Abstract
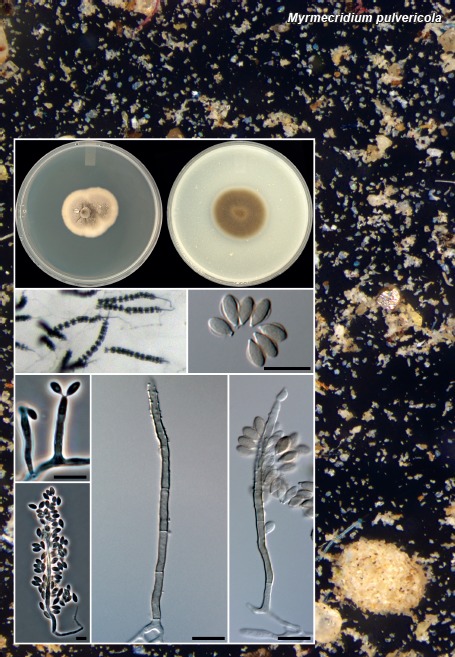
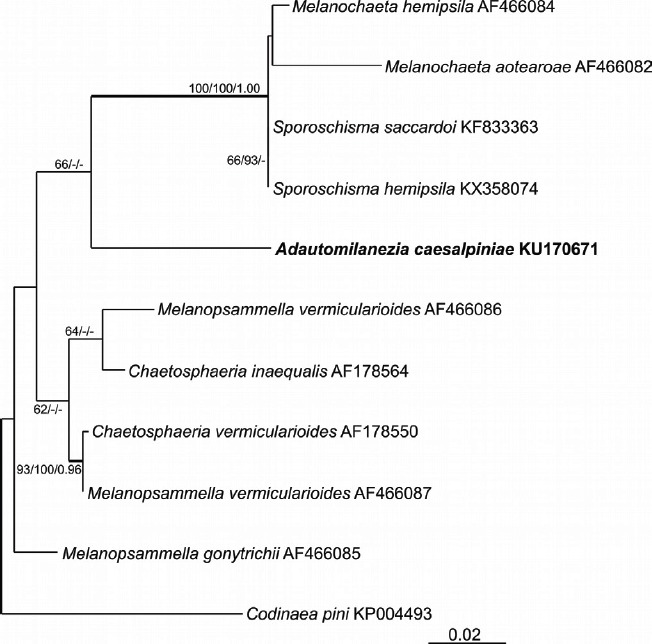
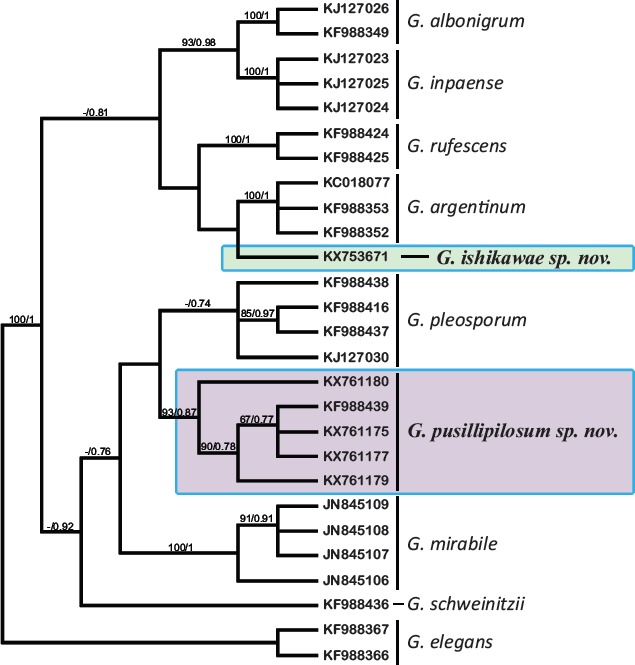
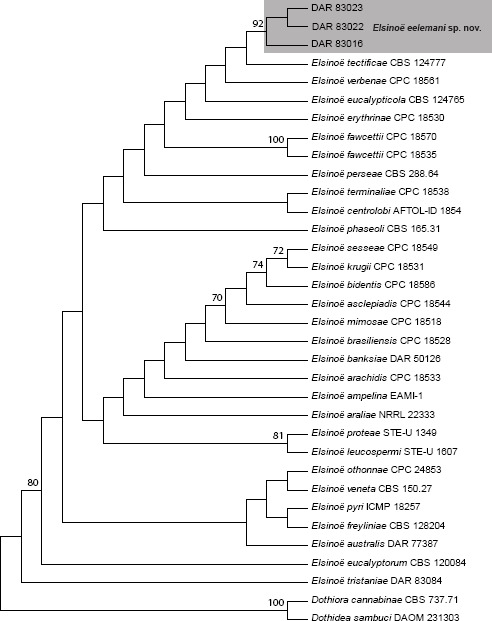
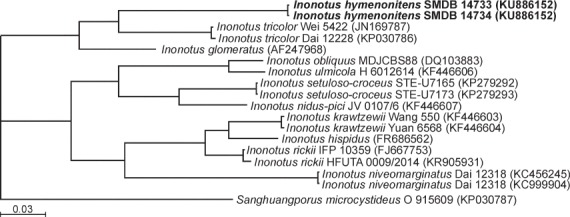
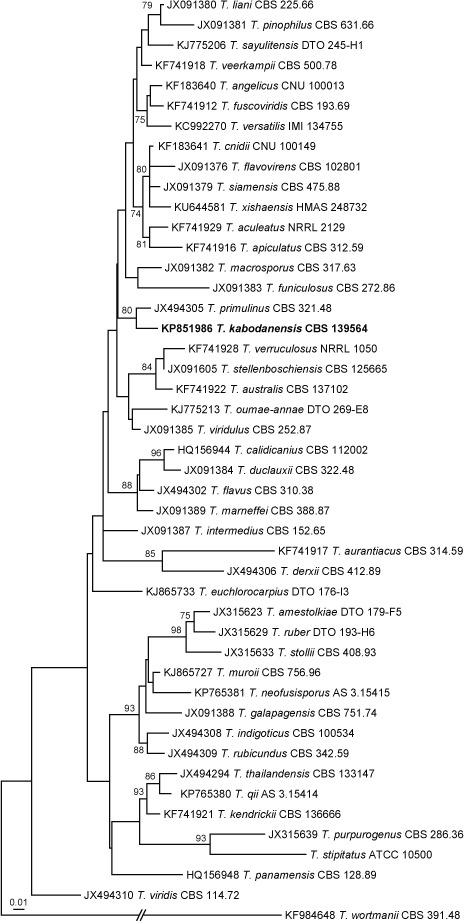
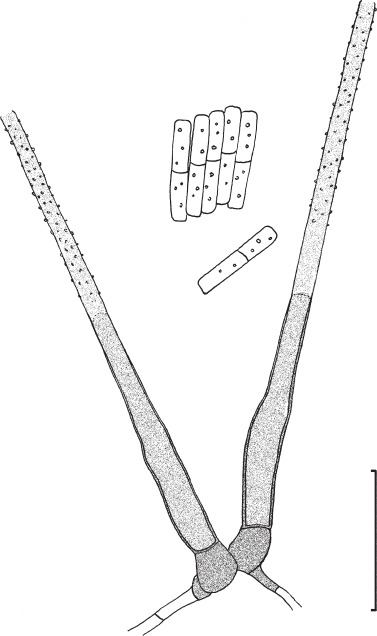
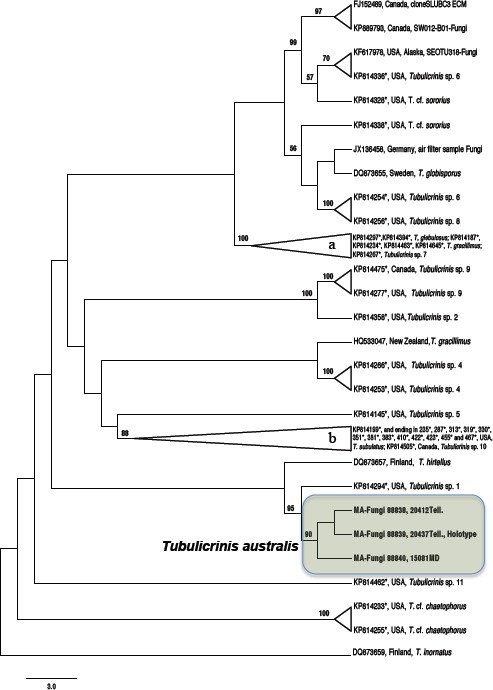
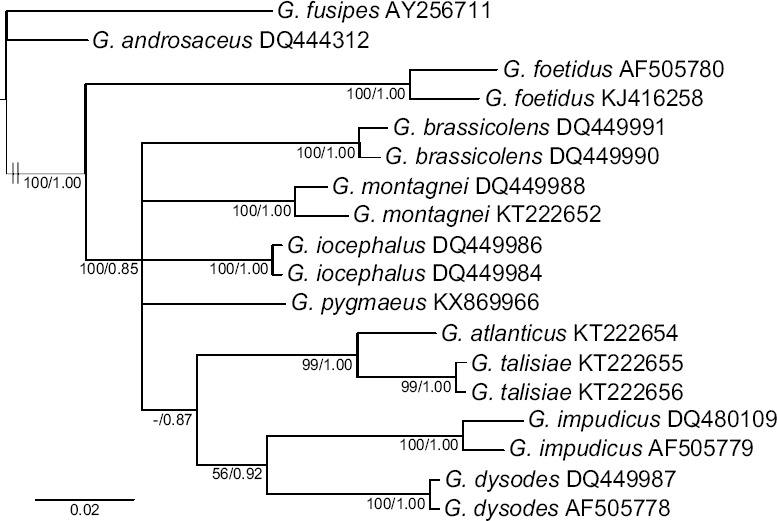
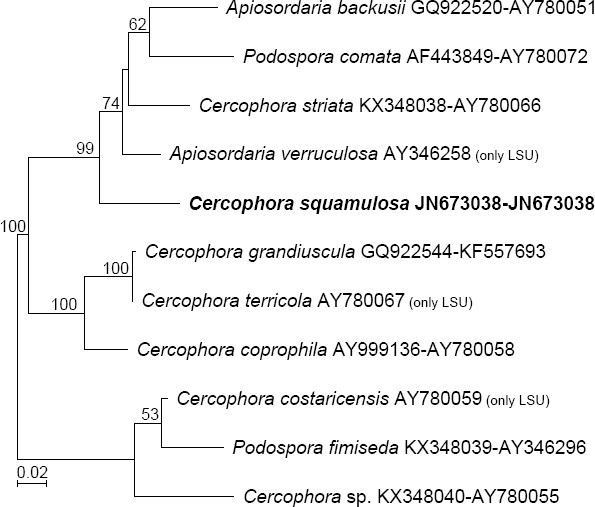
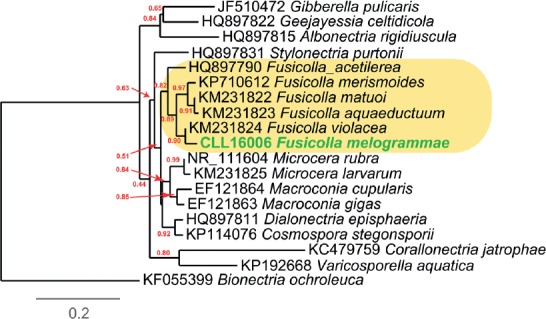
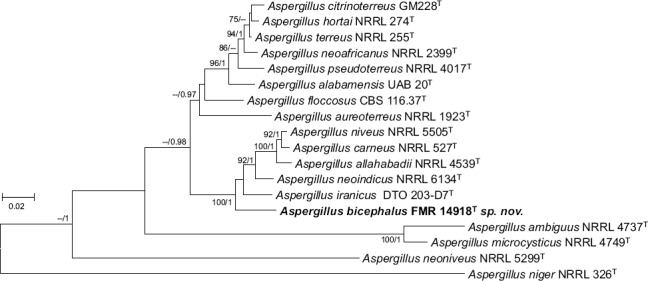
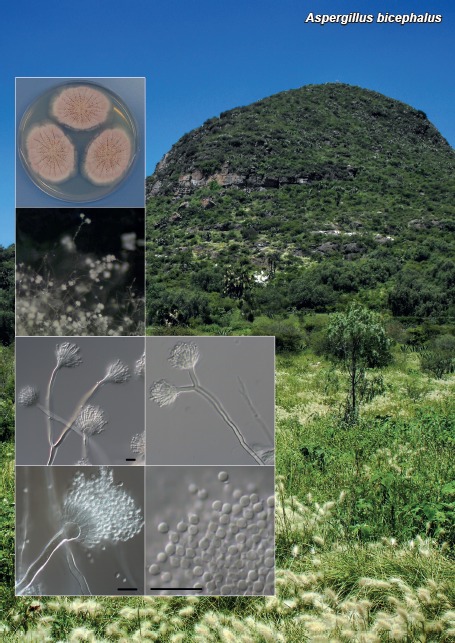
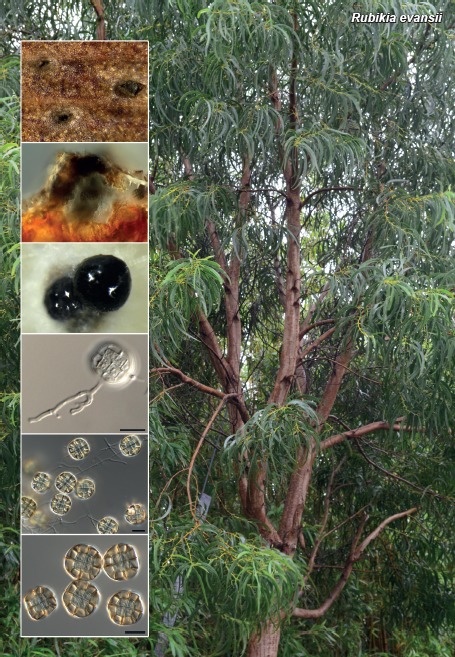
Novel species of fungi described in this study include those from various countries as follows: Australia: Apiognomonia lasiopetali on Lasiopetalum sp., Blastacervulus eucalyptorum on Eucalyptus adesmophloia, Bullanockia australis (incl. Bullanockia gen. nov.) on Kingia australis, Caliciopsis eucalypti on Eucalyptus marginata, Celerioriella petrophiles on Petrophile teretifolia, Coleophoma xanthosiae on Xanthosia rotundifolia, Coniothyrium hakeae on Hakea sp., Diatrypella banksiae on Banksia formosa, Disculoides corymbiae on Corymbia calophylla, Elsinoë eelemani on Melaleuca alternifolia, Elsinoë eucalyptigena on Eucalyptus kingsmillii, Elsinoë preissianae on Eucalyptus preissiana, Eucasphaeria rustici on Eucalyptus creta, Hyweljonesia queenslandica (incl. Hyweljonesia gen. nov.) on the cocoon of an unidentified microlepidoptera, Mycodiella eucalypti (incl. Mycodiella gen. nov.) on Eucalyptus diversicolor, Myrtapenidiella sporadicae on Eucalyptus sporadica, Neocrinula xanthorrhoeae (incl. Neocrinula gen. nov.) on Xanthorrhoea sp., Ophiocordyceps nooreniae on dead ant, Phaeosphaeriopsis agavacearum on Agave sp., Phlogicylindrium mokarei on Eucalyptus sp., Phyllosticta acaciigena on Acacia suaveolens, Pleurophoma acaciae on Acacia glaucoptera, Pyrenochaeta hakeae on Hakea sp., Readeriella lehmannii on Eucalyptus lehmannii, Saccharata banksiae on Banksia grandis, Saccharata daviesiae on Daviesia pachyphylla, Saccharata eucalyptorum on Eucalyptus bigalerita, Saccharata hakeae on Hakea baxteri, Saccharata hakeicola on Hakea victoria, Saccharata lambertiae on Lambertia ericifolia, Saccharata petrophiles on Petrophile sp., Saccharata petrophilicola on Petrophile fastigiata, Sphaerellopsis hakeae on Hakea sp., and Teichospora kingiae on Kingia australis. Brazil: Adautomilanezia caesalpiniae (incl. Adautomilanezia gen. nov.) on Caesalpina echinata, Arthrophiala arthrospora (incl. Arthrophiala gen. nov.) on Sagittaria montevidensis, Diaporthe caatingaensis (endophyte from Tacinga inamoena), Geastrum ishikawae on sandy soil, Geastrum pusillipilosum on soil, Gymnopus pygmaeus on dead leaves and sticks, Inonotus hymenonitens on decayed angiosperm trunk, Pyricularia urashimae on Urochloa brizantha, and