Until recently, options were limited for patients with non‐small cell lung cancer who progressed while receiving epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), but improvements in molecular profiling and the approval of osimertinib afford the opportunity for improved outcomes in many patients with the EGFR T790M mutation. This article explains the options available after progression on initial EGFR TKI therapy and the importance of molecular testing at progression in making treatment decisions.
Keywords: Gefitinib, Erlotinib, Afatinib, Osimertinib, Non‐small cell lung cancer, Treatment
Abstract
Molecular profiling and the discovery of drugs that target specific activating mutations have allowed the personalization of treatment for non‐small cell lung cancer (NSCLC). The epithelial growth factor receptor (EGFR) is frequently over‐expressed and/or aberrantly activated in different cancers, including NSCLC. The most common activating mutations of EGFR in NSCLC fall within the tyrosine kinase‐binding domain. Three oral EGFR tyrosine kinase inhibitors (TKIs) have been approved by the U.S. Food and Drug Administration (FDA) for first‐line use in patients with EGFR mutation‐positive NSCLC (exon 19 deletions or exon 21 [L858R] substitution mutations), as detected by an FDA‐approved test. However, disease progression is common and is often the result of secondary mutations, of which the EGFR T790M mutation is the most prevalent. Few options were available upon progression until the introduction of osimertinib, a kinase inhibitor that targets the T790M mutation, which was recently approved for use in patients with metastatic EGFR T790M mutation‐positive NSCLC, as detected by an FDA‐approved test, who progressed on or after EGFR TKI therapy. With the introduction of osimertinib, outcomes can now be improved in select patients. Therefore, performing a biopsy at progression to determine the underlying molecular cause of the acquired resistance is important for the enabling of individualized options that may provide the greatest opportunity for improved outcomes. This review discusses the latest updates in molecular testing at progression and outlines treatment options for this difficult‐to‐treat population.
Implications for Practice.
Although the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs)—gefitinib, erlotinib, and afatinib—have changed the treatment paradigm for non‐small cell lung cancer among those with EGFR mutation positive disease, most patients experience progression after approximately 12 months of treatment. Until recently, options were limited for patients who progressed, but improvements in molecular profiling and the approval of osimertinib, which targets the resistance mutation T790M, afford the opportunity for improved outcomes in many patients with this mutation. This article explains the options available after progression on initial EGFR TKI therapy and the importance of molecular testing at progression in making treatment decisions.
Introduction
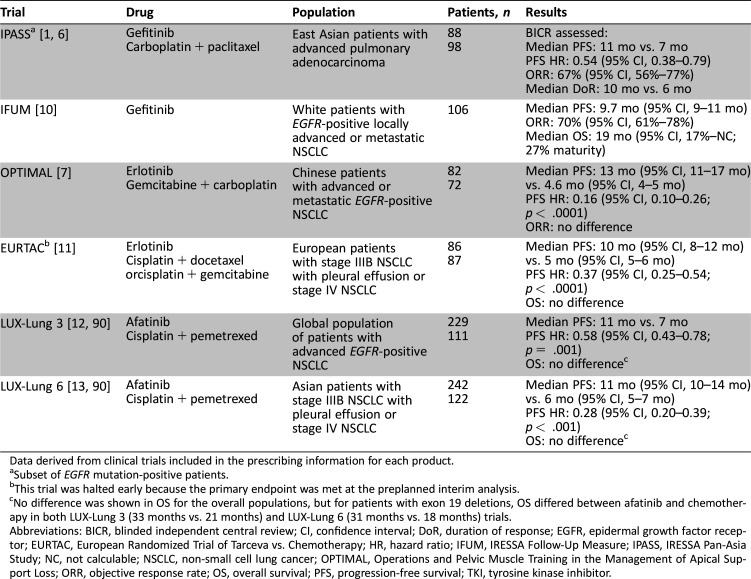
The clinical utility of using a single gene‐based biomarker as a therapeutic focus for non‐small cell lung cancer (NSCLC) was first realized with the 2004 discovery of mutations in the tyrosine kinase domain of the epithelial growth factor receptor (EGFR); this allowed identification of patients with greater sensitivity to small‐molecule tyrosine kinase inhibitors (TKIs) [1], [2], [3], [4]. This discovery, in turn, led to the subsequent regulatory approval in the United States of three oral EGFR TKIs: gefitinib, erlotinib, and afatinib. These agents are the National Comprehensive Cancer Network (NCCN)‐recommended first‐line therapies in patients with advanced or metastatic NSCLC harboring EGFR mutations (exon 19 deletions [ex19del] or exon 21 [L858R] substitution, as detected by a U.S. Food and Drug Administration [FDA]‐approved test), based on the success of clinical trials in EGFR mutation‐positive selected populations (Table 1) [1], [5], [6], [7], [8], [9], [10], [11], [12], [13] However, despite the notable efficacy achieved with EGFR TKIs, a majority of patients develop resistance after a median progression‐free survival (PFS) of approximately 1 year (range, 8–14 months) [1], [7], [10], [11], [12], [13], [14].
Table 1. Overview of pivotal studies of EGFR TKI as first‐line therapy in patients with EGFR‐positive NSCLC.
Data derived from clinical trials included in the prescribing information for each product.
Subset of EGFR mutation‐positive patients.
This trial was halted early because the primary endpoint was met at the preplanned interim analysis.
No difference was shown in OS for the overall populations, but for patients with exon 19 deletions, OS differed between afatinib and chemotherapy in both LUX‐Lung 3 (33 months vs. 21 months) and LUX‐Lung 6 (31 months vs. 18 months) trials.
Abbreviations: BICR, blinded independent central review; CI, confidence interval; DoR, duration of response; EGFR, epidermal growth factor receptor; EURTAC, European Randomized Trial of Tarceva vs. Chemotherapy; HR, hazard ratio; IFUM, IRESSA Follow‐Up Measure; IPASS, IRESSA Pan‐Asia Study; NC, not calculable; NSCLC, non‐small cell lung cancer; OPTIMAL, Operations and Pelvic Muscle Training in the Management of Apical Support Loss; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival; TKI, tyrosine kinase inhibitor.
