Abstract
An important goal in diabetes research is to understand the processes that trigger endogenous β-cell proliferation. Hyperglycemia induces β-cell replication, but the mechanism remains debated. A prime candidate is insulin, which acts locally through the insulin receptor. Having previously developed an in vivo mouse hyperglycemia model, we tested whether glucose induces β-cell proliferation through insulin signaling. By using mice lacking insulin signaling intermediate insulin receptor substrate 2 (IRS2), we confirmed that hyperglycemia-induced β-cell proliferation requires IRS2 both in vivo and ex vivo. Of note, insulin receptor activation was not required for glucose-induced proliferation, and insulin itself was not sufficient to drive replication. Glucose and insulin caused similar acute signaling in mouse islets, but chronic signaling differed markedly, with mammalian target of rapamycin (MTOR) and extracellular signal–related kinase (ERK) activation by glucose and AKT activation by insulin. MTOR but not ERK activation was required for glucose-induced proliferation. Cyclin D2 was necessary for glucose-induced β-cell proliferation. Cyclin D2 expression was reduced when either IRS2 or MTOR signaling was lost, and restoring cyclin D2 expression rescued the proliferation defect. Human islets shared many of these regulatory pathways. Taken together, these results support a model in which IRS2, MTOR, and cyclin D2, but not the insulin receptor, mediate glucose-induced proliferation.
Introduction
In the adult mouse, the primary source of new pancreatic β-cells is replication of existing β-cells (1); islet mass regulation in humans is poorly understood. Harnessing the pathways regulating β-cell proliferation could lead to therapies that restore physiologically regulated insulin secretion and thus remains a high-priority target. Glucose increases proliferation in rodent and human β-cells (2–8). The mechanisms by which glucose drives proliferation remain debated.
Glucose activates insulin signaling pathways in β-cells, including insulin receptor substrate 2 (IRS2) (9–11) and signaling mediators AKT, mammalian target of rapamycin (MTOR), and extracellular signal–related kinase (ERK) (8,12–16). IRS2 is required for proliferation induced by activating glucokinase, but whether IRS2 is required for proliferation induced by glucose itself has not been tested. Whether secreted insulin acting locally at the insulin receptor mediates glucose-induced proliferation remains contested (12,17–19). Strong data from carefully performed studies both support (20–23) and refute (24–28) a role for AKT isoforms in driving β-cell proliferation. Inhibition of MTOR with rapamycin reduces β-cell proliferation (15,29–33), but genetic manipulation of MTOR leads to less clear results, with some studies suggesting that MTOR drives β-cell proliferation (15,34–37) and others not (38–41). ERK, activated by glucose in β-cells (12), is proproliferative in other cell types but may play a paradoxical antiproliferative role in β-cells (42,43).
To bring about proliferation, signaling pathways activate the cell cycle machinery. Cell cycle regulation in β-cells resembles that of other quiescent cell types, with the transition from Gap-1 (G1) to DNA synthesis (S) phase a critical point of regulation (44,45). Glucose promotes expression of cyclin D2 (6,46–49), a key regulator of mouse β-cell proliferation (50,51). Although cyclin D2 was believed to not be expressed in human β-cells, this locus has recently been genetically linked to human insulin secretory capacity (52,53). CDK4/6, obligate partners of D-cyclins, are critically important for β-cell mass and proliferation (54,55). Although cyclin D2 is required for β-cell proliferation in response to insulin resistance (50), whether it is required for glucose-induced β-cell proliferation is not yet known.
In light of these knowledge gaps, we set out to clarify which insulin-signaling pathways promote glucose-induced β-cell proliferation, whether insulin itself might mediate this effect, and whether cyclin D2 is required. The data suggest that IRS2 is required but that insulin receptor activation is neither necessary nor sufficient to induce β-cell proliferation. Downstream of IRS2, MTOR and cyclin D2, but not ERK, mediate glucose-induced proliferation. Of note, cyclin D2 expression is lost when IRS2 or MTOR signaling is disrupted, and the proliferation defect in β-cells lacking IRS2 or MTOR signaling is rescued when cyclin D2 levels are restored. Taken together, these studies suggest that glucose induces mouse β-cell proliferation through a pathway that includes IRS2, MTOR, and cyclin D2 but not the insulin receptor.
Research Design and Methods
In Vivo Mouse Studies
Mouse studies were approved by the University of Pittsburgh and the University of Massachusetts Medical School Institutional Animal Care and Use Committees. Eight- to 12-week-old male IRS2 (B6;129-Irs2tm1Mfw/J) wild-type (WT), heterozygous (HT), and knockout (KO) mice were surgically catheterized and infused with saline (0.9% saline, 100 μL/h) or glucose (50% dextrose, 100 μL/h) containing BrdU (100μg/h; Sigma) for 96 h, as previously described (6). Arterial blood samples were taken for glucose (Ascensia Elite XL) and insulin (Millipore/Linco) measurement at 0, 24, 48, 72, and 96 h. Following infusion, mice were killed and pancreata processed for histology.
Immunofluorescence
Pancreata were fixed (Bouin’s solution; Sigma) for 4 h and embedded in paraffin. Islet cells grown on coverslips were fixed for 10 min in 4% paraformaldehyde (Sigma). β-Cell proliferation and mass were quantified on blinded images as previously described (56); 2,009 ± 119 β-cells per pancreas were counted.
Mouse Islet Experiments
Islets were isolated from C57BL/6J (adult) or IRS2-WT, -HT, and -KO (8 weeks old) mice by ductal collagenase injection and Ficoll (Histopaque-1077; Sigma) gradient (6). For direct immunoblot, islets were handpicked in cold RPMI containing 1% FBS, 5.5 mmol/L glucose, and penicillin/streptomycin; washed in PBS/100 nmol/L Na3VO4; and frozen. For culture experiments, islets rested overnight in islet growth medium (RPMI with 10% FBS, penicillin/streptomycin, and 5.5 mmol/L glucose), were dispersed by using single-use–apportioned 0.05% trypsin, and were plated on uncoated glass coverslips (Fisherbrand) for immunofluorescence or plastic coverslips for immunoblot or quantitative PCR. Immunoblot and quantitative PCR were performed on whole islets unless dispersal was required for adenoviral infection. For proliferation, dispersed mouse islet cells were cultured for 72 h, with BrdU included the last 24 h. Rapamycin (10 nmol/L; LC Laboratories), wortmannin (100 nmol/L; Sigma), PI-103 (100 nmol/L, Selleck Chemicals), PD98059 (10 μmol/L; Calbiochem), and kinetin riboside (15 μmol/L; Sigma) were added 30 min before stimulation; hydroxy-2-naphthalenylmethylphosphonic acid (HNMPA) (10 μmol/L; Santa Cruz Biotechnology) was added 1 h before stimulation; or S961 (100 nmol/L; Phoenix Pharmaceuticals) was added 2 h before stimulation. Adenovirus (Ad-shIRS2, Ad-Cre, Ad-CMV-GFP-Cyclin-D2, Ad-shCyclin-D2, and Ad-shIR; Vector Biolabs) was added the day after islet trypsinization; multiplicity of infection (MOI) is per figure legends.
