Abstract
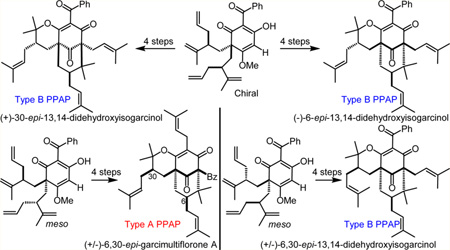
The first syntheses of 13,14-didehydroxyisogarcinol (6) and garcimultiflorone A (5) stereoisomers are reported in six steps from a commercially available phloroglucinol. Lewis acid-controlled, diastereoselective cationic oxycyclizations enabled asymmetric syntheses of (−)-6-epi-6 and (+)-30-epi-6. A similar strategy enabled production of the meso-dervied isomers (±)-6,30-epi-6 and (±)-6,30-epi-5. Finally, a convenient strategy for gram scale synthesis was developed utilizing diastereomer separation at a later stage in the synthesis that minimized the number of necessary synthetic operations to access all possible stereoisomers.
Graphical abstract
1. INTRODUCTION
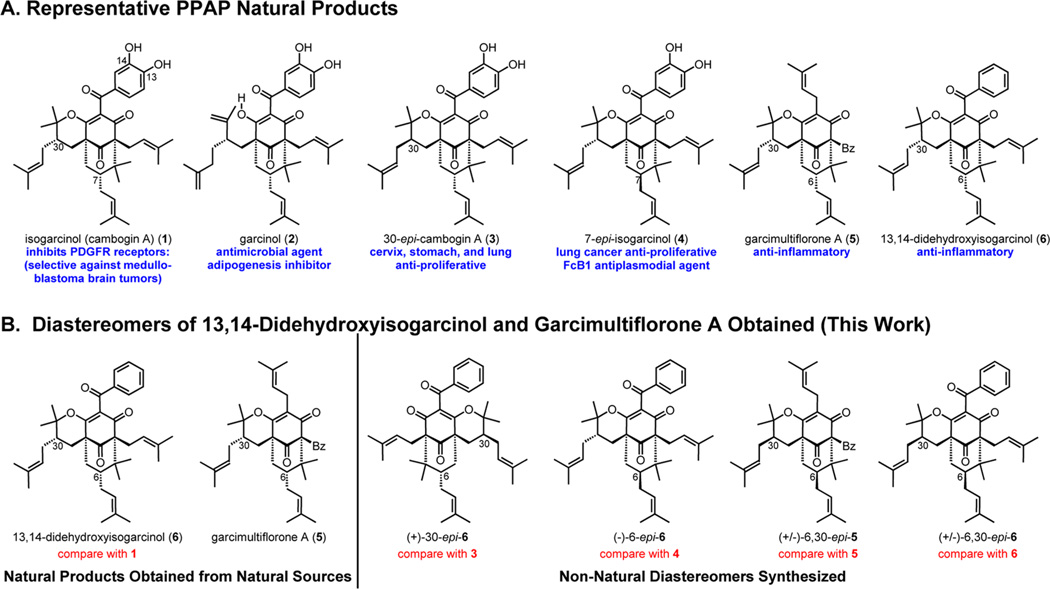
For the past 15 years, the synthetic and biological communities have been interested in polyprenylated polycyclic acylphloroglucinols (PPAPs) as natural product targets and starting points for SAR studies due to their diverse bioactivity profiles (Figure 1A). Not surprisingly, with minor structural differences leading to significant variation in bioactivity, PPAP natural products and their analogs are considered high priority targets for isolation, chemical synthesis, and biological studies.1,2 The natural products isogarcinol (1) and garcinol (2) have gained significant attention in recent years and are lead compounds for several clinical indications.3,4 The importance of synthesizing diverse analogs of both garcinol and isogarcinol to probe their biological activities was recently emphasized.5,6
Figure 1.
Representative PPAP natural products and diastereomers of 13,14-didehydroxyisogarcinol and garcimultiflorone A obtained in this work.
Isogarcinol’s dehydroxy isomer 13,14-didehydroxyisogarcinol (6) and the type A variant garcimultiflorone A (5) were isolated in 2009 from the fruits of Garcinia multiflora in South China and were reported to possess anti-inflammatory activity by inhibiting superoxide and elastase enzymes released from human neutrophils (Figure 1A).7 Apart from this single report, however, no additional information on the biological activities of natural products 5 and 6 exist further emphasizing the need for an efficient and scalable synthetic route to this class. Although studies have reported the importance of 13,14-hydroxyls with regard to isogarcinol’s histone acetyltransferase (HAT) inhibition and radical-scavenging abilities,2a,6,8c,d,11e biological data on analogs that lack the hydroxyl functionalities (e.g., 5 and 6) are limited.7 Given that we were able to procure natural products 5 and 6 from the isolation team led by Chen and co-workers,7 our goal was to develop a strategy for accessing non-natural diastereomers of 5 and/or 6 for further biological studies (Figure 1B).
We were intrigued by the marked similarity of 13,14-didehydroxyisogarcinol (6) to isogarcinol (1), a compound that has activity in numerous therapeutic indications including leukemia,8a,i colon cancer,8b,i HIV,8c,d brain tumors (medulloblastoma),8e antioxidant,8f antimicrobial,8f transplantation rejection and autoimmune diseases,8g,h,l collagen-induced and rheumatoid arthritis,8j breast cancer,8k and Parkinson’s disease.8m Interestingly, isogarcinol (1) is also available for human consumption in mangosteen drinks and capsules as a remedy for arthritis.9 Only one total synthesis of 1 has been reported since its isolation in 1980, an elegant strategy accomplished in 15 steps by Pleitker and co-workers.10,11a The compound has been isolated from numerous sources,11 and a large scale extraction procedure was developed for its procurement.11g
Stereoisomers of 5 and 6 have not yet been isolated in nature, which further emphasizes the need for a synthetic platform to access such candidates (Figure 1B),8i,23 especially given the diverse bioactivities of isogarcinol’s closely related congeners, specifically garcinol (2),11a cycloxanthochymol,12b,d 30-epi-cambogin A (3),12a,c,f,h and 7-epi-isogarcinol (4).12b,d,e,g Herein, we describe asymmetric syntheses of (+)-30-epi-6 and (−)-6-epi-6 in six steps from commercially available phloroglucinol 12 utilizing Lewis acid-dependent diastereoselective oxycyclizations (Figure 2). Using a similar strategy, we also demonstrate the syntheses of meso-derived isomers (±)-6,30-epi-6 and (±)-6,30-epi-5 (cf. Scheme 2B). Finally, a convenient strategy for the large scale synthesis of these enantiopure and racemic stereoisomers is demonstrated.
Figure 2.
Retrosynthetic Analysis for (−)-6-epi-6 and (+)-30-epi-6.
Scheme 2.
