Abstract
Disrupted sensory processing is a core feature of psychotic disorders. Auditory paired stimuli (PS) evoke a complex neural response, but it is uncertain which aspects reflect shared and/or distinct liability for the most common severe psychoses, schizophrenia (SZ) and psychotic bipolar disorder (BDP). Evoked time-voltage/time-frequency domain responses quantified with EEG during a typical PS paradigm (S1-S2) were compared among proband groups (SZ [n = 232], BDP [181]), their relatives (SZrel [259], BDPrel [220]), and healthy participants (H [228]). Early S1-evoked responses were reduced in SZ and BDP, while later/S2 abnormalities showed SZ/SZrel and BDP/BDPrel specificity. Relatives’ effects were absent/small despite significant familiality of the entire auditorineural response. This pattern suggests general and divergent biological pathways associated with psychosis, yet may reflect complications with conditioning solely on clinical phenomenology.
Keywords: Psychopathological, EEG/ERP, Sensation/Perception, Genetics
The most recent version of the Diagnostic and Statistical Manual of Mental Disorders continues the Kraepelinian tradition (Kraepelin, 1919) by distinguishing bipolar I disorder with psychosis (BDP) and schizophrenia (SZ) as categorical diseases despite substantial BDP-SZ overlap and nontrivial within-group heterogeneity on genetic disease risk (Craddock, O’Donovan, & Owen, 2009; Goes, Sanders, & Potash, 2008; Tamminga et al., 2013), clinical characteristics (Keshavan, Morris, et al., 2011; Tamminga et al., 2013), and biological profiles (Emsell & McDonald, 2009; Henry & Etain, 2010; Keshavan, Nasrallah, & Tandon, 2011; Nenadic, Gaser, & Sauer, 2012; Thaker, 2008). This distinction, therefore, may complicate understanding of etiology and disease processes underlying psychosis. Validation of empirically derived state-independent psychosis-related phenotypes may provide a valuable complementary approach. Optimally, such phenotypic markers are a heritable, index liability for illness, and are theoretically more proximal to gene transcription (“endophenotypes”; Gottesman & Shields, 1973) and thus provide a promising scaffold for unraveling the complex etiologies of psychosis pathology (Insel & Cuthbert, 2009). Given what is known about genetic risk for BDP and SZ (Smoller et al., 2013), one would expect to identify some measures that are unique to BDP, some unique to SZ, and some capturing shared BDP-SZ risk. The Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP; (Tamminga et al., 2013) study, from which this project is derived, was specifically constructed to address these issues.
Disruptions in the neural substrates of basic auditory stimulus registration and expectation have been studied extensively in psychosis using auditory paired-stimuli (or P50 gating) paradigms (Light & Braff, 1999). In the simplest version of this paradigm, identical auditory “clicks” are presented in close succession (500 ms), with stimulus pairs separated by long intervals (6–10 s). Evoked brain responses to the first (S1) and second (S2) stimuli are measured with electroencephalography (EEG), and larger differences between S1 and S2 responses have been reported for healthy subjects than those with SZ or BDP (Brockhaus-Dumke, Mueller, Faigle, & Klosterkoetter, 2008), caused by either larger ERPs to S2 (Sánchez-Morla et al., 2008) and/or an attenuated response to S1 (Clementz & Blumenfeld, 2001) among cases. Such abnormalities have been associated with dysfunctional nicotinic and adrenergic receptors (Adler, Hoffer, Griffith, Waldo, & Freedman, 1992; Adler et al., 1994), and genetic promoters thereof (Martin et al., 2007), providing potential links between the auditory paired-stimuli markers and biological mechanisms.
Traditionally, studies of auditory paired-stimuli processing in psychosis have focused on P50 and N100 peaks to S1 and S2 (positive/negative deflection at 50 ms and 100 ms, respectively) at a single vertex sensor (Cz). Wide variation in findings and effect sizes has been noted (Chang, Arfken, Sangal, & Boutros, 2011; de Wilde, Bour, Dingemans, Koelman, & Linszen, 2007), perhaps ultimately impeding the maturation of these biomarkers into clinically useful tools. Contemporary work has highlighted that neurophysiological understanding is augmented by broadening the spatial (scalp distribution; Clementz & Blumenfeld, 2001) and temporal extent of data analyses (Clementz, Dzau, Blumenfeld, Matthews, & Kissler, 2003), and through inclusion of time-frequency domain information. For instance, Hamm, Ethridge et al. (2012) identify in both BDP and SZ (a) reduced early auditory evoked responses 50–300 ms after S1 in the context of (b) augmented prestimulus gamma-band power, perhaps indicating a psychosis-related basic signal-to-noise ratio disruption in thalamocortical or sensory corticocortical circuits (Hamm, Ethridge et al., 2012). In later time ranges, SZ were differentiated from BDP and healthy subjects by a shallower recovery function immediately prior to S2 onset and more positive-going neural activations post-S2 (including the S2-P50), while BDP were unique in more excessive beta-band oscillatory power, perhaps indicating that more subtle deviations in cortical facilitation or sensorimotor excitability serve to differentiate these diagnoses.
Attempting to more fully capture the complexities of brain responses would be useful for sorting out specificity and overlap within clinical SZ and BDP diagnostic domains and may ultimately provide a powerful tool to understanding the neurophysiological disease mechanisms associated with psychosis. If early and late auditory cortical response deviations yield biomarkers of psychopathological relevance, there should be evidence of familiality and similar subgroup separation among both proband groups and their family members. The current investigations specifically investigated these predictions using spatiotemporal and frequency domain analyses of auditory paired-stimuli data with large samples of SZ and their first-degree relatives (SZrels), BDP and their first-degree relatives (BDPrels), and healthy persons collected as a part of the B-SNIP project.