Mechanisms of Acquired Resistance to EGFR TKI Therapy
There are several mechanisms of acquired resistance to EGFR TKIs (Fig. 1). The most common, encompassing approximately 60% of cases, is the result of a secondary point mutation in exon 20 of the EGFR gene, referred to as T790M [15], [16], [17], [18], [19], [20]. The T790M mutation is thought to convey resistance to EGFR TKIs through different mechanisms, including steric hindrance, which is a reduction in binding of reversible TKIs; increased binding affinity for adenosine triphosphate; and increased phosphorylation levels, which reduce the potency of the EGFR TKIs [21], [22], [23]. A less common (5%–11%) cause of acquired resistance is amplification of the mesenchymal‐epithelial transition (MET) gene, and tumors may harbor both the EGFR T790M mutation and MET amplification [15], [18], [24], [25], [26], [27]. Another mechanism, occurring in approximately 3%–20% of NSCLC cases, is transformation to small cell undifferentiated carcinoma histology (small cell lung cancer [SCLC]) [15], [27], [28]. Amplification of the ERBB2 (HER2) gene has been found in 12%–13% of patients, and mutations in the PI3KCA gene have been seen in 0%–5% of patients [15], [23]. In anywhere from 18% to 30% of patients, the cause of resistance is unknown [15], [27].
Figure 1.
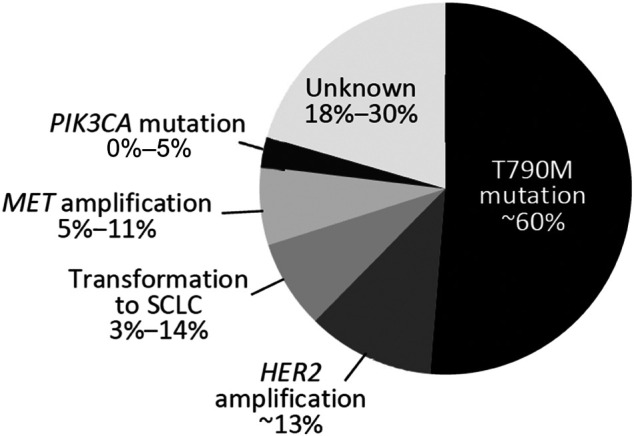
Mechanisms of acquired resistance after epidermal growth factor receptor tyrosine kinase inhibitor therapy [15], [27]. Because ranges are shown, totals do not equal 100%.
Abbreviations: MET, mesenchymal‐epithelial transition; SCLC, small cell lung cancer.
EGFR TKI resistance mutations have been hypothesized to evolve through one of two avenues [29], [30]. The selection model suggests that a very small fraction of EGFR TKI‐resistant clones is present before EGFR TKI therapy and that these clones proliferate while the sensitive clones are eradicated during treatment. The acquisition model proposes that tumor cells acquire new genetic or epigenetic defects as a result of EGFR TKI treatment by inducing a state of increased genetic and epigenetic instability. Evidence for the former includes the presence of both EGFR T790M mutations and MET‐amplified cells in pretreatment tumor samples [29], [31], [32]. In addition, EGFR T790M clones may proliferate more slowly than EGFR clones with other mutations, which may allow them to escape the effects of EGFR TKI therapy [23], [33]. However, cell culture studies and clinical trials have found that mutations can develop from selective pressure with long term repeated exposure to EGFR TKIs, thereby overcoming the effects of the drugs; this is the more widely accepted mechanism of resistance [23], [27], [29]. Furthermore, acquired resistance may result from target gene modification or activation of alternative pathways, or “bypass tracks,” that provide a compensatory signaling pathway from that of the driver mutation, allowing for continued cell growth and proliferation [30]. MET amplification in EGFR‐mutant lung cancer was the first example of the bypass track resistance mechanism identified [30].
In contrast, transformation to SCLC is less clear but is thought to involve histologic changes that result in molecular and phenotypic characteristics of SCLC, such as loss of retinoblastoma that is universally observed [34]. In one study that examined this issue, all tumors that transformed to SCLC retained the original EGFR mutation, suggesting direct development from the primary cancer [27]. Thus, this transformation may involve a pluripotent population that becomes activated upon exposure to EGFR TKIs [34], [35]. Alternatively, the same cancer stem cells could differentiate into both NSCLC and SCLC, with adenocarcinoma initially predominating, or the presence of SCLC may have been overlooked in the original testing at diagnosis [35].
Progression can also result from inadequate drug exposure against the target protein despite tumor cells remaining sensitive to drug; this is termed “pharmacologic resistance” [30]. Pharmacologic resistance generally evolves from low adherence to the treatment regimen, drug‐drug interactions, and/or pharmacokinetics that fail to evenly deliver the drug to all tumor‐infiltrating body compartments [30].
Molecular Testing upon Progression
Current guidelines state that performing a local recurrence or metastasis biopsy at progression after EGFR TKI therapy is a reasonable course of action to determine the mechanism of acquired resistance [5], and molecular profiling at this stage is increasing in importance as new targeted therapies become available. Several studies have evaluated the clinical feasibility of biopsy upon progression [15], [18], [27], [36], [37], [38], [39]. In a real‐world study of 100 patients with NSCLC who progressed after first‐ line treatment (targeted therapy or chemotherapy), biopsy at progression was possible in 82% of patients; among those who underwent rebiopsy, the sample could be histologically analyzed in 94% of cases [36]. In a second study of 39 patients who underwent biopsy at progression, samples were sufficient for histopathologic or cytologic examination in almost 90% of cases [37]. Because both tumor and plasma EGFR mutation load decrease after chemotherapy, EGFR mutation testing should not be conducted too soon after chemotherapy in order to decrease the risk for inaccurate results [40].
The College of American Pathologists (CAP), the International Association for the Study of Lung Cancer (IASLC), and the Association for Molecular Pathology (AMP) developed guidelines for molecular testing at diagnosis and progression, and they currently have open for comment a revised draft that incorporates the most recent data on sensitizing and resistance mutations in NSCLC, as well as new testing methods [41], [42]. The primary methods for acquiring tumor tissue samples in most patients who undergo biopsy at disease progression have historically been fine‐needle aspiration and core biopsy with image guidance; surgical excision has generally not been needed [15], [18], [27], [36], [37], [38], [39]. However, tumor specimens suitable for testing can also be procured by surgical resection, open biopsy, endoscopy, transthoracic needle biopsy, or thoracentesis [41]. Molecular testing is generally done on tissue samples that have been preserved as formalin‐fixed, paraffin‐embedded specimens, but testing can also be done on fresh, frozen, or alcohol‐fixed specimens [41], [43]. The most commonly used method for determining mutation status of EGFR used to be direct sequencing (Sanger sequencing); however, this method has lower sensitivity, with a risk for contamination of the post‐polymerase chain reaction (PCR) products; in addition, the method is not standardized among testing centers [44]. A more sensitive method than direct PCR sequencing is quantitative PCR or real‐time PCR (RT‐PCR; e.g., cobas EGFR Mutation Test v2; Roche Molecular Diagnostics, Pleasanton, CA, https://molecular.roche.com) that uses specific probes to amplify the DNA section to be sequenced. Other methods include commercial mutation screening assays that use multiplex PCR (e.g., MassARRAY system, Sequenom Inc., San Diego, CA, https://www.sequenom.com; SNaPshot Multiplex System, Thermo Fisher Scientific Life Sciences, Waltham, MA, http://www.thermofisher.com), which detect more than 50 point mutations, including EGFR T790M [5]. Next‐generation sequencing (NGS), which allows massive parallel analysis of a very large number of DNA fragments, is also a valid method of detecting a variety of mutations and is increasingly being used [5].
