Abstract
Cold temperatures are associated with increased prevalence of hypertension. Cold exposure increases endothelin-1 (ET1) production. The purpose of this study is to determine whether upregulation of ET1 contributes to cold-induced hypertension (CIH). In vivo RNAi silencing of the ET1 gene was achieved by adeno-associated virus 2 (AAV2) delivery of ET1 short-hairpin small interfering RNA (ET1-shRNA). Four groups of male rats were used. Three groups were given AAV.ET1-shRNA, AAV.SC-shRNA (scrambled shRNA), and phosphate-buffered saline (PBS), respectively, before exposure to a moderately cold environment (6.7 ± 2°C), while the last group was given PBS and kept at room temperature (warm, 24 ± 2°C) and served as a control. We found that systolic blood pressure of the PBS-treated and SC-shRNA–treated groups increased significantly within 2 weeks of exposure to cold, reached a peak level (145 ± 4.8 mmHg) by 6 weeks, and remained elevated thereafter. By contrast, blood pressure of the ET1-shRNA-treated group did not increase, suggesting that silencing of ET1 prevented the development of CIH. Animals were euthanized after 10 weeks of exposure to cold. Cold exposure significantly increased the left ventricle (LV) surface area and LV weight in cold-exposed rats, suggesting LV hypertrophy. Superoxide production in the heart was increased by cold exposure. Interestingly, ET1-shRNA prevented cold-induced superoxide production and cardiac hypertrophy. ELISA assay indicated that ET1-shRNA abolished the cold-induced upregulation of ET1 levels, indicating effective silencing of ET1. In conclusion, upregulation of ET1 plays a critical role in the pathogenesis of CIH and cardiac hypertrophy. AAV delivery of ET1-shRNA is an effective therapeutic strategy for cold-related cardiovascular disease.
Keywords: : gene therapy, AAV, RNAi, endothelin-1, cold-induced hypertension, cardiac hypertrophy
Introduction
Cold temperatures increase the prevalence of hypertension and cardiovascular (CV) disease. Clinical observations and epidemiological surveys have established that people who live in the cold regions have increased prevalence of hypertension and related CV diseases (e.g., stroke and myocardial infarction).1–5 Cold exposure from everyday life during winter is sufficient to induce significant and prolonged hypertension in the general population.6 Indeed, cold temperatures increase blood pressure (BP).7–8 Cold temperatures also increase the severity of hypertension in hypertensive patients,9–11 which may trigger myocardial infarction and stroke. The mortality due to ischemic heart diseases and stroke peaks during the winter season.1,9 Therefore, the cold temperature is an independent risk factor contributing to the high incidence of hypertension and related CV disease in cold regions or in winter even after adjusting for age, sex, and other known factors.9 Unfortunately, a specific intervention for cold-related cardiovascular disease is unavailable due to the unknown mechanism.
Chronic or intermittent exposure to cold increases blood pressure and causes cardiac and renal hypertrophy in rodents, namely cold-induced hypertension (CIH).9,12 There are numerous models of hypertension including genetically induced, pharmacologically induced, and surgically induced hypertension; however, few studies focus on the environmental effects of hypertension and cardiovascular hypertrophy. We found that CIH is a natural form of experimentally induced hypertension, requiring no large doses of drugs or hormones or genetic manipulations.9 A series of studies have been carried out to delineate the mechanism of CIH.9 We reported that the renin-angiotensin-aldosterone system (RAS) and endothelial nitric oxide synthase (eNOS) may be involved in the pathogenesis of CIH.13–20 We found that cold exposure increased endothelin-1 (ET1) levels in small vessels, hearts, and kidneys.21 Endothelin-1 (ET1) is the most potent vasoconstrictor through binding to ETA receptors located on smooth muscle cells. It is not known, however, whether ET1 is involved in the pathogenesis of CIH. In this study we targeted the ET1 mRNA to decrease availability of ET1 using the RNA interference (RNAi) approach. The purpose of this study is to determine if silencing of the ET1 gene provides protection against CIH.
Methods
RNAi design and AAV packaging
Design of the RNAi sequence was done through internet provided software (Invitrogen). Sequences were obtained against CDS sequence of ET1. Three ET1 and scrambled shRNA sequences were produced by Geno-Mechanix and high performance liquid chromatography purified. Optimization and specificity was determined by naked and lipofectamine-driven transfection into rat aortic smooth muscle cells, generously provided by Dr. Peter Sayeski (University of Florida), and protein levels confirmed by Western blot and ELISA. The selected sequences are GACAAGAAGTGCTGGAATT for ET1.
AAV2 carrying ET1 short-hairpin small interference RNA (ET1-shRNA) was constructed as we described previously.12,22–23 Briefly, AAV2 packaging of shRNA was done by plating AAV-293 cells into cell factories 2 days prior to packaging. Plasmid DNA containing shRNA sequences were mixed with CaCl2 and HEPES buffered saline and immediately applied to cell factories. Cells were incubated at 37°C for 6 h, at which point the medium was replaced with fresh medium and returned to 37°C for 72 h. Cells were harvested into DMEM and stored at −20°C until needed.
