Abstract
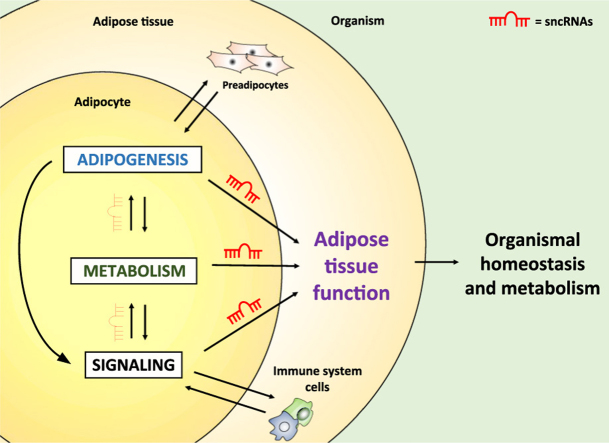
Metabolic diseases such as type 2 diabetes are a major public health issue worldwide. These diseases are often linked to a dysfunctional adipose tissue. Fat is a large, heterogenic, pleiotropic and rather complex tissue. It is found in virtually all cavities of the human body, shows unique plasticity among tissues, and harbors many cell types in addition to its main functional unit – the adipocyte. Adipose tissue function varies depending on the localization of the fat depot, the cell composition of the tissue and the energy status of the organism. While the white adipose tissue (WAT) serves as the main site for triglyceride storage and acts as an important endocrine organ, the brown adipose tissue (BAT) is responsible for thermogenesis. Beige adipocytes can also appear in WAT depots to sustain heat production upon certain conditions, and it is becoming clear that adipose tissue depots can switch phenotypes depending on cell autonomous and non-autonomous stimuli. To maintain such degree of plasticity and respond adequately to changes in the energy balance, three basic processes need to be properly functioning in the adipose tissue: i) adipogenesis and adipocyte turnover, ii) metabolism, and iii) signaling. Here we review the fundamental role of small non-coding RNAs (sncRNAs) in these processes, with focus on microRNAs, and demonstrate their importance in adipose tissue function and whole body metabolic control in mammals.
Abbreviations: 3'-UTR, 3' untranslated region; 4-HNE, 4-hydroxynonenal; AC, acetylation; ADGCR8KO, fat-specific DGCR8 knockout; ADicerKO, fat-specific Dicer knockout; ADSC, adipose-derived stem cell; AGO, Argonaute; ATGL, adipose triglyceride lipase; ATP, adenosine triphosphate; BAT, brown adipose tissue; BMI, body mass index; BMP, bone morphogenetic proteins; BMSC, bone marrow stromal cell; bp, base pair; C/EBP, CCAAT-enhancer-binding protein; CGI58, comparative gene identification-58; DGCR8, DiGeorge syndrome chromosomal [or critical] region 8; dsRNA, double stranded RNA; FFA, free fatty acid; FGF, fibroblast growth factor; FOXO1, forkhead box protein O1; GDF5, growth differentiation factor 5; GLUT4, glucose transporter type 4; GPC, G protein coupled; GPX, glutathione peroxidase; HFD, high fat diet; HIV, human immunodeficiency virus; HMGA2, high-mobility group AT-hook 2; IKK, IκB kinase; IL, interleukin; IRS1, insulin receptor substrate 1; JNK, c-Jun N-terminal kinase; KO, knockout; KSRP, KH-type splicing regulatory protein; LDL, low-density lipoprotein; miRNA, microRNA; mRNA, messenger RNA; MSC, mesenchymal stem cell; MYF5, myogenic factor 5; ncRNA, non-coding RNA; nt, nucleotide; OXPHOS, oxidative phosphorylation; PACT, Protein ACTivator of the interferon-induced protein kinase; PCOS, polycystic ovarian syndrome; PGC-1, peroxisome proliferator-activated receptor gamma coactivator 1; piRNA, piwi-interacting RNA; PKC, protein kinase C; PPARγ, peroxisome proliferator-activated receptor gamma; PRDM16, PR domain containing 16; pre-miRNA, precursor miRNA; pri-miRNA, primary miRNA; Rb, retinoblastoma protein; Rb2/p130, retinoblastoma-like protein 2; RISC, RNA-induced silencing complex; RNAi, RNA interference; ROS, reactive oxygen species; RT-qPCR, reverse transcription – quantitative polymerase chain reaction; RUNX2, Runt-related transcription factor 2; SCD-1, stearoyl-CoA desaturase-1; SGBS, Simpson-Golabi-Behmel syndrome; siRNA, small interference RNA; sncRNA, small non-coding RNA; SOD, superoxide dismutase; SREBP-1, sterol regulatory element-binding transcription factor 1; sWAT, subcutaneous white adipose tissue; T2D, Type 2 Diabetes; TCA, tricarboxylic acid; TG, triglyceride; TGFβ, transforming growth factor beta; TLR4, toll like receptor 4; TNF-α, tumor necrosis factor alpha; TRBP, HIV-1 TAR RNA binding protein; UCP1, uncoupling protein 1; VEGF, vascular endothelial growth factor; vHPA, human visceral preadipocytes; vWAT, visceral white adipose tissue; WAT, white adipose tissue; WHO, World Health Organization
Keywords: Adipose tissue, MicroRNAs, Small non-coding RNAs, Obesity, Diabetes
Graphical abstract
Highlights
-
•
Adipose tissue dysfunction is a major cause of metabolic diseases.
-
•
Adipose tissue function is determined by three hallmarks: adipogenesis, metabolism and signaling.
-
•
Small non-coding RNAs (particularly microRNAs) are causally linked to these hallmarks in adipose tissue.
-
•
Changes in microRNA expression in adipose tissue occur in physiological conditions and in disease.
-
•
Adipose tissue microRNAs control whole body metabolism and organismal homeostasis.
1. Metabolic diseases
Obesity statistics are alarming. According to the World Health Organization (WHO), more than 1.9 billion adults were overweight in 2014, and more than half a billion were obese. Once an exclusive issue of high-income countries, obesity is now prevalent in the developing world and has been considered a global epidemic [1]. This is extremely worrisome given that obesity represents one of the most important risk factors for chronic diseases, including type 2 diabetes (T2D), cardiovascular diseases and cancer – the leading causes of mortality and morbidity worldwide [2]. When appearing together, these diseases are classified as a broader syndrome often referred to as the “metabolic syndrome” [3].
The increase in the prevalence of obesity is due to many factors including genetics, diet, sedentarism and issues concerning the psychological, socioeconomic, or educational status of the individual [4]. Simplistically, obesity appears when there is a positive energetic balance, i.e. when energy consumption (food intake) overcomes energy expenditure [5], [6]. The definition and classification of obesity is still a subject of debate. The WHO divides individuals into four categories based on their body mass index (BMI, kg/m2): underweight BMI <18.50, normal weight BMI=18.50–24.99, overweight=BMI 25.00–29.99, and obese BMI≥30.00 [7]. This sole parameter often creates confusion regarding the risk of metabolic diseases, since individuals can be classified as overweight or obese but do not display increased risk of mortality nor any metabolic alteration [8], [9]. Moreover, in some cases even a negative association between BMI and mortality - in particular cardiovascular mortality – is found [10]. To avoid misdiagnosis, supporting clinical information needs to be taken into consideration, such as patient's life history (e.g. addictive behavior or body weight oscillation) and localization of excessive fat mass (e.g. upper body or lower body) [8], [11], [12]. When considering the latter, obesity can be further classified into an android syndrome (i.e. male-type or apple-shaped), where fat is deposited preferentially in intra-abdominal regions (i.e. visceral white adipose tissue - vWAT), and a gynoid syndrome (i.e. female-type or pear-shaped), where fat is deposited over the gluteofemoral region (i.e. subcutaneous white adipose tissue - sWAT) [13], [14]. The first has been more commonly linked to comorbidities such as hypertension, dyslipidemia, and T2D [15], [16], [17], while the second confers a neutral or even protective effect against metabolic diseases [17], [18]. To account for these differences, parameters like waist-to-hip ratio, magnetic resonance imaging or dual-energy X-ray absorptiometry [19], [20] have been used as bona fide predictors of metabolic diseases and as useful parameters for researchers to understand how fat accumulation determines the risk of these diseases [12], [21], [22].
The pleiotropic nature of fat depots has been extensively explored in mice. For example, transplantation of sWAT from donor mice into the intra-abdominal region of a host mouse improves metabolism by reducing body weight and overall fat mass, increasing insulin sensitivity and whole body glucose uptake [23], and alleviating diet-induced glucose intolerance and inflammation [24], [25]. Interestingly, these effects are minor when sWAT is transplanted into the subcutaneous cavity and no effect is observed when vWAT is transplanted to the intra-abdominal area [23], [24], [25], indicating that the differences between depots are determined by intrinsic characteristics as well as by anatomical localization. This is particularly highlighted in patients with lipodystrophy, where the pattern of fat accumulation differs from normal. These patients exhibit a degree of adipose tissue atrophy (whereas some depots can be hypertrophic), and often end up accumulating fat in non-adipose organs such as liver, heart, and skeletal muscle – a phenomenon that is causally linked to the metabolic syndrome [26], [27], [28]. Lipodystrophy can be classified according to fat loss topography (e.g. general or partial) or cause (e.g. congenital or acquired) [29], [30]. The most prevalent form of lipodystrophy is acquired by patients with HIV undergoing antiretroviral treatment [31], [32]. For instance, the highly active antiretroviral treatment (i.e. a combination of at least three antiretroviral drugs) inhibits mitochondrial DNA polymerase-γ causing mitochondrial toxicity, and the use of protease inhibitors up-regulates genes that inhibit adipocyte differentiation and down-regulates pro-adipogenic transcriptional factors such as PPAR-γ, C/EBP-α and SREBP-1, as well as genes involved in lipid metabolism [26], [33], [34]. These alterations are observed in white adipocytes, mainly in the subcutaneous depot either from the upper (e.g. face and shoulder) and/or lower body (e.g. gluteal and femoral). Additionally, HIV patients can also exhibit increased fat accumulation in breast, cervical, dorsocervical and visceral depots [35], [36]. Importantly, these patients have a higher risk to develop insulin resistance and cardiovascular disease, and appear to age prematurely [37], [38], [39]. Therefore, abnormal changes in adipose tissue accumulation, independently of fat gain or loss, impact on the risk of metabolic diseases, a feature that highlights the importance of adipose tissue to whole body metabolic control.
2. The etiopathogenesis of metabolic diseases
Insulin resistance is a common feature of metabolic diseases and represents the main cause of T2D [40]. Like for obesity, the prevalence of T2D in the world is extremely high (8.8% of the world's population) and is rapidly growing [41]. In 2015, T2D killed approximately 5 million people [42]. The causes of T2D involve both genetic [43] and environmental factors that progressively lead to insulin resistance, glucose intolerance, and ultimately beta-cell failure [44], [45]. Several reports support the notion that excess lipids continuously released from visceral fat into the portal vein in obese patients is a major trigger of T2D [46], [47]. These lipids expose the liver to incremental amounts of free fat acids (FFAs), inducing hepatic insulin resistance and affecting gluconeogenesis [46], [48]. Interestingly, like mentioned above, despite the association between T2D and obesity, some morbidly obese individuals as classified by BMI are significantly more glucose tolerant than leaner individuals [49]. Again, this apparent paradox might be explained by changes in the pattern of fat accumulation, since increased adipose tissue expandability and plasticity, particularly of subcutaneous depots, is linked to metabolic health [50], [51]. In essence, the proposed model evokes that every individual has a limited capacity for fat storage, and when this limit is reached, either by obesity or impaired adipocyte function, excess lipids accumulate ectopically, causing lipotoxicity, inflammation, tissue dysfunction and disease [52], [53], [54].
At the molecular level, FFAs are usually taken up by cells and esterified into long chain acyl CoA molecules [55]. The bulk of these metabolites are directed to beta-oxidation, but a small portion can be converted into two lipid intermediates: diacylglycerols and ceramides [56]. In excess, these intermediates can activate kinases such as Jun amino-terminal kinase (JNK), IκB kinase (IKK), and protein kinase C (PKC) [57], [58], [59], [60], which in turn phosphorylate the insulin receptor substrate-1 (IRS1) in serine and inhibit insulin signaling downstream of the insulin receptor [60], [61], [62]. Furthermore, lipids can signal through toll-like receptors or G protein-coupled receptors to cause insulin resistance or affect insulin secretion [63], [64]. Additionally, excessive adipocyte hypertrophy without proper neovascularization creates a pseudohypoxic state in the adipose tissue [65], [66]. Altogether, these stimuli lead to the infiltration of immune cells into the adipose tissue [67], [68]. Within the tissue, monocytes differentiate into either anti-inflammatory M2 or pro-inflammatory M1 macrophages depending on the niche [69]. M1 macrophages are more commonly present in adipose tissue under obesogenic conditions, when they are activated by fatty acids and adipocyte-derived factors, and release a variety of pro-inflammatory cytokines that signal locally or systemically to cause insulin resistance through the activation of the same group of kinases and similar mechanisms as mentioned above [70], [71].
Interleukin 1 beta (IL-1β) is one such pro-inflammatory cytokine synthesized and released from M1 macrophages in the context of obesity [72], [73]. IL-1β production and secretion is controlled by the inflammasome and contributes directly to establishment of insulin resistance [74], [75], [76]. Supporting this notion, Ehses et al. treated type 2 diabetic Goto-Kakizaki rats with IL-1 receptor antagonist and observed an improvement in insulin sensitivity and glucose tolerance [77]. M1 macrophages also produce tumor necrosis factor alpha (TNF-α) [78], [79]. TNF-α acts in cells within the adipose tissue and in non-adipose tissues to inhibit the tyrosine phosphorylation of IRS1 and therefore its activation [80], [81]. Moreover, it modifies the pattern of adipocyte differentiation [82], [83] while inducing lipolysis, which in turn increases the levels of FFAs and creates a “pro-inflammatory vicious cycle” [84], [85]. In addition to causing lipotoxicity, as mentioned before, FFAs stimulate the synthesis of pro-inflammatory cytokines through the toll-like receptor-4 (TLR4) pathway [86], [87]. IL-6 is also involved in the pathophysiology of obesity, but its role is more complex. It is increased in adipose tissue and in circulation with obesity [88], [89], and in vitro studies using adipocytes or hepatocytes chronically exposed with high levels of IL-6 show that this cytokine impairs insulin signaling [90], [91], [92]. However, transient or acute increases in IL-6 levels in healthy or type 2 diabetic patients, either by exogenous infusion or exercise, have been associated with beneficial effects, which include increased insulin sensitivity and improved glucose uptake [93], [94], [95]. Moreover, while brown adipose tissue (BAT) transplantation from a wild type donor mouse to a wild type recipient mouse improves glucose tolerance, insulin sensitivity, and decreases fat mass, when BAT isolated from a IL-6 knockout mouse is used for the transplantation these effects are lost [96]. Indeed, the period of exposure and the source of IL-6 determine its effects. While an acute increase can be beneficial, chronic exposure (as in obesity) is often deleterious [97].
Sustained cellular exposure to energetic substrates such as FFAs and glucose, in addition to systemic inflammation, also modifies the redox state of the cell and induces oxidative stress. During obesity, antioxidants enzymes [e.g. superoxide dismutase (SOD) and glutathione peroxidase (GPX)] cannot cope with the accumulation of reactive oxygen species (ROS) [e.g. superoxide (O2−), hydrogen peroxide (H2O2), and hydroxyl radical (OH−)] [98], [99], particularly in the mitochondria [100], [101]. Furthermore, TNF-α stimulates the activity of NADPH oxidase enzymes exacerbating the generation of superoxide [102]. Chronic exposure to ROS alters the expression of glucose transporters and impairs insulin-stimulated glucose uptake [103]. Interestingly, rats fed a high-fat diet are not insulin resistant when administered with the exogenous antioxidant SS31 [104], [105]. In contrast, antioxidant administration (a combination of vitamin C and E) blunts the induction of an endogenous antioxidant response and the amelioration of parameters of insulin sensitivity when healthy, non-obese people are subjected to exercise [106].
Changes in the metabolic status of the adipocyte also affect the production and secretion of adipocyte-derived factors often named adipokines. Many adipokines have been described, including the cytokines mentioned above, but two adipocyte-specific molecules are worth citing: leptin and adiponectin [107]. Leptin was the first identified adipokine. Its coding gene (Lep or ob) was found to create a phenotype of massive obesity and infertility when mutated in homozygosis in mice [108]. Leptin is secreted by adipocytes in response to a positive energy balance, and acts primarily in the brain to inhibit food intake and promote energy expenditure [109], [110]. Furthermore, leptin plays a role in immunity, energy portioning and lipid storage, as well as bone homeostasis [111]. Adiponectin is also expressed exclusively by adipocytes and acts as an anti-diabetic, cardioprotective adipokine. Adiponectin levels are extremely high in circulation (7–10 mg/L) and weight loss reduces these levels [112]. In general, adiponectin acts to improve whole body glucose homeostasis and lipid handling [107]. Adiponectin can also reduce inflammation and it is thought to be beneficial against the metabolic syndrome [107]. However, under certain conditions, adiponectin can hamper osteoblast proliferation and decrease bone mass, which can be detrimental [113].
Together, these studies evidence a complex, yet fundamental role of fatty acids, adipokines and ROS in the etiopathogenesis of metabolic diseases, reinforcing the notion that these molecules act to maintain organismal homeostasis and are required for normal physiology, but can be harmful when in excess.
3. Different types of fat
Fat is a simplistic name for a rather complex tissue that exhibits heterogeneous morphology, contains many different cell types, shows distinct functions, and localizes in all compartments of the body. The characteristic unit of the adipose tissue is the adipocyte – a cell that is specialized in storing triglycerides. In addition to adipocytes, the adipose tissue hosts stem cells, preadipocytes, fibroblasts, endothelial cells, macrophages, and other immune system cells [114]. The adipose tissue has been historically classified in two sub-classes: white (WAT) and brown (BAT). This classification is based on morphological and molecular signatures that take into account cell structure, color, localization, vascularization, gene expression and function [115].
Adipocytes in WAT are composed by a single large unilocular lipid droplet in the cytoplasm and a peripheral nucleus [116]. The vascular network of WAT is dense, providing enough substrate and oxygen delivery and facilitating the access of hormones, adipokines and other signaling molecules [117]. In humans, WAT depots are located in between visceral organs (vWAT; e.g. epicardial, mesenteric, omental, retroperitoneal, and gonadal depots), and in the subcutaneous compartment (sWAT; e.g. abdominal, gluteal, and femoral depots) [116]. Rodents have a similar pattern of WAT distribution with slight differences [118]. As mentioned before, vWAT differs from sWAT in several aspects despite being both categorized as WAT. For example, in sWAT, lipolysis is more efficiently inhibited by insulin, while the vWAT displays a great lipolytic response to catecholamines and lower response to insulin [119], [120], [121]. Furthermore, vWAT produces and releases more of the pro-inflammatory molecules that are associated with insulin resistance and T2D [88], [122], [123]. It also expresses angiotensinogen – a precursor of angiotensin II - providing a mechanistic link to cardiovascular disease [124]. On the other hand, sWAT shows a more plastic, intermediate phenotype that confers it vWAT-like or BAT-like characteristics depending on the condition [125], [126]. This aspect will be discussed later in this section.
Undoubtedly, one of the main functions of WAT is to store energy and mobilize nutrients in periods of negative energy balance. In addition, WAT has many other important roles: i) protecting organs such as eyes, gut and body parts such as heels, pads and gluteus from mechanical stress; ii) conferring insulation and thermoregulation [127]; iii) protecting the organism from lipotoxicity [128]; iv) being a major reservoir of mesenchymal stem cells [129] and immune system cells (e.g. macrophages and T cells) [130]; and v) functioning as an important endocrine organ [117]. WAT undergoes massive expansion and contraction in response to chronic alterations in energy balance, accounting for as little as 5% of the body mass in extremely lean athletes [131] or as much as 65% of the body mass in obese individuals [132], corresponding to the largest tissue of the body. Moreover, in most mammals, the WAT can completely regenerate itself following lipectomy [133], [134], [135]. This striking degree of plasticity is unique among the organs of adult mammals. At the cellular level, adipose tissue expansion is driven by both hypertrophy and hyperplasia of adipocytes [136], [137], [138]. Even in nonexpanding fat, the pool of adipocytes frequently renews itself to compensate for adipocyte death, with approximately 10% of adipocytes renewed annually in humans and 1–5% of adipocytes being replaced each day in mice [139], [140]. This is thought to be mainly accounted for by de novo adipocyte differentiation of a pool of committed adipocyte precursors (preadipocytes) existing within the adipose tissue.
In contrast to WAT, BAT is specialized in burning calories to produce heat. Brown adipocytes contain multiple lipid droplets (multilocular), display a large number of mitochondria with high respiration rates, and uniquely express uncoupling protein 1 (UCP1) - a transmembrane protein that dissipates heat by uncoupling the respiratory chain and allowing for substrate oxidation with low ATP production rate [141], [142], [143], [144], [145], [146]. BAT is highly vascularized and innervated [147]. This innervation is important to the activation of a thermogenic program that involves catecholamine signaling, nutrient mobilization and activation of UCP1 by FFA [148]. In rodents, BAT concentrates mainly in an interscapular depot, but can also be found in smaller fat pads in other anatomical regions (such as perirenal and perivascular) or within the skeletal muscle [146]. In humans, the existence of BAT was described for the first time in 1902 by Shinkishi Hatai [149]. The BAT described by Hatai was located under the sternocleidomastoid and trapezius muscles and ran laterally and in parallel to the neck (Hatai, 1902). Later on, Juliet Heaton (1972) observed the presence of BAT in adult humans – “it is localized in the neck, along the spinal cord, particularly in the para-aortic area, around the heart and infradiaphragmatic depots in the perirenal area”[150]. She added that during the first decade of life there is a wide distribution of active brown fat, but this distribution disappears gradually with age. More recently, discoveries showed that adult humans maintain an active pool of brown adipocytes located around the supra-clavicular and cervical regions of the body [151], [152], [153], [154], [155], [156], [157], [158], [159], [160].
In both rodents and humans, adipocytes with brown characteristics can also be found within WAT (particularly in sWAT) in response to cold exposure, beta-adrenergic stimulation, exercise or caloric restriction [146], [158], [161], [162], [163], [164]. Like classic (or preformed) brown adipocytes, these cells express UCP1, exhibit multilocular lipid droplets, and produce large amounts of heat. Due to their inducible/recruitable characteristics and their distinct developmental origin, these adipocytes are often distinguished from the other types by the name of inducible brown, brite (brown-in-white) or beige adipocytes.
The expression of UCP1 confers to brown and beige adipocytes the unique capacity of dissipating more heat per gram of tissue than any other cell in the body of a mammal [165], [166], [167], contributing to nearly 50% of the total oxygen consumption of a rat in the cold [168]. Ucp1 knockout mice develop obesity even under thermoneutrality due to impairment of diet-induced thermogenesis [169]. In humans, studies demonstrate that BAT-mediated cold activation increases energy expenditure by up to 28% [170], [171], [172], [173] and diet-induced thermogenesis by 32% in young men [174]. It is also estimated that 50–100 mL of BAT can increase energy expenditure by 150–300 kcal a day in cold exposed healthy men [173]. Interestingly, the amount of functional BAT or beige fat in adult humans and rodents is inversely correlated with adiposity and age, which raises the possibility of promoting brown adipogenesis to prolong metabolic healthspan and prevent obesity [146], [157], [175], [176], [177], [178].