Immunoblots
Whole islets or dispersed islet cells were sonicated in lysis buffer containing 125 mmol/L Tris (pH 6.8), 2% SDS, 1 mmol/L dithiothreitol, 20 μg/mL 4-amidinophenylmethanesulfonyl fluoride hydrochloride, and protease inhibitors (Roche). Lysates were separated by SDS-PAGE, transferred to polyvinylidene fluoride membrane, and blocked in either 5% (weight for volume) milk or BSA in PBS containing 0.1% Tween 20. Antibodies included phosphorylated (p) AKT (9271S), AKT (9272), pERK (9101S), ERK (9102S), pS6 (4856S), S6 (2217S), p4E-BP1 (2855S; Cell Signaling Technology), tubulin (Calbiochem), actin, IRS2 (Millipore), insulin receptor (Thermo Fisher Scientific), proliferating cell nuclear antigen (PCNA) (Santa Cruz Biotechnology), and cyclin D2 (NeoMarkers). Data were collected on film by using ECL or ECL Prime (Amersham) or SuperSignal West Femto Maximum Sensitivity (Thermo Fisher Scientific) and quantified by using ImageJ software.
Quantitative PCR
RNA was isolated from intact or dispersed islets with RNeasy Mini (QIAGEN) or Norgen All-in-One. cDNA (iScript; Bio-Rad) was amplified (SYBR, realplex cyclers; Eppendorf) by using the primers shown in Supplementary Table 1. Data are expressed as ΔΔCt (fold change).
Human Islet Experiments
Human islets were obtained from the Integrated Islet Distribution Program (Supplementary Tables 2 and 3). Proliferation studies were performed as described in mouse islet experiments, except experimental exposure was 96 h with BrdU for the entire time.
Statistics
Data were analyzed in GraphPad Prism software and are expressed as mean ± SEM. P values were determined by two-tailed Student t test or ANOVA with Šidák posttest for multiple comparisons. P < 0.05 was considered significant.
Results
IRS2 Is Required for Hyperglycemia-Induced β-Cell Proliferation In Vivo
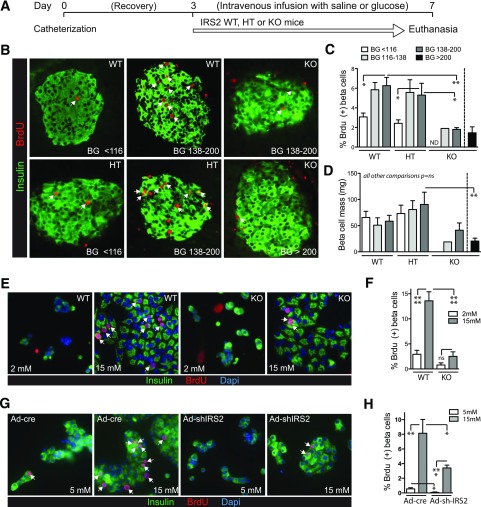
We previously developed an in vivo mouse hyperglycemia model in which blood glucose is elevated by continuous intravenous glucose infusion (6). Raising blood glucose stimulates both mouse (6,56) and human (2) β-cell proliferation. Because glucose triggers secretion of insulin, a mitogen, we considered the much debated hypothesis that glucose drives proliferation through secreted insulin acting on β-cells (12,19). To test whether insulin signaling is required for glucose-induced proliferation, we studied mice lacking IRS2, a signaling mediator of critical importance to the β-cell (57–59). Male littermates lacking zero (WT), one (HT), or both (KO) alleles of IRS2 in all tissues were infused (Fig. 1A) and stratified into groups defined by blood glucose tertiles in the WT group as follows: <116, 116–138, and 138–200 mg/dL (Supplementary Fig. 1). Glucose and insulin levels in HT mice were similar to WT mice. Seven of 13 IRS2-KO males fell into a fourth category (>200 mg/dL), but 6 KO mice had comparable blood glucose levels to hyperglycemic WT and HT mice. In WT and HT mice but not KO mice, hyperglycemia increased β-cell proliferation (Fig. 1B and C). β-Cell mass was reduced in diabetic IRS2-KO mice (Fig. 1D). Thus, in vivo glucose-driven β-cell proliferation requires insulin-signaling mediator IRS2.
Figure 1.
IRS2 is required for glucose-induced β-cell proliferation in vivo and ex vivo. A–D: Mice with generalized deletion of IRS2 or littermate controls were infused intravenously with saline or glucose for 4 days. B and C: β-Cell proliferation was increased by hyperglycemia in WT and HT controls (n = 10–16) but not in IRS2-KO mice (n = 1, 4, and 7). D: β-Cell mass was reduced in diabetic IRS2-KO mice (n = 4–9, except for KO BG 116–138 for which n = 1). E and F: Glucose induced proliferation in WT- but not KO-dispersed mouse islet cells cultured for 72 h (n = 6–9). G and H: Acute knockdown of IRS2 by adenovirus-delivered shRNA targeting IRS2 (MOI 25) in mouse islet cell cultures diminished glucose-induced β-cell proliferation (n = 4). Arrows point to BrdU-positive β-cell nuclei. Data are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. BG, blood glucose; ND, not determined; ns, not signficant.
Ex Vivo Glucose-Induced Proliferation Requires IRS2
Reduced β-cell proliferation in IRS2-KO mice might result from loss of a proproliferative signal from another tissue or from a developmental defect due to lifelong loss of IRS2. We tested whether β-cells lacking IRS2 had reduced glucose-induced proliferation when removed from the in vivo environment. Ex vivo cultured IRS2-WT and -KO β-cells had similar morphology, rounded in low glucose and spreading in high glucose (Fig. 1E). However, KO β-cells did not increase proliferation in high glucose to the same extent as WT (Fig. 1E and F), supporting an islet-intrinsic role for IRS2 in glucose-induced proliferation. To test whether the proliferation defect was due to abnormal β-cell development because of the lifelong absence of IRS2, we acutely reduced IRS2 levels with an adenovirus expressing a short hairpin RNA (shRNA) targeting mouse IRS2. Acute knockdown of IRS2 recapitulated the KO proliferation defect. Thus, IRS2 is required for glucose-induced proliferation of mouse β-cells.
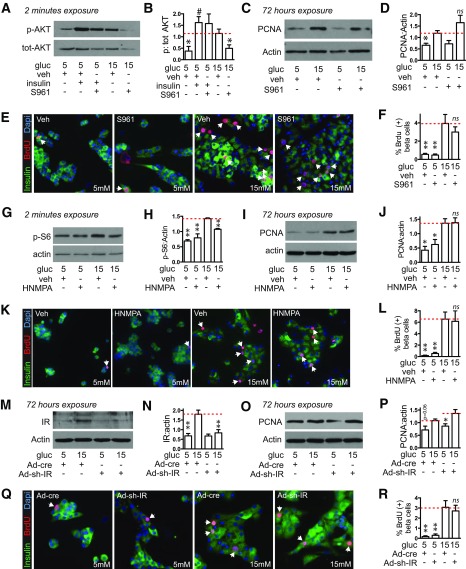
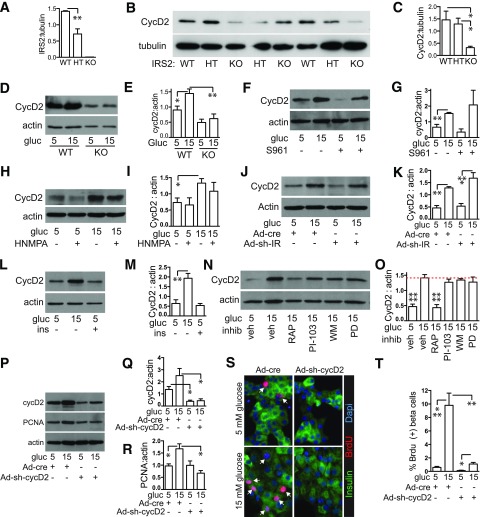
Glucose Does Not Drive Mouse β-Cell Proliferation Through the Insulin Receptor
Having confirmed that IRS2 is required for glucose-driven β-cell proliferation, we tested whether insulin acting through the insulin receptor mediates this mitogenic response. We first blocked insulin receptor activity ex vivo. At 100 nmol/L, the S961 insulin receptor antagonist effectively blocked phosphorylation of AKT in response to glucose (Fig. 2A and B). However, pretreating mouse islet cells with S961 had no effect on proliferation in either 5 or 15 mmol/L glucose as measured by immunoblot for PCNA or by BrdU incorporation (Fig. 2C–F). Blocking the insulin receptor with receptor tyrosine kinase inhibitor HNMPA produced similar results (Fig. 2G–L). To test most directly whether the insulin receptor is required for glucose-induced proliferation, we used an adenovirus expressing an shRNA targeting the mouse insulin receptor. Insulin receptor expression was induced by glucose; reduction of insulin receptor protein by ∼50% in 15 mmol/L glucose (Fig. 2M and N) failed to prevent glucose induction of proliferation as measured by PCNA or BrdU (Fig. 2O–R). These data suggest that glucose-induced β-cell proliferation requires IRS2 but not the insulin receptor.