Vector purification was done using CaCl2 and iodixanol/heparin. Harvested AAV-293 cells were frozen and thawed two times and incubated with 0.1mg DNase I and RNase A (Roche Biochemicals) for each 2.5 mL DMEM for 30 min at 37°C. After 15 min of centrifugation at 3000 rpm at 4°C the supernatant was transferred into a new tube and incubated with 0.5% deoxycholic acid (Sigma) for 30 min at 37°C. The supernatant was filtered through a 5 μm pore size filter (Millipore) and a 0.8 μm pore size filter (Millipore). The lysate was applied to a heparin column and allowed to flow at 1 drop/sec. After all the lysate had passed through the matrix was washed twice with 25 mL phosphate-buffered saline (PBS) containing 0.1 M NaCl (0.254 M NaCl final concentration). The virus was eluted with 15 mL PBS containing 0.4 M NaCl. The virus was then readjusted to physiological NaCl levels in PBS. Viral titer was determined by quantitative RT-PCR using primers and probes specific for Kanomyocin resistance gene.
AAV delivery and BP measurements
This study was performed according to the guidelines of the National Institute of Health on the care and use of laboratory animals, and was approved by the Institutional Animal Care and Use Committee of the University of Florida.
Four groups of male Sprague Dawley rats (8 rats per group) were used. Systolic blood pressure (BP) was measured using the tail cuff method as we described previously.24–27 After a stable BP was obtained, three groups received intravenous injection of AAV.ET1-shRNA, AAV.SC-shRNA, and PBS, respectively, before exposure to cold (6.4°C). The remaining group was given PBS and kept at room temperature (warm, 25°C) and served as a control. Viral injections into the tail vein were performed under isofluorane anesthesia (1.5%). The viral particles were injected intravenous at the dose of 1 × 109 infectious units per animal (0.5 mL). Following injection, BP was monitored twice during a one-week period before animals were placed in thermo-regulated chamber (6.4°C). BP was measured weekly during exposure to cold.
Magnetic resonance imaging analysis of heart size and function
Magnetic resonance imaging (MRI) analysis of heart size was performed under isoflurane (1.5%) anesthesia.28 Briefly, animals were placed feet first and prone into a 4.7T magnet. Sinoatrial images were taken from the apex of the heart to the tricuspid valve. The heart images were acquired at both systole and diastole. Images were converted to Digital Imaging and Communication in Medicine (DICOM) format and analyzed for left ventricle (LV) wall area, mass, ejection fraction, stroke volume, and cardiac output using a PIE software. MRI sessions were done for each rat prior to injections, 2, 4, and 8 weeks post cold exposure.
Measurement of ET1
After 10 weeks of cold exposure, all animals were euthanized with halothane. Blood was collected for measuring plasma levels of ET1. Rats were perfused with cold PBS. The heart and kidneys were removed and weighed. Aortas and mesenteric arteries were dissected and cleaned off extraneous tissue. A portion of these organs were saved at −80°C for measuring tissue levels of ET1.
Plasma and tissue levels of ET1 were measured using an ET1 ELISA kits (Assay Design) according to manufacturer protocol.21 ET1 levels were normalized by total protein levels determined by protein assay (BioRad).
In situ superoxide production
A portion of the frozen heart samples were embedded in optical cutting temperature for assessing superoxide levels using dihydroethidium (DHE) staining as described in our previous studies.12,22–23,29 For quantification purposes, 0.10 g of frozen heart tissue was homogenized in buffer and incubated with DHE at 37°C in a 96-well nonfluorescing plate. Samples were excited at 485/20 nm and emissions were read at 590/35 nm, sensitivity was set at 100 (Bio-Tek Synergy HTTR-1E).
Statistical analyses
BP was analyzed using one-way ANOVA repeated over time. All other data were analyzed by one-way ANOVA. The Newman–Keuls procedure was used to assess the significance of differences between groups. The significance was set at a 95% confidence limit.