UCP1–expressing adipocytes are also a “metabolic sink” for glucose and triglycerides, as they are responsible for clearing up to 75% and 50% of the total glucose and triglycerides from circulation in cold exposed mice [179], [180]. Although still debatable, recent studies have demonstrated that BAT activation in humans can contribute to the clearance of glucose from the circulation [reviewed by [179]]. These findings point to the potential of activating and/or recruiting brown or beige adipocytes to induce energy expenditure and improve glucose control. In practice, this could be achieved by the recruitment of newly formed UCP1-expressing cells from adipogenic precursor cells or by the induction of UCP1 expression in differentiated adipocytes. Both mechanisms have been shown to effectively induce energy expenditure and limit weight gain in mice when stimulated by pharmacological or genetic interventions [181], [182].
The functional identity of UCP1-expressing adipocytes is determined by both cell autonomous and cell non-autonomous mechanisms. Transcriptional regulators such as PRDM16 (PR domain containing 16), PGC1α (Peroxisome proliferator-activated receptor gamma coactivator 1-alpha), and C/EBPβ (CCAAT/enhancer-binding protein beta) [183], [184], [185], [186] are among the players required for the commitment and differentiation of brown or beige cells. Cold exposure, exercise, cancer cachexia, caloric restriction, or a rich social and physical environment are among the environmental cues that promote brown/beige fat activation and/or recruitment in mammals [161], [162], [184], [187], [188]. These conditions affect the expression of key transcriptional and post-transcriptional regulators in preadipocytes or in differentiated adipocytes, pushing these cells towards the activation of a brown-like gene expression signature, which in turn leads to UCP1 expression, mitochondrial biogenesis and energy dissipation. Some of these external cues are determined by endocrine function, i.e. by hormones or metabolites that are secreted in the circulation or locally, such as catecholamines, fibroblast growth factors (FGF), bone morphogenetic proteins (BMP), growth/differentiation factor 5 (GDF5), natriuretic peptides, prostaglandins, vascular endothelial growth factor (VEGF), and β-aminoisobutyric acid [reviewed by [179]]. For instance, the thermogenic effects of exercise have been linked to the recruitment of beige adipocytes by circulating molecules such as irisin, IL-6 and meteorin-like [161], [189], [190]. Some tumors also secrete molecules like IL-6 and PTHrP to induce beige adipocyte recruitment in WAT, a phenomenon that leads to cancer cachexia [187], [191]. Interestingly, the appearance of beige adipocytes in WAT after cold exposure or exercise requires the involvement of eosinophils and M2 macrophages, placing the immune system as a key regulator of adipocyte fate [189], [192].
The developmental origin of precursor cells also plays a role in adipocyte functional determination [193], [194], [195], [196]. In a groundbreaking study, Seale et al., 2008 demonstrated that brown but not white adipocytes arise from progenitor cells expressing Myf5 (myogenic factor 5), sharing a common precursor lineage with myoblasts [197]. Moreover, they found that the transcription regulator PRDM16 is essential for brown fat fate determination [197]. Indeed, different fat depots have different precursors with intrinsic characteristics that confer phenotypical differences to the adipose tissue [196], [198], [199], but these characteristics are not sufficient to ultimately determine the phenotype of the adipocyte upon differentiation. For example, white adipocytes located at the dorsal-anterior region in mice also derive from Myf5 progenitors [200], [201]. Differences in adipocyte characteristics can even be determined after differentiation. Rosenwald et al., 2013 showed that white adipocytes within sWAT have a bi-potential to be white or brown depending on the stimulus [126]. They also revealed that beige adipocytes rapidly acquire white-like characteristics upon removal of external stimuli without passing through an intermediate precursor stage [126], [202]. Understanding the mechanisms underlying the functional heterogeneity of adipocytes and fat depots has been a major goal of the field. Only by seeking this goal we will be able to manipulate fat cells to help people with metabolic diseases to better handle substrates and adjust their energy balance. Small non-coding RNAs (sncRNAs) appear as essential molecules in this context and therefore will be explored in details during this Review.
4. Small non-coding RNAs (sncRNAs)
The central dogma of molecular biology postulates that the genetic information contained in the DNA is transcribed into messenger molecules (e.g. messenger RNA - mRNA), which are in turn translated into proteins that exert function [203], [204]. This affirmation is not untruthful but is rather incomplete. The discovery of an abundant class of regulatory non-coding RNAs (ncRNAs) opened too many exceptions to the dogma and revolutionized molecular biology as we know it [205], [206].
SncRNAs is a sub-class of ncRNAs with maximum length of 199-nt and is further classified into at least 25 different types of molecules based on length, structure, precursor, processing machinery, organism expression, tissue expression and function [207] (Table 1). Most sncRNAs are involved in fine tuning gene expression by interacting with target mRNA or with proteins involved in mRNA translation [208], [209], and in some cases, they can interact with the chromatin to cause genomic rearrangements or epigenetic modifications [210], [211], [212], [213], [214].
Table 1.
Summary of types of sncRNAs with potential or described function across the animal kingdom. (?), function not well defined.
| Name | Abbrev | Class | Length (nt) | Function | Ref |
|---|---|---|---|---|---|
| C/D snoRNA-derived RNA snRNA | C/D sdRNA | snRNA | 17–19, 27 | Post-transcriptional gene repression, processing of pre-rRNA | [352], [353] |
| Centromere repeat-associated sRNA | crasiRNA | chRNA | 34–42 | Heterochromatin formation | [207], [354] |
| Double-strand break induced sRNA | diRNA | DSB | 21 | DNA double-strand break repair | [355], [356], [357] |
| Endogenous small interfering RNA | endo-siRNA | siRNA | 21–26 | Inhibition of retrotransposition, pos-transcriptional gene repression | [358], [359] |
| Exogeneous small interfering RNA | exo-siRNA | siRNA | 21 | Gene silencing, anti-viral | [360], [361] |
| H/ACA snoRNA-derived RNA snRNA | H/ACA sdRNA | snRNA | 20–24 | Post-transcriptional gene repression, alternative splicing | [362], [363], [364] |
| microRNA | miRNA | miRNA | 19–22 | Post-transcriptional gene repression | [221], [365] |
| miRNA-offset RNAs | moRNA | miRNA | 19–22 | Post-transcriptional gene repression | [367], [368] |
| Promoter-associated small RNAs | PASRs | 18–200 | Transcriptional regulation | [369], [370] | |
| Piwi interacting RNA or repeat-associated siRNA | piRNA or rasiRNA | piRNA | 25–33 | Germline post-transcriptional gene repression, transposon regulation, chromatin modification | [230], [242], [371] |
| Pyknon | Pyknon | miRNA/siRNA | 16–22 | Post-transcriptional gene repression | [372] |
| Small nuclear RNA or small Cajal-body RNA | snRNA or scaRNA | 128 | Pseudouridylation of U2 spliceosomal RNA (?) | [374], [375] | |
| Splice site-associated small RNA | spliRNA | CAsRNA | 17–18 | Involved in regulating epigenomic modifications and transcription (?) | [376] |
| Stem-bulge RNA | sbRNA | Y RNA | 67–133 | RNA quality control, chromosomal replication | [377], [378] |
| Small NF90-associated RNA | SNAR | Remain to be established | [379], [380] | ||
| Small vault RNA | svRNA | vRNA | 22–37 | Post-transcriptional gene regulation | [381], [382] |
| Telomere-specific sRNA | tel-sRNA | chrRNA | 24 | Epigenetic regulation | [385] |
| Transcription initiation sRNA | tiRNA | CAsRNA | 18 | Transcriptional regulation | [369], [386] |
| tRNA derived halves | tRH | tDR | 30–35 | Post-transcriptional gene repression | [387], [388] |
| tRNA-derived fragments | tRF | tDR | 20 | Post-transcriptional gene repression, translational repression | [389], [390] |
| Transcriptional start-site-miRNA | TSS-miRNA | miRNA | 20–90 | Post-transcriptional gene regulation | [369], [391] |
| Unusually small RNA | usRNA | miRNA | 15–17 | Post-transcriptional gene regulation | [392] |
| Y RNA-derived small RNAs Y RNA | yDR | Y RNA | 24–25, 30 | Ro-RNA particle to control RNA quality (?) and chromosomal replication | [393], [394] |
Despite the wide variety of sncRNAs and their function in the cell, the literature has largely focused on the role of sncRNAs in RNA interference (RNAi) - a process where sncRNA molecules inhibit gene expression and/or translation by targeting mRNAs [215]. The process of RNA-mediated silencing was first observed in experiments using plants and viruses, where scientists found transcriptional inhibition by exogenous antisense RNA [216], [217], [218]. Later on, elegant experiments performed by Craig Mello, Andrew Fire and colleagues identified the mechanisms underlying such phenomenon [219]. In brief, they found that injection of C. elegans with double-stranded RNA (dsRNA) efficiently silences endogenous genes that share complementary sequences with the exogenous RNA. Remarkably, the effect was found to be transgenerational and not to occur when single stranded mRNA or antisense RNA is administered. The biological and technological implications of these findings were immediately noticed and studies were designed to understand the role of ncRNAs in essentially all the tree of life. This was further motivated by the identification of endogenous ncRNAs with potential regulatory roles. In 1993, studies from the Ruvkun and the Ambros labs independently identified lin-4 as a tiny ncRNA that controlled developmental timing in C. elegans [220], [221]. In a series of genetic and biochemical experiments, they found that the locus of lin-4 coded for two small RNAs of approximately 22 and 61 nt, in which the sequence of the former was contained in the latter. They also identified in these molecules complementary sequences to the 3′-untranslated region (3’-UTR) of the lin-14 mRNA – a gene that was known to genetically interact with lin-4 [220], [221]. The authors predicted that lin-4 could regulate lin-14 translation through RNA-RNA interaction and anticipated a mechanism that would be characterized only several years later. Pushed by the discovery of RNAi as an endogenous and evolutionarily conserved biological process in the late 1990s, Ambros, Ruvkun and many others went on to identify similar tiny ncRNAs in different species across kingdoms and named them microRNAs (miRNAs) [220], [221], [222], [223], [224].
As of today, many other endogenous sncRNAs with silencing activity have been identified and functionally characterized. Didactically, the most studied ones compose three sub-classes: i) piwi-RNAs (piRNA); ii) endogenous small interference RNAs (endo-siRNAs) and iii) miRNAs. The first group is found in both invertebrates and vertebrates, where they are essential for fertility [225], [226], [227], [228], [229]. They are considered the longest sncRNA of the three classes, ranging from 25 to 33 nt in length [230]. PiRNAs can be found in the nucleus, where they regulate epigenetic and post-transcriptional gene silencing of retrotransposons [231], [232], or in the cytoplasm, where they target the 3’-UTR of target mRNAs to promote decay [233]. Despite elegant studies in the topic [234], [235], the mechanism of piRNA biogenesis in most species is not fully understood. What is clear is that this mechanism differs from the canonical processing of siRNAs and miRNAs since it is DICER-independent (see more below) [236]. Instead, piRNAs interact with PIWI-clade proteins, which determine their processing and mediate gene silencing [230], [236].
Endo-siRNAs are found mainly in plants and lower animals that do not have antibody or cell-mediated immunity (e.g. fungi, insects and nematodes), where their main function is to serve as a defense against viral infections [237], [238]. Nonetheless, endo-siRNAs can also regulate gene expression in mouse oocytes or stem cells [239], [240]. They derive from long dsRNA molecules such as intergenic repetitive elements, pseudogenes, and endo-siRNA clusters [241]. Endo-siRNA processing varies slightly across different species, but in all of them the long dsRNA is processed by the type III endoribonuclease DICER to generate 20–25 bp siRNA duplexes that are unwinded and loaded into the RNA-induced silencing complex (RISC) where proteins of the Argonaute family (AGO) are located [241]. The RISC complex loaded with the siRNA is guided to its target mRNA by perfect sequence complementarity, therefore directing the mRNA to degradation [241].
MiRNAs are the most studied class of sncRNAs. They differ in many aspects from piRNAs and endo-siRNAs, like in their tendency to be conserved in sequence across the evolutionary spectrum and their function as essential regulatory molecules in most cell types [207], [242]. They are similar in length and share some components of the processing/silencing machinery with siRNAs, particularly at the dicing step and downstream, but also differ in many ways [207], [242]. There are nuances when comparing the miRNA processing pathway across organisms, although in general this process is highly conserved. To avoid confusion, we will describe here the mammalian pathway unless stated otherwise (Fig. 1).
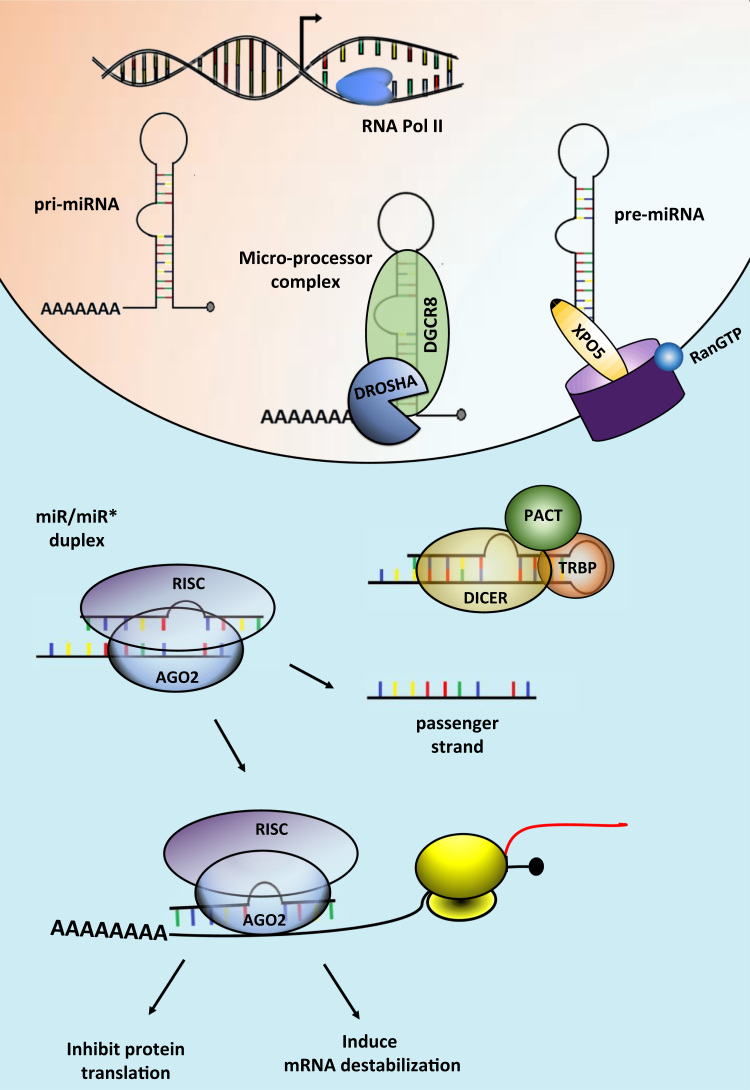
Fig. 1.
The miRNA processing pathway. miRNA genes are transcribed by RNA polymerase II (RNA Pol II) or III into primary miRNAs (pri-miRNA). In the nucleus, the pri-miRNA is cleaved by the microprocessor complex, comprised by the RNase III enzyme DROSHA and its partner DGCR8. This step will originate a precursor hairpin (pre-miRNA), which is exported to the cytoplasm by Exportin-5 (XPO5). In the cytoplasm, DICER and its partners TRBP and PACT cleaves the pre-miRNA hairpin into mature miRNA (19–22 nt). The mature miRNA will be incorporated with Argonaute 2 (AGO2) into the RNA-induced silencing complex (RISC) and will guide the RISC to silence the target mRNA by destabilizing it and/or inhibiting its translation.
MiRNA primary transcripts (pri-miRNAs) are classified as intergenic or intronic according to the genomic location where they are expressed. Intergenic pri-miRNAs are transcribed as independent transcription units, whereas intronic pri-miRNAs are processed from introns of their host transcripts [243], [244]. In general, pri-miRNAs are transcribed by the RNA polymerase II and are 5’-capped and 3’-polyadenylated [245]. In some cases, like for the cluster of miRNAs expressed in the human chromosome 19, a common pri-miRNA element is transcribed by RNA polymerase class III [246]. Pri-miRNAs are characterized by a hairpin structure that is recognized and processed by the microprocessing complex in the nucleus. This complex contains DROSHA, a type III endoribonuclease that cleaves the two strands of the hairpin at the stem approximately 22 bp away from the terminal loop, and DiGeorge syndrome critical region protein 8 (DGCR8), a dsRNA‐binding protein that ensures efficient and accurate processing by DROSHA [245]. This step gives rise to pre-miRNAs, which are exported to the cytoplasm by exportin proteins (e.g. XPO5 or Exportin-5) - a class of RanGTP-dependent dsRNA-binding proteins [247], [248].
In the cytoplasm, DICER processes the pre-miRNA at the loop to generate miRNA duplexes of approximately 22 bp with two base overhang on the 3’end [249], [250]. To do so accurately, DICER interacts with the HIV-1 TAR RNA binding protein (TRBP) and the Protein ACTivator of the interferon-induced protein kinase (PACT) [251]. The miRNA duplex is then unwinded and loaded into the RISC, where one of the strands binds to the target mRNA (the guide strand) and mediate gene silencing. The other strand is called passenger (or miRNA*) and is often degraded, although some miRNAs* are capable of down-regulating different [252] or the same gene as the guide miRNA strand [253]. What determines strand preference and whether a strand will be functionally active or be degraded is still a matter of study, but evidence demonstrates that the thermodynamic characteristic of the duplex and the position of the stem-loop in the pre-miRNA play a role in this process [250], [254], [255], [256]. Moreover, differences in the precursor transcript structure (e.g. mismatches, bulges, symmetrical and asymmetrical internal loops) determine the length diversity among miRNAs, often called isomiRs [257], [258], [259].
Once loaded in the RISC, the miRNA recognizes the target mRNA using 6–8 complementary nucleotides (the seed region) at its 5'-end, leading to translation inhibition and in some cases mRNA destabilization [260]. Recently though, Broughton et al. observed that sequences away from the seed are necessary for proper miRNA-target interaction in C. elegans [261]. Due to the short length of the seed and imperfect targeting mechanism, one miRNA can fine tune the expression of hundreds of targets, while one target can be regulated by multiple miRNAs in a combinatorial manner [262]. Thus, miRNAs take part in complex gene network regulation, where different members of the same network can be commonly targeted by one or more miRNAs [263], [264], [265]. Thousands of miRNAs have been identified in humans [262], some ubiquitously expressed and some enriched in a cell- or time-enriched fashion [266], [267], [268]. Altogether, miRNA-mediated silencing constitutes one of the most important mechanisms of gene expression regulation of animals [269]. It is predicted that about 30% of the human mRNA is targeted by miRNAs [262]. Not surprisingly, miRNAs are involved in essentially all biological processes and dysregulation of miRNA expression is linked to a wide variety of human diseases [270]. At the cellular level, miRNAs appear to confer cell robustness in response to different environmental stimuli [271], [272]. Thus, miRNA dysregulation impairs cell fate decisions [273], [274], affects metabolic processes [275], and increases stress sensitivity [271]. In adult tissues, this often results in dysfunction and disease [276], [277].
5. sncRNAs in the adipose tissue
The adipose tissue expresses a wide variety of sncRNAs with relatively high abundance. In a mouse differentiated adipocyte cell line, 68% of the sncRNA pool consist of miRNAs (our unpublished data). Given the enrichment of miRNAs in adipocytes and since the vast majority of the data on sncRNAs in adipose tissue refers to these molecules, they will be the focus of this Review. Nevertheless, we will discuss the potential role of other sncRNA species later on in topic 7.
When over 600 miRNAs were measured in mouse inguinal sWAT using RT-qPCR, 265 were detected with relative abundance [271]. Each one of these individual miRNAs is expected to exhibit its own mode of regulation that can be impacted by disease or metabolic alterations, as we will give examples in this Review. At the genomic level, the miRNA genes can undergo epigenetic modifications resulting in aberrant pri-miRNA expression through DNA methylation or histone modification [278]. Transcription factors can also influence pri-miRNA expression [279], and this form of regulation helps setting tissue specificity and pattern expression during development. For example, the transcription factor p53 stimulates pri-miR-34 expression by directly binding to its promoter region [280], while ERα inhibits pri-miR-221 serving as a transcriptional repressor [281]. Furthermore, there are critical nodes of regulation that affect the expression of a broader range of miRNAs and can have a wider impact. One such node is the miRNA processing pathway.
We and others have demonstrated that the expression of components of the miRNA processing pathway in adipose tissue is highly susceptible to regulation, particularly at the level of DICER (dicing step) [178], [271]. We found that aging leads to down-regulation of approximately 50% of all miRNAs expressed in sWAT of mice – a phenomenon that is linked to a parallel down-regulation of Dicer in adipocytes and preadipocytes, but not in other adipose tissue cells or non-adipose tissues [271]. General down-regulation of miRNAs and decreased DICER1 levels with aging are also found in human preadipocytes [271]. In humans, DICER1 levels are lower in subcutaneous fat depots of patients with partial lipodystrophy when compared to healthy individuals, and this is associated with “whitening” and dysfunction of the tissue [39], [271]. Moreover, obesity and progeria have a global impact on miRNA expression in mouse BAT [178]. In conditions of diet- and genetically-induced obesity, these changes are associated with down-regulation of Dicer [178]. Importantly, a modest reduction in BAT Dicer levels (25%) results in down-regulation of more than 75% of the most highly expressed miRNAs in the tissue [178], indicating that Dicer is a rate-limiting enzyme in miRNA processing in brown fat. These results evidence an important aspect of the miRNA processing pathway in the adipose tissue – the fact that minor expression changes in its components exert a major impact on miRNA expression. Nonetheless, not all the miRNAs are sensitive to this type of regulation and some are in one fat depot but not in others. This is consistent with the notion that each miRNA has its own mode of regulation, and context may determine whether changes in the miRNA processing machinery will affect the expression of the mature miRNA.
6. Adipose tissue miRNAs and adipose tissue function
Evidence that miRNAs are important for adipose tissue function came from studies using gain- or loss-of-function approaches to manipulate components of the miRNA processing machinery or individual miRNAs. Initial studies using tissue culture indicated a necessary role for miRNAs in preadipocyte differentiation and adipocyte fate determination [282], [283], [284]. The in vivo experiments came to support this notion and also demonstrate that adipocyte miRNAs play an important role in organismal homeostasis. For example, adipocyte-specific Dicer knockout mice (AdicerKO) exhibit a phenotype that resembles humans with partial lipodystrophy, i.e. atrophy of WAT, hypertrophy and “whitening” of BAT, insulin resistance, dyslipidemia, impaired resistance to oxidative stress and premature aging [274], [285]. Furthermore, these mice are refractory to many of the beneficial effects exerted by caloric restriction, such as improved oxidative metabolism and increased mitochondrial biogenesis in adipocytes, reduced inflammation in adipose tissue, and increased whole body insulin sensitivity [285]. Similarly, adipocyte-specific DGCR8 knockout mice (ADGCR8KO) show alterations in fat distribution, “whitening” of BAT, and insulin resistance [286]. Thus, miRNA deficiency leads to impairment of basic functions of adipocytes, such as differentiation and fate, metabolism, and signaling. This impacts on adipose tissue characteristics, cellularity and size, which in turn leads to abnormal fat accumulation and results in the metabolic syndrome. As much as it was found that global changes in miRNA biogenesis are important for adipose tissue function in a rather complex manner in the context of the metabolic syndrome (see discussion below in topic 8), the contribution of individual miRNAs to the phenotype is undeniable. We will therefore describe the mode of regulation and function of specific miRNAs in a discrete manner, as they were first identified. Some examples will be provided in the text below, but a comprehensive compilation of miRNAs with reported function in the adipose tissue is presented in Table 2. Didactically, we divided miRNAs in three topics, according to their function: a) adipogenesis, b) metabolism and c) signaling. We believe that these three cellular processes are hallmarks of a properly functional adipose tissue, and defects in any one of these processes can lead to metabolic diseases (Fig. 2).