Figure 2.
Glucose-induced proliferation in mouse islets does not require the insulin receptor (IR). A–L: IR blockers S961 (A–F; 100 nmol/L added 120 min before glucose or insulin [100 nmol/L] stimulation) or HNMPA (G–L; 10 μmol/L added 60 min before glucose stimulation) prevented activation of insulin signaling 2 min after glucose treatment (whole islets, n = 4; A and B and G and H) but did not prevent the glucose-induced increase in PCNA abundance after 72 h of glucose treatment (whole islets, n = 3–4; C and D and I and J) or the glucose-induced increase in the percent of β-cells incorporating BrdU (dispersed islets, n = 4–8; E and F and K and L). Adenovirus expressing an shRNA targeting the mouse IR (MOI 50) in dispersed islets reduced IR expression 72 h after glucose exposure (n = 5; M and N) but did not prevent the glucose-induced increase in PCNA abundance (n = 5; O and P) or β-cell BrdU incorporation (n = 4; Q and R). Arrows point to BrdU-positive β-cell nuclei. Data are mean ± SEM. *P < 0.05, **P < 0.01 vs. 15 mmol/L glucose control condition (marked by dotted line); #P < 0.05 vs. 5 mmol/L control. gluc, glucose; ns, not significant; p, phosphorylated; tot, total; veh, vehicle.
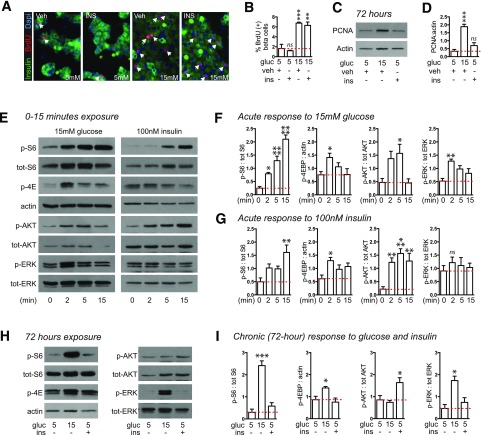
Insulin Is Not Sufficient to Drive Mouse β-Cell Proliferation
During in vivo hyperglycemia, β-cell proliferation correlated significantly with blood glucose in WT and HT mice but not in KO mice, further implicating IRS2 in the proliferative response to glucose (Supplementary Fig. 2). Plasma insulin level, however, did not correlate with β-cell proliferation in mice of any genotype. To confirm previous reports that insulin has mitogenic effects on β-cells, we assessed proliferation in mouse islets treated with insulin ex vivo. To our surprise, insulin treatment did not increase mouse β-cell BrdU incorporation in either normal or high glucose (Fig. 3A and B), whether insulin was applied daily (data shown) or once at the start of glucose exposure (data not shown). Similarly, insulin did not increase PCNA abundance in whole islets (Fig. 3C and D). We tested whether the signaling impact of insulin and glucose were different (Fig. 3E–G). Acute signaling responses to glucose and insulin were similar; however, late signaling events triggered by glucose and insulin differed markedly, with S6 and ERK robustly activated by glucose but not by insulin and AKT activated by insulin but not by glucose (Fig. 3H and I). Thus, insulin was not sufficient to drive mouse β-cell proliferation under these conditions, and MTOR and ERK but not AKT are activated by glucose at the time when proliferation is increased.
Figure 3.
Insulin is not sufficient to increase mouse β-cell proliferation; insulin and glucose have similar short-term but different long-term effects on islet signaling. A–D: Exogenous insulin (100 nmol/L, added once at experiment start) did not increase mouse β-cell proliferation as measured by BrdU incorporation (dispersed islets, n = 3; A and B) or immunoblot for PCNA (whole islets, n = 6; C and D). E–G: Acute exposure of whole mouse islets to either glucose or insulin increased phosphorylation of S6, 4E-BP1, and AKT (n = 5). H and I: Chronic exposure of whole mouse islets to glucose or insulin revealed that S6 and ERK were strongly activated by glucose but not insulin; AKT was activated by insulin but not glucose (n = 4–6). Arrows point to BrdU-positive β-cell nuclei. Data are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 relative to control condition indicated by the dotted line. gluc, glucose; ins, insulin; ns, not significant; p, phosphorylated; tot, total; veh, vehicle.
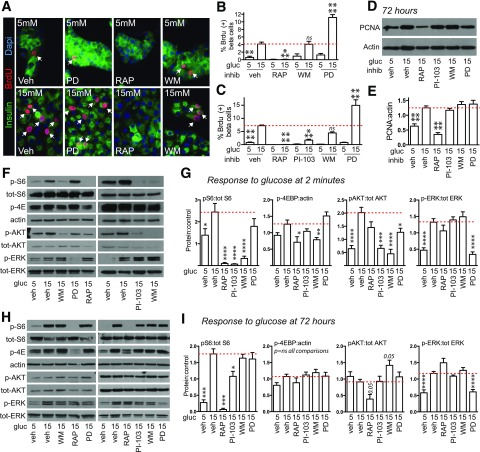
Glucose Induces Proliferation Through MTOR but Not ERK
We used insulin signaling pathway inhibitors to test whether downstream pathways mediate glucose-induced proliferation (Fig. 4A–E). To our surprise but consistent with the data suggesting that AKT was not activated by glucose after 72 h, the phosphoinositide 3-kinase (PI3K) inhibitor wortmannin had no effect on proliferation. Mitogen ERK (MEK) inhibitor PD98059 paradoxically increased proliferation. In contrast, MTOR inhibitor rapamycin eliminated the proliferative response to glucose. A second individual in the laboratory repeated this experiment, adding an additional PI3K inhibitor PI-103, and obtained similar results except that PI-103 reduced proliferation, whereas wortmannin did not. Only rapamycin prevented glucose-induced accumulation of PCNA by immunoblot. To verify that the inhibitors were blocking the intended pathways, we tested pathway activation by glucose in the presence of each inhibitor (Fig. 4F and G). Each inhibitor efficiently blocked acute activation of its target pathway. However, PI3K inhibitors also blocked acute MTOR activation. At 72 h of glucose exposure (Fig. 4H and I), rapamycin and PD98059 eliminated S6 and ERK activation, respectively. AKT was not activated by glucose, as seen previously (Fig. 3H and I), and none of the inhibitors affected this, although rapamycin showed a trend to reduce AKT phosphorylation below basal levels. PI-103 modestly reduced S6 phosphorylation. Overall, S6 pathway activation correlated best with proliferation, being highly activated by glucose but not insulin at 72 h, and inhibitors that completely (rapamycin) or partially (PI-103) blocked S6 phosphorylation reduced glucose-induced proliferation proportionally to the reduction in phosphorylated S6. Glucose increased IRS2 mRNA expression, and rapamycin did not prevent the glucose-induced increase in IRS2 mRNA (Supplementary Fig. 3). None of the inhibitors increased cell death at 72 h (Supplementary Fig. 4). These results support a model in which MEK contributes antiproliferative tone in β-cells, and chronic MTOR activation is required for glucose-induced proliferation.