Synnemellisia aurantia on Passiflora edulis. Chile: Tubulicrinis australis on Lophosoria quadripinnata. France: Cercophora squamulosa from submerged wood, and Scedosporium cereisporum from fluids of a wastewater treatment plant. Hawaii: Beltraniella acaciae, Dactylaria acaciae, Rhexodenticula acaciae, Rubikia evansii and Torula acaciae (all on Acacia koa). India: Lepidoderma echinosporum on dead semi-woody stems, and Rhodocybe rubrobrunnea from soil. Iran: Talaromyces kabodanensis from hypersaline soil. La Réunion: Neocordana musarum from leaves of Musa sp. Malaysia: Anungitea eucalyptigena on Eucalyptus grandis × pellita, Camptomeriphila leucaenae (incl. Camptomeriphila gen. nov.) on Leucaena leucocephala, Castanediella communis on Eucalyptus pellita, Eucalyptostroma eucalypti (incl. Eucalyptostroma gen. nov.) on Eucalyptus pellita, Melanconiella syzygii on Syzygium sp., Mycophilomyces periconiae (incl. Mycophilomyces gen. nov.) as hyperparasite on Periconia on leaves of Albizia falcataria, Synnemadiella eucalypti (incl. Synnemadiella gen. nov.) on Eucalyptus pellita, and Teichospora nephelii on Nephelium lappaceum. Mexico: Aspergillus bicephalus from soil. New Zealand: Aplosporella sophorae on Sophora microphylla, Libertasomyces platani on Platanus sp., Neothyronectria sophorae (incl. Neothyronectria gen. nov.) on Sophora microphylla, Parastagonospora phoenicicola on Phoenix canariensis, Phaeoacremonium pseudopanacis on Pseudopanax crassifolius, Phlyctema phoenicis on Phoenix canariensis, and Pseudoascochyta novae-zelandiae on Cordyline australis. Panama: Chalara panamensis from needle litter of Pinus cf. caribaea. South Africa: Exophiala eucalypti on leaves of Eucalyptus sp., Fantasmomyces hyalinus (incl. Fantasmomyces gen. nov.) on Acacia exuvialis, Paracladophialophora carceris (incl. Paracladophialophora gen. nov.) on Aloe sp., and Umthunziomyces hagahagensis (incl. Umthunziomyces gen. nov.) on Mimusops caffra. Spain: Clavaria griseobrunnea on bare ground in Pteridium aquilinum field, Cyathus ibericus on small fallen branches of Pinus halepensis, Gyroporus pseudolacteus in humus of Pinus pinaster, and Pseudoascochyta pratensis (incl. Pseudoascochyta gen. nov.) from soil. Thailand: Neoascochyta adenii on Adenium obesum, and Ochroconis capsici on Capsicum annuum. UK: Fusicolla melogrammae from dead stromata of Melogramma campylosporum on bark of Carpinus betulus. Uruguay: Myrmecridium pulvericola from house dust. USA: Neoscolecobasidium agapanthi (incl. Neoscolecobasidium gen. nov.) on Agapanthus sp., Polyscytalum purgamentum on leaf litter, Pseudopithomyces diversisporus from human toenail, Saksenaea trapezispora from knee wound of a soldier, and Sirococcus quercus from Quercus sp. Morphological and culture characteristics along with DNA barcodes are provided.