Results
AAV delivery of ET1-shRNA prevented the development of cold-induced hypertension
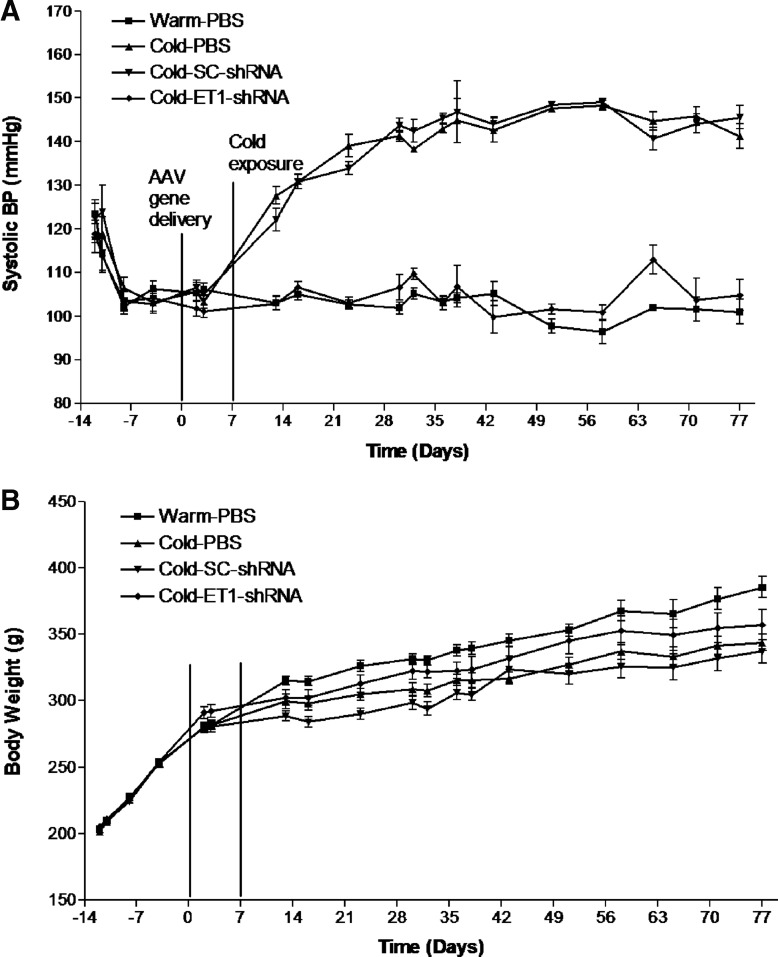
Systolic blood pressure did not differ among groups during the control period at room temperature (Fig. 1A). AAV delivery of ET1-shRNA did not affect normal BP. Exposure to moderate cold (6.7°C) resulted in a significant (p < 0.05) increase in BP in the PBS and scrambled shRNA groups within one week. BP reached a peak level (145 ± 4.8 mmHg) by 6 weeks of exposure to cold and remained elevated thereafter (Fig. 1A). Therefore, rats developed hypertension in cold, namely cold-induced hypertension (CIH). Interestingly, BP of the AAV.ET1-shRNA–treated group did not increase in response to cold exposure and remained at a level of the warm-PBS groups throughout the experiment (Fig. 1A), suggesting that AAV delivery of ET1-shRNA prevented the development of CIH.
Figure 1.
AAV delivery of ET1-shRNA prevented the development of cold-induced hypertension. (A) Systolic blood pressure. (B) Body weight. Data shown as means ± standard error of the mean (SEM); n = 8. AAV, adeno-associated virus; BP, blood pressure; ET1, endothelin-1; PBS, phosphate-buffered saline; shRNA, short-hairpin small interference RNA.
Body weights of the cold-exposed groups were decreased in relative to the warm-PBS groups (Fig. 1B). Treatment with AAV complexes did not affect body weight, suggesting that AAV may not have toxic effect on body weight gain.
In vivo MRI analysis of heart function and LV area
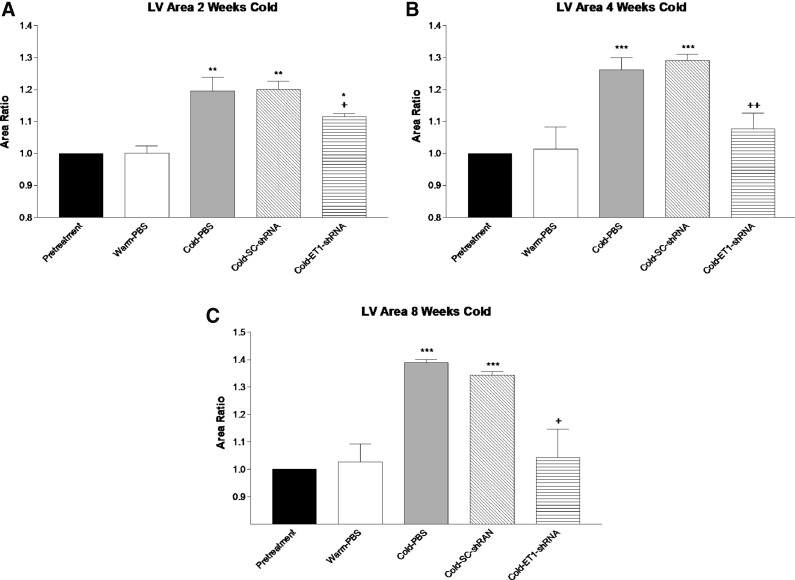
MRI analysis was used to measure cardiac function and monitor heart hypertrophy in vivo. The LV surface area was significantly increased in the PBS and Sc-shRNA groups by two weeks of exposure to cold (Fig. 2A). The LV surface area continued to increase at 4 weeks (Fig. 2B). By 8 weeks of exposure to cold, the LV area was increased by almost 40% over the warm-PBS groups (Fig. 2C). AAV delivery of ET1-shRNA significantly attenuated the LV area gain at all time points of exposure to cold (Fig. 2A–C).
Figure 2.
In vivo magnetic resonance imaging analysis of heart function and left ventricle (LV) area. (A) LV surface area by 2 weeks of cold exposure. (B) LV surface area by 4 weeks of cold exposure. (C) LV surface area by 8 weeks of cold exposure. Data were calculated as fold changes of the pretreatment level. Data shown as means ± SEM; n = 8. *p < 0.05, **p < 0.01, ***p < 0.001 vs. warm-PBS group. +p < 0.05, ++p < 0.01 vs. the cold-SC-shRNA group.