Figure 4.
Glucose-induced proliferation in mouse β-cells is prevented by inhibition of MTOR. A–C: In dispersed mouse islets, glucose-induced β-cell BrdU incorporation was prevented by rapamycin (RAP) 10 nmol/L, unaffected by wortmannin (WM) 100 nmol/L, and paradoxically increased by MEK inhibitor PD98059 (PD) 10 μmol/L, all added 30 min before glucose stimulation (n = 2–3). C: The experiment was repeated by a second individual in the laboratory, adding a second PI3K inhibitor, PI-103 (100 nmol/L) (n = 3–7). D and E: In whole islets, only RAP prevented the glucose-induced increase in PCNA abundance (n = 7–11). F and G: In whole islets treated with glucose for 2 min, RAP selectively blocked MTOR activation and PD selectively blocked ERK activation, but WM and PI-103 blocked both MTOR and AKT activation (n = 4–9). H and I: In whole islets treated with glucose for 72 h, RAP effectively blocked S6 activation, but WM, PI-103, and ERK no longer blocked their target pathways (n = 6–11). Arrows point to BrdU-positive β-cell nuclei. Data are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. control marked by dotted line. gluc, glucose; inhib, inhibitor; ns, not significant; p, phosphorylated; tot, total; veh, vehicle.
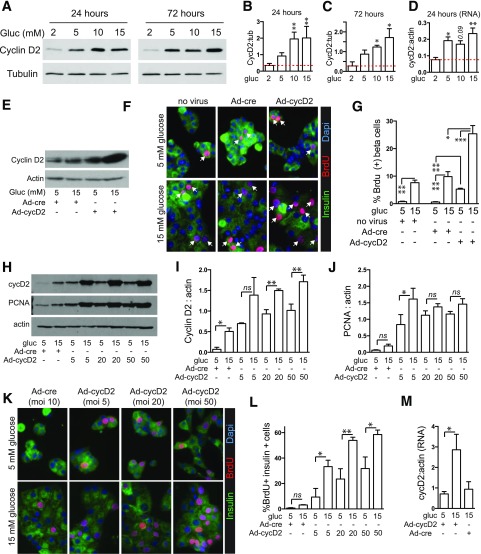
Cyclin D2 Is Sufficient to Drive β-Cell Proliferation
Excess glucose increases cyclin D2 protein in mouse β-cells both in vivo and ex vivo (6,47). In mouse islets, proliferative concentrations of glucose increased cyclin D2 protein at 24 and 72 h (Fig. 5A–C). Although low glucose reduced cyclin D2 mRNA, increasing glucose above 5 mmol/L did not further increase cyclin D2 mRNA (6,29). To test whether cyclin D2 is sufficient to drive mouse β-cell proliferation, we overexpressed cyclin D2 (Fig. 5E–G). Transduction of islet cells with Ad-CMV-GFP-Cyclin-D2 increased cyclin D2 abundance (Fig. 5E). Control adenovirus did not alter proliferation, but overexpression of cyclin D2 increased the number of β-cells entering the cell cycle at both low and high glucose (Fig. 5F and G). Of note, glucose responsiveness of proliferation was maintained even when cyclin D2 was overexpressed. To test whether glucose dependence of proliferation was due to cyclin D2 expression level, we attempted to increase cyclin D2 expression at low glucose by increasing the MOI of transduction (Fig. 5H–L). Despite increasing Ad-cyclin D2 MOI as high as 50, glucose dependence of cyclin D2 expression and BrdU incorporation were maintained. In contrast to endogenous cyclin D2 mRNA regulation (Fig. 5D), cyclin D2 mRNA expression in adenovirus overexpression was markedly induced by glucose, which is possibly related to glucose activation of the cytomegalovirus promoter (60).
Figure 5.
Cyclin D2, induced by glucose in mouse islets, is sufficient to drive β-cell proliferation. A–D: Glucose increased cyclin D2 protein abundance in whole islets at both 24 and 72 h of exposure at the protein (n = 3; A–C) and RNA (n = 3; D) level. E–G: Adenoviral overexpression of cyclin D2 (MOI 5) for 72 h in dispersed islets increased cyclin D2 abundance (E) and BrdU incorporation (n = 3; F and G) modestly in low glucose but markedly in high glucose. H–M: Glucose induction of cyclin D2 protein abundance (n = 3; H and I) and BrdU incorporation (n = 3; K and L) but not PCNA abundance (n = 3; H and J) was evident, even when MOI of Ad-cyclin D2 was increased as high as 50. M: Cyclin D2 mRNA was increased by high glucose in the context of adenoviral overexpression (dispersed islets, n = 3). Arrows point to BrdU-positive β-cell nuclei. Dotted lines in B–D mark the low glucose control. Data are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. cyc, cyclin; gluc, glucose; ns, not significant; tub, tubulin.
Glucose Induction of Cyclin D2 Protein Requires IRS2 and MTOR
Because IRS2 and MTOR are required for glucose-induced proliferation and MTOR activation increases cyclin D2 expression in islets (29), we tested whether cyclin D2 acts downstream of IRS2-MTOR in hyperglycemia. We first tested whether IRS2 affects cyclin D2 expression in vivo. Islets from IRS2-KO mice contained less cyclin D2 protein than islets from control mice (Fig. 6A–C). To learn whether glucose-mediated induction of cyclin D2 requires IRS2, WT and KO islets were cultured in 5 or 15 mmol/L glucose (Fig. 6D and E). KO islets had less cyclin D2 at baseline and failed to increase cyclin D2 protein with glucose treatment. We next tested whether the insulin receptor was required for glucose-induced cyclin D2 accumulation. Similar to the proliferation studies, inhibition of the insulin receptor using S961 (Fig. 6F and G), HNMPA (Fig. 6H and I), or shRNA (Fig. 6J and K) did not prevent the glucose-induced increase in cyclin D2 expression. Conversely, 72-h treatment with insulin did not increase cyclin D2 protein (Fig. 6L and M). To dissect which signaling pathway downstream of IRS2 mediates this effect, we tested whether blocking MEK, PI3K, or MTOR interfered with glucose induction of cyclin D2 protein. Consistent with the proliferation studies and prior work (29), MTOR inhibition reduced cyclin D2 level (Fig. 6N and O). Thus, glucose-mediated cyclin D2 induction and proliferation both require IRS2 and MTOR but not signaling through the insulin receptor.
Figure 6.
IRS2 and MTOR but not the insulin receptor are required for glucose induction of cyclin D2; cyclin D2 is required for glucose-induced β-cell proliferation. A–C: IRS2-KO islets contained less IRS2 (n = 1–4; A) and cyclin D2 (n = 4–6; B–C). D and E: IRS2-KO islets failed to induce cyclin D2 protein when cultured in high glucose (n = 5–14). F–K: Blocking insulin receptor action using S961 (whole islets, n = 4; F–G), HNMPA (whole islets, n = 3; H–I), or adenovirus with shRNA targeting the insulin receptor (MOI 50) (dispersed islets, n = 5; J–K) did not prevent glucose induction of cyclin D2 protein. L and M: Treating whole islets with insulin (100 nmol/L) did not induce cyclin D2 protein expression (n = 4). N and O: Blocking MTOR but not PI3K or ERK in whole islets prevented glucose induction of cyclin D2 protein (n = 5–8). P–T: Reducing cyclin D2 expression by adenovirus with shRNA targeting cyclin D2 in dispersed islets (MOI 5, n = 3) prevented glucose induction of cyclin D2 (P and Q), PCNA (P and R), and proliferation (S and T). Panels D–T are at 72 h. Arrows point to BrdU-positive β-cell nuclei. Dotted line in O marks the high glucose control. Data are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. cyc, cyclin; gluc, glucose; ins, insulin; IR, insulin receptor; PD, MEK inhibitor PD98059; RAP, rapamycin; veh, vehicle; WM, wortmannin.