Keywords: ITS nrDNA barcodes, LSU, novel fungal species, systematics
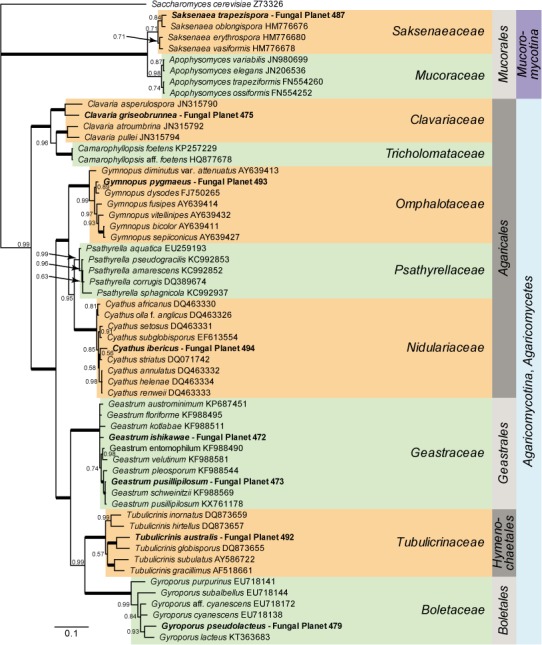
Overview Mucoromycotina and Agaricomycotina phylogeny
Consensus phylogram (50 % majority rule) of 2 394 trees resulting from a Bayesian analysis of the LSU sequence alignment (58 taxa including outgroup; 874 aligned positions; 507 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families, orders and classes are indicated with coloured blocks to the right of the tree. GenBank accession or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Saccharomyces cerevisiae (GenBank Z73326) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S20202).
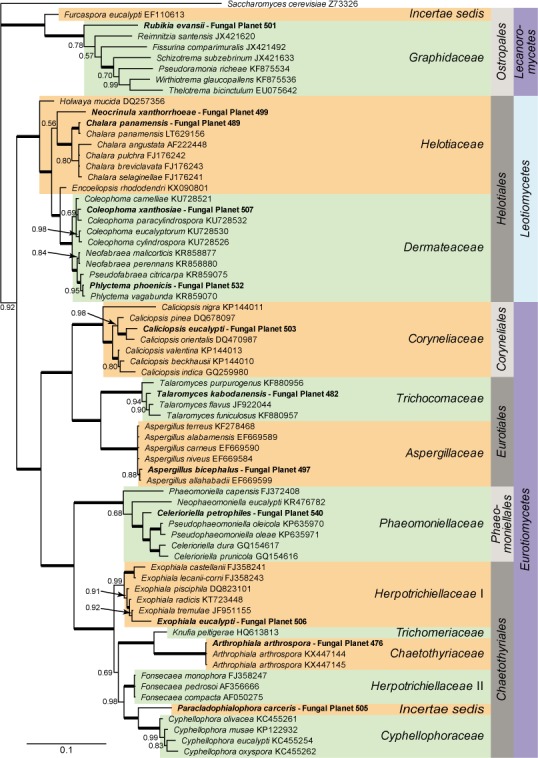
Overview Lecanoromycetes, Leotiomycetes and Eurotiomycetes, phylogeny
Consensus phylogram (50 % majority rule) of 790 trees resulting from a Bayesian analysis of the LSU sequence alignment (70 taxa including outgroup; 808 aligned positions; 386 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families, orders and classes are indicated with coloured blocks to the right of the tree. GenBank accession or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Saccharomyces cerevisiae (GenBank Z73326) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S20202).
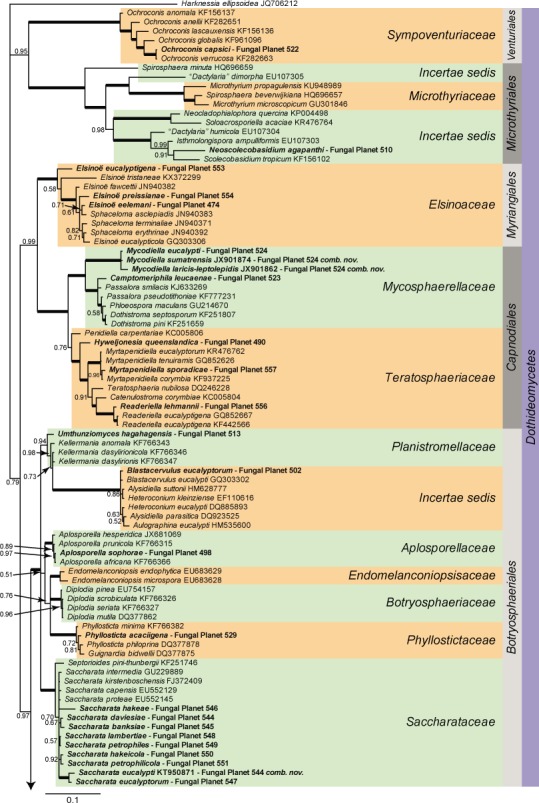
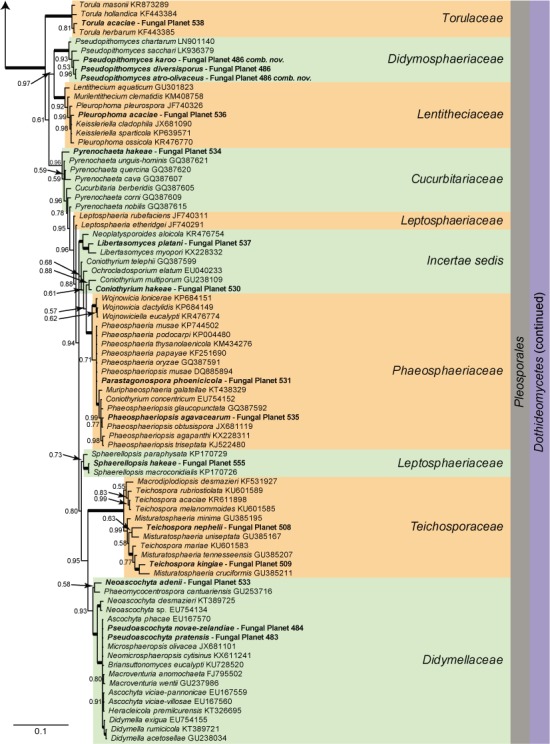
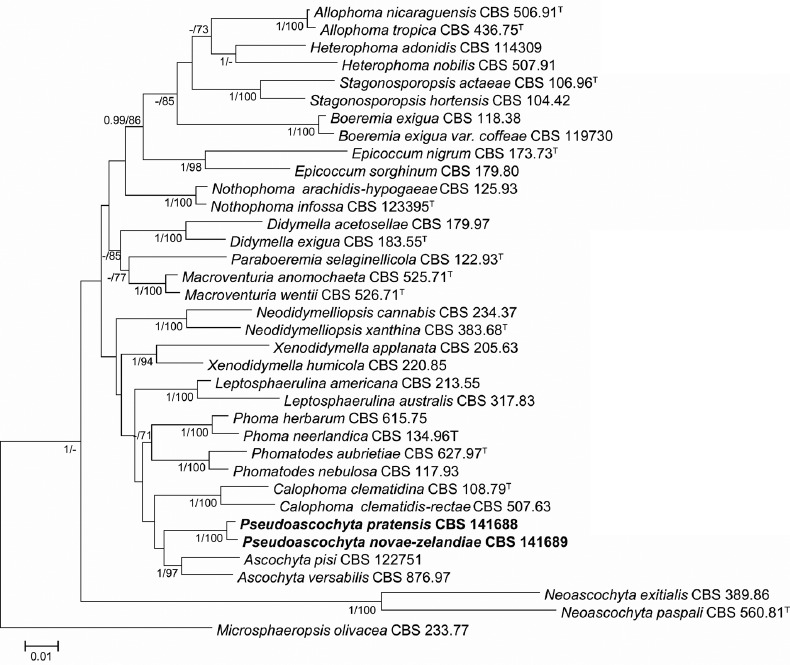
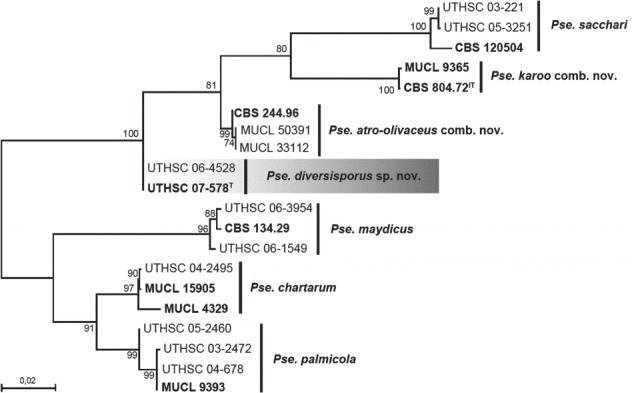
Overview Dothideomycetes phylogeny
Consensus phylogram (50 % majority rule) of 10 030 trees resulting from a Bayesian analysis of the LSU sequence alignment (167 taxa including outgroup; 806 aligned positions; 453 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families, orders and classes are indicated with coloured blocks to the right of the tree. GenBank accession or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Harknessia ellipsoidea (GenBank JQ706212) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S20202).