Method
Subject recruitment, interviews, and EEG data recording were completed at B-SNIP consortium sites: Baltimore, Chicago, Dallas, Detroit/Boston, and Hartford (full details on recruitment and clinical and demographic characteristics are available in Tamminga et al., 2013). Clinically stable participants were recruited from the community, linked community facilities and programs, community advertisements, and local National Association on Mental Illness (NAMI) or NAMI-type groups. Medical history was acquired, and participants were administered the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) diagnosis (patient or nonpatient version as appropriate). Persons meeting a DSM-IV diagnosis of SZ or BDP were rated on the Positive and Negative Symptom (PANSS; Lançon, Auquier, Nayt, & Reine, 2000), Young Mania Rating (YMRS; Young, Biggs, Ziegler, & Meyer, 1978), Montgomery-Asberg Depression Rating (MADRS; Montgomery & Asberg, 1979), and Global Assessment of Functioning (GAF) scales by trained Master’s or doctoral-level nurses, psychologists, or psychiatrists. SZrel or BDPrel recruited for the study were additionally administered the Structured Interview for DSM-IV Personality Disorders (SIDP-IV; Zanarini, Frankenburg, Sickel, & Yong, 1996) to evaluate psychosis spectrum personality traits/disorders. Exclusion criteria included serious medical, neuro-opthalmological, or neurological illness, mental retardation, head trauma with > 30 min unconsciousness, current substance use ascertained by history and urine drug screens on the day of testing, abuse in the past 3 months, dependence within 6 months, or extensive history of drug dependence. Healthy persons (H) were absent lifetime psychotic disorders or a history of psychotic or bipolar disorders in their first-degree relatives according to Family History Research Diagnostic Criteria (Andreasen, Endicott, Spitzer, & Winokur, 1977). This paper includes 1,120 total subjects.1 Age, sex, site distributions, and clinical scores are presented in Table 1. Among those with SZ, all but 40 were taking psychotropic medications. Among those with BDP, 24 were free of such medication. Detailed information regarding medication is presented in online Supplemental Table 1. The interested reader is referred to Tamminga et al. (2013) for complete details on clinical procedures and information.
Table 1.
Subject Demographic and Clinical Data
| H | SZ | BDP | SZrel | BDPrel | |
|---|---|---|---|---|---|
| Subjects in analyses (# of females) | 225 (131) | 229 (78) | 179 (109) | 255 (171) | 215 (139) |
| Age | 37.40 (12.5) | 35.11 (12.7) | 36.13 (12.8) | 43.52 (15.4) | 40.40 (15.7) |
| HU (# of females) | 53 (30) | 39 (14) | 23 (16) | 39 (27) | 17 (13) |
| UIC (# of females) | 67 (37) | 49 (19) | 64 (44) | 66 (47) | 89 (57) |
| UM (# of females) | 21 (16) | 61 (19) | 37 (20) | 50 (21) | 41 (30) |
| UTS (# of females) | 38 (24) | 26 (11) | 19 (11) | 29 (19) | 22 (11) |
| YU (# of females) | 46 (24) | 54 (15) | 36 (18) | 71 (47) | 46 (28) |
| Trials used | 138.7 (12.4) | 137.4 (14.2) | 138.5 (12.0) | 140.9 (10.9) | 139.0 (13.3) |
| GAF | 86.71 (6.61) | 49.22 (12.2) | 60.87 (12.5) | 74.13 (14.1) | 75.86 (13.5) |
| PANSS-positive | – | 17.01 (5.49, n = 220) | 12.61 (4.29, n = 176) | 16.07 (5.14, n = 30) | 13.80 (5.80, n = 25) |
| PANSS-negative | – | 16.98 (5.72, n = 220) | 12.13 (3.78, n = 176) | 13.67 (4.27, n = 30) | 12.76 (4.88, n = 25) |
| PANSS-general | – | 32.90 (8.88, n = 220) | 28.48 (8.02, n = 176) | 30.70 (8.04, n = 30) | 31.00 (7.34, n = 25) |
| MADRS | – | 8.850 (7.81, n = 207) | 10.71 (9.32, n = 168) | 6.529 (8.05, n = 51) | 7.333 (8.29, n = 39) |
| Young Mania Scale | – | 5.636 (5.81, n = 221) | 5.102 (5.80, n = 176) | 3.608 (4.47, n = 51) | 4.155 (6.29, n = 38) |
Note. Symptom scores are provided for probands and the subset of relatives of SZ and BD with a lifetime history of psychosis. HU = Harvard University; UIC = University of Illinois, Chicago; YU = Yale University; UTS = University of Texas Southwestern; UM = University of Maryland; GAF = Global Assessment of Functioning; PANSS = Positive and Negative Symptom Scale; MADRS = Montgomery-Åsberg Depression Rating Scale; APS = antipsychotic medication.
Stimuli
Recording conditions were equivalent and stimulus presentation and recording equipment identical across sites, and intersite reliability was maintained (Tamminga et al., 2013). A previous publication established absence of site effects (Hamm, Ethridge et al., 2012). While seated in a sound and electrically shielded booth (ambient sound = 61–63 dB; luminance = 0.11–0.12 foot-candles), subjects passively listened to 150 binaural broadband auditory click pairs (4-ms duration at 75 dB sound pressure level; 500-ms interclick interval) occurring an average of every 9.5 s (9–10 s interpair interval) and delivered by two 8 Ω speakers located 50 cm in front of them. Participants who were smokers refrained from smoking 1 h prior to testing.
Recording
EEG were continuously recorded from 64 Ag/AgCl sensors (impedance < 5 KΩ; Quik-Cap, Compumedics Neuroscan, El Paso, TX), positioned according to the standard 10-10 EEG system plus mastoids and CP1/2 locations to provide sampling below the canthomeatal line, with nose reference and forehead ground. Recordings were amplified (× 12,500) and digitized (1000 Hz) using Neuroscan Acquire and Synamps2 recording systems (Compumedics Neuroscan).
Data Processing
Raw EEG data were inspected for bad sensors and artifacts. Bad sensors were interpolated (< 5% for any subject) using spherical spline interpolation (BESA 5.3; MEGIS Software, Grafelfing, Germany). Data were converted to an average reference and digitally band-pass filtered from 0.5–55 Hz (zero phase filter; rolloff: 6 and 48 dB/octave, respectively). Blink and cardiac artifacts identified using independent components analysis were removed (EEGLAB 9.0; Delorme & Makeig, 2004). Data were segmented into epochs from 100 ms before to 850 ms after click-pair onset. The 100-ms pre-S1 period served as baseline. Epochs containing activity greater than 75 μV were eliminated. Subjects with fewer than 60% of total trials accepted (< 90 trials) were not included in further analyses (3 H, 3 SZ, 2 BDP, 4 SZrel, 5 BDPrel; final group numbers in Table 1). Total trials used did not significantly differ between groups (Table 1). Data from good trials were averaged across trial types within a subject to create 64-sensor event-related potentials (ERPs).