Cyclin D2 Is Required for Glucose-Induced Proliferation
Although D-cyclins and their CDK partners are critical for β-cell proliferation in mice (50,51,55), whether cyclin D2 is required for glucose-induced β-cell proliferation is not known. Kinetin riboside, a small molecule inhibitor of D-cyclin expression (61), reduced islet cyclin D2 abundance and proliferation in high glucose (Supplementary Fig. 5A and B) but also altered cell morphology, suggesting toxicity (data not shown). Targeting cyclin D2 by shRNA reduced cyclin D2 protein in mouse islet cells (Fig. 6P and Q) and decreased β-cell proliferation in high glucose by both PCNA and BrdU (Fig. 6P–T). These results suggest that cyclin D2 is required for glucose-induced mouse β-cell proliferation.
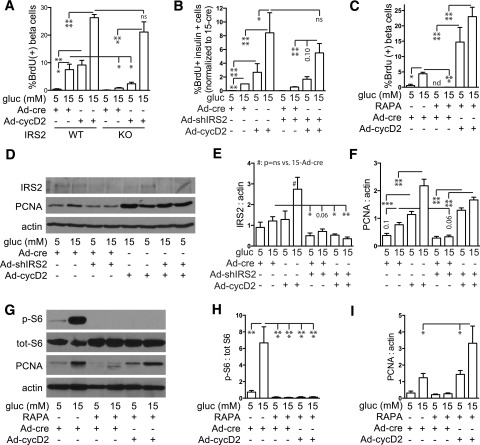
Restoring Cyclin D2 Expression Rescues Impaired Proliferation Due to Loss of IRS2 and MTOR
Because IRS2 and MTOR deficiency reduced cyclin D2 expression, we hypothesized that restoring cyclin D2 might correct the defect in glucose-induced proliferation. We first tested whether overexpression of cyclin D2 restores proliferation in β-cells lacking IRS2 (Fig. 7A and Supplementary Fig. 6). In IRS2-KO β-cells, glucose-induced proliferation was reduced with control virus, but overexpression of cyclin D2 rescued the proliferation defect. shRNA knockdown of IRS2 produced a similar result (Fig. 7B). To explore why proliferation was paradoxically glucose dependent in IRS2 knockdown cells with Ad-CMV-GFP-Cyclin-D2, we measured cyclin D2 levels (Supplementary Fig. 7). Consistent with Fig. 5, overexpressed cyclin D2 protein was increased by high glucose despite IRS2 knockdown, which is possibly related to glucose activation of the cytomegalovirus promoter (60). To test whether restoring cyclin D2 expression rescued MTOR inhibition, islet cells were transduced with control or Ad-cyclin D2 adenovirus and treated with rapamycin (Fig. 7C and Supplementary Fig. 6). With control adenovirus, rapamycin treatment strongly inhibited proliferation, but cyclin D2 overexpression reversed this proliferation defect. We confirmed that cyclin D2 rescued loss of IRS2 or MTOR activity with a second proliferation assay, immunoblot for PCNA (Fig. 7D–I). All together, these results suggest that glucose induces proliferation by a pathway in which cyclin D2 is downstream of IRS2 and MTOR.
Figure 7.
Restoring cyclin D2 expression rescues the proliferation defect due to loss of IRS2 and rapamycin (RAPA) treatment. A: In dispersed IRS2-WT islets, cyclin D2 overexpression (MOI 5) in 5 mmol/L glucose increased β-cell proliferation to levels achieved in 15 mmol/L glucose, and cyclin D2 overexpression in 15 mmol/L glucose further increased proliferation (n = 3–4). In dispersed IRS2-KO islets, 15 mmol/L glucose did not significantly increase β-cell proliferation compared with 5 mmol/L, but overexpression of cyclin D2 markedly increased proliferation in 15 mmol/L glucose. B and C: Reduced dispersed β-cell proliferation caused by adenovirus knockdown of IRS2 (MOI 50, n = 3–4; B) or RAPA treatment (n = 4–5; C) was rescued by overexpression of cyclin D2 (MOI 5). BrdU incorporation results were confirmed by a second proliferation measure, immunoblot for PCNA: loss of PCNA by IRS2 knockdown (dispersed islets, MOI 50, n = 8; D–F) or RAPA treatment (whole islets, n = 3; G–I) was rescued by overexpression of cyclin D2 (MOI 5). Data are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. cyc, cyclin; gluc, glucose; ns, not significant; p, phosphorylated; tot, total.
Human β-Cell Proliferation Shares Similarities to Mouse
Human islets differ from rodent islets in some respects (2). We and others have previously confirmed that human β-cell proliferation is induced by glucose (2,4,62); we reconfirmed this in the current culture system (Fig. 8A and B). Human β-cell proliferation was not reduced by blockade of the insulin receptor with S961 (Fig. 8C) or increased by insulin treatment in the majority of cases (Fig. 8D). We confirmed prior reports that cyclin D2 was poorly expressed in human islets by using antibodies that work well for mouse; glucose did not induce the presence of cyclin D2 in human islets (data not shown). However, four of six human islet preparations had increased cyclin D3 protein abundance when cultured in high glucose (Fig. 8E and F). Overexpression of mouse cyclin D2 protein increased human β-cell proliferation in both low and high glucose (Fig. 8G). Rapamycin treatment reduced proliferation in human islets, and proliferation was rescued by overexpression of cyclin D2 (Fig. 8H).
Figure 8.
Human β-cell proliferation regulation shows many similarities to mouse. A and B: Dispersed human islets were cultured on glass coverslips for 96 h, with BrdU included for the entire 96 h. Human β-cells had increased proliferation in 15 mmol/L glucose over 5 mmol/L glucose. C and D: Glucose-induced human β-cell proliferation was not inhibited by insulin receptor blocker S961 (100 nmol/L); insulin (100 nmol/L) did not increase proliferation in the majority of islet preparations. E and F: Glucose treatment for 72 h did not alter cyclin D2 abundance, which was poorly detected (data not shown), but increased cyclin D3 abundance in four of six preparations tested. G: Overexpression of cyclin D2 (MOI 5) increased human β-cell proliferation in both low and high glucose. H: Rapamycin reduced human β-cell proliferation (nonsignificant) but was rescued by adenoviral overexpression of cyclin D2 (MOI 5). Arrows point to BrdU-positive β-cell nuclei. Data are mean ± SEM. *P < 0.05; ***P < 0.001. cyc, cyclin; gluc, glucose; ns, not significant; RAPA, rapamycin; tub, tubulin; veh, vehicle.
Discussion
The mechanisms by which glucose regulates β-cell proliferation remain controversial. The current results suggest that signaling mediators IRS2 and MTOR are required, but ERK and the insulin receptor are not. IRS2 and MTOR were necessary for cyclin D2 expression, and cyclin D2 was necessary and sufficient to mediate glucose-induced proliferation. Strengths of this work include addressing an important controversy in the field of β-cell mass regulation, the use of in vivo and ex vivo models, and the consistent use of primary islet cells rather than transformed cell lines. This work identifies a pathway that may be useful toward designing therapies to expand β-cell mass.