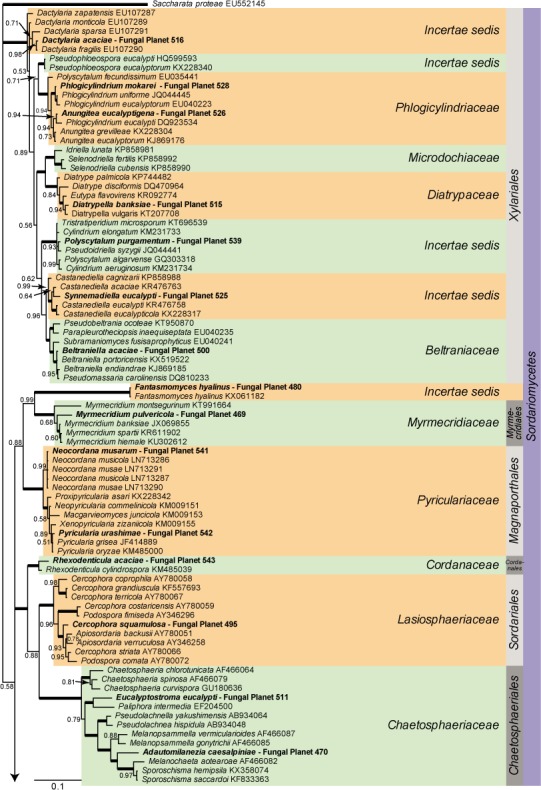
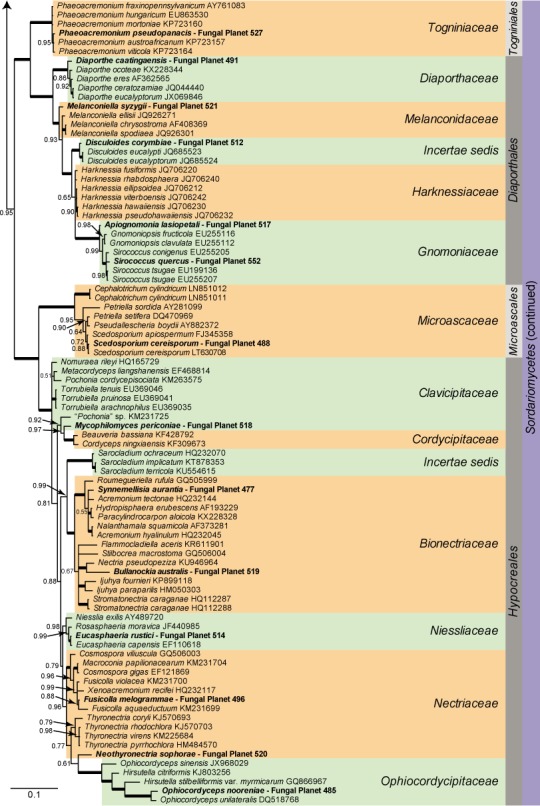
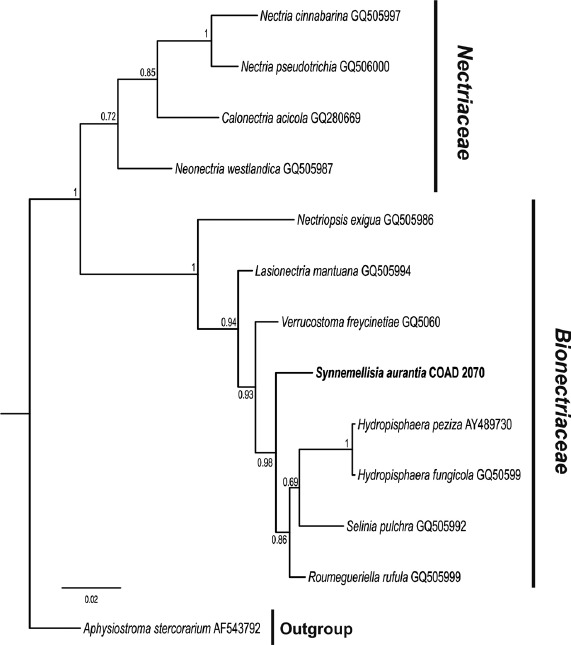
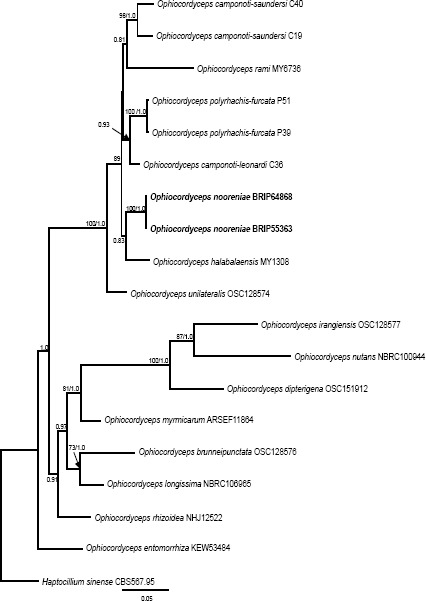
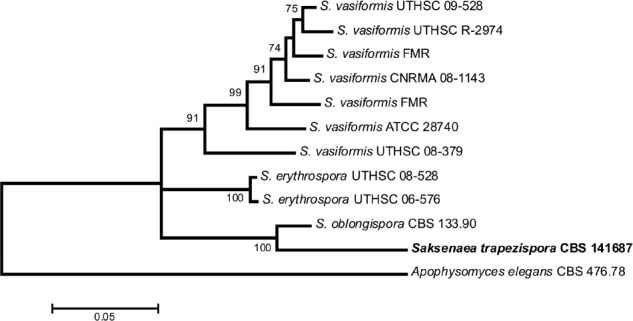
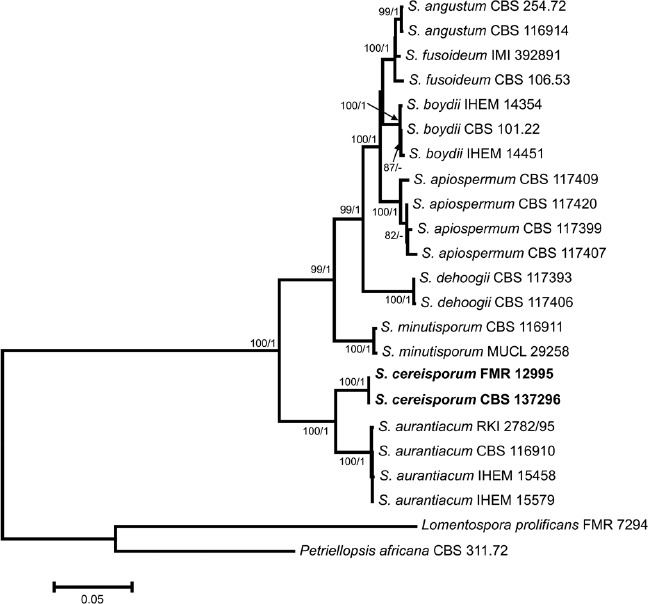
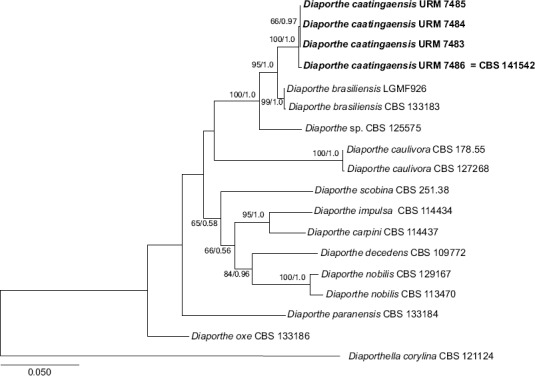
Overview Sordariomycetes phylogeny
Consensus phylogram (50 % majority rule) of 8 418 trees resulting from a Bayesian analysis of the LSU sequence alignment (174 taxa including outgroup; 788 aligned positions; 393 unique site patterns) using MrBayes v. 3.2.6 (Ronquist et al. 2012). Bayesian posterior probabilities (PP) are shown at the nodes and thickened lines represent nodes with PP = 1.00. The scale bar represents the expected changes per site. Families, orders and classes are indicated with coloured blocks to the right of the tree. GenBank accession or Fungal Planet numbers are indicated behind the species names. The tree was rooted to Saccharata proteae (GenBank EU552145) and the taxonomic novelties described in this study for which LSU sequence data were available are indicated in bold face. The alignment and tree were deposited in TreeBASE (Submission ID S20202).
