprod Immunol. 2007;58:487–96. doi: 10.1111/j.1600-0897.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 51.Hanis T, Zidek V, Sachova J, Klir P, Deyl Z. Effects of dietary trans-fatty acids on reproductive performance of Wistar rats. Br J Nutr. 1989;61:519–29. doi: 10.1079/bjn19890140. [DOI] [PubMed] [Google Scholar]
- 52.Afeiche MC, Williams PL, Gaskins AJ, Mendiola J, Jorgensen N, et al. Meat intake and reproductive parameters among young men. Epidemiology. 2014;25:323–30. doi: 10.1097/EDE.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi MR, Hekmatdoost A. Intake of food groups and idiopathic asthenozoospermia: a case-control study. Hum Reprod. 2012;27:3328–36. doi: 10.1093/humrep/des311. [DOI] [PubMed] [Google Scholar]
- 54.Allen NE, Appleby PN, Davey GK, Key TJ. Lifestyle and nutritional determinants of bioavailable androgens and related hormones in British men. Cancer Causes Control. 2002;13:353–63. doi: 10.1023/a:1015238102830. [DOI] [PubMed] [Google Scholar]
- 55.Li Y, Liu L, Wang B, Xiong J, Li Q, et al. Impairment of reproductive function in a male rat model of non-alcoholic fatty liver disease and beneficial effect of N-3 fatty acid supplementation. Toxicol Lett. 2013;222:224–32. doi: 10.1016/j.toxlet.2013.05.644. [DOI] [PubMed] [Google Scholar]
- 56.Yan L, Bai XL, Fang ZF, Che LQ, Xu SY, et al. Effect of different dietary omega-3/omega-6 fatty acid ratios on reproduction in male rats. Lipids Health Dis. 2013;12:33. doi: 10.1186/1476-511X-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vioque J. In: [Validity of the evaluation of dietary intake]. [Nutrition and Public Health. Methods, Scientific Basis and Applications] Serra-Majem L, Aranceta J, Mataix J, editors. Barcelona: Masson; 1995. Article in Spanish. [Google Scholar]
- 58.Bjornerem A, Straume B, Oian P, Berntsen GK. Seasonal variation of estradiol, follicle stimulating hormone, and dehydroepiandrosterone sulfate in women and men. J Clin Endocrinol Metab. 2006;91:3798–802. doi: 10.1210/jc.2006-0866. [DOI] [PubMed] [Google Scholar]