Table 2.
Compilation of studies that implicate miRNAs in adipose tissue biology. Up, up-regulated. Down, down-regulated. Co, co-regulated. ND, not determined. A, 6.1. miRNAs involved in adipogenesis. B, 6.2. miRNAs and adipocyte metabolism. C, 6.3. miRNAs involved in signaling.
|
Expression changes |
Function |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| miRNA | Species | Direction | Condition | Tissue | Topic | Phenotypic Association | Relation | Target Gene | Ref |
| miR-100 | Human | Down | T2D | WAT and blood | A | Adipogenesis | Induces | IGFR1 and MTOR | [395] |
| miR-103 | Mouse | Up/down/down | Adipocyte differentiation/obesity/TNF-α incubation | 3T3-L1 and isolated adipocytes | A | Adipogenesis | Induces | ND | [396] |
| miR-124 | Mouse | Up | Insulin incubation | 3T3-L1 | A | Adipogenesis | Induces | Dlx5 | [397] |
| miR-130 | Human/mouse | Down/down | Adipocyte differentiation/obesity | Primary preadipocytes, 3T3-L1 and sWAT | A | Adipogenesis | Inhibits | PPARγ | [398] |
| miR-138 | Human | Down | Adipocyte differentiation | ADSCs | A | Adipogenesis | Inhibits | EID−1 | [399] |
| miR-140 | Mouse | – | – | – | A | Adipogenesis | Induces | Neat1 (positive regulation) | [400] |
| miR-143 | Mouse | Up/down/down | Adipocyte differentiation/obesity/TNF-α incubation | 3T3-L1 and isolated adipocytes | A | Adipogenesis | Induces | ND | [396] |
| miR-143 | Mouse/human | Up | Obesity/Adipocyte differentiation | vWAT/white preadipocytes | A | Adipogenesis | Induces | Erk5 | [401], [402] |
| miR-145 | Pig | Up | Adipocyte differentiation | Primary preadipocytes | A | Adipogenesis | Inhibits | IRS1 | [403] |
| miR-146a-5p | Pig | Up | TNF-α incubation | Adipocytes | A | Adipogenesis | Inhibits | IR | [343] |
| miR-146b | Mouse | Up | Adipocyte differentiation | 3T3-L1 | A | Adipogenesis | Induces | SIRT1 | [404] |
| miR-17 | Pig | Up | Adipocyte differentiation | BMSCs | A | Adipogenesis | Induces | ND | [405] |
| miR-17–92 | Mouse | Up | Adipocyte differentiation | 3T3-L1 | A | Adipogenesis | Induces | Rb2/p130 | [310] |
| miR-181a | Pig | Up | Obesity | Adipose tissue | A | Adipogenesis | Induces | TNF | [406] |
| miR-195a | Mouse | Down | Adipocyte differentiation | C3H10T1/2 cells and 3T3-L1 | A | Adipogenesis | Inhibits | Zfp423 | [407] |
| miR-199a | Mouse | Down | Adipocyte transdifferentiation | C2C12 myoblasts | A | Adipogenesis | Inhibits | Fatp1 | [408] |
| miR-204–5p | Human | Up | Adipocyte differentiation | ADSCs | A | Adipogenesis | Induces | DVL3 | [409] |
| miR-21 | Human | Up | Adipocyte differentiation | ADSCs | A | Adipogenesis | Induces | TGFBR2 | [304] |
| miR-210 | Mouse | Up | Adipocyte differentiation | 3T3-L1 | A | Adipogenesis | Induces | Tcf7l2 | [410] |
| miR-21a-5p | Mouse | Down | Bisphenol A incubation | 3T3-L1 | A | Adipogenesis | Inhibits | Map2k3 | [411] |
| miR-224-5p | Mouse | Up | Adipocyte differentiation | 3T3-L1 | A | Adipogenesis | Inhibits | EGR2 and ACSL4 | [412] |
| miR-23a | Mouse | Down | Adipocyte differentiation | 3T3-L1 | A | Adipogenesis | Inhibits | Stat1 | [413] |
| miR-24 | Mouse | Up | Adipocyte differentiation | 3T3-L1 | A | Adipogenesis | Induces | Mapk7 | [414] |
| miR-27 | Mouse | Down/up | Adipocyte differentiation/obesity | 3T3-L1 | A | Adipogenesis | Inhibits | PPARγ and C/EBPalpha | [415], [416] |
| miR-30 | Human | Up | Adipocyte differentiation | ADSCs | A | Adipogenesis | Induces | RUNX2 | [296] |
| miR-302a | Mouse | Down | Adipocyte differentiation | 3T3-L1 | A | Adipogenesis | Inhibits | Pparγ | [417] |
| miR-33b | Human | Up | Adipocyte differentiation | SGBS cells and preadipocytes | A | Adipogenesis | Inhibits | HMGA2 | [418] |
| miR-363 | Rat | Down | Adipocyte differentiation | ADSCs | A | Adipogenesis | Inhibits | E2F3 | [311] |
| miR-369-5p | Human | Not changed | Adipocyte differentiation | MSCs | A | Adipogenesis | Inhibits | FABP4 | [419] |
| miR-375 | Mouse | Up | Adipocyte differentiation | 3T3-L1 | A | Adipogenesis | Induces | ND | [317] |
| miR-448 | Mouse | Up | Adipocyte differentiation | 3T3-L1 | A | Adipogenesis | Inhibits | Klf5 | [420] |
| miR-519d | Human | Up | Obesity | sWAT | A | Adipogenesis | Induces | PPARA | [421] |
| miR-540 | Rat | Down | Adipocyte differentiation | ADSCs | A | Adipogenesis | Inhibits | Pparγ | [422] |
| miR-125b-5p | Human | Up | Adipocyte differentiation | SGBS preadipocytes | A | Adipogenesis | Inhibits | MMP11 | [423] |
| miR-1275 | Human | Down | Obesity/adipocyte differentiation | MSCs and vWAT | A | Adipogenesis | Inhibits | ELK1 | [424], [425] |
| miR-324-3p | Human | Up | Obesity/Adipocyte differentiation | Adipose tissue, MSCs and 3T3-L1 | A | Adipogenesis | Induces | CTBP2 | [426] |
| miR-301a | Mouse | Down | Inflammation | 3T3-L1 and blood | A | Adipogenesis and inflammation | Inhibits | Pparγ | [427] |
| miR-128 | Human | Up/down | Adipocyte/osteoblast differentiation | MSCs | A | Adipogenesis/osteogenesis | Induces/inhibits | ND | [428] |
| miR-140-5p | Mouse | Up | Obesity/Adipocyte differentiation | BMSCs, 3T3-L1, and WAT | A | Adipogenesis/osteogenesis | Induces/inhibits | Tgfbr1 | [429] |
| miR-17-5p/106a | Human | Up | Adipocyte differentiation | ADSCs | A | Adipogenesis/osteogenesis | Induce/inhibit | BMP2 | [297] |
| miR-188 | Human/mouse | Up | Aging | BMSCs | A | Adipogenesis/osteogenesis | Induces/inhibits | Hdac9 and Rictor | [430] |
| miR-194 | Mouse | Down/up | Adipocyte/osteoblast differentiation | BMSCs and C3H10T1/2 cells | A | Adipogenesis/osteogenesis | Inhibits/induces | Nr2f2 | [431] |
| miR-204/211 | Mouse | Up | Adipocyte differentiation | C3H10T1/2 cells | A | Adipogenesis/osteogenesis | Induces/inhibits | RUNX2 | [295] |
| miR-22 | Human | Down/up | Adipocyte/osteoblast differentiation | ADSCs | A | Adipogenesis/osteogenesis | Inhibits/induces | HDAC6 | [432] |
| miR-223 | Mouse | Up/down | Adipocyte/osteoblast differentiation | MSCs | A | Adipogenesis/osteogenesis | Induces/inhibits | Fgfr2 | [433] |
| miR-3077-5p | Mouse | Up | Osteoporosis | MSCs | A | Adipogenesis/osteogenesis | Induces/inhibits | Runx2 | [434] |
| miR-637 | Human | Up/down | Adipocyte/osteoblast differentiation | MSCs | A | Adipogenesis/osteogenesis | Induces/inhibits | OSX | [435] |
| miR-705 | Mouse | Up | Osteoporosis | MSCs | A | Adipogenesis/osteogenesis | Induces/inhibits | Hoxa10 | [434] |
| miR-145a-5p | Mouse | Down | Obesity | Adipose tissue | A | Adipogenesis/proliferation | Inhibits/induces | ND | [436] |
| miR-146b | Human/mouse | Up | Adipocyte differentiation/obesity | MSCs and vHPA/vWAT | A | Adipogenesis/proliferation | Induces/inhibits | KLF7 | [437] |
| miR-196a | Mouse/human | Up | Cold and β-adrenergic stimulation | sWAT | A | Beige adipogenesis | Induces | Hoxc8 | [438] |
| miR-149-3p | Mouse | Up/down | Fasting/cold | sWAT | A | Beige adipogenesis and thermogenesis | Inhibits | Prdm16 | [439] |
| miR-193b/ 365 | Mouse | Up | BAT vs. WAT | Adipose tissue | A | Brown adipogenesis | Induces | Runx1t1 | [300] |
| miR-106b/ 93 | Mouse | Up | Obesity | BAT | A | Brown adipogenesis | Inhibits | ND | [440] |
| miR-182/203 | Mouse | Up | Brown adipogenesis/BAT vs. WAT | Adipose tissue and brown preadipocytes | A | Brown adipogenesis | Induces | Insig−1 and Pdgfra | [286] |
| miR-346 | Mouse | Down | Dicer KO | Preadipocytes | A | Brown adipogenesis | Induces | ND | [274] |
| miR-362 | Mouse | Down | Dicer KO | Preadipocytes | A | Brown adipogenesis | Induces | ND | [274] |
| miR-378 | Human | Up | Eicosapentaenoic acid incubation | Brown preadipocytes | A | Brown adipogenesis | Induces | ND | [441] |
| miR-328 | Mouse | Up/down/down | Brown adipogenesis/aging/obesity | BAT and brown preadipocytes | A | Brown adipogenesis/myogenesis | Induces/inhibits | Bace1 | [178] |
| miR-133 | Mouse | Down | Cold | BAT and sWAT | A | Brown/beige adipogenesis | Inhibits | Prdm16 | [305] |
| miR-155 | Mouse | Down/up | Brown adipogenesis/TGFβ incubation | Brown preadipocytes | A | Brown/beige adipogenesis | Inhibits | Cebpb | [442] |
| miR-27 | Mouse | Down | Cold and brown adipogenesis | BAT and sWAT | A | Brown/beige adipogenesis | Inhibits | Prdm16, Ppara, Creb, and Pgc1ß | [443] |
| miR-455 | Mouse/human | Up | BAT vs. WAT/cold/BMP7 | Adipose tissue and C3H10T1/2 cells | A | Brown/beige adipogenesis | Induces | Hif1an, Runx1t1 and Necdin | [444] |
| miR−34a | Mouse | Up | Obesity | Adipose tissue | A | Brown/beige adipogenesis and thermogenesis | Inhibits | Fgfr1 | [345] |
| let-7 | Mouse | Up | Adipocyte differentiation | 3T3-L1 | A | Mitotic clonal expansion and terminal differentiation | Inhibits | Hmga2 | [312] |
| miR-192* | Human | Down | Obesity | vWAT | A, B | Adipogenesis and lipid accumulation | Inhibits | SCD and ALDH3A2 | [445] |
| miR-34a | Mouse | Up | Obesity/Adipocyte differentiation | BAT, liver and macrophages/preadipocytes | A, B | Fat accumulation | Inhibits | ND | [346] |
| miR-155 | Mouse/human | Up | Obesity and TNF-α incubation | Adipose tissue and preadipocytes | A, C | Adipogenesis | Inhibits | Pparγ | [339] |
| miR-193b | Human | – | – | sWAT and 3T3-L1 | B | Adiponectin production | Induces | NFYA | [333], [303] |
| miR-378*/378 | Mouse | Co | Ppargc1b coexpression | Various tissues | B | Adiposity/oxidative metabolism | Induces/inhibits | Crat and Med13 | [326] |
| miR-378 | Mouse | Up | BAT vs. WAT | Adipose tissue | B | BAT expansion | Induces | Pde1b | [446] |
| miR-223 | Human | Up | Insulin resistance | sWAT | B | GLUT4 expression | Inhibits | GLUT4 | [335] |
| miR-93 | Human | Up | Insulin resistance, PCOS | sWAT | B | GLUT4 expression | Inhibits | GLUT4 | [319] |
| miR-10b | Human | Up | Abdominal vs. gluteofemoral | sWAT | B | Lipolysis | Induces | ND | [447] |
| miR-124a | Mouse | Down | Fasting | vWAT | B | Lipolysis | Inhibits | Atgl | [448] |
| miR-378 | Mouse | – | – | – | B | Lipolysis and energy expenditure | Induces | Akt1 and Scd1 | [449] |
| miR-200b/a/429 | Mouse | – | – | – | B | Lipolysis and energy expenditure on HFD | Induces | Eps8, Lhfp, Glis2 and Rps6kb1 | [450] |
| miR-520 | Human | Down | Obesity | vWAT | B | RAB11A expression | Inhibits | RAB11A | [451] |
| miR-141 | Human | Down | Obesity | vWAT | B | YWHAG expression | Inhibits | YWHAG | [451] |
| miR-92a | Human | Down | BAT activation | Serum exosomes | C | BAT activity | Inversely correlates | ND | [349] |
| miR-221 | Human | Up/down | Obesity/adipocyte differentiation | sWAT/white preadipocytes | C | BMI | Directly correlates | ADIPOR1 and ETS1 | [344], [302] |
| let-7a/d | Human | Down | Obesity | sWAT | C | CCL2 secretion | Inhibits | ND | [303] |
| miR-126 | Human | Down | Obesity | sWAT | C | CCL2 secretion | Inhibits | CCL2 | [303] |
| miR-193b | Human | Down | Obesity | sWAT | C | CCL2 secretion | Inhibits | ND | [303] |
| miR-146b-5p | Human | Down | Obesity | Monocytes | C | Inflammation | Inhibits | ND | [336] |
| miR-146b-5p | Human | Up | Adipocyte differentiation/TNF-α or IL−6 incubation | vHPA | C | Inflammation | Directly correlates | ND | [452] |
| miR-883b-5p | Mouse/human | Up/down | Adiponectin/obesity | WAT | C | Inflammation | Inhibits | ND | [453] |
| miR-26b | Mouse/human | Down | Obesity and insulin resistance | vWAT and preadipocytes | C | Insulin signaling | Induces | Pten | [454] |
| miR-320 | Mouse | Up | Insulin resistance | 3T3-L1 | C | Insulin signaling | Inhibits | ND | [455] |
| miR-26a | Human | Down | Obesity | sWAT | C | Lipolysis and secretion of CCL2 and TNF-α | Inhibits | ND | [303], [456] |
| miR-29b | Human | Down | Obesity/4-HNE | sWAT and ADSCs | C | TNF-α expression | Inhibits | ND | [457] |
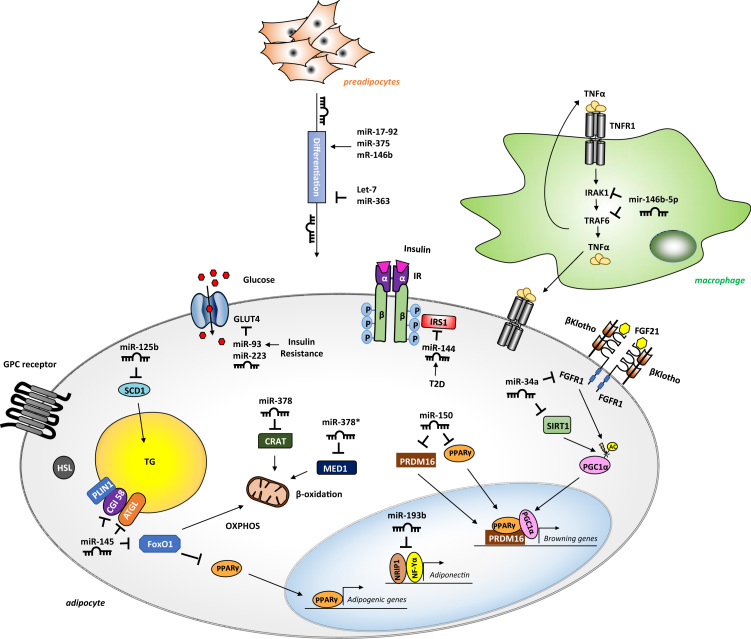
Fig. 2.
Adipocyte function and examples of miRNA-mediated regulation. MiRNAs are involved in different steps of adipocyte maturation, metabolism and signaling. They regulate adipogenesis (positively: miR-17–92, miR375 and miR-146b; negatively: let-7 and miR-363), glucose uptake (miR-93 and miR-223), lipolysis and β-oxidation (miR-145), triglyceride synthesis (miR-125b), insulin signaling (miR-144), browning (miR-150 and miR-34a), adiponectin synthesis (miR-193b) and inflammation (miR-146b-5p in macrophages).
6.1. miRNAs involved in adipogenesis
The adipogenic process is very tightly regulated [139], [287]. The total number of adipocytes is determined in the period from childhood to adolescence and is maintained constant during adulthood, in lean and obese individuals [139], [287]. Nonetheless, in 8.3 years, approximately 50% of the pool of adipocytes of an adult human is renewed [139], [287]. Adipocyte turnover is sustained by adipose-derived stem cells (ADSCs) and preadipocytes [288]. ADSCs are similar to stem cells derived from the bone marrow, i.e. they are both multipotent and can differentiate into a variety of cell types, such as osteoblasts, chondrocytes, myocytes, and adipocytes (white or brown) [288], [289], [290]. Preadipocytes are one step down in the commitment path to the adipogenic lineage, and given their high proliferation rate are usually the main source of newly formed adipocytes [291].
The pro-adipogenic transcription factors peroxisome proliferator-activated receptor-γ (PPARγ) and CCAAT-enhancer-binding proteins (C/EBPs), as well as the anti-adipogenic signaling cascade triggered by Wnt, BMPs, transforming growth factor beta (TGFβ) or hedgehog participate in the regulation of ADSC commitment into the adipose linage and control adipocyte differentiation [292]. Differentiation and maturation of healthy, functional adipocytes involve the activation of intricate molecular pathways organized in three subsequent steps: i) commitment, the stage where ADSCs differentiate into adipocyte precursor cells (including preadipocytes); ii) mitotic clonal expansion, which in tissue culture is triggered by confluence and growth arrest; and iii) terminal differentiation [293], [294]. During the first step of commitment, ADSCs can give rise to two different precursors: a) one that will differentiate into white or beige adipocytes and another that will differentiate into brown adipocytes or myocytes [127]. All these steps have been shown to be regulated by miRNAs.
For instance, a study by Hung et al. showed that incubation of a mesenchymal stem cell line (C3H10T1/2) with adipocyte differentiation medium up-regulates miR-204/211 and down-regulates Runt-related transcription factor 2 (Runx2), an osteogenic transcriptional factor stimulated by Wnt and the BMP pathways. Additionally, overexpression of miR-204 in C3H10T1/2 suppresses Runx2 expression and stimulates the commitment of these cells to the adipose lineage [295]. In a parallel study, Zaragosi et al. showed that the miR-30 family is highly up-regulated during adipogenesis of ADSCs and revealed that miR-30a and miR-30d can also target Runx2, which in turn favors adipocyte differentiation [296]. Similarly, miR-17-5p and miR-106a promote adipogenesis and inhibit osteogenesis of ADSCs by targeting the pro-osteogenic protein BMP2 [297]. Interestingly, adipose tissue miR-17-5p expression is inversely correlated with markers of hyperglycemia and insulin resistance in humans [298], [299].
MiRNAs can also silence myogenic genes to favor adipogenesis. The miR-193b-365 cluster is highly expressed in BAT, where it induces common myogenic/adipogenic precursors to commit and differentiate into brown adipocytes [300]. The miR-193b-365 cluster is induced by PRDM16 [300], a transcriptional coregulator that is necessary for brown/beige adipocyte differentiation [301]. Once up-regulated, miR-193b targets the pro-myogenic factors Cdon and Igfbp5, inhibiting myogenesis and promoting adipogenesis [300]. More recently, miR-193b (and the seed-related miR-328) was shown to target the β-secretase Bace1 to promote brown adipogenesis [178]. Importantly, miR-193b is down-regulated in subcutaneous adipose tissue of obese individuals [299], [302], [303].
In addition to inhibiting osteogenic or myogenic commitment to favor adipocyte differentiation, miRNAs can directly silence inhibitors of adipogenesis. For example, miR-21 expression is up-regulated during adipogenic differentiation of ADSCs, which leads to silencing of TGFβ receptor-2, hence suppressing the adipogenic inhibition caused by TGFβ [304]. Moreover, miRNAs can inhibit adipogenesis. MiR-133 targets PRDM16 mRNA in muscle stem cells therefore inhibiting commitment to the brown adipocyte lineage and allowing myogenesis [305]. Thus, miRNAs interact with signaling pathways and transcriptional regulators that are normally involved in developmental control to determine commitment and differentiation of ADSCs.
Once committed, adipogenic precursor cells need to proliferate. During cell proliferation, transition from G1 to S is regulated by the retinoblastoma protein (Rb)/E2F pathway [306]. Cyclin kinase-dependent hyperphosphorylation of Rb releases E2F to transcriptionally activate genes involved in the S phase [306]. The transition between these two phases of the cell cycle is extremely important and dictates whether preadipocytes will in fact reach terminal differentiation [307], [308]. During early differentiation, after proliferation and growth-arrest, preadipocytes need to reentry the cell cycle and undergo approximately two rounds of divisions (i.e. mitotic clonal expansion) in order to differentiate [307], [309]. Like the other steps in adipogenesis, miRNAs also control mitotic expansion.
Wang et al. demonstrated that the expression of the miR-17–92 cluster matches with the time of reentry and exit of the cell cycle. They observed that the miR-17–92 cluster reaches its peak after one day of the adipogenic stimulus in 3T3-L1 preadipocytes, coinciding with the down-regulation of the retinoblastoma-like protein 2 (Rb2/p130) and inducing reentry in the cell cycle [310]. After this, the miR-17–92 cluster is down-regulated and Rb2/p130 expression is increased, exiting cells from the cell cycle for terminal differentiation [310]. In contrast, miRNAs such as the members of the let-7 family (which target the mRNA of high mobility group AT-hook2 protein HMGA2) and miR-363 (which targets E2F3 mRNA) inhibit mitotic clonal expansion and terminal differentiation of adipocytes [264], [311], [312].
Fat accumulation characterizes the final stage of adipogenesis. Once the fate of the adipocyte is determined, lipids start accumulating in response to growth factor signaling and a positive energy balance [313], [314], [315]. Insulin and PPARγ are the main drivers of adipogenesis at this step [316]. Among the downstream kinases activated by insulin, it is worth mentioning the extracellular signal-regulated kinase (ERK). Tight regulation of ERK is essential for adipocyte differentiation. Whereas activation of ERK during precursor cell proliferation is required for clonal expansion, activation of ERK at the final stages of differentiation inhibits PPARγ and abrogates adipocyte differentiation [315]. Consistent with this notion, miR-375 expression increases with 3T3-L1 adipocyte differentiation to promote lipid accumulation by suppressing ERK1/2 phosphorylation [317].