Acknowledgments
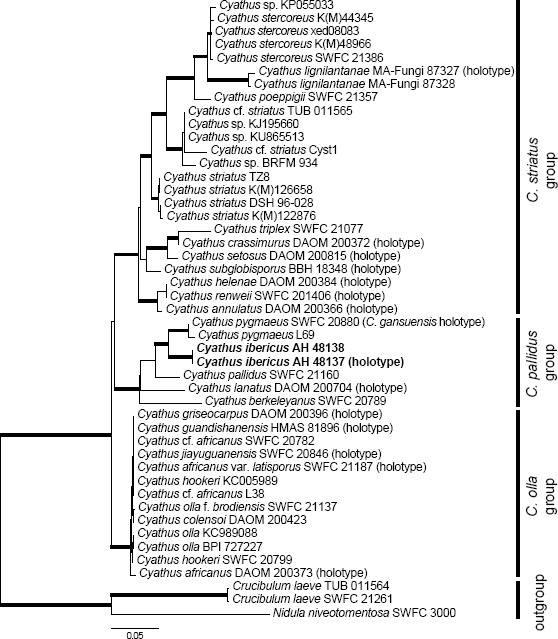
Paulo C. Ceresini acknowledges permission for scientific activities # 39131-3 from the Brazilian Ministry of Environment (MMA) / ‘Chico Mendes’ Institute for Conservation of Biodiversity (ICMBIO). Vanina L. Castroagudín is supported by a Post-Doctoral research fellowship from São Paulo Research Foundation – FAPESP / Higher Education Personnel Improvement Coordination – CAPES, Brazil (PDJ 2014/25904-2, from 2015–2016). Paulo C. Ceresini is supported by a research grant from FAPESP (2015/10453-8) and a fellowship grant from the Brazilian National Council for Scientific and Technological Development – CNPq (307295/2015-0). Santiago Català and Carlos Rojo helped by providing the DNA sequences of Cyathus ibericus used in this study. The research was on-going while J.C. Zamora was a recipient of funding from the Ministerio de Economía y Competitividad (Juan de la Cierva-formación program, FJCI-2014-19801, Spain). Christian Lechat and Nick Aplin acknowledge Amy Y. Rossman (Oregon State University, Corvallis, USA) for her advice and scientific assistance. Jacques Fournier, Las Muros, 09320 Rimont, France, is thanked for the material he collected. Thiago Accioly and co-workers acknowledge Marian Glenn (Seton Hall University, New Jersey) for the English revision of the Geastrum text. Gabriel Moreno and co-workers express their gratitude to Antonio Sánchez and Jaime de Frutos (Mycological Society of Segovia) and Celestino Gelpi (Mycological Society of Extremadura), for sending collections of Gyroporus pseudolacteus; to L. Monje and A. Pueblas of the Department of Drawing and Scientific Photography at the University of Alcalá for their help in preparing the digital photographs; to J. Rejos, curator of the AH herbarium for his assistance with the specimens examined in the present study. The survey which yielded the material considered herein was supported in part by a grant (DEB-0316284) from the National Science Foundation to the University of Arkansas. The assistance of Lal Singh in carrying out the fieldwork in India is gratefully acknowledged. K.N. Anil Raj acknowledges support from the University Grants Commission (UGC), India, in the form of a Rajiv Gandhi National Fellowship (Grant No. F. 14-2(SC)/2009 (SA-III)). K.P. Deepna Latha acknowledges support from the Kerala State Council for Science, Technology and Environment (KSCSTE) in the form of a PhD fellowship (Grant No. 001/FSHP/2011/CSTE), and is also grateful to the Principal Chief Conservator of forests, Kerala State, for granting permission (No. WL10-4937/2012, dated 03-10-2013) to collect agarics from the forests of Kerala. Margarita Dueñas and co-workers acknowledge financial support from the Agreement Endesa and San Ignacio de Huinay Foundations and Consejo Superior de Investigaciones Científicas, CSIC, 2013CL0012 and Plan Nacional I+D+I project n CGL2015-67459P, and thank Marian Glenn (Seton Hall University, USA) for revising the Tubulicrinis australis text. Gordon F. Claridge and co-workers thank Alistair McTaggart for providing the LSU sequence of Ophiocordyceps nooreniae, and Thomas Marney for his valuable advice. Josep Guarro and co-workers acknowledge financial support from the Ministerio de Economía y Competitividad, grant CGL2013-43789P. D.H. Lee acknowledges the Department of Science and Technology (DST)-National Research Foundation (NRF) Centre of Excellence in Tree Health Biotechnology, the National Research Foundation (NRF) for financial support and South African National Parks (SanParks) scientific services at Skukuza for technical and logistic assistance. Work associated with the description of Elsinoe eelemani was funded by the Rural Industries Research and Development Corporation, and the Australian Tea Tree Industry Association. Victor R.M. Coimbra acknowledges CNPq – Conselho Nacional de Desenvolvimento Científico e Tecnológico (SWE 232695/2014-8, Sisbiota 563342/2010-2, PROTAX 562106/2010-3) and FACEPE – Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (IBPG 0827-2.03/12, AMD 0218-2.00/13, APQ 0788-2.03/12) for the PhD scholarships and partially funding this research. Pedro W. Crous and Michael J. Wingfield acknowledge Murdoch University for support via the Sir Walter Murdoch Professorial appointments.
REFERENCES
- Alvarez E, Garcia-Hermoso D, Sutton DA, et al. 2010. Molecular phylogeny and proposal of two new species of the emerging pathogenic fungus Saksenaea. Journal of Clinical Microbiology 48: 4410–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonín V, Noordeloos ME. 2010. A monograph of marasmioid and collybioid fungi in Europe. IHW-Verlag, Eching. [Google Scholar]
- Araújo J, Evans H, Geiser DM, et al. 2015. Unravelling the diversity behind the Ophiocordyceps unilateralis (Ophiocordycipitaceae) complex: Three new species of zombie-ant fungi from the Brazilian Amazon. Phytotaxa 220: 224–238. [Google Scholar]
- Ariyawansa HA, Hyde KD, Jayasiri SC, et al. 2015. Fungal diversity notes 111–252 – taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 75: 27–274. [Google Scholar]
- Arzanlou M, Groenewald JZ, Gams W, et al. 2007. Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology 58: 57–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzanlou M, Samadi R, Frisvad JC, et al. 2016. Two novel Aspergillus species from hypersaline soils of The National Park of Lake Urmia, Iran. Mycological Progress: doi 10.1007/s11557-016-1230-8. [Google Scholar]
- Badali H, Gueidan C, Najafzadeh MJ, et al. 2008. Biodiversity of the genus Cladophialophora. Studies in Mycology 61: 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroni TJ. 1981. A revision of the genus Rhodocybe Maire (Agaricales). Beihefte zur Nova Hedwigia 67: 1–194. [Google Scholar]
- Baroni TJ, Halling RE. 1992. New species of Rhodocybe from South America with a key to species. Mycologia 84: 411–421. [Google Scholar]
- Baseia IG, Calonge FD. 2006. Geastrum hirsutum: a new earthstar fungus with a hairy exoperidium. Mycotaxon 95: 301–304. [Google Scholar]
- Brodie HJ. 1966. Cyathus pygmaeus from Northwestern United States. Mycologia 58: 973–975. [Google Scholar]
- Brodie HJ. 1975. The bird’s nest fungi. University of Toronto Press, Toronto, Canada. [Google Scholar]
- Brodie HJ. 1977. A key of the species of Cyathus (Nidulariaceae). Botaniska Notiser 130: 453–459. [Google Scholar]
- Brodie HJ. 1984. More bird’s nest fungi (Nidulariaceae) (a supplement to ‘the bird’s nest fungi’). Lejeunia 112: 1–69. [Google Scholar]
- Bulliard JBF. 1788. Herbier de la France 8: 337–384. [Google Scholar]
- Cabral TS, Silva BDB, Ishikawa NK, et al. 2014. A new species and new records of gasteroid fungi (Basidiomycota) from Central Amazonia, Brazil. Phytotaxa 183: 239–253. [Google Scholar]
- Calonge FD. 1998. Gasteromycetes. I. Lycoperdales, Nidulariales, Phallales, Sclerodermatales, Tulostomales. Flora Mycologica Iberica 3: 1–271. [Google Scholar]
- Calonge FD, Mata M. 2004. A new species of Geastrum from Costa Rica and Mexico. Boletín de la Sociedad Micológica de Madrid 28: 331–335. [Google Scholar]
- Castro ML, Freire L. 1995. Gyroporus ammophilus, a new poisonous bolete from the Iberian Peninsula. Persoonia 16: 123–126. [Google Scholar]
- Castroagudín VL, Moreira SI, Pereira DAS, et al. 2016. Pyricularia graminis-tritici, a new Pyricularia species causing wheat blast. Persoonia 37: 199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheewangkoon R, Groenewald JZ, Hyde KD, et al. 2012. Chocolate spot of Eucalyptus. Mycological Progress 11: 61–69. [Google Scholar]
- Cheewangkoon R, Groenewald JZ, Summerell BA, et al. 2009. Myrtaceae, a cache of fungal biodiversity. Persoonia 23: 55–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Verkley GJM, Sun G, et al. 2016. Redefining common endophytes and plant pathogens in Neofabraea, Pezicula, and related genera. Fungal Biology 120: 1291–1322. [DOI] [PubMed] [Google Scholar]
- Chen Q, Jiang JR, Zhang GZ, et al. 2015. Resolving the Phoma enigma. Studies in Mycology 82: 137–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra VRM, Pinheiro FGB, Wartchow F, et al. 2015. Studies on Gymnopus sect. Impudicae (Omphalotaceae, Agaricales) from Northern Brazil: two new species and notes on G. montagnei. Mycological Progress 14: 110. [Google Scholar]
- Condé BD, Pitkethley RN, Smith ESC, et al. 1997. Disease notes or new records: Identification of mango scab caused by Elsinoё mangiferae in Australia. Australasian Plant Pathology 26: 131. [Google Scholar]
- Cortez VG, Baseia IG, Silveira RMB. 2008. Gasteromicetos (Basidiomycota) no Parque Estadual de Itapuã, Viamão, Rio Grande do Sul, Brasil. Revista Brasileira de Biociências 6: 291–299. [Google Scholar]
- Crane C, Burgess TI. 2013. Luteocirrhus shearii gen. sp. nov. (Diaporthales, Cryphonectriaceae) pathogenic to Proteaceae in the South Western Australian Floristic Region. IMA Fungus 4: 111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane JL, Miller AN. 2016. Studies in genera similar to Torula: Bahusaganda, Bahusandhika, Pseudotorula, and Simmonsiella gen. nov. IMA Fungus 7: 29–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Carris LM, Giraldo A, et al. 2015a. The genera of fungi - fixing the application of the type species of generic names – G 2: Allantophomopsis, Latorua, Macrodiplodiopsis, Macrohilum, Milospium, Protostegia, Pyricularia, Robillarda, Rotula, Septoriella, Torula, and Wojnowicia. IMA Fungus 6: 163–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ. 2011. Why everlastings don’t last. Persoonia 26: 70–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ. 2016. They seldom occur alone. Fungal Biology 120: 1392–1415. [DOI] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Smith IW. 2007a. Phlogicylindrium eucalyptorum. Fungal Planet No. 20 Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands. [Google Scholar]
