Abstract
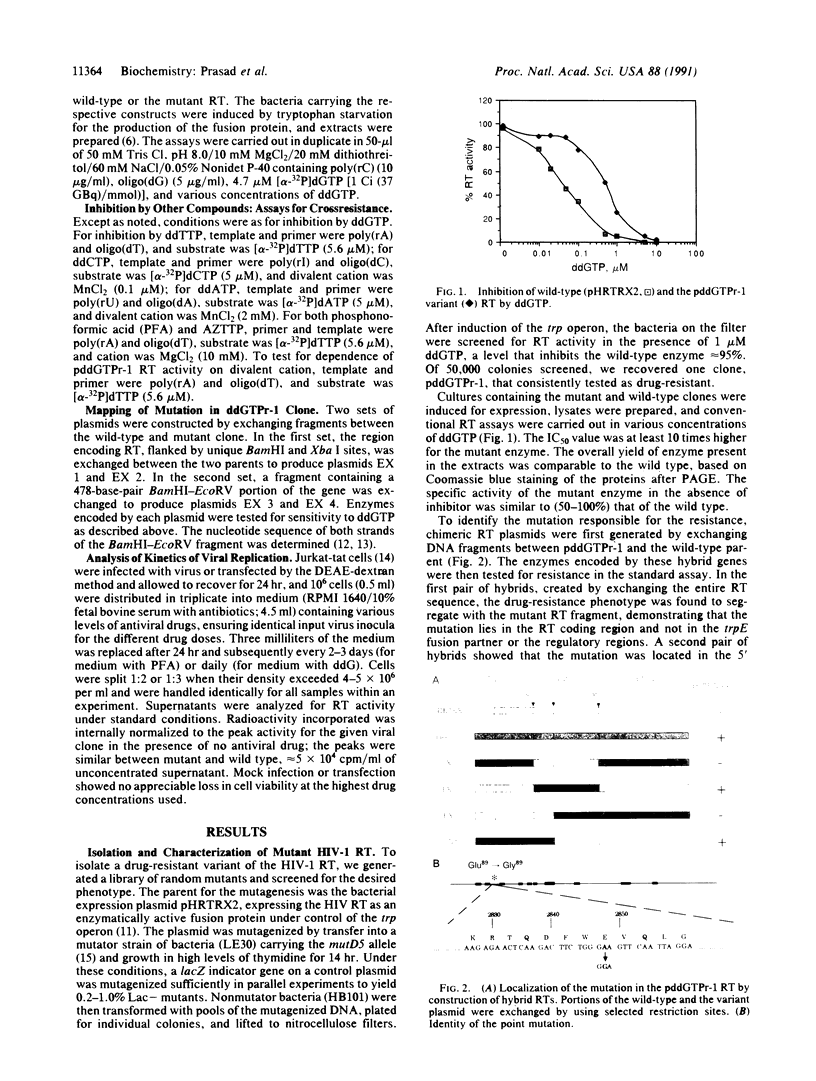
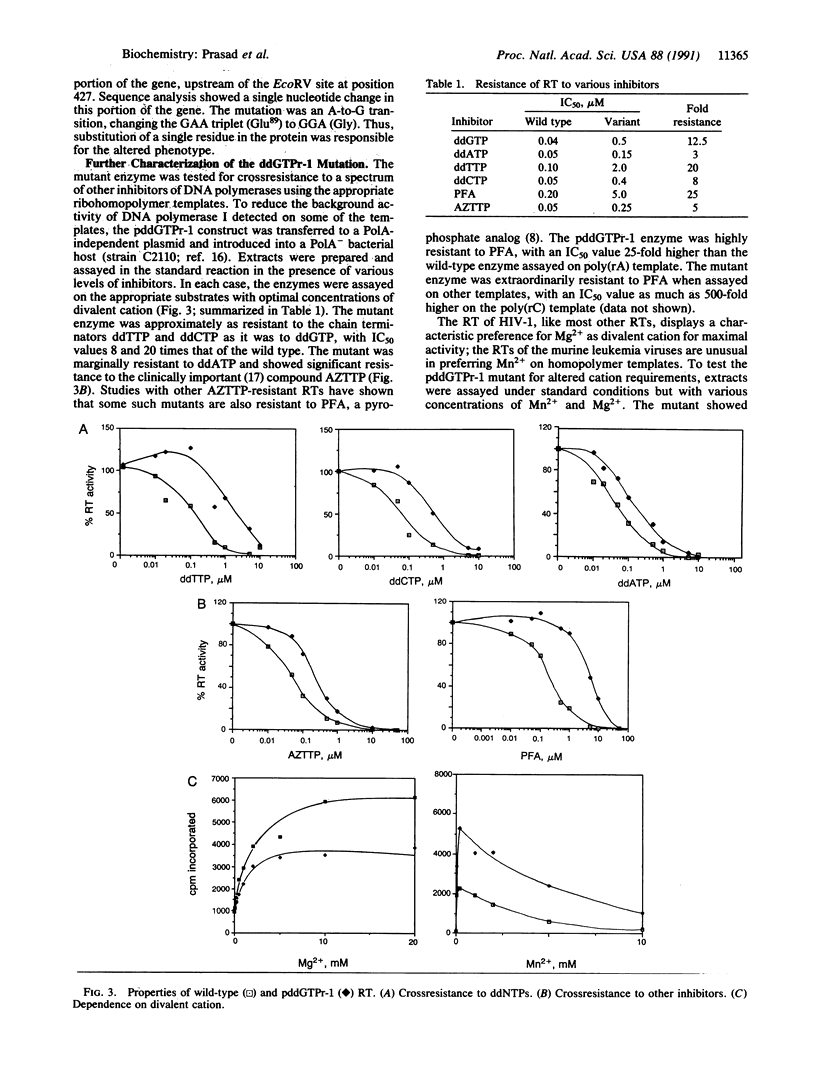
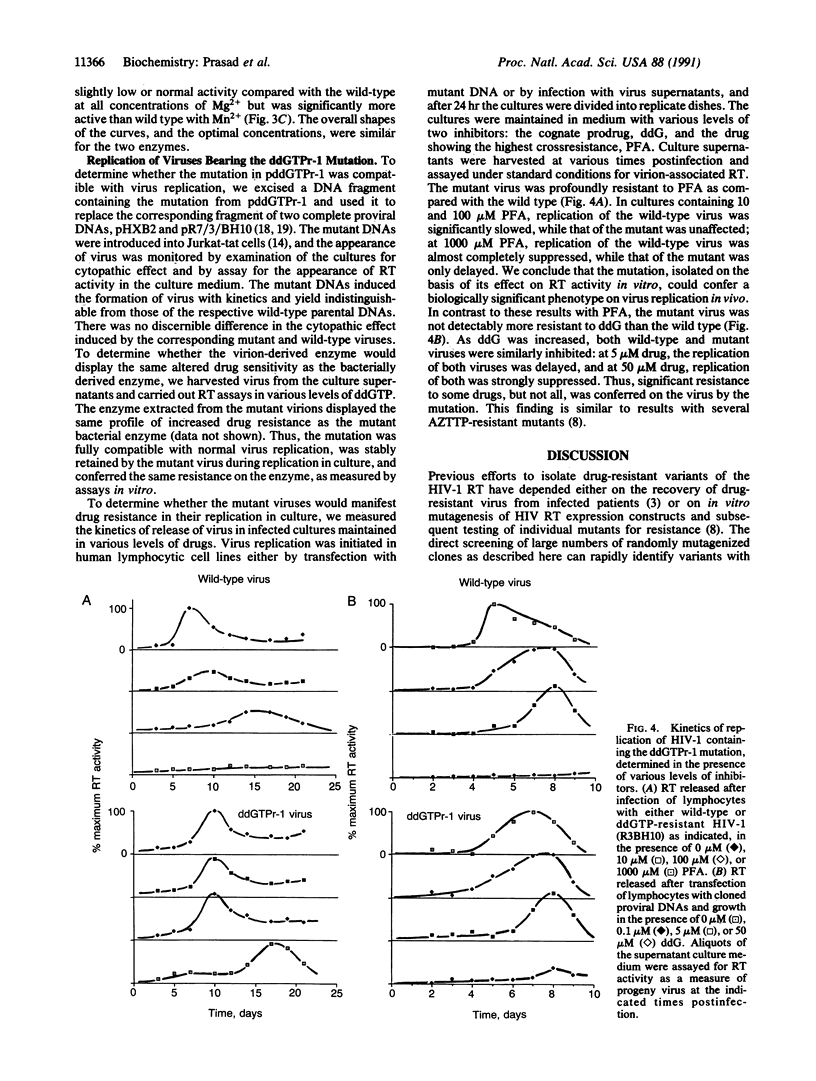
The appearance of drug-resistant strains of viral pathogens is a major difficulty confounding current efforts to block viral infections. The identification and analysis of mutations responsible for drug resistance can provide important clues helpful in understanding the mechanisms of resistance and in the eventual development of better therapies. We have used a direct screening method to scan libraries of mutagenized genes encoding the reverse transcriptase of human immunodeficiency virus type 1, and have recovered a variant enzyme that is resistant to the chain-terminator inhibitor 2',3'-dideoxyguanosine triphosphate. The single substitution mutation in this variant conferred broad crossresistance to a variety of other antiviral compounds currently in clinical trials. Virus carrying the mutation was fully infectious in cultured human lymphocytes. The replication of the mutant virus was highly resistant to phosphonoformic acid but did not show increased resistance to the prodrug dideoxyguanosine.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandra P., Vogel A., Gerber T. Inhibitors of retroviral DNA polymerase: their implication in the treatment of AIDS. Cancer Res. 1985 Sep;45(9 Suppl):4677s–4684s. [PubMed] [Google Scholar]
- Degnen G. E., Cox E. C. Conditional mutator gene in Escherichia coli: isolation, mapping, and effector studies. J Bacteriol. 1974 Feb;117(2):477–487. doi: 10.1128/jb.117.2.477-487.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F., Johnson M. S., McClure M. A. Origins and evolutionary relationships of retroviruses. Q Rev Biol. 1989 Mar;64(1):1–30. doi: 10.1086/416128. [DOI] [PubMed] [Google Scholar]
- Dougherty J. P., Temin H. M. Determination of the rate of base-pair substitution and insertion mutations in retrovirus replication. J Virol. 1988 Aug;62(8):2817–2822. doi: 10.1128/jvi.62.8.2817-2822.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Goldthorpe S. E. Antiviral drug resistance. Trends Pharmacol Sci. 1989 Aug;10(8):333–337. doi: 10.1016/0165-6147(89)90069-2. [DOI] [PubMed] [Google Scholar]