Taken these studies together, it is safe to affirm that miRNAs play a role in different aspects of adipocyte development. Not surprisingly, changes in miRNAs involved with adipogenesis have been associated with different metabolic diseases. For example, Roldan et al. observed that miRNAs such as let-7c/d/e (which target HMGA2 and inhibit cell proliferation), miR-23b and mir-27b (which are Wnt activators and PPARγ and TGFβ/Smad repressors), and miR-320, miR-542-5p, miR-140, miR-143, and miR-661 (which are associated in different manners with adipocyte differentiation) are up-regulated in ADSCs of morbidly obese individuals compared to lean individuals [318].
6.2. miRNAs and adipocyte metabolism
Once the adipocyte is terminally differentiated, miRNAs can regulate basic metabolic functions of these cells, including glucose utilization, lipid turnover, and oxidative metabolism [299]. Adipocytes use a large portion of the available blood glucose to maintain their function, particularly brown adipocytes [179]. Chen et al. observed that miR-93 overexpression in adipocytes down-regulates the glucose transporter type 4 (GLUT4) - the main insulin-sensitive glucose transporter in these cells [319]. Importantly, miR-93 expression is higher in adipose tissue of non-obese patients with insulin resistance, and inversely correlates with GLUT4 levels in these individuals [319]. These changes in miRNA-mediated glucose transport capacity in adipose tissue can therefore contribute to the pathogenesis of insulin resistance.
Once taken up by adipocytes, glucose needs to be broken down to feed the mitochondria with substrates for ATP generation. Little is known about the role of miRNAs in adipocyte glycolysis, but in other tissues the link between glucose utilization and specific miRNAs is well established [320], [321], [322]. Evidence that miRNAs control glycolysis in adipocytes comes from our study using the AdicerKO mice [285]. Adipocytes lacking Dicer induce anaerobic glycolysis even in conditions when oxidative metabolism is expected to be activated, i.e. during caloric restriction [285]. This phenotype is associated with adipocyte mitochondrial dysfunction and suggests that miRNAs are important in adipocytes to control the engagement of adequate metabolic pathways (i.e. oxidative vs. glycolytic) in response to changes in nutrient availability [285].
A defective capacity of adipocyte mitochondria to oxidize substrates has been associated with metabolic diseases [323]. The transcription co-activators of the PGC-1 family are key regulators of mitochondrial biogenesis, thermogenesis and glucose and fatty acid metabolism, and are preferentially expressed in tissues with relatively high mitochondrial content such as the BAT [324]. The pre-miR-378 hairpin resides within the first intron of the PCG-1β gene (Ppargc1b) and is co-expressed with the host gene in highly oxidative tissues, including the adipose tissue, where they counterbalance each other functions [325], [326]. Consistent with a role in fat metabolism, miR-378/378* knockout mice are resistant to diet-induced obesity [326]. miR-378 and miR-378* target the mRNAs of carnitine O-acetyltransferase and MED13, respectively, two important proteins involved in beta-oxidation [326], and therefore inhibit oxidative metabolism.
In anabolic states, intermediates of glycolysis and the tricarboxylic acid (TCA) cycle also serve as building blocks for lipid synthesis [327]. In adipocytes, newly synthesized fatty acids, or FFAs coming from the circulation can be re-esterified into triglycerides for storage, in a process called lipogenesis [327]. A rate-limiting step in lipid synthesis is catalyzed by the stearoyl-CoA desaturase1 (SCD-1). SCD-1 mRNA is a target of miR-125b in mammals, and overexpression of this miRNA in adipocytes reduces triglyceride accumulation [328]. Interestingly, miR-125b is up-regulated in mouse subcutaneous adipose tissue in response to calorie restriction, and its overexpression in preadipocytes confers protection from oxidative stress-induced cell death [271].
One of the main functions of adipocytes is to break down triglycerides to mobilize FFAs and feed the organism in conditions of negative energy balance [327]. This process, called lipolysis, is coordinated by nutrient availability, hormones and, importantly, miRNAs [327]. Lin et al. showed that miR-145 control lipolysis rate in mouse adipocytes [329]. They studied KH‐type splicing regulatory protein (KSRP) knockout mice [329], which lack an RNA-binding protein that controls the synthesis of a subclass of miRNAs, including miR-145 [330], [331]. KSRP-/- mice display higher lipolysis rate in epididymal fat due to up-regulation of forkhead box protein O1 (FOXO1), comparative gene identification-58 (CGI58) and adipose triglyceride lipase (ATGL) [329]. Ectopic expression of miR-145 in 3T3-L1 adipocytes inhibits Foxo1 and Cgi58 mRNAs by direct targeting, and thus reduces lipolysis [329]. Using the same mouse model, Chou et al. noted that KSRP-/- mice are leaner due to ”browning” of the inguinal sWAT [332]. They found that to be due to down-regulation of miR-150, which targets the mRNAs of PRDM16 and PPARγ [332].
6.3. miRNAs involved in signaling
The adipose tissue is a major site of endocrine regulation. Molecules secreted by the adipocytes and other adipose resident cells signal to the organism to control metabolic function and homeostasis [107]. On the other hand, adipocytes sense the levels of extracellular molecules that inform them of the energy status of the organism [107]. miRNAs control the expression of adipokines and components of signaling pathways, therefore contributing to intercellular communication. For example, adipose tissue miR-193b expression correlates with serum adiponectin levels in humans [333], and as previously mentioned, is down-regulated in obese individuals [299], [302], [303]. MiR-193b controls adiponectin by targeting the mRNA of nuclear transcription factor Yα and potentially nuclear receptor interacting protein 1, which are negative regulators of this adipokine [333].
MiRNAs also regulate the crosstalk between adipocytes and immune cells within the adipose tissue. Zhuang et al. showed that miR-223 knockout mice are prone to high fat diet (HFD)-induced adipose tissue inflammation and exhibit systemic insulin resistance. They observed that macrophages from the miR-223-/- mice have a skewed profile towards the pro-inflammatory M1 type and concluded that miR-223 regulates macrophage polarization during obesity [334]. Interestingly, miR-223 expression in human sWAT positively correlates with insulin resistance and TNF-α exposure induces the expression of this miRNA by nearly 2-fold in human differentiated subcutaneous adipocytes [335]. Moreover, overexpression of miR-223 blocks insulin-stimulated glucose uptake and reduces GLUT4 expression in these cells [335]. MiR-146b-5p and miR-155 are two other examples of miRNAs involved with inflammation. MiR-146b-5p is decreased in monocytes from obese patients [336] and increased in adipocytes treated with TNF-α or IL-6 [337], while miR-155 expression levels correlate with the number of macrophages infiltrating human sWAT [298], [299]. Consistent with a role in atherosclerosis, miR-155 loss-of-function intensifies the inflammatory response and the uptake of lipids by a macrophage cell line stimulated with oxidized low-density lipoprotein (LDL) [338], [339], [299]. In contrast, miRNAs can correlate with less inflammation. For instance, the levels of IL-6 in the serum of patients with non-alcoholic steatohepatitis negatively correlate with the adipose tissue expression of three miRNAs (namely miR-149, miR-574-3p, and miR-132) known to target IL-6 mRNA [340].
Some miRNAs are also potent regulators of growth factor signaling. Karolina et al. reported increased miR-144 expression in blood, liver, muscle, and adipose tissue of type 2 diabetic patients [341]. An inverse correlation between miR-144 and insulin receptor substrate 1 (IRS1) expression was observed by these authors [341]. Luciferase assays revealed that miR-144 targets the 3’-UTR of IRS1 mRNA and can therefore regulate insulin signaling [341]. MiR-139-5p also targets IRS1 in murine preadipocytes [342], while miR-146a-5p targets the mRNA of insulin receptor in primary porcine adipocytes [343]. Another miRNA that affects growth factor signaling to control adipose tissue function is miR-34a. MiR-34a is up-regulated in the subcutaneous fat depot of obese people [344] and inhibits beige and brown fat formation in obese mice partially by suppressing the FGF21 signaling pathway [345]. Inhibition of miR-34a in obese mice using systemic administration of lentivirus-expressing antisense miR-34a increases energy expenditure and induces thermogenic capacity in brown and epididymal adipose tissues [345]. In contrast, Lavery et al. showed that miR-34a knockout mice are more susceptible to weight gain under HFD and exhibit an altered macrophage phenotype [346]. It is worth mentioning that the strategies to knock out miR-34a were different between the two studies, indicating a time and/or tissue dependent function for miR-34a.
Finally, miRNAs themselves can act as signaling molecules. MiRNAs are carried in circulation by extracellular vesicles (e.g. exosomes) or associated with proteins (e.g. lipoproteins) [347]. Adipocytes are the main source of circulating miRNAs in mice (and likely in humans) and these adipocyte-secreted miRNAs can be taken up by other cells where they regulate target gene expression [348]. For instance, adipocyte-secreted miR-99b down-regulates FGF21 in liver [348]. Interestingly, BAT contributes more to the pool of circulating miRNAs than WAT [348], and this secretion is potentiated by beta-adrenergic stimulation and cold exposure [349]. Among the cold stimulated, brown fat secreted miRNAs, circulating levels of miR-92a negatively correlates with BAT activity both in mice and humans [349]. Interestingly, gastric bypass surgery in obese individuals affects the expression of 168 circulating, adipocyte-derived exosomal miRNAs, and components of the insulin signaling pathway are among the most enriched targets of these differentially expressed miRNAs [350]. Importantly, the expression of ten of these miRNAs correlates with insulin resistance [350]. Hence, miRNAs can act in cell autonomous and non-autonomous manners to regulate adipose tissue function.
7. Perspectives on the role of sncRNAs in adipose tissue
Despite the recent advances, our understanding of how sncRNAs control adipose tissue function is still in its early stages. Publications in PubMed containing the keywords “microRNA” and “adipose” have grown exponentially in the last decade and sum 478 papers as of Dec 31st, 2016 – two thirds of them published in the last 3 years (data not shown). The universe of miRNAs expressed in fat is vast, and so are their targets and interactions. Thus, much more is expected to be unveiled about adipose tissue miRNAs. For what is worth concerning redox biology, almost nothing is known regarding adipose tissue miRNAs, except for indirect links and speculation. ROS down-regulates Dicer in murine preadipocytes and Dicer knockout preadipocytes and young AdicerKO mice are more susceptible to oxidative stress-induced mortality [271]. In contrast, Dicer overexpression in the C. elegans intestine (the analog of the mammalian adipose tissue) protects worms from oxidative stress [271]. These phenotypes are partially due to changes in the expression of the lin-4/miR-125 family [271]. The downstream targets of lin-4/miR-125 that confer protection from oxidative damage are yet to be found, and so is the mechanism of how ROS regulate Dicer expression. Indeed, the regulation of miRNA expression in adipose tissue is a fascinating topic for future studies. MiRNAs in adipose tissue are globally regulated by physiological stimuli in a rather unique manner. Dicer is a rate-limiting step in miRNA biogenesis in fat and its dynamic range of regulation is intriguing. Why would adipocytes need to change Dicer expression so dramatically under physiological conditions?
Perhaps the answer to this question relies on the recent discoveries that the adipose tissue is the main source of circulating miRNAs in the mouse and potentially humans. These findings opened up a whole new dimension to the topic. Are adipose-derived circulating miRNAs relevant for physiological control in mammals? It certainly does seem so based on the most recent studies. But which miRNAs contribute to the endocrine functions of the adipose tissue and how? These are questions that remain to be answered.
Finally, miRNAs consist of only 68% of the total sncRNA pool of a murine differentiated adipocyte cell line (our unpublished data). The remaining 32% have not been characterized. Except for one match related to a report of moRNA expression in pig adipose tissue [351] and excluding miRNAs, there is no paper in PubMed associating any of the sncRNAs listed in Table 1 and the adipose tissue. Unraveling this unexplored universe is a fascinating endeavor for future studies.
8. Conclusions
SncRNAs (represented here by the well characterized and abundant class of miRNAs) play a role in a complete range of cellular processes that contribute to adipose tissue function, i.e. adipogenesis, metabolism and signaling (Fig. 2). Impaired biogenesis of DICER-dependent sncRNAs (e.g. miRNAs, siRNAs) in adipocytes leads to alterations in these processes and results in features of the metabolic syndrome. DICER is a rate limiting protein in the miRNA biogenesis pathway in adipocytes, at least for the majority of the miRNAs expressed in these cells. Given the importance of miRNAs in adipose tissue, DICER up-regulation may represent a key mechanism to accelerate sncRNA production in conditions when these molecules are necessary (as the ones reviewed here). DICER up-regulation may not necessarily increase sncRNA expression, as each sncRNA has its own mode of regulation, but it will allow miRNAs to be expressed rapidly. This will confer a more robust (less noisy) response to stimuli that control, for instance, cell fate, substrate utilization and intercellular communication. More studies are necessary to determine all the pieces in this complex puzzle, and more importantly, to find strategies to target this pathway to minimize the suffering of patients with metabolic diseases.
Acknowledgements
We thank Elzira E. Saviani for the technical support and Silas Pinto for assisting with bioinformatic analysis. This work was supported by Grants of the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (2015/01316-7 and 2015/03292-8), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (444424/2014-8) and Fundo de Apoio ao Ensino, à Pesquisa e Extensão (FAEPEX/UNICAMP) (2408/16).
References
- 1.WHO. Obesity and overweight. 2014 June 2016 [cited 2016 11/19]; Available from: 〈http://www.who.int/mediacentre/factsheets/fs311/en/〉.
- 2.Rokholm B., Baker J.L., Sorensen T.I. The levelling off of the obesity epidemic since the year 1999 – a review of evidence and perspectives. Obes. Rev. 2010;11(12):835–846. doi: 10.1111/j.1467-789X.2010.00810.x. [DOI] [PubMed] [Google Scholar]
- 3.Kaur J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.van Vliet-Ostaptchouk J.V., Snieder H., Lagou V. Gene–lifestyle interactions in obesity. Curr. Nutr. Rep. 2012;1(3):184–196. doi: 10.1007/s13668-012-0022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aronne L.J., Nelinson D.S., Lillo J.L. Obesity as a disease state: a new paradigm for diagnosis and treatment. Clin. Cornerstone. 2009;9(4):9–25. doi: 10.1016/s1098-3597(09)80002-1. (discussion 6–9) [DOI] [PubMed] [Google Scholar]
- 6.Racette S.B., Deusinger S.S., Deusinger R.H. Obesity: overview of prevalence, etiology, and treatment. Phys. Ther. 2003;83(3):276–288. [PubMed] [Google Scholar]
- 7.WHO, BMI classification, 2006 [cited; Available from: 〈http://apps.who.int/bmi/index.jsp?IntroPage=intro_3.html〉, 2016.
- 8.P.A. Ades, P.D. Savage, The obesity paradox: perception vs knowledge. Mayo Clinic Proceedings; 85(2):112–114, 2010. [DOI] [PMC free article] [PubMed]
- 9.Amundson D.E., Djurkovic S., Matwiyoff G.N. The obesity paradox. Crit. Care Clin. 2010;26(4):583–596. doi: 10.1016/j.ccc.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Romero-Corral A., Montori V.M., Somers V.K., Korinek J., Thomas R.J., Allison T.G. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. The Lancet. 2006;368(9536):666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 11.Ohlson L.-O., Larsson B., Svärdsudd K., Welin L., Eriksson H., Wilhelmsen L. The influence of body fat distribution on the incidence of diabetes mellitus: 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes. 1985;34(10):1055–1058. doi: 10.2337/diab.34.10.1055. [DOI] [PubMed] [Google Scholar]
- 12.Krotkiewski M., Björntorp P., Sjöström L., Smith U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J. Clin. Investig. 1983;72(3):1150–1162. doi: 10.1172/JCI111040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aucouturier J., Meyer M., Thivel D., Taillardat M., Duché P. Effect of android to gynoid fat ratio on insulin resistance in obese youth. Arch. Pediatr. Adolesc. Med. 2009;163(9):826–831. doi: 10.1001/archpediatrics.2009.148. [DOI] [PubMed] [Google Scholar]
- 14.Janjic D. [Android-type obesity and gynecoid-type obesity] Praxis (Bern 1994) 1996;85(49):1578–1583. [PubMed] [Google Scholar]
- 15.Després J.-P. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126(10):1301–1313. doi: 10.1161/CIRCULATIONAHA.111.067264. [DOI] [PubMed] [Google Scholar]
- 16.Björntorp P. Visceral obesity: a “Civilization Syndrome”. Obes. Res. 1993;1(3):206–222. doi: 10.1002/j.1550-8528.1993.tb00614.x. [DOI] [PubMed] [Google Scholar]
- 17.Neeland I.J., Ayers C.R., Rohatgi A.K., Turer A.T., Berry J.D., Das S.R. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity. 2013;21(9):E439–E447. doi: 10.1002/oby.20135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toss F., Wiklund P., Franks P.W., Eriksson M., Gustafson Y., Hallmans G. Abdominal and gynoid adiposity and the risk of stroke. Int J. Obes. 2011;35(11):1427–1432. doi: 10.1038/ijo.2011.9. [DOI] [PubMed] [Google Scholar]
- 19.Shen J., Baum T., Cordes C., Ott B., Skurk T., Kooijman H. Automatic segmentation of abdominal organs and adipose tissue compartments in water-fat MRI: application to weight-loss in obesity. Eur. J. Radiol. 2016;85(9):1613–1621. doi: 10.1016/j.ejrad.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Laddu D.R., Lee V.R., Blew R.M., Sato T., Lohman T.G., Going S.B. Predicting visceral adipose tissue by MRI using DXA and anthropometry in adolescents and young adults. Int. J. Body Compos. Res. 2012;10(4):93–100. [PMC free article] [PubMed] [Google Scholar]
- 21.Kissebah A.H., Vydelingum N., Murray R., Evans D.J., Hartz A.J., Kalkhoff R.K. Relation of body fat distribution to metabolic complications of obesity. J. Clin. Endocrinol. Metab. 1982;54(2):254–260. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- 22.Feller S., Boeing H., Pischon T. Body mass index, waist circumference, and the risk of type 2 diabetes mellitus: implications for routine clinical practice. Dtsch. Ärzteblatt Int. 2010;107(26):470–476. doi: 10.3238/arztebl.2010.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran T.T., Yamamoto Y., Gesta S., Kahn C.R. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7(5):410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster M.T., Shi H., Softic S., Kohli R., Seeley R.J., Woods S.C. Transplantation of non-visceral fat to the visceral cavity improves glucose tolerance in mice: investigation of hepatic lipids and insulin sensitivity. Diabetologia. 2011;54(11):2890–2899. doi: 10.1007/s00125-011-2259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foster M.T., Softic S., Caldwell J., Kohli R., de Kloet A.D., Seeley R.J. Subcutaneous adipose tissue transplantation in diet-induced obese mice attenuates metabolic dysregulation while removal exacerbates it. Physiol. Rep. 2013;1:2. doi: 10.1002/phy2.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang-Doran I., Sleigh A., Rochford J.J., O'Rahilly S., Savage D.B. Lipodystrophy: metabolic insights from a rare disorder. J. Endocrinol. 2010;207(3):245–255. doi: 10.1677/JOE-10-0272. [DOI] [PubMed] [Google Scholar]
- 27.Ajluni N., Dar M., Xu J., Neidert A.H., Oral E.A. Efficacy and safety of metreleptin in patients with partial lipodystrophy: lessons from an expanded access program. J. Diabetes Metab. 2016;7(3):659. doi: 10.4172/2155-6156.1000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan D., McIntyre A.D., Hegele R.A., Don-Wauchope A.C. Familial partial lipodystrophy presenting as metabolic syndrome. J. Clin. Lipidol. 2016;10(6):1488–1491. doi: 10.1016/j.jacl.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Herranz P., de Lucas R., Pérez-España L., Mayor M. Lipodystrophy syndromes. Dermatol. Clin. 2008;26(4):569–578. doi: 10.1016/j.det.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan J.L., Oral E.A. Clinical classification and treatment of congenital and acquired lipodystrophy. Endocr. Pract. 2010;16(2):310–323. doi: 10.4158/EP09154.RA. [DOI] [PubMed] [Google Scholar]
- 31.Mallewa J.E., Wilkins E., Vilar J., Mallewa M., Doran D., Back D. HIV-associated lipodystrophy: a review of underlying mechanisms and therapeutic options. J. Antimicrob. Chemother. 2008;62(4):648–660. doi: 10.1093/jac/dkn251. [DOI] [PubMed] [Google Scholar]
- 32.Nolis T. Exploring the pathophysiology behind the more common genetic and acquired lipodystrophies. J. Hum. Genet. 2014;59(1):16–23. doi: 10.1038/jhg.2013.107. [DOI] [PubMed] [Google Scholar]
- 33.Shikuma C.M., Hu N., Milne C., Yost F., Waslien C., Shimizu S. Mitochondrial DNA decrease in subcutaneous adipose tissue of HIV-infected individuals with peripheral lipoatrophy. AIDS. 2001;15(14):1801–1809. doi: 10.1097/00002030-200109280-00009. [DOI] [PubMed] [Google Scholar]
- 34.Carr A., Cooper D.A. Lipodystrophy associated with an HIV-protease inhibitor. New Engl. J. Med. 1998;339(18):1296. doi: 10.1056/NEJM199810293391806. [DOI] [PubMed] [Google Scholar]
- 35.Anuurad E., Bremer A., Berglund L. HIV protease inhibitors and obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2010;17(5):478–485. doi: 10.1097/MED.0b013e32833dde87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bacchetti P., Gripshover B., Grunfeld C., Heymsfield S., McCreath H., Osmond D. Fat distribution in men with HIV infection. J. Acquir. Immune Defic. Syndr. 2005;40(2):121–131. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grunfeld C., Kotler D.P., Arnett D.K., Falutz J.M., Haffner S.M., Hruz P. Contribution of metabolic and anthropometric abnormalities to cardiovascular disease risk factors. Circulation. 2008;118(2):e20–e28. doi: 10.1161/CIRCULATIONAHA.107.189623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson K.N., Hui Q., Rimland D., Xu K., Freiberg M.S., Justice A.C. Identification of HIV infection-related DNA methylation sites and advanced epigenetic aging in HIV+, treatment-naive U.S. veterans. AIDS. 2017;31(4):517–575. doi: 10.1097/QAD.0000000000001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torriani M., Srinivasa S., Fitch K.V., Thomou T., Wong K., Petrow E. Dysfunctional subcutaneous fat with reduced dicer and brown adipose tissue gene expression in HIV-infected patients. J. Clin. Endocrinol. Metab. 2016;101(3):1225–1234. doi: 10.1210/jc.2015-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saini V. Molecular mechanisms of insulin resistance in type 2 diabetes mellitus. World J. Diabetes. 2010;1(3):68–75. doi: 10.4239/wjd.v1.i3.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.WHO. World Health Day 2016: GLobal Report on Diabetes; 2016.
- 42.Federation I-ID. IDF Diabetes Atlas 7ed 2015:1–144.
