Abstract
Context
Prior studies have shown a cross-sectional association between body mass index (BMI) and salivary diurnal cortisol profile features (cortisol features); however, to our knowledge prior population-based studies have not examined the longitudinal association of body-mass index (BMI) with cortisol features.
Objective
To examine the association of (1) prior annual BMI percent change over 7 years with cortisol features, (2) baseline cortisol features with subsequent change in BMI over 6 years and (3) the association of change in cortisol features with change in BMI over 6 years.
Design
Longitudinal study
Setting
Multi-Ethnic Study of Atherosclerosis (MESA) Stress I & II Studies (2004-2006 & 2010-2012)
Participants
1,685 ethnically diverse men and women attended either MESA Stress exam (mean age 65 ± 10 years at MESA Stress I; mean age 69 ± 9 years at MESA Stress II).
Outcome Measures
Log-transformed cortisol features including wake-up cortisol, cortisol awakening response, early decline slope (30 minutes to 2 hours post-awakening), late decline slope (2 hours post-awakening to bedtime), bedtime, and total area under the curve (AUC) cortisol.
Results
Over 7 years, following multivariable adjustment, (1) a 1% higher prior annual BMI % increase was associated with a 2.9% (95% CI: −5.0%, −0.8%) and 3.0% (95% CI: −4.7%, −1.4%) lower current wake-up and total AUC cortisol, respectively; (2) there was no significant association between baseline cortisol features and subsequent change in BMI and (3) among participants with BMI ≥ 30 kg/m2, flattening of the late decline slope was associated with increases in BMI (every 1-unit increase late decline slope were associated with a 12.9% increase (95%CI: −1%, 26.8%) in BMI, respectively).
Conclusions
We found a significant association between prior annual BMI % change and cortisol features, but no significant association between baseline cortisol features and subsequent change in BMI. In participants with obesity increases in BMI were associated with less pronounced declined. Collectively, our results suggest that greater adiposity may lead to a blunted diurnal cortisol profile.
Keywords: Cortisol, Waist Circumference, Body mass index, Obesity, Adiposity, Hypothalamic-pituitary-adrenal axis
1. Introduction
The prevalence of obesity has been rising over the last twenty years and currently affects over 1 in 3 Americans [1]. Given the epidemic proportions of obesity and its involvement in many of the leading causes of death [2], it is essential to identify novel contributors to the underlying physiology of obesity. The role of dysregulated cortisol in obesity is one such novel mechanism. The HPA axis is a major component of the neuroendocrine system that controls the response to stress and contributes to the regulation of energy storage and expenditure. The terminal response of the HPA axis is production of cortisol from the adrenal gland with a classic circadian rhythm (diurnal cortisol profile): cortisol rapidly rising after awakening, reaching a peak after 30-45 minutes and then gradually declining over the course of the day [3,4].
Perturbations of the diurnal cortisol profile are cross-sectionally associated with obesity and conditions linked to obesity including insulin resistance, type 2 diabetes, metabolic syndrome and cardiovascular disease [5–7], as well as cardiovascular and all-cause mortality [8]. Cross-sectional associations of the diurnal cortisol profile with measures of obesity include: (1) higher BMI, WHR and WC are associated with lower wakeup and morning cortisol [9–15]; (2) CAR is positively associated with WHR [16] and WC [17]; and (3) higher WHR is associated with lower diurnal cortisol variability [18,19], and lower total AUC cortisol [15]. In MESA Stress I, BMI and WC are negatively correlated with wake-up cortisol and CAR AUC and positively correlated with the early decline slope (indicating a flatter slope) [20]. Despite the extensive cross-sectional literature on the HPA axis and obesity, longitudinal associations of diurnal cortisol curve features (Figure 1) with obesity are lacking limiting understanding of the directionality and temporality of these associations [6]. Thus, we used data from MESA Exams 1-5 and both MESA Stress exams to assess the temporality of the association between cortisol features and BMI/WC. Specifically, we investigated 3 aims: (1) the association of prior annual BMI and WC percent change with cortisol features over 7 years; (2) the association of baseline cortisol feature at MESA Stress I with subsequent change in BMI and WC over 6 years; (3) the association of changes in cortisol features with simultaneous changes in BMI and WC over 6 years.
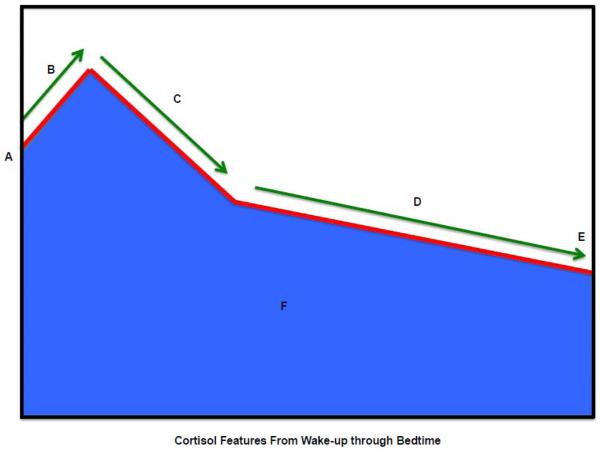
Figure 1.
Diurnal Cortisol Profile: Summary of diurnal cortisol features.
Key:
A. Wake-up cortisol (Time 0)
B. Cortisol awakening response (0 minutes to 30 minutes)
C. Early decline slope cortisol (30 minutes to 2 hours)
D. Late decline slope cortisol (2 hours to bedtime)
E. Bedtime cortisol
F. Total area under the curve (0 minutes to bedtime) cortisol
2. Methods
2.1 Study Population
MESA is a multi-center, longitudinal cohort study of the prevalence and correlates of subclinical cardiovascular disease and the factors influencing its progression [21]. Between July 2000 and August 2002, 6814 men and women, 45-84 years of age, without clinical cardiovascular disease who identified themselves as White, Black, Hispanic or Chinese, were recruited from six U.S. communities: Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; and St. Paul, Minnesota. Details on the sampling frames and the cohort examination procedures have been published previously[21]. The MESA Stress I Study collected detailed measures of stress hormones, including salivary cortisol measures between 2004 and 2006 (during period of MESA Exam 3 and Exam 4) at the New York and Los Angeles MESA study sites. The MESA Stress II Study collected similar data, on a subsample of 1,082 participants at the New York, Los Angeles and Baltimore MESA study sites between 2010-2012 during MESA Exam 5 (Supplementary Fig. A.1). Informed consent was obtained from each participant and the institutional review boards of all the participating institutions approved the study and consent procedures.
2.2 Hormonal Measures
In MESA Stress I, salivary cortisol measures were collected over 3 days with 6 time points measured per day. The first sample was taken immediately after waking (and before getting out of bed), the second sample 30 min later, the third sample at around 10:00 AM, the fourth sample at around noon (or before lunch if lunch occurred before noon), the fifth sample at around 6 PM (or before dinner if dinner occurred before 6 PM), and the sixth sample right before bedtime. In MESA Stress II, salivary cortisol measures were collected over 2 days and sample collection times corresponded to those in MESA Stress I, with the following exception—two additional samples were collected at 1 hour after breakfast and 1800h (8 time points) to better characterize the early and late decline slopes, respectively [22]. Participants were given similar instructions in MESA Stress I and II including not to eat or drink or brush their teeth 15 min before collecting the salivary samples. They were also instructed to leave the cotton swab in their mouths for less than 2 min until soaked, moving it around inside their mouth. In MESA Stress I, participants recorded collection time on special cards; in addition, a time-tracking device (Track Caps) automatically registered the time at which cotton swabs were extracted to collect each sample. The participants were informed of this time-tracking device.
From our work in MESA Stress I [22], we learned that we could adequately characterize the diurnal cortisol curve with 2 days of sample collection. Thus in MESA Stress II the third day was eliminated to reduce participant burden. In MESA Stress II, participants were instructed to record the exact time of sample collection on a special card, which was facilitated by a provided alarm clock. Saliva samples were stored at −20° C until analysis. Before biochemical analysis, samples were thawed and centrifuged at 3000 rpm for 3 minutes to obtain clear saliva with low viscosity. Cortisol levels were determined using a commercially available chemiluminescence assay with a high sensitivity of 0.16 ng/mL (IBL, Hamburg, Germany). Intra- and inter-assay coefficients of variation were less than 8%. As previously published, participants were generally compliant with sample collection times (Supplementary Methods A.1)[23].