Asymmetric Syntheses of (−)-6-epi-6 and (+)-30-epi-6 and Racemic Syntheses of (±)-6,30-epi-6 and (±)-6,30-epi-5
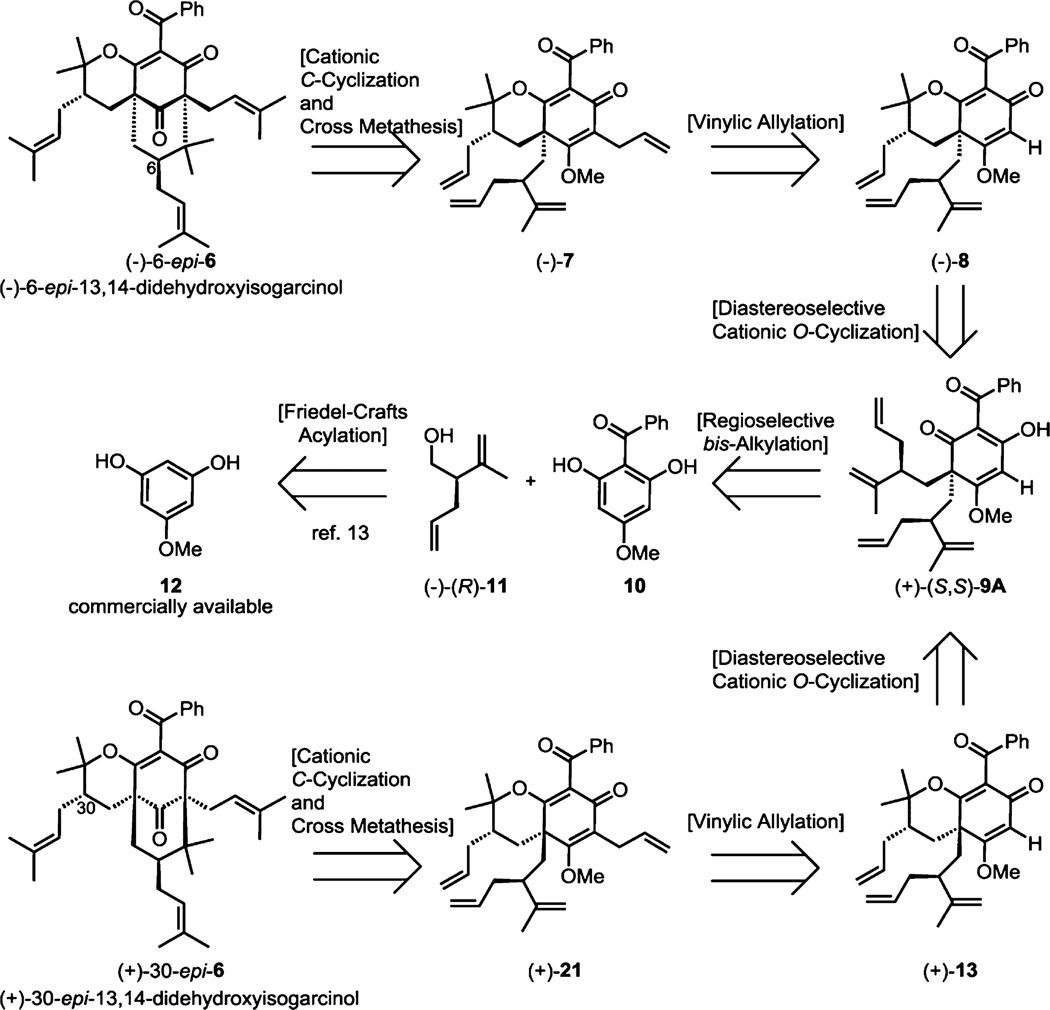
Retrosynthetic analysis of enantiopure isomers (−)-6-epi-6 and (+)-30-epi-6 from commercially available phloroglucinol 12 is shown in Figure 2. Isomers (−)-6-epi-6 and (+)-30-epi-6 may be derived from cross metathesis and cationic cyclization of precursors (−)-7 and (+)-21, respectively. Vinylic allylation of the unsubstituted precursors (−)-8 and (+)-13 should enable access to respective pyranodienones (−)-7 and (+)-21. We envisioned that diastereoselective, cationic oxycyclization mediated through Lewis-acid chelation of (+)-(S,S)-9A may furnish (−)-8 and/or (+)-13 in reasonable diastereoselectivity. Regioselective bis-alkylation of acylphloroglucinol 10 using the triflate derived from alcohol (−)-(R)-11 may provide dearomatized adduct (+)-(S,S)-9A.13 Finally, our laboratory has previously shown that compound 10 is conveniently derived from the commercially available monomethylphloroglucinol 12 via Friedel–Crafts acylation.13
2. RESULTS AND DISCUSSION
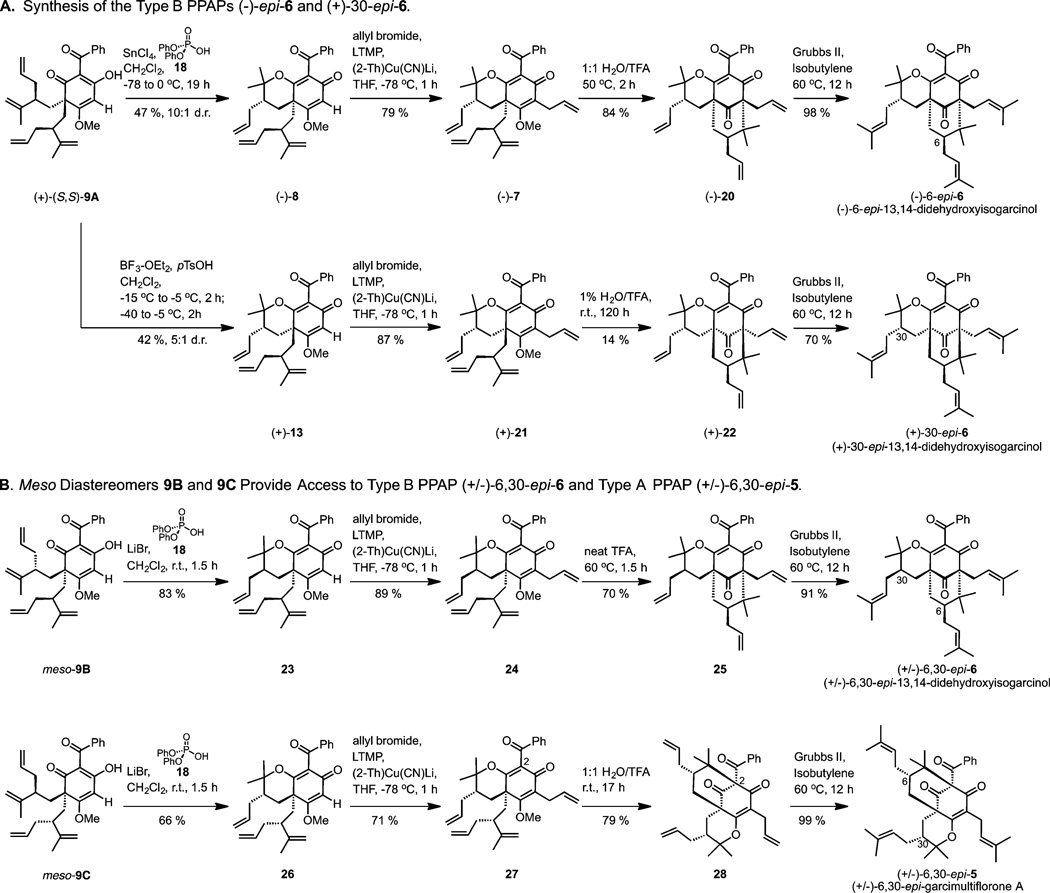
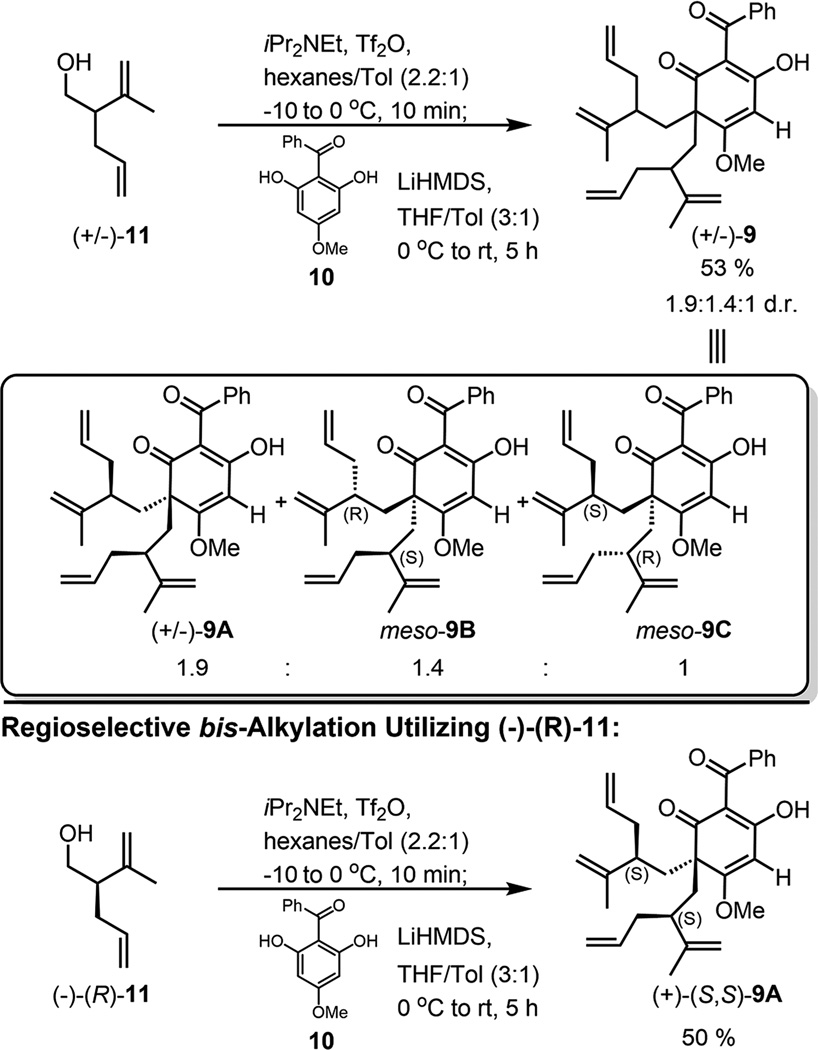
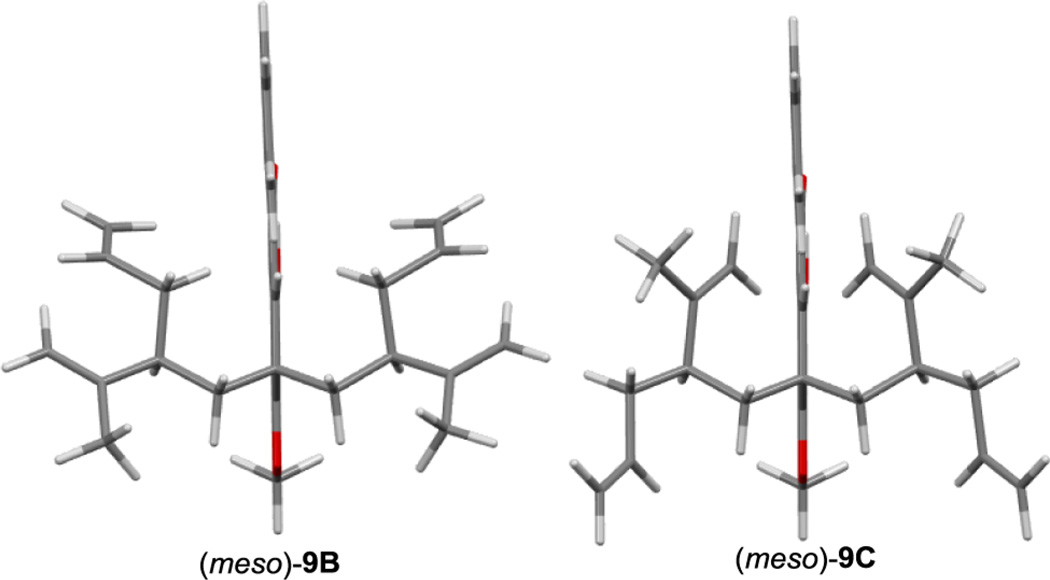
At the outset of our investigation, racemic alcohol (±)-11 was prepared in the same manner previously described by our group in a single step from commercially available starting materials.13 Pleasingly, triflation of (±)-11 was followed by regioselective bis-alkylation of acylphloroglucinol 10 in the presence of LiHMDS providing the dearomatized enols (±)-9A, (meso)-9B, and (meso)-9C in 53% yield (Scheme 1). The (R) and (S) alkyl substituents of (meso)-9B and (meso)-9C create a mirror plane of symmetry in each structure rendering them achiral/meso (Figure 3). Alternatively, compound (+)-(S,S)-9A could be produced as a single, enantiopure diastereomer via bis-alkylation of acylphloroglucinol 