Spatiotemporal Data Reduction
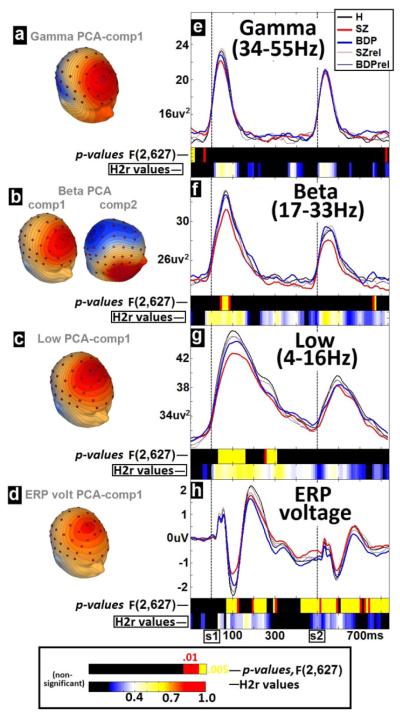
In order to maximize use of available spatial, temporal, and oscillatory information in the evoked auditory response, a frequency-wise principal component analysis (PCA) of evoked power (Ivleva et al., 2013) was first conducted across all subjects to define frequency bands for analysis (see Supplemental Methods): (a) LOW, 4–16 Hz; (b) BETA, 17–33 Hz; and (c) GAMMA, 34–55 Hz. Next, a spatial PCA (Carroll et al., 2008; Dien, Khoe, & Mangun, 2007; Hamm, Ethridge et al., 2012) was completed on the broadband grand-averaged ERP waveforms (for traditional ERP analyses) and then once for each frequency band (see Supplemental Methods for details). Figure 1a–d displays sPCA weights (topographies) for each waveform (ERP-voltage, LOW, BETA, and GAMMA). Weights were then multiplied by multisensor broadband ERP waveforms at each time point and summed across sensors, yielding a single “virtual sensor.” An additional step for LOW, BETA, and GAMMA involved convolving the virtual sensor with modified Morlet wavelets (4–55 Hz, 4-ms steps, 1 cycle at lowest to 8 cycles at highest) (Ethridge et al., 2012; Hamm, Dyckman, McDowell, & Clementz, 2012; Hamm, Ethridge et al., 2012) to derive oscillatory power waveforms for each frequency bin (Supplemental Methods). For BETA, two sPCA components were derived; weighted averages of the two power waveforms were summed to derive a single waveform for analysis. This resulted in four sets of component scores (Figure 1e–h), which were analyzed instead of 64 separate sensors, efficiently summarizing the spatial distributions, minimizing the number of statistical comparisons necessary, and maximizing the signal/noise ratio of the ERP data (Carroll et al., 2008; Clementz & Blumenfeld, 2001; Ethridge et al., 2012; Hamm, Ethridge et al., 2012).
Figure 1.
Waveform comparisons. Left (a–d): Spatial PCA component distributions for GAMMA, BETA, LOW, and ERP waveforms. Right (e–f): Group average waveforms, SZ-BDP-H ANOVA p values (above bar), and familiality values (, below bar; e–f).
Waveform Group Comparisons
As an initial step to determine time-voltage and time-frequency periods of psychopathological interest while considering possible age and gender effects between groups (Table 1), comparisons were completed as follows. First, healthy aging effects were modeled by regressing time-bin amplitudes on age for H. When beta coefficients for age effects were significant (p < .05), data for all subjects within the time bin were adjusted by removing the predicted impact of age on waveform amplitude prior to group comparisons (Dukart, Schroeter, & Mueller, 2011). Healthy aging effects were equivalent between genders, generally small (all r2 < .14), and were scarcely significant except for P50 and early LOW amplitudes (< 100 ms) to S1/S2. Data from each of the four waveforms (ERP-voltage, LOW, BETA, GAMMA) were then grouped into 10-ms bins and averaged within each bin across the entire epoch (95 separate bins per waveform). Three × 2 analyses of variance (ANOVAs) (Diagnosis × Gender) were calculated to determine H versus proband differences and Group × Gender interactions on waveform amplitudes. To account for significant effects due to a small number of large voltage values within a bin, nonparametric probability estimates were calculated via a bootstrap procedure. Monte Carlo simulations determined that two adjacent time bins significant at p < .005 or three at p < .01 were required to maintain familywise alpha at p < .01 (see Supplemental Methods for details). Data from significant time-bin clusters were extracted for an analysis of independence and examination of H versus relative effects.
Variable Reduction and Relatives Analysis
To efficiently summarize variables that uniquely discriminated proband and H (constituting unique candidate psychosis-related biomarkers), values from consecutive significant time bins were averaged for each subject and submitted to linear discriminant analysis with group as the dependent variable (H, SZ, BDP). Variables minimizing the overall Wilks’ lambda with individual multiple F statistics significant at p < .01 were entered in a stepwise fashion (Mardia, Kent, & Bibby, 1980), leaving a parsimonious selection of neurophysiological measures (Ethridge et al., 2012; Hamm, Ethridge et al., 2012). Next, effect sizes comparing each proband and relative group to H were examined for each of the surviving variables. Glass’s Δ, means, and standard deviations for all groups are reported in Table 2. Significance was assessed based on bootstrapped 95% confidence intervals. Initially, SZrel and BDPrel group statistics in Table 2 were computed including individuals with (a) history of any psychosis spectrum disorder (SZrels = 31, BDPrels = 18), and (b) meeting all or all but one criterion for an Axis II Cluster A or B personality disorder (SZrel = 52, BDPrel = 36). Computed means, SDs, and effects sizes for relatives after excluding (a) and then (a + b) are displayed in Supplemental Table 2.
Table 2.
Primary Effects
| Pre-S1 GAMMA | S1 N100 | LOW early | S1 P200 | S2 P50 | ||
|---|---|---|---|---|---|---|
| Mean (standard deviation) | H | 12.76 (4.90) | −1.85 (1.33) | 46.70 (4.58) | 1.39 (1.50) | −0.58 (0.82) |
| SZ | 14.52 (4.80) | −1.11 (1.42) | 43.87 (5.69) | 0.98 (1.29) | −0.39 (0.80) | |
| BDP | 14.04 (4.71) | −1.43 (1.40) | 45.38 (4.91) | 0.67 (1.38) | −0.74 (0.80) | |
| SZrel | 13.22 (4.98) | −1.70 (1.55) | 46.26 (5.54) | 1.27 (1.52) | −0.41 (0.95) | |
| BDPrel | 13.58 (4.61) | −1.74 (1.48) | 46.07 (5.29) | 1.07 (1.56) | −0.57 (0.87) | |
| Glass’s Δ (vs. H) | SZ | 0.36 | 0.557 | −0.618 | −0.271 | 0.232 |
| BDP | 0.261 | 0.320 | −0.288 | −0.475 | −0.19 | |
| SZrel | 0.095 | 0.113 | −0.095 | −0.08 | 0.207 | |
| BDPrel | 0.167 | 0.089 | −0.137 | −0.214 | 0.009 |
Note. Means, standard deviations (above), and effect sizes (below) for simple comparisons of variables uniquely discriminating proband groups. Effects with bootstrapped confidence intervals (2.5% and 97.5%) not including zero are indicated by bold type.