2.3 Cortisol Features
We investigated six features of the diurnal cortisol profile: Wake-up cortisol levels, CAR, standardized total AUC, early decline slope, late decline slope and bedtime cortisol (Figure 1). Due to its skewed distribution, cortisol was log-transformed before the cortisol features were calculated [20,23,24]. Wake-up cortisol was defined as the salivary cortisol obtained at time 0. CAR was the cortisol rise from time 0 to 30 minutes post-awakening. Early decline in cortisol was defined as the decline in cortisol from 30 minutes post-awakening to 2 hours post-awakening. Late decline in cortisol was the decline in cortisol from 2 hours post-awakening to bedtime.
2.4 Assessment of waist circumference and body mass index
WC was measured at the minimum abdominal girth. Weight and height were measured using a balanced beam scale and a vertical ruler, respectively, with participants wearing light clothing and no shoes. Height was recorded to the nearest 0.5 cm and weight to the nearest 0.5 lb. BMI was calculated as weight (kg) divided by height squared (m2). For stratified analysis, BMI categories were defined by the World Health Organization classification as normal (<25 kg/m2), overweight (25-29.9 kg/m2) or obese (≥ 30 kg/m2) [25]. All anthropometric measures were taken in duplicate and averaged.
2.5 Assessment of Covariates
We adjusted for variables considered as potential confounders in the obesity-cortisol association. According to the different aims, the covariates were assessed at different time points. Aim 1 and Aim 2 the covariates were assessed at the corresponding MESA Stress Exam where cortisol features were ascertained; Aim 3: baseline covariates were measured at MESA Stress I and time-varying covariates were measured at MESA exams 3-5. Covariates such as age, sex, race/ethnicity and cigarette smoking, were self-reported using protocols as previously published [20,21]. Socioeconomic status was assessed by total gross family annual income ($) in the past 12 months prior to MESA Stress exam. Participants were categorized as current or not current smokers, as it has been shown that current smokers have higher cortisol levels than not current smokers, where there are no differences in cortisol values among ex-smokers or never smokers [26]. Over the counter and prescription medication history including use of beta blockers, hormone replacement therapy, aspirin and steroids was collected in each office visit [20,21]. Physical activity was obtained from an interviewer-administered questionnaire adapted from the Cross-Cultural Activity Participation Study [27,28] and measured as moderate-to-vigorous physical activity in metabolic equivalent minutes per week [29]. Depressive symptoms were assessed using the Center for Epidemiological Studies Depression (CES-D) scale as previously described [30]. Diabetes status was defined according to the 2003 American Diabetes Association criteria as fasting glucose ≥ 7.0 nmol/l (126 mg/dl) or use of hypoglycemic medication (oral agents and/or insulin), as previous described [23,31].
2.6 Statistical Analysis
2.6.1 Aim 1: Association of prior annual BMI and WC percent change in the prior 7 years with cortisol features
Aim 1 analyses included participants who attended either MESA Stress Exam (Supplementary Fig. A.1). 1002 participants were enrolled in MESA Stress I and 1082 participants enrolled in MESA Stress II. We first excluded samples with missing or invalid cortisol features (i.e., unreliable cortisol values of 0 or >100 nmol/L or missing sample collection time; N=119 at Stress I and N=72 at Stress II). We then excluded individuals missing BMI and WC at either visit (N=19 from Stress I and N=45 from Stress II) and missing data on important covariates including gross family annual income, beta blocker use, steroid use, hormone replacement therapy, CES-D, aspirin use, physical activity, current smoking status and diabetes status (N=35 from Stress I and N=37 from Stress II). We also excluded individuals using steroids (N=29 from Stress I and N=43 from Stress II). The final analytic dataset (N=1,685) included data from 800 and 885 participants from MESA Stress I and II, respectively, followed for an average of 6.8 years (SD 2.9), with 443 participants having data from both stress exams.
We calculated BMI and WC average annual change prior to each exam at which cortisol was assessed for each subject using the formula (BMI as example):
N = number of visits prior to Exam when cortisol was collected
rBMI = average annual % change
We used linear mixed effects models including an individual level random intercept to account for the within-person correlation in the samples from people who attended both MESA Stress exams. Separately for each cortisol feature, the model included the cortisol feature as outcome variable and the BMI average annual % change (calculated using the formula above) as exposure adjusting for an exam indicator for MESA Stress Exam (I or II) at which cortisol samples were collected, and sociodemographic and health-related factors. In addition, we adjusted for BMI at MESA Exam 1 as baseline BMI is a potential confounding variable [32,33]. Robust standard errors were reported.
We fit a sequence of multivariate adjustment models to examine the association of prior BMI rate of annual change (as exposure variable) with the current cortisol features (as outcome variable) as follows: Model 0 only includes exam indicator in the model unadjusted for any demographic or individual medical conditions; Model 1 further adjusted for BMI at MESA Exam 1; Model 2 further adjusted for demographic variables including age at MESA Stress exam, sex and race/ethnicity; Model 3 additionally adjusted for individual health conditions at the time which cortisol was assessed including CES-D, diabetes status, smoking status, physical activity and use of medications including beta-blockers and hormone replacement therapy.
2.6.2 Aim 2: Association of baseline cortisol feature at MESA Stress I with subsequent change in BMI and WC over 6 years
Aim 2 included participants who attended both MESA Stress I and either MESA Stress II or MESA Exam 5 and who had valid cortisol curve features at baseline, BMI/WC at baseline (MESA Exam 1) and MESA Exam 5 or MESA Stress II, and data on other demographic and health-related factors at baseline (MESA Exam 1) (Supplementary Fig. A.2). Of the 1002 participants enrolled in MESA Stress I, 810 of them also attended either MESA Stress II Exam or MESA Exam 5. We excluded participants with missing BMI and WC measures at baseline or MESA Stress II/MESA Exam 5 (N=1). We also excluded participants who did not have valid baseline diurnal cortisol features (N=84) or were missing data on important covariates (N=41), as described above in section 2.6.1; and were using steroids at baseline (N=21). After applying these exclusions, the final analytic dataset included 663 participants.
Fixed effects models [34] were used to estimate the association of time and time invariant predictors with within person changes in outcome (BMI/WC) over 6 years. Fixed effects models estimates the associations of within person changes in predictors with within person changes in outcomes, tightly controlling for all known and unknown individual-level time invariant confounders, which means the estimation on the within person outcome change (BMI/WC) over time is fully adjusted for individual characteristics at baseline. Main effects of time invariant predictors cannot be estimated. However, by including an interaction term between a time invariant exposure (e.g. baseline cortisol) with time (where the value is zero at MESA Stress I and years between the two studies at MESA Stress II) the fixed effects model allows estimation of the association of a time invariant exposure with within-person annual change in the outcome (i.e. whether the rate of change per year in BMI/WC differs by levels of the cortisol feature at baseline). To control for potential confounding of the temporal trends on the outcome (BMI/WC) due to individual characteristics at baseline, we included interactions of baseline covariates including demographics (age, sex, race/ethnicity, wealth index), medication use (beta-blocker, HRT, aspirin), health behavior (smoking, physical activity) and depressive symptoms score (CES-D) with the time (since baseline MESA Stress I). Log-transformed BMI/WC were used as outcome variables in the analysis in order to obtain estimates of the associations of cortisol with percent change in BMI/WC rather than the absolute change in BMI/WC. Sequential multivariable adjustment modeling was performed: Model 1 (unadjusted model) used BMI/WC at MESA Stress exam as outcome variables and included time (years since MESA Stress I) and the interaction term of baseline cortisol feature with time. Model 2 included Model 1 adjustments and the interaction terms of baseline demographic variables (age, race, sex, self-reported gross family annual income) with time. Model 3 included Model 2 adjustments and the interaction terms of baseline diabetes status, depression symptoms (CES-D), medications (beta-blocker, HRT, aspirin), and health behavior (current smoking status and physical activity) with time.
2.6.3 Aim 3: Association of change in cortisol feature with change in BMI and WC over 6 years
After applying the exclusion criteria outlined for Aim 2 (n=663), we also excluded participants who did not attend MESA Stress II or had invalid cortisol features at MESA Stress II (N=193), were missing data on key adjustment covariates (N=13), and were using steroids at MESA Stress II (N=12) (Supplementary Fig. A.2). The final sample for this analysis included 445 participants.