- 43.Fuchsberger C., Flannick J., Teslovich T.M., Mahajan A., Agarwala V., Gaulton K.J. The genetic architecture of type 2 diabetes. Nature. 2016;536(7614):41–47. doi: 10.1038/nature18642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas C.C., Philipson L.H. Update on diabetes classification. Med. Clin. North Am. 2015;99(1):1–16. doi: 10.1016/j.mcna.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 45.Dedoussis G.V.Z., Kaliora A.C., Panagiotakos D.B. Genes, diet and type 2 diabetes mellitus: a review. Rev. Diabet. Stud. 2007;4(1):13–24. doi: 10.1900/RDS.2007.4.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rytka J.M., Wueest S., Schoenle E.J., Konrad D. The portal theory supported by venous drainage-selective fat transplantation. Diabetes. 2011;60(1):56–63. doi: 10.2337/db10-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Item F., Konrad D. Visceral fat and metabolic inflammation: the portal theory revisited. Obes. Rev. 2012;13(Suppl 2):30–39. doi: 10.1111/j.1467-789X.2012.01035.x. [DOI] [PubMed] [Google Scholar]
- 48.Perry R.J., Samuel V.T., Petersen K.F., Shulman G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510(7503):84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muscelli E., Pereira J.A., Lazarin M.A., da Silva C.A., Pareja J.C., Saad M.J. Lack of insulin inhibition on insulin secretion in non-diabetic morbidly obese patients. Int J. Obes. Relat. Metab. Disord. 2001;25(6):798–804. doi: 10.1038/sj.ijo.0801607. [DOI] [PubMed] [Google Scholar]
- 50.Gray S.L., Vidal-Puig A.J. Adipose tissue expandability in the maintenance of metabolic homeostasis. Nutr. Rev. 2007;2:S7–S12. doi: 10.1111/j.1753-4887.2007.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 51.McLaughlin T., Sherman A., Tsao P., Gonzalez O., Yee G., Lamendola C. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia. 2007;50(1707) doi: 10.1007/s00125-007-0708-y. [DOI] [PubMed] [Google Scholar]
- 52.Unger R.H. Lipid overload and overflow: metabolic trauma and the metabolic syndrome. Trends Endocrinol. Metab. 2003;14(9):398–403. doi: 10.1016/j.tem.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 53.Mittendorfer B. Origins of metabolic complications in obesity: adipose tissue and free fatty acid trafficking. Curr. Opin. Clin. Nutr. Metab. Care. 2011;14(6):535–541. doi: 10.1097/MCO.0b013e32834ad8b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreno-Indias I., Tinahones F.J. Impaired adipose tissue expandability and lipogenic capacities as ones of the main causes of metabolic disorders. J. Diabetes Res. 2015;2015:970375. doi: 10.1155/2015/970375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edens N.K., Leibel R.L., Hirsch J. Mechanism of free fatty acid re-esterification in human adipocytes in vitro. J. Lipid Res. 1990;31(8):1423–1431. [PubMed] [Google Scholar]
- 56.Ghosh N., Patel N., Jiang K., Watson J.E., Cheng J., Chalfant C.E. Ceramide-activated protein phosphatase involvement in insulin resistance via Akt, Serine/Arginine-Rich Protein 40, and Ribonucleic Acid Splicing in L6 skeletal muscle cells. Endocrinology. 2007;148(3):1359–1366. doi: 10.1210/en.2006-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masharani U.B., Maddux B.A., Li X., Sakkas G.K., Mulligan K., Schambelan M. Insulin resistance in non-obese subjects is associated with activation of the JNK pathway and impaired insulin signaling in skeletal muscle. PLoS One. 2011;6(5):e19878. doi: 10.1371/journal.pone.0019878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bourbon N.A., Sandirasegarane L., Kester M. Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J. Biol. Chem. 2002;277(5):3286–3292. doi: 10.1074/jbc.M110541200. [DOI] [PubMed] [Google Scholar]
- 59.Lam T.K., Yoshii H., Haber C.A., Bogdanovic E., Lam L., Fantus I.G. Free fatty acid-induced hepatic insulin resistance: a potential role for protein kinase C-delta. Am. J. Physiol. Endocrinol. Metab. 2002;283(4):E682–E691. doi: 10.1152/ajpendo.00038.2002. [DOI] [PubMed] [Google Scholar]
- 60.Zhang L., Keung W., Samokhvalov V., Wang W., Lopaschuk G. Role of fatty acid uptake and fatty acid β-oxidation in mediating insulin resistance in heart and skeletal muscle. Biochim. Biophys. Acta. 2010;1801(1):1–22. doi: 10.1016/j.bbalip.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 61.Hsieh C.T., Chuang J.H., Yang W.C., Yin Y., Lin Y. Ceramide inhibits insulin-stimulated Akt phosphorylation through activation of Rheb/mTORC1/S6K signaling in skeletal muscle. Cell Signal. 2014;26(7):1400–1408. doi: 10.1016/j.cellsig.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 62.Summers S.A. Ceramides in insulin resistance and lipotoxicity. Prog. Lipid Res. 2006;45:42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 63.Tschop M., Thomas G. Fat fuels insulin resistance through Toll-like receptors. Nat. Med. 2006;12(12):1359–1361. doi: 10.1038/nm1206-1359. [DOI] [PubMed] [Google Scholar]
- 64.Jia L., Vianna C.R., Fukuda M., Berglund E.D., Liu C., Tao C. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat. Commun. 2014;5:3878. doi: 10.1038/ncomms4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yin J., Gao Z., He Q., Zhou D., Guo Z., Ye J. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2009;296(2):E333–E342. doi: 10.1152/ajpendo.90760.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 2013;93(1):1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 67.Rausch M.E., Weisberg S., Vardhana P., Tortoriello D.V. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int. J. Obes. 2005;32(3):451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 68.Bricambert J., Favre D., Brajkovic S., Bonnefond A., Boutry R., Salvi R. Impaired histone deacetylases 5 and 6 expression mimics the effects of obesity and hypoxia on adipocyte function. Mol. Metab. 2016;5(12):1200–1207. doi: 10.1016/j.molmet.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cinti S., Mitchell G., Barbatelli G., Murano I., Ceresi E., Faloia E. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005;46(11):2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 71.Kanda H., Tateya S., Tamori Y., Kotani K., Hiasa K-i, Kitazawa R. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006;116(6):1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sims J.E., Smith D.E. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 2010;10(2):89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 73.Bing C. Is interleukin-1β a culprit in macrophage-adipocyte crosstalk in obesity? Adipocyte. 2015;4(2):149–152. doi: 10.4161/21623945.2014.979661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wen H., Gris D., Lei Y., Jha S., Zhang L., Huang M.T. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011;12(5):408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gao D., Madi M., Ding C., Fok M., Steele T., Ford C. Interleukin-1beta mediates macrophage-induced impairment of insulin signaling in human primary adipocytes. Am. J. Physiol. Endocrinol. Metab. 2014;307(3):E289–E304. doi: 10.1152/ajpendo.00430.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mori M.A., Bezy O., Kahn C.R. Metabolic syndrome: is Nlrp3 inflammasome a trigger or a target of insulin resistance? Circ. Res. 2011;108(10):1160–1162. doi: 10.1161/RES.0b013e318220b57b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ehses J.A., Lacraz G., Giroix M.H., Schmidlin F., Coulaud J., Kassis N. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc. Natl. Acad. Sci. USA. 2009;106(33):13998–14003. doi: 10.1073/pnas.0810087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carswell E.A., Old L.J., Kassel R.L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl. Acad. Sci. USA. 1975;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fain J.N., Bahouth S.W., Madan A.K. TNFalpha release by the nonfat cells of human adipose tissue. Int J. Obes. Relat. Metab. Disord. 2004;28(4):616–622. doi: 10.1038/sj.ijo.0802594. [DOI] [PubMed] [Google Scholar]
- 80.Hotamisligil G.S. The role of TNFα and TNF receptors in obesity and insulin resistance. J. Intern. Med. 1999;245(6):621–625. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 81.Csehi S.-B., Mathieu S., Seifert U., Lange A., Zweyer M., Wernig A. Tumor necrosis factor (TNF) interferes with insulin signaling through the p55 TNF receptor death domain. Biochem. Biophys. Res. Commun. 2005;329(1):397–405. doi: 10.1016/j.bbrc.2005.01.140. [DOI] [PubMed] [Google Scholar]
- 82.Cawthorn W.P., Heyd F., Hegyi K., Sethi J.K. Tumour necrosis factor-[alpha] inhibits adipogenesis via a [beta]-catenin//TCF4(TCF7L2)-dependent pathway. Cell Death Differ. 2007;14(7):1361–1373. doi: 10.1038/sj.cdd.4402127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kurebayashi S., Sumitani S., Kasayama S., Jetten A.M., Hirose T. TNF-alpha inhibits 3T3-L1 adipocyte differentiation without downregulating the expression of C/EBPbeta and delta. Endocr. J. 2001;48(2):249–253. doi: 10.1507/endocrj.48.249. [DOI] [PubMed] [Google Scholar]
- 84.Laurencikiene J., van Harmelen V., Arvidsson Nordstrom E., Dicker A., Blomqvist L., Naslund E. NF-kappaB is important for TNF-alpha-induced lipolysis in human adipocytes. J. Lipid Res. 2007;48(5):1069–1077. doi: 10.1194/jlr.M600471-JLR200. [DOI] [PubMed] [Google Scholar]
- 85.Cawthorn W.P., Sethi J.K. TNF-α and adipocyte biology. FEBS Lett. 2008;582(1):117–131. doi: 10.1016/j.febslet.2007.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi H., Kokoeva M.V., Inouye K., Tzameli I., Yin H., Flier J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006;116(11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun K., Kusminski C.M., Scherer P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011;121(6):2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maachi M., Pieroni L., Bruckert E., Jardel C., Fellahi S., Hainque B. Systemic low-grade inflammation is related to both circulating and adipose tissue TNF[alpha], leptin and IL-6 levels in obese women. Int J. Obes. Relat. Metab. Disord. 2004;28(8):993–997. doi: 10.1038/sj.ijo.0802718. [DOI] [PubMed] [Google Scholar]
- 89.Park E.J., Lee J.H., Yu G.-Y., He G., Ali S.R., Holzer R.G. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140(2):197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lagathu C., Bastard J.P., Auclair M., Maachi M., Capeau J., Caron M. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: prevention by rosiglitazone. Biochem. Biophys. Res. Commun. 2003;311(2):372–379. doi: 10.1016/j.bbrc.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 91.Rotter V., Nagaev I., Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J. Biol. Chem. 2003;278(46):45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 92.Senn J.J., Klover P.J., Nowak I.A., Mooney R.A. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51(12):3391–3399. doi: 10.2337/diabetes.51.12.3391. [DOI] [PubMed] [Google Scholar]
- 93.Steensberg A., Fischer C.P., Sacchetti M., Keller C., Osada T., Schjerling P. Acute interleukin-6 administration does not impair muscle glucose uptake or whole-body glucose disposal in healthy humans. J. Physiol. 2003;548(2):631–638. doi: 10.1113/jphysiol.2002.032938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petersen E.W., Carey A.L., Sacchetti M., Steinberg G.R., Macaulay S.L., Febbraio M.A. Acute IL-6 treatment increases fatty acid turnover in elderly humans in vivo and in tissue culture in vitro. Am. J. Physiol. Endocrinol. Metab. 2005;288(1):E155–E162. doi: 10.1152/ajpendo.00257.2004. [DOI] [PubMed] [Google Scholar]
- 95.Ellingsgaard H., Hauselmann I., Schuler B., Habib A.M., Baggio L.L., Meier D.T. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 2011;17(11):1481–1489. doi: 10.1038/nm.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stanford K.I., Middelbeek R.J.W., Townsend K.L., An D., Nygaard E.B., Hitchcox K.M. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 2013;123(1):215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nieto-Vazquez I., Fernández-Veledo S., de Alvaro C., Lorenzo M. Dual role of interleukin-6 in regulating insulin sensitivity in murine skeletal muscle. Diabetes. 2008;57(12):3211–3221. doi: 10.2337/db07-1062. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Sies H. Oxidative stress: oxidants and antioxidants. Exp. Physiol. 1997;82(2):291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 99.Banerjee A.K., Mandal A., Chanda D., Chakraborti S. Oxidant, antioxidant and physical exercise. Mol. Cell Biochem. 2003;253(1–2):307–312. doi: 10.1023/a:1026032404105. [DOI] [PubMed] [Google Scholar]
- 100.Furukawa S., Fujita T., Shimabukuro M., Iwaki M., Yamada Y., Nakajima Y. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barazzoni R., Zanetti M., Gortan Cappellari G., Semolic A., Boschelle M., Codarin E. Fatty acids acutely enhance insulin-induced oxidative stress and cause insulin resistance by increasing mitochondrial reactive oxygen species (ROS) generation and nuclear factor-kappaB inhibitor (IkappaB)-nuclear factor-kappaB (NFkappaB) activation in rat muscle, in the absence of mitochondrial dysfunction. Diabetologia. 2012;55(3):773–782. doi: 10.1007/s00125-011-2396-x. [DOI] [PubMed] [Google Scholar]
- 102.Kim Y.-S., Morgan M.J., Choksi S., Liu Z.-g. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol. Cell. 2007;26(5):675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 103.Pessler D., Rudich A., Bashan N. Oxidative stress impairs nuclear proteins binding to the insulin responsive element in the GLUT4 promoter. Diabetologia. 2001;44(12):2156–2164. doi: 10.1007/s001250100024. [DOI] [PubMed] [Google Scholar]
- 104.Besse-Patin A., Estall J.L. An intimate relationship between ros and insulin signalling: implications for antioxidant treatment of fatty liver disease. Int J. Cell Biol. 2014;2014:519153. doi: 10.1155/2014/519153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Anderson E.J., Lustig M.E., Boyle K.E., Woodlief T.L., Kane D.A., Lin C.-T. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J. Clin. Investig. 2009;119(3):573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ristow M., Zarse K., Oberbach A., Kloting N., Birringer M., Kiehntopf M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA. 2009;106(21):8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stern J.H., Rutkowski J.M., Scherer P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016;23(5):770–784. doi: 10.1016/j.cmet.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 109.Halaas J.L., Gajiwala K.S., Maffei M., Cohen S.L., Chait B.T., Rabinowitz D. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 110.Schwartz M.W., Seeley R.J., Campfield L.A., Burn P., Baskin D.G. Identification of targets of leptin action in rat hypothalamus. J. Clin. Investig. 1996;98(5):1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abella V., Scotece M., Conde J., Pino J., Gonzalez-Gay M.A., Gomez-Reino J.J. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 2017;13(2):100–109. doi: 10.1038/nrrheum.2016.209. [DOI] [PubMed] [Google Scholar]
- 112.Coppola A., Marfella R., Coppola L., Tagliamonte E., Fontana D., Liguori E. Effect of weight loss on coronary circulation and adiponectin levels in obese women. Int J. Cardiol. 2009;134(3):414–416. doi: 10.1016/j.ijcard.2007.12.087. [DOI] [PubMed] [Google Scholar]
- 113.Kajimura D., Lee H.W., Riley K.J., Arteaga-Solis E., Ferron M., Zhou B. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell Metab. 2013;17(6):901–915. doi: 10.1016/j.cmet.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011;11(2):85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Peirce V., Carobbio S., Vidal-Puig A. The different shades of fat. Nature. 2014;510(7503):76–83. doi: 10.1038/nature13477. [DOI] [PubMed] [Google Scholar]
- 116.Wronska A., Kmiec Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol. 2012;205(2):194–208. doi: 10.1111/j.1748-1716.2012.02409.x. [DOI] [PubMed] [Google Scholar]
- 117.Tilg H., Moschen A.R. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006;6(10):772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 118.Chusyd D.E., Wang D., Huffman D.M., Nagy T.R. Relationships between rodent white adipose fat pads and human white adipose fat depots. Front. Nutr. 2016;3:10. doi: 10.3389/fnut.2016.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kurioka S., Murakami Y., Nishiki M., Sohmiya M., Koshimura K., Kato Y. Relationship between visceral fat accumulation and anti-lipolytic action of insulin in patients with type 2 diabetes mellitus. Endocr. J. 2002;49(4):459–464. doi: 10.1507/endocrj.49.459. [DOI] [PubMed] [Google Scholar]
- 120.Meek S.E., Nair K.S., Jensen M.D. Insulin regulation of regional free fatty acid metabolism. Diabetes. 1999;48(1):10–14. doi: 10.2337/diabetes.48.1.10. [DOI] [PubMed] [Google Scholar]
- 121.Lafontan M., Berlan M. Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol. Sci. 2003;24(6):276–283. doi: 10.1016/S0165-6147(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 122.Fontana L., Eagon J.C., Trujillo M.E., Scherer P.E., Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 123.Qiang G., Kong H.W., Fang D., McCann M., Yang X., Du G. The obesity-induced transcriptional regulator TRIP-Br2 mediates visceral fat endoplasmic reticulum stress-induced inflammation. Nat. Commun. 2016;7:11378. doi: 10.1038/ncomms11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mathieu P., Poirier P., Pibarot P., Lemieux I., Després J.-P. Visceral obesity. the link among inflammation, hypertension, and cardiovascular disease. 2009;53(4):577–584. doi: 10.1161/HYPERTENSIONAHA.108.110320. [DOI] [PubMed] [Google Scholar]
- 125.Lee Y.-H., Petkova Anelia P., Mottillo Emilio P., Granneman James G. In Vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012;15(4):480–491. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rosenwald M., Perdikari A., Rulicke T., Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 2013;15(6):659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- 127.Rosen Evan D., Spiegelman Bruce M. What we talk about when we talk about fat. Cell. 2014;156(1):20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Virtue S., Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome – an allostatic perspective. Biochim. Biophys. Acta. 2010;1801(3):338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 129.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ferrante A.W., Jr. The immune cells in adipose tissue. Diabetes Obes. Metab. 2013;15(3):S34–S38. doi: 10.1111/dom.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fleck S.J. Body composition of elite American athletes. Am. J. Sports Med. 1983;11(6):398–403. doi: 10.1177/036354658301100604. [DOI] [PubMed] [Google Scholar]
- 132.Ortega F.J., Mayas D., Moreno-Navarrete J.M., Catalan V., Gomez-Ambrosi J., Esteve E. The gene expression of the main lipogenic enzymes is downregulated in visceral adipose tissue of obese subjects. Obesity (Silver Spring) 2010;18(1):13–20. doi: 10.1038/oby.2009.202. [DOI] [PubMed] [Google Scholar]
- 133.Larson K.A., Anderson D.B. The effects of lipectomy on remaining adipose tissue depots in the Sprague Dawley rat. Growth. 1978;42(4):469–477. [PubMed] [Google Scholar]
- 134.Reyne Y., Nougues J., Vezinhet A. Adipose tissue regeneration in 6-month-old and adult rabbits following lipectomy. Proc. Soc. Exp. Biol. Med. 1983;174(2):258–264. doi: 10.3181/00379727-174-41734. [DOI] [PubMed] [Google Scholar]
- 135.Kaneko H., Dridi S., Tarallo V., Gelfand B.D., Fowler B.J., Cho W.G. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471(7338):325–330. doi: 10.1038/nature09830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hellman B., Hellerstrom C. Cell renewal in the white and brown fat tissue of the rat. Acta Pathol. Microbiol. Scand. 1961;51:347–353. doi: 10.1111/j.1699-0463.1961.tb00373.x. [DOI] [PubMed] [Google Scholar]
- 137.Hirsch J., Han P.W. Cellularity of rat adipose tissue: effects of growth, starvation, and obesity. J. Lipid Res. 1969;10(1):77–82. [PubMed] [Google Scholar]
- 138.Bertrand H.A., Masoro E.J., Yu B.P. Increasing adipocyte number as the basis for perirenal depot growth in adult rats. Science. 1978;201(4362):1234–1235. doi: 10.1126/science.151328. [DOI] [PubMed] [Google Scholar]
- 139.Spalding K.L., Arner E., Westermark P.O., Bernard S., Buchholz B.A., Bergmann O. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 140.Rigamonti A., Brennand K., Lau F., Cowan C.A. Rapid cellular turnover in adipose tissue. PLoS One. 2011;6(3):e17637. doi: 10.1371/journal.pone.0017637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cannon B., Nedergaard J. Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans) Int J. Obes. 2010;34(1):S7–S16. doi: 10.1038/ijo.2010.177. [DOI] [PubMed] [Google Scholar]
- 142.Nedergaard J., Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab. 2010;11(4):268–272. doi: 10.1016/j.cmet.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 143.Nedergaard J., Bengtsson T., Cannon B. Three years with adult human brown adipose tissue. Ann. N. Y. Acad. Sci. 2010;1212:E20–E36. doi: 10.1111/j.1749-6632.2010.05905.x. [DOI] [PubMed] [Google Scholar]
- 144.Richard D., Carpentier A.C., Dore G., Ouellet V., Picard F. Determinants of brown adipocyte development and thermogenesis. Int J. Obes. 2010;34(2):S59–S66. doi: 10.1038/ijo.2010.241. [DOI] [PubMed] [Google Scholar]
- 145.Ravussin E., Kozak L.P. Have we entered the brown adipose tissue renaissance? Obes. Rev. 2009;10(3):265–268. doi: 10.1111/j.1467-789X.2008.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Townsend K., Tseng Y.H. Brown adipose tissue: recent insights into development, metabolic function and therapeutic potential. Adipocyte. 2012;1(1):13–24. doi: 10.4161/adip.18951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gomez-Hernandez A., Beneit N., Diaz-Castroverde S., Escribano O. Differential role of adipose tissues in obesity and related metabolic and vascular complications. Int. J. Endocrinol. 2016;2016:1216783. doi: 10.1155/2016/1216783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fedorenko A., Lishko P.V., Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151(2):400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hatai S. On the presence in human embryos of an interscapular gland corresponding to the so-called hibernating Glanf of lower mammals. Anatoinischer Anz. 1902;21:369. [Google Scholar]
- 150.Heaton J.M. The distribution of brown adipose tissue in the human. J. Anat. 1972;112(1):35–39. [PMC free article] [PubMed] [Google Scholar]
- 151.Hany T.F., Gharehpapagh E., Kamel E.M., Buck A., Himms-Hagen J., von Schulthess G.K. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur. J. Nucl. Med. Mol. Imaging. 2002;29(10):1393–1398. doi: 10.1007/s00259-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 152.Nedergaard J., Bengtsson T., Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 2007;293(2):E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 153.Goetze S., Lavely W.C., Ziessman H.A., Wahl R.L. Visualization of brown adipose tissue with 99mTc-methoxyisobutylisonitrile on SPECT/CT. J. Nucl. Med. 2008;49(5):752–756. doi: 10.2967/jnumed.107.048074. [DOI] [PubMed] [Google Scholar]