The current findings do not support a model in which glucose drives β-cell proliferation by increasing mitogenic signaling through the insulin receptor. Insulin and the insulin receptor have been postulated to drive β-cell proliferation in response to insulin resistance and hyperglycemia (63). In some cell culture systems, insulin promotes β-cell mitogenesis (12,18). Mice with β-cell deletion of the insulin receptor had normal islet morphology under basal conditions (64) but impaired β-cell proliferation in response to insulin resistance (65). These in vivo experiments may be complicated by insulin receptor deletion in the hypothalamus because deletion was performed by using the rat insulin promoter. The current ex vivo system, while directly assessing effects on β-cells, also has caveats, including the presence of other cell types in islet cultures, potentially high concentrations of insulin or other islet hormones due to regulated and unregulated secretion, possible activity of insulin through insulin-like growth factor 1 receptor, and possible off-target effects of S961 and HNMPA. Insulin receptors may have been downregulated by insulin in culture media or cleaved by trypsin in dispersed cell experiments, although the AKT response to insulin and effectiveness of S961 and HNMPA argue against this. The finding is consistent with evidence that in vivo pharmacological blockade of the insulin receptor causes rather than restricts β-cell mass expansion (66).
How glucose activates IRS2 independent of the insulin receptor requires further study. Glucose increases IRS2 mRNA and protein abundance in β-cells (9,10), but increasing IRS2 expression is not sufficient to drive proliferation in low glucose (67). IRS activity is modulated by other signals, such as hepatocyte growth factor, insulin-like growth factor 1, and glucagon-like peptide 1, although not all signals activating IRS2 are proproliferative in β-cells, and hepatocyte growth factor–driven proliferation does not require IRS2 (67–70). The current findings in primary islet cells differ from some published results in transformed cell lines, possibly illustrating caveats of studying proliferation regulation in transformed cells (13,67,71).
Short-term signaling induced by glucose and insulin were similar, but longer-term signaling differed substantially. At the late time point when proliferation was increased, glucose activated MTOR and ERK but not AKT, and insulin activated AKT but not MTOR or ERK. Because wortmannin blocked both AKT and MTOR acutely but did not prevent proliferation, the early signaling induced by glucose appears to be less critical to the proliferation decision than the longer-term changes. Lack of AKT activation by glucose at 72 h and failure of insulin to increase proliferation despite activating AKT suggest that AKT may not be directly driving proliferation. Because the PI3K inhibitor effect was not sustained over long-term culture, the current studies were not able to test whether PI3K inhibition is required for proliferation. However, we conclude that acute AKT activation is not required. The finding confirms that inhibition of MTOR with rapamycin reduces β-cell proliferation (15,29–31,33,34,72), although to our knowledge, it is the first to show that MTOR is required for glucose-induced proliferation.
Although MEK/ERK is mitogenic in a wide variety of cell types, we find MEK inhibition to paradoxically increase β-cell proliferation. This observation is consistent with one prior study (43) but somewhat inconsistent with the model that MEK/ERK is proproliferative in β-cells but tonically inhibited by menin (42). That study suggested that rat sarcoma is antiproliferative in β-cells, but reduces proliferation through RASSF1A rather than through an antiproliferative effect of MEK. In that system, MEK inhibition did not alter β-cell proliferation; proliferation in response to glucose was not tested.
Although glucose can affect cell biology without metabolism (73), the glucose effect on β-cell IRS2 expression and proliferation requires metabolism through glucokinase (GK) (8,10). GK activity is required for IRS2 expression and β-cell proliferation in response to high-fat feeding, and restoring IRS2 expression corrects β-cell mass and proliferation (11). GK activators were considered a potential therapeutic approach to increase β-cell mass and function, but disappointingly, long-term GK activation does not increase mouse β-cell mass or proliferation (49,74,75). These studies suggest that if physiological pathways regulating β-cell proliferation in response to hyperglycemia are to be useful therapeutically, pathways must be targeted further downstream.
The current results show that in mouse islets, cyclin D2 protein abundance closely parallels proliferation. Cyclin D2 was necessary and sufficient for glucose-induced mouse β-cell proliferation. Islet regulation of cyclin D2 is at the protein level (6,29,76); cyclin D2 is stabilized through phosphorylation of threonine 280. PI3K- and MTOR-dependent kinases are implicated in cyclin D2 regulation in β-cells (20,29). The current data support a requirement for MTOR in glucose-induced cyclin D2 protein abundance. More studies are needed to determine whether cyclin D3 fulfills this role in human islets. Cyclin D3 overexpression drives human β-cell proliferation when combined with active CDK4 (54). Whether cyclin D2 plays a role in human islets remains unclear, although reports have suggested that cyclin D2 is not expressed in human islets (54,77). The recent genetic link between the cyclin D2 genomic locus and type 2 diabetes risk through insulin secretory capacity (52,53) suggests that more research is needed in this area.
Supplementary Material
Article Information
Acknowledgments. The authors thank the National Institute of Diabetes and Digestive and Kidney Diseases and the Integrated Islet Distribution Program for supplying human islets. The authors also thank the Beta Cell Biology Group at the University of Massachusetts Medical School for helpful discussions.
Funding. This work was supported by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grants R01-DK-077096 (to A.G.-O.), K08-DK-076562 (to L.C.A.), and R01-DK-095140 (to L.C.A.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.E.S., R.B.S., and Y.K. contributed to the performance of experiments, data analysis, and review and editing of the manuscript. P.E., D.P., P.R., B.Z., H.L., and N.A.P. contributed to the performance of experiments and review and editing of the manuscript. C.P.O. and A.G.-O. contributed to the study design and review and editing of the manuscript. L.C.A. contributed to the study design, data analysis, and writing, review, and editing of the manuscript. L.C.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-0529/-/DC1.
R.E.S., R.B.S., and Y.K. contributed equally to this work.