Fixed effects model [34] were also used in this aim to estimate the association of within-person change in outcome (BMI/WC) with within-person change in exposure (cortisol curve feature) over time. The model is similar to the one used in Aim 2 except that it includes a time-varying exposure (cortisol curve feature) instead of the baseline cortisol feature. Since the change in outcome by the change in exposure can be confounded by other time-varying factors, we adjusted for time (where the value is zero at MESA Stress I and years between the two studies at MESA Stress II) and its interaction with demographic variables (age, race, sex) (to account for BMI/WC change due to aging), as well as time varying CES-D, diabetes, smoking status, beta-blocker use, hormone replacement therapy (HRT) use, aspirin use, and physical activity at each MESA Stress exam. Log-transformed BMI/WC were also used as the outcome variables in the analysis to obtain the estimates of associations of changes in cortisol with percent changes in in BMI/WC.
Sequential multivariable adjustment modeling was performed: Model 1 (unadjusted) used BMI/WC (log-transformed) at MESA Stress Exam as outcome variables and included time (years since MESA Stress I) and the time varying cortisol curve feature. Model 2 included Model 1 adjustments and the interaction terms for baseline demographic variables (age, race, sex) with time and the time-varying measures of self-reported annual gross family income. Model 3 included Model 2 adjustments plus adjustments for time-varying diabetes status, depression symptoms (CES-D), medication use (beta-blocker, HRT, aspirin), and health behaviors (current smoking status and physical activity). We performed sensitivity analysis stratifying the results by baseline WHO BMI classifications (BMI < 25 (normal), 25-29.9 (overweight) and ≥ 30 kg/m2 (obese)) to test for effect modification by baseline BMI status [14]. Analyses were performed using SAS version 9.3 software.
3. Results
The 1685 participants in Aim 1 were racially/ethnically diverse: African American (Stress 1 [S1]: 26.9%; Stress 2 [S2]: 32.2%), Hispanic (S1: 53.4%; S2: 40.6%), and non-Hispanic white (S1: 19.8%; S2: 27.2%); they were approximately evenly distributed across sexes (S1: 52.0% women and 48.0% men; S2: 54.7% women and 45.3% men). The average age in Stress 1 was: 65.2 ± 9.8 years and in Stress 2 was 69.4 ± 8.9 years (Table 1). We further compared the baseline (Stress 1) characteristics of samples included and excluded in Aim 1 analyses. In general, the excluded sample was older with more African Americans and participants with lower income and in worse health (Supplementary Table A.1). The descriptive data for the 663 and 445 participants included in Aims 2 and 3, respectively, are found in Supplementary Table A.2.
Table 1.
Characteristics of participants at MESA Stress I (2004-2006) and MESA Stress II (2010-2012)
| MESA Stress I Included = 800 / 1002 |
MESA Stress II Included = 885 / 1082 |
||||
|---|---|---|---|---|---|
| Continuous | Mean | STD | Mean | STD | |
| Age (years) | 65.2 | 9.8 | 69.4 | 8.9 | |
| CES-D scorea | 8.56 | 8.62 | 8.50 | 7.85 | |
| Gross family annual income (US Dollars) | 41363 | 31188 | 49019 | 33713 | |
| Physical Activity (MET min/week) | 4918 | 4395 | 5320 | 6907 | |
| Body-mass index (kg/m2) | 29.0 | 5.6 | 29.4 | 5.4 | |
| Waist Circumference (cm) | 100.1 | 14.6 | 100.9 | 13.9 | |
| Categorical | N | % | N | % | |
| Age (years) | 45-54 | 141 | 17.6 | 7 | 0.8 |
| 55-64 | 247 | 30.9 | 288 | 32.5 | |
| 65-74 | 266 | 33.3 | 317 | 35.8 | |
| 75-84 | 129 | 16.1 | 227 | 25.6 | |
| ≥85 | 17 | 2.1 | 46 | 5.2 | |
| Cancer – n (%) | No | 720 | 90.0 | 762 | 86.1 |
| Yes | 80 | 10.0 | 123 | 13.9 | |
| Hormone replacement therapy – n (%) |
No | 767 | 95.9 | 862 | 97.4 |
| Yes | 33 | 4.1 | 23 | 2.6 | |
| Aspirin therapy – n (%) | No | 546 | 68.3 | 485 | 54.8 |
| Yes | 254 | 31.8 | 400 | 45.2 | |
| Beta-blocker – n (%) | No | 677 | 84.6 | 714 | 80.7 |
| Yes | 123 | 15.4 | 171 | 19.3 | |
| Diabetes – n (%) | No | 669 | 83.6 | 709 | 80.1 |
| Yes | 131 | 16.4 | 176 | 19.9 | |
| Sex – n (%) | Female | 416 | 52.0 | 484 | 54.7 |
| Male | 384 | 48.0 | 401 | 45.3 | |
| Race/Ethnicity a | non- Hispanic white |
158 | 19.8 | 241 | 27.2 |
| African- American |
215 | 26.9 | 285 | 32.2 | |
| Hispanic American |
427 | 53.4 | 359 | 40.6 | |
| Current Smoking – n (%) | No | 731 | 91.4 | 832 | 94.0 |
| Yes | 69 | 8.6 | 53 | 6.0 | |
| Steroid use – n (%) | No | 800 | 100.0 | 885 | 100.0 |
Center for Epidemiologic Studies Depression Scale
Race/ethnicity was self-reported.
3.1 Association of 7 years of prior annual BMI and WC percent change with cortisol features
The estimated difference in cortisol features associated with prior annual change in BMI is shown in Table 2. Significant associations of BMI prior annual change were found with total AUC cortisol and wakeup cortisol in all adjusted models. The fully adjusted model (Model 3) revealed a 3.0% lower total AUC cortisol (95% CI: −0.047, −0.014) and 2.9% lower wakeup cortisol (95% CI: −0.05, −0.008) per 1-percent higher prior BMI annual % change. Bedtime cortisol was significantly associated with prior BMI annual % change in models adjusted for baseline BMI at MESA exam 1, but was non-significant after adjustment for demographic or health factors. Similar results were seen for WC (−2.4%, 95% CI: (−0.039, −0.009) for total AUC cortisol and −2.0%, 95% CI: (−0.037, −0.003) for wakeup cortisol), except that bedtime cortisol remained significant in fully adjusted models with a 2.8% lower bedtime cortisol (95% CI: −0.052, −0.003) per 1-percent increase in prior WC annual % change (Figure 2).
Table 2.
Associations of prior changes in body-mass index (BMI) and waist circumference (WC) with cortisol features
| Difference in cortisol feature associated with a 1 percent higher annual % change in BMI prior to the cortisol measurement | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 0 | Model 1 | Model 2 | Model 3 | ||||||||||
| Parameter | Feature | EST.a | 95% CI | p- value |
EST. | 95% CI | p- value |
EST. | 95% CI | p- value |
EST. | 95% CI | p- value |
| BMI | Total AUC (16- hours) cortisolb |
−0.035 | (−0.052,−0.018) | 0.000 | −0.038 | (−0.055,−0.021) | 0.000 | −0.029 | (−0.046,−0.013) | 0.001 | −0.030 | (−0.047, −0.014) | 0.000 |
| Bedtime cortisol | −0.041 | (−0.074,−0.009) | 0.013 | −0.042 | (−0.074,−0.009) | 0.012 | −0.027 | (−0.058,0.004) | 0.084 | −0.028 | (−0.059, 0.003) | 0.078 | |
| Cortisol awakening response |
−0.006 | (−0.026,0.014) | 0.556 | −0.006 | (−0.026,0.014) | 0.555 | −0.009 | (−0.029,0.011) | 0.390 | −0.009 | (−0.028, 0.011) | 0.396 | |
| Early decline slope | −0.010 | (−0.025,0.005) | 0.182 | −0.008 | (−0.023,0.007) | 0.297 | −0.006 | (−0.021,0.009) | 0.457 | −0.006 | (−0.021, 0.009) | 0.400 | |
| Late decline slope | 0.002 | (−0.001,0.004) | 0.220 | 0.002 | (−0.001,0.004) | 0.195 | 0.002 | (0,0.005) | 0.092 | 0.002 | (0, 0.005) | 0.089 | |
| Wakeup cortisol | −0.028 | (−0.049,−0.007) | 0.009 | −0.035 | (−0.056,−0.013) | 0.002 | −0.028 | (−0.05,−0.007) | 0.009 | −0.029 | (−0.05,−0.008) | 0.007 | |
| Difference in cortisol feature associated with a 1 percent higher annual % change in WC prior to the cortisol measurement | |||||||||||||
| Waist Circumference |
Total AUC (16- hours) cortisolb |
−0.028 | (−0.043,−0.012) | 0.000 | −0.030 | (−0.045,−0.014) | 0.000 | −0.022 | (−0.037,−0.008) | 0.003 | −0.024 | (−0.039,−0.009) | 0.002 |
| Bedtime cortisol | −0.037 | (−0.063,−0.011) | 0.006 | −0.035 | (−0.061,−0.009) | 0.008 | −0.026 | (−0.051,−0.002) | 0.034 | −0.028 | (−0.052,−0.003) | 0.027 | |
| Cortisol awakening response |
0.002 | (−0.018,0.023) | 0.842 | 0.000 | (−0.02,0.02) | 0.997 | −0.001 | (−0.022,0.019) | 0.908 | −0.001 | (−0.021, 0.02) | 0.948 | |
| Early decline slope | −0.011 | (−0.026,0.003) | 0.133 | −0.005 | (−0.019, 0.008) | 0.439 | −0.004 | (−0.019,0.01) | 0.563 | −0.006 | (−0.021, 0.008) | 0.390 | |
| Late decline slope | 0.000 | (−0.002,0.002) | 0.974 | 0.000 | (−0.002, 0.002) | 0.857 | 0.000 | (−0.002,0.003) | 0.715 | 0.000 | (−0.002, 0.003) | 0.683 | |
| Wakeup cortisol | −0.017 | (−0.035,0.001) | 0.068 | −0.025 | (−0.043,−0.007) | 0.006 | −0.019 | (−0.036,−0.002) | 0.029 | −0.020 | (−0.037,−0.003) | 0.024 | |
Models:
Model 0 (unadjusted model): cortisol feature at stress exam as outcome variables and include BMI prior annual change (%) and MESA Stress study indicator as covariates.