- 154.van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Drossaerts J.M., Kemerink G.J., Bouvy N.D. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009;360(15):1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 155.Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B. Identification and importance of brown adipose tissue in adult humans. New Engl. J. Med. 2009;360(15):1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Saito M., Okamatsu-Ogura Y., Matsushita M., Watanabe K., Yoneshiro T., Nio-Kobayashi J. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58(7):1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cypess A.M., Chen Y.C., Sze C., Wang K., English J., Chan O. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc. Natl. Acad. Sci. USA. 2012;109(25):10001–10005. doi: 10.1073/pnas.1207911109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ouellet V., Labbé S.M., Blondin D.P., Phoenix S., Guérin B., Haman F. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. The Journal of Clinical Investigation. 2012;122(2):545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vijgen G.H., Sparks L.M., Bouvy N.D., Schaart G., Hoeks J., van Marken Lichtenbelt W.D. Increased oxygen consumption in human adipose tissue from the "brown adipose tissue" region. J. Clin. Endocrinol. Metab. 2013;98(7):E1230–E1234. doi: 10.1210/jc.2013-1348. [DOI] [PubMed] [Google Scholar]
- 160.Cypess A.M., Doyle A.N., Sass C.A., Huang T.L., Mowschenson P.M., Rosen H.N. Quantification of human and rodent brown adipose tissue function using 99mTc-methoxyisobutylisonitrile SPECT/CT and 18F-FDG PET/CT. J. Nucl. Med. 2013;54(11):1896–1901. doi: 10.2967/jnumed.113.121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bostrom P., Wu J., Jedrychowski M.P., Korde A., Ye L., Lo J.C. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fabbiano S., Suárez-Zamorano N., Rigo D., Veyrat-Durebex C., Stevanovic Dokic A., Colin Didier J. Caloric restriction leads to browning of white adipose tissue through type 2 immune signaling. Cell Metabolism. 2016;24(3):434–446. doi: 10.1016/j.cmet.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 163.Lee P., Werner C.D., Kebebew E., Celi F.S. Functional thermogenic beige adipogenesis is inducible in human neck fat. Int. J. Obese. 2005;38(2):170–176. doi: 10.1038/ijo.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bartesaghi S., Hallen S., Huang L., Svensson P.A., Momo R.A., Wallin S. Thermogenic activity of UCP1 in human white fat-derived beige adipocytes. Mol. Endocrinol. 2015;29(1):130–139. doi: 10.1210/me.2014-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Foster D.O., Frydman M.L. Nonshivering thermogenesis in the rat. II. Measurements of blood flow with microspheres point to brown adipose tissue as the dominant site of the calorigenesis induced by noradrenaline. Can. J. Physiol. Pharmacol. 1978;56(1):110–122. doi: 10.1139/y78-015. [DOI] [PubMed] [Google Scholar]
- 166.Thurlby P.L., Trayhurn P. Regional blood flow in genetically obese (ob/ob) mice. The importance of brown adipose tissue to the reduced energy expenditure on non-shivering thermogenesis. Pflug. Arch. 1980;385(3):193–201. doi: 10.1007/BF00647457. [DOI] [PubMed] [Google Scholar]
- 167.Puchalski W., Bockler H., Heldmaier G., Langefeld M. Organ blood flow and brown adipose tissue oxygen consumption during noradrenaline-induced nonshivering thermogenesis in the Djungarian hamster. J. Exp. Zool. 1987;242(3):263–271. doi: 10.1002/jez.1402420304. [DOI] [PubMed] [Google Scholar]
- 168.Foster D.O., Frydman M.L. Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can. J. Physiol. Pharmacol. 1979;57(3):257–270. doi: 10.1139/y79-039. [DOI] [PubMed] [Google Scholar]
- 169.Feldmann H.M., Golozoubova V., Cannon B., Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9(2):203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 170.Yoneshiro T., Aita S., Matsushita M., Kameya T., Nakada K., Kawai Y. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity. 2011;19(1):13–16. doi: 10.1038/oby.2010.105. [DOI] [PubMed] [Google Scholar]
- 171.Yoneshiro T., Aita S., Matsushita M., Kayahara T., Kameya T., Kawai Y. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Investig. 2013;123(8):3404–3408. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.van der Lans A.A., Hoeks J., Brans B., Vijgen G.H., Visser M.G., Vosselman M.J. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Investig. 2013;123(8):3395–3403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chondronikola M., Volpi E., Borsheim E., Porter C., Annamalai P., Enerback S. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63(12):4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee P., Smith S., Linderman J., Courville A.B., Brychta R.J., Dieckmann W. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 2014;63(11):3686–3698. doi: 10.2337/db14-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wu J., Bostrom P., Sparks L.M., Ye L., Choi J.H., Giang A.H. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rogers N.H., Landa A., Park S., Smith R.G. Aging leads to a programmed loss of brown adipocytes in murine subcutaneous white adipose tissue. Aging Cell. 2012;11(6):1074–1083. doi: 10.1111/acel.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Graja A., Schulz T.J. Mechanisms of aging-related impairment of brown adipocyte development and function. Gerontology. 2015;61(3):211–217. doi: 10.1159/000366557. [DOI] [PubMed] [Google Scholar]
- 178.Oliverio M., Schmidt E., Mauer J., Baitzel C., Hansmeier N., Khani S. Dicer1-miR-328-Bace1 signalling controls brown adipose tissue differentiation and function. Nat. Cell Biol. 2016;18(3):328–336. doi: 10.1038/ncb3316. [DOI] [PubMed] [Google Scholar]
- 179.Sidossis L., Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J. Clin. Investig. 2015;125(2):478–486. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bartelt A., Bruns O.T., Reimer R., Hohenberg H., Ittrich H., Peldschus K. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011;17(2):200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 181.Seale P., Conroe H.M., Estall J., Kajimura S., Frontini A., Ishibashi J. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Investig. 2011;121(1):96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kozak L.P., Anunciado-Koza R. UCP1: its involvement and utility in obesity. Int. J. Obes. 2005;32(7):S32–S38. doi: 10.1038/ijo.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Puigserver P., Wu Z., Park C.W., Graves R., Wright M., Spiegelman B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 185.Karamanlidis G., Karamitri A., Docherty K., Hazlerigg D.G., Lomax M.A. C/EBPbeta reprograms white 3T3-L1 preadipocytes to a Brown adipocyte pattern of gene expression. J. Biol. Chem. 2007;282(34):24660–24669. doi: 10.1074/jbc.M703101200. [DOI] [PubMed] [Google Scholar]
- 186.Carmona M.C., Hondares E., Rodriguez de la Concepcion M.L., Rodriguez-Sureda V., Peinado-Onsurbe J., Poli V. Defective thermoregulation, impaired lipid metabolism, but preserved adrenergic induction of gene expression in brown fat of mice lacking C/EBPbeta. Biochem. J. 2005;389(1):47–56. doi: 10.1042/BJ20050009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kir S., White J.P., Kleiner S., Kazak L., Cohen P., Baracos V.E. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513(7516):100–104. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cao L., Choi E.Y., Liu X., Martin A., Wang C., Xu X. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14(3):324–338. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rao R.R., Long J.Z., White J.P., Svensson K.J., Lou J., Lokurkar I. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157(6):1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Knudsen J.G., Murholm M., Carey A.L., Bienso R.S., Basse A.L., Allen T.L. Role of IL-6 in exercise training- and cold-induced UCP1 expression in subcutaneous white adipose tissue. PloS One. 2014;9(1):e84910. doi: 10.1371/journal.pone.0084910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Petruzzelli M., Schweiger M., Schreiber R., Campos-Olivas R., Tsoli M., Allen J. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20(3):433–447. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 192.Qiu Y., Nguyen K.D., Odegaard J.I., Cui X., Tian X., Locksley R.M. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157(6):1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gesta S., Bluher M., Yamamoto Y., Norris A.W., Berndt J., Kralisch S. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc. Natl. Acad. Sci. USA. 2006;103(17):6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yamamoto Y., Gesta S., Lee K.Y., Tran T.T., Saadatirad P., Kahn C.R. Adipose depots possess unique developmental gene signatures. Obesity. 2010;18(5):872–878. doi: 10.1038/oby.2009.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gesta S., Tseng Y.-H., Kahn C.R. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131(2):242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 196.Macotela Y., Emanuelli B., Mori M.A., Gesta S., Schulz T.J., Tseng Y.H. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61(7):1691–1699. doi: 10.2337/db11-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chau Y.-Y., Bandiera R., Serrels A., Martínez-Estrada O.M., Qing W., Lee M. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat. Cell Biol. 2014;16(4):367–375. doi: 10.1038/ncb2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vishvanath L., MacPherson K.A., Hepler C., Wang Q.A., Shao M., Spurgin S.B. Pdgfrbeta+ Mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab. 2016;23(2):350–359. doi: 10.1016/j.cmet.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sanchez-Gurmaches J., Guertin D.A. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat. Commun. 2014;5:4099. doi: 10.1038/ncomms5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sanchez-Gurmaches J., Hung C.M., Sparks C.A., Tang Y., Li H., Guertin D.A. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 2012;16(3):348–362. doi: 10.1016/j.cmet.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Altshuler-Keylin S., Shinoda K., Hasegawa Y., Ikeda K., Hong H., Kang Q. Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell Metab. 2016;24(3):402–419. doi: 10.1016/j.cmet.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Crick F.H.C. Central dogma of molecular biology. Nature. 1970:227. doi: 10.1038/227561a0. [DOI] [PubMed] [Google Scholar]
- 204.Crick F.H.C. On protein synthesis. Symp. Soc. Exp. Biol. 1956:139–163. [PubMed] [Google Scholar]
- 205.Scherrer K. Historical review: the discovery of "gian" RNA and RNA processing: 40 years of enigma. Trends Biochem. Sci. 2003;28(10):566–571. doi: 10.1016/j.tibs.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 206.Cech Thomas R., Steitz Joan A. The noncoding RNA revolution – trashing old rules to forge new ones. Cell. 2014;157(1):77–94. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 207.Vickers K.C., Roteta L.A., Hucheson-Dilks H., Han L., Guo Y. Mining diverse small RNA species in the deep transcriptome. Trends Biochem. Sci. 2015;40(1):4–7. doi: 10.1016/j.tibs.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ivanov P., Emara M.M., Villen J., Gygi S.P., Anderson P. Angiogenin-induced tRNA fragments inhibit translation initiation. Mol. Cell. 2011;43(4):613–623. doi: 10.1016/j.molcel.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Groszhans H., Filipowicz W. Molecular biology: the expanding world of small RNAs. Nature. 2008;451(7177):414–416. doi: 10.1038/451414a. [DOI] [PubMed] [Google Scholar]
- 210.Mochizuki K. RNA-directed epigenetic regulations of DNA rearrangements. Essays Biochem. 2010;48(1):89–100. doi: 10.1042/bse0480089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Borges F., Martienssen R.A. The expanding world of small RNAs in plants. Nat. Rev. Mol. Cell Biol. 2015;16(12):727–741. doi: 10.1038/nrm4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Reinhart B.J., Bartel D.P. Small RNAs correspond to centromere heterochromatic repeats. Science. 2002;297(5588):1831. doi: 10.1126/science.1077183. [DOI] [PubMed] [Google Scholar]
- 213.Ream T.S., Haag J.R., Wierzbicki A.T., Nicora C.D., Norbeck A.D., Zhu J.K. Subunit compositions of the RNA-silencing enzymes Pol IV and Pol V reveal their origins as specialized forms of RNA polymerase II. Mol. Cell. 2009;33(2):192–203. doi: 10.1016/j.molcel.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Claycomb J.M., Batista P.J., Pang K.M., Gu W., Vasale J.J., van Wolfswinkel J.C. The Argonaute CSR-1 and its 22G-RNA cofactors are required for holocentric chromosome segregation. Cell. 2009;139(1):123–134. doi: 10.1016/j.cell.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kim D.H., Rossi J.J. RNAi mechanisms and applications. BioTechniques. 2008;44(5):613–616. doi: 10.2144/000112792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ratcliff F., Harrison B.D., Baulcombe D.C. A similarity between viral defense and gene silencing in plants. Science. 1997;276(5318):1558–1560. doi: 10.1126/science.276.5318.1558. [DOI] [PubMed] [Google Scholar]
- 217.Ecker J.R., Davis R.W. Inhibition of gene expression in plant cells by expression of antisense RNA. Proc. Natl. Acad. Sci. USA. 1986;83(15):5372–5376. doi: 10.1073/pnas.83.15.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Napoli C., Lemieux C., Jorgensen R. Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell. 1990;2(4):279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391(6669):806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 220.Wightman B., Ha I., Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75(5):855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 221.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 222.Hutvagner G., McLachlan J., Pasquinelli A.E., Balint E., Tuschl T., Zamore P.D. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293(5531):834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 223.Grishok A., Pasquinelli A.E., Conte D., Li N., Parrish S., Ha I. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell. 2001;106(1):23–34. doi: 10.1016/s0092-8674(01)00431-7. [DOI] [PubMed] [Google Scholar]
- 224.O'Donnell K.A., Wentzel E.A., Zeller K.I., Dang C.V., Mendell J.T. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435(7043):839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 225.Aravin A., Gaidatzis D., Pfeffer S., Lagos-Quintana M., Landgraf P., Iovino N. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442(7099):203–207. doi: 10.1038/nature04916. [DOI] [PubMed] [Google Scholar]
- 226.Carmell M.A., Girard A., van de Kant H.J., Bourc'his D., Bestor T.H., de Rooij D.G. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev. Cell. 2007;12(4):503–514. doi: 10.1016/j.devcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 227.Cox D.N., Chao A., Baker J., Chang L., Qiao D., Lin H. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 1998;12(23):3715–3727. doi: 10.1101/gad.12.23.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Das P.P., Bagijn M.P., Goldstein L.D., Woolford J.R., Lehrbach N.J., Sapetschnig A. Piwi and piRNAs act upstream of an endogenous siRNA pathway to suppress Tc3 transposon mobility in the Caenorhabditis elegans germline. Mol. Cell. 2008;31(1):79–90. doi: 10.1016/j.molcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chuma S., Nakano T. piRNA and spermatogenesis in mice. Philos. Trans. R. Soc. B: Biol. Sci. 2013;368(1609) doi: 10.1098/rstb.2011.0338. (20110338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Meister G. Argonaute proteins: functional insights and emerging roles. Nat. Rev. Genet. 2013;14(7):447–459. doi: 10.1038/nrg3462. [DOI] [PubMed] [Google Scholar]
- 231.Klenov M.S., Lavrov S.A., Korbut A.P., Stolyarenko A.D., Yakushev E.Y., Reuter M. Impact of nuclear Piwi elimination on chromatin state in Drosophila melanogaster ovaries. Nucleic Acids Res. 2014;42(10):6208–6218. doi: 10.1093/nar/gku268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Klenov M.S., Sokolova O.A., Yakushev E.Y., Stolyarenko A.D., Mikhaleva E.A., Lavrov S.A. Separation of stem cell maintenance and transposon silencing functions of Piwi protein. Proc. Natl. Acad. Sci. USA. 2011;108(46):18760–18765. doi: 10.1073/pnas.1106676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rouget C., Papin C., Boureux A., Meunier A.-C., Franco B., Robine N. Maternal mRNA deadenylation and decay by the piRNA pathway in the early Drosophila embryo. Nature. 2010;467(7319):1128–1132. doi: 10.1038/nature09465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Czech B., Hannon G.J. One Loop to Rule Them All: the Ping-Pong Cycle and piRNA-Guided Silencing. Trends Biochem. Sci. 2016;41(4):324–337. doi: 10.1016/j.tibs.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Aravin A.A., Sachidanandam R., Bourc'his D., Schaefer C., Pezic D., Toth K.F. A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol. Cell. 2008;31(6):785–799. doi: 10.1016/j.molcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hayashi R., Schnabl J., Handler D., Mohn F., Ameres S.L., Brennecke J. Genetic and mechanistic diversity of piRNA 3'-end formation. Nature. 2016;539(7630):588–592. doi: 10.1038/nature20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ghildiyal M., Seitz H., Horwich M.D., Li C., Du T., Lee S. Endogenous siRNAs derived from transposons and mRNAs in drosophila somatic cells. Science. 2008;320(5879):1077–1081. doi: 10.1126/science.1157396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Cao M., Du P., Wang X., Yu Y.Q., Qiu Y.H., Li W. Virus infection triggers widespread silencing of host genes by a distinct class of endogenous siRNAs in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2014;111(40):14613–14618. doi: 10.1073/pnas.1407131111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Tam O.H., Aravin A.A., Stein P., Girard A., Murchison E.P., Cheloufi S. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature. 2008;453(7194):534–538. doi: 10.1038/nature06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Stein P., Rozhkov N.V., Li F., Cardenas F.L., Davydenko O., Vandivier L.E. Essential role for endogenous siRNAs during meiosis in mouse oocytes. PLoS Genet. 2015;11(2):e1005013. doi: 10.1371/journal.pgen.1005013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Piatek M.J., Werner A. Endogenous siRNAs, regulators of internal affairs. Biochem. Soc. Trans. 2014;42(4):1174–1179. doi: 10.1042/BST20140068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kim V.N., Han J., Siomi M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009;10(2):126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 243.Kim Y.K., Kim V.N. Processing of intronic microRNAs. EMBO J. 2007;26(3):775–783. doi: 10.1038/sj.emboj.7601512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Saini H.K., Griffiths-Jones S., Enright A.J. Genomic analysis of human microRNA transcripts. Proc. Natl. Acad. Sci. 2007;104(45):17719–17724. doi: 10.1073/pnas.0703890104. (2007 November 6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15(8):509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 246.Borchert G.M., Lanier W., Davidson B.L. RNA polymerase III transcribes human microRNAs. Nat. Struct. Mol. Biol. 2006;13(12):1097–1101. doi: 10.1038/nsmb1167. [DOI] [PubMed] [Google Scholar]
- 247.Sun H.L., Cui R., Zhou J., Teng K.Y., Hsiao Y.H., Nakanishi K. ERK activation globally downregulates miRNAs through phosphorylating exportin-5. Cancer Cell. 2016;30(5):723–736. doi: 10.1016/j.ccell.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Yi R., Doehle B.P., Qin Y., Macara I.G., Cullen B.R. Overexpression of exportin 5 enhances RNA interference mediated by short hairpin RNAs and microRNAs. RNA. 2005;11(2):220–226. doi: 10.1261/rna.7233305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rybak-Wolf A., Jens M., Murakawa Y., Herzog M., Landthaler M., Rajewsky N. A variety of dicer substrates in human and C. elegans. Cell. 2014;159(5):1153–1167. doi: 10.1016/j.cell.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 250.Khvorova A., Reynolds A., Jayasena S.D. Functional siRNAs and miRNAs exhibit strand bias. Cell. 2003;115(2):209–216. doi: 10.1016/s0092-8674(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 251.Wilson R.C., Tambe A., Kidwell M.A., Noland C.L., Schneider C.P., Doudna J.A. Dicer-TRBP complex formation ensures accurate mammalian microRNA biogenesis. Mol. Cell. 2015;57(3):397–407. doi: 10.1016/j.molcel.2014.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Shan S.W., Fang L., Shatseva T., Rutnam Z.J., Yang X., Du W. Mature miR-17-5p and passenger miR-17-3p induce hepatocellular carcinoma by targeting PTEN, GalNT7 and vimentin in different signal pathways. J. Cell Sci. 2013;126(6):1517–1530. doi: 10.1242/jcs.122895. [DOI] [PubMed] [Google Scholar]
- 253.Yang X., Du W.W., Li H., Liu F., Khorshidi A., Rutnam Z.J. Both mature miR-17-5p and passenger strand miR-17-3p target TIMP3 and induce prostate tumor growth and invasion. Nucleic Acids Res. 2013;41(21):9688–9704. doi: 10.1093/nar/gkt680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Krol J., Sobczak K., Wilczynska U., Drath M., Jasinska A., Kaczynska D. Structural features of microRNA (miRNA) precursors and their relevance to miRNA biogenesis and small interfering RNA/short hairpin RNA design. J. Biol. Chem. 2004;279(40):42230–42239. doi: 10.1074/jbc.M404931200. [DOI] [PubMed] [Google Scholar]
- 255.Schwarz D.S., Hutvagner G., Du T., Xu Z., Aronin N., Zamore P.D. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115(2):199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 256.Lin S.L., Chang D., Ying S.Y. Asymmetry of intronic pre-miRNA structures in functional RISC assembly. Gene. 2005;356:32–38. doi: 10.1016/j.gene.2005.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wu H., Ye C., Ramirez D., Manjunath N. Alternative processing of primary microRNA transcripts by Drosha generates 5' end variation of mature microRNA. PloS One. 2009;4(10):e7566. doi: 10.1371/journal.pone.0007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Starega-Roslan J., Witkos T.M., Galka-Marciniak P., Krzyzosiak W.J. Sequence features of Drosha and Dicer cleavage sites affect the complexity of isomiRs. Int. J. Mol. Sci. 2015;16(4):8110–8127. doi: 10.3390/ijms16048110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Starega-Roslan J., Krol J., Koscianska E., Kozlowski P., Szlachcic W.J., Sobczak K. Structural basis of microRNA length variety. Nucleic Acids Res. 2010:2010. doi: 10.1093/nar/gkq727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kim D., Sung Y.M., Park J., Kim S., Kim J., Park J. General rules for functional microRNA targeting. Nat. Genet. 2016;48(12):1517–1526. doi: 10.1038/ng.3694. [DOI] [PubMed] [Google Scholar]
- 261.Broughton J.P., Lovci M.T., Huang J.L., Yeo G.W., Pasquinelli A.E. Pairing beyond the seed supports MicroRNA targeting specificity. Mol. Cell. 2016;64(2):320–333. doi: 10.1016/j.molcel.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lewis B.P., Burge C.B., Bartel D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 263.Lal A., Navarro F., Maher C.A., Maliszewski L.E., Yan N., O'Day E. miR-24 Inhibits cell proliferation by targeting E2F2, MYC, and other cell-cycle genes via binding to "seedless" 3'UTR microRNA recognition elements. Mol. Cell. 2009;35(5):610–625. doi: 10.1016/j.molcel.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ouyang D., Ye Y., Guo D., Yu X., Chen J., Qi J. MicroRNA-125b-5p inhibits proliferation and promotes adipogenic differentiation in 3T3-L1 preadipocytes. Acta Biochim. Biophys. Sin. 2015;47(5):355–361. doi: 10.1093/abbs/gmv024. [DOI] [PubMed] [Google Scholar]