Although a greater quantity of specimen is preferred, the laboratory performing the EGFR mutation testing ultimately determines the requirements for tumor content, fixation, and quality and may have validated methods for testing smaller specimens [41]. Nevertheless, small specimens increase the risk for false‐negative results, as do samples with low tumor cell content. Immunohistochemistry (IHC) for EGFR protein overexpression and EGFR copy number analysis (by fluorescent in situ hybridization) should not be used because these are not predictive markers of susceptibility to EGFR TKIs [41].
Testing for means of acquired resistance is warranted when patients progress after EGFR TKI therapy. Pathologist review of biopsy specimens using hematoxylin and eosin staining is performed, along with neuroendocrine IHC with synaptophysin, chromogranin, and/or CD56, to determine whether transformation to SCLC has occurred [27]. In a study of 37 patients with drug‐resistant NSCLC, of the 14% (n = 5) of patients who transformed to SCLC, all still harbored the original EGFR mutation, and 1 patient additionally acquired a PIK3CA mutation [27]. MET amplification can be identified through dual‐color in situ hybridization assays and can also be detected by NGS [26], [45], [46], [47]. Both in situ hybridization and NGS are also used to detect HER2 amplification [48]. Commercial mutation screening tests that identify multiple point mutations are generally designed to detect PIK3CA alterations. NGS can also detect PIK3CA mutations, along with PIK3CA amplifications and alterations in other PIK3CA pathway genes [49], [50].
As with the initial biopsy and molecular testing at diagnosis, several factors should be considered in weighing the risks and benefits of testing at progression. Because lung biopsy is a fairly invasive procedure, there is a risk for complications. In a study of NSCLC patients who progressed while receiving chemotherapy or an EGFR TKI, 14% experienced a post‐procedural complication, the most common being intrapulmonary hemorrhage (7%) and pneumothorax (6%); most cases of pneumothorax resolved spontaneously [38]. In the real‐world study by Chouaid et al. (2014), of 82 patients, there were 1 case of pneumothorax that required chest drainage and 2 cases of hemoptysis that required minor prolongation of the hospital stay [36].
Misinterpretation of the biopsy results is also a concern because of intratumor (different areas of the same tumor with different genetic profiles) and intermetastatic (differences in genetic profiles between the primary tumor and metastases) heterogeneity of mutations [19], [51], [52]. The clinical implications of this heterogeneity are profound because the risk for false‐negative or false‐positive results regarding a given genomic marker may affect treatment decisions and therefore outcomes [51]. EGFR mutation heterogeneity may also reflect the use of an insensitive assay, which itself can produce erroneous results. Careful sampling and handling of specimens are critical to assist in maximizing tumor content and improving molecular testing results.
EGFR mutation heterogeneity may also reflect the use of an insensitive assay, which itself can produce erroneous results. Careful sampling and handling of specimens are critical to assist in maximizing tumor content and improving molecular testing results.
Less invasive methods for detecting mutations have been introduced to overcome the limitations of tissue biopsy. Such substitute sample types include circulating tumor cells (CTCs) and circulating cell‐free tumor DNA (ctDNA), which are both isolated from blood. Although the term “liquid biopsy” is used for both tests, CTCs are cells released from the primary tumor as viable or apoptotic cells, whereas ctDNA is cell‐free material released from CTCs or the primary tumor as DNA fragments [53], [54]. There is insufficient evidence to support the prognostic value of CTCs, and this method is not recommended in the revised CAP/IASLC/AMP guidelines in the setting of NSCLC [42]. However, many studies have shown the utility of using ctDNA to assess EGFR mutation status, and it is a clinically and analytically validated method approved by the FDA (e.g., cobas EGFR Mutation Test v2) [55], [56], [57], [58], [59]. The most recent study of ctDNA analysis at diagnosis found 94.3% concordance in identifying EGFR mutations between 1,033 tissue samples and 803 plasma samples from the same patients, with 65.7% sensitivity and 99.8% specificity [55]. A meta‐analysis found that the overall sensitivity and specificity for blood (pooled plasma and serum) versus tissue testing in detecting EGFR mutations in all exons were 61% and 90%, respectively, and the concordance rate was 79% [60]. In addition, several recent studies have shown that the T790M mutation could effectively be detected by using plasma DNA in patients with NSCLC who progressed after EGFR TKI therapy; in one study, the T790M mutation was detected as early as 2–12 months before radiologic progression [61], [62], [63]. Additionally, a urine‐based test is under development, and the positive percentage agreement for T790M status between urine and tissue was 83% in a preliminary study [64].
Although these tests hold great promise and are gaining strong use in the clinical trial and community settings, there are a few limitations. Perhaps the most critical is that few standard tests are available in the United States, and most are not approved for use in NSCLC [5], [53]. Tumor heterogeneity is another issue, with differences between different ctDNA samples taken from circulation and between a ctDNA sample and a sample from a biopsy of the primary tumor. It is difficult to control the various steps that occur between blood sampling and ctDNA evaluation, such as DNA extraction from the sample, quantification of amount recovered, and contamination from genomic DNA, and this variability can affect the quality and accuracy of the test [53]. Although false‐positive results are rare, they are possible, given the small amounts of DNA normally available in blood samples as compared with tissue [63]. False negatives are also possible, and it has been suggested that one way to improve identification of true false negatives is to assess the presence of other EGFR‐sensitizing mutations as an internal control for circulating tumor DNA. If they are present but T790M is not, then the result is likely a true negative; if none are present, then it is likely a false negative. In general, when there is a negative result, reflex testing of a tissue biopsy specimen to confirm T790M status may be warranted. Ongoing investigations are studying new methods, including more sensitive RT‐PCR and digital PCR assays [63], [65]. In addition, plasma‐based molecular testing is being investigated to monitor disease status and clinical response to therapy, which may assist in making more rapid and targeted treatment decisions [66]. Overall, liquid biopsies are a substantial advancement in managing patients with NSCLC.