Model 1 (adjust for BMI at MESA Exam 1): In addition to Model 0, include BMI at MESA Exam 1.
Model 2 (adjust for demographics): in addition to Model 1, include demographic variables (Age, Race, Sex) and family gross annual income at stress exam when cortisol feature was measured.
Model 3 (fully adjusted model): in addition to Model 2, include diabetes, depression symptom (Center for Epidemiologic Studies Depression Scale), medications (beta-blocker, hormone replacement therapy, aspirin), and health behavior (current smoking status, physical activity) at stress exam when cortisol feature was measured.
The estimates in cortisol features are based on log-transformed cortisol values; therefore, they can be back-transformed by exponentiation to obtain an estimate of the percent change in the feature associated with 1 percent higher in BMI prior annual change. For example, Model 3 suggests that by 1 percent higher in BMI prior annual change, the change in wakeup (based on log-transformed cortisol value) is −0.029 which can be interpreted as exp(−0.029) −1= −2.9% lower in wakeup (in the original cortisol scale) associated with 1 percent higher in BMI prior annual change. Positive values for decline slopes indicate flatter slopes, whereas positive values for the cortisol awakening response indicate a steeper slope.
AUC = area under the curve
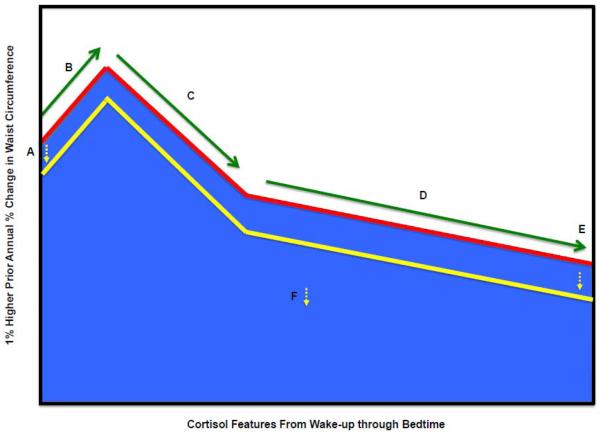
Figure 2.
Associations of 7 years of prior percent change in waist circumference with cortisol features
Key:
A. Wake-up cortisol (Time 0)
B. Cortisol awakening response (0 minutes to 30 minutes)
C. Early decline slope cortisol (30 minutes to 2 hours)
D. Late decline slope cortisol (2 hours to bedtime)
E. Bedtime cortisol
F. Total area under the curve (0 minutes to bedtime) cortisol
Red Line - Reference Diurnal Cortisol Profile
Yellow Line – Change in diurnal cortisol profile per 1% higher prior annual waist circumference change (significant features denoted by yellow dashed arrow)
Fully adjusted for cortisol feature at stress exam as outcome variables and include waist circumference prior annual percent change and MESA Stress study indicator as covariates, waist circumference at MESA Exam 1, demographic variables (Age, Race, Sex) and Socioeconomic Status (gross family annual income) at stress exam when cortisol feature was measured, diabetes, depression symptoms (Center for Epidemiologic Studies Depression), medications (beta-blocker, hormone replacement therapy, aspirin), and health behaviors (current smoking status, physical activity) at stress exam when cortisol feature was measured.
3.2 Association of baseline cortisol feature at MESA Stress I with subsequent change in BMI and WC over 6 years
We found no association of baseline cortisol features with subsequent change in BMI or WC over 6 years (Table 3; Figure 3). Stratifying the results by baseline BMI classifications (BMI < 25, 25-29.9 and ≥ 30 kg/m2) did not alter the findings.
Table 3.
Associations of baseline cortisol features with subsequent changes in body-mass index (BMI) or waist circumference (WC) over 6 years.
| Mean differences in subsequent annual BMI change (%) associated with a 1 unit higher cortisol feature value at baseline | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||||
| Feature | % Diff | 95% CI | p-value | % Diff | 95% CI | p-value | % Diff | 95% CI | p- value |
| Total AUC (16-hours) cortisola | −0.061 | (−0.3,0.1) | 0.57 | 0.083 | (−0.1,0.3) | 0.44 | 0.072 | (−0.1,0.3) | 0.51 |
| Bedtime cortisol | −0.133 | (−0.3,0) | 0.03 | −0.030 | (−0.2,0.1) | 0.64 | −0.037 | (−0.2,0.1) | 0.56 |
| Cortisol awakening response | 0.167 | (0,0.3) | 0.06 | 0.149 | (0,0.3) | 0.09 | 0.138 | (0,0.3) | 0.12 |
| Early decline slope | −0.172 | (−0.4,0) | 0.13 | −0.161 | (−0.4,0.1) | 0.15 | −0.155 | (−0.4,0.1) | 0.17 |
| Late decline slope | −0.330 | (−1.7,1.1) | 0.65 | −0.085 | (−1.5,1.3) | 0.90 | −0.170 | (−1.6,1.2) | 0.81 |
| Wakeup cortisol | −0.024 | (−0.2,0.1) | 0.77 | 0.053 | (−0.1,0.2) | 0.52 | 0.057 | (−0.1,0.2) | 0.49 |
| Mean differences in subsequent annual WC change (%) associated with a 1 unit higher cortisol feature value at baseline | |||||||||
| Model 1 | Model 2 | Model 3 | |||||||
| Feature | % Diff | 95% CI | p-value | % Diff | 95% CI | p-value | % Diff | 95% CI |
p-
value |
| Total AUC (16-hours) cortisola | 0.06 | (−0.2,0.3) | 0.60 | 0.10 | (−0.1,0.3) | 0.38 | 0.09 | (−0.1,0.3) | 0.43 |
| Bedtime cortisol | −0.06 | (−0.2,0.1) | 0.30 | −0.02 | (−0.1,0.1) | 0.74 | −0.03 | (−0.2,0.1) | 0.66 |
| Cortisol awakening response | 0.11 | (−0.1,0.3) | 0.20 | 0.11 | (−0.1,0.3) | 0.21 | 0.10 | (−0.1,0.3) | 0.24 |
| Early decline slope | −0.09 | (−0.3,0.1) | 0.44 | −0.11 | (−0.3,0.1) | 0.34 | −0.10 | (−0.3,0.1) | 0.37 |
| Late decline slope | 0.12 | (−1.3,1.5) | 0.87 | 0.13 | (−1.3,1.5) | 0.86 | 0.07 | (−1.3,1.5) | 0.93 |
| Wakeup cortisol | 0.02 | (−0.1,0.2) | 0.84 | 0.06 | (−0.1,0.2) | 0.49 | 0.06 | (−0.1,0.2) | 0.47 |
Models:
Model 1 (unadjusted model): BMI/WC at stress exam as outcome variables and include time (years since MESA Stress I) and the interaction term of baseline cortisol feature with time.
Model 2 (adjust for demographics): in addition to Model 1, include the interaction terms of baseline demographic variables (Age, Race, Sex, SES (gross family annual income)) with time.