- Crous PW, Groenewald JZ, Summerell B. 2007b. Exophiala placitae. Fungal Planet No. 17 Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands. [Google Scholar]
- Crous PW, Mohammed C, Glen M, et al. 2007c. Eucalyptus microfungi known from culture. 3. Eucasphaeria and Sympoventuria genera nova, and new species of Furcaspora, Harknessia, Heteroconium and Phacidiella. Fungal Diversity 25: 19–36. [Google Scholar]
- Crous PW, Schubert K, Braun U, et al. 2007d. Opportunistic, human-pathogenic species in the Herpotrichiellaceae are phenotypically similar to saprobic or phytopathogenic species in the Venturiaceae. Studies in Mycology 58: 185–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Wingfield MJ, et al. 2015b. Fungal Systematics and Evolution: FUSE 1. Sydowia 67: 81–118. [Google Scholar]
- Crous PW, Shivas RG, Quaedvlieg W, et al. 2014. Fungal Planet description sheets: 214–280. Persoonia 32: 184–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Shivas RG, Wingfield MJ, et al. 2012a. Fungal Planet description sheets: 128–153. Persoonia 29: 146–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Alfenas AC, et al. 2012b. Genera of diaporthalean coelomycetes associated with leaf spots of tree hosts. Persoonia 28: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Carnegie AJ, et al. 2009. Unravelling Mycosphaerella: do you believe in genera? Persoonia 23: 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, et al. 2011a. Fungal Planet description sheets: 92–106. Persoonia 27: 130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Shivas RG, et al. 2012c. Fungal Planet description sheets: 107–127. Persoonia 28: 138–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Summerell BA, Swart L, et al. 2011b. Fungal pathogens of Proteaceae. Persoonia 27: 20–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, et al. 2013. Fungal Planet description sheets: 154–231. Persoonia 31: 188–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J, et al. 2015c. Fungal Planet description sheets: 320–370. Persoonia 34: 167–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Le Roux JJ, et al. 2015d. Fungal Planet description sheets: 371–399. Persoonia 35: 264–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Mansilla JP, et al. 2006. Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. II. Studies in Mycology 55: 99–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Richardson DM, et al. 2016. Fungal Planet description sheets: 400–468. Persoonia 36: 316–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham GH. 1963. The Thelephoraceae of Australia and New Zealand. Bulletin 145: 1–359. New Zealand, Department of Scientific and Industrial Research. [Google Scholar]
- Da Cunha KC, Sutton DA, Gené J, et al. 2014. Pithomyces species (Montagnulaceae) from clinical specimens: identification and antifungal susceptibility profiles. Medical Mycology 52: 748–757. [DOI] [PubMed] [Google Scholar]
- Dai YC. 2010. Hymenochaetaceae (Basidiomycota) in China. Fungal Diversity 45: 131–343. [Google Scholar]
- Damm U, Fourie PH, Crous PW. 2007. Aplosporella prunicola, a novel species of anamorphic Botryosphaeriaceae. Fungal Diversity 27: 35–43. [Google Scholar]
- De Beer ZW, Duong TA, Wingfield MJ. 2016a. The divorce of Sporothrix and Ophiostoma: solution to a problematic relationship. Studies in Mycology 83: 165–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beer ZW, Marincowitz S, Duong TA, et al. 2016b. Hawksworthiomyces gen. nov. (Ophiostomatales), illustrates the urgency for a decision on how to name novel taxa known only from environmental nucleic acid sequences (ENAS). Fungal Biology 120: 1323–1340. [DOI] [PubMed] [Google Scholar]
- De Beer ZW, Seifert KA, Wingfield MJ. 2013. The Ophiostomatoid fungi: their dual position in the Sordariomycetes. In: Seifert KA, De Beer ZW, Wingfield MJ. (eds), The Ophiostomatoid fungi: expanding frontiers. CBS Biodiversity Series 12: 1–19. CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands. [Google Scholar]
- De Beer ZW, Wingfield MJ. 2013. Emerging lineages in the Ophiostomatales. In: Seifert KA, De Beer ZW, Wingfield MJ. (eds), The Ophiostomatoid fungi: expanding frontiers. CBS Biodiversity Series 12: 21–46. CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands. [Google Scholar]
- De Gruyter J, Woudenberg JHC, Aveskamp MM, et al. 2013. Redisposition of Phoma-like anamorphs in Pleosporales. Studies in Mycology 75: 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hoog GS. 1985. Taxonomy of the Dactylaria complex, IV. Dactylaria, Neta, Subulispora and Scolecobasidium. Studies in Mycology 26: 1–60. [Google Scholar]
- De Hoog GS, Guarro J, Gené J, et al. 2000. Atlas of Clinical Fungi, 2nd ed Centraalbureau voor Schimmelcultures / Universitat Rovira i Virgili, Utrecht / Reus. [Google Scholar]
- De Hoog GS, Nishikaku AS, Fernández Zeppenfeldt G, et al. 2007. Molecular analysis and pathogenicity of the Cladophialophora carrionii complex, with the description of a novel species. Studies in Mycology 58: 219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVay JE, Lukezic FL, English WH, et al. 1965. Controlling Ceratocystis canker of stone fruit trees. California Agriculture 19: 2–4. [Google Scholar]
- Dulymamode R, Minter DW, Peerally A. 1998. Fungi from Mauritius: Rubikia splendida sp. nov., a coelomycete with unusual features. Mycological Research 102: 1242–1244. [Google Scholar]
- Dumortier BC. 1822. Commentationes botanicae. Gasterman-Dieu, Tournay. [Google Scholar]
- Ekanayaka AH, Dissanayake AJ, Jayasiri SC, et al. 2016. Aplosporella thailandica; a novel species revealing the sexual-asexual connection in Aplosporellaceae (Botryosphaeriales). Mycosphere 7: 440–447. [Google Scholar]
- Ellis MB. 1960. Dematiaceous Hyphomycetes. I. Mycological Papers 76: 1–36. [Google Scholar]
- Ellis MB. 1971. Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew, Surrey, UK. [Google Scholar]
- Evans HC, Elliot SL, Hughes DP. 2011. Hidden diversity behind the zombie-ant fungus Ophiocordyceps unilateralis: four new species described from carpenter ants in Minas Gerais, Brazil. PLoS ONE 6: e17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans HC, Minter DW. 1985. Two remarkable new fungi on pine from Central America. Transactions of the British Mycological Society 84: 57–78. [Google Scholar]
- Evans HC, Samson RA. 1982. Entomogenous fungi from the Galapagos islands. Canadian Journal of Botany 60: 2325–2333. [Google Scholar]
- Evans HC, Samson RA. 1984. Cordyceps species and their anamorphs pathogenic on ants (Formicidae) in tropical forest ecosystems II. The Camponotus (Formicinae) complex. Transactions of the British Mycological Society 82: 127–150. [Google Scholar]
- Galindo-Leal C, Câmara IG. 2005. Mata Atlântica: biodiversidade, ameaças e perspectivas. Fundação SOS Mata Atlântica – Belo Horizonte, Conservação Internacional. [Google Scholar]
- Garrido-Benavent I, Pérez-Ortega S. 2015. Unravelling the diversity of European Caliciopsis (Coryneliaceae, Ascomycota): Caliciopsis valentina sp. nov. and C. beckhausii comb. nov., with a worldwide key to Caliciopsis. Mycological Progress 14: 1–10. [Google Scholar]
- Gilgado F, Cano J, Gené J, et al. 2005. Molecular phylogeny of the Pseudallescheria boydii species complex: proposal of two new species. Journal of Clinical Microbiology 43: 4930–4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilgado F, Cano J, Gené J, et al. 2008. Molecular and phenotypic data supporting distinct species statuses for Scedosporium apiospermum and Pseudallescheria boydii and the proposed new species Scedosporium dehoogii. Journal of Clinical Microbiology 46: 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes RR, Glienke C, Videira CIR, et al. 2013. Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 31: 1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M, Guindon S, Gascuel O. 2010. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution 27: 221–224. [DOI] [PubMed] [Google Scholar]
- Gramaje D, Mostert L, Groenewald JZ, et al. 2015. Phaeoacremonium: From esca disease to phaeohyphomycosis. Fungal Biology 119: 759–783. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Sato G, Matsuda T, et al. 2015. Taxonomic revision of Pseudolachnea and Pseudolachnella and establishment of Neopseudolachnella and Pseudodinemasporium gen. nov. Mycologia 107: 383–408 [DOI] [PubMed] [Google Scholar]
- Hernández-Restrepo M, Groenewald JZ, Crous PW. 2015. Neocordana gen. nov., the causal organism of Cordana leaf spot of banana. Phytotaxa 205: 229–238. [Google Scholar]
- Hernández-Restrepo M, Groenewald JZ, Crous PW. 2016. Taxonomic and phylogenetic re-evaluation of Microdochium, Monographella and Idriella. Persoonia 36: 57–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirooka Y, Rossman AY, Samuels GJ, et al. 2012. A monograph of Allantonectria, Nectria, and Pleonectria (Nectriaceae, Hypocreales, Ascomycota) and their pycnidial, sporodochial, and synnematous anamorphs. Studies in Mycology 71: 1–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjortstam K, Larsson K-H, Ryvarden L. 1988. The Corticiaceae of North Europe. Vol. 8 Phlebiella, Thanatephorus – Ypsilonidium. Fungiflora, Oslo, Norway. [Google Scholar]