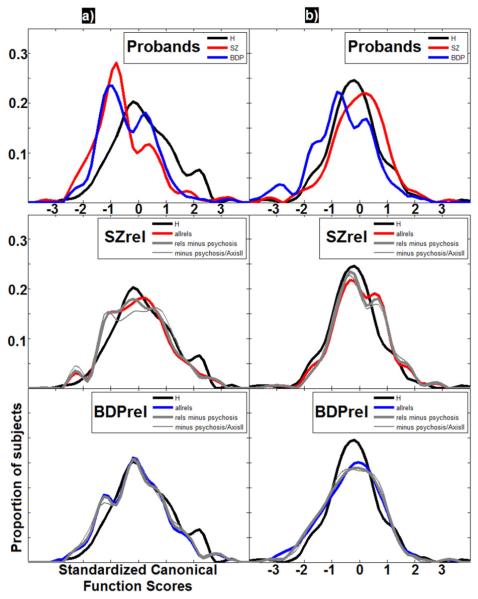
Finally, to identify how EEG variables interrelate in their descriptions of group differences, multivariate functions were calculated with a canonical discriminate analysis (CDA). CDA is similar to PCA but uses pooled within-group covariance matrices and pits group means as variables and measurements as observations (Kshirsagar, 1972; Lawley, 1959). Thus, the ngroups-1 components (or functions) are extracted (in our case, two), which are uncorrelated and maximize group differences. For each function, means and SDs were calculated and distributions were plotted for each group as a linear frequency plot (Figure 2).
Figure 2.
Frequency plots of canonical functions. Proportion of the total group membership for probands (above) and each relative group (below; plotting also for exclusion of psychosis history and Axis II pathology) for (a) early, and (b) late ERP canonical functions.
Familiality Analyses
Because strong claims of traditional genetic heritability in the current sample are problematic given the absence of monozygotic twin pairs or second-degree relatives (Kendler & Neale, 2009), the more conservative term “familiality” was chosen to refer to the degree to which ERP measures are predicted by family membership. Familiality was assessed in proband-relative pedigrees via estimates calculated using SOLAR (Sequential Oligogenic Linkage Analysis Routines; Almasy & Blangero, 1998). This approach is the same used to report “heritability” in previous reports with the same kind of family sample (Turetsky et al., 2008). Total phenotypic variance was partitioned into additive polygenic and random environmental components. We assessed effects of age and sex on each phenotype and, when significant, adjusted for their effects in the familiality analyses. Statistical significance of was determined by comparing the log likelihoods between the polygenic model and the sporadic model, where the was constrained at zero (Hong et al., 2008) (blacked out bins in Figure 1e–h represent p > .01).
Results
Proband Effects
Average waveforms for all H, SZ, and BDP (Figure 1e–h) along with relatives and plots of p values for DX main effects (upper color strip below waveforms in Figure 1e–h) reveal several time bins that significantly differentiated groups. Seven separate bin clusters exceeded adjusted p < .01 for the ERP-voltage waveform:
S1_N100: 70 to 130 ms after S1, peaking at 105 ms, F(2,627) = 14.6
S1_P200: 200 to 260 ms, peaking at 235 ms, F(2,627) = 14.6
pre_S2: 420 to 510 ms, peaking at 465 ms, F(2,627) = 11.4
S2_P50: 530 to 600 ms, peaking at 555 ms, F(2,627) = 15.4
S2_N1/P200: 600 to 710 ms, peaking at 685 ms, F(2,627) = 9.00
S2_N200: 730 to 750 ms, peaking at 735 ms, F(2,627) = 6.28
S2_late: 770 to 850 ms, peaking at 785 ms, F(2,627) = 13.1
Four periods reached threshold for oscillatory power waveforms:
LOW_early: 40 to 160 ms after S1, peaking at 105 ms, F(2,627) = 14.4
LOW_mid: 260 to 310 ms, peaking at 275 ms, F(2,627) = 7.46
BETA_early: 40 to 90 ms, peaking at 55 ms, F(2,627) = 5.76
preS1_GAMMA: −100 to −70 ms, peaking at −85 ms, F(2,627) = 9.80
No time bins had significant DX × Gender interactions. Adding a collection site factor revealed no Group × Site interactions.
Discriminant Analysis
Linear discriminant analyses using the 11 variables with significant effects indicated that five of these variables explained unique group discrimination variance, thereby accounting for the between-group variance explained by the 11 significant effects: preS1_GAMMA, N100_S1, LOW_early, P200_S1, and P50_S2. Group comparisons meeting significance level are bolded in Table 2.
Prior to S1, SZ and BDP had higher baseline GAMMA amplitude than H with similar effect sizes (Table 2). Amplitudes of N100_S1 and LOW_early were significantly reduced in both proband groups compared to H but with larger effect sizes in SZ than BDP (75%–115% larger). None of these measures showed significant reductions in relatives (all Glass’s Δ < 0.17/ > −0.17).
Amplitudes of the S1_P200 were also reduced in both proband groups but showed the opposite pattern: BDP effect sizes were 75% larger than SZ. Relatives of BDP, but not of SZ, showed significant reductions of S1_P200. This effect was attenuated, but was still statistically significant, by exclusion of relatives with history of psychosis, dropping the effect size from −0.21 to −0.18.
SZ and BDP both deviated from H on S2_P50, but SZ had significantly more positive amplitudes than H in this time range while BDP were significantly more negative. Importantly, relatives of SZ also had more positive S2_P50 amplitudes than H (Δ = .21); this effect remained unchanged after the exclusion of subjects with psychosis and Axis II personality disorder history (Δ = .22). BDPrel did not deviate from H in this time range.
Multivariate Canonical Analysis
The five variables surviving discriminant analysis were used to construct the two CDA functions, each of which significantly reduced Wilks’ lambda estimates for overall variance (p < .001) (Lawley, 1959). Loadings (correlation) for the remaining variables describe the nature of the extracted functions (Table 3). The first function, called “early ERP,” was most heavily associated with activity before 300 ms post-S1, including N100, lower frequency evoked amplitude (LOW_early/mid, BETA_early), and pre-stimulus GAMMA activity. For probands, this function showed a pattern of H > BDP > SZ, while relative groups were intermediate. The second function, called “late ERP,” was associated with ERP-voltage activity after 300 ms post-S1, including the pre-S2 recovery (negativity) and the S2_P50. For probands and relatives, this function showed a pattern of BDP(rel) < H < SZ(rel). Interestingly, the S1_P200, being intermediate in latency, loaded equally on both functions (Table 3).
Table 3.