- 265.Lim L.P., Lau N.C., Garrett-Engele P., Grimson A., Schelter J.M., Castle J. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433(7027):769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 266.Sood P., Krek A., Zavolan M., Macino G., Rajewsky N. Cell-type-specific signatures of microRNAs on target mRNA expression. Proc. Natl. Acad. Sci. USA. 2006;103(8):2746–2751. doi: 10.1073/pnas.0511045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ludwig N., Leidinger P., Becker K., Backes C., Fehlmann T., Pallasch C. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016:2016. doi: 10.1093/nar/gkw116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Lagos-Quintana M., Rauhut R., Yalcin A., Meyer J., Lendeckel W., Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 2002;12(9):735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 269.Guo H., Ingolia N.T., Weissman J.S., Bartel D.P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466(7308):835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Stenvang J., Silahtaroglu A.N., Lindow M., Elmen J., Kauppinen S. The utility of LNA in microRNA-based cancer diagnostics and therapeutics. Semin. Cancer Biol. 2008;18(2):89–102. doi: 10.1016/j.semcancer.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 271.Mori Marcelo A., Raghavan P., Thomou T., Boucher J., Robida-Stubbs S., Macotela Y. Role of MicroRNA processing in adipose tissue in stress defense and longevity. Cell Metab. 2012;16(3):336–347. doi: 10.1016/j.cmet.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Isik M., Blackwell T.K., Berezikov E. MicroRNA mir-34 provides robustness to environmental stress response via the DAF-16 network in C. elegans. Sci. Rep. 2016;6:36766. doi: 10.1038/srep36766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ivey K.N., Srivastava D. MicroRNAs as regulators of differentiation and cell fate decisions. Cell. Stem Cell. 2010;7(1):36–41. doi: 10.1016/j.stem.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 274.Mori M.A., Thomou T., Boucher J., Lee K.Y., Lallukka S., Kim J.K. Altered miRNA processing disrupts brown/white adipocyte determination and associates with lipodystrophy. J. Clin. Investig. 2014;124(8):3339–3351. doi: 10.1172/JCI73468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Vienberg S., Geiger J., Madsen S., Dalgaard L.T. MicroRNAs in metabolism. Acta Physiol. 2017;219(2):346–361. doi: 10.1111/apha.12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.van Rooij E., Sutherland L.B., Liu N., Williams A.H., McAnally J., Gerard R.D. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc. Natl. Acad. Sci. USA. 2006;103(48):18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Jiang Q., Wang Y., Hao Y., Juan L., Teng M., Zhang X. miR2Disease: a manually curated database for microRNA deregulation in human disease. Nucleic Acids Res. 2009;37:D98–D104. doi: 10.1093/nar/gkn714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Suzuki H., Maruyama R., Yamamoto E., Kai M. Epigenetic alteration and microRNA dysregulation in cancer. Front. Genet. 2013;4(258) doi: 10.3389/fgene.2013.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Schanen B.C., Li X. Transcriptional regulation of mammalian miRNA genes. Genomics. 2011;97(1):1–6. doi: 10.1016/j.ygeno.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Christoffersen N.R., Shalgi R., Frankel L.B., Leucci E., Lees M., Klausen M. p53-independent upregulation of miR-34a during oncogene-induced senescence represses MYC. Cell Death Differ. 2009;17(2):236–245. doi: 10.1038/cdd.2009.109. [DOI] [PubMed] [Google Scholar]
- 281.Di Leva G., Gasparini P., Piovan C., Ngankeu A., Garofalo M., Taccioli C. MicroRNA cluster 221-222 and estrogen receptor α interactions in breast cancer. J. Natl. Cancer Inst. 2010;102(10):706–721. doi: 10.1093/jnci/djq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Mudhasani R., Imbalzano A.N., Jones S.N. An essential role for Dicer in adipocyte differentiation. J. Cell Biochem. 2010;110(4):812–816. doi: 10.1002/jcb.22625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Mudhasani R., Puri V., Hoover K., Czech M.P., Imbalzano A.N., Jones S.N. Dicer is required for the formation of white but not brown adipose tissue. J. Cell Physiol. 2011;226(5):1399–1406. doi: 10.1002/jcp.22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Fujimoto Y., Nakagawa Y., Shingyouchi A., Tokushige N., Nakanishi N., Satoh A. Dicer has a crucial role in the early stage of adipocyte differentiation, but not in lipid synthesis, in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2012;420(4):931–936. doi: 10.1016/j.bbrc.2012.03.110. [DOI] [PubMed] [Google Scholar]
- 285.Reis F.C., Branquinho J.L., Brandao B.B., Guerra B.A., Silva I.D., Frontini A. Fat-specific Dicer deficiency accelerates aging and mitigates several effects of dietary restriction in mice. Aging. 2016;8(6):1201–1222. doi: 10.18632/aging.100970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kim H.-J., Cho H., Alexander R., Patterson H.C., Gu M., Lo K.A. MicroRNAs are required for the feature maintenance and differentiation of brown adipocytes. Diabetes. 2014;63(12):4045–4056. doi: 10.2337/db14-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Arner P., Spalding K.L. Fat cell turnover in humans. Biochem. Biophys. Res. Commun. 2010;396(1):101–104. doi: 10.1016/j.bbrc.2010.02.165. [DOI] [PubMed] [Google Scholar]
- 288.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2004;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 289.Huang J.I., Zuk P.A., Jones N.F., Zhu M., Lorenz H.P., Hedrick M.H. Chondrogenic potential of multipotential cells from human adipose tissue. Plast. Reconstr. Surg. 2004;113(2):585–594. doi: 10.1097/01.PRS.0000101063.27008.E1. [DOI] [PubMed] [Google Scholar]
- 290.Heydarkhan-Hagvall S., Schenke-Layland K., Yang J.Q., Heydarkhan S., Xu Y., Zuk P.A. Human adipose stem cells: a potential cell source for cardiovascular tissue engineering. Cells Tissues Organs. 2008;187(4):263–274. doi: 10.1159/000113407. [DOI] [PubMed] [Google Scholar]
- 291.Cawthorn W.P., Scheller E.L., MacDougald O.A. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J. Lipid Res. 2012;53(2):227–246. doi: 10.1194/jlr.R021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Moseti D., Regassa A., Kim W.K. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int. J. Mol. Sci. 2016;17(1) doi: 10.3390/ijms17010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Rosen E.D., MacDougald O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006;7(12):885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 294.Cristancho A.G., Lazar M.A. Forming functional fat: a growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011;12(11):722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Huang J., Zhao L., Xing L., Chen D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells. 2010;28(2):357–364. doi: 10.1002/stem.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Zaragosi L.E., Wdziekonski B., Brigand K.L., Villageois P., Mari B., Waldmann R. Small RNA sequencing reveals miR-642a-3p as a novel adipocyte-specific microRNA and miR-30 as a key regulator of human adipogenesis. Genome Biol. 2011;12(7):R64. doi: 10.1186/gb-2011-12-7-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Li H., Li T., Wang S., Wei J., Fan J., Li J. miR-17-5p and miR-106a are involved in the balance between osteogenic and adipogenic differentiation of adipose-derived mesenchymal stem cells. Stem Cell Res. 2013;10(3):313–324. doi: 10.1016/j.scr.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 298.Kloting N., Berthold S., Kovacs P., Schon M.R., Fasshauer M., Ruschke K. MicroRNA expression in human omental and subcutaneous adipose tissue. PloS One. 2009;4(3):e4699. doi: 10.1371/journal.pone.0004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Arner P., Kulyte A. MicroRNA regulatory networks in human adipose tissue and obesity. Nat. Rev. Endocrinol. 2015;11(5):276–288. doi: 10.1038/nrendo.2015.25. [DOI] [PubMed] [Google Scholar]
- 300.Sun L., Xie H., Mori M.A., Alexander R., Yuan B., Hattangadi S.M. MiR-193b-365, a brown fat enriched microRNA cluster, is essential for brown fat differentiation. Nat. Cell Biol. 2011;13(8):958–965. doi: 10.1038/ncb2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Seale P., Kajimura S., Yang W., Chin S., Rohas L.M., Uldry M. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6(1):38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Meerson A., Traurig M., Ossowski V., Fleming J.M., Mullins M., Baier L.J. Human adipose microRNA-221 is upregulated in obesity and affects fat metabolism downstream of leptin and TNF-α. Diabetologia. 2013;56(9):1971–1979. doi: 10.1007/s00125-013-2950-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Arner E., Mejhert N., Kulyté A., Balwierz P.J., Pachkov M., Cormont M. Adipose tissue MicroRNAs as regulators of CCL2 production in human obesity. Diabetes. 2012;61(8):1986–1993. doi: 10.2337/db11-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kim Y.J., Hwang S.J., Bae Y.C., Jung J.S. MiR-21 regulates adipogenic differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells. 2009;27(12):3093–3102. doi: 10.1002/stem.235. [DOI] [PubMed] [Google Scholar]
- 305.Trajkovski M., Ahmed K., Esau C.C., Stoffel M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat. Cell Biol. 2012;14(12):1330–1335. doi: 10.1038/ncb2612. [DOI] [PubMed] [Google Scholar]
- 306.Nevins J.R. The Rb/E2F pathway and cancer. Hum. Mol. Genet. 2001;10(7):699–703. doi: 10.1093/hmg/10.7.699. (2001 April 1) [DOI] [PubMed] [Google Scholar]
- 307.Tang Q.Q., Otto T.C., Lane M.D. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc. Natl. Acad. Sci. USA. 2003;100(1):44–49. doi: 10.1073/pnas.0137044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Cho Y.C., Jefcoate C.R. PPARgamma1 synthesis and adipogenesis in C3H10T1/2 cells depends on S-phase progression, but does not require mitotic clonal expansion. J. Cell. Biochem. 2004;91(2):336–353. doi: 10.1002/jcb.10743. [DOI] [PubMed] [Google Scholar]
- 309.Kim S.J., Kim T., Choi H.N., Kim H.-W., Park J.B., Jeon B.H. TonEBP/NFAT5 inhibits adipocyte differentiation via modulation of mitotic clonal expansion during early phase of differentiation in 3T3-L1 cells. FASEB J. 2016;30(1):1292.8. [Google Scholar]
- 310.Wang Q., Li Y.C., Wang J., Kong J., Qi Y., Quigg R.J. miR-17-92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc. Natl. Acad. Sci. USA. 2008;105(8):2889–2894. doi: 10.1073/pnas.0800178105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Chen L., Cui J., Hou J., Long J., Li C., Liu L. A novel negative regulator of adipogenesis: microrna-363. Stem Cells. 2014;32(2):510–520. doi: 10.1002/stem.1549. [DOI] [PubMed] [Google Scholar]
- 312.Sun T., Fu M., Bookout A.L., Kliewer S.A., Mangelsdorf D.J. MicroRNA let-7 regulates 3T3-L1 adipogenesis . Mol. Endocrinol. 2009;23(6):925–931. doi: 10.1210/me.2008-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Qiu Z., Wei Y., Chen N., Jiang M., Wu J., Liao K. DNA synthesis and mitotic clonal expansion is not a required step for 3T3-L1 preadipocyte differentiation into adipocytes. J. Biol. Chem. 2001;276(15):11988–11995. doi: 10.1074/jbc.M011729200. [DOI] [PubMed] [Google Scholar]
- 314.Prusty D., Park B.-H., Davis K.E., Farmer S.R. Activation of MEK/ERK Signaling Promotes Adipogenesis by Enhancing Peroxisome Proliferator-activated Receptor γ (PPARγ) and C/EBPα Gene Expression during the Differentiation of 3T3-L1 Preadipocytes. J. Biol. Chem. 2002;277(48):46226–46232. doi: 10.1074/jbc.M207776200. [DOI] [PubMed] [Google Scholar]
- 315.Bost F., Aouadi M., Caron L., Binétruy B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie. 2005;87(1):51–56. doi: 10.1016/j.biochi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 316.Tseng Y.H., Butte A.J., Kokkotou E., Yechoor V.K., Taniguchi C.M., Kriauciunas K.M. Prediction of preadipocyte differentiation by gene expression reveals role of insulin receptor substrates and necdin. Nat. Cell Biol. 2005;7(6):601–611. doi: 10.1038/ncb1259. [DOI] [PubMed] [Google Scholar]
- 317.Ling H.Y., Wen G.B., Feng S.D., Tuo Q.H., Ou H.S., Yao C.H. MicroRNA-375 promotes 3T3-L1 adipocyte differentiation through modulation of extracellular signal-regulated kinase signalling. Clin. Exp. Pharmacol. Physiol. 2011;38(4):239–246. doi: 10.1111/j.1440-1681.2011.05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Roldan M., Macias-Gonzalez M., Garcia R., Tinahones F.J., Martin M. Obesity short-circuits stemness gene network in human adipose multipotent stem cells. Faseb J. 2011;25(12):4111–4126. doi: 10.1096/fj.10-171439. [DOI] [PubMed] [Google Scholar]
- 319.Chen Y.-H., Heneidi S., Lee J.-M., Layman L.C., Stepp D.W., Gamboa G.M. miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes. 2013;62(7):2278–2286. doi: 10.2337/db12-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Kornfeld J.-W., Baitzel C., Konner A.C., Nicholls H.T., Vogt M.C., Herrmanns K. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature. 2013;494(7435):111–115. doi: 10.1038/nature11793. [DOI] [PubMed] [Google Scholar]
- 321.Tang H., Lee M., Sharpe O., Salamone L., Noonan E.J., Hoang C.D. Oxidative stress-responsive microRNA-320 regulates glycolysis in diverse biological systems. FASEB J. 2012;26(11):4710–4721. doi: 10.1096/fj.11-197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Liu A.M., Xu Z., Shek F.H., Wong K.F., Lee N.P., Poon R.T. miR-122 targets pyruvate kinase M2 and affects metabolism of hepatocellular carcinoma. PloS One. 2014;9(1):e86872. doi: 10.1371/journal.pone.0086872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Vernochet C., Damilano F., Mourier A., Bezy O., Mori M.A., Smyth G. Adipose tissue mitochondrial dysfunction triggers a lipodystrophic syndrome with insulin resistance, hepatosteatosis, and cardiovascular complications. Faseb J. 2014;28(10):4408–4419. doi: 10.1096/fj.14-253971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Lin J., Handschin C., Spiegelman B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1(6):361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 325.Eichner L.J., Perry M.C., Dufour C.R., Bertos N., Park M., St-Pierre J. miR-378(*) mediates metabolic shift in breast cancer cells via the PGC-1beta/ERRgamma transcriptional pathway. Cell Metab. 2010;12(4):352–361. doi: 10.1016/j.cmet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 326.Carrer M., Liu N., Grueter C.E., Williams A.H., Frisard M.I., Hulver M.W. Control of mitochondrial metabolism and systemic energy homeostasis by microRNAs 378 and 378*. Proc. Natl. Acad. Sci. 2012;109(38):15330–15335. doi: 10.1073/pnas.1207605109. (2012 September 18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Luo L., Liu M. Adipose tissue in control of metabolism. J. Endocrinol. 2016;231(3) doi: 10.1530/JOE-16-0211. (R77-r99) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Cheng X., Xi Q.Y., Wei S., Wu D., Ye R.S., Chen T. Critical role of miR-125b in lipogenesis by targeting stearoyl-CoA desaturase-1 (SCD-1) J. Anim. Sci. 2016;94(1):65–76. doi: 10.2527/jas.2015-9456. [DOI] [PubMed] [Google Scholar]
- 329.Lin Y.Y., Chou C.F., Giovarelli M., Briata P., Gherzi R., Chen C.Y. KSRP and MicroRNA 145 are negative regulators of lipolysis in white adipose tissue. Mol. Cell. Biol. 2014;34(12):2339–2349. doi: 10.1128/MCB.00042-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Trabucchi M., Briata P., Garcia-Mayoral M., Haase A.D., Filipowicz W., Ramos A. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature. 2009;459(7249):1010–1014. doi: 10.1038/nature08025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Kurzynska-Kokorniak A., Koralewska N., Pokornowska M., Urbanowicz A., Tworak A., Mickiewicz A. The many faces of Dicer: the complexity of the mechanisms regulating Dicer gene expression and enzyme activities. Nucleic Acids Res. 2015:2015. doi: 10.1093/nar/gkv328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Chou C.-F., Lin Y.-Y., Wang H.-K., Zhu X., Giovarelli M., Briata P. KSRP Ablation enhances brown fat gene program in white adipose tissue through reduced miR-150 expression. Diabetes. 2014;63(9):2949–2961. doi: 10.2337/db13-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Belarbi Y., Mejhert N., Lorente-Cebrian S., Dahlman I., Arner P., Ryden M. MicroRNA-193b controls adiponectin production in human white adipose tissue. J. Clin. Endocrinol. Metab. 2015;100(8):E1084–E1088. doi: 10.1210/jc.2015-1530. [DOI] [PubMed] [Google Scholar]
- 334.Zhuang G., Meng C., Guo X., Cheruku P.S., Shi L., Xu H. A novel regulator of macrophage activation: mir-223 in obesity-associated adipose tissue inflammation. Circulation. 2012;125(23):2892–2903. doi: 10.1161/CIRCULATIONAHA.111.087817. [DOI] [PubMed] [Google Scholar]
- 335.Chuang T.Y., Wu H.L., Chen C.C., Gamboa G.M., Layman L.C., Diamond M.P. MicroRNA-223 expression is upregulated in insulin resistant human adipose tissue. J. Diabetes Res. 2015;2015:943659. doi: 10.1155/2015/943659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Hulsmans M., Van Dooren E., Mathieu C., Holvoet P. Decrease of miR-146b-5p in monocytes during obesity is associated with loss of the anti-inflammatory but not insulin signaling action of adiponectin. PloS One. 2012;7(2):e32794. doi: 10.1371/journal.pone.0032794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Chunmei S., Lijun Z., Xiaohui C., Nan G., Ling C., Lu Z. IL-6 and TNF-α induced obesity-related inflammatory response through transcriptional regulation of miR-146b. J. Interferon Cytokine Res. 2014;34(5):342–348. doi: 10.1089/jir.2013.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Huang R.-s., Hu G.-q., Lin B., Lin Z.-y., Sun C.-c. MicroRNA-155 silencing enhances inflammatory response and lipid uptake in oxidized low-density lipoprotein-stimulated human THP-1 macrophages. J. Investig. Med. 2010;58(8):961–967. doi: 10.231/JIM.0b013e3181ff46d7. [DOI] [PubMed] [Google Scholar]
- 339.Karkeni E., Astier J., Tourniaire F., Abed M.E., Romier B., Gouranton E. Obesity-associated inflammation induces microRNA-155 expression in adipocytes and adipose tissue: outcome on adipocyte function. J. Clin. Endocrinol. Metab. 2016;101(4):1615–1626. doi: 10.1210/jc.2015-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Estep M., Armistead D., Hossain N., Elarainy H., Goodman Z., Baranova A. Differential expression of miRNAs in the visceral adipose tissue of patients with non-alcoholic fatty liver disease. Aliment Pharm. Ther. 2010;32(3):487–497. doi: 10.1111/j.1365-2036.2010.04366.x. [DOI] [PubMed] [Google Scholar]
- 341.Karolina D.S., Armugam A., Tavintharan S., Wong M.T., Lim S.C., Sum C.F. MicroRNA 144 impairs insulin signaling by inhibiting the expression of insulin receptor substrate 1 in type 2 diabetes mellitus. PloS One. 2011;6(8):e22839. doi: 10.1371/journal.pone.0022839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Mi L., Chen Y., Zheng X., Li Y., Zhang Q., Mo D. MicroRNA-139-5p suppresses 3T3-L1 preadipocyte differentiation through notch and IRS1/PI3K/Akt insulin signaling pathways. J. Cell Biochem. 2015;116(7):1195–1204. doi: 10.1002/jcb.25065. [DOI] [PubMed] [Google Scholar]
- 343.Wu D., Xi Q.-Y., Cheng X., Dong T., Zhu X.-T., Shu G. miR-146a-5p inhibits TNF-α-induced adipogenesis via targeting insulin receptor in primary porcine adipocytes. J. Lipid Res. 2016;57(8):1360–1372. doi: 10.1194/jlr.M062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Ortega F.J., Moreno-Navarrete J.M., Pardo G., Sabater M., Hummel M., Ferrer A. MiRNA expression profile of human subcutaneous adipose and during adipocyte differentiation. PloS One. 2010;5(2):e9022. doi: 10.1371/journal.pone.0009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Fu T., Seok S., Choi S., Huang Z., Suino-Powell K., Xu H.E. MicroRNA 34a inhibits beige and brown fat formation in obesity in part by suppressing adipocyte fibroblast growth factor 21 signaling and SIRT1 function. Mol. Cell Biol. 2014;34(22):4130–4142. doi: 10.1128/MCB.00596-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Lavery C.A., Kurowska-Stolarska M., Holmes W.M., Donnelly I., Caslake M., Collier A. miR-34a−/− mice are susceptible to diet-induced obesity. Obesity. 2016;24(8):1741–1751. doi: 10.1002/oby.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Boon R.A., Vickers K.C. Intercellular transport of microRNAs. Arterioscler Thromb. Vasc. Biol. 2013;33(2):186–192. doi: 10.1161/ATVBAHA.112.300139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.T. Thomou, M. Mori, J. Dreyfuss, M. Konishi, M. Sakaguchi, C. Wolfrum, et al. Adipose-derived circulating mirnas regulate gene expression in other tissues. in press, 2017 (doi: 10.1038/nature21365). [DOI] [PMC free article] [PubMed]
- 349.Chen Y., Buyel J.J., Hanssen M.J.W., Siegel F., Pan R., Naumann J. Exosomal microRNA miR-92a concentration in serum reflects human brown fat activity. Nat. Commun. 2016;7:11420. doi: 10.1038/ncomms11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Hubal M.J., Nadler E.P., Ferrante S.C., Barberio M.D., Suh J.-H., Wang J. Circulating adipocyte-derived exosomal MicroRNAs associated with decreased insulin resistance after gastric bypass. Obesity. 2017;25(1):102–110. doi: 10.1002/oby.21709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Gaffo E., Zambonelli P., Bisognin A., Bortoluzzi S., Davoli R. miRNome of Italian large white pig subcutaneous fat tissue: new miRNAs, isomiRs and moRNAs. Anim. Genet. 2014;45(5):685–698. doi: 10.1111/age.12192. [DOI] [PubMed] [Google Scholar]
- 352.Brameier M., Herwig A., Reinhardt R., Walter L., Gruber J. Human box C/D snoRNAs with miRNA like functions: expanding the range of regulatory RNAs. Nucleic Acids Res. 2011;39(2):675–686. doi: 10.1093/nar/gkq776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Hokii Y., Sasano Y., Sato M., Sakamoto H., Sakata K., Shingai R. A small nucleolar RNA functions in rRNA processing in Caenorhabditis elegans. Nucleic Acids Res. 2010;38(17):5909–5918. doi: 10.1093/nar/gkq335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.van Steensel B. Chromatin: constructing the big picture. EMBO J. 2011;30(10):1885–1895. doi: 10.1038/emboj.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Wei W., Ba Z., Gao M., Wu Y., Ma Y., Amiard S. A role for small RNAs in DNA double-strand break repair. Cell. 2012;149(1):101–112. doi: 10.1016/j.cell.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 356.d’Adda di Fagagna F. A direct role for small non-coding RNAs in DNA damage response. Trends Cell Biol. 2014;24(3):171–178. doi: 10.1016/j.tcb.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 357.Sharma V., Misteli T. Non-coding RNAs in DNA damage and repair. FEBS Lett. 2013;587(13):1832–1839. doi: 10.1016/j.febslet.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Flemr M., Malik R., Franke V., Nejepinska J., Sedlacek R., Vlahovicek K. A retrotransposon-driven dicer isoform directs endogenous small interfering RNA production in mouse oocytes. Cell. 2013;155(4):807–816. doi: 10.1016/j.cell.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 359.Watanabe T., Totoki Y., Toyoda A., Kaneda M., Kuramochi-Miyagawa S., Obata Y. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature. 2008;453:539–543. doi: 10.1038/nature06908. [DOI] [PubMed] [Google Scholar]