Model 3 (fully adjusted model): in addition to Model 2, include the interaction terms of baseline diabetes, depression symptom (Center for Epidemiologic Studies Depression Scale), medications (beta-blocker, hormone replacement therapy, aspirin), and health behavior (current smoking status and physical activity) with time.
AUC = area under the curve
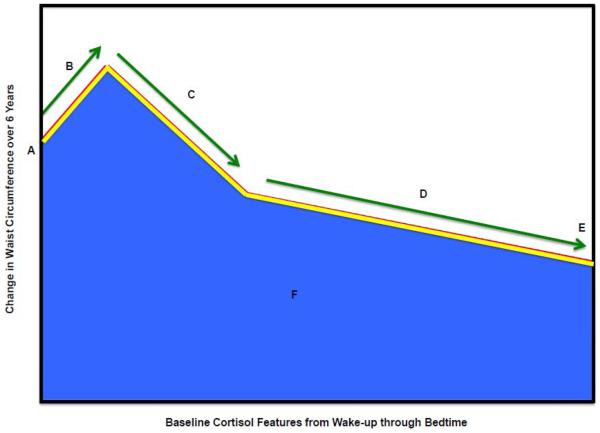
Figure 3.
Associations of baseline cortisol features with subsequent changes in waist circumference over 6 years.
Key:
A. Wake-up cortisol (Time 0)
B. Cortisol awakening response (0 minutes to 30 minutes)
C. Early decline slope cortisol (30 minutes to 2 hours)
D. Late decline slope cortisol (2 hours to bedtime)
E. Bedtime cortisol
F. Total AUC (0 minutes to bedtime) cortisol
Red Line – Reference Diurnal Cortisol Profile
Yellow Line – Change in diurnal cortisol profile per 1% higher prior annual waist circumference change (significant features denoted by yellow dashed arrow)
Adjusted for waist circumference at stress exam as outcome variables and includes time (years since MESA Stress I) and the interaction term of baseline cortisol feature with time, the interaction terms of baseline demographic variables (Age, Race, Sex, Socioeconomic Status (gross family annual income)) with time and the interaction terms of baseline diabetes, depression symptom (Center for Epidemiologic Studies Depression), medications (beta-blocker, hormone replacement therapy, aspirin), and health behavior (current smoking status and physical activity) with time.
3.3 Association of change in cortisol feature with change in BMI and WC over 6 years
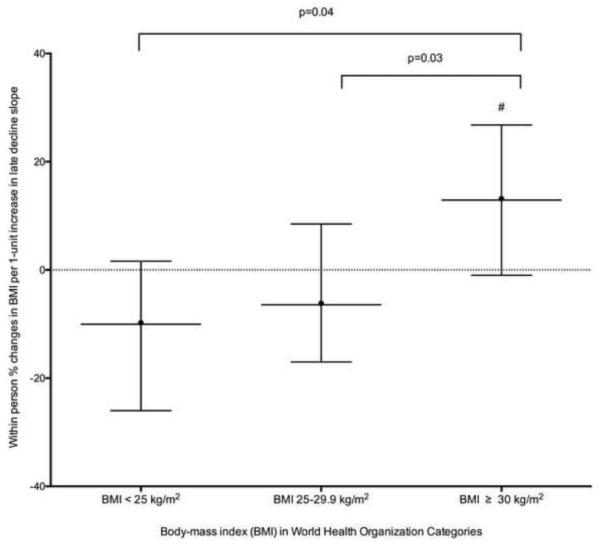
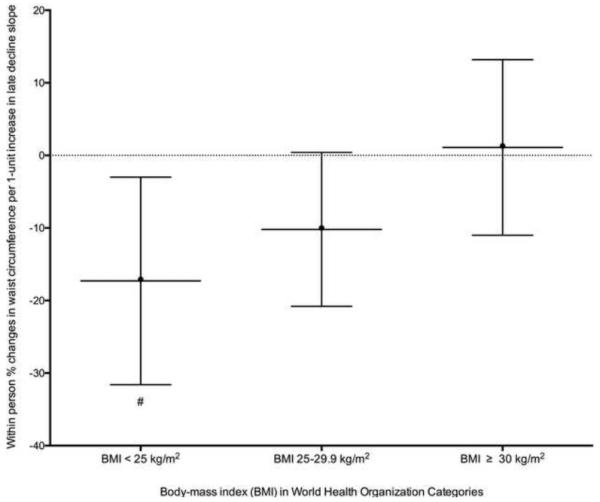
We did not find significant associations of change in any cortisol features with the change in BMI in analyses including participants throughout the full spectrum of BMI (Table 4). Stratification by WHO BMI classifications (BMI < 25, 25-29.9 and ≥ 30 kg/m2) revealed a positive association of a 1-unit increase in late decline slope with an increase in BMI over 6 years, whereas among participants with BMI 25-29.9 and < 25 kg/m2, every 1-unit increase in late decline slope was associated with a decrease in BMI with significant differences between the BMI categories (p<0.05) (Table 5; Figure 4A).
Table 4.
Associations of within person percent changes in body-mass index (BMI) and waist circumference (WC) with a 1-unit within person increase in cortisol features
| % Change in BMI associated with a 1 unit increase in cortisol features over time | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||||
| Feature | % Change | 95% CI | p-value | % Change | 95% CI | p-value | % Change | 95% CI | p-value |
| Total AUC (16-hours) cortisola | −0.088 | (−1,0.8) | 0.85 | −0.117 | (−1,0.8) | 0.80 | −0.069 | (−1,0.8) | 0.88 |
| Bedtime cortisol | 0.070 | (−0.6,0.7) | 0.83 | −0.006 | (−0.6,0.6) | 0.98 | 0.008 | (−0.6,0.6) | 0.98 |
| Cortisol awakening response | −0.193 | (−0.9,0.5) | 0.58 | −0.154 | (−0.8,0.5) | 0.65 | −0.063 | (−0.7,0.6) | 0.86 |
| Early decline slope | 0.090 | (−0.9,1.1) | 0.85 | −0.031 | (−1,0.9) | 0.95 | −0.186 | (−1.1,0.8) | 0.70 |
| Late decline slope | 0.190 | (−7.5,7.8) | 0.96 | 0.056 | (−7.4,7.5) | 0.99 | 0.111 | (−7.4,7.6) | 0.98 |
| Wakeup cortisol | −0.058 | (−0.7,0.6) | 0.86 | −0.025 | (−0.7,0.6) | 0.94 | −0.028 | (−0.7,0.6) | 0.93 |
| % Change in WC associated with a 1 unit increase in cortisol features over time | |||||||||
| Model 1 | Model 2 | Model 3 | |||||||
| Feature | % Change | 95% CI | p-value | % Change | 95% CI | p-value | % Change | 95% CI | p-value |
| Total AUC (16-hours) cortisola | 0.49 | (−0.4,1.4) | 0.29 | 0.54 | (−0.3,1.4) | 0.22 | 0.60 | (−0.3,1.5) | 0.18 |
| Bedtime cortisol | 0.27 | (−0.4,0.9) | 0.40 | 0.20 | (−0.4,0.8) | 0.53 | 0.21 | (−0.4,0.8) | 0.49 |
| Cortisol awakening response | 0.29 | (−0.4,1) | 0.40 | 0.32 | (−0.3,1) | 0.35 | 0.42 | (−0.2,1.1) | 0.21 |
| Early decline slope | 0.45 | (−0.5,1.4) | 0.36 | 0.28 | (−0.7,1.2) | 0.55 | 0.18 | (−0.8,1.1) | 0.71 |
| Late decline slope | −5.69 | (−12.8,1.4) | 0.13 | −7.20 | (−14,−0.4) | 0.046 | −7.31 | (−14.1,−0.5) | 0.043 |
| Wakeup cortisol | −0.13 | (−0.8,0.5) | 0.68 | 0.03 | (−0.6,0.6) | 0.94 | 0.00 | (−0.6,0.6) | 0.99 |
Models:
Model 1 (unadjusted model): BMI/WC at stress exam as outcome variables and include time (years between two stress studies) and the time varying cortisol feature
Model 2 (adjust for demographics): in addition to Model 1, include the interaction terms of baseline demographic variables (Age, Race, Sex) with time, and the time varying SES (gross family annual income).
Model 3 (fully adjusted model): in addition to Model 2, include other time-varying factors including diabetes, depression symptom (Center for Epidemiologic Studies Depression Scale), medications (beta-blocker, hormone replacement therapy, aspirin), and health behavior (current smoking status, physical activity).