Cardiac function was determined based on ejection fraction and cardiac output. The ejection fraction was 72 ± 6.8% in the warm-PBS group at 8 weeks. Cold exposure slightly but not significantly decreased the ejection fraction (65 ± 5.6%) in the PBS or Sc-shRNA groups (data not shown). The ejection fraction was 68 ± 4.8% for the ET1-shRNA group. Cardiac output was not significantly different between groups at any time points although ET1-shRNA-treated animals showed a slightly higher cardiac output in relative to the PBS and Sc-shRNA groups by 8 weeks of exposure to cold. Thus, AAV delivery did not affect cardiac output significantly, suggesting the safety of the AAV complex.
AAV delivery attenuated cold-induced cardiac hypertrophy
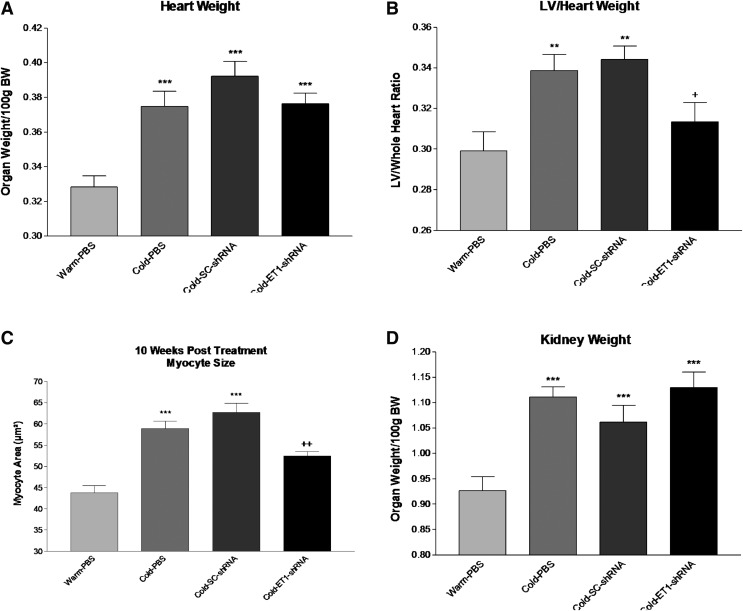
Cold increased heart weight significantly vs. the Warm-PBS group (Fig. 3A), indicating cardiac hypertrophy. ET1-shRNA slightly but not significantly decreased heart weight. The LV was dissected out and weighed. Interestingly, ET1-shRNA significantly decreased the increase in LV weight in cold-exposed rats (Fig. 3B), suggesting that RNAi silencing of ET1 attenuates cold-induced cardiac hypertrophy. Myocytes size was measured in 5 μm sections of the LV. Cold exposure significantly increased the myocyte size (Fig. 3C), indicating myocyte hypertrophy. ET1-shRNA prevented cold-induced myocyte hypertrophy (Fig. 3C).
Figure 3.
AAV delivery attenuated cold-induced cardiac hypertrophy. (A) Heart weight. (B) LV:heart weight ratio. (C) Myocyte area. (D) Kidney weight. These parameters were measured at 10 weeks after exposure to cold. Data shown as means ± SEM; n = 8. **p < 0.01, ***p < 0.001 vs. warm-PBS group. +p < 0.05, ++p < 0.01 vs. the cold-SC-shRNA group.
Cold exposure also significantly increased kidney weights (Fig. 3D), indicating renal hypertrophy. Unexpectedly, ET1-shRNA did not affect cold-induced renal hypertrophy.
AAV delivery ET1-shRNA attenuated the cold-induced increase in ET1 levels
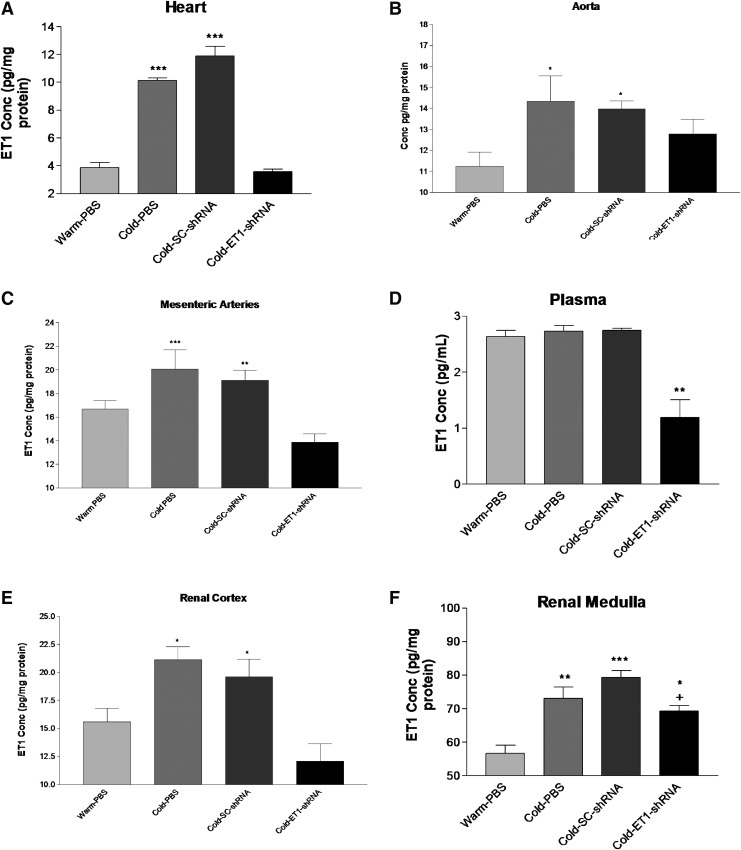
ET1 protein levels were measured in several tissues and plasma using ELISA. Cold exposure significantly increased ET1 levels in the heart, aorta, and mesenteric arteries (Fig. 4A–C), indicating that cold exposure induces ET1 production. ET1-shRNA prevented the cold-induced increases in ET1 generation in these organs, suggesting effective silencing of ET1 gene. By contrast, plasma concentration of ET1 was not affected by cold exposure although it was decreased significantly by ET1-shRNA (Fig. 4D). ET1-shRNA abolished the cold-induced increase in ET1 levels in the renal cortex (Fig. 4E). The cold-induced increase in ET1 level in the renal medulla was also significantly attenuated but not prevented by ET1-shRNA (Fig. 4F).
