Abstract
Few cases of IgA nephropathy with angioimmunoblastic T-cell lymphoma (AITL) have been reported. We herein present the case of a 79-year-old Japanese man with AITL and IgA nephropathy. The patient presented with generalized edema, fatigue, and fever. Laboratory investigations revealed polyclonal gammopathy with a high level of IgA, microscopic hematuria, proteinuria, and some other immunological abnormalities. Computed tomography revealed generalized lymphadenopathy. A diagnosis of AITL and IgA nephropathy was made based on inguinal lymph node and renal biopsies. Following chemotherapy for AITL, the patient's edema, microscopic hematuria, and proteinuria were alleviated. These findings indicate that IgA nephropathy may occur in AITL patients.
Keywords: angioimmunoblastic T-cell lymphoma, immunoglobulin A
Introduction
Angioimmunoblastic T-cell lymphoma (AITL) is a rare tumor that accounts for 1% of all lymphoma cases. It is characterized by the loss of the lymphoid architecture, the presence of pleomorphic cellular infiltrates, and the proliferation of microvasculature in the lymph nodes. Patients usually present with fever, generalized lymphadenopathy, skin rash, polyclonal hypergammaglobulinemia, Coombs-positive anemia, thrombocytopenia, and hypocomplementemia (1). Renal involvement is rare in patients with AITL. However, in some cases involving proteinuria (2), nephrotic syndrome (3), acute renal failure (4,5), and membranous nephropathy (6) have been reported. However, IgA nephropathy with AITL has never been reported. We herein report the case of a Japanese man presenting with AITL and IgA nephropathy.
Case Report
A 79-year-old Japanese man was admitted to our department with generalized edema, fatigue, fever, and weight gain of one week in duration. Two years prior to admission, the patient was diagnosed with IgM-κ monoclonal gammopathy. Follow-up examinations that were performed in another hospital once every six months showed no signs of progression. Aside from monoclonal gammopathy, the patient had a history of diabetes mellitus, hypertension, and benign prostatic hyperplasia. The patient had no family history of renal disease, leukemia, or lymphoma. Amlodipine and sitagliptin were prescribed and were taken on a regular basis. He did not report any recent changes in medications or their dosages, and he experienced no other systemic symptoms.
On physical examination, his blood pressure was 140/88 mmHg, his pulse rate was 100/min, his respiratory rate was 24/min with an O2 saturation of 95% on room air, and his body temperature was 37.1°C. Generalized lymphadenopathy and edema were detected. The results of a cardiovascular examination were normal, and auscultation revealed decreased bilateral breath sounds in the lower lung fields. An abdominal examination was unremarkable with no obvious hepatosplenomegaly; a neurological examination was also unremarkable.
Laboratory investigations revealed normal complete blood counts and normal bilirubin levels (total bilirubin, 0.7 mg/dL); however, he had elevated levels of transaminases (alanine transaminase, 50 IU/L; aspartate transaminase, 39 IU/L), lactate dehydrogenase (466 IU/L), serum creatinine (1.80 mg/dL), and C-reactive protein (2.08 mg/dL). These findings were consistent with hypergammaglobulinemia (total protein, 9.0 g/dL; albumin, 2.7 g/dL; IgG, 4,161 mg/dL; IgA, 828 mg/dL; and IgM, 525 mg/dL); serum and urine electrophoresis did not detect any monoclonal proteins, including IgM-κ. The other findings included a positive direct Coombs' test, hypocomplementemia (complement component 3, 39 mg/dL; complement component 4, 5 mg/dL; 50% complement hemolysis, <14 U/mL), cryoglobulinemia, antinuclear antibody positivity, a biological false positive reaction, and elevated soluble interleukin-2 receptor (7,810 U/mL) levels. There was no evidence of chronic viral infection (the patient was negative for hepatitis B surface antigen; hepatitis B surface, hepatitis C virus, human immunodeficiency virus, human T-cell leukemia virus type 1, and treponema pallidum antibodies). The patient was negative for anti-neutrophil cytoplasmic antibodies. A spot urinalysis revealed proteinuria (urine protein, 3+, 2.48 g/gCr) and microscopic hematuria (urine blood, 3+). Urinary sediments showed 20-29 red blood cells per high-power field.