Canonical Functions
| Canonical functions |
||
|---|---|---|
| Early ERP: loadings | Late ERP: loadings | |
| LOW_early | 0.70 | −0.31 |
| S1_N100 | −0.70 | 0.19 |
| preS1_GAMMA | −0.50 | 0.03 |
| LOW_mid | 0.45 | −0.12 |
| BETA_early | 0.34 | −0.13 |
| S2_P50 | 0.18 | 0.69 |
| S1_P200 | 0.51 | 0.53 |
| S2_P200 | 0.03 | 0.56 |
| S2_late | −0.06 | 0.52 |
| preS2 | −0.24 | 0.49 |
| S2_N100 | −0.03 | 0.47 |
| Group | Early ERP: Means (SD) | Late ERP: Means (SD) |
| H | 0.42 (1.04) | 0.07 (0.95) |
| SZ | −0.31 (1.03) | 0.22 (0.99) |
| BDP | −0.14 (0.90) | −0.36 (1.07) |
| SZrel | 0.27 (1.07) | 0.18 (1.09) |
| BDPrel | 0.17 (1.07) | −0.02 (1.05) |
| Familiality () | 0.31, p < .001 | 0.17, p < .05 |
Note. Above: loadings (correlations) for each canonical function with each ERP variable. Below: Means and standard deviations for each group and function. Bottom: Familialities for each function.
Use of SKUMIX (Hamm, Dyckman, Ethridge, McDowell, & Clementz, 2010; Maclean, Morton, Elston, & Yee, 1976), which employs a maximum likelihood approach to test for significant skewness and multimodality, identified that both SZ and BDP distributions for the multivariate early ERP function were skewed (γSZ = 0.87; γBDP = 0.56), but not multimodal.
Familiality
Amplitudes of all waveforms displayed consistently significant estimates throughout (lower color strip below waveforms in Figure 1e–h). in the current context indexes the proportion (bounded from 0.0–1.0) of phenotypic variance not due to age or gender that is attributable to factors shared with first-degree relatives. For ERP-voltage waveforms, most time points in the P50/N100/P200 complex to both S1 and S2 had significant ranging from .2 to .5 and peaking in the early P200 range to S1 () and again in the N100 range to S2 ().
LOW frequency waveforms showed significant across the entire epoch post-trial onset. The largest for this frequency band coincided with peak amplitude values to S1 onset (120–200 ms) and reflected a substantial level of familiality (peak ). BETA frequency waveforms showed significant for most post-trial onset time points. The largest for this frequency band coincided with (a) the S1-onset amplitude increase (−20–150 ms) peaking at at 140 ms, and (b) post-S2 amplitude peak (30–70 ms) peaking at . Overall, LOW frequency amplitude peaks to S1 and BETA frequency amplitude peaks to both S1 and S2 showed the greatest familiality. GAMMA frequency waveforms showed that were more similar to ERP-voltage . Early time points post-S1 and S2 (in the vicinity of amplitude peaks) showed significant in the low to moderate range (.2–.45).
Discussion
The current investigation comprehensively leveraged EEG information to detail abnormalities of basic auditory neural processing in large samples of SZ and BDP groups. The strongly familial ERP deviations neither perfectly separated psychotic subgroups nor conclusively united them, suggesting the same pattern of complex inheritance reported in recent large-scale genetic studies (Owen, 2012; Smoller et al., 2013). Multivariate analyses indicated that early peaks and low-frequency oscillations constituted a broad, shared neuropathology between SZ and BDP, while late, slow-developing neural activities showed both disease specificity as well as the strongest differentiation of relative subgroups.
Reduced N100 to S1 is one of the most replicated heritable biomarkers of psychosis (Rosburg, Boutros, & Ford, 2008; Turetsky et al., 2008) and indexes disruption of early sensory cortical feed-forward circuitry (Hamm, Gilmore, Picchetti, Sponheim, & Clementz, 2011; Sweet et al., 2007) that may underlie disordered perception (Heinks-Maldonado et al., 2007). The current results reiterated N100’s psychosis pertinence and familiality, abnormality in BDP, and overlap and differentiation from low-frequency evoked oscillations in the same time range. Although N100 and low-frequency evoked oscillations to S1 did share similar group discriminations, the results of the linear discriminant analysis highlight that each captures some degree of unique pathology variance. Compared to the temporally and spatially more focal N100 indexing primary/secondary auditory cortical registration of a stimulus (Godey, Schwartz, de Graaf, Chauvel, & Liégeois-Chauvel, 2001; Yvert, Fischer, Bertrand, & Pernier, 2005), low-frequency evoked oscillatory power may encompass the spread of information across a spatially more broad cortical network (Kopell, Ermentrout, Whittington, & Traub, 2000). Early S1 ERPs also showed SZ effects that were 75–115% larger than BDP, echoing previous findings (Hamm, Ethridge et al., 2012; Ivleva et al., 2013), dimensional conceptualizations of psychosis (Ivleva et al., 2012), and supporting an additive inheritance model for early auditory psychosis biomarkers.
These N100 and low-frequency effects were not present among relatives, departing from some (Hall, Taylor, Salisbury, & Levy, 2010; Hong et al., 2008; Turetsky et al., 2008) but not all previous studies (Waldo, Adler, & Freedman, 1988; Winterer, Egan, Rädler, Coppola, & Weinberger, 2001). Both measures, along with similar BETA effects in the same time range, however, did show substantial familiality, with evoked beta and LOW oscillations approaching . This discrepancy between familiarity and lack of statistical effects among relatives begs for clarification. One possibility is that early auditory processing phenotypes follow a relatively complex genetic inheritance, with some relatives at risk for, but not expressing, psychosis demonstrating enhanced auditory cortical circuitry as a protective factor (see, e.g., Hamm et al., 2013).
Prior to the onset of S1, both SZ and BDP displayed augmented gamma band power that covaried with diminished N100 and evoked low-frequency oscillations. Increased intrinsic high-frequency activity is a common finding in psychotic populations (Reinhart, Mathalon, Roach, & Ford, 2011; Rolls, Loh, Deco, & Winterer, 2008; Spencer, 2011), theorized to indicate reduced NMDA receptor modulation of inhibitory interneuron activity (Curley & Lewis, 2012; Hamm, Gilmore, & Clementz, 2012) and consequent disruption of signal-to-noise ratio (Rolls et al., 2008). Given that gamma was neither significantly augmented among relatives nor showed familiality, excessive gamma power may be related to disease expression and/or neural compensation in psychosis.
In contrast to intrinsic and basic auditory processing functions, later occurring evoked responses, which most likely index higher-level cognitive and contextual processing, showed disease specificity in probands and relatives. First, the P200 peak to S1 was reduced in both SZ and BDP, but had a 75% larger effect size in BDP. P200 was also abnormal in BDPrel but not SZrel, and demonstrated significant familiality. While the precise relationship between auditory P200 and earlier ERPs is not well characterized, some evidence indicates independence of P200 and N100 sensitivity to arousal (Crowley & Colrain, 2004).