Figure 4.
AAV delivery ET1-shRNA attenuated the cold-induced increase in ET1 levels. Concentrations of ET1 in heart (A), aorta (B), mesenteric arteries (C), plasma (D), renal cortex (E), and renal medulla (F). These parameters were measured at 10 weeks after exposure to cold. Data shown as means ± SEM; n = 8. *p < 0.05, **p < 0.01, ***p < 0.001 vs. warm-PBS group. +p < 0.05 vs. the cold-SC-shRNA group.
AAV delivery ET1-shRNA attenuated the cold-induced increase in superoxide production
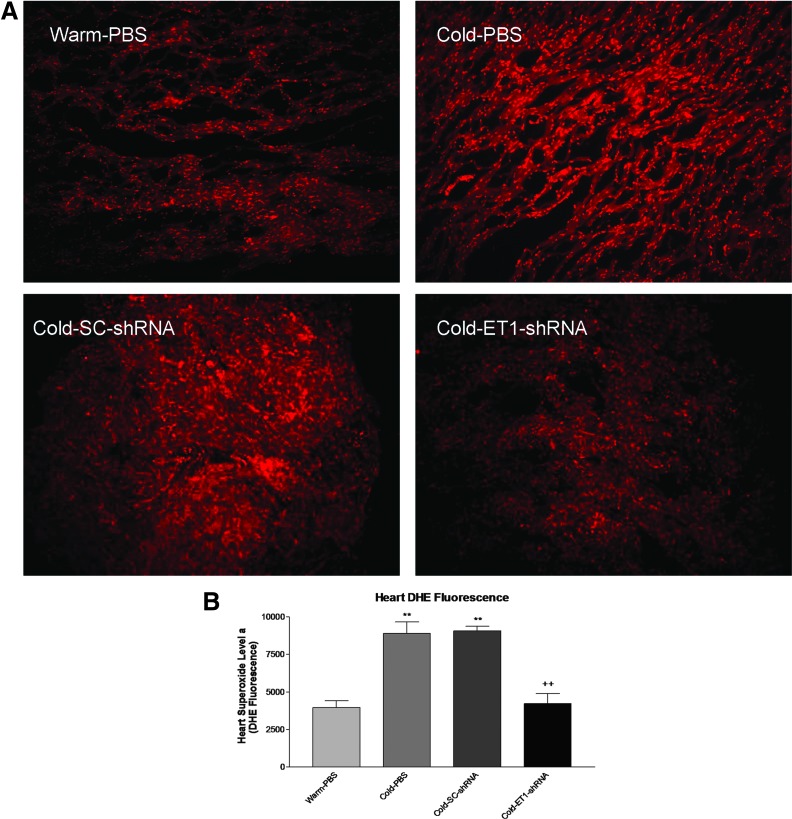
We assessed the levels of in situ superoxide production in the heart. Frozen heart sections were stained with DHE and visualized when excited. Cold exposure increased superoxide levels (brighter red ethidium fluorescence) which was mitigated by ET1-shRNA (Fig. 5A). To further quantify the amount of superoxide levels, we performed an assay using freshly homogenized tissue incubated with DHE and read in a fluorescent plate reader. The data confirmed that ET1-shRNA abolished the cold-induced increase in superoxide production in the heart (Fig. 5B).
Figure 5.
AAV delivery ET1-shRNA attenuated the cold-induced increase in superoxide production. (A) In situ superoxide levels in the heart (red, DHE staining). (B) Quantification of superoxide levels. These parameters were measured at 10 weeks after exposure to cold. Data shown as means ± SEM; n = 8. **p < 0.01 vs. warm-PBS group. ++p < 0.05 vs. the cold-SC-shRNA group.