Computed tomography revealed the swelling of the cervical, axillary, upper mediastinal, abdominal, and inguinal lymph nodes. In addition, hepatosplenomegaly and thoracoabdominal fluids were observed. A histopathological examination of inguinal lymph node and renal biopsy specimens and bone marrow aspiration was performed for further evaluation.
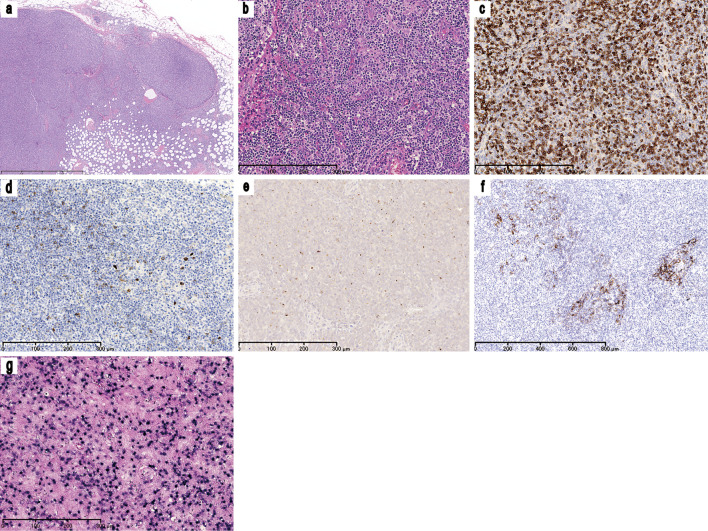
As shown in Fig. 1, the near-complete effacement of the normal lymph node architecture was observed; this was associated with marked vascular proliferation and aggregates of medium-sized atypical lymphoid cells in the inguinal lymph nodes. These atypical lymphoid cells showed clear to pale cytoplasm and had convoluted nuclei with dispersed chromatin. The immunophenotype of these cells was CD3+, CD20-, CD5+, CD4+, and CD8-. Although the cells were negative for CD10 expression, some of these cells also expressed C-X-C motif chemokine ligand 13. CD21 immunohistochemistry highlighted the expansion of follicular dendritic cells. Epstein-Barr virus-encoded small RNA (EBER) in situ hybridization revealed the marked infiltration of Epstein-Barr virus-positive B cells. The distribution of these EBER+ cells was consistent with the distribution of CD20+ cells and not consistent with the distribution of CD3+ cells. Although some EBER+ and CD20+ B-cells were medium to large in size, the majority were small. In addition, the abnormal morphology of the EBER+ and CD20+ B-cells and the architecture of these proliferated cells did not extend the range of reactive B-cell proliferation. Although no chromosomal abnormalities were detected, T-cell receptor rearrangement was found using Southern blotting. These findings were consistent with the diagnosis of AITL. A histological evaluation of the bone marrow aspirate revealed the lymphoma cell involvement.
Figure 1.