References
- 1.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46 [DOI] [PubMed] [Google Scholar]
- 2.Levitt HE, Cyphert TJ, Pascoe JL, et al. Glucose stimulates human beta cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia 2011;54:572–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diiorio P, Jurczyk A, Yang C, et al. Hyperglycemia-induced proliferation of adult human beta cells engrafted into spontaneously diabetic immunodeficient NOD-Rag1null IL2rγnull Ins2Akita mice. Pancreas 2011;40:1147–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metukuri MR, Zhang P, Basantani MK, et al. ChREBP mediates glucose-stimulated pancreatic β-cell proliferation. Diabetes 2012;61:2004–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kassem S, Bhandari S, Rodríguez-Bada P, et al. Large islets, beta-cell proliferation, and a glucokinase mutation. N Engl J Med 2010;362:1348–1350 [DOI] [PubMed] [Google Scholar]
- 6.Alonso LC, Yokoe T, Zhang P, et al. Glucose infusion in mice: a new model to induce beta-cell replication. Diabetes 2007;56:1792–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Ocana A, Alonso L. Glucose mediated regulation of beta cell proliferation. Open Endocrinol J 2010;4:55–65 [Google Scholar]
- 8.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, et al. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab 2011;13:440–449 [DOI] [PubMed] [Google Scholar]
- 9.Demozay D, Tsunekawa S, Briaud I, Shah R, Rhodes CJ. Specific glucose-induced control of insulin receptor substrate-2 expression is mediated via Ca2+-dependent calcineurin/NFAT signaling in primary pancreatic islet β-cells. Diabetes 2011;60:2892–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lingohr MK, Briaud I, Dickson LM, et al. Specific regulation of IRS-2 expression by glucose in rat primary pancreatic islet β-cells. J Biol Chem 2006;281:15884–15892 [DOI] [PubMed] [Google Scholar]
- 11.Terauchi Y, Takamoto I, Kubota N, et al. Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest 2007;117:246–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assmann A, Ueki K, Winnay JN, Kadowaki T, Kulkarni RN. Glucose effects on beta-cell growth and survival require activation of insulin receptors and insulin receptor substrate 2. Mol Cell Biol 2009;29:3219–3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickson LM, Lingohr MK, McCuaig J, et al. Differential activation of protein kinase B and p70(S6)K by glucose and insulin-like growth factor 1 in pancreatic β-cells (INS-1). J Biol Chem 2001;276:21110–21120 [DOI] [PubMed] [Google Scholar]
- 14.Cousin SP, Hügl SR, Myers MG Jr, White MF, Reifel-Miller A, Rhodes CJ. Stimulation of pancreatic beta-cell proliferation by growth hormone is glucose-dependent: signal transduction via janus kinase 2 (JAK2)/signal transducer and activator of transcription 5 (STAT5) with no crosstalk to insulin receptor substrate-mediated mitogenic signalling. Biochem J 1999;344:649–658 [PMC free article] [PubMed] [Google Scholar]
- 15.Bartolomé A, Guillén C, Benito M. Role of the TSC1-TSC2 complex in the integration of insulin and glucose signaling involved in pancreatic β-cell proliferation. Endocrinology 2010;151:3084–3094 [DOI] [PubMed] [Google Scholar]
- 16.Martinez SC, Cras-Méneur C, Bernal-Mizrachi E, Permutt MA. Glucose regulates Foxo1 through insulin receptor signaling in the pancreatic islet β-cell. Diabetes 2006;55:1581–1591 [DOI] [PubMed] [Google Scholar]
- 17.Beith JL, Alejandro EU, Johnson JD. Insulin stimulates primary β-cell proliferation via Raf-1 kinase. Endocrinology 2008;149:2251–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohsugi M, Cras-Méneur C, Zhou Y, et al. Reduced expression of the insulin receptor in mouse insulinoma (MIN6) cells reveals multiple roles of insulin signaling in gene expression, proliferation, insulin content, and secretion. J Biol Chem 2005;280:4992–5003 [DOI] [PubMed] [Google Scholar]
- 19.Rhodes CJ, White MF, Leahy JL, Kahn SE. Direct autocrine action of insulin on β-cells: does it make physiological sense? Diabetes 2013;62:2157–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fatrai S, Elghazi L, Balcazar N, et al. Akt induces β-cell proliferation by regulating cyclin D1, cyclin D2, and p21 levels and cyclin-dependent kinase-4 activity. Diabetes 2006;55:318–325 [DOI] [PubMed] [Google Scholar]
- 21.Bernal-Mizrachi E, Wen W, Stahlhut S, Welling CM, Permutt MA. Islet β cell expression of constitutively active Akt1/PKB α induces striking hypertrophy, hyperplasia, and hyperinsulinemia. J Clin Invest 2001;108:1631–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao P, Roccisana J, Takane KK, et al. Gene transfer of constitutively active Akt markedly improves human islet transplant outcomes in diabetic severe combined immunodeficient mice. Diabetes 2005;54:1664–1675 [DOI] [PubMed] [Google Scholar]
- 23.Blandino-Rosano M, Alejandro EU, Sathyamurthy A, et al. Enhanced beta cell proliferation in mice overexpressing a constitutively active form of Akt and one allele of p21Cip. Diabetologia 2012;55:1380–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buzzi F, Xu L, Zuellig RA, et al. Differential effects of protein kinase B/Akt isoforms on glucose homeostasis and islet mass. Mol Cell Biol 2010;30:601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen WS, Peng X-D, Wang Y, et al. Leptin deficiency and beta-cell dysfunction underlie type 2 diabetes in compound Akt knockout mice. Mol Cell Biol 2009;29:3151–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernal-Mizrachi E, Fatrai S, Johnson JD, et al. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet β cells. J Clin Invest 2004;114:928–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuttle RL, Gill NS, Pugh W, et al. Regulation of pancreatic β-cell growth and survival by the serine/threonine protein kinase Akt1/PKBalpha. Nat Med 2001;7:1133–1137 [DOI] [PubMed] [Google Scholar]
- 28.Garofalo RS, Orena SJ, Rafidi K, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB β. J Clin Invest 2003;112:197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balcazar N, Sathyamurthy A, Elghazi L, et al. mTORC1 activation regulates β-cell mass and proliferation by modulation of cyclin D2 synthesis and stability. J Biol Chem 2009;284:7832–7842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraenkel M, Ketzinel-Gilad M, Ariav Y, et al. mTOR inhibition by rapamycin prevents β-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes 2008;57:945–957 [DOI] [PubMed] [Google Scholar]
- 31.Velazquez-Garcia S, Valle S, Rosa TC, et al. Activation of protein kinase C-ζ in pancreatic β-cells in vivo improves glucose tolerance and induces β-cell expansion via mTOR activation. Diabetes 2011;60:2546–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aly H, Rohatgi N, Marshall CA, et al. A novel strategy to increase the proliferative potential of adult human β-cells while maintaining their differentiated phenotype. PLoS One 2013;8:e66131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zarrouki B, Benterki I, Fontés G, et al. Epidermal growth factor receptor signaling promotes pancreatic β-cell proliferation in response to nutrient excess in rats through mTOR and FOXM1. Diabetes 2014;63:982–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rachdi L, Balcazar N, Osorio-Duque F, et al. Disruption of Tsc2 in pancreatic β cells induces β cell mass expansion and improved glucose tolerance in a TORC1-dependent manner. Proc Natl Acad Sci U S A 2008;105:9250–9255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alliouachene S, Tuttle RL, Boumard S, et al. Constitutively active Akt1 expression in mouse pancreas requires S6 kinase 1 for insulinoma formation. J Clin Invest 2008;118:3629–3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu Y, Lindner J, Kumar A, Yuan W, Magnuson MA. Rictor/mTORC2 is essential for maintaining a balance between β-cell proliferation and cell size. Diabetes 2011;60:827–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Treins C, Alliouachene S, Hassouna R, Xie Y, Birnbaum MJ, Pende M. The combined deletion of S6K1 and Akt2 deteriorates glycemic control in a high-fat diet. Mol Cell Biol 2012;32:4001–4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briaud I, Dickson LM, Lingohr MK, McCuaig JF, Lawrence JC, Rhodes CJ. Insulin receptor substrate-2 proteasomal degradation mediated by a mammalian target of rapamycin (mTOR)-induced negative feedback down-regulates protein kinase B-mediated signaling pathway in β-cells. J Biol Chem 2005;280:2282–2293 [DOI] [PubMed] [Google Scholar]