10 utilizing the enantioenriched triflate derived from alcohol (−)-(R)-11 (Scheme 1). A multigram scale synthesis of alcohol (−)-(R)-11 was achieved from a modified and improved 3-step protocol.14 Because of their tendency to form calcium salts on silica gel,13 enol (+)-(S,S)-9A and racemic variants (±)-9A, (meso)-9B, and (meso)-9C were conveniently purified by potassium salt formation without the need for column chromatography.14 The structure of (meso)-9C was confirmed by X-ray crystal structure analysis.14
Scheme 1.
Regioselective bis-Alkylation of Acylphloroglucinol 10: Racemic and Enantioenriched Variants
Figure 3.
Symmetry of meso isomers 9B and 9C represented by 3D models.
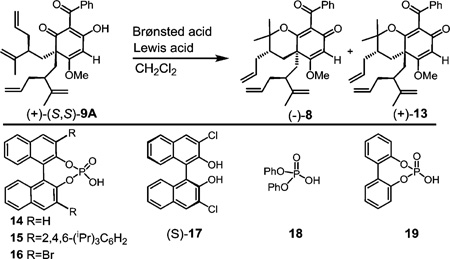
We next evaluated the possibility for diastereoselective oxycyclization of (+)-(S,S)-9A to provide the enantiopure pyranodienones (−)-8 and (+)-13 (Table 1). The diastereoselectivity in this case would result if the two 1,1-disubstituted alkenes of (+)-(S,S)-9A are protonated at different rates. A number of acidic conditions were evaluated for cyclization including HCl in CH2Cl2, which provided (−)-8 and (+)-13 in 1:1.1 d.r. (Table 1, entry 1). After considerable experimentation, we discovered that binary acid combinations15 of LiBr with either chiral or achiral phosphoric acids provided high yields of (−)-18 and (+)-13 in roughly 1:2 d.r. in favor of (+)-13 (Table 1, entries 2–8). In the absence of LiBr, the reaction did not proceed as the acidity of the phosphoric acid was presumably enhanced through lithium coordination (Table 1, entry 10). Because both chiral (14–17) and achiral phosphoric acids (18 and 19) provided similar outcomes with LiBr (Table 1, entries 2–8), we anticipated that the observed diastereoselectivity may be the result of Lewis acid coordination to enol (+)-(S,S)-9A which was supported by the fact that LiBr alone favored (+)-13 in 1:1.6 d.r. (Table 1, entry 11).