Second, the current results substantiate reduced pre-S2 negativity in SZ (Hamm, Ethridge et al., 2012) but not BDP. While this effect has been uncommonly described in the paired-stimuli context, related impairments in top-down sensory cortical anticipation modulations, including impaired corollary discharge (Heinks-Maldonado et al., 2007), have been theorized to underlie sensory disruptions in SZ (Ford & Mathalon, 2012). Pre-S2 negativity, however, was not abnormal in SZrel and did not demonstrate substantial familiality, suggesting that it marks some aspect of SZ disease expression rather than risk. Finally, responses to S2 in the P50 time range were more positive in both SZ and SZrel than H, replicating numerous previous paired-stimuli reports of augmented S2 evoked responses in SZ (Chang et al., 2011). The current findings nonetheless add nuance when considered in context of the overall evoked response. BDP/BDPrel diverged significantly from SZ/SZrel in this time range but had more negative voltage throughout the post-S2 time period. In addition, when considered in the context of a higher pre-S2 baseline among SZ, it is uncertain whether the more positive voltage P50-range responses among SZ are best described as indexing poor stimulus filtering. In contrast to early ERPs, however, these later-occurring deviations highlight SZ-and BDP-specific alterations in cortical responses and may be indexing distinct inherited biomarkers for these psychoses (Gottesman & Gould, 2003).
Medication effects are a concern for any study comparing different diagnostic groups. These groups nonetheless showed more similarities than differences in medication treatment, a feature that likely reflects the similarities in psychosis manifestations. Most late ERP effects in the current study demonstrated both familiality and reductions in unmedicated, nonsymptomatic relatives, under-girding the utility of these measures in future etiological studies and ruling out a pure medication-based explanation. Exclusion of psychosis history and Axis II pathology mostly did not significantly alter the early or late ERP means for SZrel or BDPrel, indicating that comorbid pathology does not likely account for the relatives effects. A remaining limitation of the current study is the age difference between relatives groups and H. Age effects were modeled in healthy subjects and applied, when statistically signifi-cant, to the entire sample to adjust for these discrepancies. Future efforts should be made to include more siblings and, further, to include unaffected monozygotic twins to directly assess heritability instead of simply familiality.
The current report examined complete time-voltage and time-frequency waveforms using information from the entire scalp in a parsimonious manner via spatial PCA-aided data reduction and tie binning. This empirically driven, comprehensive approach deviates from traditional auditory paired-stimuli studies of psychosis that have focused primarily on magnitudes and difference/ratio scores of P50 and N100 peaks, employing a series of data processing steps and filtering prior to peak measurements. In order to allow a more direct comparison of the current data set with previous reports, we scored P50 gating with the historical procedure (Olincy & Martin, 2005) and the less-often-studied N100 gating with the procedure employed by the Consortium on the Genetics of Schizophrenia (Turetsky et al., 2008) and others (Rentzsch, Jockers-Scherübl, Boutros, & Gallinat, 2008; see Supplemental Methods). A few outcomes of these analyses are worthy of note (see Supplemental Tables 3 and 4). The identification of S1-N100 peak reduction in SZ and SZrel, along with significant familiality for this measure and a significant S1-S2 difference in probands but not relatives, corroborates previous reports (Turetsky et al., 2008), and suggests, through an attenuation of effects in BDP/BDPrel, a specificity toward nonaffective psychosis for these abnormalities (Hamm, Ethridge et al., 2012). S1-P50 peak reductions were present in SZ with small effect sizes (Δ = −.196) and were heritable as traditionally reported (Brenner et al., 2009; Chang et al., 2011; Clementz & Blumenfeld, 2001; Thaker, 2008), yet proband and relatives effects with regard to S2-P50 amplitudes, S2/S1 ratio, and S1-S2 difference scores showed largely nonsignificant deviations from H. Psychosis P50 gating abnormalities have traditionally shown variability across reports and specific metrics, perhaps due to methodological variance (Chang et al., 2011; de Wilde et al., 2007).
The combination of early evoked response deviations shared between psychosis subgroups and later, slow, and S2 voltage deviations differentiating affective from nonaffective psychoses provide clues towards the underlying neuropathology of SZ and BDP along with a more narrow, detailed set of targets for future genetic and epidemiological studies. Examinations of multivariate composite measures highlighted clear heterogeneity and overlap present both within and between these diagnostic categories (e.g., probands in Figure 2). This pattern of deviations together with the strong familiality of these measures at once suggest the utility of auditory neurophysiological phenotypes, while high-lighting the challenge that significant biological heterogeneity poses to research in the context of the current diagnostic systems. Future studies that cut across multiple psychotic and nonpsychotic diagnostic groups, and that seek to identify homogenous patient groups with respect to these familial auditory neurophysiological phenotypes, may be fruitful (Kapur, Phillips, & Insel, 2012; Keshavan, Clementz, Pearlson, Sweeney, & Tamminga, 2013).
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01s MH077945, MH077862, MH077851, MH078113, and MH085485).
Footnotes
Data from 180 of the subjects (H and probands only) appeared in Hamm, Ethridge et al. (2012).