- 360.Fire A., Xu S., Montgomery M.K., Kostas S.A., Driver S.E., Mello C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998:391. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 361.Zeng Y., Yi R., Cullen B.R. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc. Natl. Acad. Sci. 2003;100(17):9779–9784. doi: 10.1073/pnas.1630797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Reichow S.L., Hamma T., Ferré-D'Amaré A.R., Varani G. The structure and function of small nucleolar ribonucleoproteins. Nucleic Acids Res. 2007;35(5):1452–1464. doi: 10.1093/nar/gkl1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Kiss T. Small nucleolar RNA-guided post-transcriptional modification of cellular RNAs. EMBO J. 2001;20(14):3617–3622. doi: 10.1093/emboj/20.14.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Ender C., Krek A., Friedlander M.R., Beitzinger M., Weinmann L., Chen W. A human snoRNA with microRNA-like functions. Mol. Cell. 2008;32(4):519–528. doi: 10.1016/j.molcel.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 365.Wahid F., Shehzad A., Khan T., Kim Y.Y. MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim. Biophys. Acta – Mol. Cell Res. 2010;1803(11):1231–1243. doi: 10.1016/j.bbamcr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 367.Bortoluzzi S., Bisognin A., Biasiolo M., Guglielmelli P., Biamonte F., Norfo R. Characterization and discovery of novel miRNAs and moRNAs in JAK2V617F-mutated SET2 cells. Blood. 2012;119(13):e120–e130. doi: 10.1182/blood-2011-07-368001. [DOI] [PubMed] [Google Scholar]
- 368.Zhao J., Schnitzler G.R., Iyer L.K., Aronovitz M.J., Baur W.E., Karas R.H. MicroRNA-offset RNA alters gene expression and cell proliferation. PloS One. 2016;11(6):e0156772. doi: 10.1371/journal.pone.0156772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Taft R.J., Kaplan C.D., Simons C., Mattick J.S. Evolution, biogenesis and function of promoter-associated RNAs. Cell Cycle. 2009;8(15):2332–2338. doi: 10.4161/cc.8.15.9154. [DOI] [PubMed] [Google Scholar]
- 370.Fejes-Toth K., Sotirova V., Sachidanandam R., Assaf G., Hannon G.J., Kapranov A.P. Post-transcriptional processing generates a diversity of 5'-modified long and short RNAs. Nature. 2009;457(7232):1028–1032. doi: 10.1038/nature07759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Czech B., Hannon G.J. One loop to rule them all: the ping-pong Cycle and piRNA-guided silencing. Trends Biochem. Sci. 2016;41(4):324–337. doi: 10.1016/j.tibs.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Rigoutsos I., Huynh T., Miranda K., Tsirigos A., McHardy A., Platt D. Short blocks from the noncoding parts of the human genome have instances within nearly all known genes and relate to biological processes. Proc. Natl. Acad. Sci. 2006;103(17):6605–6610. doi: 10.1073/pnas.0601688103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Deryusheva S., Gall J.G. Small cajal body–specific RNAs of Drosophila function in the absence of cajal bodies. Mol. Biol. Cell. 2009;20(24):5250–5259. doi: 10.1091/mbc.E09-09-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Deryusheva S., Gall J.G. Novel small Cajal-body-specific RNAs identified in Drosophila: probing guide RNA function. RNA. 2013;19(12):1802–1814. doi: 10.1261/rna.042028.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Qureshi I.A., Mehler M.F. Emerging roles of non-coding RNAs in brain evolution, development, plasticity and disease. Nat. Rev. Neurosci. 2012;13(8):528–541. doi: 10.1038/nrn3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Kowalski M.P., Baylis H.A., Krude T. Non-coding stem-bulge RNAs are required for cell proliferation and embryonic development in C. elegans. J. Cell Sci. 2015;128(11):2118–2129. doi: 10.1242/jcs.166744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Boria I., Gruber A.R., Tanzer A., Bernhart S.H., Lorenz R., Mueller M.M. Nematode sbRNAs: homologs of vertebrate Y RNAs. J. Mol. Evol. 2010;70(4):346–358. doi: 10.1007/s00239-010-9332-4. [DOI] [PubMed] [Google Scholar]
- 379.Parrott A.M., Tsai M., Batchu P., Ryan K., Ozer H.L., Tian B. The evolution and expression of the snaR family of small non-coding RNAs. Nucleic Acids Res. 2011;39(4):1485–1500. doi: 10.1093/nar/gkq856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Parrott A.M., Mathews M.B. Novel rapidly evolving hominid RNAs bind nuclear factor 90 and display tissue-restricted distribution. Nucleic Acids Res. 2007;35(18):6249–6258. doi: 10.1093/nar/gkm668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Persson H., Kvist A., Vallon-Christersson J., Medstrand P., Borg A., Rovira C. The non-coding RNA of the multidrug resistance-linked vault particle encodes multiple regulatory small RNAs. Nat. Cell Biol. 2009;11(10):1268–1271. doi: 10.1038/ncb1972. [DOI] [PubMed] [Google Scholar]
- 382.Hussain S., Sajini Abdulrahim A., Blanco S., Dietmann S., Lombard P., Sugimoto Y. NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep. 2013;4(2):255–261. doi: 10.1016/j.celrep.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Cao F., Li X., Hiew S., Brady H., Liu Y., Dou Y. Dicer independent small RNAs associate with telomeric heterochromatin. RNA. 2009;15(7):1274–1281. doi: 10.1261/rna.1423309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Taft R.J., Glazov E.A., Cloonan N., Simons C., Stephen S., Faulkner G.J. Tiny RNAs associated with transcription start sites in animals. Nat. Genet. 2009;41(5):572–578. doi: 10.1038/ng.312. [DOI] [PubMed] [Google Scholar]
- 387.Van Goethem A., Yigit N., Everaert C., Moreno-Smith M., Mus L.M., Barbieri E. Depletion of tRNA-halves enables effective small RNA sequencing of low-input murine serum samples. Sci. Rep. 2016;6:37876. doi: 10.1038/srep37876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Nientiedt M., Deng M., Schmidt D., Perner S., Müller S.C., Ellinger J. Identification of aberrant tRNA-halves expression patterns in clear cell renal cell carcinoma. Sci. Rep. 2016;6:37158. doi: 10.1038/srep37158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Goodarzi H., Liu X., Nguyen H.C.B., Zhang S., Fish L., Tavazoie S.F. Endogenous tRNA-derived fragments suppress breast cancer progression via YBX1 displacement. Cell. 2015;161(4):790–802. doi: 10.1016/j.cell.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Pliatsika V., Loher P., Telonis A.G., Rigoutsos I. MINTbase: a framework for the interactive exploration of mitochondrial and nuclear tRNA fragments. Bioinformatics. 2016;32(16):2481–2489. doi: 10.1093/bioinformatics/btw194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Seila A.C., Calabrese J.M., Levine S.S., Yeo G.W., Rahl P.B., Flynn R.A. Divergent transcription from active promoters. Science. 2008;322(5909):1849–1851. doi: 10.1126/science.1162253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Li Z., Kim S.W., Lin Y., Moore P.S., Chang Y., John B. Characterization of viral and human RNAs smaller than canonical microRNAs. J. Virol. 2012;83(24) doi: 10.1128/JVI.01325-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Nicolas F.E., Hall A.E., Csorba T., Turnbull C., Dalmay T. Biogenesis of Y RNA-derived small RNAs is independent of the microRNA pathway. FEBS Lett. 2012;586(8):1226–1230. doi: 10.1016/j.febslet.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 394.Christov C.P., Gardiner T.J., Szuts D., Krude T. Functional requirement of noncoding Y RNAs for human chromosomal DNA replication. Mol. Cell. Biol. 2006;26(18):6993–7004. doi: 10.1128/MCB.01060-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Pek S.L., Sum C.F., Lin M.X., Cheng A.K., Wong M.T., Lim S.C. Circulating and visceral adipose miR-100 is down-regulated in patients with obesity and Type 2 diabetes. Mol. Cell Endocrinol. 2016;427:112–123. doi: 10.1016/j.mce.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 396.Xie H., Lim B., Lodish H.F. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes. 2009;58(5):1050–1057. doi: 10.2337/db08-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Qadir A.S., Woo K.M., Ryoo H.M., Baek J.H. Insulin suppresses distal-less homeobox 5 expression through the up-regulation of microRNA-124 in 3T3-L1 cells. Exp. Cell Res. 2013;319(14):2125–2134. doi: 10.1016/j.yexcr.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 398.Lee E.K., Lee M.J., Abdelmohsen K., Kim W., Kim M.M., Srikantan S. miR-130 suppresses adipogenesis by inhibiting peroxisome proliferator-activated receptor gamma expression. Mol. Cell Biol. 2011;31(4):626–638. doi: 10.1128/MCB.00894-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Yang Z., Bian C., Zhou H., Huang S., Wang S., Liao L. MicroRNA hsa-miR-138 inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells through adenovirus EID-1. Stem Cells Dev. 2011;20(2):259–267. doi: 10.1089/scd.2010.0072. [DOI] [PubMed] [Google Scholar]
- 400.Gernapudi R., Wolfson B., Zhang Y., Yao Y., Yang P., Asahara H. MicroRNA 140 promotes expression of long noncoding RNA NEAT1 in adipogenesis. Mol. Cell Biol. 2015;36(1):30–38. doi: 10.1128/MCB.00702-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Esau C., Kang X., Peralta E., Hanson E., Marcusson E.G., Ravichandran L.V. MicroRNA-143 regulates adipocyte differentiation. J. Biol. Chem. 2004;279(50):52361–52365. doi: 10.1074/jbc.C400438200. [DOI] [PubMed] [Google Scholar]
- 402.Takanabe R., Ono K., Abe Y., Takaya T., Horie T., Wada H. Up-regulated expression of microRNA-143 in association with obesity in adipose tissue of mice fed high-fat diet. Biochem. Biophys. Res. Commun. 2008;376(4):728–732. doi: 10.1016/j.bbrc.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 403.Guo Y., Chen Y., Zhang Y., Zhang Y., Chen L., Mo D. Up-regulated miR-145 expression inhibits porcine preadipocytes differentiation by targeting IRS1. Int. J. Biol. Sci. 2012;8(10):1408–1417. doi: 10.7150/ijbs.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Ahn J., Lee H., Jung C.H., Jeon T.I., Ha T.Y. MicroRNA‐146b promotes adipogenesis by suppressing the SIRT1‐FOXO1 cascade. EMBO Mol. Med. 2013;5(10):1602–1612. doi: 10.1002/emmm.201302647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.An X., Ma K., Zhang Z., Zhao T., Zhang X., Tang B. miR-17, miR-21, and miR-143 enhance adipogenic differentiation from porcine bone marrow-derived mesenchymal stem cells. DNA Cell Biol. 2016;35(8):410–416. doi: 10.1089/dna.2015.3182. [DOI] [PubMed] [Google Scholar]
- 406.Li H., Chen X., Guan L., Qi Q., Shu G., Jiang Q. MiRNA-181a regulates adipogenesis by targeting tumor necrosis factor-alpha (TNF-alpha) in the porcine model. PloS One. 2013;8(10):e71568. doi: 10.1371/journal.pone.0071568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Yun U.J., Song N.J., Yang D.K., Kwon S.M., Kim K., Kim S. miR-195a inhibits adipocyte differentiation by targeting the preadipogenic determinator Zfp423. J. Cell. Biochem. 2015;116(11):2589–2597. doi: 10.1002/jcb.25204. [DOI] [PubMed] [Google Scholar]
- 408.Qi R., Long D., Wang J., Wang Q., Huang X., Cao C. MicroRNA-199a Targets the fatty acid transport protein 1 gene and inhibits the adipogenic trans-differentiation of C2C12 myoblasts. Cell Physiol. Biochem. 2016;39(3):1087–1097. doi: 10.1159/000447817. [DOI] [PubMed] [Google Scholar]
- 409.He H., Chen K., Wang F., Zhao L., Wan X., Wang L. miR-204-5p promotes the adipogenic differentiation of human adipose-derived mesenchymal stem cells by modulating DVL3 expression and suppressing Wnt/beta-catenin signaling. Int. J. Mol. Med. 2015;35(6):1587–1595. doi: 10.3892/ijmm.2015.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Qin L., Chen Y., Niu Y., Chen W., Wang Q., Xiao S. A deep investigation into the adipogenesis mechanism: profile of microRNAs regulating adipogenesis by modulating the canonical Wnt/beta-catenin signaling pathway. BMC Genom. 2010;11:320. doi: 10.1186/1471-2164-11-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Xie X., Song J., Li G. MiR-21a-5p suppresses bisphenol A-induced pre-adipocyte differentiation by targeting map2k3 through MKK3/p38/MAPK. Biochem. Biophys. Res. Commun. 2016;473(1):140–146. doi: 10.1016/j.bbrc.2016.03.066. [DOI] [PubMed] [Google Scholar]
- 412.Peng Y., Xiang H., Chen C., Zheng R., Chai J., Peng J. MiR-224 impairs adipocyte early differentiation and regulates fatty acid metabolism. Int. J. Biochem. Cell Biol. 2013;45(8):1585–1593. doi: 10.1016/j.biocel.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 413.Huang Y., Huang J., Qi R., Wang Q., Wu Y., Wang J. Effects of MicroRNA-23a on differentiation and gene expression profiles in 3T3-L1 adipocytes. Genes. 2016;7(10) doi: 10.3390/genes7100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Jin M., Wu Y., Wang J., Chen J., Huang Y., Rao J. MicroRNA-24 promotes 3T3-L1 adipocyte differentiation by directly targeting the MAPK7 signaling. Biochem. Biophys. Res. Commun. 2016;474(1):76–82. doi: 10.1016/j.bbrc.2016.04.073. [DOI] [PubMed] [Google Scholar]
- 415.Lin Q., Gao Z., Alarcon R.M., Ye J., Yun Z. A role of miR-27 in the regulation of adipogenesis. FEBS J. 2009;276(8):2348–2358. doi: 10.1111/j.1742-4658.2009.06967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Kim S.Y., Kim A.Y., Lee H.W., Son Y.H., Lee G.Y., Lee J.W. miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARgamma expression. Biochem. Biophys. Res. Commun. 2010;392(3):323–328. doi: 10.1016/j.bbrc.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 417.Jeong B.C., Kang I.H., Koh J.T. MicroRNA-302a inhibits adipogenesis by suppressing peroxisome proliferator-activated receptor gamma expression. FEBS Lett. 2014;588(18):3427–3434. doi: 10.1016/j.febslet.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 418.Price N.L., Holtrup B., Kwei S.L., Wabitsch M., Rodeheffer M., Bianchini L. SREBP-1c/MicroRNA 33b genomic loci control adipocyte differentiation. Mol. Cell. Biol. 2016;36(7):1180–1193. doi: 10.1128/MCB.00745-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Bork S., Horn P., Castoldi M., Hellwig I., Ho A.D., Wagner W. Adipogenic differentiation of human mesenchymal stromal cells is down-regulated by microRNA-369-5p and up-regulated by microRNA-371. J. Cell. Physiol. 2011;226(9):2226–2234. doi: 10.1002/jcp.22557. [DOI] [PubMed] [Google Scholar]
- 420.Kinoshita M., Ono K., Horie T., Nagao K., Nishi H., Kuwabara Y. Regulation of adipocyte differentiation by activation of serotonin (5-HT) receptors 5-HT2AR and 5-HT2CR and involvement of microRNA-448-mediated repression of KLF5. Mol. Endocrinol. 2010;24(10):1978–1987. doi: 10.1210/me.2010-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Martinelli R., Nardelli C., Pilone V., Buonomo T., Liguori R., Castano I. miR-519d overexpression is associated with human obesity. Obesity. 2010;18(11):2170–2176. doi: 10.1038/oby.2009.474. [DOI] [PubMed] [Google Scholar]
- 422.Chen L., Chen Y., Zhang S., Ye L., Cui J., Sun Q. MiR-540 as a novel adipogenic inhibitor impairs adipogenesis via suppression of PPARgamma. J. Cell. Biochem. 2015;116(6):969–976. doi: 10.1002/jcb.25050. [DOI] [PubMed] [Google Scholar]
- 423.Rockstroh D., Loffler D., Kiess W., Landgraf K., Korner A. Regulation of human adipogenesis by miR125b-5p. Adipocyte. 2016;5(3):283–297. doi: 10.1080/21623945.2016.1195044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Pang L., You L., Ji C., Shi C., Chen L., Yang L. miR-1275 inhibits adipogenesis via ELK1 and its expression decreases in obese subjects. J. Mol. Endocrinol. 2016;57(1):33–43. doi: 10.1530/JME-16-0007. [DOI] [PubMed] [Google Scholar]
- 425.Shi C., Huang F., Gu X., Zhang M., Wen J., Wang X. Adipogenic miRNA and meta-signature miRNAs involved in human adipocyte differentiation and obesity. Oncotarget. 2016;7(26):40830–40845. doi: 10.18632/oncotarget.8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Wang L., Xu L., Xu M., Liu G., Xing J., Sun C. Obesity-associated MiR-342-3p promotes adipogenesis of mesenchymal stem cells by suppressing CtBP2 and releasing C/EBPalpha from CtBP2 binding. Cell Physiol. Biochem. 2015;35(6):2285–2298. doi: 10.1159/000374032. [DOI] [PubMed] [Google Scholar]
- 427.Li H., Xue M., Xu J., Qin X. MiR-301a is involved in adipocyte dysfunction during obesity-related inflammation via suppression of PPARgamma. Die Pharm. 2016;71(2):84–88. [PubMed] [Google Scholar]
- 428.Zhang W., Yao C., Wei Z., Dong Q. miR-128 promoted adipogenic differentiation and inhibited osteogenic differentiation of human mesenchymal stem cells by suppression of VEGF pathway. J. Recept. Signal Transduct. Res. 2016:1–7. doi: 10.1080/10799893.2016.1212375. [DOI] [PubMed] [Google Scholar]
- 429.Zhang X., Chang A., Li Y., Gao Y., Wang H., Ma Z. miR-140-5p regulates adipocyte differentiation by targeting transforming growth factor-beta signaling. Sci. Rep. 2015;5:18118. doi: 10.1038/srep18118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Li C.-J., Cheng P., Liang M.-K., Chen Y.-S., Lu Q., Wang J.-Y. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J. Clin. Investig. 2015;125(4):1509–1522. doi: 10.1172/JCI77716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Jeong B.C., Kang I.H., Hwang Y.C., Kim S.H., Koh J.T. MicroRNA-194 reciprocally stimulates osteogenesis and inhibits adipogenesis via regulating COUP-TFII expression. Cell Death Dis. 2014;5:e1532. doi: 10.1038/cddis.2014.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Huang S., Wang S., Bian C., Yang Z., Zhou H., Zeng Y. Upregulation of miR-22 promotes osteogenic differentiation and inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells by repressing HDAC6 protein expression. Stem Cells Dev. 2012;21(13):2531–2540. doi: 10.1089/scd.2012.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Guan X., Gao Y., Zhou J., Wang J., Zheng F., Guo F. miR-223 Regulates adipogenic and osteogenic differentiation of mesenchymal stem cells through a C/EBPs/miR-223/FGFR2 regulatory feedback loop. Stem Cells. 2015;33(5):1589–1600. doi: 10.1002/stem.1947. [DOI] [PubMed] [Google Scholar]
- 434.Liao L., Yang X., Su X., Hu C., Zhu X., Yang N. Redundant miR-3077-5p and miR-705 mediate the shift of mesenchymal stem cell lineage commitment to adipocyte in osteoporosis bone marrow. Cell Death Dis. 2013;4:e600. doi: 10.1038/cddis.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Zhang J.F., Fu W.M., He M.L., Wang H., Wang W.M., Yu S.C. MiR-637 maintains the balance between adipocytes and osteoblasts by directly targeting Osterix. Mol. Biol. Cell. 2011;22(21):3955–3961. doi: 10.1091/mbc.E11-04-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Du J., Cheng X., Shen L., Tan Z., Luo J., Wu X. Methylation of miR-145a-5p promoter mediates adipocytes differentiation. Biochem. Biophys. Res. Commun. 2016;475(1):140–148. doi: 10.1016/j.bbrc.2016.05.057. [DOI] [PubMed] [Google Scholar]
- 437.Chen L., Dai Y.M., Ji C.B., Yang L., Shi C.M., Xu G.F. MiR-146b is a regulator of human visceral preadipocyte proliferation and differentiation and its expression is altered in human obesity. Mol. Cell. Endocrinol. 2014;393(1–2):65–74. doi: 10.1016/j.mce.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 438.Mori M., Nakagami H., Rodriguez-Araujo G., Nimura K., Kaneda Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS Biol. 2012;10(4):e1001314. doi: 10.1371/journal.pbio.1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Ding H., Zheng S., Garcia-Ruiz D., Hou D., Wei Z., Liao Z. Fasting induces a subcutaneous-to-visceral fat switch mediated by microRNA-149-3p and suppression of PRDM16. Nat. Commun. 2016;7:11533. doi: 10.1038/ncomms11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Wu Y., Zuo J., Zhang Y., Xie Y., Hu F., Chen L. Identification of miR-106b-93 as a negative regulator of brown adipocyte differentiation. Biochem. Biophys. Res. Commun. 2013;438(4):575–580. doi: 10.1016/j.bbrc.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 441.Kim J., Okla M., Erickson A., Carr T., Natarajan S.K., Chung S. EPA potentiates brown thermogenesis through FFAR4-dependent upregulation of miR-30b and miR-378. J. Biol. Chem. 2016;291(39):20551–20562. doi: 10.1074/jbc.M116.721480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Chen Y., Siegel F., Kipschull S., Haas B., Fröhlich H., Meister G. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat. Commun. 2013;4:1769. doi: 10.1038/ncomms2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Sun L., Trajkovski M. MiR-27 orchestrates the transcriptional regulation of brown adipogenesis. Metab. Clin. Exp. 2014;63(2):272–282. doi: 10.1016/j.metabol.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 444.Zhang H., Guan M., Townsend K.L., Huang T.L., An D., Yan X. MicroRNA‐455 regulates brown adipogenesis via a novel HIF1an‐AMPK‐PGC1α signaling network. EMBO Rep. 2015;16(10):1378–1393. doi: 10.15252/embr.201540837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Mysore R., Zhou Y., Sadevirta S., Savolainen-Peltonen H., Nidhina Haridas P.A., Soronen J. MicroRNA-192* impairs adipocyte triglyceride storage. Biochim. Biophys. Acta. 2016;1861(4):342–351. doi: 10.1016/j.bbalip.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 446.Pan D., Mao C., Quattrochi B., Friedline R.H., Zhu L.J., Jung D.Y. MicroRNA-378 controls classical brown fat expansion to counteract obesity. Nat. Commun. 2014;5:4725. doi: 10.1038/ncomms5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Tsiloulis T., Pike J., Powell D., Rossello F.J., Canny B.J., Meex R.C. Impact of endurance exercise training on adipocyte microRNA expression in overweight men. FASEB J. 2017;31(1):161–171. doi: 10.1096/fj.201600678R. [DOI] [PubMed] [Google Scholar]
- 448.Das S.K., Stadelmeyer E., Schauer S., Schwarz A., Strohmaier H., Claudel T. Micro RNA-124a regulates lipolysis via adipose triglyceride lipase and comparative gene identification 58. Int. J. Mol. Sci. 2015;16(4):8555–8568. doi: 10.3390/ijms16048555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Zhang Y., Li C., Li H., Song Y., Zhao Y., Zhai L. miR-378 activates the pyruvate-PEP futile cycle and enhances lipolysis to ameliorate obesity in mice. EBioMedicine. 2016;5:93–104. doi: 10.1016/j.ebiom.2016.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Tao C., Ren H., Xu P., Cheng J., Huang S., Zhou R. Adipocyte miR-200b/a/429 ablation in mice leads to high-fat-diet-induced obesity. Oncotarget. 2016;7(42):67796–67807. doi: 10.18632/oncotarget.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Capobianco V., Nardelli C., Ferrigno M., Iaffaldano L., Pilone V., Forestieri P. miRNA and protein expression profiles of visceral adipose tissue reveal miR-141/YWHAG and miR-520e/RAB11A as two potential miRNA/protein target pairs associated with severe obesity. J. Proteome Res. 2012;11(6):3358–3369. doi: 10.1021/pr300152z. [DOI] [PubMed] [Google Scholar]
- 452.Shi C., Zhu L., Chen X., Gu N., Chen L., Zhu L. IL-6 and TNF-alpha induced obesity-related inflammatory response through transcriptional regulation of miR-146b. J. Interferon Cytokine Res. 2014;34(5):342–348. doi: 10.1089/jir.2013.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Ge Q., Gerard J., Noel L., Scroyen I., Brichard S.M. MicroRNAs regulated by adiponectin as novel targets for controlling adipose tissue inflammation. Endocrinology. 2012;153(11):5285–5296. doi: 10.1210/en.2012-1623. [DOI] [PubMed] [Google Scholar]
- 454.Xu G., Ji C., Song G., Zhao C., Shi C., Song L. MiR-26b modulates insulin sensitivity in adipocytes by interrupting the PTEN/PI3K/AKT pathway. Int. J. Obes. 2005;39(10):1523–1530. doi: 10.1038/ijo.2015.95. [DOI] [PubMed] [Google Scholar]
- 455.Ling H.Y., Ou H.S., Feng S.D., Zhang X.Y., Tuo Q.H., Chen L.X. Changes in microRNA (miR) profile and effects of miR-320 in insulin-resistant 3T3-L1 adipocytes. Clin. Exp. Pharmacol. Physiol. 2009;36(9):e32–e39. doi: 10.1111/j.1440-1681.2009.05207.x. [DOI] [PubMed] [Google Scholar]
- 456.Lorente-Cebrian S., Mejhert N., Kulyte A., Laurencikiene J., Astrom G., Heden P. MicroRNAs regulate human adipocyte lipolysis: effects of miR-145 are linked to TNF-alpha. PloS One. 2014;9(1):e86800. doi: 10.1371/journal.pone.0086800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Zhang X.M., Guo L., Huang X., Li Q.M., Chi M.H. 4-Hydroxynonenal regulates TNF-alpha gene transcription indirectly via ETS1 and microRNA-29b in human adipocytes induced from adipose tissue-derived stromal cells. Anat. Rec. 2016;299(8):1145–1152. doi: 10.1002/ar.23371. [DOI] [PubMed] [Google Scholar]