Inguinal lymphoid biopsy. (a) A low power view of an inguinal lymph node biopsy specimen [Hematoxylin and Eosin (H&E) staining, ×25]. The normal lymph node architecture is almost completely effaced and the diffuse infiltration of lymphoid cells is observed. (b) A moderate-power view of the inguinal lymph node biopsy specimen (H&E staining, ×100). The aggregation of medium-sized atypical lymphoid cells and marked vascular proliferation can be observed. The neoplastic lymphoid cells have a clear to pale cytoplasm and convoluted nuclei with dispersed chromatin. Immunohistochemical staining for (c) CD3 (×100), (d) CD10 (×100), (e) CXCL13 (×100), and (f) CD21 (×50) revealed that most of the neoplastic cells showed CD3 positivity; the cells were negative for CD10 expression but some expressed CXCL13. The CD21 immunohistochemistry highlights the expansion of follicular dendritic cells. (g) EBV EBERin situ hybridization (×100) showing the marked infiltration of EBV-positive B cells. CD: Cluster of differentiation, CXCL: C-X-C motif chemokine ligand, EBER: Epstein-Barr-virus-encoded small RNA, EBV: Epstein-Barr virus
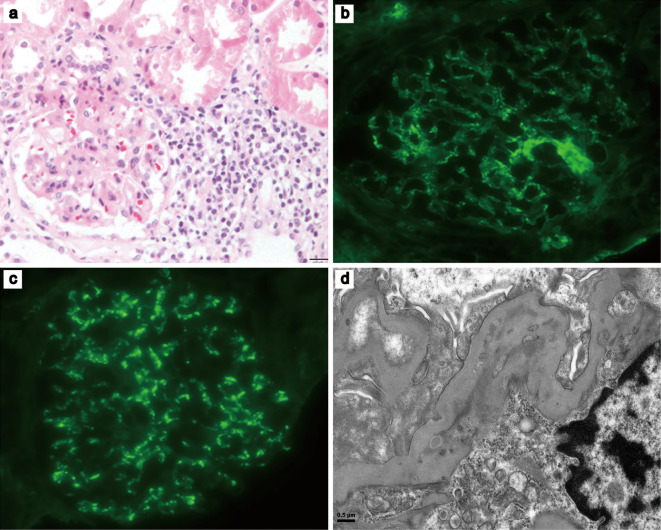
Renal biopsy showed malignant lymphoma invasion and an IgA nephropathy pattern (Fig. 2). Light microscopy showed the focal infiltration of small to medium sized lymphoid cells, with convoluted nuclei in the periglomerular and peritubular regions. The majority of these lymphoid cells demonstrated immunoreactivity to CD3+, interspersed with some CD20+ cells. Some EBER+ cells were also detected in the interstitium. An immunofluorescence examination revealed mesangial deposits of IgA and complement component 3. Small, dense deposits in the mesangial matrix were visualized using electron microscopy. The diagnosis of AITL and IgA nephropathy was made based on the clinical and histopathological findings and the results of the laboratory examinations. Before the diagnosis, the patient was initially treated with prednisolone (60 mg) per day. Five days later, the patient's edema was alleviated and his urinary protein level decreased to 0.58 g/gCr, but the hematuria persisted. After the diagnosis of AITL was made, steroid therapy was administered, followed by chemotherapy with intravenous cyclophosphamide (750 mg/m2), pirarubicin, (50 mg/m2) and vincristine (1.4 mg/m2); oral prednisolone (60 mg per day) was administered from days 1 to 5; the duration of one treatment cycle was 21 days. Following the completion of six chemotherapy courses, the patient's symptoms gradually improved, and the generalized lymphadenopathy and edema were alleviated. Following the completion of chemotherapy, the patient's immunoglobulin and serum creatinine levels, proteinuria, and hematuria normalized. Repeated computed tomography examinations showed that the lymphadenopathy and other abnormal findings were resolved. This patient was considered to have achieved complete remission; no subsequent recurrences of lymphadenopathy or edema have been observed.
Figure 2.