Table 1.
Diastereoselective Oxycyclization and Chelation Studies
 | |||||
|---|---|---|---|---|---|
| entry | Brønsted Acid | Lewis acid | conditions | yield | d.r. (8:13)b |
| 1 | HCla | none | −10 °C to r.t., 72 h | 25%b | 1:1.1 |
| 2 | 19 (10 equiv) | LiBr (10 equiv) | 0 °C, 48 h | 99% | 1:1.8 |
| 3 | 18 (5 equiv) | LiBr (5 equiv) | r.t., 1.5 h | 79% | 1:1.8 |
| 4 | (R)-14 (10 equiv) | LiBr (10 equiv) | 0 °C, 48 h | 91% | 1:2 |
| 5 | (S)-14 (10 equiv) | LiBr (10 equiv) | 0 °C, 48 h | 91% | 1:3 |
| 6 | (R)-15 (5 equiv) | LiBr (5 equiv) | r.t., 48 h | 89% | 1:2.2 |
| 7 | (S)-15 (5 equiv) | LiBr (5 equiv) | r.t., 48 h | 86% | 1:1.8 |
| 8 | (S)-16 (10 equiv) | LiBr (10 equiv) | 0 °C, 48 h | 99% | 1:1.5 |
| 9 | (S)-17 (1 equiv) | SbCl5 (1 equiv) | −78 °C, 2 h | 18% | 9:1 |
| 10 | (S)-14 (5 equiv) | none | −5 to 10 °C, 48 h | 0% | none |
| 11 | none | LiBr (10 equiv) | r.t., 5 d | 19% | 1:1.6 |
| 12 | none | SnCl4 (2 equiv) | −78 to −20 °C, 48 h | 36% | 7:1 |
| 13c | (±)-14 (2 equiv) | SnCl4 (1 equiv) | −78 to 50 °C, 36 h | 58% | 1:1.1 |
| 14 | pTsOH (10 equiv) | BF3OEt2 (79 equiv) | −78 to −5 °C, 1 h | 32%d (57% brsm) | 1:2 |
CH2Cl2/4 M HCl in dioxane (1:1).
Determined by 1H NMR analysis of the crude reaction mixture.
SnCl4 saturated with (±)-14 prior to addition of substrate (+)-(S,S)-9A.
54% starting material also recovered.
As it is known that Lewis acid coordination to Brønsted acid restricts the orientational flexibility of the proton,16,17 metal coordination to the β-keto enol functionality of (+)-(S,S)-9A may restrict the orientation of its enol proton in addition to making it more acidic and prone to react with the proximal 1,1-disubstituted olefin.16 Indeed, we discovered that stronger Lewis acids (e.g., SbCl5 and SnCl4) improved the overall diastereoselectivity. For example, evaluation of the Lewis acid-assisted Brønsted acid (LBA) system developed by Corey and co-workers,19 SbCl5 with 2,2′-dichloro-(S)-BINOL (S)-17 in CH2Cl2 at −78 °C, led to the production of (−)-8 in 18% yield and 9:1 d.r. (Table 1, entry 9).
The effect of chelation on diastereoselectivity was also evaluated by saturating the open coordination sites on SnCl4 with (±)-14 to generate the corresponding LBA and prevent substrate chelation.14,18 In this case, reaction was not observed until the temperature reached 50 °C providing clean formation of products (−)-8 and (+)-13 in 58% yield and 1:1.1 d.r (Table 1, entry 13).
After screening an array of Lewis acids with variations in size and coordination ability along with different phosphoric acid promoters, optimized conditions for diastereoselective cyclization to (−)-8 or (+)-13 were identified (Table 2). The combination of SnCl4 with the inexpensive, achiral diphenylphosphoric acid 18 ($3.18/gram) required lower reaction times and provided a cost-effective approach for the production of (−)-8 (47%, 10:1 d.r., Table 2, entry 4). Interestingly, the opposite selectivity for cyclization was highly favored only in the case of BF3-OEt2 and p-TsOH which provided (+)-13 in 42% yield and 1:5 d.r. (Table 2, entry 6).
Table 2.
Optimized Lewis/Brønsted Acid Combinations for Diastereoselective Cyclization
 | |||||
|---|---|---|---|---|---|
| entry | Brønsted Acid | Lewis acid | conditions | yield | d.r. (8:13)b |
| 1 | (S)-14 (2 equiv) | SnCl4 (2 equiv) | −78 to −30 °C, 6 d | 68% | 14:1 |
| 2 | (R)-14 (2 equiv) | SnCl4 (2 equiv) | −78 to −30 °C, 6 d | 9% | 1:0 |
| 3a | (±)-14 (2 equiv) | SnCl4 (2 equiv) | −78 to −20 °C, 17 h | 52% | 14:1 |
| 4 | 18 (2 equiv) | SnCl4 (2 equiv) | −78 to 0 °C, 19 h | 47% | 10:1 |
| 5 | 18 (10 equiv) | InCl3 (10 equiv) | r.t., 1 h | 99% | 5.4:1 |
| 6 | pTsOH (10 equiv) | BF3OEt (79 equiv) | −15 to −5 °C, 2 h; −40 to −5 °C, 2 h | 42% | 1:5 |
Substrate (±)-9A was used.
Determined by 1H NMR analysis.