Discussion
Cold temperatures have adverse effects on the human cardiovascular system. The prevalence of hypertension and ischemic heart disease is higher in the cold regions, suggesting that cold temperatures are an important risk factor for cardiovascular disease. The mortality from cardiovascular disease peaks in winter. Cold temperatures trigger myocardial infarction and stroke which are largely attributed to CIH. Unfortunately, there is no specific intervention recommended for CIH. We have previously shown that ET1 levels are significantly increased due to cold exposure.21 In this study, we demonstrated that silencing of the ET1 gene by RNA interference (RNAi) prevented the development of CIH (Fig. 1). To the best of our knowledge, this is the first report showing that upregulation of ET1 production plays a critical role in the pathogenesis of CIH. This finding is significant because it provides a potential therapeutic target for CIH and CIH-related cardiovascular disease. Interestingly, one single dose of AAV.ET1-shRNA controls hypertension for up to 10 weeks (length of the study), indicating the long-lasting therapeutic effect of the AAV complex. The AAV vector can deliver target genes to the nuclei of cells, where the therapeutic gene then integrates into the host genome.30 Therefore, AAV delivery of ET1-shRNA provides a powerful and long-lasting therapy for hypertension, overcoming the disadvantages of the current pharmacological therapy.
The effect of ET1 is mediated by ET receptors. There are two major types of ET receptors (ETA and ETB), each has several subtypes.21,31 Activation of ETA and ETB receptors often has opposite effects on blood pressure. The purpose of this study is to investigate if the ET system plays a role in the pathogenesis of CIH. Therefore, the best approach for achieving this objective is to test whether inhibition of ET1 generation attenuates the development of CIH. Future studies are required to assess the contribution of individual ET receptors to CIH.
ET1-shRNA effectively silenced the ET1 gene as it abolished cold-induced upregulation of ET1 production in hearts, aortas, and small arteries (Fig. 4). Our previous studies showed that AAV is an effective vector for delivering therapeutic genes to cardiomyocytes, renal cells, and vascular cells.12,32–34 AAV-mediated delivery of mineralocorticoid receptor (MR) shRNA decreased MR protein expression in renal tubules, attenuated CIH, and improved kidney structure and function.34 In vivo expression of anti-aging gene Klotho attenuated hypertension and improved kidney function and structure in spontaneous hypertensive rats.33 By contrast, AAV-based RNAi inhibition of brain klotho activates the sympathetic nervous system and potentiates cold-induced elevation of BP though the endothelin pathway,32 implicating that there exists a cross-talk between Klotho and ET1 in the pathogenesis of CIH.
We chose to use AAV to carry therapeutic genes because AAV is safe, nonpathogenic, noninflammatory, and extremely stable.35–36 AAV has been approved by the Food and Drug Administration as a vector for clinical gene therapy.37–44 In this study we found that the virus had no effect on growth rate, food consumption (data not shown), or heart function. There were no deaths following the tail vein injection and the rats developed no abnormalities for the duration of the treatment.
In this study, we monitored the heart size by measuring in vivo heart surface area using MRI. Animals also developed cardiac hypertrophy during exposure to cold as evidenced by significant increases in LV surface areas and heart weight (Figs. 2, 3). Cardiac hypertrophy is an important risk factor for myocardial infarction. Interestingly, RNAi silencing of ET1 attenuated cold-induced LV hypertrophy but does not significantly decrease the heart weight, suggesting that LV hypertrophy is likely due to increased afterload (i.e., hypertension). This finding also suggests that cold-induced cardiac hypertrophy is whole heart hypertrophy. It was reported that increased thyroid hormone (TH) secretion contributes to cold-induced cardiac hypertrophy.45 TH promotes protein synthesis and myocyte growth.45 Cold-induced kidney hypertrophy was not due to upregulation of ET1 because ET1-shRNA did not attenuate the increase in kidney weight in cold-exposed rats (Fig. 3).
Cold exposure activates the RAS and downregulates eNOS expression contributing to the development of CIH.13–20 We found that the cold-induced increase in ET1 production in the heart is likely due to activation of the RAS via AT1A receptors.9 It was reported that an increase in ET1 suppressed eNOS expression because inhibition of ET actions by Bosentan (ET receptor antagonist) abolished the downregulation of eNOS expression in diabetic rats.46 Thus, we believe that cold-induced overactivation of the RAS increases ET1 production which suppresses eNOS expression thus playing a role in the pathogenesis of CIH.
Cold exposure increased superoxide levels in the heart (Fig. 5), which is likely due to increased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity.12 We reported that suppression of superoxide production by inhibition of NADPH oxidase attenuates the development of cardiac hypertrophy,12 suggesting that overproduction of superoxide contributes to cardiac hypertrophy. It is noteworthy that silencing of ET1 abolished cold-induced upregulation of superoxide production. Thus, this study provides the first evidence that upregulation of ET1 is a critical mediator of cold-induced generation of superoxide. It was reported that ET1 activates NADPH oxidases.47 On the other hand, cold exposure increases secretion of thyroid hormones,45 which stimulates Nox1 NADPH oxidase activity.48 A future study is required to assess the relationship of ET1 and TH in the regulation of NADPH oxidase activity and superoxide production.