Renal biopsy. (a) Light microscopy, focal infiltration by small to medium-sized atypical lymphoid cells can be seen in periglomerular and peritubular regions (Hematoxylin and Eosin staining, ×40). (b and c) An immunofluorescence examination showing mesangial deposits of (b) IgA (×40) and (c) C3 (×40). (d) An electron microscopic image (×3,000) showing a small amount of dense deposits in the mesangial matrix. C3: complement component 3, IgA: immunoglobulin A
Discussion
We described the case of a patient with AITL with concomitant IgA nephropathy. We propose that these two diseases could be pathologically associated from two points of view. First, the serum IgA in AITL patients can be pathogenic and may cause extranodal involvement. The phenomenon of excessive serum IgA production in AITL patients has also been reported and is believed to result from the excessive differentiation of IgA-plasmablasts, which is induced by transforming growth factor-β1 and interleukin-21, which are released by neoplastic T follicular helper cells (7). Furthermore, elevated serum IgA levels have been shown to be a novel prognostic factor in patients with AITL, although the underlying mechanism remains to be clarified (8,9). With respect to the direct pathological contribution of IgA in AITL patients, several IgA-related extranodal diseases have been reported to be associated with AITL. These include IgA-related leukocytoclastic vasculitis (10,11), atypical linear IgA dermatosis (12), and IgA pemphigus (13). These findings suggest that AITL patients may produce pathogenic IgA, which may result in extranodal diseases, including nephropathy.
Second, there was chronological coincidence in the duration of the urinary abnormalities and the systemic lymphadenopathy in the present case. This patient's microscopic hematuria, proteinuria, and lymphadenopathy were observed at the same time, and these abnormalities gradually diminished with the repeated chemotherapy. We therefore assumed that the patient's IgA nephropathy was associated with AITL.
It is possible that the patient had idiopathic IgA nephropathy because the high circulating levels of IgA alone could not have caused the disease in the majority of these patients. Elevated circulating levels of IgA have, however, been reported in some patients with IgA nephropathy (14). Furthermore, we did not have any direct evidence of the relationship between the IgA deposits in the mesangium and circulating IgA. However, since mesangial IgA is probably derived from a circulating pool of pathogenic IgA (15), and since the polyclonal immune activation observed in many multisystemic autoimmune diseases like AITL could cause IgA nephropathy (14), we could not deny the association between IgA nephropathy and AITL. The further understanding of this relationship requires an investigation to determine the precise pathological mechanisms underlying the tissue damage that is caused by serum IgA in AITL patients.
The direct invasion of the kidney, in association with AITL, also seemed to be a possible cause of the microscopic hematuria, proteinuria, and renal failure that were observed in the present case; however, the pathological findings of the kidney biopsy in differed from those of a previously reported case of AITL with direct kidney invasion (16). Moreover, kidney enlargement, which is frequently seen in cases with the massive infiltration of lymphoid cells into the renal parenchyma (17,18), was not observed in our patient. We therefore believe that IgA nephropathy was the main disease in our patient.
The severity of IgA-related extranodal disease in patients with AITL may be a useful marker for evaluating the efficacy of chemotherapy. In fact, the grade of microscopic hematuria gradually decreased and finally disappeared after the completion of six courses of chemotherapy, which was paralleled by a reduction in tumor volume. Other cases of extranodal disease associated with IgA in AITL have also shown a gradual response to chemotherapy (10-13). Furthermore, the reappearance of purpura was observed along with the recurrence of AITL in a case of IgA-associated leukocytoclastic vasculitis in AITL (10). Considering the simplicity of the evaluation methods for patients with reported extranodal disease, such as screening for microscopic hematuria or skin lesions, the recognition of IgA-related extranodal disease in patients with AITL seems to be important in the follow-up of AITL.