Treatment Options at Disease Progression
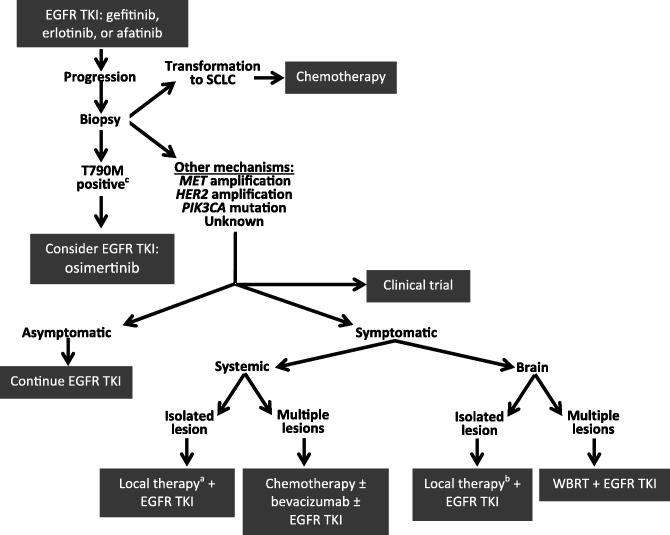
Historically, few effective options were available for patients who progressed after EGFR TKI therapy. Thus, there was a significant unmet clinical need for agents that could provide benefit in the setting of progression after first‐line TKI therapy. The fact that the T790M mutation is responsible for most cases of progression made it an ideal target to study [15], [16], [17], [18], [19], [20]. The 2015 introduction to the worldwide market of osimertinib, a third‐generation TKI that specifically targets the EGFR T790M mutation, represented a treatment breakthrough that offers an effective option for a difficult‐to‐treat population. The NCCN provides guidelines regarding progression after EGFR TKI and has incorporated osimertinib into their most current recommendations [5]. Treatment options are based on the mechanism of resistance to the initial EGFR TKI treatment and whether the patient is symptomatic or asymptomatic [5]. An overview of current recommendations for different progression types is presented in Figure 2, and participation in a clinical trial should always be considered.
Figure 2.
Current treatment algorithm for EGFR mutation‐positive NSCLC [5], [23], [27], [91]. a, As determined by a U.S. Food and Drug Administration‐approved test; b, options include stereotactic body radiation, surgical resection, radiofrequency ablation, cryotherapy, and stereotactic ablative radiotherapy; c, options include surgical resection and stereotactic radiosurgery.
Abbreviations: EGFR, epithelial growth factor receptor; MET, mesenchymal‐epithelial transition; NSCLC, non‐small cell lung cancer; SCLC, small cell lung cancer; TKI, tyrosine kinase inhibitor; WBRT, whole‐brain radiotherapy.
Focus on T790M
Osimertinib is approved in the U.S., Europe, and elsewhere for the treatment of patients with metastatic EGFR T790M mutation‐positive NSCLC, as detected by an FDA‐approved test, who progressed on or after EGFR TKI therapy. It is a once‐daily, oral, potent, irreversible inhibitor that is selective for both EGFR TKI‐sensitizing mutations and the T790M resistance mutation [67], [68]. Because osimertinib is approved for use in patients with metastatic EGFR T790M mutation‐positive NSCLC, a companion diagnostic test was developed. The cobas EGFR Mutation Test v2 uses tumor tissue or plasma to detect 42 mutations in exons 18–21 of the EGFR gene, including T790M.
Approval of osimertinib was based on two multicenter, single‐arm, open‐label trials in patients whose disease progressed after previous treatment with an EGFR TKI [67]. In a pooled analysis of patients (n = 411) who received the recommended dose of 80 mg/day, the blinded independent central review objective response rate (ORR) was 59% (95% confidence interval, 54%–64%), and 96% of patients with confirmed ORR had ongoing responses ranging from 1.1 to 5.6 months after a median follow‐up duration of 4.2 months for the first study and 4.0 months for the second study [67].
The most common adverse events in patients treated with osimertinib 80 mg/day (n = 411) were diarrhea (42% overall; 1.0% grade ≥3) and rash (41% overall; 0.5% grade ≥3) [67]. The incidences of diarrhea and rash with osimertinib were not dissimilar, and were arguably improved, compared with those observed with gefitinib (diarrhea, 27%–47% overall and 3%–4% grade ≥3; rash, 37%–66% overall and 2%–3% grade ≥3), erlotinib (diarrhea, 26%–57% overall and 1%–5% grade ≥3; rash, 75%–80% overall and 2%–13% grade ≥3), and afatinib (diarrhea, 88%–95% overall and 5%–14% grade ≥3; rash, 81%–89% overall and 15%–16% grade ≥3) [1], [7], [10], [11], [12], [13], [69]. Although further investigation is needed before osimertinib can be universally recommended for all patients who express the T790M mutation, these results suggest that osimertinib should be considered for these patients unless there are contraindications, including weighing the risks for adverse events.
Other Alternatives
The current NCCN guidelines (version 4.2016) recommend that for asymptomatic patients who are not candidates for osimertinib, first‐line EGFR TKI therapy can be continued upon progression [5]. This is supported by a study in patients with EGFR TKI failure that stratified progression as dramatic (rapid progression of multiple target lesions, progressive involvement of nontarget lesions, symptom score of 2), gradual (no significant increase in tumor burden, symptom score ≤1), or local (progression due to solitary extracranial lesion or limitation in intracranial lesions, symptom score ≤1) [70]. In the gradual progression group, continuation with EGFR TKI therapy resulted in a longer overall survival (39.4 months) compared with switching to chemotherapy (17.8 months; p = .02) [70]. In a phase II study (ASPIRATION [Asian‐Pacific trial of Tarceva as first‐line therapy in EGFR mutation]) investigating continuation of erlotinib after progression with first‐line erlotinib, an additional 3 months of PFS was observed, but results suggested that outcomes may have been better for patients who had better initial responses [71]. However, in the phase III gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR mutation‐positive non‐small cell lung cancer after progression on first‐line gefitinib (IMPRESS) trial of patients treated with first‐line gefitinib plus cisplatin/pemetrexed, no benefit was observed with continuation of gefitinib plus the doublet chemotherapy regimen at progression versus continuing on chemotherapy alone [72]. Nevertheless, cessation of EGFR TKI therapy is associated with a “disease flare” that may develop in some (9%–23%) patients within a median of 7–8 days of discontinuation [73], [74]. Patients who experience a disease flare have a poorer prognosis than those who do not [73]. Although continuing targeted therapy beyond progression is common practice in other molecularly defined tumors (e.g., trastuzumab in HER2‐positive breast cancer), the decision to continue EGFR TKI after acquired resistance in NSCLC should be made on an individual basis considering the nature of the progression, the tolerability of the current treatment regimen, and the patient's preferences.