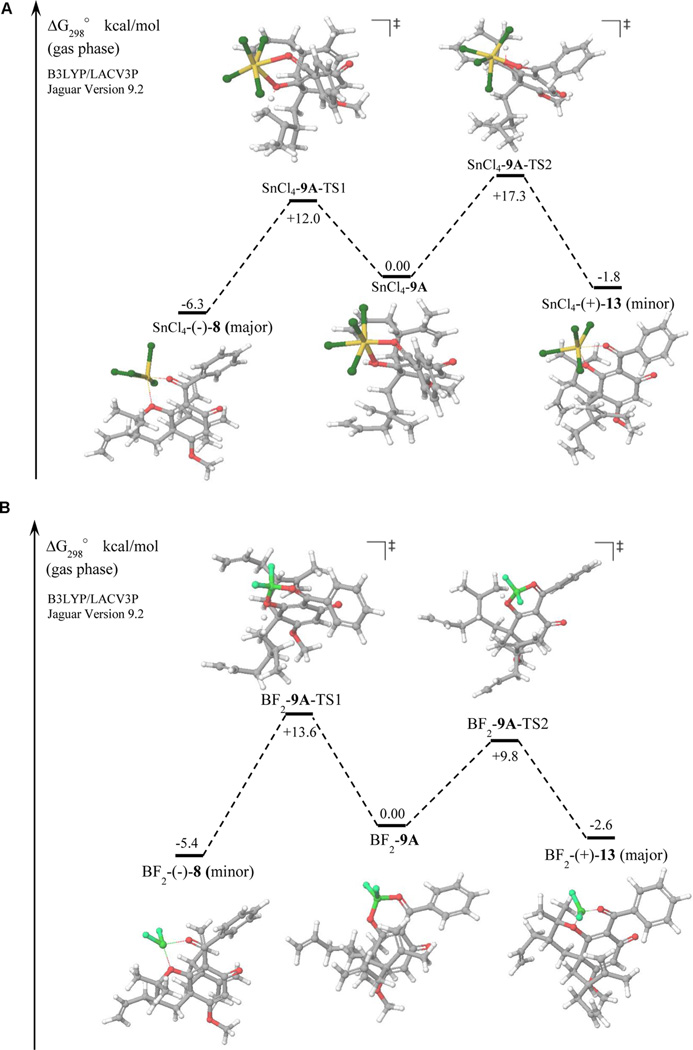
It has been reported that SnCl4 and BF3-OEt2 can bind in a bidentate fashion to β-keto enol substrates resulting in the formation of hexacoordinate Sn- and tetrahedral BF2-complexes.16a,20,21 We hypothesized that the observed diastereoselectivity may be governed by an intramolecular protonation event following complex formation wherein the proximal olefin approach is perpendicular to the acidic enol proton.16a,17e As SnCl4 alone provided (−)-8 in 7:1 d.r. and 36% yield (Table 1, entry 12), we simplified our transition state analysis by examining bidentate complexes without Brønsted acid ligand (Figure 4A,B). Indeed, analysis of transition states indicated that complex SnCl4-9A-TS1 appears to favor cyclization to (−)-8 by an energy difference of 5.3 kcal/mol in comparison with transition state SnCl4-9A-TS2 leading to (+)-13 (Figure 4A). This discrimination may be explained in part by improved stabilization of SnCl4-9A-TS1 via coordination of the two chlorine ligands to the acidic enol proton and to the emerging carbocation.14,17a,24 In contrast, complex BF2-9A-TS2 favors the formation of (+)-13 by an energy difference of 3.8 kcal/mol relative to BF2-9A-TS1 leading to (−)-8 which correlates with our experimental results (Figure 4B). To the best of our knowledge, this represents the first example of a SnCl4- or BF3-OEt2-phosphoric acid LBA system and the first Lewis acid-controlled diastereoselective oxycyclization of dearomatized acylphloroglucinols.
Figure 4.
(A) Relative transition state energies for intramolecular protonation of SnCl4-9A. (B) Relative transition state energies for intramolecular protonation of BF2-9A.
With optimized methods for accessing either (−)-8 or (+)-13 from (+)-(S,S)-9A (Scheme 2A), oxycyclization of meso-9B and meso-9C was also achieved in short reaction times and reasonable yield with use of LiBr and diphenyl phosphate (Scheme 2B). Vinylic allylation of pyranodienones (−)-8, (+)-13, 23, and 26 proceeded smoothly utilizing lithium 2-thienylcyanocuprate to provide the cyclization precursors (−)-7, (+)-21, 24, and 27 respectively in good yields (Scheme 2A,B).22
In our efforts to cyclize compound (−)-7 to the PPAP core (−)-20, we observed undesired conversion to product 25 in dry TFA. However, we discovered that isomerization could be completely avoided in the presence of water allowing for efficient cyclization to cycloadduct (−)-20 with 1:1 H2O/TFA (Scheme 2A). Compound 24 seamlessly cyclized at 60 °C after 1.5 h in dry TFA to its respective [3.3.1]-bicyclic core 25 in good yield (Scheme 2B). The relative stereochemical assignments of compound 25 were determined by detailed NOE studies and were later confirmed by X-ray crystal structure analysis (Scheme 3B).14
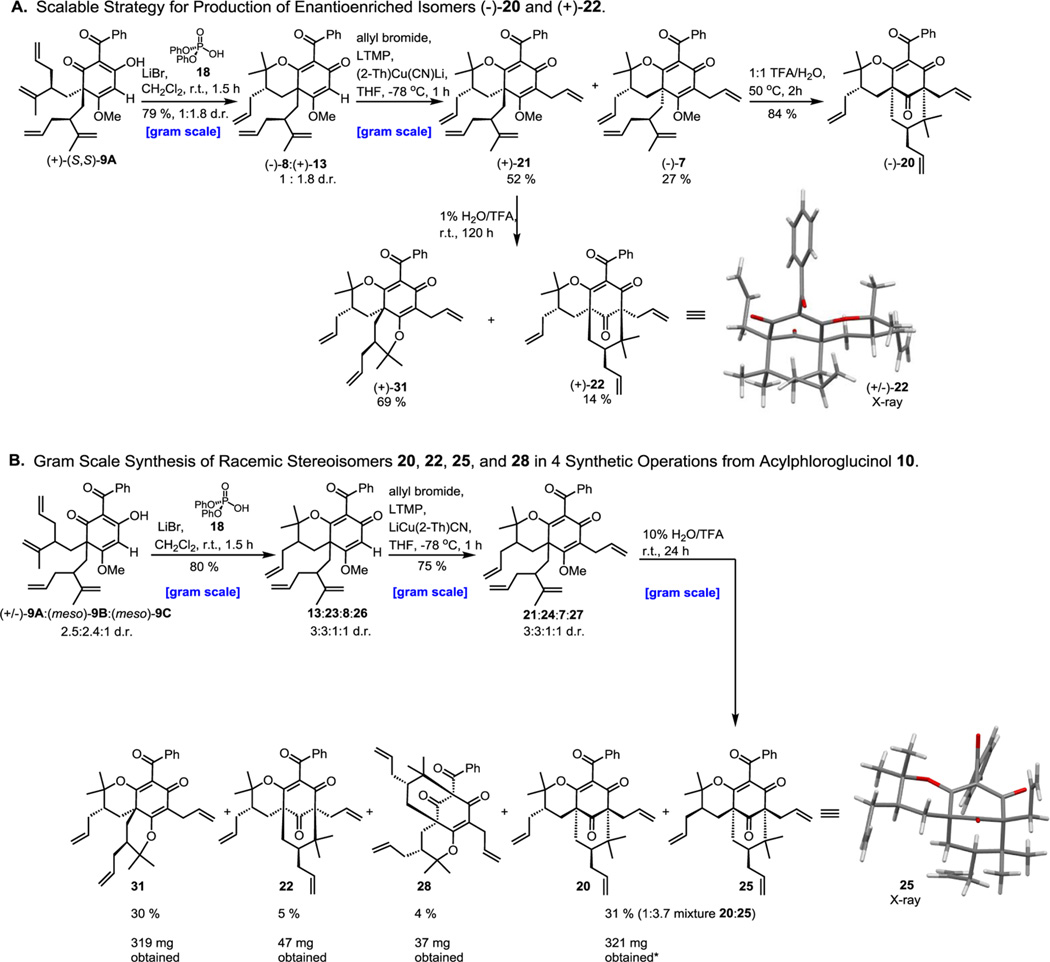
Scheme 3.