In conclusion, AITL can be associated with IgA nephropathy. As several IgA-related extranodal diseases seem to be useful markers of the severity of AITL, clinicians should assess IgA-related lesions in patients with AITL.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Dogan A, Gaulard P, Jaffe ES, Ralfkiaer E, Müller-Hermelink HK. Angioimmunoblastic T-cell lymphoma. In: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Swerdlow SH, Campo E, Harris NL et al. , Eds. International Agency for Research on Cancer, Lyon, 2008: 309-311. [Google Scholar]
- 2. Staszewski H, Kumar G, Mishriki Y. Minimal change disease as the etiology of the nephrotic syndrome in a patient with angioimmunoblastic lymphadenopathy. Med Pediatr Oncol 16: 206-209, 1988. [DOI] [PubMed] [Google Scholar]
- 3. De Samblanx H, Verhoef G, Zachée P, Vandenberghe P. A male with angioimmunoblastic T-cell lymphoma and proliferative glomerulonephritis. Ann Hematol 83: 455-459, 2004. [DOI] [PubMed] [Google Scholar]
- 4. Wood WG, Harkins MM. Nephropathy in angioimmunoblastic lymphadenopathy. Am J Clin Pathol 71: 58-63, 1979. [DOI] [PubMed] [Google Scholar]
- 5. Duwaji MS, Shemin DG, Medeiros LJ, Esparza AR. Proliferative glomerulonephritis with unusual, organized, cylindrical deposits associated with angioimmunoblastic lymphadenopathy-like T-cell lymphoma. Arch Pathol Lab Med 119: 377-380, 1995. [PubMed] [Google Scholar]
- 6. Togashi M, Wakui H, Kodama K, et al. Angioimmunoblastic T-cell lymphoma and membranous nephropathy: a still unreported association. Clin Exp Nephrol 14: 288-293, 2010. [DOI] [PubMed] [Google Scholar]
- 7. Dullaers M, Li D, Xue Y, et al. A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity 30: 120-129, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tokunaga T, Shimada K, Yamamoto K, et al. Retrospective analysis of prognostic factors for angioimmunoblastic T-cell lymphoma: a multicenter cooperative study in Japan. Blood 119: 2837-2843, 2012. [DOI] [PubMed] [Google Scholar]
- 9. Kato A, Imai Y, Aoki K, et al. Serum IgA level, monocyte count, and international prognostic index are independently associated with overall survival in patients with HTLV-I-negative nodal peripheral T cell lymphoma. Ann Hematol 93: 1185-1191, 2014. [DOI] [PubMed] [Google Scholar]
- 10. Sugaya M, Nakamura K, Asahina A, Tamaki K. Leukocytoclastic vasculitis with IgA deposits in angioimmunoblastic T cell lymphoma. J Dermatol 28: 32-37, 2001. [DOI] [PubMed] [Google Scholar]
- 11. Endo Y, Tsuji M, Shirase T, et al. Angioimmunoblastic T-cell lymphoma presenting with both IgA-related leukocytoclastic vasculitis and mucous membrane pemphigoid. Eur J Dermatol 21: 274-276, 2011. [DOI] [PubMed] [Google Scholar]
- 12. Nassar D, Gabillot-Carré M, Ortonne N, et al. Atypical linear IgA dermatosis revealing angioimmunoblastic T-cell lymphoma. Arch Dermatol 145: 342-343, 2009. [DOI] [PubMed] [Google Scholar]
- 13. Yashiro M, Nakano T, Taniguchi T, et al. IgA paraneoplastic pemphigus in angioimmunoblastic T-cell lymphoma with antibodies to desmocollin 1, type VII collagen and laminin 332. Acta Derm Venereol 94: 235-236, 2014. [DOI] [PubMed] [Google Scholar]
- 14. Pouria S, Barratt J. Secondary IgA nephropathy. Semin Nephrol 28: 27-37, 2008. [DOI] [PubMed] [Google Scholar]
- 15. Floege J. Recurrent IgA nephropathy after renal transplantation. Semin Nephrol 24: 287-291, 2004. [DOI] [PubMed] [Google Scholar]
- 16. Goto A, Takada A, Yamamoto S, Notoya A, Mukai M. Angioimmunoblastic T-cell lymphoma with renal involvement: a case report of direct bilateral kidney invasion by lymphoma cells. Ann Hematol 83: 731-732, 2004. [DOI] [PubMed] [Google Scholar]
- 17. Törnroth T, Heiro M, Marcussen N, Franssila K. Lymphomas diagnosed by percutaneous kidney biopsy. Am J Kidney Dis 42: 960-971, 2003. [DOI] [PubMed] [Google Scholar]
- 18. Li SJ, Chen HP, Chen YH, Zhang LH, Tu YM, Liu ZH. Renal involvement in non-Hodgkin lymphoma: proven by renal biopsy. PLoS One 9: e95190, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]