For patients who exhibit symptomatic brain metastases after first‐line EGFR TKI therapy, the NCCN (version 4.2016) recommends considering local treatment while continuing EGFR TKI therapy for an isolated lesion or whole‐brain radiotherapy plus continuation of EGFR TKI therapy for multiple lesions. It is thought that central nervous system relapse may be the result of poor penetration of the EGFR TKI into the brain as opposed to the emergence of resistant clones; thus, continuing EGFR TKI therapy could potentially maintain the systemic remission [75].
For symptomatic extracranial lesions, local therapy should be added to the EGFR TKI for an isolated lesion, whereas chemotherapy with or without an EGFR TKI should be considered for multiple systemic lesions [5]. Because systemic progression after EGFR TKI is thought to result from the emergence of EGFR TKI‐resistant clones, which may be confirmed upon molecular testing at progression, switching to chemotherapy after progression is common [75], [76]. A reasonable approach for oligometastases is stereotactic body radiation or surgical resection with continuation of the EGFR TKI, provided the systemic remission is maintained [75].
Despite the risk for a disease flare with discontinuation of EGFR TKI therapy, some patients have experienced benefits with reintroduction of the same EGFR TKI after a drug holiday. In a study of 23 patients who took a median 7‐month break from gefitinib, during which time they received cytotoxic anticancer therapy, retreatment with gefitinib resulted in a partial response in 22% of patients, with a disease control rate of 65% [77]. A similar study (n = 14) with erlotinib showed that after reintroduction of erlotinib following a median 9.5‐month holiday, 36% of patients experienced a partial response and 50% had stable disease [78]. These were small studies, and further investigation in this area is needed.
Areas Under Investigation
Other agents that target T790M are under investigation. A recent study of olmutinib (HM61713), another third‐generation EGFR TKI for patients who harbor the T790M mutation and who progressed on prior EGFR TKI therapy, demonstrated a confirmed ORR of 44% and a median duration of response of 8.3 months in this population [79]. Olmutinib is approved in South Korea for this indication. Interim results of a phase I trial of ASP8273, another agent that targets the T790M mutation, showed a disease control rate of 65% in patients with the T790M mutation and previous treatment with an EGFR TKI (n = 40) [80]. Nazartinib (EGF816) binds and inhibits the most common mutant forms of EGFR, including L858R and ex19del, as well as T790M, with minimal inhibition of wild‐type EGFR [81]. Phase I study results in patients with T790M mutation positive NSCLC (n = 132) showed that nazartinib was well tolerated, and the confirmed ORR was 44% for a disease control rate of 91%; phase II and III studies are ongoing [82]. Another third‐generation TKI, rociletinib, showed early promise in a phase I/II trial [83], but further analyses showed that efficacies were not as great as in initial reports, and almost half of patients experienced a serious adverse event [84]; therefore, development was subsequently halted.
For patients who progress through a pathway other than T790M mutation, research has been aimed at adding agents to the first‐line EGFR TKI. MET amplification is the second most common cause of acquired resistance (approximately 5%–11% of cases), and investigations into adding a MET inhibitor with an EGFR TKI inhibitor are ongoing [15], [18], [26], [27]. A phase II study of cabozantinib, a dual MET/vascular endothelial growth factor receptor 2 inhibitor, plus erlotinib in patients who progressed on EGFR TKI therapy demonstrated significant tumor growth rate reduction [85]. Studies (NCT01610336 and NCT01911507) of capmatinib plus either gefitinib or erlotinib in patients with acquired resistance to gefitinib or erlotinib and with MET amplification are underway, with results expected in 2017.
Immune checkpoint inhibitors (e.g., nivolumab and pembrolizumab) that target the programmed cell death protein‐1 (PD‐1) receptor to restore antitumor immunity have been approved for NSCLC, with response rates up to 20% in heavily pretreated patients [86], [87]. However, results have been less impressive in EGFR mutation‐positive patients, with outcomes potentially favoring chemotherapy over PD‐1 inhibitors [86], [87], [88], [89]. This may be because EGFR‐driven NSCLC tends to have a lower total mutational burden, and investigations have suggested that sensitivity to immune checkpoint inhibitors may be greater in tumors with high levels of somatic mutations [88]. In addition, the majority of EGFR mutation‐positive tumors lack concurrent programmed death‐ligand 1 (PD‐L1) expression and have low levels of CD8 tumor infiltrating lymphocytes, which is not conducive to creating an inflammatory microenvironment and thereby may limit the effectiveness of PD‐1 and PD‐L1 inhibitors [89]. Further characterization of these agents in patients with EGFR mutation‐positive disease is ongoing.
Immune checkpoint inhibitors (e.g., nivolumab and pembrolizumab) that target the programmed cell death protein‐1 (PD‐1) receptor to restore antitumor immunity have been approved for NSCLC, with response rates up to 20% in heavily pretreated patients. However, results have been less impressive in EGFR mutation positive patients, with outcomes potentially favoring chemotherapy over PD‐1 inhibitors.
Conclusion
Progression of EGFR‐driven NSCLC after EGFR TKI therapy presents a significant challenge for clinicians. With more than half of cases of progression attributed to acquired resistance with the T790M mutation, osimertinib, a new agent that targets the EGFR T790M mutation, represents a significant advancement for this difficult‐to‐treat population and should be considered for patients who progress on first‐line EGFR TKI therapy and who are found to harbor the T790M mutation.
Thus, performing molecular testing at progression is critically important to identify patients whom osimertinib would benefit. Furthermore, plasma‐based testing for the T790M mutation is a viable option that may prevent the need for a metastasis biopsy in a significant subset of patients, making testing at progression easier to accomplish. As understanding of the underlying genomics of acquired resistance increases, further treatments that target these mechanisms of progression will be developed, coming closer to the promise of personalized medicine for optimal outcomes.
Acknowledgments
We thank Meredith Rogers, M.S., C.M.P.P., of The Lockwood Group, for providing writing and editorial assistance funded by AstraZeneca.
Footnotes
For Further Reading: Glenwood D. Goss, Johanna N. Spaans. Epidermal Growth Factor Receptor Inhibition in the Management of Squamous Cell Carcinoma of the Lung. The Oncologist 2016; 21:205–213.