- Fischl M. A., Richman D. D., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Schooley R. T. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- Hizi A., Barber A., Hughes S. H. Effects of small insertions on the RNA-dependent DNA polymerase activity of HIV-1 reverse transcriptase. Virology. 1989 May;170(1):326–329. doi: 10.1016/0042-6822(89)90389-9. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Darby G., Richman D. D. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989 Mar 31;243(4899):1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Darby G. Virus drug-resistance: mechanisms and consequences. Antiviral Res. 1984 Apr;4(1-2):1–42. doi: 10.1016/0166-3542(84)90023-8. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT). Science. 1989 Dec 1;246(4934):1155–1158. doi: 10.1126/science.2479983. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D., Purifoy D. J. Infectious potential of human immunodeficiency virus type 1 reverse transcriptase mutants with altered inhibitor sensitivity. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4803–4807. doi: 10.1073/pnas.86.13.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larder B. A., Purifoy D. J., Powell K. L., Darby G. Site-specific mutagenesis of AIDS virus reverse transcriptase. 1987 Jun 25-Jul 1Nature. 327(6124):716–717. doi: 10.1038/327716a0. [DOI] [PubMed] [Google Scholar]
- Leider J. M., Palese P., Smith F. I. Determination of the mutation rate of a retrovirus. J Virol. 1988 Sep;62(9):3084–3091. doi: 10.1128/jvi.62.9.3084-3091.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McCune J. M., Rabin L. B., Feinberg M. B., Lieberman M., Kosek J. C., Reyes G. R., Weissman I. L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988 Apr 8;53(1):55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- Prasad V. R., Goff S. P. A novel in situ colony screening method to detect human immunodeficiency virus reverse transcriptase activity expressed in bacteria. Isolation of pseudorevertants of reverse transcriptase mutants. J Biol Chem. 1989 Oct 5;264(28):16689–16693. [PubMed] [Google Scholar]