Scalable Strategy for Production of (−)-20 and (+)-22 and Gram Scale Synthesis of Racemic Stereoisomers 20, 22, 25, and 28 in a Single Synthetic Sequence
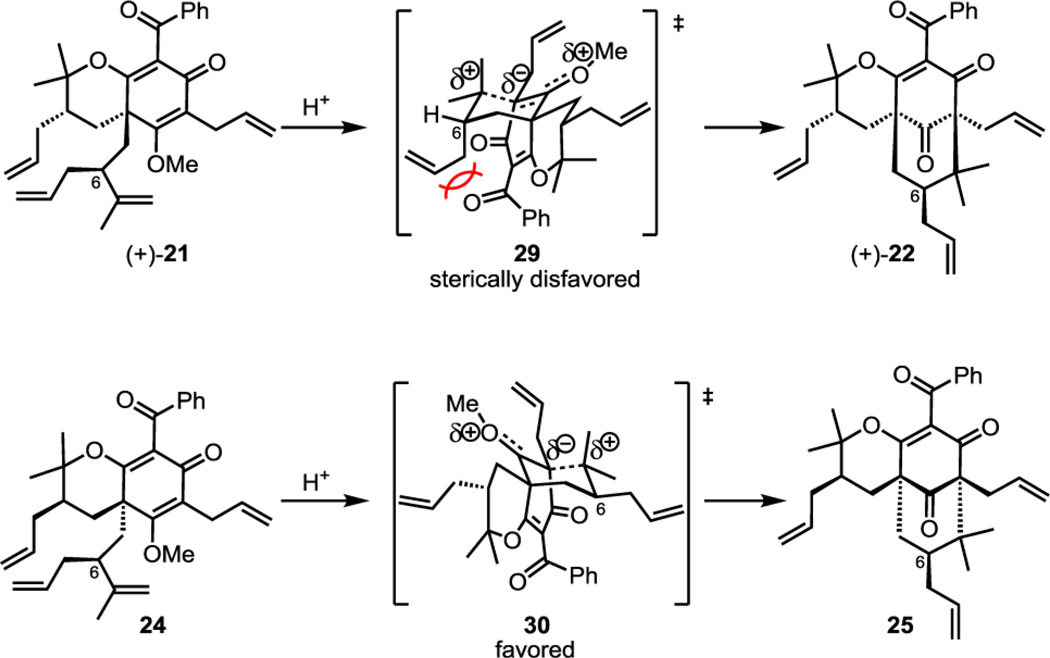
The cyclization of compound (+)-21 to (+)-22 proved more challenging, which may be due to unfavorable steric interactions shown in transition state 29 (Figure 5). The axially oriented allyl group at C6 likely experiences destabilizing interactions with the proximal benzoyl or cyclohexadienone functionalities disfavoring cyclization to product (+)-22.13 In contrast, the C6 allyl group in transition state 30 is equatorially oriented and avoids steric influences that would deter cyclization to the desired stereoisomer 25.
Figure 5.
Comparison of transition state models for cyclization to (+)-22 and 25.
After considerable experimentation, we discovered that the formation of the C-cyclization adduct (+)-22 could only be achieved in the presence of water, where 1% H2O/TFA provided an optimized yield of 14% (Scheme 2A) along with 69% of byproduct (+)-31 (cf. Scheme 3A). The relative stereochemical assignment of compound 22 was determined by X-ray crystal structure analysis (Scheme 3A).14 Formation of the undesired O-cyclization product (+)-31 may result from hydrolysis of methyl enol ether (+)-21 in H2O/TFA as further increasing the water content provided higher yields of byproduct (+)-31. It is also notable that compound (+)-21 did not cyclize in dry TFA even after stirring for 24 h at room temperature. Furthermore, in the presence of 1:1 H2O/TFA, exclusive formation of (+)-31 was observed.
Pyranodienone 27, shown in Scheme 2B, proved unsuitable for cyclization to its corresponding type B PPAP core which was likely impeded by undesired steric interactions in the transition state analogous to those noted in Figure 5. Surprisingly, we discovered that 1:1 H2O/TFA induced unexpected cyclization at C2 of compound 27 cleanly furnishing type A PPAP core 28 in 79% yield (Scheme 2B). As one possibility, the cyclization of 27 may proceed through a cationic rearrangement wherein acid-induced pyran opening leads to cyclization at C2 and a formal 1,3-shift25 followed by an oxycyclization/demethylation sequence. Pleasingly, olefin metathesis proceeded efficiently for all stereoisomers providing type B PPAPs (−)-6-epi-6, (+)-30-epi-6, (±)-6,30-epi-6, and type A PPAP (±)-6,30-epi-5 in excellent yields (Scheme 2A,B).
Finally, a convenient strategy for large scale production of all stereoisomers was developed by enabling diastereomer separation at a later stage in the synthesis which minimized the number of necessary synthetic operations (Scheme 3). A scalable strategy for the synthesis of enantiopure bicyclo[3.3.1]-nonanes (−)-20 and (+)-22 is demonstrated in Scheme 3A. Gram scale oxycyclization of enol (+)-(S,S)-9A with LiBr and diphenyl phosphate 18 provided (−)-8 and (+)-13 in 79% yield (1:1.8 d.r. as determined by 1H NMR analysis). Furthermore, phosphoric acid 18 could be recovered after workup.14 Vinylic allylation served as the most convenient stage for diastereomer separation where column chromatography was sufficient to provide ample quantities of products (+)-21 and (−)-7 for subsequent cyclization.
A convenient strategy for gram scale synthesis of racemic stereoisomers 20, 22, 25, and 28 is shown in Scheme 3B. In comparison, their synthesis would require a total of 13 steps (4 steps for each isomer) by the strategy presented in Schemes 1 and 2. Hence, our modified gram scale sequence in Scheme 3B accomplishes the same goal in 9 fewer steps providing a superior alternative for the synthesis of (±)-6-epi-6, (±)-30-epi-6, (±)-6,30-epi-6, and (±)-6-epi-5. To produce modest yields of each diastereomer in the final cyclization step of Scheme 3B, it was necessary to use 10% H2O/TFA as 1% H2O/TFA would improve the yield of stereoisomer 22 but minimize formation of 28 (Scheme 3B). Moreover, substantially raising the water content employing 1:1 H2O/TFA as solvent would prevent synthesis of product 22 and maximize formation of the type A PPAP core 28. This gram scale strategy demonstrated that all stereoisomers 20, 22, 25, and 28 could be achieved in only 4 synthetic operations from acylphloroglucinol 10 in a time frame of 72 h (Scheme 3B).14 It should be noted that compounds 20 and 25 were isolated as a mixture by column chromatography and could be further separated for characterization and biological evaluation by preparative TLC.14
3. CONCLUSION
In conclusion, all non-natural diastereomers of 13,14-didehydroxyisogarcinol (6) and one diastereomer of garcimultiflorone A (5) were synthesized for the first time by an efficient six step strategy from the commercially available phloroglucinol 12 using regioselective bis-alkylation, Lewis acid-controlled oxycyclization, and cationic C-cyclization as key steps. Type B stereoisomers (−)-6-epi-6, (+)-30-epi-6, and (±)-6,30-epi-6 were obtained in up to 22%, 2%, and 16% respective overall yields from acylphloroglucinol 10. Notably, our synthesis also provided the type A PPAP (±)-6,30-epi-garcimultiflorone A ((±)-6,30-epi-5) in 8.4% overall yield from 10. Moreover, diastereoselective oxycyclization of (+)-(S,S)-9A utilized novel SnCl4 and BF3-OEt2 LBA systems to induce stereoselective, intramolecular protonation resulting in selective formation of either pyranodienone (−)-8 or (+)-13. Finally, a gram scale strategy was developed to allow for production of ample quantities of each isomer in a single synthetic sequence which minimized step count and purification events. Notably, a synthesis of bicyclo[3.3.1]nonanes (−)-20 and (+)-22 was achieved without the need for preparative TLC and the production of stereoisomers 20, 22, 25, and 28 could be accomplished in only 4 synthetic operations from acylphloroglucinol 10. Further studies on the chemistry and biology of garcimultiflorone A (5), 13,14-didehydroxyisogarcinol (6), and their stereoisomers are in progress and will be reported in future publications.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (R01 GM-073855 and R35 GM118173), and the Deutsche Forschungsgemeinschaft (postdoctoral fellowship to V.E.-L.) for research support, Dr. Jeffrey Bacon (Boston University) for X-ray crystal structure analyses, and Dr. Norman Lee (Boston University) for high-resolution mass spectrometry data. Work at the BU-CMD was supported by the National Institutes of Health (NIH) (R24GM111625). We thank Lin-Yang Cheng and Professor Dr. Jih-Jung Chen for providing natural product samples of 5 and 6. We also thank Mr. Xiaowei Wu, Drs. Adam Scharf and Paul Ralifo (Boston University), and Dr. Neil Lajkiewicz (Incyte Pharmaceuticals) for helpful discussions.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The authors declare no competing financial interest.