Implications for Practice: Anti‐epidermal growth factor receptor (EGFR) therapies remain controversial in unselected/wild‐type EGFR squamous nonsmall cell lung cancer (NSCLC). Recent meta‐analyses and squamous‐only NSCLC EGFR‐inhibition trials have overcome the power limitations of early trials and can now inform the management of squamous NSCLC with anti‐EGFR therapies. With the approval of immunotherapeutics in the second‐line management of squamous NSCLC, there exists an opportunity for novel combination therapies to improve efficacy and durable tumor control. The optimal timing and sequencing of available second‐line targeted therapies, however, have yet to be defined. This review analyzes randomized clinical trials of EGFR inhibition in NSCLC and meta‐analyses of these trials, with a focus on patients with squamous histology.
Author Contributions
Conception/Design: Mark A. Socinski, Liza C. Villaruz, Jeffrey Ross
Manuscript writing: Mark A. Socinski, Liza C. Villaruz, Jeffrey Ross
Final approval of manuscript: Mark A. Socinski, Liza C. Villaruz, Jeffrey Ross
Disclosures
Mark A. Socinski: Celgene, Genentech, Novartis (H), Celgene, Genentech, Novartis, GlaxoSmithKline, Pfizer, Synta, Boehringer Ingelheim, Clovis (RF); Jeffrey Ross: AstraZeneca (C/A, H), Foundation Medicine, Inc. (E). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/ inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Mok TS, Wu YL, Thongprasert S et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–957. [DOI] [PubMed] [Google Scholar]
- 2. Lynch TJ, Bell DW, Sordella R et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–2139. [DOI] [PubMed] [Google Scholar]
- 3. Paez JG, Jänne PA, Lee JC et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004;304:1497–1500. [DOI] [PubMed] [Google Scholar]
- 4. Pao W, Miller V, Zakowski M et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA 2004;101:13306–13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Care Network . NCCN clinical practice guidelines in oncology: Non‐small cell lung cancer v4. 2016. Available at: https:// http://www.nccn.org/. Accessed August 15, 2016.
- 6.AstraZeneca . Iressa (Gefitinib) Prescribing Information. Wilmington, DE: AstraZeneca, 2015. [Google Scholar]
- 7. Zhou C, Wu YL, Chen G et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): A multicentre, open‐label, randomised, phase 3 study. Lancet Oncol 2011;12:735–742. [DOI] [PubMed] [Google Scholar]
- 8.Boehringer Ingelheim Pharmaceuticals . Gilotrif (Afatinib) Prescribing Information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, 2014. [Google Scholar]
- 9.Genentech . Tarceva (Erlotinib) Prescribing Information. San Francisco, CA: Genentech, 2015. [Google Scholar]
- 10. Douillard JY, Ostoros G, Cobo M et al. First‐line gefitinib in Caucasian EGFR mutation‐positive NSCLC patients: A phase‐IV, open‐label, single‐arm study. Br J Cancer 2014;110:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosell R, Carcereny E, Gervais R et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EUR‐TAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012;13:239–246. [DOI] [PubMed] [Google Scholar]
- 12. Sequist LV, Yang JC, Yamamoto N et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–3334. [DOI] [PubMed] [Google Scholar]
- 13. Wu Y‐L, Zhou C, Hu C‐P et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐Lung 6): An open‐label, randomised phase 3 trial. Lancet Oncol 2014;15:213–222. [DOI] [PubMed] [Google Scholar]
- 14. Langer CJ. Epidermal growth factor receptor inhibition in mutation‐positive non‐small‐cell lung cancer: Is afatinib better or simply newer? J Clin Oncol 2013;31:3303–3306. [DOI] [PubMed] [Google Scholar]
- 15. Yu HA, Arcila ME, Rekhtman N et al. Analysis of tumor specimens at the time of acquired resistance to EGFR‐TKI therapy in 155 patients with EGFR‐ mutant lung cancers. Clin Cancer Res 2013;19: 2240–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oxnard GR, Arcila ME, Sima CS et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR‐mutant lung cancer: Distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun JM, Ahn MJ, Choi YL et al. Clinical implications of T790M mutation in patients with acquired resistance to EGFR tyrosine kinase inhibitors. Lung Cancer 2013;82:294–298. [DOI] [PubMed] [Google Scholar]
- 18. Arcila ME, Oxnard GR, Nafa K et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid‐based assay. Clin Cancer Res 2011;17:1169–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuiper JL, Heideman DA, Thunnissen E et al. Incidence of T790M mutation in (sequential) rebiopsies in EGFR‐mutated NSCLC‐patients. Lung Cancer 2014;85:19–24. [DOI] [PubMed] [Google Scholar]
- 20. Li W, Ren S, Li J et al. T790M mutation is associated with better efficacy of treatment beyond progression with EGFR‐TKI in advanced NSCLC patients. Lung Cancer 2014;84:295–300. [DOI] [PubMed] [Google Scholar]
- 21. Kobayashi S, Boggon TJ, Dayaram T et al. EGFR mutation and resistance of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2005;352:786–792. [DOI] [PubMed] [Google Scholar]
- 22. Yun CH, Mengwasser KE, Toms AV et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci USA 2008;105:2070–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cortot AB, Jänne PA. Molecular mechanisms of resistance in epidermal growth factor receptor‐ mutant lung adenocarcinomas. Eur Respir Rev 2014; 23:356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bean J, Brennan C, Shih JY et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA 2007;104:20932–20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Engelman JA, Zejnullahu K, Mitsudomi T et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316:1039–1043. [DOI] [PubMed] [Google Scholar]
- 26. Frampton GM, Ali SM, Rosenzweig M et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov 2015;5:850–859. [DOI] [PubMed] [Google Scholar]
- 27. Sequist LV, Waltman BA, Dias‐Santagata D et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oser MG, Niederst MJ, Sequist LV et al. Transformation from non‐small‐cell lung cancer to small‐cell lung cancer: Molecular drivers and cells of origin. Lancet Oncol 2015;16:e165–e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suda K, Mizuuchi H, Maehara Y et al. Acquired resistance mechanisms to tyrosine kinase inhibitors in lung cancer with activating epidermal growth factor receptor mutation—diversity, ductility, and destiny. Cancer Metastasis Rev 2012;31:807–814. [DOI] [PubMed] [Google Scholar]
- 30. Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: Learning from lung cancer. Nat Rev Clin Oncol 2014;11:473–481. [DOI] [PubMed] [Google Scholar]
- 31. Inukai M, Toyooka S, Ito S et al. Presence of epidermal growth factor receptor gene T790M mutation as a minor clone in non‐small cell lung cancer. Cancer Res 2006;66:7854–7858. [DOI] [PubMed] [Google Scholar]
- 32. Pao W, Miller VA, Politi KA et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chmielecki J, Foo J, Oxnard GR et al. Optimization of dosing for EGFR‐mutant non‐small cell lung cancer with evolutionary cancer modeling. Sci Transl Med 2011;3:90ra59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Niederst MJ, Sequist LV, Poirier JT et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small‐cell lung cancer. Nat Commun 2015;6:6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jiang SY, Zhao J, Wang MZ et al. Small‐cell lung cancer transformation in patients with pulmonary adenocarcinoma: A case report and review of literature. Medicine (Baltimore) 2016;95:e2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chouaid C, Dujon C, Do P et al. Feasibility and clinical impact of re‐biopsy in advanced non small‐cell lung cancer: A prospective multicenter study in a real‐world setting (GFPC study 12‐01). Lung Cancer 2014;86:170–173. [DOI] [PubMed] [Google Scholar]
- 37. Bosc C, Ferretti GR, Cadranel J et al. Rebiopsy during disease progression in patients treated by TKI for oncogene‐addicted NSCLC. Target Oncol 2015; 10:247–253. [DOI] [PubMed] [Google Scholar]
- 38. Yoon HJ, Lee HY, Lee KS et al. Repeat biopsy for mutational analysis of non‐small cell lung cancers resistant to previous chemotherapy: Adequacy and complications. Radiology 2012;265:939–948. [DOI] [PubMed] [Google Scholar]
- 39. Overman MJ, Modak J, Kopetz S et al. Use of research biopsies in clinical trials: Are risks and benefits adequately discussed? J Clin Oncol 2013; 31:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bai H, Wang Z, Chen K et al. Influence of chemotherapy on EGFR mutation status among patients with non‐small‐cell lung cancer. J Clin Oncol 2012;30:3077–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lindeman NI, Cagle PT, Beasley MB et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: Guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol 2013;8:823–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.College of American Pathologists, International Association for the Study of Lung Cancer, Association for Molecular Pathology. Draft molecular guideline recommendations now open for public comment. 2016. Available at: http://www.iaslc.org/articles/capiaslcamp-molecular-testing-guideline-open-comment-period. Accessed August 15, 2016.