Cold exposure increases ET1 levels in the heart, aorta, small vessels, and kidneys. In vivo silencing of the ET1 gene by AAV delivery of ET1-shRNA prevented the development of cold-induced hypertension and cardiac hypertrophy. This study provides the first evidence that upregulation of ET1 production contributes to the pathogenesis of cold-induced hypertension and cardiac hypertrophy. The finding of this study will help develop an effective strategy for the control of cold-related cardiovascular disease.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 HL116863, HL122166, DK093403, HL118558, AG049780, and HL077490.
We would like to thanks Dr. Xiuqing Wang, Dr. Mahajoub Bello Roufai, and Lucile Skelley for technical support.
Author Disclosure
No competing financial interests exist.
References
- 1.Gorjanc ML, Flanders WD, VanDerslice J, et al. . Effects of temperature and snowfall on mortality in Pennsylvania. Am J Epidemiol 1999;149:1152–1160 [DOI] [PubMed] [Google Scholar]
- 2.Baker-Blocker A. Winter weather and cardiovascular mortality in Minneapolis-St. Paul. Am J Public Health 1982;72:261–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thorvaldsen P, Asplund K, Kuulasmaa K, et al. . Stroke incidence, case fatality, and mortality in the WHO MONICA project. World Health Organization Monitoring Trends and Determinants in Cardiovascular Disease. Stroke 1995;26:361–367 [DOI] [PubMed] [Google Scholar]
- 4.Fu S, Cao Y, Li Y, et al. . Hypertensive epidemiology in Heilongjiang Province in China. Chin Med J (Engl) 2002;115:498–501 [PubMed] [Google Scholar]
- 5.Velazquez Monroy O, Rosas Peralta M, Lara Esqueda A, et al. [Arterial hypertension in Mexico: results of the National Health Survey 2000]. Arch Cardiol Mex 2002;72:71–84 [PubMed] [Google Scholar]
- 6.Donaldson GC, Robinson D, Allaway SL. An analysis of arterial disease mortality and BUPA health screening data in men, in relation to outdoor temperature. Clin Sci (Lond) 1997;92:261–268 [DOI] [PubMed] [Google Scholar]
- 7.Heller RF, Rose G, Pedoe HD, et al. . Blood pressure measurement in the United Kingdom Heart Disease Prevention Project. J Epidemiol Community Health 1978;32:235–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prineas RJ, Gillum RF, Horibe H, et al. . The Minneapolis children's blood pressure study. Part 1: standards of measurement for children's blood pressure. Hypertension 1980;2:I18–24 [DOI] [PubMed] [Google Scholar]
- 9.Sun Z. Cardiovascular responses to cold exposure. Front Biosci (Elite Ed) 2010;2:495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verdon F, Boudry JF, Chuat M, et al. [Seasonal variations in arterial pressure in hypertensive patients]. Schweiz Med Wochenschr 1993;123:2363–2369 [PubMed] [Google Scholar]
- 11.Minami J, Kawano Y, Ishimitsu T, et al. . Seasonal variations in office, home and 24 h ambulatory blood pressure in patients with essential hypertension. J Hypertens 1996;14:1421–1425 [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Skelley L, Wang B, et al. . AAV-based RNAi silencing of NADPH oxidase gp91(phox) attenuates cold-induced cardiovascular dysfunction. Hum Gene Ther 2012;23:1016–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Cade R, Sun Z. Human eNOS gene delivery attenuates cold-induced elevation of blood pressure in rats. Am J Physiol Heart Circ Physiol 2005;289:H1161–1168 [DOI] [PubMed] [Google Scholar]
- 14.Sun Z, Wang X, Wood CE, et al. . Genetic AT1A receptor deficiency attenuates cold-induced hypertension. Am J Physiol Regul Integr Comp Physiol 2005;288:R433–439 [DOI] [PubMed] [Google Scholar]
- 15.Sun Z, Zhang Z, Cade R. Renal responses to chronic cold exposure. Can J Physiol Pharmacol 2003;81:22–27 [DOI] [PubMed] [Google Scholar]
- 16.Sun Z, Cade R, Morales C. Role of central angiotensin II receptors in cold-induced hypertension. Am J Hypertens 2002;15:85–92 [DOI] [PubMed] [Google Scholar]
- 17.Sun Z, Cade R, Katovich MJ, et al. . Body fluid distribution in rats with cold-induced hypertension. Physiol Behav 1999;65:879–884 [DOI] [PubMed] [Google Scholar]
- 18.Sun Z, Cade JR, Fregly MJ, et al. . Effect of chronic treatment with propranolol on the cardiovascular responses to chronic cold exposure. Physiol Behav 1997;62:379–384 [DOI] [PubMed] [Google Scholar]
- 19.Sun Z, Cade JR, Fregly MJ. Cold-induced hypertension. A model of mineralocorticoid-induced hypertension. Ann N Y Acad Sci 1997;813:682–688 [DOI] [PubMed] [Google Scholar]
- 20.Sun Z, Fregly MJ, Rowland NE, et al. . Comparison of changes in blood pressure and dipsogenic responsiveness to angiotensin II in male and female rats chronically exposed to cold. Physiol Behav 1996;60:1543–1549 [DOI] [PubMed] [Google Scholar]
- 21.Chen GF, Sun Z. Effects of chronic cold exposure on the endothelin system. J Appl Physiol (1985) 2006;100:1719–1726 [DOI] [PubMed] [Google Scholar]