AUC = area under the curve
Table 5.
Associations of within person percent changes in body mass index (BMI) and waist circumference (WC) with a 1-unit within person increase in cortisol features stratified by baseline World Health Organization BMI status
| Effect Modification by Baseline BMI on change BMI and change in late decline slope over 6 years* | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |||||||
| % Diff | 95% CI | p-value | % Diff | 95% CI | p-value | % Diff | 95% CI | p-value | |
| Association of late decline slope with BMI (BMI < 25 kg/m2) |
−10.868 | (−25.7,4) | 0.1771 | −8.695 | (−23.9,6.5) | 0.2838 | −10.043 | (−26.1,6) | 0.2451 |
| Association of late decline slope with BMI (BMI 25-29.9 kg/m2) |
−7.687 | (−18.9,3.5) | 0.1979 | −7.921 | (−18.9,3.1) | 0.1776 | −6.438 | (−17.8,5) | 0.2853 |
| Association of late decline slope with BMI (BMI ≥ 30 kg/m2) |
15.109 | (1.3,28.9) | 0.0222 | 13.942 | (0.2,27.7) | 0.0346 | 12.902 | (−1,26.8) | 0.0540 |
| Difference in association of late decline slope with BMI (Overweight vs. Normal) |
0.7394 | 0.9356 | 0.7215 | ||||||
| Difference in association of late decline slope with BMI (Obese vs. Normal) |
0.0152 | 0.0351 | 0.0404 | ||||||
| Difference in association of late decline slope with BMI (Obese vs. Overweight) |
0.0118 | 0.0144 | 0.0341 | ||||||
| Effect Modification by Baseline BMI on change WC and change in late decline slope over 6 years | |||||||||
| Model 1 | Model 2 | Model 3 | |||||||
| % Diff | 95% CI | p-value | % Diff | 95% CI | p-value | % Diff | 95% CI | p-value | |
| Association of late decline slope with WC (BMI < 25 kg/m2) |
−14.992 | (−29,−1) | 0.0535 | −14.189 | (−28.1,−0.3) | 0.0654 | −17.287 | (−31.6,−3) | 0.0321 |
| Association of late decline slope with WC (BMI 25-29.9 kg/m2) |
−9.669 | (−20.5,1.2) | 0.0971 | −11.942 | (−22.2,−1.6) | 0.0337 | −10.204 | (−20.8,0.4) | 0.0754 |
| Association of late decline slope with WC (BMI ≥ 30 kg/m2) |
3.313 | (−8.9,15.6) | 0.5901 | 1.488 | (−10.5,13.5) | 0.8061 | 1.084 | (−11,13.2) | 0.8596 |
| Difference in association of late decline slope with WC (Overweight vs. Normal) |
0.5589 | 0.8003 | 0.4427 | ||||||
| Difference in association of late decline slope with WC (Obese vs. Normal) |
0.0600 | 0.1019 | 0.0622 | ||||||
| Difference in association of late decline slope with WC (Obese vs. Overweight) |
0.1191 | 0.0947 | 0.1682 | ||||||
Model 1 (unadjusted) used BMI/WC (log-transformed) at MESA Stress Exam as outcome variables and included time (years since MESA Stress I) and the time varying cortisol curve feature.
Model 2 included Model 1 adjustments and the interaction terms for baseline demographic variables (age, race, sex) with time and the time-varying measures of self-reported annual gross family income.
Model 3 included Model 2 adjustments plus adjustments for time-varying diabetes status, depression symptoms (Center for Epidemiologic Studies Depression Scale), medication use (beta-blocker, hormone replacement therapy, aspirin), and health behaviors (current smoking status and physical activity).
Figure 4A.
Associations of within person percent changes in body mass index with a 1-unit within person increase in the late decline slope over 6 years stratified by baseline World Health Organization body mass index category.
Fully Adjusted for body mass index (log-transformed) at MESA Stress Exam as outcome variables and included time (years since MESA Stress I) and the time varying cortisol curve feature, the interaction terms for baseline demographic variables (age, race, sex) with time and the time-varying measures of self-reported annual gross family income, time-varying diabetes status, depression symptoms (Center for Epidemiologic Studies Depression), medication use (beta-blocker, hormone replacement therapy, aspirin), and health behaviors (current smoking status and physical activity).
# p=0.05
We found a significant association of change in late decline slope with change in WC in the fully adjusted model. A one-unit increase in the late decline slope was associated with a 7.3% lower WC (95% CI: −14.1%, −0.5%) (Table 4). Stratifying the results by WHO BMI classifications (BMI < 25, 25-29.9 and ≥ 30 kg/m2) revealed that findings were driven primarily by participants with a BMI < 25 kg/m2 (Table 5; Figure 4B).
Figure 4B.
Associations of within person percent changes in waist circumference with a 1-unit within person increase in the late decline slope over 6 years stratified by baseline World Health Organization body mass index category
Fully Adjusted for waist circumference (log-transformed) at MESA Stress Exam as outcome variables and included time (years since MESA Stress I) and the time varying cortisol curve feature, the interaction terms for baseline demographic variables (age, race, sex) with time and the time-varying measures of self-reported annual gross family income, time-varying diabetes status, depression symptoms (Center for Epidemiologic Studies Depression), medication use (beta-blocker, hormone replacement therapy, aspirin), and health behaviors (current smoking status and physical activity).
4. Discussion
The MESA stress study is one of few population-based cohort studies with well-characterized, rigorously collected longitudinal data on diurnal cortisol curve characteristics and BMI/WC . This enabled us to examine longitudinal associations of various cortisol features with BMI/WC, revealing a novel association of prior annual BMI and WC percent change with cortisol curve features.
4.1 Association of 7 years of prior annual BMI and WC percent change with cortisol curve features
In this first report of the relation of prior annual BMI and WC percent change with the full diurnal cortisol curve in a multi-ethnic cohort, we found an inverse association between wake-up cortisol, bedtime cortisol and total cortisol AUC with prior annual BMI and WC percent change.
The inverse association between wake-up cortisol and prior annual BMI and WC percent change is consistent with prior cross-sectional studies showing an inverse association of WHR, WC and BMI with wake-up and morning cortisol levels [9,10,12–14,20]. The inverse association of bedtime salivary cortisol with prior annual BMI and WC percent change is consistent with cross-sectional cohort studies suggesting lower bedtime cortisol with obesity [20,35], but inconsistent with the hypothesis that the diurnal rhythm of plasma cortisol is lost in obesity such that plasma cortisol is higher than in lean subjects during the evening [9,18,36].
The inverse association of total AUC cortisol with prior annual BMI and WC is consistent with the cross-sectional inverse associations between CAR AUC and BMI/WC in MESA Stress I [20] and inverse associations between BMI 12 years prior and WHR at time of collection with total cortisol levels in the 1958 British birth cohort follow-up study [15]. Previous cross-sectional analyses of cardiometabolic processes linked to obesity have also shown an inverse association with total AUC cortisol including insulin resistance [23], metabolic syndrome [37] and men with diabetes [38].
4.2 Association of change in cortisol feature with change in BMI and WC over 6 years
The novel analysis of the longitudinal association of change in diurnal cortisol features with change in BMI and WC over 6 years, was divergent by WHO BMI classifications. Participants with normal BMI had decreases in BMI/WC with flattening (more positive) of the late decline slope, whereas participants with an obese BMI had increases in BMI with flattening of the late decline slope.
The increasing late decline slope (flattening) association with increasing BMI over 6 years among obese participants is consistent with prior cross-sectional large cohort studies showing a flattening of the diurnal cortisol profile with obesity [14,20]. The decrease in WC with increasing late decline slope among participants with normal BMI has two potential explanations. First, a study by Rosmond and Bjorntorp [39] found that the flattest (i.e. most adverse) salivary cortisol slopes were significantly associated with BMI extremes (>31 and <21) and second, given that we are assessing middle-aged individuals it could be that those with BMI < 25 kg/m2 had a normal BMI at study entry due to other pre-existing conditions including anorexia, cancer, etc., which would be associated with a flatter slope [6].