- Prasad V. R., Goff S. P. Linker insertion mutagenesis of the human immunodeficiency virus reverse transcriptase expressed in bacteria: definition of the minimal polymerase domain. Proc Natl Acad Sci U S A. 1989 May;86(9):3104–3108. doi: 10.1073/pnas.86.9.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston B. D., Poiesz B. J., Loeb L. A. Fidelity of HIV-1 reverse transcriptase. Science. 1988 Nov 25;242(4882):1168–1171. doi: 10.1126/science.2460924. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Roberts J. D., Bebenek K., Kunkel T. A. The accuracy of reverse transcriptase from HIV-1. Science. 1988 Nov 25;242(4882):1171–1173. doi: 10.1126/science.2460925. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Campbell K., Haseltine W. A. Construction of recombinant murine retroviruses that express the human T-cell leukemia virus type II and human T-cell lymphotropic virus type III trans activator genes. J Virol. 1986 Jan;57(1):379–384. doi: 10.1128/jvi.57.1.379-384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanese N., Goff S. P. Domain structure of the Moloney murine leukemia virus reverse transcriptase: mutational analysis and separate expression of the DNA polymerase and RNase H activities. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1777–1781. doi: 10.1073/pnas.85.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanese N., Prasad V. R., Goff S. P. Structural requirements for bacterial expression of stable, enzymatically active fusion proteins containing the human immunodeficiency virus reverse transcriptase. DNA. 1988 Jul-Aug;7(6):407–416. doi: 10.1089/dna.1.1988.7.407. [DOI] [PubMed] [Google Scholar]
- Tanese N., Roth M., Goff S. P. Expression of enzymatically active reverse transcriptase in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4944–4948. doi: 10.1073/pnas.82.15.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarchoan R., Pluda J. M., Perno C. F., Mitsuya H., Thomas R. V., Wyvill K. M., Broder S. Initial clinical experience with dideoxynucleosides as single agents and in combination therapy. Ann N Y Acad Sci. 1990;616:328–343. doi: 10.1111/j.1749-6632.1990.tb17853.x. [DOI] [PubMed] [Google Scholar]