Additional supporting information may be found in the online version of this article at the publisher’s web-site:
References
- Adler LE, Hoffer LJ, Griffith J, Waldo MC, Freedman R. Normalization by nicotine of deficient auditory sensory gating in the relatives of schizophrenics. Biological Psychiatry. 1992;32:607–616. doi: 10.1016/0006-3223(92)90073-9. [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer L, Nagamoto HT, Waldo MC, Kisley MA, Giffith JM. Yohimbine impairs P50 auditory sensory gating in normal subjects. Neuropsychopharmacology. 1994;10:249–257. doi: 10.1038/npp.1994.28. [DOI] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. American Journal of Human Genetics. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria: Reliability and validity. Archives of General Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Kieffaber PD, Clementz BA, Johannesen JK, Shekhar A, O’Donnell BF, Hetrick WP. Event-related potential abnormalities in schizophrenia: A failure to “gate in” salient information? Schizophrenia Research. 2009;113:332–338. doi: 10.1016/j.schres.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Mueller R, Faigle U, Klosterkoetter J. Sensory gating revisited: Relation between brain oscillations and auditory evoked potentials in schizophrenia. Schizophrenia Research. 2008;99:238–249. doi: 10.1016/j.schres.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Carroll CA, Kieffaber PD, Vohs JL, O’Donnell BF, Shekhar A, Hetrick WP. Contributions of spectral frequency analyses to the study of P50 ERP amplitude and suppression in bipolar disorder with or without a history of psychosis. Bipolar Disorders. 2008;10:776–787. doi: 10.1111/j.1399-5618.2008.00622.x. [DOI] [PubMed] [Google Scholar]
- Chang W-P, Arfken CL, Sangal MP, Boutros NN. Probing the relative contribution of the first and second responses to sensory gating indices: A meta-analysis. Psychophysiology. 2011;48:980–992. doi: 10.1111/j.1469-8986.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld LD. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Experimental Brain Research. 2001;139:377–390. doi: 10.1007/s002210100744. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Dzau JR, Blumenfeld LD, Matthews S, Kissler J. Ear of stimulation determines schizophrenia—Normal brain activity differences in an auditory paired-stimuli paradigm. Neuroscience. 2003;18:2853–2858. doi: 10.1046/j.1460-9568.2003.03027.x. [DOI] [PubMed] [Google Scholar]
- Craddock N, O’Donovan MC, Owen MJ. Psychosis genetics: Modeling the relationship between schizophrenia, bipolar disorder, and mixed (or “schizoaffective”) psychoses. Schizophrenia Bulletin. 2009;3:482–490. doi: 10.1093/schbul/sbp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley KE, Colrain IM. A review of the evidence for P2 being an independent component process: Age, sleep and modality. Clinical Neurophysiology. 2004;115:732–744. doi: 10.1016/j.clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Curley AA, Lewis DA. Cortical basket cell dysfunction in schizophrenia. Journal of Physiology. 2012;590:715–724. doi: 10.1113/jphysiol.2011.224659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde OM, Bour LJ, Dingemans PM, Koelman JHTM, Linszen DH. A meta-analysis of P50 studies in patients with schizophrenia and relatives: Differences in methodology between research groups. Schizophrenia Research. 2007;97:137–151. doi: 10.1016/j.schres.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dien J, Khoe W, Mangun GR. Evaluation of PCA and ICA of simulated ERPs: Promax vs. infomax rotations. Human Brain Mapping. 2007;28:742–763. doi: 10.1002/hbm.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukart J, Schroeter ML, Mueller K. Age correction in dementia—Matching to a healthy brain. PLoS One. 2011;6:e22193. doi: 10.1371/journal.pone.0022193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsell L, McDonald C. The structural neuroimaging of bipolar disorder. International Review of Psychiatry. 2009;21:297–313. doi: 10.1080/09540260902962081. [DOI] [PubMed] [Google Scholar]
- Ethridge LE, Hamm JP, Shapiro JR, Summerfelt AT, Keedy SK, Stevens MC, Clementz BA. Neural activations during auditory oddball processing discriminating schizophrenia and psychotic bipolar disorder. Biological Psychiatry. 2012;72:766–774. doi: 10.1016/j.biopsych.2012.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH. Anticipating the future: Automatic prediction failures in schizophrenia. International Journal of Psychophysiology. 2012;83:232–239. doi: 10.1016/j.ijpsycho.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godey B, Schwartz D, de Graaf JB, Chauvel P, Liégeois-Chauvel C. Neuromagnetic source localization of auditory evoked fields and intracerebral evoked potentials: A comparison of data in the same patients. Clinical Neurophysiology. 2001;112:1850–1859. doi: 10.1016/s1388-2457(01)00636-8. [DOI] [PubMed] [Google Scholar]
- Goes FS, Sanders LLO, Potash JB. The genetics of psychotic bipolar disorder. Current Psychiatry Reports. 2008;10:178–189. doi: 10.1007/s11920-008-0030-5. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Shields J. Genetic theorizing and schizophrenia. British Journal of Psychiatry. 1973;122:15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Hall M-H, Taylor G, Salisbury DF, Levy DL. Sensory gating event-related potentials and oscillations in schizophrenia patients and their unaffected relatives. Schizophrenia Bulletin. 2010;37:1187–1199. doi: 10.1093/schbul/sbq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Dyckman KA, McDowell JE, Clementz BA. Pre-cue fronto-occipital alpha phase and distributed cortical oscillations predict failures of cognitive control. Journal of Neuroscience. 2012;32:7034–7041. doi: 10.1523/JNEUROSCI.5198-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Dyckman KA, Ethridge LE, McDowell JE, Clementz BA. Preparatory activations across a distributed cortical network determine production of express saccades in humans. Journal of Neuroscience. 2010;30:7350–7357. doi: 10.1523/JNEUROSCI.0785-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Ethridge LE, Shapiro JR, Pearlson GD, Tamminga CA, Sweeney JA, Clementz BA. Family history of psychosis moderates early auditory cortical response abnormalities in non-psychotic bipolar disorder. Bipolar Disorders. 2013;15:774–786. doi: 10.1111/bdi.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Ethridge LE, Shapiro JR, Stevens MC, Boutros NN, Summerfelt AT, Clementz BA. Spatiotemporal and frequency domain analysis of auditory paired stimuli processing in schizophrenia and bipolar disorder with psychosis. Psychophysiology. 2012;49:522–530. doi: 10.1111/j.1469-8986.2011.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Gilmore CS, Clementz BA. Augmented gamma band auditory steady-state responses: Support for NMDA hypofunction in schizophrenia. Schizophrenia Research. 2012;138:1–7. doi: 10.1016/j.schres.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Gilmore CS, Picchetti NAM, Sponheim SR, Clementz BA. Abnormalities of neuronal oscillations and temporal integration to low- and high-frequency auditory stimulation in schizophrenia. Biological Psychiatry. 2011;69:989–96. doi: 10.1016/j.biopsych.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinks-Maldonado TH, Mathalon DH, Houde JF, Gray M, Faustman WO, Ford JM. Relationship of imprecise corollary discharge in schizophrenia to auditory hallucinations. Archives of General Psychiatry. 2007;64:286–296. doi: 10.1001/archpsyc.64.3.286. [DOI] [PubMed] [Google Scholar]
- Henry C, Etain B. New ways to classify bipolar disorders: Going from categorical groups to symptom clusters or dimensions. Current Psychiatry Reports. 2010;12:505–511. doi: 10.1007/s11920-010-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Mitchell BD, McMahon RP, Wonodi I, Buchanan RW, Thaker GK. Sensory gating endophenotype based on its neural oscillatory pattern and heritability estimate. Archives of General Psychiatry. 2008;65:1008–1016. doi: 10.1001/archpsyc.65.9.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN. Endophenotypes: Bridging genomic complexity and disorder heterogeneity. Biological Psychiatry. 2009;66:988–989. doi: 10.1016/j.biopsych.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Ivleva EI, Bidesi AS, Thomas BP, Meda SA, Francis A, Moates AF, Tamminga CA. Brain gray matter phenotypes across the psychosis dimension. Psychiatry Research. 2012;204:13–24. doi: 10.1016/j.pscychresns.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivleva EI, Moates AF, Hamm JP, Bernstein IH, O’Neill HB, Cole D, Tamminga CA. Smooth pursuit eye movement, prepulse inhibition, and auditory paired stimuli processing endophenotypes across the schizophrenia-bipolar disorder psychosis dimension. Schizophrenia Bulletin. 2013 doi: 10.1093/schbul/sbt047. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Molecular Psychiatry. 2012;17:1174–1179. doi: 10.1038/mp.2012.105. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC. “Familiality” or heritability. Archives of General Psychiatry. 2009;66:452–453. doi: 10.1001/archgenpsychiatry.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Clementz BA, Pearlson GD, Sweeney JA, Tamminga CA. Reimagining psychoses: An agnostic approach to diagnosis. Schizophrenia Research. 2013;146:10–16. doi: 10.1016/j.schres.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Morris DW, Sweeney JA, Pearlson G, Thaker G, Seidman LJ, Tamminga C. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: The Schizo-Bipolar Scale. Schizophrenia Research. 2011;133:250–254. doi: 10.1016/j.schres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Nasrallah HA, Tandon R. Schizophrenia, “just the facts” 6. Moving ahead with the schizophrenia concept: From the elephant to the mouse. Schizophrenia Research. 2011;127:3–13. doi: 10.1016/j.schres.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. Dementia praecox and paraphrenia. E & S Livingstone; Edinburgh, Scotland: 1919. [Google Scholar]
- Kshirsagar A. Multivariate analysis. 1972 Retrieved from http://www.lavoisier.fr/livre/notice.asp?ouvrage=1668465.