4.3 Mechanisms of the Association of Generalized and Abdominal Obesity with the Diurnal Cortisol Profile
Our data suggests that generalized obesity (BMI) and abdominal obesity (WC) may drive changes in diurnal cortisol curve features. We showed that prior change in BMI/WC was significantly associated with multiple cortisol curve features, but did not find any associations between baseline cortisol curve features and subsequent change in BMI or WC. Given the association of change in cortisol features with change in BMI/WC over time in those with obesity at baseline, we suspect that generalized obesity may perpetuate HPA axis dysfunction over time with an overall flattening of the diurnal cortisol profile as suggested by the flatter late decline slope in this study. There is one prior study of the longitudinal association of morning serum cortisol (two serum cortisol measures collected 30-minutes apart within 4 hours of waking, and pooled in equal aliquots to smooth episodic secretion) and measures of obesity among older non-Hispanic white (96%) men [11]. They noted inverse cross-sectional associations of serum cortisol with BMI and an inverse association of change in cortisol with BMI over 7 years and no association of baseline cortisol with change in BMI over 7 years [11]. These results are strikingly similar to our results and further suggest that the temporality favors obesity and adiposity leading to HPA axis dysfunction.
The underlying mechanisms potentially explaining the association of increasing BMI and WC with lower wakeup, bedtime and total AUC cortisol, may be related to differences between whole-body HPA axis cortisol metabolism and adipocyte cortisol metabolism. Incollingo Rodriguez et al [6] noted that hypocortisolism in whole-body cortisol metabolism in obesity may result from simultaneous hyperactivity in the adipocyte metabolic system and might identify dysregulation at the level of the adipocyte. Adipocytes synthesize cortisol from conversion of cortisone to cortisol via 11β-HSD1 and this is important for intracellular pre-receptor metabolism. Studies in rodent models of obesity have confirmed increased 11β-HSD1 activity and mRNA levels in adipocytes and decreased 11β-HSD1 activity in the liver [6]. In studies of overexpression of 11β-HSD1 in rodent adipose tissue, non-stressed serum corticosterone was lower with overexpression of 11β-HSD1 but adipocyte levels of corticosterone were elevated in the 11β-HSD1 overexpression mice vs. wild type [40]. In humans, 11β-HSD1 is increased in abdominal obesity and generalized obesity ranging from 2-13 fold higher and increases with higher BMI [6]. These studies suggest that adipose tissue 11β-HSD1 may be a significant contributor to the relationship between abdominal obesity, general cortisol activity and adipocyte cortisol metabolism and this may explain the association of lower morning, total cortisol AUC and bedtime salivary cortisol with greater prior increases in BMI and WC in our study.
4.4 Translational Implications
Our study reveals that increasing BMI over time was generally associated with a more blunted diurnal cortisol profile. Our observational findings suggest that prevention of weight gain and/or weight loss might restore the normal diurnal cortisol profile. Data from 3 prior weight loss studies, using surgical and non-surgical methods, resulted in changes to the diurnal cortisol profile shifting it toward more normal dynamics (e.g. higher awakening and 30 minute cortisol) and decreased overall HPA axis drive [41–43]. Given that the blunted cortisol diurnal profile has been associated with other metabolic (e.g. diabetes) and depressive disorders [7], preventing weight gain-associated alternations in the HPA axis may minimize the impact of the diurnal cortisol profile on the development of metabolic and depressive disorders.
4.5 Future Directions
Obesity has been classically defined as a state of psuedo-cushing’s or subclinical hypercortisolism, consisting of the clinical features of Cushing’s syndrome combined with laboratory evidence of hypercortisolism, caused by an underlying primary condition not related to the HPA axis including stress both from illness or emotional disturbances, depression, intense aerobic exercise, or caloric restriction [44–47]. This has been recently challenged by Abraham et al [48], who found no association of urinary free cortisol or dexamethasone suppression testing among overweight or obese versus normal BMI participants in a clinical study nor in a literature review. Our current understanding of the role of cortisol in obesity physiology is limited by the lack of simultaneous measurement of diurnal cortisol profiles together with serum, urine, and hair cortisol, glucocorticoid receptor status, and 11-beta hydroxylase activity. Including these measures together in observational studies and randomized clinical trials, would allow for a comprehensive assessment, interpretation and understanding of the HPA axis activity and its association with obesity.
4.6 Strengths/Weaknesses
Our study has several strengths. First, MESA is a large population-based multi-ethnic cohort. Second, we used standardized measurements of weight, height and WC and did not rely on self-report. Third, salivary cortisol collection occurred over multiple days in MESA Stress I and II, allowing a more accurate determination of each participant’s diurnal cortisol pattern. Fourth, we were able to adjust for potential confounders including, smoking, diabetes, medications and depressive symptoms.
Our study should be considered with some limitations. First, the participants were middle to older age at baseline and the relationship between cortisol curve characteristics and BMI/WC may differ in younger individuals. Second, we did not have data to more objectively assess visceral fat via abdominal imaging. Third, we were unable to assess adipocyte cortisol metabolism, which may have a significant effect on generalized and abdominal obesity not reflected by saliva levels. Fourth, the statistical associations were interpreted without correction for multiple comparisons. Typical multiple comparison corrections assume tests are independent, and are too conservative for correlated hypotheses as we have here. Hence, some caution is warranted in the interpretation of our study results.
5. Conclusion
In summary, this study provides insight into the temporal relations between diurnal cortisol curve features and BMI/WC suggesting that increases in BMI/WC may drive the associations with the diurnal cortisol profile. Given the global obesity epidemic, further longitudinal exploration of the role of abdominal and generalized obesity with the HPA axis and adipocyte cortisol metabolism remains a significant area for further research to understand the physiology of obesity and develop future pharmacological interventions.
Supplementary Material
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding
This research was supported by contracts HHSN268201500003I, N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR. MESA Stress Study was supported by RO1 HL10161-01A1 and R21 DA024273 (PI: Dr. Diez Roux). Dr. Joshua J. Joseph MD was supported by an institutional training grant from the National Institute of Diabetes, Digestive, and Kidney Diseases (T32 DK062707).
Abbreviations
- 11β-HSD1
11 beta-hydroxysteroid dehydrogenase type 1
- AUC
area under the curve
- BMI
body-mass index
- CAR
cortisol awakening response
- HRT
hormone replacement therapy
- HPA
hypothalamic-pituitary-adrenal
- MESA
Multi-Ethnic Study of Atherosclerosis
- WC
waist circumference
- WHR
waist-to-hip ratio
Footnotes
Disclosure Statement
The authors have no relevant conflicts of interest to disclose.