REFERENCES
- 1.(a) Piaz FD, Tosco A, Eletto D, Piccinelli AL, Moltedo O, Franceschelli S, Sbardella G, Remondelli P, Rastrelli L, Vesci L, Pisano C, Tommasi ND. Chem Bio Chem. 2010;11:818. doi: 10.1002/cbic.200900721. [DOI] [PubMed] [Google Scholar]; (b) Zhu L, Qi J, Chiao CY, Zhang Q, Porco JA, Jr, Faller DV, Dai Y. Int. J. Oncol. 2014;45:2128. doi: 10.3892/ijo.2014.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sell TS, Belkacemi T, Flockerzi V, Beck A. Sci. Rep. 2014;4:7500. doi: 10.1038/srep07500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Ravindra KC, Selvi BR, Arif M, Reddy BAA, Thanuja GR, Agrawal S, Pradhan SK, Nagashayana N, Dasgupta D, Kundu TK. J. Biol. Chem. 2009;284:24453. doi: 10.1074/jbc.M109.023861. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhang H, Zhang D-D, Lao Y-Z, Fu W-W, Liang S, Yuan Q-H, Yang L, Xu H-X. J. Nat. Prod. 2014;77:1700. doi: 10.1021/np5003498. [DOI] [PubMed] [Google Scholar]
- 3.(a) Majeed M. 2008/0207952 A1. Patent US. 2008 Aug 28;; (b) Taito N, Atsushi I. 2011/158806 A1. Patent WO. 2011 Dec 22;; (c) Nishino T, Wang C, Mochizuki-Kashio M, Osawa M, Nakauchi H, Iwama A. PLoS One. 2011;6:e24298. doi: 10.1371/journal.pone.0024298. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Majeed M. 2012/0178801 A1. Patent US. 2012 Jul 12;
- 4.Padhye S, Ahmad A, Oswal N, Sarkar FH. J. Hematol. Oncol. 2009;2:38. doi: 10.1186/1756-8722-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baliga MS, Bhat HP, Pai RJ, Boloor R, Palatty PL. Food Res. Int. 2011;44:1790. [Google Scholar]
- 6.Arif M, Pradhan SK, Thanuja GR, Vedamurthy BM, Agrawal S, Dasgupta D, Kundu TK. J. Med. Chem. 2009;52:267. doi: 10.1021/jm800657z. [DOI] [PubMed] [Google Scholar]
- 7.Chen J-J, Ting C-W, Hwang T-L, Chen I-S. J. Nat. Prod. 2009;72:253. doi: 10.1021/np8006364. [DOI] [PubMed] [Google Scholar]
- 8.For bioactivity studies on isogarcinol, see: Matsumoto K, Akao Y, Kobayashi E, Ito T, Ohguchi K, Tanaka T, Iinuma M, Nozawa Y. Biol. Pharm. Bull. 2003;26:569. doi: 10.1248/bpb.26.569. Hong J, Kwon SJ, Sang S, Ju J, Zhou J, Ho C-T, Huang M-T, Yang CS. Free Radical Biol. Med. 2007;42:1211. doi: 10.1016/j.freeradbiomed.2007.01.016. Sarli V, Giannis A. Chem. Biol. 2007;14:605. doi: 10.1016/j.chembiol.2007.06.001. Mantelingu K, Reddy BAA, Swaminathan V, Kishore AH, Siddappa NB, Kumar GVP, Nagashankar G, Natesh N, Roy S, Sadhale PP, Ranga U, Narayana C, Kundu TK. Chem. Biol. 2007;14:645. doi: 10.1016/j.chembiol.2007.04.011. Tian Z, Shen J, Wang F, Xiao P, Yang J, Lei H, Kazlauskas A, Kohane IS, Wu E. PLoS One. 2011;6:e21370. doi: 10.1371/journal.pone.0021370. Tchakam PD, Lunga PK, Kowa TK, Lonfouo AHN, Wabo HK, Tapondjou LA, Tane P, Kuiate J-R. BMC Complementary Altern. Med. 2012;12:136. doi: 10.1186/1472-6882-12-136. Cao S, Cen J. 103275048 A. Patent CN. 2013 Sep 4; Cen J, Shi M, Yang Y, Fu Y, Zhou H, Wang M, Su Z, Wei Q. PLoS One. 2013;8:e66503. doi: 10.1371/journal.pone.0066503. Kuete V, Tchakam PD, Wiench B, Ngameni B, Wabo HK, Tala MF, Moungang ML, Ngadjui BT, Murayama T, Efferth T. Phytomedicine. 2013;20:528. doi: 10.1016/j.phymed.2013.02.003. Fu Y, Zhou H, Wang M, Cen J, Wei Q. J. Agric. Food Chem. 2014;62:4127. doi: 10.1021/jf405790q. Hongxi X, Kaikai S, Zhijie D, Hong Z. 103536588 A. Patent CN. 2014 Jan 29; Cen J, Wang M, Jiang G, Yin Y, Su Z, Tong L, Iuo J, Ma Y, Gao Y, Wei Q. Biochimie. 2015;111:119. doi: 10.1016/j.biochi.2015.02.004. Park G, Tan J, Garcia G, Kang Y, Salvesen G, Zhang Z. J. Biol. Chem. 2016;291:3531. doi: 10.1074/jbc.M115.675488.