- 43. Thomas A, Rajan A, Lopez‐Chavez A et al. From targets to targeted therapies and molecular profiling in non‐small cell lung carcinoma. Ann Oncol 2013;24:577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Angulo B, Conde E, Suárez‐Gauthier A et al. A comparison of EGFR mutation testing methods in lung carcinoma: Direct sequencing, real‐time PCR and immunohistochemistry. PLoS One 2012;7: e43842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jia P, Jin H, Meador CB et al. Next‐generation sequencing of paired tyrosine kinase inhibitor‐sensitive and ‐resistant EGFR mutant lung cancer cell lines identifies spectrum of DNA changes associated with drug resistance. Genome Res 2013;23:1434–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sholl LM, Aisner DL, Varella‐Garcia M et al. Multi‐institutional oncogenic driver mutation analysis in lung adenocarcinoma: The lung cancer mutation consortium experience. J Thorac Oncol 2015;10:768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yamamoto S, Tsuda H, Miyai K et al. Gene amplification and protein overexpression of MET are common events in ovarian clear‐cell adenocarcinoma: Their roles in tumor progression and prognostication of the patient. Mod Pathol 2011;24:1146–1155. [DOI] [PubMed] [Google Scholar]
- 48. Heinmöller P, Gross C, Beyser K et al. HER2 status in non‐small cell lung cancer: Results from patient screening for enrollment to a phase II study of herceptin. Clin Cancer Res 2003;9:5238–5243. [PubMed] [Google Scholar]
- 49. Oxnard GR, Paweletz CP, Kuang Y et al. Noninvasive detection of response and resistance in EGFR‐mutant lung cancer using quantitative next‐generation genotyping of cell‐free plasma DNA. Clin Cancer Res 2014;20:1698–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scheffler M, Bos M, Gardizi M et al. PIK3CA mutations in non‐small cell lung cancer (NSCLC): Genetic heterogeneity, prognostic impact and incidence of prior malignancies. Oncotarget 2015;6:1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bedard PL, Hansen AR, Ratain MJ et al. Tumour heterogeneity in the clinic. Nature 2013;501:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Diaz LA Jr., Bardelli A. Liquid biopsies: Genotyping circulating tumor DNA. J Clin Oncol 2014;32:579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ilie M, Hofman V, Long E et al. Current challenges for detection of circulating tumor cells and cell‐free circulating nucleic acids, and their characterization in non‐small cell lung carcinoma patients. What is the best blood substrate for personalized medicine? Ann Transl Med 2014;2:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alix‐Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer 2014;14:623–631. [DOI] [PubMed] [Google Scholar]
- 55. Douillard JY, Ostoros G, Cobo M et al. Gefitinib treatment in EGFR mutated caucasian NSCLC: Circulating‐free tumor DNA as a surrogate for determination of EGFR status. J Thorac Oncol 2014;9:1345–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rosell R, Moran T, Queralt C et al; Spanish Lung Cancer Group . Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958–967. [DOI] [PubMed] [Google Scholar]
- 57. Yung TK, Chan KC, Mok TS et al. Single‐molecule detection of epidermal growth factor receptor mutations in plasma by microfluidics digital PCR in non‐small cell lung cancer patients. Clin Cancer Res 2009;15:2076–2084. [DOI] [PubMed] [Google Scholar]
- 58. Aung KL, Board RE, Ellison G et al. Current status and future potential of somatic mutation testing from circulating free DNA in patients with solid tumours. HUGO J 2010;4:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Philip R. Developing diagnostics: Perspective from the FDA. Presented at: 2016 American Society of Clinical Oncology Annual Meeting; June 3–7, 2016; Chicago, IL.