Author Contributions
All authors contributed to the design and conduct of the study, data collection and analysis, data interpretation, manuscript writing and gave final approval of the version to be published.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of Childhood and Adult Obesity in the United States, 2011-2012. JAMA. 2014;311:806. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22:150–69. doi: 10.1159/000118611. doi:118611. [DOI] [PubMed] [Google Scholar]
- [4].Golden SH, Sánchez BN, DeSantis AS, Wu M, Castro C, Seeman TE, et al. Salivary cortisol protocol adherence and reliability by socio-demographic features: The Multi-Ethnic Study of Atherosclerosis. Psychoneuroendocrinology. 2014;43:30–40. doi: 10.1016/j.psyneuen.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. The Pathogenetic Role of Cortisol in the Metabolic Syndrome: A Hypothesis. J Clin Endocrinol Metab. 2009;94:2692–701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- [6].Incollingo Rodriguez AC, Epel ES, White ML, Standen EC, Seckl JR, Tomiyama AJ. Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: A systematic review. Psychoneuroendocrinology. 2015;62:301–18. doi: 10.1016/j.psyneuen.2015.08.014. [DOI] [PubMed] [Google Scholar]
- [7].Joseph JJ, Golden SH. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus: Role of cortisol in stress, depression, and diabetes. Ann N Y Acad Sci. 2016 doi: 10.1111/nyas.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kumari M, Shipley M, Stafford M, Kivimaki M. Association of Diurnal Patterns in Salivary Cortisol with All-Cause and Cardiovascular Mortality: Findings from the Whitehall II Study. J Clin Endocrinol Metab. 2011;96:1478–85. doi: 10.1210/jc.2010-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Walker BR, Soderberg S, Lindahl B, Olsson T. Independent effects of obesity and cortisol in predicting cardiovascular risk factors in men and women. J Intern Med. 2000;247:198–204. doi: 10.1046/j.1365-2796.2000.00609.x. [DOI] [PubMed] [Google Scholar]
- [10].Duclos M, Pereira PM, Barat P, Gatta B, Roger P. Increased cortisol bioavailability, abdominal obesity, and the metabolic syndrome in obese women. Obes Res. 2005;13:1157–1166. doi: 10.1038/oby.2005.137. [DOI] [PubMed] [Google Scholar]
- [11].Travison TG, O’Donnell AB, Araujo AB, Matsumoto AM, McKinlay JB. Cortisol levels and measures of body composition in middle-aged and older men. Clin Endocrinol (Oxf) 2007;67:71–7. doi: 10.1111/j.1365-2265.2007.02837.x. [DOI] [PubMed] [Google Scholar]
- [12].Ward A, Fall CH, Stein CE, Kumaran K, Veena SR, Wood PJ, et al. Cortisol and the metabolic syndrome in South Asians. Clin Endocrinol (Oxf) 2003;58:500–505. doi: 10.1046/j.1365-2265.2003.01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Boyle SH, Surwit RS, Georgiades A, Brummett BH, Helms MJ, Williams RB, et al. Depressive Symptoms, Race, and Glucose Concentrations: The role of cortisol as mediator. Diabetes Care. 2007;30:2484–8. doi: 10.2337/dc07-0258. [DOI] [PubMed] [Google Scholar]
- [14].Kumari M, Chandola T, Brunner E, Kivimaki M. A Nonlinear Relationship of Generalized and Central Obesity with Diurnal Cortisol Secretion in the Whitehall II Study. J Clin Endocrinol Metab. 2010;95:4415–23. doi: 10.1210/jc.2009-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Power C, Li L, Hertzman C. Associations of Early Growth and Adult Adiposity with Patterns of Salivary Cortisol in Adulthood. J Clin Endocrinol Metab. 2006;91:4264–70. doi: 10.1210/jc.2006-0625. [DOI] [PubMed] [Google Scholar]
- [16].Wallerius S, Rosmond R, Ljung T, Holm G, Björntorp P. Rise in morning saliva cortisol is associated with abdominal obesity in men: a preliminary report. J Endocrinol Invest. 2003;26:616–9. doi: 10.1007/BF03347017. [DOI] [PubMed] [Google Scholar]
- [17].Therrien F, Drapeau V, Lalonde J, Lupien SJ, Beaulieu S, Tremblay A, et al. Awakening cortisol response in lean, obese, and reduced obese individuals: effect of gender and fat distribution. Obesity. 2007;15:377–385. doi: 10.1038/oby.2007.509. [DOI] [PubMed] [Google Scholar]
- [18].Rosmond R, Dallman MF, Björntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998;83:1853–9. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- [19].Björntorp P, Holm G, Rosmond R. Hypothalamic arousal, insulin resistance and Type 2 diabetes mellitus. Diabet Med J Br Diabet Assoc. 1999;16:373–83. doi: 10.1046/j.1464-5491.1999.00067.x. [DOI] [PubMed] [Google Scholar]
- [20].Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, DeSantis AS, et al. Diurnal salivary cortisol is associated with body mass index and waist circumference: The multiethnic study of atherosclerosis. Obesity. 2013;21:E56–63. doi: 10.1002/oby.20047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bild DE. Multi-Ethnic Study of Atherosclerosis: Objectives and Design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- [22].Wang X, Sánchez BN, Golden SH, Shrager S, Kirschbaum C, Karlamangla AS, et al. Stability and predictors of change in salivary cortisol measures over six years: MESA. Psychoneuroendocrinology. 2014;49:310–20. doi: 10.1016/j.psyneuen.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Joseph JJ, Wang X, Spanakis E, Seeman T, Wand G, Needham B, et al. Diurnal salivary cortisol, glycemia and insulin resistance: The multi-ethnic study of atherosclerosis. Psychoneuroendocrinology. 2015;62:327–35. doi: 10.1016/j.psyneuen.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- [26].Badrick E, Kirschbaum C, Kumari M. The Relationship between Smoking Status and Cortisol Secretion. J Clin Endocrinol Metab. 2007;92:819–24. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- [27].Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999;8:805–13. doi: 10.1089/152460999319129. [DOI] [PubMed] [Google Scholar]
- [28].LaMonte MJ, Durstine JL, Addy CL, Irwin ML, Ainsworth BE. Physical activity, physical fitness, and Framingham 10-year risk score: the cross-cultural activity participation study. J Cardpulm Rehabil. 2001;21:63–70. doi: 10.1097/00008483-200103000-00001. [DOI] [PubMed] [Google Scholar]
- [29].Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, et al. The Association Between Physical Activity and Subclinical Atherosclerosis: The Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2008;169:444–54. doi: 10.1093/aje/kwn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- [31].American Diabetes Association Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2010;33:S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Myrskylä M, Chang VW. Weight Change, Initial BMI, and Mortality Among Middle- and Older-aged Adults. Epidemiology. 2009;20:840–8. doi: 10.1097/EDE.0b013e3181b5f520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Attard SM, Herring AH, Howard AG, Gordon-Larsen P. Longitudinal trajectories of BMI and cardiovascular disease risk: The national longitudinal study of adolescent health: BMI Trajectories and CVD Risk. Obesity. 2013;21:2180–8. doi: 10.1002/oby.20569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Allison PD. Fixed effects regression models. SAGE; Los Angeles: 2009. [Google Scholar]
- [35].Ranjit N, Young EA, Raghunathan TE, Kaplan GA. Modeling cortisol rhythms in a population-based study. Psychoneuroendocrinology. 2005;30:615–24. doi: 10.1016/j.psyneuen.2005.02.003. [DOI] [PubMed] [Google Scholar]
- [36].Ljung T, Andersson B, Bengtsson B-\AAke, Björntorp P, M\a arin P. Inhibition of Cortisol Secretion by Dexamethasone in Relation to Body Fat Distribution: A Dose-Response Study. Obes Res. 1996;4:277–282. doi: 10.1002/j.1550-8528.1996.tb00546.x. [DOI] [PubMed] [Google Scholar]
- [37].DeSantis AS, DiezRoux AV, Hajat A, Golden SH, Jenny NS, Sanchez BN, et al. Associations of Salivary Cortisol Levels with Metabolic Syndrome and Its Components: The Multi-Ethnic Study of Atherosclerosis. J Clin Endocrinol Metab. 2011;96:3483–92. doi: 10.1210/jc.2011-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, Roux AD, et al. Diurnal salivary cortisol and urinary catecholamines are associated with diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis. Metabolism. 2012;61:986–95. doi: 10.1016/j.metabol.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rosmond R, Björntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J Intern Med. 2000;247:188–97. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- [40].Masuzaki H. A Transgenic Model of Visceral Obesity and the Metabolic Syndrome. Science. 2001;294:2166–70. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- [41].Valentine AR, Raff H, Liu H, Ballesteros M, Rose JM, Jossart GH, et al. Salivary Cortisol Increases After Bariatric Surgery in Women. Horm Metab Res. 2011;43:587–90. doi: 10.1055/s-0031-1279777. [DOI] [PubMed] [Google Scholar]
- [42].Tam CS, Frost EA, Xie W, Rood J, Ravussin E, Redman LM. No Effect of Caloric Restriction on Salivary Cortisol Levels in Overweight Men and Women. Metabolism. 2014;63:194–8. doi: 10.1016/j.metabol.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fontana L, Villareal DT, Das SK, Smith SR, Meydani SN, Pittas AG, et al. Effects of 2-year calorie restriction on circulating levels of IGF-1, IGF-binding proteins and cortisol in nonobese men and women: a randomized clinical trial. Aging Cell. 2016;15:22–7. doi: 10.1111/acel.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Newell-Price J, Trainer P, Besser M, Grossman A. The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr Rev. 1998;19:647–72. doi: 10.1210/edrv.19.5.0346. [DOI] [PubMed] [Google Scholar]
- [45].Peeke PM, Chrousos GP. Hypercortisolism and obesity. Ann N Y Acad Sci. 1995;771:665–676. doi: 10.1111/j.1749-6632.1995.tb44719.x. [DOI] [PubMed] [Google Scholar]
- [46].Stalder T, Steudte S, Alexander N, Miller R, Gao W, Dettenborn L, et al. Cortisol in hair, body mass index and stress-related measures. Biol Psychol. 2012;90:218–23. doi: 10.1016/j.biopsycho.2012.03.010. [DOI] [PubMed] [Google Scholar]
- [47].Wester VL, Staufenbiel SM, Veldhorst MAB, Visser JA, Manenschijn L, Koper JW, et al. Long-term cortisol levels measured in scalp hair of obese patients: Obesity and Hair Cortisol. Obesity. 2014;22:1956–8. doi: 10.1002/oby.20795. [DOI] [PubMed] [Google Scholar]
- [48].Abraham SB, Rubino D, Sinaii N, Ramsey S, Nieman LK. Cortisol, obesity, and the metabolic syndrome: A cross-sectional study of obese subjects and review of the literature. Obesity. 2013;21:E105–17. doi: 10.1002/oby.20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.