- 9.Xie Z, Sintara M, Chang T, Ou B. Food Sci. Nutr. 2015;3:342. doi: 10.1002/fsn3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Socolsky C, Plietker B. Chem.—;Eur. J. 2005;21:3053. doi: 10.1002/chem.201406077. [DOI] [PubMed] [Google Scholar]
- 11.For isolation and structure of isogarcinol, see: Rao AVR, Venkatswamy G, Pendse AD. Tetrahedron Lett. 1980;21:1975. Krishnamurthy N, Ravindranath B, Row TNG, Venkatesan K. 1982;23:2233. Krishnamurthy N, Lewis YS, Ravindranath B. Tetrahedron Lett. 1981;22:793. Iinuma M, Tosa H, Tanaka T, Kanamaru S, Asai F, Kobayashi Y, Miyauchi K, Shimano R. Biol. Pharm. Bull. 1996;19:311. doi: 10.1248/bpb.19.311. Ito C, Itoigawa M, Miyamoto Y, Onoda S, Rao KS, Mukainaka T, Tokuda H, Nishino H, Furukawa H. J. Nat. Prod. 2003;66:206. doi: 10.1021/np020372g. Hartati S, Kadono LBS, Kosela S, Harrison LJ. J. Biol. Sci. 2008;8:137. Kaur R, Chattopadhyay SK, Tandon S, Sharma S. Ind. Crops Prod. 2012;37:420. Wabo HK, Kowa TK, Lonfouo AHN, Tchinda AT, Tane P, Kikuchi H, Frédérich M, Oshima Y. Rec. Nat. Prod. 2012;6:94. Kaur R, Vasudev PG, Chattopadhyay SK. Acta Crystallogr., Sect. E: Struct. Rep. Online. 2012;68:o1861. doi: 10.1107/S1600536812020788. Ngameni B, Fotso GW, Ngachussi E, Poumale HMP, Ngadjui BT, Shiono Y, Murayama T. Asian J. Chem. 2014;26:6943.
- 12.For isolation and structure of 7-epi-isogarcinol (4), 30-epi-cambogin (2), cycloxanthochymol (5), and garcinol (6), see: Fuller RW, Blunt JW, Boswell JL, Cardellina JH, Boyd MR. J. Nat. Prod. 1999;62:130. doi: 10.1021/np9801514. Marti G, Eparvier V, Moretti C, Susplugas S, Prado S, Grellier P, Retailleau P, Guéritte F, Litaudon M. Phytochemistry. 2009;70:75. doi: 10.1016/j.phytochem.2008.10.005. Xu G, Kan WLT, Zhou Y, Song J-Z, Han Q-B, Qiao C-F, Cho C-H, Rudd JA, Lin G, Xu H-X. J. Nat. Prod. 2010;73:104. doi: 10.1021/np9004147. Marti G, Eparvier V, Moretti C, Prado S, Grellier P, Hue N, Thoison O, Delpech B, Guéritte F, Litaudon M. Phytochemistry. 2010;71:964. doi: 10.1016/j.phytochem.2010.03.008. Marti G, Eparvier V, Litaudon M, Grellier P, Guéritte F. Molecules. 2010;15:7106. doi: 10.3390/molecules15107106. Gao X-M, Yu T, Lai FSF, Zhou Y, Liu X, Qiao C-F, Song J-Z, Chen S-L, Luo KQ, Xu H-X. Bioorg. Med. Chem. 2010;18:4957. doi: 10.1016/j.bmc.2010.06.014. Xia Z-X, Zhang D-D, Liang S, Lao Y-Z, Zhang H, Tan H-S, Chen S-L, Wang X-H, Xu H-X. J. Nat. Prod. 2012;75:1459. doi: 10.1021/np3003639. Tala MF, Wabo HK, Zeng G-Z, Ji C-J, Tane P, Tan N-H. Phytochem. Lett. 2013;6:326.
- 13.Boyce JH, Porco JA., Jr Angew. Chem., Int. Ed. 2014;53:7832. doi: 10.1002/anie.201404437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.See the Supporting Information for complete experimental details.
- 15.(a) Lv J, Li X, Zhong L, Luo S, Cheng J-P. Org. Lett. 2010;12:1096. doi: 10.1021/ol1000928. [DOI] [PubMed] [Google Scholar]; (b) Lv J, Luo S. Chem. Commun. 2013;49:847. doi: 10.1039/c2cc34288j. [DOI] [PubMed] [Google Scholar]
- 16.(a) Ishihara K, Nakashima D, Hiraiwa Y, Yamamoto H. J. Am. Chem. Soc. 2003;125:24. doi: 10.1021/ja021000x. [DOI] [PubMed] [Google Scholar]; (b) Cheon CH, Yamamoto H. J. Am. Chem. Soc. 2008;130:9246. doi: 10.1021/ja8041542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.For pioneering studies on the use of Lewis acid-assisted Brønsted acid catalysis for polyene cyclizations, see: Ishihara K, Nakamura S, Yamamoto H. J. Am. Chem. Soc. 1999;121:4906. Nakamura S, Ishihara K, Yamamoto H. J. Am. Chem. Soc. 2000;122:8131. Ishihara K, Ishibashi H, Yamamoto H. J. Am. Chem. Soc. 2001;123:1505. Ishihara K, Ishibashi H, Yamamoto H. J. Am. Chem. Soc. 2002;124:3647. doi: 10.1021/ja0124865. Ishibashi H, Ishihara K, Yamamoto H. J. Am. Chem. Soc. 2004;126:11122. doi: 10.1021/ja0472026.
- 18.Wassef MA, Hessin S. Commun. Fac. Sci. Univ. Ankara, Ser. B: Chem. Chem. Eng. 1981;27:153. [Google Scholar]
- 19.(a) Surendra K, Corey EJ. J. Am. Chem. Soc. 2012;134:11992. doi: 10.1021/ja305851h. [DOI] [PubMed] [Google Scholar]; (b) Surendra K, Rajendar G, Corey EJ. J. Am. Chem. Soc. 2014;136:642. doi: 10.1021/ja4125093. [DOI] [PubMed] [Google Scholar]
- 20.For examples of relevant octahedral Sn-complexes, see: Zinov’eva EG, Efimov VA, Kol’tsov NI, Musin RZ, Dimukhametov MN, Gubaidullin AT, Krivolapov DB. Russ. J. Gen. Chem. 2008;78:1509. Feshin VP, Feshina EV. Russ. J. Gen. Chem. 2011;81:497.
- 21.For examples of relevant BF2-complexes, see: Maeda H, Haketa Y. Org. Biomol. Chem. 2008;6:3091. doi: 10.1039/b806161k. Štephane B. Org. Lett. 2010;12:2900.
- 22.Uwamori M, Saito A, Nakada M. J. Org. Chem. 2012;77:5098. doi: 10.1021/jo300646j. [DOI] [PubMed] [Google Scholar]
- 23.Dandapani S, Jeske M, Curran DP. Proc. Natl. Acad. Sci. U. S. A. 2004;101:12008. doi: 10.1073/pnas.0401677101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(a) Ishibashi H, Ishihara K, Yamamoto H. Chem. Rec. 2002;2:177. doi: 10.1002/tcr.10020. [DOI] [PubMed] [Google Scholar]; (b) Kumazawa K, Ishihara K, Yamamoto H. Org. Lett. 2004;6:2551. doi: 10.1021/ol049126h. [DOI] [PubMed] [Google Scholar]
- 25.(a) Nasveschuk CG, Rovis T. Angew. Chem., Int. Ed. 2005;44:3264. doi: 10.1002/anie.200500088. [DOI] [PubMed] [Google Scholar]; (b) Nasveschuk CG, Rovis T. Org. Biomol. Chem. 2008;6:240. doi: 10.1039/b714881j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.