- Lançon C, Auquier P, Nayt G, Reine G. Stability of the five-factor structure of the Positive and Negative Syndrome Scale (PANSS) Schizophrenia Research. 2000;42:231–239. doi: 10.1016/s0920-9964(99)00129-2. [DOI] [PubMed] [Google Scholar]
- Lawley D. Tests of significance in canonical analysis. Biometrika. 1959;46:59. [Google Scholar]
- Light GA, Braff DL. Human and animal studies of schizophrenia-related gating deficits. Current Psychiatry Reports. 1999;1:31–40. doi: 10.1007/s11920-999-0008-y. [DOI] [PubMed] [Google Scholar]
- Maclean CJ, Morton NE, Elston RC, Yee S. Skewness in commingled distributions. Biometrics. 1976;32:695–699. [PubMed] [Google Scholar]
- Mardia K, Kent J, Bibby J. Multivariate analysis. Academic Press; London, UK: 1980. [Google Scholar]
- Martin LF, Leonard S, Hall M-H, Tregellas JR, Freedman R, Olincy A. Sensory gating and alpha-7 nicotinic receptor gene allelic variants in schizoaffective disorder, bipolar type. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2007;144:611–614. doi: 10.1002/ajmg.b.30470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. The British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Nenadic I, Gaser C, Sauer H. Heterogeneity of brain structural variation and the structural imaging endophenotypes in schizophrenia. Neuropsychobiology. 2012;66:44–49. doi: 10.1159/000338547. [DOI] [PubMed] [Google Scholar]
- Olincy A, Martin L. Diminished suppression of the P50 auditory evoked potential in bipolar disorder subjects with a history of psychosis. American Journal of Psychiatry. 2005;162:43–49. doi: 10.1176/appi.ajp.162.1.43. [DOI] [PubMed] [Google Scholar]
- Owen MJ. Implications of genetic findings for understanding schizophrenia. Schizophrenia Bulletin. 2012;38:904–907. doi: 10.1093/schbul/sbs103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart RMG, Mathalon DH, Roach BJ, Ford JM. Relationships between pre-stimulus γ power and subsequent P300 and reaction time breakdown in schizophrenia. International Journal of Psychophysiology. 2011;79:16–24. doi: 10.1016/j.ijpsycho.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzsch J, Jockers-Scherübl MC, Boutros NN, Gallinat J. Test-retest reliability of P50, N100 and P200 auditory sensory gating in healthy subjects. International Journal of Psychophysiology. 2008;67:81–90. doi: 10.1016/j.ijpsycho.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nature Reviews. Neuroscience. 2008;9:696–709. doi: 10.1038/nrn2462. [DOI] [PubMed] [Google Scholar]
- Rosburg T, Boutros NN, Ford JM. Reduced auditory evoked potential component N100 in schizophrenia—A critical review. Psychiatry Research. 2008;161:259–274. doi: 10.1016/j.psychres.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Sánchez-Morla EM, García-Jiménez MA, Barabash A, Martínez-Vizcaíno V, Mena J, Cabranes-Díaz JA, Santos JL. P50 sensory gating deficit is a common marker of vulnerability to bipolar disorder and schizophrenia. Acta Psychiatrica Scandinavica. 2008;117:313–318. doi: 10.1111/j.1600-0447.2007.01141.x. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Craddock N, Kendler K, Lee PH, Neale BM, Nurnberger JI, Sullivan PF. Identification of risk loci with shared effects on five major psychiatric disorders: A genome-wide analysis. Lancet. 2013;381:1371–1379. doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM. Baseline gamma power during auditory steady-state stimulation in schizophrenia. Frontiers in Human Neuroscience. 2011;5:190. doi: 10.3389/fnhum.2011.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Bergen SE, Sun Z, Marcsisin MJ, Sampson AR, Lewis DA. Anatomical evidence of impaired feedforward auditory processing in schizophrenia. Biological Psychiatry. 2007;61:854–864. doi: 10.1016/j.biopsych.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Iveleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Sweeney JA. Clinical phenotypes of psychosis in the bipolar and schizophrenia network on intermediate phenotpes (B-SNIP) American Journal of Psychiatry. 2013;170:1263–1274. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PubMed] [Google Scholar]
- Thaker GK. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophrenia Bulletin. 2008;34:760–773. doi: 10.1093/schbul/sbn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Greenwood TA, Olincy A, Radant AD, Braff DL, Cadenhead KS, Calkins ME. Abnormal auditory N100 amplitude: A heritable endophenotype in first-degree relatives of schizophrenia probands. Biological Psychiatry. 2008;64:1051–1059. doi: 10.1016/j.biopsych.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo MC, Adler LE, Freedman R. Defects in auditory sensory gating and their apparent compensation in relatives of schizophrenics. Schizophrenia Research. 1988;1:19–24. doi: 10.1016/0920-9964(88)90035-7. [DOI] [PubMed] [Google Scholar]
- Winterer G, Egan MF, Rädler T, Coppola R, Weinberger DR. Event-related potentials and genetic risk for schizophrenia. Biological Psychiatry. 2001;50:407–417. doi: 10.1016/s0006-3223(01)01072-1. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yvert B, Fischer C, Bertrand O, Pernier J. Localization of human supratemporal auditory areas from intracerebral auditory evoked potentials using distributed source models. NeuroImage. 2005;28:140–153. doi: 10.1016/j.neuroimage.2005.05.056. [DOI] [PubMed] [Google Scholar]
- Zanarini M, Frankenburg F, Sickel A, Yong L. The Diagnostic Interview for DSM-IV Personality Disorders. McLean Hospital; Belmont, MA: 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.