- 22.Lin Y, Sun Z. In vivo pancreatic beta-cell-specific expression of antiaging gene klotho: a novel approach for preserving beta-cells in type 2 diabetes. Diabetes 2015;64:1444–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y, Sun Z. Antiaging gene klotho attenuates pancreatic beta-cell apoptosis in type 1 diabetes. Diabetes 2015;64:4298–4311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen K, Zhou X, Sun Z. Haplodeficiency of klotho gene causes arterial stiffening via upregulation of scleraxis expression and induction of autophagy. Hypertension 2015;66:1006–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y, Chen J, Sun Z. Antiaging gene klotho deficiency promoted high-fat diet-induced arterial stiffening via inactivation of AMP-activated protein kinase. Hypertension 2016;67:564–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou X, Chen K, Lei H, et al. . Klotho gene deficiency causes salt-sensitive hypertension via monocyte chemotactic protein-1/CC chemokine receptor 2-mediated inflammation. J Am Soc Nephrol 2015;26:121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, Chen K, Wang Y, et al. . Antiaging gene klotho regulates adrenal CYP11B2 expression and aldosterone synthesis. J Am Soc Nephrol 2016;27:1765–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bello Roufai M, Li H, Sun Z. Heart-specific inhibition of protooncogene c-myc attenuates cold-induced cardiac hypertrophy. Gene Ther 2007;14:1406–1416 [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Wang Q, Sun Z. Normal IgG downregulates the intracellular superoxide level and attenuates migration and permeability in human aortic endothelial cells isolated from a hypertensive patient. Hypertension 2012;60:818–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu P, Phillips MI, Bui J, et al. . Adeno-associated virus vector-mediated transgene integration into neurons and other nondividing cell targets. J Virol 1998;72:5919–5926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Sun Z. Antiaging gene Klotho regulates endothelin-1 levels and endothelin receptor subtype B expression in kidneys of spontaneously hypertensive rats. J Hypertens 2014;32:1629–1636 [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Sun Z. RNAi silencing of brain klotho potentiates cold-induced elevation of blood pressure via the endothelin pathway. Physiol Genomics 2010;41:120–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Sun Z. Klotho gene delivery prevents the progression of spontaneous hypertension and renal damage. Hypertension 2009;54:810–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Skelley L, Cade R, et al. . AAV delivery of mineralocorticoid receptor shRNA prevents progression of cold-induced hypertension and attenuates renal damage. Gene Ther 2006;13:1097–1103 [DOI] [PubMed] [Google Scholar]
- 35.Phillips MI, Kimura B. Gene therapy for hypertension: antisense inhibition of the renin-angiotensin system. Methods Mol Med 2005;108:363–379 [DOI] [PubMed] [Google Scholar]
- 36.Phillips MI, Tang Y, Schmidt-Ott K, et al. . Vigilant vector: heart-specific promoter in an adeno-associated virus vector for cardioprotection. Hypertension 2002;39:651–655 [DOI] [PubMed] [Google Scholar]
- 37.Pacak CA, Byrne BJ. AAV vectors for cardiac gene transfer: experimental tools and clinical opportunities. Mol Ther 2011;19:1582–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet 2011;12:341–355 [DOI] [PubMed] [Google Scholar]
- 39.High KA. Gene therapy for haemophilia: a long and winding road. J Thromb Haemost 2011;9 Suppl 1:2–11 [DOI] [PubMed] [Google Scholar]
- 40.Flotte TR, Trapnell BC, Humphries M, et al. . Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin: interim results. Hum Gene Ther 2011;22:1239–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashtari M, Cyckowski LL, Monroe JF, et al. . The human visual cortex responds to gene therapy-mediated recovery of retinal function. J Clin Invest 2011;121:2160–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li C, Narkbunnam N, Samulski RJ, et al. . Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther 2012;19:288–294 [DOI] [PubMed] [Google Scholar]
- 43.Allay JA, Sleep S, Long S, et al. . Good manufacturing practice production of self-complementary serotype 8 adeno-associated viral vector for a hemophilia B clinical trial. Hum Gene Ther 2011;22:595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCown TJ. Adeno-associated virus (AAV) vectors in the CNS. Curr Gene Ther 2011;11:181–188 [DOI] [PubMed] [Google Scholar]
- 45.Fregly MJ, Rossi F, Cade JR. A role for thyroid hormones in cold-induced elevation of blood pressure and cardiac hypertrophy. Can J Physiol Pharmacol 1994;72:1066–1074 [DOI] [PubMed] [Google Scholar]
- 46.Alkan E, Ugan RA, Basar MM, et al. . Role of endothelin receptors and relationship with nitric oxide synthase in impaired erectile response in diabetic rats. Andrologia 2016 [DOI] [PubMed] [Google Scholar]
- 47.Dammanahalli KJ, Sun Z. Endothelins and NADPH oxidases in the cardiovascular system. Clin Exp Pharmacol Physiol 2008;35:2–6 [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Sun Z. Thyroid hormone induces artery smooth muscle cell proliferation: discovery of a new TRalpha1-Nox1 pathway. J Cell Mol Med 2010;14:368–380 [DOI] [PMC free article] [PubMed] [Google Scholar]