- 39.Mori H, Inoki K, Opland D, et al. Critical roles for the TSC-mTOR pathway in β-cell function. Am J Physiol Endocrinol Metab 2009;297:E1013–E1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shigeyama Y, Kobayashi T, Kido Y, et al. Biphasic response of pancreatic β-cell mass to ablation of tuberous sclerosis complex 2 in mice. Mol Cell Biol 2008;28:2971–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elghazi L, Balcazar N, Blandino-Rosano M, et al. Decreased IRS signaling impairs β-cell cycle progression and survival in transgenic mice overexpressing S6K in β-cells. Diabetes 2010;59:2390–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chamberlain CE, Scheel DW, McGlynn K, et al. Menin determines K-RAS proliferative outputs in endocrine cells. J Clin Invest 2014;124:4093–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasavada RC, Wang L, Fujinaka Y, et al. Protein kinase C-ζ activation markedly enhances β-cell proliferation: an essential role in growth factor mediated β-cell mitogenesis. Diabetes 2007;56:2732–2743 [DOI] [PubMed] [Google Scholar]
- 44.Cozar-Castellano I, Harb G, Selk K, et al. Lessons from the first comprehensive molecular characterization of cell cycle control in rodent insulinoma cell lines. Diabetes 2008;57:3056–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiaschi-Taesch NM, Kleinberger JW, Salim FG, et al. Human pancreatic β-cell G1/S molecule cell cycle atlas. Diabetes 2013;62:2450–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salpeter SJ, Klochendler A, Weinberg-Corem N, et al. Glucose regulates cyclin D2 expression in quiescent and replicating pancreatic β-cells through glycolysis and calcium channels. Endocrinology 2011;152:2589–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salpeter SJ, Klein AM, Huangfu D, Grimsby J, Dor Y. Glucose and aging control the quiescence period that follows pancreatic beta cell replication. Development 2010;137:3205–3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arumugam R, Fleenor D, Lu D, Freemark M. Differential and complementary effects of glucose and prolactin on islet DNA synthesis and gene expression. Endocrinology 2011;152:856–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakamura A, Togashi Y, Orime K, et al. Control of beta cell function and proliferation in mice stimulated by small-molecule glucokinase activator under various conditions. Diabetologia 2012;55:1745–1754 [DOI] [PubMed] [Google Scholar]
- 50.Georgia S, Hinault C, Kawamori D, et al. Cyclin D2 is essential for the compensatory β-cell hyperplastic response to insulin resistance in rodents. Diabetes 2010;59:987–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kushner JA, Ciemerych MA, Sicinska E, et al. Cyclins D2 and D1 are essential for postnatal pancreatic beta-cell growth. Mol Cell Biol 2005;25:3752–3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steinthorsdottir V, Thorleifsson G, Sulem P, et al. Identification of low-frequency and rare sequence variants associated with elevated or reduced risk of type 2 diabetes. Nat Genet 2014;46:294–298 [DOI] [PubMed] [Google Scholar]
- 53.Yaghootkar H, Stancáková A, Freathy RM, et al. Association analysis of 29,956 individuals confirms that a low-frequency variant at CCND2 halves the risk of type 2 diabetes by enhancing insulin secretion. Diabetes 2015;64:2279–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fiaschi-Taesch NM, Salim F, Kleinberger J, et al. Induction of human β-cell proliferation and engraftment using a single G1/S regulatory molecule, cdk6. Diabetes 2010;59:1926–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rane SG, Dubus P, Mettus RV, et al. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in β-islet cell hyperplasia. Nat Genet 1999;22:44–52 [DOI] [PubMed] [Google Scholar]
- 56.Pascoe J, Hollern D, Stamateris R, et al. Free fatty acids block glucose-induced β-cell proliferation in mice by inducing cell cycle inhibitors p16 and p18. Diabetes 2012;61:632–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat Genet 1999;23:32–40 [DOI] [PubMed] [Google Scholar]
- 58.Cantley J, Choudhury AI, Asare-Anane H, et al. Pancreatic deletion of insulin receptor substrate 2 reduces beta and alpha cell mass and impairs glucose homeostasis in mice. Diabetologia 2007;50:1248–1256 [DOI] [PubMed] [Google Scholar]
- 59.Kubota N, Tobe K, Terauchi Y, et al. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory beta-cell hyperplasia. Diabetes 2000;49:1880–1889 [DOI] [PubMed] [Google Scholar]
- 60.Kimura M, Namba H, Okubo M, et al. Enhancive effects of d-glucose and its analogs on expression of d-glucose-unrelated transgenes in mammalian cells. J Biosci Bioeng 2011;112:194–201 [DOI] [PubMed] [Google Scholar]
- 61.Tiedemann RE, Mao X, Shi C-X, et al. Identification of kinetin riboside as a repressor of CCND1 and CCND2 with preclinical antimyeloma activity. J Clin Invest 2008;118:1750–1764 [DOI] [PMC free article] [PubMed]
- 62.Sharma RB, O’Donnell AC, Stamateris RE, et al. Insulin demand regulates β cell number via the unfolded protein response. J Clin Invest 2015;125:3831–3846 [DOI] [PMC free article] [PubMed]
- 63.Flier SN, Kulkarni RN, Kahn CR. Evidence for a circulating islet cell growth factor in insulin-resistant states. Proc Natl Acad Sci U S A 2001;98:7475–7480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kulkarni RN, Brüning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 1999;96:329–339 [DOI] [PubMed] [Google Scholar]
- 65.Okada T, Liew CW, Hu J, et al. Insulin receptors in β-cells are critical for islet compensatory growth response to insulin resistance. Proc Natl Acad Sci U S A 2007;104:8977–8982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiao Y, Le Lay J, Yu M, Naji A, Kaestner KH. Elevated mouse hepatic betatrophin expression does not increase human β-cell replication in the transplant setting. Diabetes 2014;63:1283–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lingohr MK, Dickson LM, McCuaig JF, Hugl SR, Twardzik DR, Rhodes CJ. Activation of IRS-2-mediated signal transduction by IGF-1, but not TGF-α or EGF, augments pancreatic β-cell proliferation. Diabetes 2002;51:966–976 [DOI] [PubMed] [Google Scholar]
- 68.Alvarez-Perez JC, Rosa TC, Casinelli GP, et al. Hepatocyte growth factor ameliorates hyperglycemia and corrects β-cell mass in IRS2-deficient mice. Mol Endocrinol 2014;28:2038–2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park S, Dong X, Fisher TL, et al. Exendin-4 uses Irs2 signaling to mediate pancreatic β cell growth and function. J Biol Chem 2006;281:1159–1168 [DOI] [PubMed] [Google Scholar]
- 70.Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012;55:2565–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schuppin GT, Pons S, Hügl S, et al. A specific increased expression of insulin receptor substrate 2 in pancreatic beta-cell lines is involved in mediating serum-stimulated beta-cell growth. Diabetes 1998;47:1074–1085 [DOI] [PubMed] [Google Scholar]
- 72.Xie J, El Sayed NM, Qi C, Zhao X, Moore CE, Herbert TP. Exendin-4 stimulates islet cell replication via the IGF1 receptor activation of mTORC1/S6K1. J Mol Endocrinol 2014;53:105–115 [DOI] [PubMed] [Google Scholar]
- 73.Wauson EM, Zaganjor E, Lee A-Y, et al. The G protein-coupled taste receptor T1R1/T1R3 regulates mTORC1 and autophagy. Mol Cell 2012;47:851–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakamura A, Terauchi Y, Ohyama S, et al. Impact of Small-molecule glucokinase activator on glucose metabolism and β-cell mass. Endocrinology 2009;150:1147–1154 [DOI] [PubMed]
- 75.Tornovsky-Babeay S, Dadon D, Ziv O, et al. Type 2 diabetes and congenital hyperinsulinism cause DNA double-strand breaks and p53 activity in β cells. Cell Metab 2014;19:109–121 [DOI] [PubMed] [Google Scholar]
- 76.He LM, Sartori DJ, Teta M, et al. Cyclin D2 protein stability is regulated in pancreatic β-cells. Mol Endocrinol 2009;23:1865–1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davis DB, Lavine JA, Suhonen JI, et al. FoxM1 is up-regulated by obesity and stimulates β-cell proliferation. Mol Endocrinol 2010;24:1822–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.