- Honrubia M, Calonge FD, Demoulin V, et al. 1982. Aportación al conocimiento de los hongos del SE. de España VI: Esclerodermatales, Licoperdales, Nidulariales, Falales, Himenogasterales, Podaxales (Gasteromicetes, Basidiomicetes). Anales de la Universidad de Murcia - Ciencias 38: 101–132. [Google Scholar]
- Hyde KD, Hongsanan S, Jeewon R, et al. 2016. Fungal diversity notes 367–490: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 80: 1–270. [Google Scholar]
- Ito K, Sato K, Ota N. 1957. Studies on the needle cast of Japanese larch. I. Life history of the causal fungus, Mycosphaerella larici-leptolepis. Bulletin of the Government Forest Experiment Station, Meguro 96: 69–88. [Google Scholar]
- Jaklitsch WM, Olariaga I, Voglmayr H. 2016. Teichospora and Teichosporaceae. Mycological Progress 15: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Voglmayr H. 2014. Persistent hamathecial threads in the Nectriaceae, Hypocreales: Thyronectria revisited and re-instated. Persoonia 33: 182–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie C-Y, Zhou Q-X, Zhao W-S, et al. 2013. A new Myrmecridium species from Guizhou, China. Mycotaxon 124: 1–8. [Google Scholar]
- Johnston PR, Seifert KA, Stone JK, et al. 2014. Recommendations on generic names competing for use in Leotiomycetes (Ascomycota). IMA Fungus 5: 91–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten PA. 1876. Symbolae ad mycologiam Fennicam. III. Meddelanden af Societas pro Fauna et Flora Fennica 1: 55–59. [Google Scholar]
- Kile GA. 1993. Plant diseases caused by species of Ceratocystis sensu stricto and Chalara. In: Wingfield MJ, Seifert KA, Webber JF. (eds), Ceratocystis and Ophiostoma: taxonomy, ecology and pathogenicity: 173–183. American Phytopathological Society Press, St. Paul, Minnesota, USA. [Google Scholar]
- Klaubauf S, Tharreau D, Fournier E, et al. 2014. Resolving the polyphyletic nature of Pyricularia (Pyriculariaceae). Studies in Mycology 79: 85–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klich MA. 2002. Identification of common Aspergillus species. 1st ed Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands. [Google Scholar]
- Kobmoo N, Mongkolsamrit S, Tasanathai K, et al. 2012. Molecular phylogenies reveal host-specific divergence of Ophiocordyceps unilateralis sensu lato following its host ants. Molecular Ecology 21: 3022–3031. [DOI] [PubMed] [Google Scholar]
- Kornerup A, Wanscher JH. 1978. Methuen handbook of colour. 3rd ed Eyre Methuen, London. [Google Scholar]
- Kurose D, Evans HC, Djeddour DH, et al. 2009. Systematics of Mycosphaerella species associated with the invasive weed Fallopia japonica, including the potential biological control agent M. polygoni-cuspidati. Mycoscience, 50: 179–189. [Google Scholar]
- Lackner M, De Hoog GS, Yang L, et al. 2014. Proposed nomenclature for Pseudallescheria, Scedosporium and related genera. Fungal Diversity 67: 1–10. [Google Scholar]
- Lakhanpal TN, Mukerji KG. 1981. Taxonomy of the Indian Myxomycetes. Bibliotheca Mycologica 78: 1–531. [Google Scholar]
- Lechat C, Lesaga-Meessen L, Favel A. 2015. A new species of Ijuhya, I. fournieri, from French Guiana. Ascomycete.org 7: 101–104. [Google Scholar]
- Léveillé JH. 1848. Fragments mycologiques. Annales des Sciences Naturelles Botanique 9: 119–144. [Google Scholar]
- Li DW, Chen JY, Wang YX. 2011. Rhexodenticula zhengii sp. nov. from fallen leaves from China. Mycotaxon 117: 287–290. [Google Scholar]
- Li Y, Li HZ, Wang Q, et al. 2007. Flora fungorum sinicorum. Myxomycetes II: 1–131. Science Press, Beijing. [Google Scholar]
- Lombard L, Van der Merwe NA, Groenewald JZ, et al. 2015. Generic concepts in Nectriaceae. Studies in Mycology 80: 189–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasas WFO, Schumann IH. 1972. The genus Pithomyces in South Africa. Bothalia 10: 509–516. [Google Scholar]
- Marincowitz S, Crous PW, Groenewald JZ, et al. 2008. Microfungi occurring on the Proteaceae in the fynbos. CBS Biodiversity Series 7: 1–166. [Google Scholar]
- Mchau GRA, Crous PW, Philips AJL. 1998. Molecular characterization of some Elsinoë isolates from leguminous hosts. Plant Pathology 47: 773–779. [Google Scholar]
- McKenzie EHC, Pinnoi A, Wong MKM, et al. 2002. Two new hyaline Chalara species, and a key to species described since 1975. Fungal Diversity 11: 129–139. [Google Scholar]
- Mel’nik V, Lee S, Groenewald JZ, et al. 2004. New hyphomycetes from Restionaceae in the fynbos: Parasarcopodium ceratocaryi gen. et sp. nov. and Rhexodenticulata elegiae sp. nov. Mycological Progress 3: 19–28. [Google Scholar]
- Melo Santos AM, Cavalcanti DR, Da Silva JMC, et al. 2007. Biogeographical relationships among tropical forests in north-eastern Brazil. Journal of Biogeography 34: 437–446. [Google Scholar]
- Miadlikowska J, Kauff F, Högnabba F, et al. 2014. A multigene phylogenetic synthesis for the class Lecanoromycetes (Ascomycota): 1307 fungi representing 1139 infrageneric taxa, 317 genera and 66 families. Molecular Phylogenetics and Evolution 79: 132–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles AK, Tan YP, Shivas RG, et al. 2015. Novel pathotypes of Elsinoë australis associated with Citrus australasica and Simmondsia chinensis in Australia. Tropical Plant Pathology 40: 26–34. [Google Scholar]
- Miller AN, Huhndorf SM. 2001. Neotropical Ascomycetes 10. New and interesting Cercophora species. Sydowia 53: 211–226. [Google Scholar]
- Milne I, Lindner D, Bayer M, et al. 2009. TOPALi v2: a rich graphical interface for evolutionary analyses of multiple alignments on HPC clusters and multi-core desktops. Bioinformatics 25: 126–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnis AM, Kennedy AH, Grenier DB, et al. 2012. Phylogeny and taxonomic revision of the Planistromellaceae including its coelomycetous anamorphs: contributions towards a monograph of the genus Kellermania. Persoonia 29: 11–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostert L, Groenewald JZ, Summerbell RC, et al. 2005. Species of Phaeoacremonium associated with human infections and environmental reservoirs in infected woody plants. Journal of Clinical Microbiology 43: 1752–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostert L, Groenewald JZ, Summerbell RC, et al. 2006. Taxonomy and pathology of Togninia (Diaporthales) and its Phaeoacremonium anamorphs. Studies in Mycology 54: 1–115. [Google Scholar]
- Mugambi GK, Huhndorf SM. 2009. Molecular phylogenetics of Pleosporales: Melanommataceae and Lophiostomataceae recircumscribed (Pleosporomycetidae, Dothideomycetes, Ascomycota). Studies in Mycology 64: 103–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz JA. 2005. Boletus s.l. (excl. Xerocomus). Fungi Europaei 2. Edizioni Candusso, Alassio. [Google Scholar]
- Nag Raj TR, Kendrick WB. 1976. A monograph of Chalara and allied genera. Wilfred Laurier University Press, Waterloo. [Google Scholar]
- Olariaga I, Salcedo I, Daniëls PP, et al. 2015. Taxonomy and phylogeny of yellow Clavaria species with clamped basidia – Clavaria flavostellifera sp. nov. and the typification of C. argillacea, C. flavipes and C. sphagnicola. Mycologia 107: 104–122. [DOI] [PubMed] [Google Scholar]
- Olson DM, Dinerstein E, Wikramanayake ED, et al. 2001. Terrestrial ecoregions of the world: A new map of life on earth. BioScience 51: 933–938. [Google Scholar]
- Pascoe I, Crous PW, Groenewald JZ. 2007. Sphaceloma banksiicola. Fungal Planet description sheet no. 14. CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands. [Google Scholar]
- Pereira JM, Barreto RW, Ellison C, et al. 2003. Corynespora cassiicola f. sp. lantanae: a potential biocontrol agent for Lantana camara from Brazil. Biological Control 26: 21–31. [Google Scholar]
- Pratibha J, Prabhugaonkar A. 2015. Multi-gene phylogeny of Pithomyces with the sexual morph of P. flavus Berk. & Broome. Phytotaxa 218: 84–90. [Google Scholar]
- Quaedvlieg W, Binder M, Groenewald JZ, et al. 2014. Introducing the Consolidated Species Concept to resolve species in the Teratosphaeriaceae. Persoonia 33: 1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedvlieg W, Verkley GJM, Shin H-D, et al. 2013. Sizing up Septoria. Studies in Mycology 75: 307–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt CA, Kepler RM, Gams W, et al. 2014. Phylogenetic-based nomenclatural proposals for Ophiocordycipitaceae (Hypocreales) with new combinations in Tolypocladium. IMA Fungus 5: 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeshkumar KC, Crous PW, Groenewald JZ, et al. 2016. Resolving the phylogenetic placement of Porobeltraniella and allied genera in the Beltraniaceae. Mycological Progress: doi: 10.1007/s11557-016-1234-4. [Google Scholar]
- Ramaley AW. 1997. New Paraphaeosphaeria species and their anamorphs. Mycotaxon 61: 347–358. [Google Scholar]
- Rao NK, Manoharachary C, Goos RD. 1988. Forest litter Hyphomycetes from Andhra Pradesh, India. III. A new synnematous Hyphomycete. Mycologia 80: 896–899. [Google Scholar]
- Rayner RW. 1970. A mycological colour chart. Commonwealth Mycological Institute and British Mycological Society, Kew, Surrey, UK. [Google Scholar]
- Réblová M. 2006. Molecular systematics of Ceratostomella sensu lato and morphologically similar fungi. Mycologia 98: 68–93. [DOI] [PubMed] [Google Scholar]
- Réblová M. 2013. Two taxonomic novelties in the Sordariomycetidae: Ceratolenta caudata gen. et sp. nov. and Platytrachelan abietis gen. et comb. nov. for Ceratosphaeria abietis. Mycologia 105: 462–475. [DOI] [PubMed] [Google Scholar]