- 60. Mao C, Yuan JQ, Yang ZY et al. Blood as a substitute for tumor tissue in detecting EGFR mutations for guiding EGFR TKIs treatment of nonsmall cell lung cancer: A systematic review and meta‐analysis. Medicine (Baltimore) 2015;94:e775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sueoka‐Aragane N, Katakami N, Satouchi M et al. Monitoring EGFR T790M with plasma DNA from lung cancer patients in a prospective observational study. Cancer Sci 2016;107:162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee JY, Qing X, Xiumin W et al. Longitudinal monitoring of EGFR mutations in plasma predicts outcomes of NSCLC patients treated with EGFR TKIs: Korean Lung Cancer Consortium (KLCC‐12‐02). Oncotarget 2016;7:6984–6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thress KS, Brant R, Carr TH et al. EGFR mutation detection in ctDNA from NSCLC patient plasma: A cross‐platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer 2015;90:509–515. [DOI] [PubMed] [Google Scholar]
- 64. Wakelee HA, Gadgeel SM, Goldman JW et al. Epidermal growth factor receptor (EGFR) genotyping of matched urine, plasma and tumor tissue from non‐small cell lung cancer (NSCLC) patients (pts) treated with rociletinib. J Clin Oncol 2016;34(suppl):9001a. [Google Scholar]
- 65. Zhu G, Ye X, Dong Z et al. Highly sensitive droplet digital PCR method for detection of EGFR‐ activating mutations in plasma cell‐free DNA from patients with advanced non‐small cell lung cancer. J Mol Diagn 2015;17:265–272. [DOI] [PubMed] [Google Scholar]
- 66. Marchetti A, Palma JF, Felicioni L et al. Early prediction of response to tyrosine kinase inhibitors by quantification of EGFR mutations in plasma of NSCLC patients. J Thorac Oncol 2015;10:1437–1443. [DOI] [PubMed] [Google Scholar]
- 67.AstraZeneca . Tagrisso (osimertinib) package insert. Wilmington, DE: AstraZeneca, 2015. [Google Scholar]
- 68. Jänne PA, Yang JC, Kim DW et al. AZD9291 in EGFR inhibitor‐resistant non‐small‐cell lung cancer. N Engl J Med 2015;372:1689–1699. [DOI] [PubMed] [Google Scholar]
- 69. Thatcher N, Chang A, Parikh P et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non‐small‐cell lung cancer: Results from a randomised, placebo‐ controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 2005;366:1527–1537. [DOI] [PubMed] [Google Scholar]
- 70. Yang JJ, Chen HJ, Yan HH et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non‐small cell lung cancer. Lung Cancer 2013;79:33–39. [DOI] [PubMed] [Google Scholar]
- 71. Park K, Yu CJ, Kim SW et al. First‐line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in Asian patients with epidermal growth factor receptor mutation positive non‐small‐cell lung cancer: The ASPIRATION study. JAMA Oncol 2016;2:305–312. [DOI] [PubMed] [Google Scholar]
- 72. Soria JC, Wu YL, Nakagawa K et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR‐mutation‐positive non‐small‐cell lung cancer after progression on first‐line gefitinib (IMPRESS): A phase 3 randomised trial. Lancet Oncol 2015;16:990–998. [DOI] [PubMed] [Google Scholar]
- 73. Chen HJ, Yan HH, Yang JJ et al. Disease flare after EGFR tyrosine kinase inhibitor cessation predicts poor survival in patients with non‐small cell lung cancer. Pathol Oncol Res 2013;19:833–838. [DOI] [PubMed] [Google Scholar]
- 74. Chaft JE, Oxnard GR, Sima CS et al. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR‐mutant lung cancer and acquired resistance to erlotinib or gefitinib: Implications for clinical trial design. Clin Cancer Res 2011; 17:6298–6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gandara DR, Li T, Lara PN et al. Acquired resistance to targeted therapies against oncogene‐ driven non‐small‐cell lung cancer: Approach to subtyping progressive disease and clinical implications. Clin Lung Cancer 2014;15:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Song T, Yu W, Wu SX. Subsequent treatment choices for patients with acquired resistance to EGFR‐TKIs in non‐small cell lung cancer: Restore after a drug holiday or switch to another EGFR‐TKI? Asian Pac J Cancer Prev 2014;15:205–213. [DOI] [PubMed] [Google Scholar]
- 77. Oh IJ, Ban HJ, Kim KS et al. Retreatment of gefitinib in patients with non‐small‐cell lung cancer who previously controlled to gefitinib: A single‐arm, open‐label, phase II study. Lung Cancer 2012;77: 121–127. [DOI] [PubMed] [Google Scholar]
- 78. Becker A, Crombag L, Heideman DA et al. Retreatment with erlotinib: Regain of TKI sensitivity following a drug holiday for patients with NSCLC who initially responded to EGFR‐TKI treatment. Eur J Cancer 2011;47:2603–2606. [DOI] [PubMed] [Google Scholar]
- 79. Park K, Lee JS, Kim JH et al. BI 1482694 (HM61713), an EGFR mutant‐specific inhibitor, in T790M + NSCLC: Efficacy and safety at the RP2D. J Clin Oncol 2016;34(suppl):9055a. [Google Scholar]
- 80. Yu HA, Spira AI, Horn L et al. Antitumor activity of ASP8273 300 mg in subjects with EGFR mutation positive non‐small cell lung cancer: Interim results from an ongoing phase 1 study. J Clin Oncol 2016;34(suppl):9050a. [Google Scholar]
- 81. Jia Y, Juarez J, Li J et al. EGF816 exerts anticancer effects in non‐small cell lung cancer by irreversibly and selectively targeting primary and acquired activating mutations in the EGFR receptor. Cancer Res 2016;76:1591–1602. [DOI] [PubMed] [Google Scholar]
- 82. Tan DS, Yang JC, Leighl NB et al. Updated results of a phase 1 study of EGF816, a third‐generation, mutant‐selective EGFR tyrosine kinase inhibitor (TKI), in advanced non‐small cell lung cancer (NSCLC) harboring T790M. J Clin Oncol 2016;34(suppl):9044a. [Google Scholar]
- 83. Sequist LV, Soria JC, Goldman JW et al. Rociletinib in EGFR‐mutated non‐small‐cell lung cancer. N Engl J Med 2015;372:1700–1709. [DOI] [PubMed] [Google Scholar]
- 84.Oncology Drugs Advisory Committee . Rociletinib for the treatment of T790M‐positive mutant epidermal growth factor receptor (EGFR) non‐small cell lung cancer (NSCLC) briefing document, 2016. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM494785.pdf. Accessed August 15, 2016.
- 85. Reckamp K, Frankel PH, Gitilz J et al. Phase II trial of XL184 (cabozantinib) plus erlotinib in patients (pts) with advanced EGFR‐mutant non‐small cell lung cancer (NSCLC) with progressive disease (PD) on epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) therapy: A California Cancer Consortium phase II trial (NCI 9303). J Clin Oncol 2014;32(suppl):8014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Herbst RS, Baas P, Kim DW et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐ positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 87. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rizvi NA, Hellmann MD, Snyder A et al. Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015;348:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gainor JF, Shaw AT, Sequist LV et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD‐1 pathway blockade in non‐small cell lung cancer (NSCLC): A retrospective analysis. Clin Cancer Res 2016;22:4585–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yang JC, Wu YL, Schuler M et al. Afatinib versus cisplatin‐based chemotherapy for EGFR mutation‐positive lung adenocarcinoma (LUX‐Lung 3 and LUX‐Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141–151. [DOI] [PubMed] [Google Scholar]
- 91. Yu HA, Sima CS, Huang J et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR‐mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol 2013;8:346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]