- Réblová M, Fournier J, Štěpánek V. 2016. Two new lineages of aquatic ascomycetes: Atractospora gen. nov. and Rubellisphaeria gen. et sp. nov., and a sexual morph of Myrmecridium montsegurinum sp. nov. Mycological Progress 15: 21. [Google Scholar]
- Réblová M, Réblová K, Štěpánek V. 2015. Molecular systematics of Barbatosphaeria (Sordariomycetes): multigene phylogeny and secondary ITS structure. Persoonia 35: 21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder R, Kelly PL, Hill AA St, et al. 2009. Superelongation disease, caused by Elsinoë brasiliensis, confirmed on cassava in Trinidad and Tobago. Plant Pathology 58: 800. [Google Scholar]
- Reges JTL, Negrisoli MM, Dorigan AF, et al. 2016. Pyricularia pennisetigena and P. zingibericola from invasive grasses infect signal grass, barley and wheat. Pesquisa Agropecuaria Tropical 46: 206–214. [Google Scholar]
- Rodrigues A, Passarini MRZ, Ferro M, et al. 2014. Fungal communities in the garden chamber soils of leaf-cutting ants. Journal of Basic Microbiology 54: 1186–1196. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Mark P, et al. 2012. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman AY, Adams GC, Cannon PF, et al. 2015. Recommendations of generic names in Diaporthales competing for protection or use. IMA Fungus 6: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman AY, Castlebury LA, Farr DF, et al. 2008. Sirococcus conigenus, Sirococcus piceicola sp. nov. and Sirococcus tsugae sp. nov. on conifers: anamorphic fungi in the Gnomoniaceae, Diaporthales. Forest Pathology 38: 47–60. [Google Scholar]
- Rossman AY, Samuels GJ, Rogerson CT, et al. 1999. Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Studies in Mycology 42: 1–248. [Google Scholar]
- Roumeguère C. 1880. Publication des ‘Reliquiae Libertianae’. Pars 1. Revue Mycologique Toulouse 2: 7–24. [Google Scholar]
- Ryvarden L. 2002. Studies in neotropical polypores 17. New neotropical Inonotus species. Synopsis Fungorum 15: 70–80. [Google Scholar]
- Ryvarden L. 2005. The Genus Inonotus, a synopsis. Synopsis Fungorum 21: 1–149. [Google Scholar]
- Samerpitak K, Van der Linde E, Choi H-J, et al. 2014. Taxonomy of Ochroconis, genus including opportunistic pathogens on humans and animals. Fungal Diversity 65: 89–126. [Google Scholar]
- Samson RA, Peterson SW, Frisvad JC, et al. 2011. New species in Aspergillus section Terrei. Studies in Mycology 69: 39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert K, Morgan-Jones G, Gams W, et al. 2011. The genera of Hyphomycetes. CBS Biodiversity Series 9. CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands. [Google Scholar]
- Seifert KA, De Beer ZW, Wingfield MJ. 2013. The Ophiostomatoid fungi: expanding frontiers. In: Seifert KA, De Beer ZW, Wingfield MJ. (eds), The Ophiostomatoid fungi: expanding frontiers. CBS Biodiversity Series 12: 21–46. CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands. [Google Scholar]
- Seifert KA, Okada G. 1990. Taxonomic implications of conidiomatal anatomy in synnematous Hyphomycetes. Studies in Mycology 32: 29–40. [Google Scholar]
- Simmons DR, Kepler RM, Rehner SA, et al. 2015a. Phylogeny of Hirsutella species (Ophiocordycipitaceae) from the USA: remedying the paucity of Hirsutella sequence data. IMA Fungus 6: 345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DR, Lund J, Levitsky T, et al. 2015b. Ophiocordyceps myrmicarum, a new species infecting invasive Myrmica rubra in Maine. Journal of Invertebrate Pathology 125: 23–30. [DOI] [PubMed] [Google Scholar]
- Slippers B, Boissin E, Phillips AJL, et al. 2013. Phylogenetic lineages in the Botryosphaeriales: A systematic and evolutionary framework. Studies in Mycology 76: 31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slippers B, Roux J, Wingfield MJ, et al. 2014. Confronting the constraints of morphological taxonomy in the Botryosphaeriales. Persoonia 33: 155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares DJ, Barreto RW, Braun U. 2009. Brazilian mycobiota of the aquatic weed Sagittaria montevidensis. Mycologia 101: 401–416. [DOI] [PubMed] [Google Scholar]
- Sousa JO, Silva BDB, Alfredo DS, et al. 2014. New records of Geastraceae (Basidiomycota: Phallomycetidae) from Atlantic rainforest remnants and relicts of northeastern Brazil. Darwiniana, Nueva Serie 2: 207–221. [Google Scholar]
- Stamatakis A, Alchiotis N. 2010. Time and memory efficient likelihood-based tree searches on phylogenomic alignments with missing data. Bioinformatics 26: i132–i139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerell BA, Groenewald JZ, Carnegie AJ, et al. 2006. Eucalyptus microfungi known from culture. 2. Alysidiella, Fusculina and Phlogicylindrium genera nova, with notes on some other poorly known taxa. Fungal Diversity 23: 323–350. [Google Scholar]
- Sunhede S. 1989. Geastraceae (Basidiomycotina). Morphology, ecology and systematics with special emphasis on the North European species. Synopsis Fungorum 1: 1–534. [Google Scholar]
- Sutton BC. 1980. The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. CMI, Kew, UK. [Google Scholar]
- Swart HJ. 1988. Australian leaf-inhabiting fungi. XXVI. Some noteworthy Coelomycetes on Eucalyptus. Transactions of the British Mycological Society 90: 279–291. [Google Scholar]
- Swart L, Crous PW, Kang JC, et al. 2001. Differentiation of species of Elsinoë associated with scab disease of Proteaceae based on morphology, symptomatology, and ITS sequence phylogeny. Mycologia 93: 366–379. [Google Scholar]
- Swofford DL. 2003. PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods). Sinauer Associates, Sunderland, Massachusetts. [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. 2013. MEGA 6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thind KS. 1977. The Myxomycetes of India. Indian Council of Agricultural Research, New Delhi. [Google Scholar]
- Trakunyingcharoen T, Lombard L, Groenewald JZ, et al. 2014. Mycoparasitic species of Sphaerellopsis, and allied lichenicolous and other genera. IMA Fungus 5: 391–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trakunyingcharoen T, Lombard L, Groenewald JZ, et al. 2015. Caulicolous Botryosphaeriales from Thailand. Persoonia 34: 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouillas FP, Pitt WM, Sosnowski MR, et al. 2011. Taxonomy and DNA phylogeny of Diatrypaceae associated with Vitis vinifera and other woody plants in Australia. Fungal Diversity 49: 203–223. [Google Scholar]
- Untereiner WA, Gueidan C, Orr MJ, et al. 2011. The phylogenetic position of the lichenicolous ascomycete Capronia peltigerae. Fungal Diversity 49: 225–233. [Google Scholar]
- Van der Aa HA, Vanev S. 2002. A revision of the species described in Phyllosticta. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands. [Google Scholar]
- Videira SIR, Groenewald JZ, Braun U, et al. 2016. All that glitters is not Ramularia. Studies in Mycology 83: 49–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Kolecka A, et al. 2015a. Elucidating the Ramularia eucalypti species complex. Persoonia 34: 50–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videira SIR, Groenewald JZ, Verkley GJM, et al. 2015b. The rise of Ramularia from the Mycosphaerella labyrinth. Fungal Biology 119: 823–843. [DOI] [PubMed] [Google Scholar]
- Vizzini A, Angelini C, Ercole E. 2015. Molecular confirmation of Gyroporus lacteus and typification of Boletus cyanescens. Phytotaxa 226: 27–38. [Google Scholar]
- Voglmayr H, Rossman AY, Castlebury LA, et al. 2012. Multigene phylogeny and taxonomy of the genus Melanconiella (Diaporthales). Fungal Diversity 57: 1–44. [Google Scholar]
- Wikee S, Lombard L, Crous PW, et al. 2013a. Phyllosticta capitalensis, a widespread endophyte of plants. Fungal Diversity 60: 91–105. [Google Scholar]
- Wikee S, Lombard L, Nakashima C, et al. 2013b. A phylogenetic re-evaluation of Phyllosticta (Botryosphaeriales). Studies in Mycology 76: 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield MJ, De Beer C, Visser C, et al. 1996. A new Ceratocystis species defined using morphological and ribosomal DNA sequence comparisons. Systematic and Applied Microbiology 19: 191–202. [Google Scholar]
- Wingfield MJ, Seifert KA, Webber J. 1993. Ceratocystis and Ophiostoma: Taxonomy, Ecology and Pathogenicity. APS Press, St. Paul, Minnesota, USA. [Google Scholar]
- Wood AR, Damm U, Van der Linde EJ, et al. 2016. Finding the missing link: Resolving the Coryneliomycetidae within Eurotiomycetes. Persoonia 37: 37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y. 1998. The Myxomycete biota of Japan. Toyo Shorin Publishing Co., Ltd; Japan. [Google Scholar]
- Yilmaz N, Visagie CM, Houbraken J, et al. 2014. Polyphasic taxonomy of the genus Talaromyces. Studies in Mycology 78: 175–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora JC, Calonge FD, Hosaka K, et al. 2014. Systematics of the genus Geastrum (Fungi: Basidiomycota) revisited. Taxon 63: 477–497. [Google Scholar]
- Zamora JC, Calonge FD, Martín MP. 2015. Integrative taxonomy reveals an unexpected diversity in Geastrum section Geastrum (Geastrales, Basidiomycota). Persoonia 34: 130–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamora JC, Kuhar F, Castiglia V, et al. 2014. On Geastrum argentinum, a forgotten species. Mycoscience 55: 177–182. [Google Scholar]
- Zhao R-L, Desjardin DE, Soytong D, et al. 2006. Proposed synonyms in Cyathus. Mycotaxon 97: 327–335. [Google Scholar]
- Zhao R-L, Jeewon R, Desjardin DE, et al. 2007. Ribosomal DNA phylogenies: Is the current infrageneric classification accurate? Mycologia 99: 385–395. [DOI] [PubMed] [Google Scholar]